Abstract
Within dry inner Alpine environments climate warming is expected to affect the development of forest ecosystems by changing species composition and inducing shifts in forest distribution. By applying dendroecological techniques we evaluated climate sensitivity of radial growth and establishment of Picea abies in a drought-prone mixed-coniferous forest in the Austrian Alps. Time series of annual increments were developed from > 220 trees and assigned to four age classes. While radial growth of old P. abies trees (mean age 121 and 174 yr) responded highly significant to May-June precipitation, young trees (mean age 28 and 53 yr) were insensitive to precipitation in the current year. Because tree age was closely correlated to height and diameter (r2 = 0.709 and 0.784, respectively), we relate our findings to the increase in tree size rather than age per se. Synchronicity found among trend in basal area increment and tree establishment suggests that canopy openings increased light and water availability, which favoured growth and establishment of moderately shade-tolerant P. abies. We conclude that although P. abies is able to regenerate at this drought prone site, increasing inter-tree competition for water in dense stands gradually lowers competitive strength and restricts scattered occurrence to dry-mesic sites.
Introduction
Climate sensitivity of tree growth and establishment will affect the development of forest ecosystems under a warmer and drier climate by changing species composition and inducing shifts in forest distribution, whereby an extensive literature supports the hypothesis that natural disturbance plays an important role in determining the development of structure and function of forest ecosystems (for reviews see Attiwill 1994). Disturbances affect population dynamics by causing mortality, altering resource availability, providing recruitment opportunities and influencing the relative competitive status of individuals. Drought is one of the main climatic constraints for tree growth and an important factor triggering both temporary declines and tree mortality in forests (Allen et al. 2010). Climate change scenarios predict that forests in Europe are likely to experience more severe droughts and higher summer temperatures in the future (IPCC 2007), whereby changes not only in species distribution but also in community composition can be expected to occur (Suarez and Kitzberger 2008). Because future forest structure and composition will be mainly a consequence of current recruitment, understanding environmental factors controlling natural regeneration, i.e., dependence of seedling establishment and early growth on climate variability is important to predict dynamics in mixed forests.
While temporal changes in tree establishment can be reconstructed using dendroecological techniques (e.g., Lorimer and Frelich 1989) the climatic factors most closely associated with variations in tree-ring parameters can be identified by applying dendroclimatological methods (Hughes 2002). In central alpine dry valleys several dendroclimatological studies revealed that extensive drought periods limit radial tree growth and play a major role in increasing the mortality of forest trees (Oberhuber et al. 1998; Bigler et al. 2006). In these studies climate-growth relationships were analyzed on dominant mature trees to minimize non-climatic signals due to competition (Fritts 1976). However, there is evidence from ecophysiological studies that functional processes, e.g., photosynthetic performance and water use efficiency, change with tree age and/or size (Mencuccini et al. 2005; Rossi et al. 2008). Tree age and/or size are also known to modulate sensitivity of radial tree growth to climate (Szeicz and MacDonald 1994; Mérian and Lebourgeois 2011), whereby larger/older trees were found to be more sensitive to growth limiting climate factors than younger/smaller trees. On the other hand, drought periods during the growing season are expected to be a major threat to the establishment of conifer seedlings (Castro et al. 2005; Moser et al. 2010), whereby species-specific microsite requirements, e.g., concerning light intensity and water availability, exist (Hunziker and Brang 2005).
In drought-exposed mixed coniferous stands in a dry inner Alpine environment Scots pine (Pinus sylvestris L.), European larch (Larix decidua Mill.) and Norway spruce (Picea abies (L.) Karst.) co-occur at dry-mesic sites, whereby drought tolerant P. sylvestris dominates the canopy. In these forests natural regeneration of light-demanding pioneer species (P. sylvestris and L. decidua) is generally restricted to canopy gaps (Ellenberg and Leuschner 2010), which might be related to the finding that light-demanding species require more ‘growing space’ (i.e., low density stands) than shade-tolerant species (Simard and Sachs 2004). Consistently, P. abies, which is a moderately shade-tolerant species, is frequently occurring in the understorey and shows scattered regeneration at dry-mesic sites under closed canopy, indicating a possibly climate-induced shift in forest species composition, which was reported from other drought exposed sites in the Central Alps (Moser et al. 2010; Rigling et al. 2012). Because we also detected increasing drought sensitivity of mature P. abies in recent decades (Schuster and Oberhuber 2012), the objectives of this study were to determine (i) climate sensitivity of P. abies in relationship to tree age and/or size and (ii) whether establishment and growth of P. abies is impaired at this drought prone site. We hypothesized that tree age modulates growth response to climate, i.e., young small trees show lower sensitivity to drought stress than old large trees and that shade-tolerance and shallow root system of P. abies favour continuous establishment and growth at dry-mesic sites.
Material and Methods
Study area
The study site is part of a postglacial rock-slide area situated in the montane belt (c. 750 m a.s.l.) within the inner Alpine dry valley of the Inn River (Tyrol, Austria, 47° 13′ 53″ N, 10° 50′ 51″ E) and has a relatively continental climate with mean annual precipitation and temperature of 716 mm and 7.3 °C, respectively (long-term mean during 1911-2009 at Ötz, 812 m a.s.l., 5 km from the study area). Precipitation records at the study site revealed that during 2007 – 2011 the amount of rainfall was < 5 mm on 70.9 and 65.1 % of rainy days during April – June and April – September, respectively. The dominating plant community is an open Spring Heath-Pine wood (Erico-Pinetum typicum), whereby on scattered dry-mesic sites mixed stands are developed (Pinus sylvestris 60 %, Picea abies 20 % and Larix decidua 20 %). Stand height and canopy coverage are 15 to 18 m and c. 70 %, respectively. Within selected plots P. sylvestris and L. decidua are 152 ± 17 yr (n = 42) and 150 ± 17 yr (n = 39) old, respectively (mean cambial age at breast height). P. abies is the only tree species which rejuvenates naturally under closed canopy, whereby the number of saplings and trees < 10 m height amounts to c. 30 individuals in an area covering 30 × 30 m, i.e. c. 333 individuals per ha. Saplings < 0.2 m height and seedlings occurred only sporadically. Site topographic conditions included primarily hollows and north facing slopes (slope angle 5 – 20 °). Shallow soils of protorendzina type, i.e., rendzic leptosols according to the FAO classification system (FAO 2006), are developed and consist of unconsolidated, coarse-textured materials with low water holding capacity (soil depth 10 – 20 cm). A thick moss layer dominates the understorey, indicating slightly moist conditions.
