Abstract
BACKGROUND
Catheter ablation of the left atrium (LA) is associated with potential collateral injury to surrounding structures, especially the esophagus and the right phrenic nerve (PN).
OBJECTIVES
The purpose of this study was to evaluate the efficacy and feasibility of intrapericardial balloon placement (IPBP) for protection of collateral structures adjacent to the LA.
METHODS
Electroanatomic mapping was performed in porcine hearts using a transseptal endocardial approach in eight swine weighing 40–50kg. An intrapericardial balloon was inflated in the oblique sinus, via percutaneous epicardial access, to displace the esophagus. Similarly, with the balloon positioned in the transverse sinus, IPBP was used to displace the right PN. Esophageal temperature was monitored while endocardial radiofrequency (RF) energy was delivered to the distal inferior PV.
RESULTS
In all cases, balloon placement was successful with no significant effects on hemodynamic function. Balloon inflation increased the distance between the esophagus and posterior LA by 12.3±4.0mm. IPBP significantly attenuated increases in luminal esophageal temperature during endocardial RF application (6.1±2.4°C vs 1.2±1.1°C, p<0.0001). High-output endocardial pacing from the RSPV ostium stimulated PN activity. Following displacement of the right PN with IPBP, PN capture was abolished in 30 of 33 sites (91%).
CONCLUSIONS
These findings demonstrate that in an animal model, IPBP is feasible in the setting of catheter ablation procedures and has the potential to decrease the risk of collateral damage to the esophagus and PN during LA ablation.
Keywords: Catheter ablation, Esophageal injury, Phrenic nerve injury
Pulmonary vein (PV) isolation by endocardial catheter ablation is an established treatment for atrial fibrillation (AF).1 Supplemental linear ablations have improved efficacy in some cases, particularly for persistent AF.2,3 However, a major risk associated with endocardial ablation is collateral damage to tissues near the posterior left atrium (LA), which can have lethal consequences.4 Collateral damage from endocardial ablation may result in atrio-esophageal fistula5 or phrenic nerve (PN) injury.6,7 The risk of collateral damage limits application of radiofrequency (RF) energy in certain areas such as the posterior LA wall and near the right superior PV (RSPV).
Intrapericardial balloon retraction of the LA for prevention of esophageal injury during endocardial catheter ablation was recently reported.8 Balloon inflation9,10 or infusion of air11 into the pericardial space has also been used for protection of the left PN during epicardial VT ablation. Despite these case reports, no systematic assessment of an intrapericardial balloon protection strategy has been performed to demonstrate protection of collateral tissues during LA ablation. The purpose of this study was to assess the efficacy and feasibility of a novel intrapericardial balloon placement (IPBP) technique to displace and protect the esophagus and right PN during LA ablation.
METHODS
This protocol was approved by the UCLA Animal Research Committee and was performed according to institutional guidelines.
Animals and preoperative preparation
Eight pigs weighing 40–50kg were anesthetized with 1.4mg/kg Telazol (intramuscular), and then intubated. Artificial respiration was maintained via endotracheal tube and mechanical ventilator (Summit Medical, Bend, OR). General anesthesia was maintained with inhaled 1.5% to 2.5% isoflurane. Femoral venous and arterial catheters were inserted using modified Seldinger technique. Electrocardiogram (ECG) and arterial pressure were monitored continually during procedures. Luminal esophageal temperature was monitored as previously described,12–14 by inserting an esophageal catheter (Blazer II 4mm tip catheter, EP Technologies/Boston Scientific) orally and advancing to the level of the LA under fluoroscopic guidance. Epicardial access was obtained via subxiphoid puncture using a Tuohy needle as previously described;8 an 8 French sheath (SL0, St. Jude Medical, Inc.) or an 8.5 French deflectable sheath (Agilis™, St. Jude Medical, Inc.) was advanced over a guidewire into the pericardial space. After administration of intravenous heparin, a single transseptal puncture was performed to pass an 8 French SL0 sheath into the LA. A circular mapping catheter (Optima™, St. Jude Medical, Inc.) and a 4mm steerable electrophysiology catheter were used for mapping the LA and PVs endocardially.
