Abstract
Total joint arthroplasty has revolutionized the treatment of arthritic and degenerative conditions for many joints in the body; however, wear debris is continuously generated with day-to-day use of an artificial joint. Excessive production of wear by-products induces a foreign body and chronic inflammatory reaction that accelerates periprosthetic bone destruction and inhibits bone formation. The specific biologic reaction is dependent on the type, amount, and characteristics of the byproducts of wear, along with individual genetic variations. For polymeric and ceramic particles, the inflammatory reaction is generally nonspecific and nonimmune; however, with metallic by-products, a type IV, T lymphocyte-mediated, antigen-dependent immune reaction can occur in some patients. The production of proinflammatory cytokines, chemokines, reactive oxygen species, and other mediators is upregulated by wear particles. Animal models have shown that the biologic reaction to wear particles is systemic in nature, not a localized event. Mechanical stimuli and the presence of endotoxin also appear to be important. Efficacious biologic treatments of periprosthetic osteolysis are not yet available. Research continues with the hope that viable strategies for preventing and treating particle-induced osteolysis will be introduced in the future, thus mitigating the need for revision surgery.
Despite the advent of more modern implants and novel bearing surfaces for joint arthroplasty, periprosthetic osteolysis and adverse tissue responses to the by-products of wear are still of great interest to arthroplasty sur geons.1 This statement is realistic based on several facts. (1) Highly cross-linked polyethylene was introduced in North America approximately 10 years ago. There are millions of total hip replacements with conventional polyethylene that may have to be revised because of progressive wear and osteolysis. Many total knee replacements performed during the previous decade and beyond often used very thin polyethylene inserts that were sterilized in air and had suboptimal locking mechanisms and designs; this has led to increased wear. (2) As the population ages and continues to be physically active, implants for joint arthroplasty will be subjected to higher stresses for longer periods. (3) Hard-on-hard bearings have created a new set of concerns. Metal-on-metal articulations may be associated with the increased production of metal ions, wear particulates, corrosion by-products, and adverse tissue reactions.2 Ceramic-on-ceramic articulations may be associated with breakage, chipping, squeaking, edge loading, and stripe wear.3 These and other tribologic-related issues underscore the continued importance of wear by-products. (4) With the increased use of larger femoral heads to decrease the dislocation rate of hip replacements, there is some evidence (although controversial) that volumetric wear of polyethylene may be increased.4 These larger femoral heads often use thinner polyethylene liners than were previously used. (5) Disk replacement in the spine and other joint replacements are in their infancy compared with hip and knee arthroplasty. Wear particle-related inflammation and osteolysis have already been noted in these joints.5,6
How Do Cellular By-products Activate Cells?
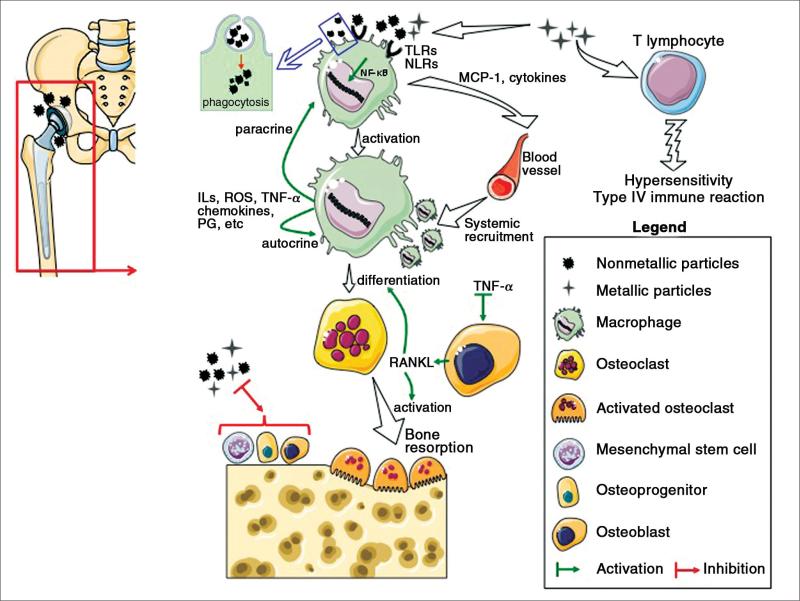
When wear by-products are generated, ionic moieties become solubilized and smaller particles form dispersions and aggregative complexes with serum proteins. Nearby cells are presented with small nano-sized material (up to approximately 300 nm in diameter) that can be pinocytosed (without cellular activation), particle-protein complexes that can be phagocytosed (usually smaller than 10 μm in size), or larger composites that are not phagocytosable. Particle phagocytosis leads to cellular activation; however, particle-protein complexes do not have to be phagocytosed to activate cells.7,8 In the latter case, cell surface receptors become stimulated by larger nonphagocytosable particle-protein complexes, resulting in cellular activation. The inflammatory cascade is further stimulated by autocrine and paracrine processes resulting from released proinflammatory factors and by the stimulation of toll-like receptors and related pathways9,10 (Figure 1). The presence of endotoxin on particles may also play an important exacerbating role.11,12
Figure 1.
