Epidural patient-controlled analgesia after total knee arthroplasty (TKA), helps patients by decreasing postoperative pain and making early ambulation [1-3]. Ropivacaine is a newly developed amide form of local anesthetics, and is composed of pure S (-) isomers, compared with bupivacaine. Therefore, ropivacaine is free from cardiac toxicity and is widely used in epidural PCA combined with opioids [4,5].
Many studies were conducted to find the optimal concentration of ropivacaine in epidural infusion after general anesthesia; however, not much combined with opioids after combined epidural-spinal anesthesia. We studied the effective and safe concentration of ropivacaine combined with fentanyl after total knee arthroplasty for 5 days.
The protocol was approved by the Institutional Review Board of our hospital and all patients provided written informed consent. Sixty American Society of Anesthesiologists physical status class 1-3 patients (aged 60-80 years), who were scheduled for elective unilateral TKA under combined epidural-spinal anesthesia, were enrolled in this study. In all patients, epidural-spinal combined anesthesia was performed with 0.5% hyperbaric bupivacaine 10-12 mg, and 20 gauge epidural catheter was inserted at the L3/4 or L4/5 lumbar spinal level. On completion of surgery, patients were randomized to receive an epidural PCA: Group I (n = 20, 0.2% ropivacaine with fentanyl 0.625%), Group II (n = 20, 0.25% ropivacaine with fentanyl 0.625%), Group III (n = 20, 0.3% ropivacaine with fentanyl 0.625%). The PCA pump was set to deliver 2 ml/hr and bolus dose 0.5 ml with lockout time of 15 min, and infused 5 days after surgery. Anesthesiologist who was not aware of this study visited patients and measured the pain score, additional opioids use, motor function test, and sensory test in lower extremities in each day. Pain score was measured using a 0-100 mm visual analogue scale (VAS), where a score of 0 meant no pain and a score of 100 meant the worst pain imaginable. Motor function test was measured via a modified bromage scale (0 = no motor block, 1 = motor block with difficulty in hip joint flexion, 2 = motor block with difficulty in knee flexion, 3 = motor block with difficulty in ankle flexion). Sensory test measured the skin prick test in the lower extremities, using a 25-gauge needle.
The distribution of all continuous variables, which followed a normal distribution, such as, age, height, weight, anesthesia time and VAS score, were compared using the two sample Student's t-test. Non-normally-distributed continuous data, such as, and additional opioids use were compared with the non-parametric Mann-Whitney U test. Categorical data for each group, such as, gender distribution and complications were compared using a chi-square test or Fisher's exact test. For all statistical tests, two-tailed value of P < 0.05 were considered statistically significant. Statistical analysis was conducted using the SPSS package version 19.0 (SPSS Inc. /IBM).
Patients in Group III (0.3% ropivacaine with 0.625 % fentanyl) showed serious side effects from the first day of surgery, such as drowsy mental status, dizziness, hypotension, and uncontrolled nausea/vomiting, Therefore, they removed an epidural catheter and received intermittent IV morphine for pain control, and were excluded from this study.
In VAS score, Group II (0.25% ropivacaine with fentanyl) showed lower VAS score at the 1st postoperative day than Group I (0.2% ropivacaine with fentanyl) (Table 1). But, there were no other differences between the two groups. And Group II showed a lower rate of additional analgesics than Group I on the 3rd postoperative day (0.8 ± 0.1 vs 0.2 ± 0.1, P = 0.01).
Table 1.
Pain Score during Five Days by Visual Analog Scale
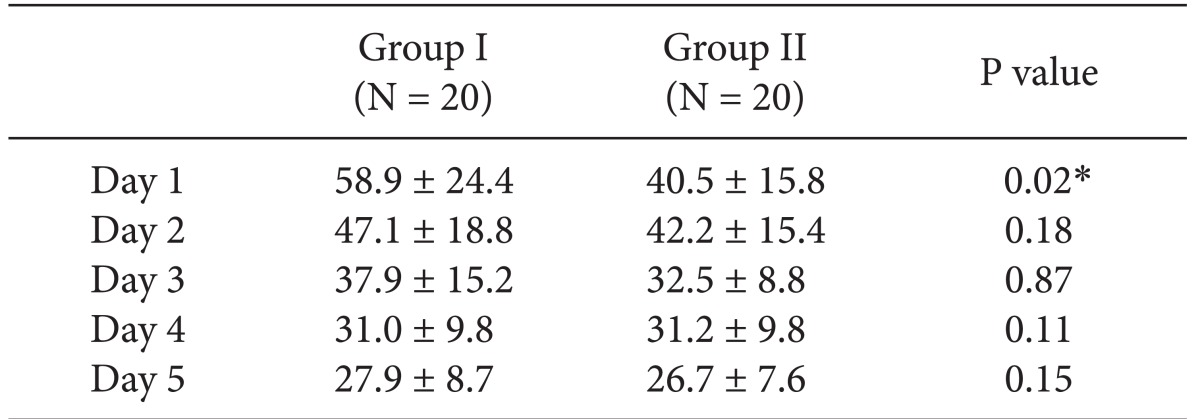
Values are expressed as means ± SD. Group I is ropivacaine 0.2%/fentanyl group, Group II is ropivacaine 0.25%/fentanyl group, *P < 0.05 between groups.