Intrapericardial balloon placement
Through the epicardial sheath, Meditech 4cm balloon catheters (diameter: 12mm to 18 mm; Meditech, Boston Scientific) were advanced over a guidewire to the oblique sinus, immediately adjacent to the posterior LA. When positioned between the LA and esophagus, the balloon was inflated with a contrast agent using an insufflator until complete inflation was observed radiographically. In addition to fluoroscopy, electroanatomic (EA) mapping (NavX system, St. Jude Medical, Inc.) was used to determine LA geometry and analyze movement of the common inferior PV (CIPV) antrum and the esophagus, with three-dimensional geometry created by a quadripolar electrophysiology catheter before and after balloon inflation. Mean arterial pressure and heart rate were continuously monitored for the duration of the procedure.
In porcine hearts, the CIPV is the cardiac structure situated closest to the esophagus. A non-irrigated 4mm-tip ablation catheter (Celsius, Biosense Webster) was positioned endocardially at the distal CIPV to deliver RF energy near the esophagus with and without IPBP. The balloon catheter was positioned in the intrapericardial space between the ablation catheter and the esophagus. To verify reproducibility of the findings, high power RF energy was delivered from three different positions in the CIPV. Power was limited to 50W with a maximal tip temperature of 60°C for 30sec with each application. The esophageal catheter was positioned against the anterior esophageal wall, directly opposite the ablation catheter in the distal CIPV, guided by the EA map and fluoroscopic views. Following balloon inflation, CIPV and esophageal catheters were repositioned, if necessary, to minimize the distance between them. The temperature probe’s position was verified by EA map and fluoroscopy in three planes (LAO, RAO and AP) and adjusted frequently to compensate for any movements of the catheter tips. The temperature probe output was time-tagged and annotated to the procedure log every 3 seconds before, during and after RF catheter ablation.
Catheter ablation near the right PVs can cause collateral damage to the right PN, which runs along the pericardium.6,7 To separate the pericardium and the PN, we positioned the intrapericardial balloon adjacent to the anterior aspect of the right superior PV. After creating LA and PV geometry by EA mapping, location of the right PN was identified around the anterior aspect of the RSPV by eliciting diaphragmatic stimulation with bipolar pacing (10mA, 2ms pulse width) from the distal pole of the endocardial catheter. These locations were marked on the EA map. After each balloon placement and inflation, endocardial high-output pacing was repeated at these sites to assess for PN capture.
Statistical analysis
All continuous data are expressed as mean ± standard deviation. The Pearson correlation coefficient was used to quantify the relationship between variables. Changes in esophageal temperature were analyzed initially by repeated measure of ANOVA, followed by Student’s t-test to compare temperatures with- and without- IPBP. A two-sided value of < 0.05 was considered statistically significant.
RESULTS
Approaches for positioning the intrapericardial balloon
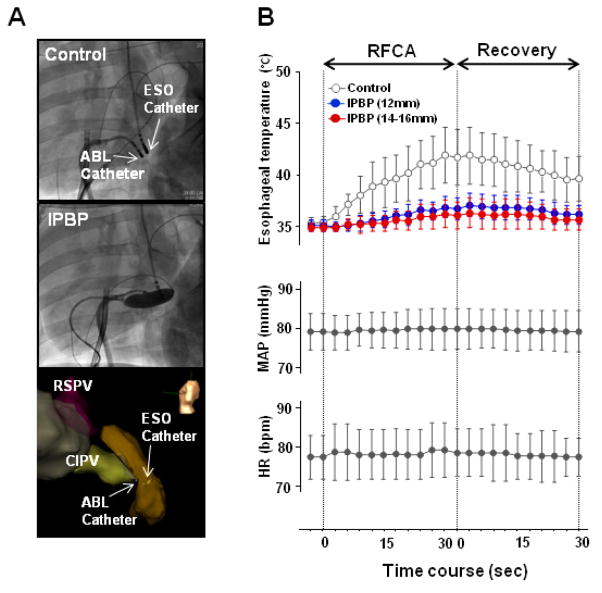
Anesthesia was uneventful in all animals, and all procedures were completed as planned. In all animals, intrapericardial balloons were successfully positioned and inflated in the oblique sinus, adjacent to the posterior LA. Following subxiphoid puncture and access to the pericardial space, two approaches were used to position the balloon near the CIPV ostial region: direct posterior access, and anterior access with deflectable sheath guidance. The Tuohy needle was directed towards the apex of the heart and advancement of the guidewire indicated whether posterior or anterior access was achieved. Movement of the guidewire to the posterior wall allowed a direct posterior approach, in which the balloon catheter was advanced and positioned behind the posterior LA (Figure 1A). In the event of anterior advancement of the guidewire, catheter guidance from the anterior wall towards the posterior LA was facilitated by use of a deflectable sheath (Figure 1B). Balloon placement within the pericardial space is shown in Figure 1C.
