Abstract
BACKGROUND & AIMS
Serum fibrosis marker levels during antiviral treatment in chronic hepatitis C (CHC) patients enrolled in the lead-in phase of the Hepatitis C Antiviral Long-term Treatment against Cirrhosis (HALT-C) Trial were determined.
METHODS
Week 0, 24, 48 and 72 serum samples were analyzed for YKL-40, TIMP-1, PIIINP, and HA levels. All 456 CHC patients received peginterferon alfa2a and ribavirin for 24 to 48 weeks.
RESULTS
Mean age was 49.2 years, 71% were male, and 39% had cirrhosis. Lower pretreatment serum YKL-40, TIMP-1, PIIINP, and HA levels were significantly associated with week 20 virological response (p < 0.0001). In multivariate analysis, non-1 CHC genotype, non-black race, prior Interferon monotherapy, and lower baseline serum AST/ALT and log10 YKL-40 levels were independently associated with week 20 virological response. Statistically significant declines in all marker levels were observed at week 72 compared to baseline in the 81 patients with a sustained virological response (SVR) but not in the 72 subjects with breakthrough or relapse. At weeks 24 and 48, significant increases were observed in serum PIIINP and HA levels compared to baseline in virological responders and non-responders (p < 0.0001).
CONCLUSIONS
Pretreatment YKL-40 levels are an independent predictor of initial virological response to pegInterferon and ribavirin treatment. Levels of all 4 serum fibrosis markers significantly decreased in the SVR patients, consistent with reduced hepatic fibrogenesis. Measuring serum fibrosis marker levels before and after antiviral therapy may provide important prognostic information in CHC patients.
Combination pegylated Interferon- ribavirin therapy is associated with a sustained virological response (SVR) in 40 to 50% of patients with chronic hepatitis C (CHC) who have genotype 1 and 70 to 80% of patients who have genotype 2/3 infection 1,2. In addition, as many as 20% of prior nonresponders to standard interferon +/- ribavirin may respond to retreatment with pegylated interferon and ribavirin 3,4. Subjects with a virological response during treatment usually demonstrate improved serum aminotransferase levels as well as reduced hepatic necroinflammatory and fibrosis scores even in the absence of viral clearance 5,6.
In cross-sectional studies, several serum fibrosis markers have been shown to correlate with hepatic fibrosis severity. These markers are direct or indirect reflections of liver fibrogenesis including the resorption of low-density extracellular matrix and the deposition of high density matrix with cross-linked collagen 7. For example, significant associations between fibrosis severity and serum tissue inhibitor of matrix metalloproteinase inhibitor-1 (TIMP-1), amino-terminal peptide of type III procollagen (PIIINP), YKL-40 and hyaluronic acid (HA) levels have been shown in cross-sectional studies of CHC and fatty liver disease patients 8–11. TIMP-1 expression is enhanced by proinflammatory stimuli such as chronic hepatitis C virus infection and can inhibit matrix metalloproteinases involved in fibrolysis resulting in increased hepatic collagen deposition 12,13. Similarly, YKL-40 expression in human liver has been correlated with the rate of fibrosis progression in untreated CHC patients10. In addition, longitudinal studies have demonstrated increases in some serum marker levels with fibrosis progression as well as reductions following successful antiviral treatment of CHC 10, 11, 14. However, there are limited data regarding the utility of serum fibrosis markers in a large group of well-characterized CHC patients treated with pegylated Interferon and ribavirin 15,16. The Hepatitis C Antiviral Long-term Treatment against Cirrhosis (HALT-C) Trial is a prospective, multicenter study of maintenance pegylated interferon in CHC patients with advanced fibrosis who failed to respond to prior treatment. During the lead-in phase of the study, all patients were treated with full dose peginterferonα2a and ribavirin 17. Patients with persistently detectable HCV RNA at week 20 were eligible for randomization while virological responders continued on treatment for a full 48 weeks of combination therapy. The primary aim of this study is to describe the relationship of baseline serum fibrosis markers with virological response during the lead-in phase of HALT-C. In addition, among patients in the responder arm, changes in the serum fibrosis marker levels during 48 weeks of treatment and their relationship to sustained virological response (SVR) were determined. Our hypothesis was that HALT-C Trial patients with virological suppression during treatment would have improved serum fibrosis marker levels while non-responders were expected to have stable or worsening serum fibrosis marker levels.
Methods
Patient population
To be included in the HALT-C Trial, subjects had to have detectable serum HCV RNA and bridging hepatic fibrosis (i.e. Ishak fibrosis score ≥ 3) or cirrhosis on liver biopsy obtained within 12 months of enrollment and a lack of a sustained response to a prior course of interferon with or without ribavirin 3,17. Subjects were retreated with peginterferon alfa-2a and ribavirin for 24 weeks in the “lead-in phase” of the study. Subjects who remained viremic at week 20 were eligible for randomization to maintenance peginterferon versus no treatment for 3.5 years; subjects with undetectable HCV RNA at week 20, as determined by polymerase chain reaction (PCR) assay (Roche Molecular Systems, COBAS Amplicor v 2.0, sensitivity of 100 IU/ml) continued in the “responder arm” of the study and completed a 48 week course of combination antiviral treatment. All HALT-C Trial participants entering the lead-in phase at the University of Michigan, University of Massachusetts/University of Connecticut, Massachusetts General Hospital and the Virginia Commonwealth University, had serum collected for this ancillary study. Serum isolated from whole blood samples was frozen immediately at −80° C and stored at a central repository (Seracare, Washington, DC). The study was approved by local Institutional Review Boards and all patients provided written informed consent.
Serum fibrosis marker assays
Stored serum samples were tested for serum TIMP-1 (normal range: 80 to 500 ng/ml) (Quantikine, R & D Systems, Minneapolis, MN), YKL-40 (normal range: 24 to 125 ug/l) (Metra YKL-40, Quidel Corp, San Diego, CA), and PIIINP (normal range: 2 to 4 ug/l) (UniQ, Orion Diagnostica, Espoo, Finland) using commercially available ELISA’s at the University of Michigan as previously described 8. Samples that exceeded the upper limit of quantitation were retested using a 1:10 dilution. Serum HA levels were determined by an automated liquid-phase immunoassay using the LiBASys Analyzer (Wako Diagnostics, Richmond, VA) (normal range: 10 to 100 ng/ml) 8.