Illustration of the main biologic principles involved in wear particle-induced periprosthetic osteolysis. IL = interleukin, PG = prostaglandins, TLRs = toll-like receptors, NLRs = nod-like receptors, ROS = reactive oxygen species, NF-kB = nuclear factor kappaB, MCP-1 = macrophage chemotactic protein-1, TNF-α = tumor necrosis factor-α, RANKL = receptor activator of nuclear factor-kappaB ligand.
Willert and Semlitsch13,14 were the first to recognize the importance of the biologic response to wear debris and the association with adverse tissue responses, including periprosthetic osteolysis. The local and regional biologic reaction to the presence of excessive wear debris leads to a state of decompensation or tissue dysregulation. The macrophage has traditionally been the focus of much research concerning the biology of wear particulates; however, several observations have broadened the scope of the cellular response. Tissues retrieved from revised joint arthroplasties with and without osteolysis have many different cell types, which constitute a chronic inflammatory infiltrate. These cells include monocyte/macrophage lineage cells (macrophages, foreign body giant cells, and osteoclasts), fibroblasts, lymphocytes, and cells associated with vascular structures. Histologic evidence also shows active bone remodeling in the adjacent bone bed, including the presence of osteoprogenitor cells and osteoblasts. Kadoya et al15 reported that active bone formation was prominent in interface tissues and the adjacent bone of revised aseptically loose joint arthroplasties, indicating ongoing repair. This finding is consistent with a chronic inflammatory reaction, which consists of concurrent acute inflammation, local tissue destruction, fibrosis, and active repair. Interestingly, different cell types (in addition to cells of the monocyte/macrophage lineage) are capable of phagocytosis of wear particles, including fibroblasts and osteoblasts.
Wear particle-associated aseptic chronic inflammation is also referred to as granulomatous inflammation and may be of two general types. Nonimmune, nonspecific granulomatous inflammation is generally associated with polymeric, ceramic, and other debris and results in a histologic reaction dominated by macrophages and fibroblasts, with few lymphocytes. This reaction is believed to be dependent on the general particle characteristics, including size, shape, topography, and surface area.16 T lymphocytes are not a prominent histologic finding.
Immune granulomas are associated with excessive metallic by-products and have a more prominent widespread lymphocytic reaction that also may be located in a perivascular location (so-called perivascular cuffing).17 It is believed that in some patients, the metal particle-protein complex can function as a hapten, evoking a type IV T lymphocyte-mediated immune reaction. Nonmetallic wear debris (such as polymers and ceramics) primarily stimulate the innate immune system and are nonspecific, whereas metal particulates and ions can stimulate both the innate and the adaptive (T lymphocyte-mediated) immune systems.18 Recently, it has become apparent that there are idiosyncratic, genetically based differences in an individual patient's response to different particle burdens.19,20
The Particle-Induced Inflammatory Cascade
After stimulation, cells of the monocyte/macrophage lineage, polymorphonuclear leukocytes, fibroblasts, osteoblasts, and other cells increase the transcription of proinflammatory substances, including cytokines, chemokines (chemotactic cytokines), reactive oxygen intermediates (nitric oxide and peroxide), prostaglandins, metalloproteinases, lysosomal enzymes, and other factors.19,21-25 These inflammatory mediators are under direct transcriptional control of nuclear factor kappa-light-chain-enhancer of activated B cells, nuclear factor interleukin-6 (IL-6), and other factors.8,24 In addition to activation of the innate immune system by toll-like receptors on the cell surface, some particle types (especially metals) can activate the inflammasome, a subset of the nucleotide oligomerization domain (NOD)-like receptors (NLRs). These moieties assemble into a complex multimolecular structure called NLRP (nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain-containing), which activates the caspase-1 cascade, leading to proinflammatory factor production, especially IL-1 and IL-18.22,26