Field collection, sample preparation, chronology development and statistics
Within six selected plots, which showed no disturbance by anthropogenic influences (logging, livestock grazing), 15 to 20 trees belonging to height classes ≥ 0.2 < 2 m (saplings) and ≥ 2 m (mature trees) were sampled, summarizing to a total of > 220 trees. Sampling bias was avoided by collecting about equal number of saplings and mature trees. Stem disks were taken close to the soil surface from saplings having a stem diameter < 5 cm. All other trees were cored at 0.3 m stem height. Because large diameter trees on steeper slopes sporadically showed irregular radial growth at lower stem height, cores were taken at 1 m above ground. Tree height and stem diameter at the soil surface were recorded from every sampled tree. Cores were taken parallel to the contour line to avoid stem areas with reaction wood, which generally occurs in stems of trees growing on slopes (Fritts 1976). In the laboratory all samples were air-dried and increment cores were glued in grooved wooden mounts. The surface of stem disks and increment cores was prepared with a sharp razor-blade and ring widths were measured along two opposing radii per tree using an incremental measuring table (resolution 1 μm) under up to 60x magnification. To estimate the age of individual trees cored above root collar at 30 and 100 cm stem height, the age-height relationship was determined from harvested saplings (n = 135). The correct dating of tree ring series was checked using COFECHA (Grissino-Mayer 2001), which identifies segments within each ring series that may have erroneous cross-dating or measurement errors.
Climate-growth relationships were explored based on residual ring width chronologies, which were calculated using ARSTAN (Cook and Holmes 1984). A two-stage detrending procedure was chosen to remove most of the low-frequency variability in each ring series that is assumed to be unrelated to climate, i.e., tree aging and forest stand development. In the first step a negative exponential curve or linear regression was fit to the ring-series. The second step used a cubic smoothing spline with a frequency-response cutoff set at two-thirds of the length of each series. Dimensionless indices were formed by dividing the observed ring width value by the predicted ring width value. This process creates stationary time series for each tree with a mean of 1 and a homogeneous variance. Residual chronologies were derived from ARMA modelling, with a robust mean value function applied to discount the effect of statistical outliers (Holmes 1994). Residual chronologies are commonly used in dendroclimatic studies because removal of serial autocorrelation is required for some statistical analysis.
Several statistics were calculated for the standardized chronologies, prior to autoregressive modelling. The standard deviation (SD) measures the variability of the measurements at all wave lengths. Mean sensitivity is a measure of the mean relative change between adjacent ring-widths and is calculated as the absolute differences between adjacent indices divided by the mean of the two indices. Higher values of mean sensitivity and higher standard deviations are indicative of more climatically responsive chronologies (Fritts 1976). The first-order autocorrelation quantifies the temporal persistence in growth among consecutive years.
To estimate the signal strength, i.e. the amount of climatic information included in the developed chronologies, the expressed population signal (EPS; Wigley et al. 1984) was calculated. The EPS considers the inter-series correlation and the sample size. Because the EPS estimates how well a finite number of samples represents the theoretical population mean, the EPS quantifies the degree to which a particular sample chronology portrays the hypothetically perfect chronology, which may in turn be regarded as the potential climate signal. Though a specific range of EPS values cannot be given, Wigley et al. (1984) suggest a threshold of 0.85 as an acceptable statistical quality.
Common variance in tree ring chronologies was estimated by the percentage of variance explained by the first component in principal component analysis (PCA). PCA is a data reduction technique that transforms the original variables (here, the standardized ring width chronologies of selected vitality classes) into a smaller set of uncorrelated variables (eigenvectors or principal components) in such a way that the first few components encompass most of the variability in the original variables. Higher common variance indicates a greater climatic influence on tree growth (Briffa and Jones 1990).
Computation of basal area increment (BAI)
The use of mean ring width data to study long-term growth suffers from negative trend in ring series due to increasing circumference of the tree (“age-trend”; Fritts 1976). Furthermore, BAI is more closely related to biomass increment than ring width. Therefore, ring width was converted into BAI to remove variation in radial growth attributable to increasing circumference according to the formula:
where R is the radius of the tree inside bark and n is the year of tree ring formation. An estimate of bark thickness (5 and 10 mm for stem diameter ≤ 5 and > 5 cm, respectively) was used to estimate radius inside bark. Diameter at breast height (DBH) was measured at the time of core sampling. To examine the mean growth trend of the different age classes, BAI for each year was averaged over all individuals belonging to the same class. The BAI data were not standardized to preserve the long-term growth trend. Unlike in ring width series, age-related trends in BAI are generally positive and do not show a decreasing trend until trees begin to senesce (LeBlanc 1990).
Climate data set and evaluation of influence of climate on radial growth
Total monthly precipitation and mean monthly temperatures were collected at a meteorological station in Ötz (750 m a.s.l.; < 5 km from the study area), reaching back to 1911. Because climatic data showed clear trends during the study period (Fig. 6), all temperature and precipitation series were detrended by applying a 32 yr smoothing spline, i.e., one-third of series length, to prevent biasing of climate-growth analyses. Climate-growth relationships have been tested by means of response function analysis (Fritts 1976) using the software ‘denRFcpb’ of the package biondic (Guiot et al. 2012) for platform R (R core team 2012). A bootstrap procedure with 1000 replications was used to assess the 95 % confidence intervals of the regression coefficients (Guiot 1991). Climate-growth relationships were determined using residual ring width chronologies calculated by program ARSTAN. The climatic data set included mean monthly air temperature (°C) and total monthly precipitation (mm) from May of the year prior growth to September of the year of growth. Significantly influencing months were determined at the 95 % confidence level. Coefficients must also be computed between ring indices and climate variables for several months before the growing season, because the width of an annual ring is an integration of climatically influenced processes occurring over a longer period (Fritts 1976). Additionally, Pearson product-moment correlation coefficients (r) were calculated between seasonal climate variables and radial growth. Seasonal and bimonthly climate variables were calculated by averaging mean monthly air temperatures and adding up monthly precipitation sums during bi- and trimonthly periods (December-February, March-May, June-August, September-November; May-June).