Figure 1. Intrapericardial balloon position.
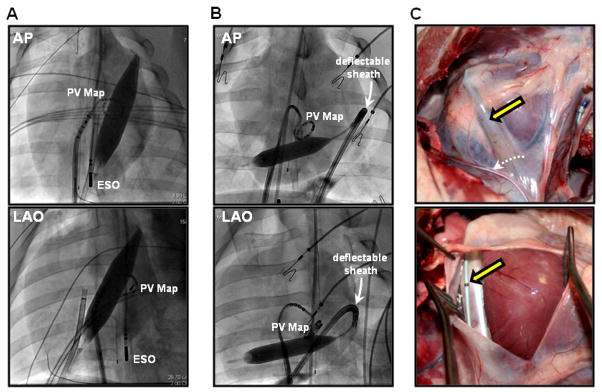
Anterior-posterior (AP) and left anterior oblique (LAO) views of the intrapericardial balloon positioned using different approaches: (A) direct puncture to the posterior pericardial space, (B) anterior access with deflectable sheath guidance. (C) Post-procedural, in vivo photographs of inflated intrapericardial balloon (yellow arrow). Phrenic nerve along on the pericardium is indicated by dashed white arrow. ESO = esophageal mapping catheter; PV map = pulmonary vein mapping catheter.
Esophageal Displacement
Figure 2 and Table 1 show typical results of IPBP on LA-esophageal distance. After pericardial balloon inflation, the average distance between the esophagus and atrial endocardium increased by 12.3 ± 4.0 mm calculated by EA mapping and confirmed fluoroscopically (Table 1). In three animals, simultaneous CIPV and esophageal movement was documented after balloon inflation (Figure 2A–B). During balloon inflation, mean arterial blood pressures and heart rates did not significantly change (80.4 ± 4.4 versus 76.4 ± 6.4mmHg, and 78.5 ± 5.0 versus 79.8 ± 7.2bpm, respectively, p=ns). After balloon inflation, changes in mean arterial pressure (−3.8 ± 6.9 versus −4.3 ± 4.7 mmHg, p=ns) and heart rate (1.4 ± 3.3 versus 1.0 ± 4.6 bpm, p=ns) were not significantly different between small (12–14 mm) and large (16–18 mm) balloons.
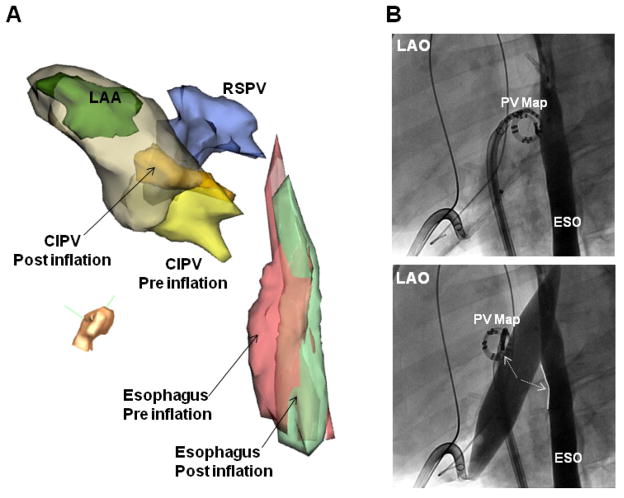
Figure 2. Separation of the left atrium and esophagus during IPBP.
(A) Modified left lateral view of electroanatomic images and (B) Left anterior oblique (LAO) fluoroscopic view of the left atrium and contrast-filled esophagus, before and after balloon inflation in the same animal. In this case, balloon inflation caused simultaneous movement of the CIPV and esophagus away from each other. Esophagus and PV map catheter separation increased with balloon inflation. (C) Right anterior oblique (RAO) view and (D) posterior view of electroanatomic maps in the same porcine heart and esophagus shown in Figure 1A. During balloon inflation within the oblique sinus, the esophagus was markedly flattened (D) and shifted away from the posterior left atrium (LA). The minimum distance between the LA and esophagus increased by 21mm. ESO= esophagus; RSPV= right superior PV; LSPV= left superior PV; CIPV= common inferior PV; LAA= left atrial appendage; EPI = epicardial shell. PV map= pulmonary vein mapping catheter located at the CIPV ostium.