Statistical methods
Individual serum fibrosis marker data were imported into a secure internet-based website maintained by the HALT-C Trial data coordinating center (New England Research Institute, Watertown, MA). Non-normally distributed variables were log-transformed when necessary. Using logistic-regression analysis, we tested the following variables as univariate predictors of week 20 and week 72 virological response: age, gender, patient race, diabetes, HCV genotype, baseline levels of HCV RNA, serum ALT level, AST/ALT ratio, Ishak fibrosis score, prior treatment with Interferon and ribavirin, albumin, platelet count, compliance with pegIFN and ribavirin, and baseline serum YKL-40, HA, PIIINP, and TIMP-1 levels. Variables with p < 0.05 on univariate testing were entered into a multivariate regression analysis. In the first multivariate analysis, we compared 3 groups of lead-in patients according to their week 20 virological response; complete responders (undetectable HCV RNA), partial responders (> 1 log 10 reduction in HCV RNA), and null-responders (< 1 log 10 reduction in HCV RNA). In the second multivariate analysis, we compared the SVR patients with the breakthrough/relapser patients. For continuous variables, odds ratios reflect the relative odds of SVR or week 20 virological response per 1 standard deviation increase in the predictor variable.
RESULTS
Lead-in patient population
At the 4 participating HALT-C Trial sites, 456 patients entered the lead-in phase and had serum samples available at baseline and after 24 weeks of combination antiviral therapy (Table 1). The mean age was 49.2 + 7.1 years, 71% were male, 39% had cirrhosis, and 89% had HCV genotype 1. Because the YKL-40 and HA data were not normally distributed, log transformation was undertaken.
Table 1.
Baseline characteristics of the 456 HALT-C Lead-in patients enrolled in serum fibrosis marker study
| Parameter | Total patients | Wk 20 Complete responder | Wk 20 Partial responder | Wk 20 Non-responder | P value (across 3 groups) |
|---|---|---|---|---|---|
| n | 456 | 169 | 143 | 144 | |
| Age (yrs) | 49.2 + 7.1 | 47.9 + 5.9 | 50.5 + 8.0 | 49.4 + 7.1 | 0.07 |
| Male | 71% | 75% | 73% | 65% | 0.0424 |
| White (non-hispanic) Black or Black + hispanic Hispanic |
74% 20% 4% |
86% 7% 5% |
74% 20% 3% |
60% 36% 3% |
< 0.0001 |
| Lifetime alcohol (drinks) | 17,440 + 27,778 | 18, 173 + 23,813 | 14,576 + 17,620 | 19,434 + 38,275 | 0.72 |
| Lifetime smoking (pack-yrs) | 15 + 17 | 16 + 17 | 16 + 18 | 13 + 16 | 0.19 |
| Diabetes | 26% | 19% | 24% | 35% | 0.001 |
| BMI > 30 kg/m2 | 41% | 41% | 42% | 39% | 0.75 |
| Prior IFN + rib | 68% | 59% | 76% | 71% | 0.0105 |
| Log HCV RNA (IU/ml) | 6.44 + 0.54 | 6.41 + 0.63 | 6.53 + 0.43 | 6.39 + 0.51 | 0.83 |
| Genotype 1 | 89% | 78% | 94% | 97% | < 0.0001 |
| AST (U/l) | 93 + 70 | 96 + 90 | 85 + 52 | 99 + 58 | 0.77 |
| ALT (U/l) | 115 + 81 | 130 + 103 | 104 + 63 | 107 + 63 | 0.0056 |
| AST/ALT ratio | 0.85 + 0.27 | 0.75 + 0.24 | 0.86 + 0.27 | 0.97 + 0.27 | < 0.0001 |
| Alk phosphatase (x ULN) | 0.87 + 0.41 | 0.77 + 0.3 | 0.87 + 0.4 | 0.98 + 0.5 | < 0.0001 |
| Total bilirubin (mg/dl) | 0.77 + 0.43 | 0.77 + 0.43 | 0.74 + 0.39 | 0.79 + 0.46 | 0.80 |
| INR | 1.03 + 0.1 | 1.03 + 0.1 | 1.03 + 0.1 | 1.04 + 0.1 | 0.23 |
| Albumin (g/dl) | 3.87 + 0.36 | 3.98 + 0.31 | 3.88 + 0.37 | 3.72 + 0.37 | < 0.0001 |
| Platelets (1000/mm3) | 174 + 66 | 180 + 59 | 179 + 74 | 162 + 63 | 0..0272 |
| APRI | 1.6 + 1.8 | 1.5 + 2.2 | 1.5 +1.4 | 1.8 + 1.6 | 0.16 |
| Log YKL-40 (ug/l) | 2.39 + 0.44 | 2.20 + 0.38 | 2.46 + 0.44 | 2.55 + 0.43 | < 0.0001 |
| Log HA (ng/ml) | 1.93 + 0.47 | 1.79 + 0.45 | 1.97 + 0.45 | 2.06 + 0.48 | < 0.0001 |
| Serum PIIINP (ug/l) | 6.14 + 2.73 | 5.45 + 2.43 | 6.28 + 2.66 | 6.81 + 2.97 | < 0.0001 |
| Serum TIMP-1 (ng/ml) | 237.9 + 71.0 | 221.2 + 63.9 | 239.0 + 65.0 | 256.7 + 79.8 | < 0.0001 |
| Algorithm score* | −0.7 + 1.39 | −1.13 + 1.22 | −0.68 + 1.33 | −0.24 + 1.5 | < 0.0001 |
| Mean Ishak fibrosis score | 4.0 + 1.2 | 3.8 + 1.2 | 4.1 + 1.2 | 4.2 + 1.3 | 0.0013 |
| Ishak 5/6 (cirrhosis) | 37% | 27% | 40% | 45% | 0.0010 |
| Took > 80% Peg and rib to wk 20 | 59% | 68% | 57% | 50% | 0.0011 |
consists of HA, TIMP-1, and platelets per baseline paper (ref 8).
All results reported as mean +/− standard deviation.
Serum fibrosis markers during the Lead-in phase
At treatment week 20, 169 patients (37%) had undetectable HCV RNA (complete responders), 143 patients (31%) suppressed HCV RNA by at least 1 log 10 (partial responders), and 144 (32%) failed to suppress HCV RNA by 1 log 10 (null-responders) 17. As in the overall HALT-C cohort, complete responders were significantly more likely to be Caucasian (86%), female (25%), non-diabetic (19%), have a non-1 HCV genotype (22%) and have less severe fibrosis compared to partial and null-responders 3. In addition, laboratory markers showed less advanced liver disease in complete responders compared to others including lower pretreatment alkaline phosphatase and AST/ALT ratios and higher serum albumin levels. Finally, complete responders were significantly less likely to have received prior interferon and ribavirin combination therapy and more likely to adhere to the prescribed dose of peginterferon and ribavirin during the lead-in phase of HALT-C (Table 1).