Particle-associated cross-talk among macrophages, fibroblasts, osteoblasts and their progenitors, and other cells leads to the production of inflammatory factors that act in an autocrine and paracrine fashion. Osteoclast differentiation, maturation, and function are upregulated by these released factors, leading to the local destruction of bone. Some of the main inflammatory factors involved in these processes include tumor necrosis factor-α, IL-1, IL-6, IL-8, receptor activator of nuclear factor-kappaB ligand (RANKL), macrophage chemotactic protein-1, and others.27,28
Numerous in vitro, in vivo, and tissue retrieval studies have demonstrated that wear particles stimulate acute and chronic inflammation, as well as bone destruction (osteolysis).29,30 Recent studies have shown that retrieved interface tissues from loose implants demonstrate an imbalance in the receptor activator of nuclear factor-kappaB (RANK)-RANKL–osteoprotegerin axis, favoring bone resorption.31 Particle-induced upregulation of the inflammatory cascade is also influenced by other factors, including mechanical forces and endotoxin.11,32 The relative contribution of wear particles, endotoxin, mechanical forces, and other factors to the production of osteolysis is controversial; it is probably most useful to consider these factors as co-contributory, not as separate entities.33
Wear particles not only stimulate bone resorption but also interfere with bone formation. Recent studies have shown that both metallic and polymeric particles interfere with the proliferation, differentiation, and function of mesenchymal stem cells, osteoprogenitor cells, and osteoblasts.34-39 These adverse effects also have been shown in animal models of bone ingrowth in the presence of wear particles.40
Wear Particles Induce a Systemic Immune Response
It had been surmised that wear particles from joint implants stimulate a local or perhaps a limited regional inflammatory reaction. However, based on recent animal models, it has been concluded that wear debris stimulates a systemic immune reaction, with the mobilization and trafficking of remote macrophages to the local area of particle deposition.41-43 Macrophage chemotactic protein-1 appears to play a critical role in the chemotactic response of macrophages to wear debris. The depletion of macrophages diminishes this immune response and osteolysis.44 These findings suggest potential opportunities for mitigation of the macrophage-associated chronic inflammatory response.
Do Wear Particles Stimulate Antigen-Specific Immune Reactions?
The subject of immune reactions to wear particles has been briefly mentioned in this chapter and reviewed in detail elsewhere.45 However, recent evidence suggests that metallic ions and complexes may stimulate different types of tissue reactions, ranging from benign-appearing localized fibrosis and inflammation to a type IV immune reaction that can lead to severe pain, as well as bone and soft-tissue destruction.17,18,46-48 More recent accounts of pseudotumors associated with metal-on-metal implants, especially specific types of resurfacing and total hip arthroplasties, have caused concern.18,48 Patients with these implants should be followed closely, with serial clinical, radiographic, and laboratory examinations as indicated.
Summary
Wear in total joint arthroplasties is inevitable and dependent on the use of the joint during daily activities. Excessive wear particles stimulate a cascade of biologic events that may lead to degradation of bone and inhibition of bone formation (osteolysis). Newer, more wear-resistant bearing surfaces and implant designs have introduced new opportunities for limiting the production of particulate debris and subsequent adverse biologic reactions. However, concerns exist with some hard-on-hard bearing couples that have led to severe adverse reactions in some patients. Successful biologic treatments for managing osteolysis are not yet available. Continued mechanistic preclinical research studies may potentially yield viable strategies for prevention and treatment of particle-associated osteolysis.
Acknowledgment
This work was supported in part by NIH Grant 1R01AR055650-04 and the Ellenburg Chair in Surgery at Stanford University.