Table 1.
Separation of esophagus and atrium before and after IPBP
| Animal (No.) | approach | Balloon size (mm) | Maximum displacement of esophagus (mm) | Mean pressure (mmHg) | Heart Rate (bpm) | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Control | During IPBP | Control | During IPBP | ||||
| 1 | Anterior, Deflectable sheath | 12 | 10 | 86 | 86 | 82 | 84 |
| 2 | Posterior puncture | 12 | 11 | 76 | 70 | 75 | 76 |
| 3 | Anterior, Deflectable sheath | 12 | 10 | 80 | 86 | 88 | 92 |
| 4 | Anterior, Deflectable sheath | 14 | 8 | 88 | 76 | 78 | 74 |
| 5 | Anterior, Deflectable sheath | 14 | 11 | 79 | 72 | 78 | 82 |
| 6 | Posterior puncture | 16 | 13 | 76 | 77 | 80 | 82 |
| 7 | Anterior, Deflectable sheath | 16 | 14 | 78 | 72 | 72 | 68 |
| 8 | Posterior puncture | 18 | 21 | 80 | 72 | 75 | 80 |
| Average ± SD | 14.3 ± 2.3 | 12.3 ± 4.0 | 80.4±4.4 | 76.4±6.4 | 78.5±5.0 | 79.8±7.2 | |
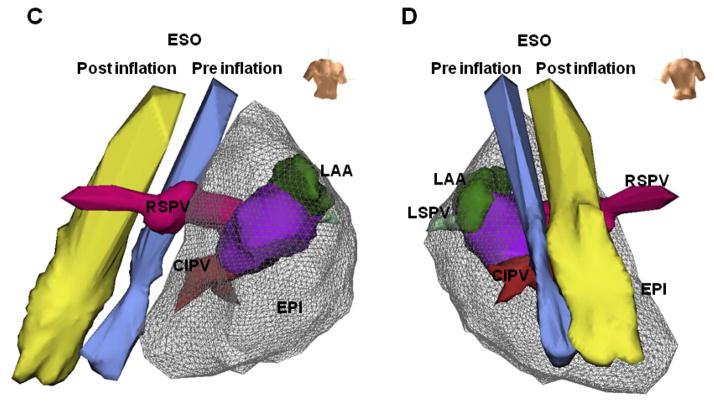
Balloon protection of esophagus during RF energy application
In six animals, an ablation catheter was positioned in the distal CIPV close to the esophageal catheter, and an intrapericardial balloon was successfully placed (12mm in three, 14mm in two, and 16mm in one animal) between these catheters (Figure 3A). During ablation and recovery, esophageal temperature increased by 6.1 ± 2.4°C under control conditions, significantly more than in the presence of IPBP (1.2 ± 1.1°C, p<0.0001, Figure 3B, top). At each time point during ablation and recovery, balloon inflation also significantly attenuated the rise in esophageal temperature (p<0.004). Temperature attenuation during IPBP was not significantly different (p=0.64) between smaller (12mm) and larger balloon sizes (14–16mm). Balloon inflation did not affect mean arterial pressures or heart rates during RFCA application (Figure 3B, bottom).
Figure 3. Luminal esophageal temperature.
(A) Fluoroscopic images of the positions of each electrode without IPBP (control, upper panel) and with balloon inflation (IPBP, middle panel) in the LAO fluoroscopic views. LAO electroanatomic images (lower panel). ESO= esophageal catheter; ABL= endocardial ablation catheter. (B) Time courses of luminal esophageal temperature, mean arterial pressure (MAP) and heart rate (HR) during radiofrequency catheter ablation (RFCA) at the distal CIPV and during recovery. Temperature increased during control conditions (no IPBP, white circles), but were unchanged with balloon inflation. Overall temperature increased significantly in control conditions compared to IPBP groups for all balloon sizes (p<0.0001, ANOVA). At each time point during RFCA application and over 30 seconds of recovery, control temperatures were significantly greater than IPBP groups (p<0.004, Student’s T-test). There were no significant differences in the esophageal temperature between the smaller balloon (12mm, blue circles) and larger balloons (14–16mm, red circles) (p=0.64, ANOVA). During RFCA application and recovery phases, MAP and HR did not change significantly.