Prior to treatment, mean serum log10 HA, log10 YKL-40, TIMP-1, and PIIINP levels were significantly lower in complete responders compared to partial and non-responders (Table 1). The magnitude of change in the log10 YKL-40 levels between week 0 and 24 was significantly different amongst the HALT-C patients based upon their response to antiviral therapy (p=0.019) (Figure 1A). The mean week 24 log10 YKL-40 levels were significantly reduced in the partial responders compared to baseline (2.20 vs 2.46, p = 0.0008) and even more so in the non-responders (2.20 vs 2.55, p = 0.0005). However, contrary to our expectations, the mean log10 YKL-40 levels did not substantially change during the lead-in phase in the complete virological responders (2.20 vs 2.20, p = 0.53). In contrast, serum TIMP-1 levels at week 24 were significantly lower in the complete responders compared to baseline (210 vs 221, p= 0.048) while they remained unchanged in the partial responders (232 vs 239, p=0.15) and non-responders (257 vs 257, p=0.78) (Figure 1C).
Figure 1.
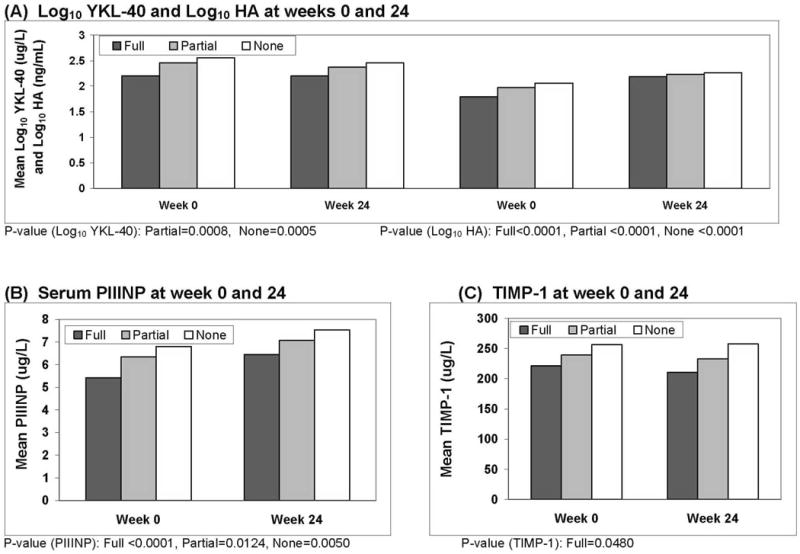
Serum fibrosis marker levels at weeks 0 and 24. A). The log10 YKL-40 levels were significantly lower at week 24 in the partial (p=0.0008) and non-responders (p=0.0005) compared to week 0 but were unchanged in the complete responders. In contrast, the week 24 log10 hyaluronic acid levels were all significantly increased compared to week 0 in all 3 patient groups (p < 0.0001). B). The serum PIIINP levels were significantly higher at week 24 compared to week 0 in all 3 patient groups; complete responders (p < 0.0001), partial responders (p=0.012), non-responders (p=0.005). C) The serum TIMP-1 levels only significantly declined at week 24 compared to week 0 in the complete responders (p=0.048).
NOTE: Only P-values for significant differences, comparing Week 0 to 24, are shown for each marker.
Although the magnitude of change in serum PIIINP levels between weeks 0 and 24 was not significantly different amongst the 3 groups of patients (p=0.43), the PIIINP levels consistently increased in all 3 groups during treatment (Figure 1B). Furthermore, the increase at week 24 was greatest in the complete responders (6.45 vs 5.42, p< 0.0001). Similarly, log10 HA levels significantly increased between weeks 0 and 24 in all 3 patient groups and complete responders experienced the greatest increase during treatment (Figure 1A).
Responder arm patient population
Week 20 complete virological responders were continued on peginterferon and ribavirin for a full 48 weeks and then had additional testing at week 72. In this study, 153 of the 169 eligible week 20 virological responders underwent testing for the 4 serum fibrosis markers at week 48 and week 72 (Table 2). At week 72, the patients were classified as sustained virological responders (SVR) if they had undetectable HCV RNA or as breakthrough/relapsers if they had a breakthrough on treatment or relapsed after week 48. The 81 SVR patients were more likely to have a non-1 HCV genotype, milder hepatic fibrosis, received prior IFN monotherapy, and have a lower pretreatment HCV RNA level than the breakthrough/relapsers (Table 2). However, baseline laboratory markers of disease severity including platelet counts, albumin levels, and total bilirubin levels were similar. In addition, the pretreatment levels of log10 YKL-40, log10 HA, serum PIIINP, and TIMP-1 were similar in the two patient groups.
Table 2.