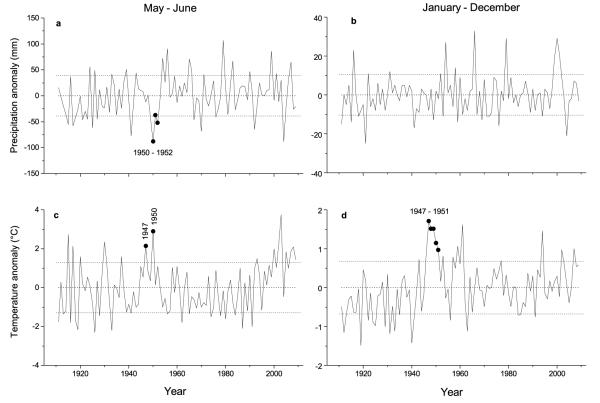
Fig. 6.
Time series plots of May – June and January – December precipitation (a, b) and temperature anomalies (c, d) relative to 1911-2009 average. Long-term means for precipitation and temperature are 158 ± 39 mm (May – June) and 716 ± 104 mm (year) and 13.6 ± 1.3 °C (May – June) and 7.3 ± 0.7 °C (year), respectively. Closed symbols indicate anomalies in climate variables during 1947-1952. Horizontal dashed lines indicate standard deviation.
To analyse long-term changes in environmental conditions within the study area, we developed biological growth curves for each age class by aligning annual increments of individual trees to the biological (cambial) age at sampling height instead of calendar year. Although biological growth curves are independent of the annual variation of climate, long-term changes in climate or stand structure and history can be deduced, when growth rates or growth trends in the early lifespan of different age classes of trees are compared (e.g., Dulamsuren et al. 2010).
Height and diameter growth with tree age were modelled with a Gompertz function, which proved its versatility in describing growth-limiting processes (Zeide 1993). The nonlinear regression procedure included in the Origin software package (OriginLab Corporation, Northampton, MA) was used.
Temporal development of tree establishment
Tree establishment in the past was deduced from first dated annual increment of individual tree ring series, whereby missing rings to the pith in increment cores were estimated via the curvature of inner rings and assuming that stems had a circular shape (Applequist 1958). Missing rings up to coring height at 0.3 and 1 m were approximated based on a linear relationship found between age and tree height of samples taken at the stem base close to the soil surface (Pearson product-moment correlation r = 0.594, P < 0.0001, n = 135). Based on this relationship 13 and 44 yr were added to number of years counted on cores, which were sampled at 0.3 and 1 m stem height, respectively. Although we are aware that there is some inaccuracy inherent in determination of temporal development of tree establishment, the expected shifts along the abscissa in Fig. 4b only amount to a few years and thus do not affect the basic information that can be gathered from this analysis.
Fig. 4.
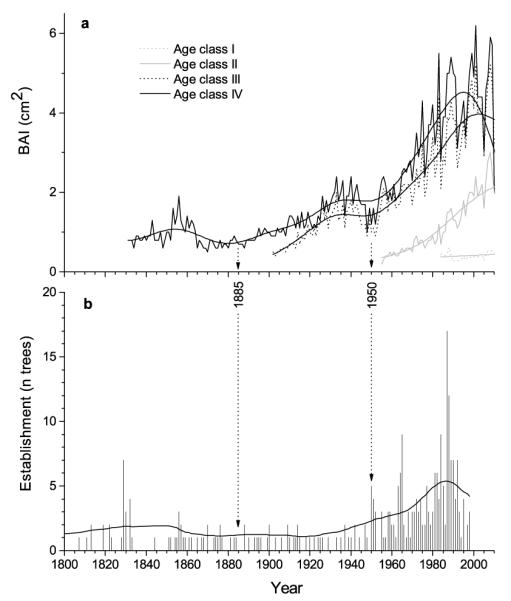
Comparison of a unstandardized basal area chronologies of Picea abies age classes with b tree establishment. Sample size in a is ≥ 10 trees in each age class. Data were smoothed based on fast Fourier transform low-pass filter, whereby the number of points was set to 20.
Results
Chronology descriptive statistics
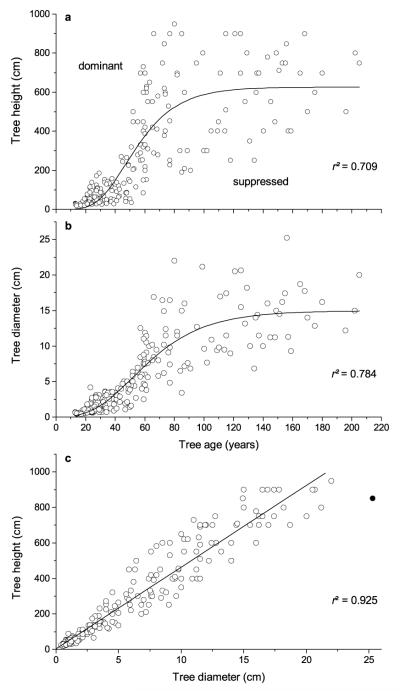
Four ring-width chronologies were developed with the focus on establishing homogeneous age classes (Table 1). Age classes I through IV have mean age of 28, 53, 121 and 173 yr, respectively, at the stem base. Mean ring width ranged from c. 0.4 to 0.7 mm and showed high variability among trees in the youngest age class. Statistics of ring width series revealed lowest values of mean sensitivity, signal-to-noise ratio and common variance accounted for by the first EV in the youngest age class. Age classes II – IV show quite similar SNR, EPS and EV-values when a common time interval was analysed. EPS-values exceeded the suggested threshold of 0.85 in all age classes indicating a strong climate signal in ring-width chronologies. Low first-order autocorrelations indicate that radial growth of age classes I and II was not or was slightly (age classes III and IV) influenced by conditions in the preceding year. Mean height of trees included in age classes I – IV was 1.53, 4.46, 9.10 and 11.29 m, respectively. Tree height and diameter plotted against tree age show a sigmoidal relationship (Fig. 1a-b), whereby data scattering indicate growth-age relationships of dominant and suppressed trees (i.e., data above and below Gompertz curve, respectively). A close linear relationship was found between tree height and diameter (Fig. 1c).
