Abstract
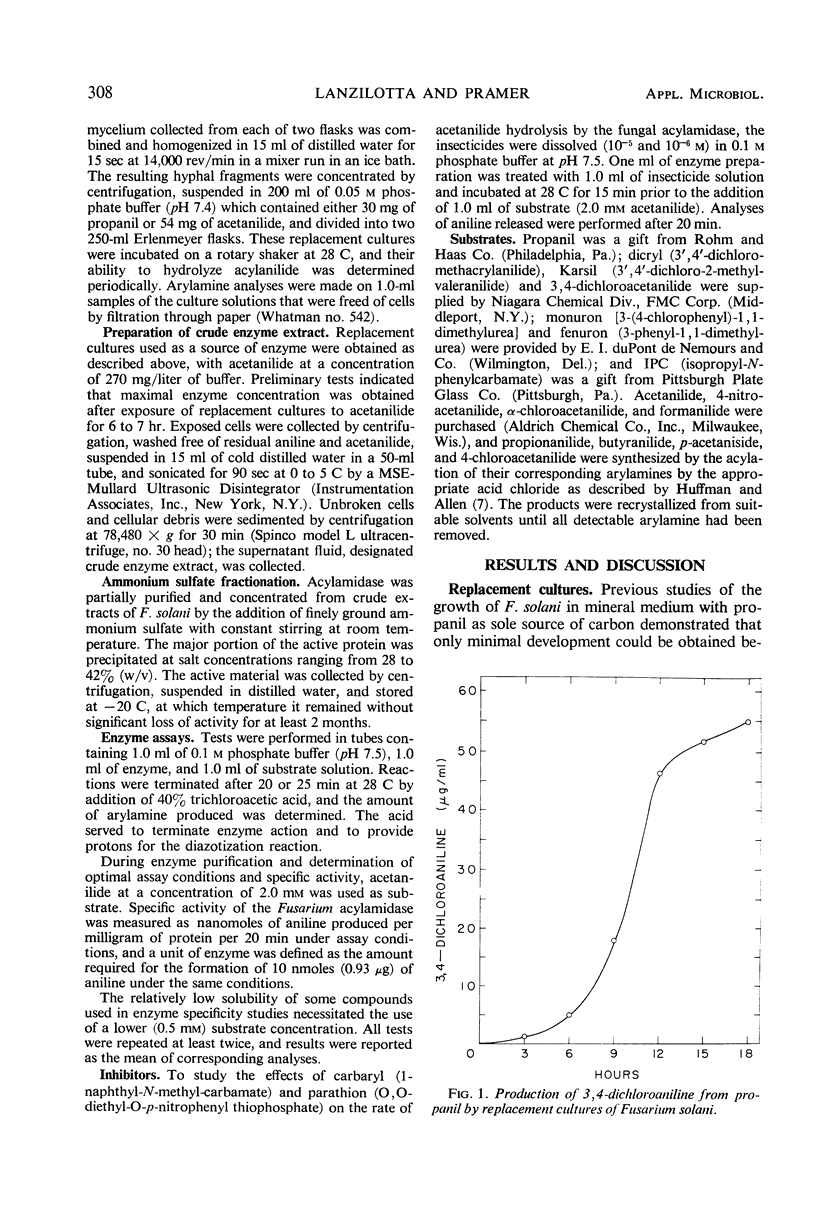
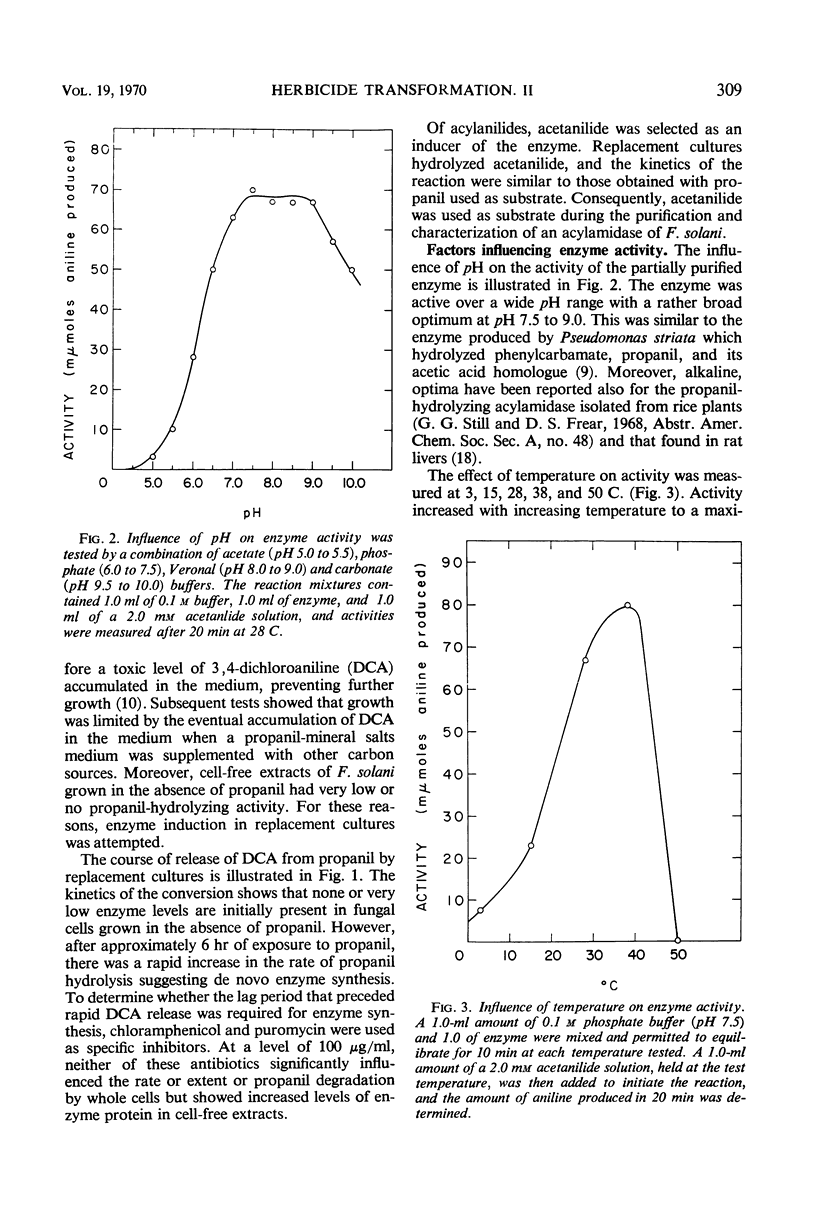
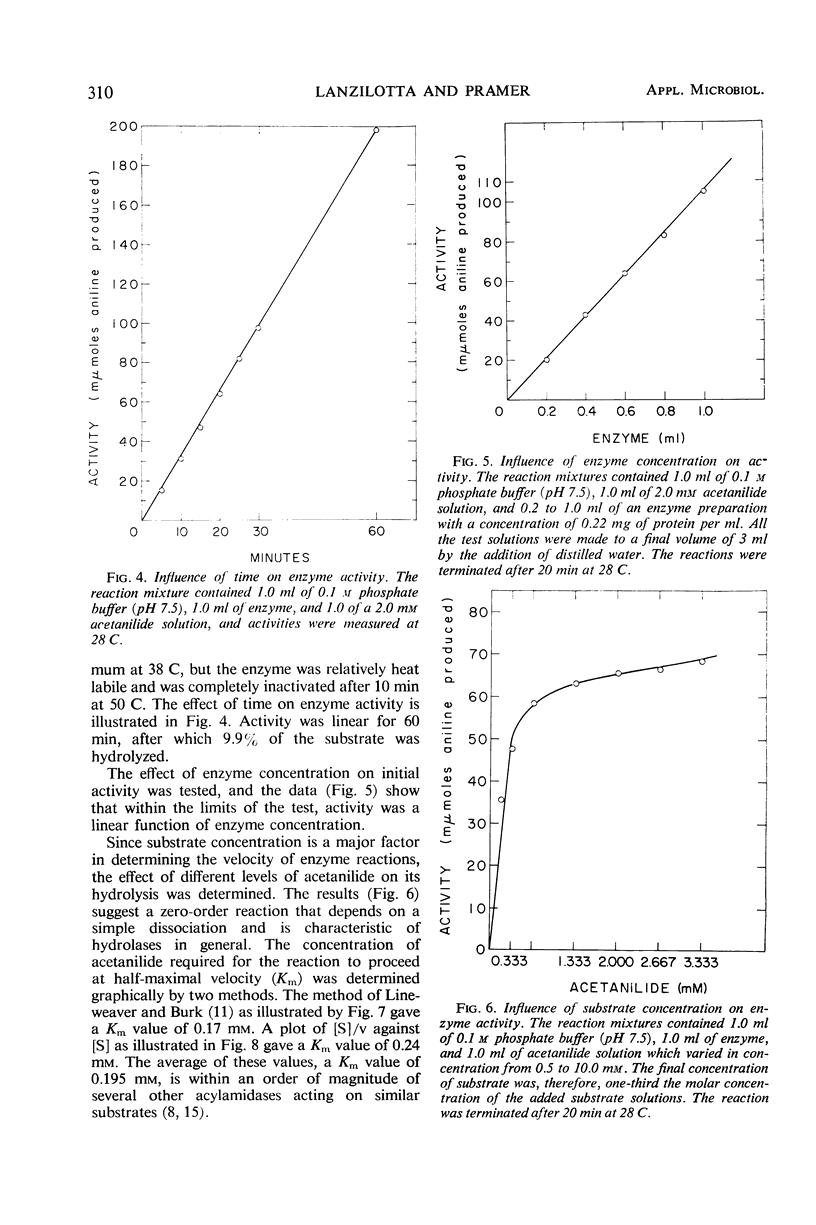
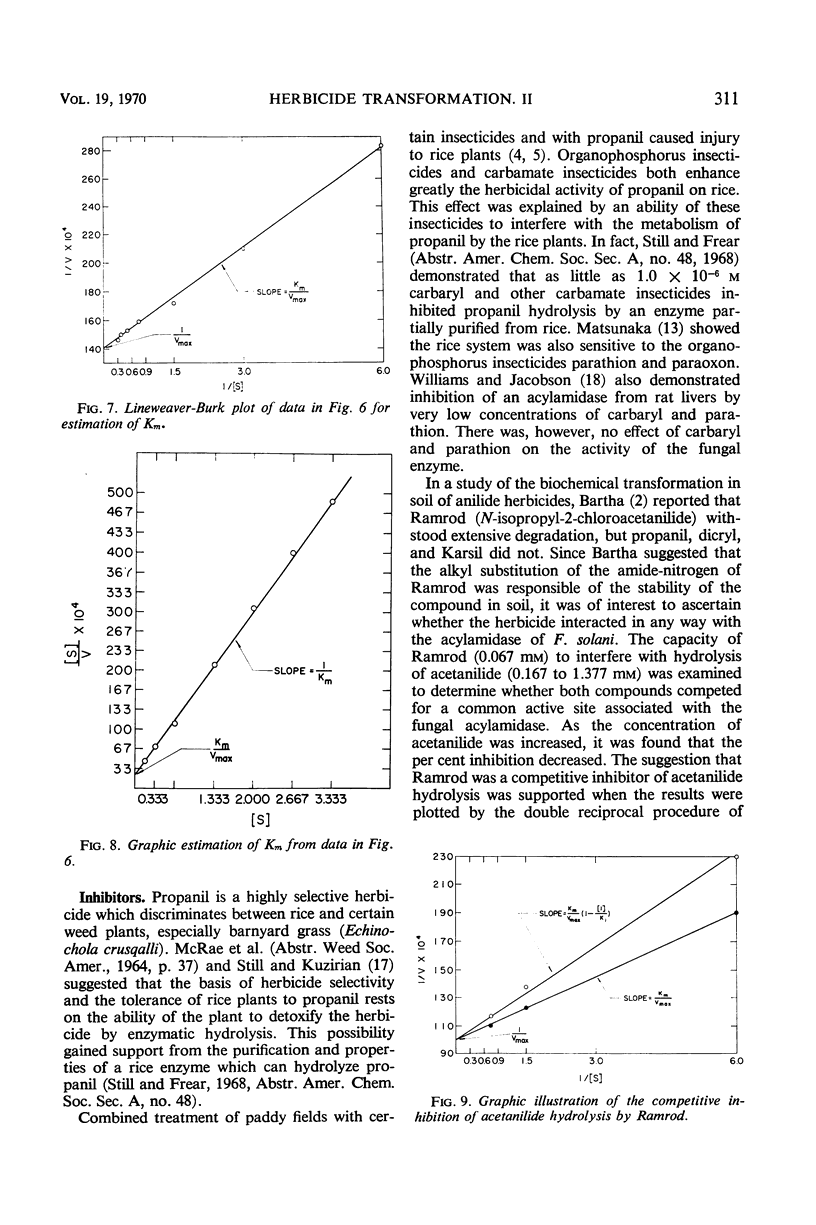
Replacement cultures liberated 3,4-dichloroaniline (DCA) from 3,4-dichloropropionanilide (propanil). The kinetics of the conversion suggest a requirement for de novo enzyme synthesis, but the system was not influenced by chloramphenicol or puromycin. Enzyme activity was detected when acetanilide (Km = 0.195 mm) was used to replace propanil as substrate. Fungal acylamidase (E.C. 3.5.1., an aryl acylamine amidohydrolase) was concentrated by salt precipitation and characterized. The Fusarium solani acylamidase exhibited an optimum at pH 7.5 to 9.0 and was inactivated in 10 min at 50 C. The enzyme was not sensitive to methyl-carbamate or organophosphate insecticides, but the herbicide, Ramrod (N-isopropyl-2-chloroacetanilide), acted as a competitive inhibitor