Baseline characteristics of the week 20 virological responders who received 48 weeks of antiviral treatment
| Parameter | Total patients | W72 SVR patients | W72 Breakthrough/relapser patients | P value between groups |
|---|---|---|---|---|
| N | 153** | 81 | 72 | |
| Age (yrs) | 48.0 + 5.8 | 47.3 + 5.6 | 48.6 + 6.0 | 0.16 |
| Male | 75% | 73% | 78% | 0.48 |
| White (non-hispanic) Black or Black + Hispanic Hispanic | 88% 7% 4% | 86% 7% 5% | 89% 7% 3% | 0.63 |
| Lifetime alcohol (drinks) | 18,938 + 24,561 | 17743 + 25,168 | 20,320 + 23,946 | 0.52 |
| Lifetime smoking (pack-yrs) | 16.3 + 18 | 17.0 + 16.9 | 15.4 + 19.2 | 0.59 |
| Diabetes | 20% | 21% | 19% | 0.81 |
| BMI > 30 kg/m2 | 41% | 38% | 44% | 0.44 |
| Splenomegaly on USN | 31% | 33% | 29% | 0.58 |
| Prior IFN + ribavirin | 58% | 47% | 69% | 0.0054 |
| Log HCV RNA (IU/mL) | 6.43 + 0.64 | 6.24 + 0.72 | 6.64 + 0.45 | 0.0002 |
| Genotype 1 | 78% | 69% | 88% | 0.0080 |
| AST (U/l) | 94 + 88 | 90 + 57 | 99 + 114 | 0.55 |
| ALT (U/l) | 128 + 96 | 131 + 78 | 124 + 112 | 0.64 |
| AST/ALT | 0.75 + 0.25 | 0.71 + 0.19 | 0.79 + 0.29 | 0.0512 |
| Alk phosphatase (x ULN) | 0.76 + 0.30 | 0.76 + 0.32 | 0.76 + 0.29 | 0.95 |
| Total bilirubin (mg/dl) | 0.77 + 0.44 | 0.73 + 0.37 | 0.82 + 0.5 | 0.22 |
| INR | 1.02 + 0.09 | 1.01 + 0.09 | 1.03 + 0.09 | 0.48 |
| Albumin (g/dl) | 3.98 + 0.32 | 4.01 + 0.32 | 3.95 + 0.31 | 0.22 |
| Platelets | 179 + 59 | 177 + 59 | 180 + 58 | 0.77 |
| APRI | 1.5 + 2.2 | 1.3 + 1.0 | 1.7 + 3.1 | 0.35 |
| Log YKL-40 (ug/l) | 2.2 + 0.38 | 2.2 + 0.37 | 2.2 + 0.39 | 0.96 |
| Log HA (ng/ml) | 1.78 + 0.45 | 1.73 + 0.40 | 1.83 + 0.49 | 0.21 |
| Serum PIIINP (ug/l) | 5.44 + 2.46 | 5.19 + 1.94 | 5.72 + 2.92 | 0.18 |
| TIMP-1 (ng/ml) | 221.2 + 63.9 | 224.9 + 70.5 | 218.3 + 55.8 | 0.52 |
| Algorithim score * | −1.14 + 1.22 | −1.16 + 1.18 | −1.12 + 1.27 | 0.87 |
| Ishak 5/6 (cirrhosis) | 25.5% | 22% | 29% | 0.33 |
| Mean Ishak fibrosis | 3.7 + 1.2 | 3.6 + 1.2 | 3.9 + 1.2 | 0.18 |
| Took > 80% peg and ribavirin to wk 20 | 69% | 72% | 65% | 0.40 |
16 of the 169 week 20 virological responders from Table 1 are excluded due to lack of follow-up data
Serum fibrosis markers during the responder arm
As seen in figure 2A, both the SVR and breakthrough/relapser patients had insignificant decreases in log10 YKL-40 levels at weeks 24 and 48 compared to week 0. However, the two groups, SVR and breakthrough/relapsers are significantly different for change in log10YKL-40 levels from baseline to week 72 (p=0.0022) with a greater and significant decline in the SVR group (p< 0.0001). This decline was greater in the SVR patients compared to the breakthrough/relapsers (p < 0.0001). A similar pattern of change in serum TIMP-1 levels was also noted when comparing the SVR and breakthrough/relapser patients (Figure 2D). During treatment, neither group experienced a significant reduction in serum TIMP-1 levels, however, at week 72 a significant decrease was noted between the two groups (p=0.018). As with YKL-40, the decline from baseline was greater and significant in the SVR patients at week 72 compared to baseline (185 vs 225 ng/ml, p < 0.0001) and no significant difference was observed for the breakthrough/relapsers (208 vs 218, p = 0.43).
Figure 2.
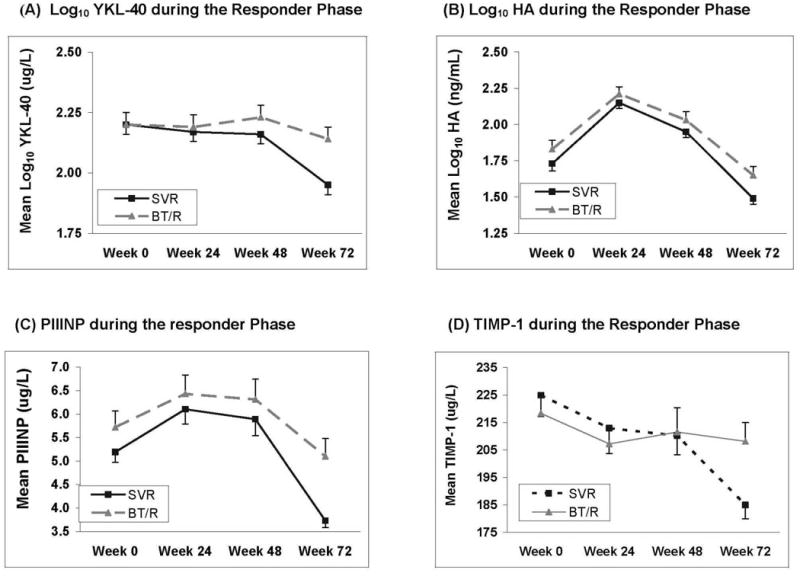
Serum fibrosis marker levels before, during and after 48 weeks of pegylated interferon and ribavirin treatment. (□ = Sustained virological responders; ▲ = Breakthru/relapsers). A). Log10 YKL-40 levels were significantly lower at week 72 compared to baseline in the SVR patients (p=0.0002) and remained unchanged in the breakthrough/relapsers B). Log10 hyaluronic acid levels were significantly lower at week 72 in the SVR patients (p < 0.0001) as well as the breakthrough/relapsers compared to week 0 (p= 0.007). C). Serum PIIINP levels significantly declined at week 72 compared to week 0 in the SVR patients (p = 0.0004) but were unchanged in the breakthrough/relapsers (p= 0.21) D). Serum TIMP-1 levels only significantly declined at week 72 compared to baseline in the SVR patients (p=0.0022).
Significant differences in serum PIIINP levels over time between the SVR and breakthrough/relapser patients were noted as well (Figure 2C). Both groups had significant increases in serum PIIINP levels at week 24 compared to baseline. However, the SVR patients had significant declines in serum PIIINP levels at week 72 compared to week 0 (3.73 vs 5.19, p= 0.0004) while serum PIIINP levels in the breakthrough/relapsers were unchanged (5.1 vs 5.72, p=0.21). Qualitatively, a similar pattern in log10 HA levels was noted over time (Figure 2B). Both the SVR and breakthrough/relapsers experienced significant increases in log10 HA levels at weeks 24 and 48 compared to baseline. However, patients with SVR eventually had significant reductions from baseline in log10 HA levels at week 72 (1.49 vs 1.73, p < 0.0001) and the breakthrough/relapsers also had significant but less dramatic reductions (1.65 vs 1.83, p = 0.007).