Displacement of right phrenic nerve
Figure 4 and Table 2 show typical results of IPBP protection of the right PN. At a total of 33 total sites (4.1 sites/animal), endocardial high-output pacing around the RSPV anterior ostium resulted in PN capture prior to IPBP inflation (Table 2). These sites were tagged as PN capture sites. In all animals, positioning of the balloon catheter for protection of the PN was achieved via a superior approach through the transverse sinus, or via an inferior approach using a deflectable sheath (Figure 4). Slight anatomic differences between animals dictated which approach (superior or inferior) was appropriate. Balloon inflation prevented PN capture at 30 of 33 (91%) tagged sites (see online movie). All animals had normal PN capture at the end of the study, as confirmed by repeat high-output pacing and fluoroscopy after removal of the intrapericardial balloon, demonstrating that inflation of the intrapericardial balloon did not cause PN injury.
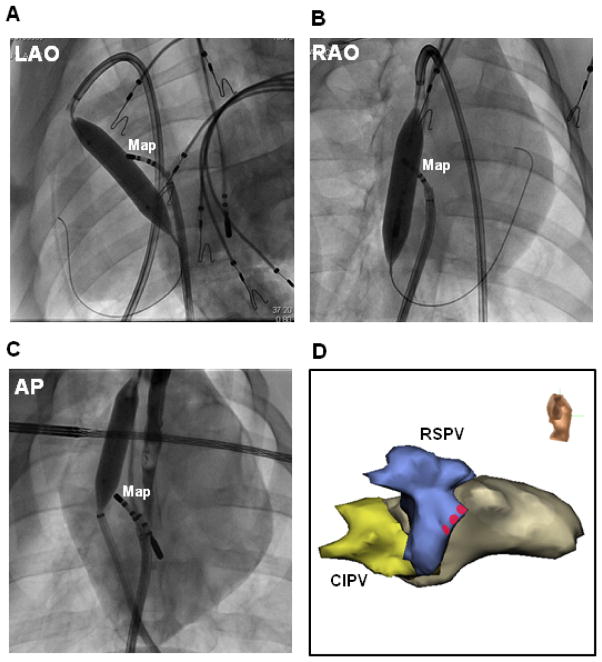
Figure 4. Phrenic nerve protection.
(A) LAO and (B) RAO fluoroscopic images of the same porcine heart shown in Figure 1B. A 16mm by 4cm balloon was inflated near the anterior aspect of the RSPV via the transverse sinus (superior approach). A mapping catheter (Map) was positioned at the endocardial RSPV ostium for high-output pacing. Fluoroscopic AP (C) and right lateral electroanatomic (D) images of a second porcine heart show a 12mm by 4cm balloon inflated near the anterior aspect of the RSPV via an inferior approach. Red circles indicate sites with phrenic nerve capture prior to balloon inflation.
Table 2.
Displacement of phrenic nerve
| Animal (No.) | Balloon size (mm) | Sites with PN capture | Sites with PN displacement | PN injury after IPBP | |
|---|---|---|---|---|---|
| Superior approach | Inferior approach | ||||
| 1 | 12 | 5 | 5 | 5 | None |
| 2 | 12 | 4 | 4 | 4 | None |
| 3 | 12 | 4 | 3 | 4 | None |
| 4 | 14 | 3 | 3 | 3 | None |
| 5 | 14 | 5 | 3 | 0 | None |
| 6 | 16 | 3 | 3 | 2 | None |
| 7 | 16 | 4 | 4 | 4 | None |
| 8 | 18 | 5 | 3 | 4 | None |
| Total | 33 | 28 | 26 | ||
PN = phrenic nerve
Complications
There were no cases of hemopericardium, cardiac tamponade, or intrapericardial balloon rupture. No other acute complications were observed.
DISCUSSION
To our knowledge, this is the first in vivo animal study to systematically examine the feasibility and efficacy of intrapericardial balloon placement (IPBP) in the setting of LA ablation. The major findings of this study are: (i) IPBP was technically feasible in this animal model and did not result in acute complications; (ii) IPBP effectively displaced vulnerable collateral tissues, and (iii) IPBP prevented significant esophageal heating from endocardial RF delivery, as well as PN capture from endocardial pacing.