Table 1.
Statistics of developed ring-width chronologies (Autocorr = first-order autocorrelation, MS = mean sensitivity, SD = standard deviation, SNR = signal to noise ratio, EPS = expressed population signal, EV = variance first eigenvector). Mean ± values standard deviation.
| Age class | Trees (n) | Age1 (years) | Mean ring width (μm) | Autocorr2 | MS2 (%) | SD2 | SNR2 | EPS2 | EV2 (%) |
|---|---|---|---|---|---|---|---|---|---|
| I | 52 | 28 ± 9 | 395±307 | 0.051 | 13 | 0.112 | 5.8 | 0.852 | 38.2 |
| II | 58 | 53 ± 12 | 701±393 | 0.011 | 17 | 0.141 | 11.5 | 0.920 | 46.1 |
| III | 37 | 121 ± 24 | 625±317 | 0.203 | 19 | 0.179 | 12.6(9.8) | 0.927(0.907) | 46.5(49.3) |
| IV | 34 | 173 ± 24 | 584±216 | 0.268 | 18 | 0.187 | 19.6(10.1) | 0.951(0.910) | 41.2(54.7) |
Cambial age including estimated age at sampling height
Calculated on the basis of detrended ‘standard’ chronologies, i.e. prior to removing of serial autocorrelation (see Methods); SNR, EPS and EV-values shown for age class II and inside parentheses for age classes III and IV cover the common time interval 1976 to 2011.
Fig. 1.
a Height and b diameter growth with age of Picea abies modelled by applying the Gompertz function. c Linear relationship between tree height and diameter. Closed symbol in c was treated as outlier and not included in Pearson correlation. Corresponding r2 are indicated.
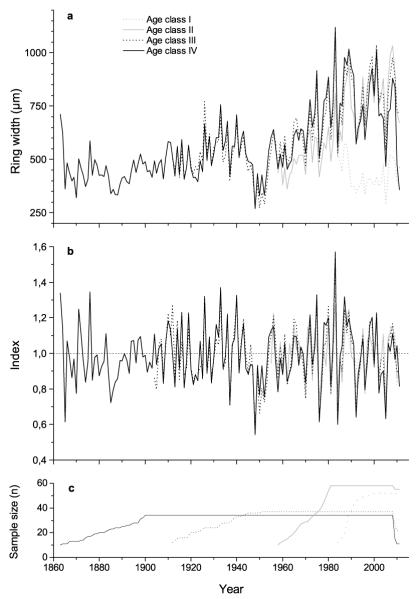
Radial growth of P. abies ring series is characterized by growth depression occurring around 1950, and strikingly increasing growth between c. 1955 and 1990, which is followed by a steady-state in annual increments afterwards. An age-related exponential decrease in ring width is missing (Fig. 2a).
Fig. 2.
Tree ring data of Picea abies age classes. a Ring width series and b residual chronologies used in response function analysis. c Sample size represents the accumulation of number of trees into the sample population over time. Ring series are shown for n ≥ 10 trees.
Effects of climatic factors on radial growth variation
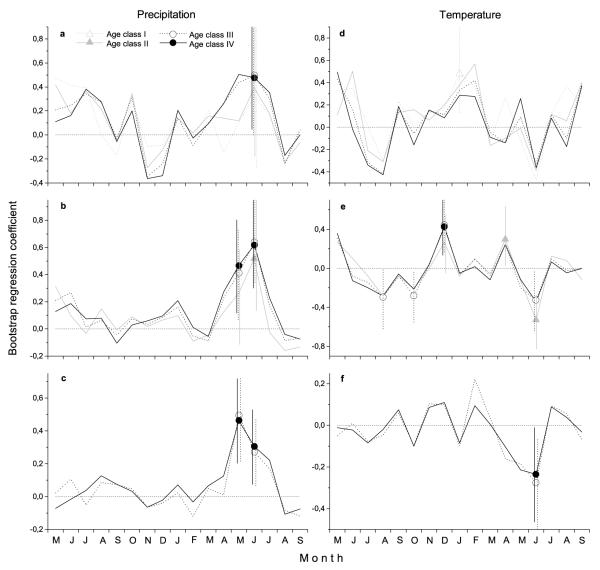
Residual ring width chronologies used for calculation of response functions are depicted in Fig. 2b. Bootstrapped regression coefficients indicated that precipitation (direct relationship) and temperature (inverse relationship) in current May - June significantly influenced radial growth variation of P. abies (Fig. 3). Climate response during similar time periods revealed lower coefficients to May – June precipitation of age classes I and II compared to older trees (Fig. 3a-b, Table 2). While above average June temperature significantly negatively affected annual increments of all but the youngest age class (Fig. 3 e-f), coefficient for January temperature was positive in age class I (Fig. 3d). However, broadly overlapping confidence intervals indicate missing significant difference among age classes in response to climate variables (Fig. 3). Table 2 summarizes Pearson correlation coefficients between seasonal and bimonthly climate variables and P. abies radial growth of different age classes. In the time period covered by all chronologies, i.e. 1984 – 2009, correlation coefficients for May – June precipitation and radial growth were positive and increased with tree age; they were significant for trees older 100 yr (age classes III and IV). Correspondingly, summer precipitation significantly influenced growth variation of oldest P. abies trees only. Coefficients of winter temperatures (positive relationship) declined with tree age. In the period 1955 – 2009 correlation coefficients for spring and May – June precipitation (positive relationships) and May – June temperature (negative relationship) slightly increased with tree age. When climate-growth relationships were evaluated for the maximum period covered by climate data and ring series of trees older then 100 yr (age class III and IV), the climatic factors that most strongly affected growth variation were precipitation and temperature in May – June (positive and negative relationship, respectively). Highly significant correlation coefficients were also detected for spring precipitation (positive relationship) and summer temperature (negative relationship).