Predictors of antiviral response
To determine if pretreatment serum fibrosis marker levels were independent predictors of viral suppression, multivariate logistic regression was used to test the variables with a significant association with week 20 response on univariate testing. In the final model, non-1 HCV genotype (OR= 7.98, 95% CI:3.63, 17.5, p<0.0001), non- Black race (OR= 3.35, 95% CI: 1.68, 6.69, p=0.0006), prior Interferon monotherapy (OR: 1.83, 95% CI: 1,14, 2.93, p=0.012) and lower pretreatment serum AST/ALT (OR=1.53, 95% CI: 1.14, 2.05, p=0.005) and log10 YKL-40 (OR=1.86, 95% CI: 1.43, 2.45 p < 0.0001) levels were independent predictors of week 20 viral clearance (See Appendix for Model equations). The performance of this model (Model 2) for predicting week 20 response can be seen in figure 3 where the c-statistic = 0.80 (95% CI: 0.76, 0.84). In comparison, prior analyses of the entire HALT-C cohort identified prior Interferon monotherapy, non-black race, HCV genotype, HCV viral load, and cirrhosis as predictors of week 20 viral clearance (Model 1) 3. When Model 1 was applied to the current cohort of 453 patients, the c-statistic was 0.68 (95% CI: 0.63, 0.73) and the model was significantly improved when the pretreatment log10 YKL-40 level was added with a c-statistic of 0.76 (95% CI: 0.72, 0.81). Therefore, the baseline log10 YKL-40 levels provide important incremental information regarding the likelihood of a week 20 virological response compared to what was previously known.
Figure 3.
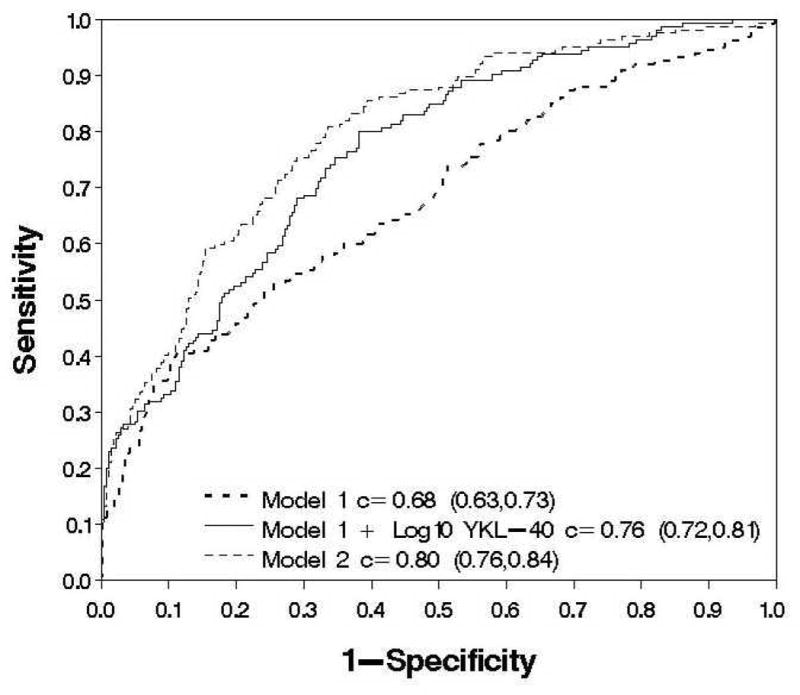
Multivariate models of week 20 virological response in 456 HALT-C Trial patients. Model 1 which was derived from the overall HALT-C cohort consists of non-1 HCV genotype, HCV RNA level, non-black race, prior interferon monotherapy, and cirrhosis had a c-statistic of 0.68 (95% CI: 0.63, 0.73). The addition of baseline log10 YKL-40 levels to Model 1 had a c-statistic of 0.76 (95% CI: 0.72, 0.81) and was significantly better than model 1 alone in predicting week 20 virological response (p= 0.0004). In comparison, Model 2 which was derived from the current dataset and includes baseline log10 YKL-40, serum AST/ALT, non-black race, interferon monotherapy, and non-1 HCV genotype had a c-statistic of 0.80 (95% CI: 0.76, 0.84) and was significantly better at predicting week 20 virological response compared to Model 1 (p < 0.0001) and Model 1 with log10 YKL-40 (p=0.0080)
In order to determine the importance of pretreatment serum fibrosis marker levels on the likelihood of achieving an SVR following 48 weeks of treatment, a multivariate model was constructed. This analysis identified prior interferon monotherapy (OR= 2.33, 95% CI: 1.12, 4.88, p =0.024), baseline levels of log10 HCV RNA (OR=2.42, 95% CI: 1.56, 3.78, P<0.0001), baseline AST/ALT ratio (OR=1.63, 95% CI: 1.04, 2.56, p=0.034) and non-1 HCV genotype-1 (OR=4.39, 95% CI: 1.75, 11.0, p=0.0016) as independent predictors of SVR. The overall c-statistic for this model was 0.77 (95% CI: 0.70, 0.84)
Discussion
In light of the increasing prevalence of chronic liver disease in the general US population, the development of sensitive and specific serum markers that accurately reflect hepatic fibrogenesis and disease stage has the potential to transform and streamline the diagnosis of advanced liver disease 7. Reliable markers of fibrosis may also help facilitate the development of anti-fibrotic therapies 7. The primary aim of this study was to determine the relationship between baseline serum fibrosis marker levels and response to treatment with pegylated Interferon and ribavirin as well as to study the effect of antiviral therapy on the serum marker levels during and after treatment. The pretreatment level of each serum fibrosis marker tested was significantly lower in patients who completely cleared HCV RNA at week 20 compared to partial and non-responders (Figure 1). Because associations between all of these markers with disease severity have been reported previously, the significant relationships observed may, in part, reflect the importance of baseline disease severity in predicting response to antiviral treatment 1, 2, 8. However, these markers may also provide incremental information regarding treatment response as it relates to the presence of profibrogenic signalling and extracellular matrix turnover in the liver 19. In support of this, the pretreatment log10 YKL-40 level was an independent predictor of week 20 response on multivariate analysis. This laboratory parameter also provided incremental ability to predict response beyond HCV genotype and viral load (Figure 3). If confirmed in additional studies of CHC patients, measurement of baseline YKL-40 levels may prove useful in predicting antiviral response.
The magnitude in change of serum log10 YKL-40 levels during the lead-in phase of the HALT-C Trial differed significantly in the 3 virologic response groups. However, only the partial and null-responders experienced significant reductions in YKL-40 levels while the full responder patients had essentially unchanged log10 YKL-40 levels on treatment (Figure 1A). This observation was unexpected since a prior study had demonstrated significant reductions in serum YKL-40 levels in 23 SVR patients following interferon therapy14. However, significant reductions in serum YKL-40 levels occurred in the 44 non-responders and a trend emerged towards lower pretreatment levels in the SVR group compared to non-responders as noted in our study 14. Absence of improvement at week 20 in the serum log10 YKL-40 levels among HALT-C complete responders may, in part, be explained by their significantly lower pretreatment level compared to the other patient groups. In addition, further follow-up of these patients showed significant reductions in YKL-40 levels after sustained viral clearance had been achieved (Figure 2A). In contrast, changes in serum TIMP-1 levels correlated with virological response during the lead-in phase as expected (Figure 1C). However, the magnitude of decline in the serum TIMP-1 levels in the complete responders was substantially lower than that reported in prior studies of standard Interferon and ribavirin treatment which may be a reflection of the more severe hepatic fibrosis in the HALT-C Trial patients 20,21.