Esophageal Displacement and Protection
Over the past decade, the use of catheter ablation for the treatment of AF has increased.15 In an effort to improve procedural outcomes, extensive LA lesion sets have been proposed.2,3 Esophageal injury and atrio-esophageal fistula are potentially life-threatening complications that may arise from RF energy applied to the posterior LA wall.5 One recent study showed that an esophageal ulcer was observed by endoscopy 1 to 3 days post AF ablation in 36% of patients without luminal esophageal temperature monitoring.16 Multiple strategies have been proposed to detect esophageal heating and to protect the esophagus during ablation. The esophagus can be filled with radio-opaque contrast media to determine its position fluoroscopically and limit RF application adjacent to the esophagus.17 Temperature monitoring within the esophagus has also been used to detect esophageal heating during ablation12,16 Active protection techniques have also been proposed, including an esophageal cooling system18 and mechanical displacement of the esophagus away from the LA using an endoscopic transesophageal probe.19,20
More recently, other strategies aimed at increasing the distance between the heart and collateral structures have been proposed. Methods using intrapericardial air and saline11 or an intrapericardial balloon9 to separate the heart from the PN during ablation have been described. Our group published a case report describing the use of an intrapericardial balloon to protect the esophagus in a patient undergoing repeat AF ablation;8 the findings suggested that IPBP may allow safe and effective delivery of RF energy to the targeted tissues. The present study is the first systematic experimental evaluation of such an intrapericardial balloon protection (IPBP) technique.
These data demonstrate that the IPBP approach is technically feasible in porcine hearts. Appropriate balloon alignment was achieved using one of two approaches, and balloon position within the pericardial space was stable after balloon inflation. In all animals, the esophagus was displaced from its original position by intrapericardial balloon inflation. In some cases, both the esophagus and the PV were displaced. Nevertheless, no significant hemodynamic consequences resulted from impaired LA filling, and no significant complications were observed. Optimization of size and shape for intrapericardial balloons warrants further study.
The mechanism of esophageal injury or fistula during RF ablation is not completely understood. Thermal injury is the most likely cause (with an area of necrosis surrounded by inflammatory cells), although an ischemic mechanism has also been proposed.5,21,22 Shorter distance and increased contact force might increase the risk of esophageal injury.23 The IPBP method has potential advantages for the prevention of esophageal injury during RF application. In addition to displacement of the esophagus, the presence of the intervening liquid-filled balloon likely conferred additional shielding of surrounding tissues from RF energy. For difficult balloon catheter approaches, the deflectable sheath proved valuable for precise positioning in the pericardial space.
Right Phrenic Nerve Displacement
The PN has an epicardial course (Figure 1C), and PN injury has been reported after percutaneous catheter ablation procedures for accessory pathways,24 inappropriate sinus tachycardia,25 and AF.6,7,26 The anterior wall of the RSPV is less than 2mm from the right phrenic nerve in 32% of subjects based on autopsy data.27 The reported prevalence of PN injury as a complication of AF ablation is 0.11% to 0.48%.7,28 Methods to prevent left PN injury during epicardial catheter ablation for ventricular tachycardia have been proposed.9–11 To our knowledge, there have been no reports describing an effective displacement technique of the right PN in the setting of LA ablation. Our experimental observations suggest that two balloon approaches (superior and inferior) with a deflectable sheath appear to be feasible for displacement and protection of the right PN in this animal model. In addition, the balloon catheter was easily repositioned from an esophageal protection site in the oblique sinus, to the anterior aspect of the RSPV for protection of the right PN, suggesting that IPBP provides a flexible approach for collateral protection during LA ablation.
Limitations
The present study was performed in a limited number of porcine hearts, which have anatomic differences from human hearts. The porcine esophagus is usually positioned slightly further away from the posterior LA than in humans. However, it can be argued that the closer proximity between the esophagus and posterior LA in humans suggests that IPBP may be even more useful in humans than in our animal model. For this technique, epicardial access is required, which is not performed in all ablation centers and carries its own inherent risks. Our studies used an impedance-based electroanatomical mapping system. Balloon inflation may have caused a small change in the thoracic impedance field which could alter the electroanatomical map. However, no changes in cardiac geometry (PVs, left appendage, and left atrial wall) were detected, and catheter position was confirmed by fluoroscopic imaging. Our study did not address the minimum volume or ideal shape of the balloon for attenuating the rise in temperature in the esophagus. Further studies are warranted to elucidate the potential significance of IPBP in a clinical setting and to optimize balloon shapes and sizes.