Fig. 3.
Bootstrap regression coefficients from prior May to current September based on residual chronologies showing effects of a – c precipitation and d – f mean air temperature on ring-width indices for the time periods 1984-2009 (a, d), 1955-2009 (b, e) and 1911-2009 (c, f). Significantly influencing months (P < 0.05) and 95 % confidence intervals are indicated by symbols and vertical lines, respectively.
Table 2.
Pearson product-moment correlation coefficients (r) between residual ring width chronologies of Picea abies age classes and detrended seasonal and bimonthly climatic variables (P = precipitation, T = mean temperature) for different periods (* P < 0.05, ** P < 0.01, *** P < 0.001).
| Climate variable/Age class | I | II | III | IV | II | III | IV | III | IV |
|---|---|---|---|---|---|---|---|---|---|
| 1984 - 2009 | 1955 - 2009 | 1911 -2009 | |||||||
| Winter P (Dec - Feb) | 0.09 | 0.06 | 0.06 | 0.12 | −0.04 | 0.03 | 0.10 | −0.05 | 0.01 |
| Spring P (Mar - May) | 0.02 | 0.05 | 0.15 | 0.22 | 0.18 | 0.29* | 0.35* | 0.36*** | 0.40*** |
| Summer P (Jun - Aug) | 0.08 | 0.23 | 0.35 | 0.42* | 0.14 | 0.27 | 0.29* | 0.14 | 0.15 |
| May - June P | 0.21 | 0.28 | 0.49*** | 0.55** | 0.45*** | 0.60*** | 0.59*** | 0.57*** | 0.58*** |
| May - June P, prior year | 0.41* | 0.29 | 0.23 | 0.11 | 0.18 | 0.24 | 0.13 | 0.15 | −0.02 |
| Winter T | 0.58** | 0.49** | 0.41* | 0.33 | 0.31* | 0.37** | 0.31* | 0.20* | 0.16 |
| Spring T | 0.05 | −0.12 | −0.06 | −0.06 | −0.02 | 0.02 | 0.01 | −0.09 | −0.11 |
| Summer T | −0.13 | −0.25 | −0.24 | −0.30 | −0.18 | −0.21 | −0.23 | −0.31** | −0.30** |
| May - June T | −0.36 | −0.42* | −0.34 | −0.28 | −0.45** | −0.39** | −0.34* | −0.47*** | −0.46*** |
| May - June T, prior year | 0.39* | 0.37 | 0.41* | 0.35 | 0.32* | 0.29* | 0.25 | 0.04 | 0.11 |
Growth trends and tree establishment
While a temporary release in BAI occurred around 1850, all but the youngest age class showed slightly and strikingly increasing trend in BAI after 1885 and 1950, respectively (Fig. 4a). In early 1990s trend in BAI levelled off and started to decrease in oldest trees. No increasing trend was found in age class I. While tree establishment was predominantly regular during 19th and first half of the 20th century, number of tree establishment strikingly rose consistently with BAI increase after about 1950 until early 1990s, when a decrease in tree recruitment is obvious (Fig. 4b). Tree establishment peaked in 1987 and 1988. Although cones on mature trees were observed, seedlings and saplings < 20 cm height could be found only sporadically, indicating that recent establishment of P. abies is restricted.
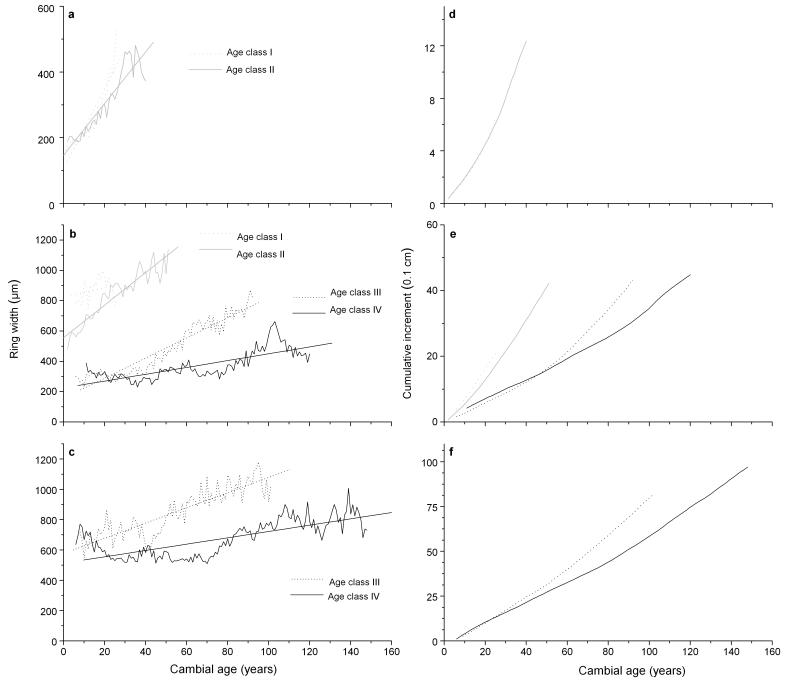
Annual and cumulative biological growth curves (Fig. 5a-f), where the increment is related to the age of the cambium at the sampling height instead of the calendar year show that in more recently established young trees radial growth was predominantly higher and/or increased faster during the first decades of the lifespan than in older trees, irrespective of sampling location (Fig. 5a-c). The slope of linear regression trend lines decrease with mean tree age. All linear regression models describing the relationship between annual increment and cambial age of different age classes were highly significant (P < 0.0001) except for age class I at 0.3 m sampling height (Table 3).