Contrary to expectations, significant increases in both serum PIIINP and log10 HA levels were noted in all patients during the lead-in phase of HALT-C (Figure 1A & 1B). The magnitude of increase in both analytes was similar across the 3 patient groups and has previously been reported for HA during standard Interferon treatment 22, 23. Hyaluronic acid is known to be cleared by liver sinusoids and the enhanced hepatic blood flow as a result of ribavirin induced anemia could have led to increased levels during treatment as has been noted in septic patients with a hyperdynamic circulation 27. Alternatively, interferon treatment could reduce endothelial cell uptake of HA in the liver 22. Finally, systemic effects of Interferon to increase hyaluronic acid generation from extrahepatic sites such as adipose tissue could potentially play a role 28. Possible explanations for the increase in serum PIIINP levels during treatment include effects of the antiviral therapy on bone resorption leading to increased extrahepatic release of PIIINP into the systemic circulation 24,25. Despite the unexpected increases during treatment, a significant and consistent decrease in both serum PIIINP and HA levels were observed at week 72 in the patients with viral clearance compared to baseline. These observations suggest that hepatic fibrogenesis is truly reduced in SVR patients compared to breakthrough/relapsers as previously demonstrated in the long-term follow-up of CHC patients with a SVR 29,30.
When analyzing the data from the CHC patients who completed a 48 week course of antiviral therapy, subtle differences in serum YKL-40 and TIMP-1 levels begin to emerge as early as week 24 and become more prominent over time. In addition, significant declines in log10 YKL-40 levels and TIMP-1 levels become apparent after stopping therapy only in SVR patients at week 72 compared to baseline while the breakthrough/relapsers essentially remained unchanged (Figure 2A & 2D). This finding supports other prior studies demonstrating a reduction in fibrogenic markers from liver biopsy samples in interferon treated patients and particularly those who achieve an SVR31. However, measuring the serum markers during antiviral therapy provided minimal incremental information beyond virological response. As with the lead-in cohort, significant increases in serum PIIINP and log10 HA levels were observed in both the SVR and breakthrough/relapsers at weeks 24 and 48 compared to baseline (Figure 2B and 2C). However, as with TIMP-1 and YKL-40, the markers were significantly reduced at week 72 compared to baseline in the SVR group while serum PIIINP levels were essentially unchanged at week 72 in the breakthrough/relapsers and the log10 HA levels declined in the breakthrough/relapsers but to a lesser extent. The lack of an association between the baseline serum fibrosis marker levels with SVR may be due to the fact that the week 20 non-responders were not treated or followed beyond 24 weeks per study protocol. Therefore, the SVR analysis was restricted to a subgroup of patients who all had an on-treatment virological response.
The current study has several strengths and limitations. Distinguishing this study from most prior longitudinal studies of serum fibrosis markers in CHC patients is the large number of well-characterized patients enrolled 10–14. Furthermore, all of the patients received the same antiviral therapy in a prospective clinical trial and had collection and processing of serum samples in a standardized manner at the 4 participating sites. Finally, highly reproducible commercial assays were used for each analyte in a single laboratory with careful attention to quality control. On the other hand, the generalizability of our findings may be limited by the restriction for entry into the HALT-C Trial of patients with bridging fibrosis or cirrhosis. In addition, the requirement for prior non-response to Interferon therapy may have influenced our study results because antiviral treatment can lead to persistently lower serum ALT levels and reduce stellate cell activity; potentially, the hepatic fibrogenesis cascade may be intrinsically altered in prior non-responders compared to treatment naïve patients 31–33. Therefore, additional studies of the serum fibrosis markers in other groups of treatment naïve CHC patients with a broader distribution of disease severity would prove worthwhile to confirm or refute our findings. In addition, comparing the results obtained with the 4 serum fibrosis markers chosen for this study with other proposed serum marker panels in additional patient cohorts may prove worthwhile 15,34.
In summary, lower baseline levels of all 4 serum fibrosis markers tested were significant predictors of week 20 virological response in HALT-C Trial patients receiving pegylated interferon and ribavirin. On multivariate analysis, the pretreatment log10 YKL-40 level was a significant and independent predictor of week 20 virological response suggesting a potential role for measuring this analyte prior to treatment not only to help assess disease severity but also as a potential predictor of response. On the other hand, measurement of the 4 serum markers during combination antiviral treatment in the HALT-C Trial provided limited clinically useful information. Furthermore, the significant increases in serum PIIINP and HA levels during treatment could be misleading or confusing to clinicians and create undue worry for patients. These observations reinforce the need for validating proposed fibrosis markers in future interventional studies of CHC before, during, and after antiviral treatment. Following treatment, statistically significant reductions in all 4 serum markers occurred in patients with an SVR. Therefore, although the utility of these tests is clearly limited during pegylated Interferon and ribavirin treatment, our findings suggest that these serum markers may prove useful in the long-term monitoring of CHC patients. Additional assessment of these markers in the randomized phase of the HALT-C Trial comparing maintenance pegylated interferon to no treatment in relationship to clinical outcomes and disease progression are ongoing.
Acknowledgments
This study was supported by the National Institute of Diabetes & Digestive & Kidney Diseases (contract numbers are listed below). Additional support was provided by the National Institute of Allergy and Infectious Diseases (NIAID), the National Cancer Institute, the National Center for Minority Health and Health Disparities and by General Clinical Research Center grants from the National Center for Research Resources, National Institutes of Health (grant numbers are listed below). Additional funding to conduct this study was supplied by Hoffmann-La Roche, Inc., through a Cooperative Research and Development Agreement (CRADA) with the National Institutes of Health.