In the bromage scale, one patient in Group I and three patients in Group II showed scale 1 (P = 0.60), and in the skin prick test, one patient in Group I and eight patients in group II showed hypothesia (P = 0.14). No difference was found in the rate of nausea and vomiting between the two groups. No patients in both group showed urinary retention and inability of walking.
Badner et al. [1] reported that epidural infusions of ropivacaine 0.1%, 0.2%, and 0.3% at 10 ml/hr was safe, improved post-operative analgesia, and decreased PCA morphine requirements in patients undergoing major orthopedic surgery. Furthermore, they reported that 0.1% and 0.2% concentrations produced similar sensory anesthesia with less motor blockade, when compared with 0.3%. However, this study is a dose-finding study of ropivacaine, used in epidural infusion, not combined with opioids, the dose is relatively high (10 ml/hr), and infusion duration is 21 hours.
As is used in our hospital, patients was epidurally infused for five days PCA, and their infusion rate is relatively small (2 ml/hr). Seven patients in Group III (0.3 % ropivacaine with fentanyl) showed continuous hypotension and 5 patients showed serious nausea/vomiting, and dizziness; therefore, we decided to stop infusion and excluded from our study. It is probably that epidural infusion of high concentration of local anesthetics with opioids produces more profound vessel dilatation by more blocks of sympathetic tones. We thought that epidural infusion of 0.3% ropivacaine with opioids is not safe after a major orthopedic surgery.
Group II (ropivacaine 0.25% with fentanyl) showed lower VAS score than Group I (ropivacaine 0.2% with fentanyl) at the first postoperative day (P < 0.05), and required less additional opioids at the third postoperative day (P < 0.05). No difference was found between the two groups, in terms of side effects. All patients showed minimal sensory and motor block at the 2 ml/hr infusion rate.
Therefore, we concluded that 0.25% ropivacaine mixed with fentanyl is suitable when infused in the epidural PCA at 2 ml/hr for patients undergoing unilateral total knee arthroplasty.
References
- 1.Badner NH, Reid D, Sullivan P, Ganapathy S, Crosby ET, Mekenna J, et al. Continuous epidural infusion of ropivacaine for the prevention of postoperative pain after major orthopedic surgery: a dose-finding study. Can J Anaesth. 1996;43:17–22. doi: 10.1007/BF03015952. [DOI] [PubMed] [Google Scholar]
- 2.Etches RC, Writer WD, Ansley D, Nydahl PA, Ong BY, Lui A, et al. Continuous epidural ropivacaine 0.2% for analgesia after lower abdominal surgery. Anesth Analg. 1997;84:784–790. doi: 10.1097/00000539-199704000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Scott DA, Chamley DM, Mooney PH, Deam RK, Mark AH, Hagglof B. Epidural ropivacaine infusion for postoperative analgesia after major lower abdominal surgery-a dose finding study. Anesth Analg. 1995;81:982–986. doi: 10.1097/00000539-199511000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Feldman HS, Arthur GR, Govino BG. Comparative systemic toxicity of convulsant and supraconvulsant doses of intravenous ropivacaine, bupivacaine, and lidocaine in the conscious dog. Anesth Analg. 1989;69:794–801. [PubMed] [Google Scholar]
- 5.Moller R, Covino BG. Cardiac electrophysiologic properties of bupivacaine and lidocaine compared with ropivacaine, a new amide local anesthetic. Anesthesiology. 1990;72:322–329. doi: 10.1097/00000542-199002000-00019. [DOI] [PubMed] [Google Scholar]