Fig. 5.
a – c Growth curves and d – f cumulative increments of Picea abies age classes related to cambial age. Sample size is ≥ 10 trees in each age class. Model statistics are given in Table 3. Growth curves and cumulative increments were calculated from discs taken at stem base (a, d) and core samples taken at 0.3 m (b, e) and 1 m (c, f).
Table 3.
Regression model statistics of linear trend in growth curves of Picea abies age classes at different sampling height
| Age class | Sampling height | r2 | P | Slope | Constant |
|---|---|---|---|---|---|
| I | stem base | 0.86 | <0.001 | 12.076 ± 1.009 | 107.33 |
| II | stem base | 0.86 | <0.001 | 7.856 ± 0.524 | 147.22 |
| I | 0.3 m | 0.26 | 0.247 | 3.317 ± 2.775 | 820.74 |
| II | 0.3 m | 0.87 | <0.001 | 10.721 ± 0.586 | 556.68 |
| III | 0.3 m | 0.90 | <0.001 | 6.632 ± 0.236 | 156.93 |
| IV | 0.3 m | 0.58 | <0.001 | 2.255 ± 0.187 | 224.70 |
| III | 1 m | 0.77 | <0.001 | 5.013 ± 0.286 | 576.97 |
| IV | 1 m | 0.53 | <0.001 | 2.090 ± 0.165 | 513.19 |
Time-series plots of the key climate variables, i.e., May – June precipitation and temperature indicated that several warm-dry years were recorded from 1950 through 1952, which were followed by cool-moist years until mid 1960s (Fig. 6a). While no long-term trend in precipitation is detectable during 1911 - 2010 (Fig. 6a-b), an increasing trend in May – June temperature is obvious after early 1990s (Fig. 6c). Mean annual temperature was > 1.5 °C above long-term average during 1947 through 1949 (Fig. 6d). In 1950 the second hottest summer (June through August) was recorded since 1911, exceeding long-term average by 2.4 °C (data not shown).
Discussion
Age/size dependence of climate-growth relationship
Consistent with our first hypothesis, climate-growth relationships revealed differences among P. abies age classes in response to climatic factors. Although we could not detect statistically significant differences among P. abies age classes in response to monthly climate variables, correlation coefficients of current May – June precipitation sum (i.e., the climatic factor that most closely is related to growth variation) increased with tree age, indicating more pronounced radial growth limitation by water availability in older trees. Ageing is associated with increase in size and eco-physiological studies have shown that size-related changes in functional processes occur in trees (Mencuccini et al. 2005). Hence, different drought sensitivity, i.e., the relative impact of precipitation and temperature on radial growth of young vs. old trees found in our study might be related to tree size, rather than tree age per se (i.e., cellular senescence). De Luis et al. (2009) found that climate–growth relationship of Pinus halepensis and Pinus pinea on a semiarid site are modulated by tree-size. Similarly, Carrer and Urbinati (2004) reported that climate variables become more limiting for radial growth with age of timberline conifers. In a multi-species analysis of size-mediated climate-growth relationships Mérian and Lebourgeois (2011) found that generally larger trees were more sensitive to drought than smaller trees especially under xeric climate. However, authors found no significant differences in sensitivity to climate between two size classes of P. abies (c. 40 and 50 cm DBH). This discrepancy compared to our results is most likely due to quite larger range of tree size analyzed in our study (c. 1 - 20 cm diameter at stem base) and/or more xeric conditions prevailing at our study site, where P. abies exists at the boundary of drought tolerance. Within the study area radial growth reduction of P. abies to heat-wave in 2003 was also more pronounced in large than small diameter trees, i.e. > 25 and 10 - 25 cm DBH, respectively (Pichler and Oberhuber 2007). We suggest that lower water demand of small trees and reduced transpiration forcing due to lower vapour pressure deficit and wind velocity below canopy, are responsible for these findings. That trees of different sizes are affected by micro-climatic variations and smaller trees take advantage of canopy atmosphere was proposed by Mérian and Lebourgeois (2011). It was also reported by several authors that hydraulic constraints and nutrient limitation increase as trees grow taller (Martínez-Vilalta et al. 2007), whereby these effects increase with climatic xericity (Mérian and Lebourgeois 2011). Additionally, decreasing growing space above ground and below ground due to successive canopy closure and shallow stony soils with low water holding capacity, respectively, most likely increased inter-tree competition for scarce resources (water, nutrients). These findings explain higher climate sensitivity of older, i.e., larger trees, within the drought-prone and nutrient poor study area.
Change in timing or duration of cambial activity and/or xylem cell formation with increasing tree age might be another explanation of our findings. Rossi et al. (2008) reported that younger conifers (< 80 yr) at the timberline show longer xylogenetic activity than older trees (> 250 yr), which produces a dilution (attenuation) of the climatic signal over a longer period. Consistently, Szeicz and MacDonald (1995) found that the period with significant positive responses to climate variables became shorter with tree age.
High sensitivity of old P. abies trees (mean age 121 and 174 yr) to May – June precipitation found in this study is in agreement with previous results on the same species (Pichler and Oberhuber 2007) and indicates that radial tree growth is primarily limited by precipitation. That at lower altitudes P. abies is sensitive to summer drought was also reported by e.g., Battipaglia et al. (2009). Several authors documented that a decrease in water status of a tree affects cell expansion and the frequency of cambial cell division (Abe and Nakai 1999). The inverse relationship between May – June temperature and growth can be interpreted as an indirect influence of water deficit on annual increments, because high temperatures impair soil water balance because of increased evapotranspiration. The lagged response to previous May - June precipitation might be related to more favourable conditions for carbohydrate storage, growth of fine roots and mycorrhiza and/or bud development (Pallardy 2008).
The size dependent direct response to winter temperature (December-February) might indicate influence of early snow melt and soil thawing on start of the growing season. Earlier warming of upper soil layers might be favourable to root and shoot growth of small trees, which possibly develop a more superficial root system than large old trees. Repo et al. (2008) reported that delayed soil thawing can reduce growth of Scots pine saplings. On the other hand, mild temperatures in winter and absence of frosts in understorey could favor early photosynthesis, increasing carbohydrate storage for current year growth.