In addition to the authors of this manuscript, the following individuals were instrumental in the planning, conduct and/or care of patients enrolled in this study at each of the participating institutions as follows:
University of Massachusetts Medical Center, Worcester, MA: (Contract N01-DK-9-2326) Gyongyi Szabo, MD, Maureen Cormier, RN, Donna Giansiracusa, RN
University of Connecticut Health Center, Farmington, CT: (Grant M01RR-06192) Gloria Borders, RN
Massachusetts General Hospital, Boston, MA: (Contract N01-DK-9-2319, Grant M01RR-01066) Raymond T. Chung, MD, Andrea E. Reid, MD, Atul K. Bhan, MD, Wallis A. Molchen
University of Michigan Medical Center, Ann Arbor, MI: (Contract N01-DK-9-2323, Grant M01RR-00042) Pamela A. Richtmyer, LPN, CCRC, R. Tess Bonham, BS, Mita Ghosh, Leslie Giudotti, Emily Anderson
Virginia Commonwealth University Health System, Richmond, VA: (Contract N01-DK-9-2322, Grant M01RR-00065) Mitchell L. Shiffman, MD, Charlotte Hofmann, RN, Paula Smith, RN
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition, Bethesda, MD: James E. Everhart, MD, MPH, Jay H. Hoofnagle, MD, Leonard B. Seeff, MD, Elizabeth C. Wright, PhD
New England Research Institutes, Watertown, MA: (Contract N01-DK-9-2328) Kristin K. Snow, MSc, ScD, Linda Massey, Teresa M. Curto, MSW, MPH
Armed Forces Institute of Pathology, Washington, DC: Zachary D. Goodman, MD
Data and Safety Monitoring Board Members: (Chair) Gary L. Davis, MD, Guadalupe Garcia-Tsao, MD, Michael Kutner, PhD, Stanley M. Lemon, MD, Robert P. Perrillo, MD
Abbreviations
- ALT
Alanine aminotransferase
- APRI
AST to platelet ratio index
- AST
Aspartate aminotransferase
- BMI
Body mass index
- CHC
Chronic hepatitis C
- HA
Hyaluronic acid
- HALT-C
Hepatitis C antiviral long-term treatment against cirrhosis
- HCV
Hepatitis C virus
- INR
International normalized ratio
- OR
Odds ratio
- PCR
Polymerase chain reaction
- PIIINP
Amino-terminal peptide of type III procollagen
- SVR
Sustained virological response
- TIMP-1
Tissue inhibitor of matrix metalloproteinase-1
Appendix
Models to predict week 20 virological response
Model 1
Log (p/1-p) = −0.43 −0.59*Prior-treatment +1.83*(Genotypes 2 or 3) −0.02*(HCV RNA count >=1.5 mil) + 0.003*(Alt at baseline) −0.71*(Cirrhosis at baseline)
Model 1 with YKL-40
Log (p/1-p) = 3.85 −1.85*(log10YKL-40 at baseline) −0.49*Prior-treatment +2.1*(Genotypes2 or 3) - 0.26*(HCV RNA count >=1.5 mil) +0.003*(ALT at baseline) −0.35*(Cirrhosis at baseline)
Model 2
Log (p/1-p) = 6.47 −1.21* Black race −0.60* Prior-treatment −1.55*(Ast-Alt Ratio at baseline) – 2.08*(Genotype 1) −1.40*(log10YKL-40 at baseline)
Model to predict SVR
Log (p/1-p) = −12.2 +0.85*Prior Treatment +1.39* Log HCVRNA count +1.99*(AST/ALT Ratio at baseline) +1.48*(Genotype 1)
Footnotes
Financial Disclosures
Financial relationships of the authors with Hoffmann-La Roche, Inc., are as follows: R.J. Fontana is on the speaker’s bureau; H.L. Bonkovsky receives research support; R.K. Sterling is a consultant, receives research support, and is on the speaker’s bureau; and A.S. Lok is a consultant. Authors with no financial relationships related to this project are: J.L. Dienstag, D. Naishadham, and G.L. Su.
References
- 1.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferona2a plus ribavirin for chronic hepatitis C virus infection. NEJM. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 2.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomized trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 3.Shifffman ML, DiBisceglie AM, Lindsay KL, et al. Peginterferon Alfa-2a and ribavirin in patients with chronic hepatitis C who failed prior treatment. Gastroenterology. 2004;126:1015–1023. doi: 10.1053/j.gastro.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Poynard T, Schiff E, Terg R, et al. Results from the EPIC3 program: Platelet counts are strong predictors of sustained viral response (SVR) in the re-treatment of previous Interferon- ribavirin non-responders. (Abstract) Gastroenterology. 2008;134(Suppl 1):A772. [Google Scholar]
- 5.Shiffman ML, Hoffman CM, Contos MJ, et al. A randomized controlled trial of maintenance interferon therapy for patients with chronic hepatitis C virus and persistent viremia. Gastroenterology. 1999;117:1164–1171. doi: 10.1016/s0016-5085(99)70402-6. [DOI] [PubMed] [Google Scholar]
- 6.Poynard T, McHutchison J, Manns M, et al. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303–1313. doi: 10.1053/gast.2002.33023. [DOI] [PubMed] [Google Scholar]
- 7.Friedman SL, Rockey DC, Bissell DM. Hepatic fibrosis 2006: Report of the third AASLD Single Topic Conference. Hepatology. 2007;45:242–249. doi: 10.1002/hep.21459. [DOI] [PubMed] [Google Scholar]
- 8.Fontana RJ, Goodman ZD, Dienstag JL, et al. Relationship of serum fibrosis markers with liver fibrosis stage and collagen content in patients with advanced chronic hepatitis C. Hepatology. 2008;47:789–798. doi: 10.1002/hep.22099. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg WMC, Voelker M, Thiel R, et al. Serum markers detect the presence of liver fibrosis: A cohort study. Gastroenterology. 2004;127:1704–1713. doi: 10.1053/j.gastro.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 10.Kamal SM, Turner B, He Q, et al. Progression of fibrosis in hepatitis C with and without schistosomiasis: Correlation with serum markers of fibrosis. Hepatology. 2006;43:771–779. doi: 10.1002/hep.21117. [DOI] [PubMed] [Google Scholar]
- 11.Mehta P, Ploutz-Snyder R, Nandi J, et al. Diagnostic accuracy of serum hyaluronic acid, FIBROSpect II, and YKL-40 for discriminating fibrosis stages in chronic hepatitis C. Am J Gastroenterol. 2008;103:1–9. doi: 10.1111/j.1572-0241.2007.01761.x. [DOI] [PubMed] [Google Scholar]