References
- 1.Marshall A, Ries MD. Paprosky W; Implant Wear Symposium 2007 Clinical Work Group: How prevalent are implant wear and osteolysis, and how has the scope of osteolysis changed since 2000? J Am Acad Orthop Surg. 2008;16(suppl 1):S1–S6. doi: 10.5435/00124635-200800001-00003. [DOI] [PubMed] [Google Scholar]
- 2.Malviya A, Ramaskandhan J, Holland JP. Lingard EA: Metal-on-metal total hip arthroplasty. J Bone Joint Surg Am. 2010;92(7):1675–1683. doi: 10.2106/JBJS.I.01426. [DOI] [PubMed] [Google Scholar]
- 3.Walter WL, Yeung E. Esposito C: A review of squeaking hips. J Am Acad Orthop Surg. 2010;18(6):319–326. doi: 10.5435/00124635-201006000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Lachiewicz PF, Heckman DS, Soileau ES, Mangla J. Martell JM: Femoral head size and wear of highly cross-linked polyethylene at 5 to 8 years. Clin Orthop Relat Res. 2009;467(12):3290–3296. doi: 10.1007/s11999-009-1038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Punt IM, Austen S, Cleutjens JP, et al. Are periprosthetic tissue reactions observed after revision of total disc replacement comparable to the reactions observed after total hip or knee revision surgery? Spine (Phila Pa 1976) 2012;37(2):150–159. doi: 10.1097/BRS.0b013e3182154c22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kepler CK, Nho SJ, Bansal M, et al. Radiographic and histopathologic analysis of osteolysis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(4):588–595. doi: 10.1016/j.jse.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Sun DH, Trindade MC, Nakashima Y, et al. Human serum opsonization of orthopedic bio-material particles: Protein-binding and monocyte/macrophage activation in vitro. J Biomed Mater Res A. 2003;65(2):290–298. doi: 10.1002/jbm.a.10477. [DOI] [PubMed] [Google Scholar]
- 8.Nakashima Y, Sun DH, Trindade MC, et al. Signaling pathways for tumor necrosis factor-alpha and interleukin-6 expression in human macrophages exposed to titanium-alloy particulate debris in vitro. J Bone Joint Surg Am. 1999;81(5):603–615. doi: 10.2106/00004623-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Pearl JI, Ma T, Irani AR, et al. Role of the toll-like receptor pathway in the recognition of orthopedic implant wear-debris particles. Biomaterials. 2011;32(24):5535–5542. doi: 10.1016/j.biomaterials.2011.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamaki Y, Takakubo Y, Goto K, et al. Increased expression of toll-like receptors in aseptic loose periprosthetic tissues and septic synovial membranes around total hip implants. J Rheumatol. 2009;36(3):598–608. doi: 10.3899/jrheum.080390. [DOI] [PubMed] [Google Scholar]
- 11.Greenfield EM, Bechtold J. Implant Wear Symposium 2007 Biologic Work Group: What other biologic and mechanical factors might contribute to osteolysis? J Am Acad Orthop Surg. 2008;16(suppl 1):S56–S62. doi: 10.5435/00124635-200800001-00012. [DOI] [PubMed] [Google Scholar]
- 12.Hirayama T, Tamaki Y, Takakubo Y, et al. Toll-like receptors and their adaptors are regulated in macrophages after phagocytosis of lipopolysaccharide-coated titanium particles. J Orthop Res. 2011;29(7):984–992. doi: 10.1002/jor.21369. [DOI] [PubMed] [Google Scholar]
- 13.Willert HG, Semlitsch M. Reactions of the articular capsule to wear products of artificial joint prostheses. J Biomed Mater Res. 1977;11(2):157–164. doi: 10.1002/jbm.820110202. [DOI] [PubMed] [Google Scholar]
- 14.Willert HG, Semlitsch M. Tissue reactions to plastic and metallic wear products of joint endopros-theses. Clin Orthop Relat Res. 1996;333:4–14. [PubMed] [Google Scholar]
- 15.Kadoya Y, Revell PA, al-Saffar N, Kobayashi A, Scott G, Freeman MA. Bone formation and bone resorption in failed total joint arthroplasties: Histomorphometric analysis with histochemical and immunohistochemical technique. J Orthop Res. 1996;14(3):473–482. doi: 10.1002/jor.1100140318. [DOI] [PubMed] [Google Scholar]
- 16.Shanbhag AS, Jacobs JJ, Black J, Galante JO, Glant TT. Macrophage/particle interactions: Effect of size, composition and surface area. J Biomed Mater Res. 1994;28(1):81–90. doi: 10.1002/jbm.820280111. [DOI] [PubMed] [Google Scholar]