Tree establishment and growth release
Successively more favourable conditions for ‘invasion’ of P. abies in mixed stands within the study area in recent decades are indicated by (i) synchronous increase in BAI and tree establishment and (ii) faster radial growth increase in early lifespan of more recently established trees, than in trees that established > 100 yr ago. Trees that originated in the understorey are expected to have low initial growth rates with an increasing trend afterwards, while trees with a declining or no trend are considered to have originated in large gaps, with unobstructed access to the canopy (Frelich and Graumlich 1994). Canopy disturbances become apparent in tree ring series by subsequent growth release (Lorimer and Frelich 1989). Correspondingly, decades-long suppressed growth of trees belonging to age classes III and IV most likely indicate that they established under higher stand density, i.e., closed canopy, compared to more recently established trees (age classes I and II). However, changing climate conditions as a cause of different growth trends have also to be considered. Slight release of BAI after mid-1880s might be related to loss of vitality of canopy trees as a result of a continuous drought period lasting 30 days from March through April 1885 (data not shown). This is supported by striking abrupt growth reductions in 1885 found in dominant P. sylvestris at xeric sites (Oberhuber 2001). Consistently, the distinct increase in BAI starting in early 1950s of all except the youngest age class and concurrent with increase in tree establishment most likely indicates gap formation related to drought induced mortality in canopy trees as a result of warm-dry conditions prevailing from late 1940s through early 1950s. This interpretation remains speculative, however, because rapid decay of dead trees precludes exact dating and determining causes of gap formation.
Canopy openings result in changed environmental conditions, including improved light conditions and increased soil water content by positively influencing throughfall, reducing total evapo-transpiration at stand level and retention of rainfall in tree canopy (Aussenac and Granier 1988). Several authors have related increased tree growth of thinned stands to reduced soil water stress due to apportioning available soil water among fewer trees (e.g., Aussenac and Granier 1988). Furthermore, although P. abies is a moderately shade-tolerant species, photosynthate production is improved through canopy openings, while seedling establishment is strongly favoured by slightly increased light levels in small canopy gaps (Drobyshev 1999). It is well known that P. abies as a late-successional species is able to regenerate in cool and shady conditions under the forest canopy (Hunziker and Brang 2005), while light demanding pioneer species P. sylvestris and L. decidua can only establish in large gaps within the study area. Since establishment of P. abies before 1950 occurred only sporadically, the present study indicates that seed germination requires sufficient soil moisture and/or light supply. This interpretation is consistent with findings that occurrence of P. abies seedlings is related to the presence of mosses (Hanssen 2003; Hunziker and Brang 2005) and that establishment success of tree species is affected by water balance in the upper soil layers (Ditmarová et al. 2010). Hence, our results are in contrast to our starting hypothesis, which stated that a continuous recruitment rather than disturbance driven establishment of P. abies occurred in these mixed forest stands. Decrease in root competition after gap formation most likely enabled surviving trees to expand their root system into a larger area to exploit water and nutrients, which favoured successive increase in BAI after 1950. Because soil water content throughout the growing season was found to be constantly higher close to the soil surface than in deeper soil layers (data not shown), fine roots of P. abies, which are distributed primarily in upper soil layers (Schmid and Kazda 2002), might be very efficient in absorbing scattered low rainfall events. Although ecological conditions after gap formation are suitable for early seedling establishment and further growth into sapling stage, most saplings are more likely to die than to grow up and develop into mature dominant trees due to increase in inter-tree competition for light and/or soil moisture (Abrams and Mostoller 1995). Consistently, recent decrease in tree establishment in early 1990s and levelling of BAI during last decade in all age classes are related to canopy closure and increased inter-tree competition for scarce resources (water, nutrients), possibly aggravated by recent temperature increase in May-June.
It is implausible that an increase in atmospheric nitrogen deposition by precipitation and/or increased N-mineralization rate due to climate warming caused improvement of nutrient regime, which favoured P. abies establishment and BAI because co-occurring P. sylvestris and L. decidua showed only a temporary increase in BAI and a distinct decreasing trend in recent decades, respectively (Schuster and Oberhuber 2012). It is also well known that tree rejuvenation is strongly affected by browsing pressure. However, wildlife and livestock grazing is negligible within the study area (S. Vogl, personal communication). Therefore, a reduction in number of animals as a possible cause of increased establishment of P. abies after 1950 can be excluded. Furthermore, striking BAI increase of large trees (age classes III-IV) for several decades can not be explained by reduced browsing pressure in the understorey.
Conclusion
Coupling of analyses of climate-growth relationships, basal area increment and establishment dates greatly increased our understanding of regeneration and growth of P. abies in a mixed-conifer forest exposed to drought. Our results illustrate that in coniferous stands of the central Alps composed of late-successional P. abies and the pioneer species P. sylvestris and L. decidua establishment and growth of P. abies are not counteracted by drought. We conclude that stand dynamics at dry-mesic sites within the study area is controlled by competition for light and water, whereby shade-tolerance, a protecting canopy and shallow rooting provide P. abies a competitive advantage over more drought-tolerant P. sylvestris, until increase in inter-tree competition for water in dense stands exceeds physiological thresholds for drought resistance. Analyses of competitive interactions among co-occurring conifers below ground, i.e., species-specific morphological and physiological responses of the root system to drought, are needed to prove suggested processes in our study.
Acknowledgments
This work was supported by the Austrian Science Fund (FWF Project No. P22280-B16 “Conifer radial stem growth in response to drought”). We thank Barbara Zeisler for help with measurement of tree-ring width, Benjamin Dietre for introduction to R-statistics and Sepp Vogl, Imst, for details regarding wildlife stock and anthropogenic influences within the study area. We also thank anonymous reviewers for their valuable suggestions and comments to improve the manuscript. We acknowledge Hydrographischer Dienst, Innsbruck, for providing us the climate data.