Conclusions
Our experimental observations suggest that the IPBP concept is feasible and might decrease the risk of collateral damage that would otherwise occur during RF catheter ablation of the LA. Deflectable sheath guidance may facilitate the optimal positioning of the intrapericardial balloon. If the results in this animal study are borne out in humans, the IPBP method might one day serve as a tool to improve the safety and efficacy and catheter ablation procedures for LA arrhythmias such as atrial fibrillation.
Supplementary Material
Acknowledgments
Support: Supported by NIH RO1-HL084261 and HL067647 grants to Dr. Shivkumar
We express our gratitude to Dr. Tara Bourke, Nikhil Sunny Patel and Kelly A. Walker for their assistance with porcine experiments.
Abbreviations and Acronyms
- AF
Atrial Fibrillation
- IPBP
intrapericardial balloon protection
- PV
pulmonary vein
- CIPV
ommon inferior pulmonary vein
- RSPV
right superior pulmonary vein
- PN
phrenic nerve
Footnotes
Disclosure: The University of California, Los Angeles has intellectual property relating to this area of work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 2.Jais P, Hocini M, Hsu LF, et al. Technique and results of linear ablation at the mitral isthmus. Circulation. 2004;110:2996–3002. doi: 10.1161/01.CIR.0000146917.75041.58. [DOI] [PubMed] [Google Scholar]
- 3.Hocini M, Jais P, Sanders P, et al. Techniques, evaluation, and consequences of linear block at the left atrial roof in paroxysmal atrial fibrillation: a prospective randomized study. Circulation. 2005;112:3688–3696. doi: 10.1161/CIRCULATIONAHA.105.541052. [DOI] [PubMed] [Google Scholar]
- 4.Cappato R, Calkins H, Chen SA, et al. Prevalence and causes of fatal outcome in catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2009;53:1798–1803. doi: 10.1016/j.jacc.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 5.Cummings JE, Schweikert RA, Saliba WI, et al. Brief communication: atrial-esophageal fistulas after radiofrequency ablation. Ann Intern Med. 2006;144:572–574. doi: 10.7326/0003-4819-144-8-200604180-00007. [DOI] [PubMed] [Google Scholar]
- 6.Bai R, Patel D, Di Biase L, et al. Phrenic nerve injury after catheter ablation: should we worry about this complication? J Cardiovasc Electrophysiol. 2006;17:944–948. doi: 10.1111/j.1540-8167.2006.00536.x. [DOI] [PubMed] [Google Scholar]
- 7.Sacher F, Monahan KH, Thomas SP, et al. Phrenic nerve injury after atrial fibrillation catheter ablation: characterization and outcome in a multicenter study. J Am Coll Cardiol. 2006;47:2498–2503. doi: 10.1016/j.jacc.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 8.Buch E, Nakahara S, Shivkumar K. Intra-pericardial balloon retraction of the left atrium: a novel method to prevent esophageal injury during catheter ablation. Heart Rhythm. 2008;5:1473–1475. doi: 10.1016/j.hrthm.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buch E, Vaseghi M, Cesario DA, et al. A novel method for preventing phrenic nerve injury during catheter ablation. Heart Rhythm. 2007;4:95–98. doi: 10.1016/j.hrthm.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan R, Cano O, Ho SY, et al. Characterization of the phrenic nerve course within the epicardial substrate of patients with nonischemic cardiomyopathy and ventricular tachycardia. Heart Rhythm. 2009;6:59–64. doi: 10.1016/j.hrthm.2008.09.033. [DOI] [PubMed] [Google Scholar]
- 11.Matsuo S, Jais P, Knecht S, et al. Images in cardiovascular medicine. Novel technique to prevent left phrenic nerve injury during epicardial catheter ablation. Circulation. 2008;117:e471. doi: 10.1161/CIRCULATIONAHA.107.748335. [DOI] [PubMed] [Google Scholar]
- 12.Redfearn DP, Trim GM, Skanes AC, et al. Esophageal temperature monitoring during radiofrequency ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2005;16:589–593. doi: 10.1111/j.1540-8167.2005.40825.x. [DOI] [PubMed] [Google Scholar]