- 17.Willert HG, Buchhorn GH, Fayyazi A, et al. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints: A clinical and histomorphological study. J Bone Joint Surg Am. 2005;87(1):28–36. doi: 10.2106/JBJS.A.02039pp. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs JJ, Campbell PA, T Konttinen Y. Implant Wear Symposium 2007 Biologic Work Group: How has the biologic reaction to wear particles changed with newer bearing surfaces? J Am Acad Orthop Surg. 2008;16(suppl 1):S49–S55. doi: 10.5435/00124635-200800001-00011. [DOI] [PubMed] [Google Scholar]
- 19.Catelas I, Jacobs JJ. Biologic activity of wear particles. Instr Course Lect. 2010;59:3–16. [PubMed] [Google Scholar]
- 20.Gordon A, Kiss-Toth E, Stockley I, Eastell R, Wilkinson JM. Polymorphisms in the interleukin-1 receptor antagonist and interleukin-6 genes affect risk of osteolysis in patients with total hip arthroplasty. Arthritis Rheum. 2008;58(10):3157–3165. doi: 10.1002/art.23863. [DOI] [PubMed] [Google Scholar]
- 21.Goodman SB, Lind M, Song Y, Smith RL. In vitro, in vivo, and tissue retrieval studies on particulate debris. Clin Orthop Relat Res. 1998;352:25–34. [PubMed] [Google Scholar]
- 22.Hallab NJ, Jacobs JJ. Biologic effects of implant debris. Bull NYU Hosp Jt Dis. 2009;67(2):182–188. [PubMed] [Google Scholar]
- 23.Purdue PE, Koulouvaris P, Nestor BJ, Sculco TP. The central role of wear debris in periprosthetic osteolysis. HSS J. 2006;2(2):102–113. doi: 10.1007/s11420-006-9003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purdue PE, Koulouvaris P, Potter HG, Nestor BJ, Sculco TP. The cellular and molecular biology of periprosthetic osteolysis. Clin Orthop Relat Res. 2007;454:251–261. doi: 10.1097/01.blo.0000238813.95035.1b. [DOI] [PubMed] [Google Scholar]
- 25.Hukkanen M, Corbett SA, Platts LA, et al. Nitric oxide in the local host reaction to total hip replacement. Clin Orthop Relat Res. 1998;352:53–65. [PubMed] [Google Scholar]
- 26.Caicedo MS, Desai R, McAllister K, Reddy A, Jacobs JJ, Hallab NJ. Soluble and particulate Co-Cr-Mo alloy implant metals activate the inflammasome danger signaling pathway in human macrophages: A novel mechanism for implant debris reactivity. J Orthop Res. 2009;27(7):847–854. doi: 10.1002/jor.20826. [DOI] [PubMed] [Google Scholar]
- 27.Granchi D, Ciapetti G, Amato I, et al. The influence of alumina and ultra-high molecular weight polyethylene particles on osteoblast-osteoclast cooperation. Biomaterials. 2004;25(18):4037–4045. doi: 10.1016/j.biomaterials.2003.10.100. [DOI] [PubMed] [Google Scholar]
- 28.Kaufman AM, Alabre CI, Rubash HE, Shanbhag AS. Human macrophage response to UHMWPE, TiAlV, CoCr, and alumina particles: Analysis of multiple cytokines using protein arrays. J Biomed Mater Res A. 2008;84(2):464–474. doi: 10.1002/jbm.a.31467. [DOI] [PubMed] [Google Scholar]
- 29.Bostrom M, O'Keefe R. Implant Wear Symposium 2007 Biologic Work Group: What experimental approaches (eg, in vivo, in vitro, tissue retrieval) are effective in investigating the biologic effects of particles? J Am Acad Orthop Surg. 2008;16(suppl 1):S63–S67. doi: 10.5435/00124635-200800001-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuan RS, Lee FY, Konttinen Y, Wilkinson JM, Smith RL. Implant Wear Symposium 2007 Biologic Work Group: What are the local and systemic biologic reactions and mediators to wear debris, and what host factors determine or modulate the biologic response to wear particles? J Am Acad Orthop Surg. 2008;16(suppl 1):S42–S48. doi: 10.5435/00124635-200800001-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mandelin J, Li TF, Liljeström M, et al. Imbalance of RANKL/RANK/OPG system in interface tissue in loosening of total hip replacement. J Bone Joint Surg Br. 2003;85(8):1196–1201. doi: 10.1302/0301-620x.85b8.13311. [DOI] [PubMed] [Google Scholar]