- 12.Lichtinghagen R, Michels D, Haberkorn CI, et al. Matrix metalloproteinase (MMP-2, MMP-7 and tissue inhibitor of metalloproteinase-1 are closely related to the fibroprolferative process in the liver during chronic hepatitis C. J Hepatol. 2001;34:239–247. doi: 10.1016/s0168-8278(00)00037-4. [DOI] [PubMed] [Google Scholar]
- 13.Lichtinghagen R, Bahr MJ, Wehmeier M, et al. Expression and coordinated regulation of matrix metalloproteinases in chronic hepatitis C and hepatitis C virus-induced liver cirrhosis. Clinical Science. 2003;105:373–82. doi: 10.1042/CS20030098. [DOI] [PubMed] [Google Scholar]
- 14.Saitou Y, Shiraki K, Yamanaka Y, et al. Non-invasive estimation of liver fibrosis and response to interferon therapy by a serum fibrogenesis marker, YKL-40, in patients with HCV-associated disease. World J Gastroenterol. 2005;11:476–481. doi: 10.3748/wjg.v11.i4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poynard T, McHutchison J, Manns M, et al. Biochemical surrogate markers of liver fibrosis and activity in a randomized trial of peginterferon alfa-2b and ribavirin. Hepatology. 2003;38:481–492. doi: 10.1053/jhep.2003.50319. [DOI] [PubMed] [Google Scholar]
- 16.Paradis V, Asselah T, Dargere D, et al. Serum proteome to predict virologic response in patients with hepatitis C treated by pegylated interferon plus ribavirin. Gastroenterology. 2006;130:2189–2197. doi: 10.1053/j.gastro.2006.02.059. [DOI] [PubMed] [Google Scholar]
- 17.Lee WM, Dienstag JL, Lindsay KL, et al. Evolution of the HALT-C trial: Pegylated interferon as a maintenance therapy for chronic hepatitis C in previous Interferon nonresponders. Control Clin Trials. 2004;25:472–492. doi: 10.1016/j.cct.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Lindsay KL, Morishima C, Wright EC, et al. Blunted cytopenias and weight loss: New correlates of virologic null response to re-treatment of chronic hepatitis C. Clin Gastro & Hepatology. 2008;6:234–241. doi: 10.1016/j.cgh.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Malinda KM, Ponce L, Kleinman H, et al. GP38k, a protein synthesized by vascular smooth muscle cells, stimulates directional migration of human umbilical vein endothelial cells. Exp Cell Res. 1999;250:168–173. doi: 10.1006/excr.1999.4511. [DOI] [PubMed] [Google Scholar]
- 20.Mitsuda A, Suou T, Ikuta Y, Kawasaki H. Changes in serum tissue inhibitor of matrix metalloproteinase-1 after interferon alpha treatment in chronic hepatitis C. J Hepatology. 2000;32:666–672. doi: 10.1016/s0168-8278(00)80230-5. [DOI] [PubMed] [Google Scholar]
- 21.Nojgaard C, Johansen JS, Krarup HB, et al. Effect of antiviral therapy on markers of fibrogenesis in patients with chronic hepatitis C. Scan J Gastroenterol. 2003;6:659–665. doi: 10.1080/00365520310002300. [DOI] [PubMed] [Google Scholar]
- 22.Ishibashi K, Kashiwagi T, Ito A, et al. Changes in serum fibrogenesis markers during interferon therapy for chronic hepatitis C. Hepatology. 1996;24:27–31. doi: 10.1053/jhep.1996.v24.pm0008707274. [DOI] [PubMed] [Google Scholar]
- 23.Patel K, Lajoie A, Heaton S, et al. Clinical use of hyaluronic acid as a predictor of fibrosis change in hepatitis C. J Gastroenterol Hepatol. 2003;18:253–257. doi: 10.1046/j.1440-1746.2003.02930.x. [DOI] [PubMed] [Google Scholar]
- 24.Solis-Herruzo JA, Castellano G, Fernandez I, et al. Decreased bone mineral density after therapy with alpha interferon in combination with ribavirin for chronic Hepatitis C. J Hepatology. 2000;33:812–817. doi: 10.1016/s0168-8278(00)80314-1. [DOI] [PubMed] [Google Scholar]
- 25.Framarin L, Avataneo T, Salzedo E, et al. Vertebral osteopenia due to bone marrow hyperplasia during interferon-a and ribavirin therapy for chronic Hepatitis C. Dig Liver Dis. 2003;35:732–734. doi: 10.1016/s1590-8658(03)00418-3. [DOI] [PubMed] [Google Scholar]
- 26.Moreira RO, Balduino A, Martins HSL, et al. Ribavirin, but not interferon alpha-2b, is associated with impaired osteoblast proliferation and differentiation in vitro. Calcified Tissue International. 2004;75:160–168. doi: 10.1007/s00223-004-0175-0. [DOI] [PubMed] [Google Scholar]
- 27.Berg S. Hyaluronan turnover in relation to infection and sepsis. J Int Med. 1997;242:73–77. doi: 10.1046/j.1365-2796.1997.00177.x. [DOI] [PubMed] [Google Scholar]
- 28.Han CY, Subramanian S, Chan CK, et al. Adipocyte-derived serum amyloid A3 and hyaluronan play a role in monocyte recruitment and adhesion. Diabetes. 2007;56:2260–1173. doi: 10.2337/db07-0218. [DOI] [PubMed] [Google Scholar]
- 29.Marcellin P, Boyer N, Gervais A, et al. Long-term histologic improvement and loss of detectable HCV RNA in patients with chronic hepatitis C and sustained response to Interferon-a therapy. Ann Intern Med. 1997;127:875–881. doi: 10.7326/0003-4819-127-10-199711150-00003. [DOI] [PubMed] [Google Scholar]
- 30.Lau DTY, Kleiner DE, Ghany MG, et al. 10-year follow-up after interferon-a therapy for chronic hepatitis C. Hepatology. 1998;28:1121–1127. doi: 10.1002/hep.510280430. [DOI] [PubMed] [Google Scholar]
- 31.Guido M, Rugge M, Chemello L, et al. Liver stellate cells in chronic viral hepatitis: the effect of interferon therapy. J Hepatology. 1996;24:301–307. doi: 10.1016/s0168-8278(96)80008-0. [DOI] [PubMed] [Google Scholar]
- 32.Pradat P, Alberti A, Poynard T, et al. Predictive value of ALT levels for histologic findings in chronic hepatitis C: A European collaborative study. Hepatology. 2002;36:973–7. doi: 10.1053/jhep.2002.35530. [DOI] [PubMed] [Google Scholar]
- 33.Persico M, Persico E, Suozzo R, et al. Natural history of hepatitis C virus carriers with persistently normal aminotransferase levels. Gastroenterology. 2000;118:760–4. doi: 10.1016/s0016-5085(00)70145-4. [DOI] [PubMed] [Google Scholar]
- 34.Cales P, Obert F, Michalak S, et al. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology. 2005;42:1373–81. doi: 10.1002/hep.20935. [DOI] [PubMed] [Google Scholar]