- 13.Perzanowski C, Teplitsky L, Hranitzky PM, et al. Real-time monitoring of luminal esophageal temperature during left atrial radiofrequency catheter ablation for atrial fibrillation: observations about esophageal heating during ablation at the pulmonary vein ostia and posterior left atrium. J Cardiovasc Electrophysiol. 2006;17:166–170. doi: 10.1111/j.1540-8167.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- 14.Cummings JE, Schweikert RA, Saliba WI, et al. Assessment of temperature, proximity, and course of the esophagus during radiofrequency ablation within the left atrium. Circulation. 2005;112:459–464. doi: 10.1161/CIRCULATIONAHA.104.509612. [DOI] [PubMed] [Google Scholar]
- 15.Noheria A, Kumar A, Wylie JV, Jr, et al. Catheter ablation vs antiarrhythmic drug therapy for atrial fibrillation: a systematic review. Arch Intern Med. 2008;168:581–586. doi: 10.1001/archinte.168.6.581. [DOI] [PubMed] [Google Scholar]
- 16.Singh TM, d’Avila A, Doshi SK, et al. Esophageal Injury and Temperature Monitoring During Atrial Fibrillation Ablation. Circ Arrhythmia Electrophysiol. 2008;1:162–168. doi: 10.1161/CIRCEP.107.789552. [DOI] [PubMed] [Google Scholar]
- 17.Yamane T, Matsuo S, Date T, et al. Visualization of the esophagus throughout left atrial catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17:105. doi: 10.1111/j.1540-8167.2005.00286.x. [DOI] [PubMed] [Google Scholar]
- 18.Berjano EJ, Hornero F. A cooled intraesophageal balloon to prevent thermal injury during endocardial surgical radiofrequency ablation of the left atrium: a finite element study. Phys Med Biol. 2005;50:N269–279. doi: 10.1088/0031-9155/50/20/N03. [DOI] [PubMed] [Google Scholar]
- 19.Herweg B, Johnson N, Postler G, et al. Mechanical esophageal deflection during ablation of atrial fibrillation. Pacing Clin Electrophysiol. 2006;29:957–961. doi: 10.1111/j.1540-8159.2006.00470.x. [DOI] [PubMed] [Google Scholar]
- 20.Chugh A, Rubenstein J, Good E, et al. Mechanical displacement of the esophagus in patients undergoing left atrial ablation of atrial fibrillation. Heart Rhythm. 2009;6:319–322. doi: 10.1016/j.hrthm.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Dixit S, Marchlinski FE. How to recognize, manage, and prevent complications during atrial fibrillation ablation. Heart Rhythm. 2007;4:108–115. doi: 10.1016/j.hrthm.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Quintana D, Cabrera JA, Climent V, et al. Anatomic relations between the esophagus and left atrium and relevance for ablation of atrial fibrillation. Circulation. 2005;112:1400–1405. doi: 10.1161/CIRCULATIONAHA.105.551291. [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa H, Ikeda A, Shah DC, et al. Role of contact force in esophageal injury during left atrial radiofrequency ablation. Heart Rhythm. 2008;15:S68. [Google Scholar]
- 24.Rumbak MJ, Chokshi SK, Abel N, et al. Left phrenic nerve paresis complicating catheter radiofrequency ablation for Wolff-Parkinson-White syndrome. Am Heart J. 1996;132:1281–1285. doi: 10.1016/s0002-8703(96)90477-9. [DOI] [PubMed] [Google Scholar]
- 25.Durante-Mangoni E, Del Vecchio D, Ruggiero G. Right diaphragm paralysis following cardiac radiofrequency catheter ablation for inappropriate sinus tachycardia. Pacing Clin Electrophysiol. 2003;26:783–784. doi: 10.1046/j.1460-9592.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- 26.Lee BK, Choi KJ, Kim J, et al. Right phrenic nerve injury following electrical disconnection of the right superior pulmonary vein. Pacing Clin Electrophysiol. 2004;27:1444–1446. doi: 10.1111/j.1540-8159.2004.00652.x. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez-Quintana D, Cabrera JA, Climent V, et al. How close are the phrenic nerves to cardiac structures? Implications for cardiac interventionalists. J Cardiovasc Electrophysiol. 2005;16:309–313. doi: 10.1046/j.1540-8167.2005.40759.x. [DOI] [PubMed] [Google Scholar]
- 28.Cappato R, Calkins H, Chen SA, et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111:1100–1105. doi: 10.1161/01.CIR.0000157153.30978.67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.