of acetanilide hydrolysis (Ki = 0.167 mm). Hydrolysis rates were decreased by various para substitutions of acetanilide. Chloro substitution in the acyl moiety of acetanilide also reduced the rate of hydrolysis. 3,4-Dichloroacetanilide was less susceptible to enzyme action than acetanilide, but 3,4-dichloropropionanilide was hydrolyzed much more rapidly than propionanilide. The fungal acylamidase was highly specific for N-acetylarylamines. It did not catalyze hydrolysis of formanilide, butyranilide, dicryl, Karsil, fenuron, monuron, or isopropyl-N-phenylcarbamate. It appears to differ from acylamidases that have been isolated from rice, rat liver, chick kidney, and Neurospora.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M. Biodegradation: problems of molecular recalcitrance and microbial fallibility. Adv Appl Microbiol. 1965;7:35–80. doi: 10.1016/s0065-2164(08)70383-6. [DOI] [PubMed] [Google Scholar]
- BIRNBAUM S. M., LEVINTOW L., KINGSLEY R. B., GREENSTEIN J. P. Specificity of amino acid acylases. J Biol Chem. 1952 Jan;194(1):455–470. [PubMed] [Google Scholar]
- GOLDBARG J. A., FRIEDMAN O. M., PINEDA E. P., SMITH E. E., CHATTERJI R., STEIN E. H., RUTENBURG A. M. The colorimetric determination of gamma-glutamyl transpeptidase with a synthetic substrate. Arch Biochem Biophys. 1960 Nov;91:61–70. doi: 10.1016/0003-9861(60)90455-0. [DOI] [PubMed] [Google Scholar]
- JAKOBY W. B. Kynurenine formamidase from Neurospora. J Biol Chem. 1954 Apr;207(2):657–663. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lanzilotta R. P., Pramer D. Herbicide transformation. I. Studies with whole cells of Fusarium solani. Appl Microbiol. 1970 Feb;19(2):301–306. doi: 10.1128/am.19.2.301-306.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEHLER A. H., KNOX W. E. The conversion of tryptophan to kynurenine in liver. II. The enzymatic hydrolysis of formylkynurenine. J Biol Chem. 1950 Nov;187(1):431–438. [PubMed] [Google Scholar]
- Matsunaka S. Propanil hydrolysis: inhibition in rice plants by insecticides. Science. 1968 Jun 21;160(3834):1360–1361. doi: 10.1126/science.160.3834.1360. [DOI] [PubMed] [Google Scholar]
- NIMMO-SMITH R. H. Aromatic N-deacylation by chick-kidney mitochondria. Biochem J. 1960 May;75:284–293. doi: 10.1042/bj0750284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharabi N. E., Bordeleau L. M. Biochemical decomposition of the herbicide N-(3,4-dichlorophenyl)-2-methylpentanamide and related compounds. Appl Microbiol. 1969 Sep;18(3):369–375. doi: 10.1128/am.18.3.369-375.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Still C. C., Kuzirian O. Enzyme detoxication of 3',4'-dichloropropionanilide in rice and barnyard grass, a factor in herbicide selectivity. Nature. 1967 Nov 25;216(5117):799–800. doi: 10.1038/216799a0. [DOI] [PubMed] [Google Scholar]