- 32.Bechtold JE, Kubic V, Søballe K. Bone ingrowth in the presence of particulate polyethylene: Synergy between interface motion and particulate polyethylene in periprosthetic tissue response. J Bone Joint Surg Br. 2002;84(6):915–919. doi: 10.1302/0301-620x.84b6.12111. [DOI] [PubMed] [Google Scholar]
- 33.Aspenberg P, Van der Vis H. Migration, particles, and fluid pressure: A discussion of causes of prosthetic loosening. Clin Orthop Relat Res. 1998;352:75–80. [PubMed] [Google Scholar]
- 34.Chiu R, Ma T, Smith RL, Goodman SB. Kinetics of polymethylmethacrylate particle-induced inhibition of osteoprogenitor differentiation and proliferation. J Orthop Res. 2007;25(4):450–457. doi: 10.1002/jor.20328. [DOI] [PubMed] [Google Scholar]
- 35.Chiu R, Ma T, Smith RL, Goodman SB. Ultrahigh molecular weight polyethylene wear debris inhibits osteoprogenitor proliferation and differentiation in vitro. J Biomed Mater Res A. 2009;89(1):242–247. doi: 10.1002/jbm.a.32001. [DOI] [PubMed] [Google Scholar]
- 36.Lozito TP, Tuan RS. Mesenchymal stem cells inhibit both endogenous and exogenous MMPs via secreted TIMPs. J Cell Physiol. 2011;226(2):385–396. doi: 10.1002/jcp.22344. [DOI] [PubMed] [Google Scholar]
- 37.Wang ML, Nesti LJ, Tuli R, et al. Titanium particles suppress expression of osteoblastic phenotype in human mesenchymal stem cells. J Orthop Res. 2002;20(6):1175–1184. doi: 10.1016/S0736-0266(02)00076-1. [DOI] [PubMed] [Google Scholar]
- 38.Ramachandran R, Goodman SB, Smith RL. The effects of titanium and polymethylmethacrylate particles on osteoblast phenotypic stability. J Biomed Mater Res A. 2006;77(3):512–517. doi: 10.1002/jbm.a.30649. [DOI] [PubMed] [Google Scholar]
- 39.Yao J, Cs-Szabó G, Jacobs JJ, Kuettner KE, Glant TT. Suppression of osteoblast function by titanium particles. J Bone Joint Surg Am. 1997;79(1):107–112. doi: 10.2106/00004623-199701000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Goodman SB. The effects of micromotion and particulate materials on tissue differentiation: Bone chamber studies in rabbits. Acta Orthop Scand Suppl. 1994;258:1–43. doi: 10.3109/17453679409155227. [DOI] [PubMed] [Google Scholar]
- 41.Zhang K, Jia TH, McQueen D, et al. Circulating blood monocytes traffic to and participate in the periprosthetic tissue inflammation. Inflamm Res. 2009;58(12):837–844. doi: 10.1007/s00011-009-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ren PG, Lee SW, Biswal S, Goodman SB. Systemic trafficking of macrophages induced by bone cement particles in nude mice. Biomaterials. 2008;29(36):4760–4765. doi: 10.1016/j.biomaterials.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ren PG, Huang Z, Ma T, Biswal S, Smith RL, Goodman SB. Surveillance of systemic trafficking of macrophages induced by UHMWPE particles in nude mice by noninvasive imaging. J Biomed Mater Res A. 2010;94(3):706–711. doi: 10.1002/jbm.a.32744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ren W, Markel DC, Schwendener R, Ding Y, Wu B, Wooley PH. Macrophage depletion diminishes implant-wear-induced inflammatory osteolysis in a mouse model. J Biomed Mater Res A. 2008;85(4):1043–1051. doi: 10.1002/jbm.a.31665. [DOI] [PubMed] [Google Scholar]
- 45.Goodman SB. Wear particles, periprosthetic osteolysis and the immune system. Biomaterials. 2007;28(34):5044–5048. doi: 10.1016/j.biomaterials.2007.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davies AP, Willert HG, Campbell PA, Learmonth ID, Case CP. An unusual lymphocytic perivascular infiltration in tissues around contemporary metal-on-metal joint replacements. J Bone Joint Surg Am. 2005;87(1):18–27. doi: 10.2106/JBJS.C.00949. [DOI] [PubMed] [Google Scholar]
- 47.Hallab NJ, Caicedo M, Finnegan A, Jacobs JJ. Th1 type lymphocyte reactivity to metals in patients with total hip arthroplasty. J Orthop Surg Res. 2008;3:6. doi: 10.1186/1749-799X-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas P, Braathen LR, Dörig M, et al. Increased metal allergy in patients with failed metal-on-metal hip arthroplasty and peri-implant T-lymphocytic inflammation. Allergy. 2009;64(8):1157–1165. doi: 10.1111/j.1398-9995.2009.01966.x. [DOI] [PubMed] [Google Scholar]