References
- Abe H, Nakai T. Effect of the water status within a tree on tracheid morphogenesis in Cryptomeria japonica D. Don. Trees. 1999;14:124–129. [Google Scholar]
- Abrams MD, Mostoller SA. Gas exchange, leaf structure and nitrogen in contrasting successional tree species growing in open and understory sites during a drought. Tree Physiol. 1995;15:361–370. doi: 10.1093/treephys/15.6.361. [DOI] [PubMed] [Google Scholar]
- Allen CD, Macalady AK, Chenchouni H, Bachelet D, McDowell N, Vennetier M, Kitzberger T, Rigling A, Breshears DD, Hogg EH, Gonzalez P, Fensham R, Zhang Z, Castro J, Demidova N, Lim J-H, Allard G, Running SW, Semerci A, Cobb N. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manage. 2010;259:660–684. [Google Scholar]
- Applequist MB. A simple pith locator for using with off-center increment cores. J. Forestry. 1958;56:141. [Google Scholar]
- Attiwill PM. The disturbance of forest ecosystems: the ecological basis for conservative management. For. Ecol. Manage. 1994;63:247–300. [Google Scholar]
- Aussenac G, Granier A. Effects of thinning on water stress and growth in Douglas-fir. Can. J. For. Res. 1988;18:100–105. [Google Scholar]
- Battipaglia G, Saurer M, Cherubini P, Siegwolf RTW, Cotrufo MF. Tree rings indicate different drought resistance of a native (Abies alba Mill.) and a nonnative (Picea abies (L.) Karst.) species co-occurring at a dry site in Southern Italy. For. Ecol. Manage. 2009;257:820–828. [Google Scholar]
- Bigler C, Bräker OU, Bugmann H, Dobbertin M, Rigling A. Drought as an inciting mortality factor in Scots pine stands of the Valais, Switzerland. Ecosystems. 2006;9:330–343. [Google Scholar]
- Briffa KR, Jones PD. Basic chronology statistics and assessment. In: Cook ER, Kairiukstis L, editors. Methods of Dendrochronology. Applications in the Environmental Sciences. Kluwer Academic Publishers; Dordrecht: 1990. pp. 137–153. [Google Scholar]
- Carrer M, Urbinati C. Age-dependent tree-ring growth responses to climate in Larix decidua and Pinus cembra. Ecology. 2004;85(3):730–740. [Google Scholar]
- Castro J, Zamora R, Hódar JA, Gómez JM. Alleviation of summer drought boosts establishment success of Pinus sylvestris in a Mediterranean mountain: an experimental approach. Plant Ecol. 2005;181:191–202. [Google Scholar]
- Cook ER, Holmes RL. Program ARSTAN User Manual. Laboratory of Tree Ring Research, University of Arizona; Tucson, USA: 1984. [Google Scholar]
- De Luis M, Novak K, Cufar K, Raventos J. Size mediated climate-growth relationships in Pinus halepensis and Pinus pinea. Trees. 2009;23:1065–1073. [Google Scholar]
- Ditmarová L, Kurjak D, Palmroth S, Kmet J, Střelcová K. Physiological responses of Norway spruce (Picea abies) seedlings to drought stress. Tree Physiol. 2010;30:205–213. doi: 10.1093/treephys/tpp116. [DOI] [PubMed] [Google Scholar]
- Drobyshev IV. Regeneration of Norway spruce in canopy gaps in Sphagnum-Myrtillus old-growth forests. For. Ecol. Manage. 1999;115:71–83. [Google Scholar]
- Dulamsuren C, Hauck M, Khishigjargal M, Leuschner HH, Leuschner C. Diverging climate trends in Mongolian taiga forests influence growth and regeneration of Larix sibirica. Oecologia. 2010;163:1091–1102. doi: 10.1007/s00442-010-1689-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberg H, Leuschner C. Vegetation Mitteleuropas mit den Alpen in ökologischer, dynamischer und historischer Sicht. Ulmer; Stuttgart: 2010. [Google Scholar]
- FAO . World reference base for soil resources. Vol. 103. FAO, World Soil Resources Reports; Rome: 2006. [Google Scholar]
- Frelich LE, Graumlich LJ. Age-class distribution and spatial patterns in an old-growth hemlock-hardwood forest. Can. J. For. Res. 1994;24:1939–1947. [Google Scholar]
- Fritts HC. Tree rings and climate. Academic Press; London: 1976. [Google Scholar]
- Grissino-Mayer HD. Evaluating crossdating accuracy: a manual and tutorial for the computer program COFECHA. Tree-Ring Res. 2001;57:205–221. [Google Scholar]
- Guiot J. The bootstrapped response function. Tree-Ring Bull. 1991;51:39–41. [Google Scholar]
- Guiot J, Brewer S, Gally Y. Bioindic: Statistic analyses for environmental bioindicators. 2012 R package version 4.0.4. URL http://www.cerege.fr/?id_rubrique=3&masque=inc-presentation&id_article=29165.
- Hanssen KH. Natural regeneration of Picea abies on small clear-cuts in SE Norway. For. Ecol. Manage. 2003;180:199–213. [Google Scholar]
- Holmes RL. Dendrochronology program library user’s manual. Laboratory of Tree-Ring Research University of Arizona; Tucson, USA: 1994. [Google Scholar]
- Hughes MK. Dendrochronology in climatology – the state of the art. Dendrochronologia. 2002;20(1-2):95–116. [Google Scholar]
- Hunziker U, Brang P. Microsite patterns of conifer seedling establishment and growth in a mixed stand in the southern Alps. For. Ecol. Manage. 2005;210:67–79. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC) Climate Change 2007: The Physical Science Basis: Contribution of Working Group I to the Fourth Assessment Report of the IPCC. Cambridge University Press; Cambridge: 2007. [Google Scholar]
- LeBlanc DC. Relationships between breast-height and whole-stem growth indices for red spruce on Whiteface mountain, New York. Can. J. For. Res. 1990;20:1399–1407. [Google Scholar]
- Lorimer CG, Frelich LE. A method for estimating canopy disturbance frequency and intensity in dense temperate forests. Can. J. For. Res. 1989;19:651–663. [Google Scholar]
- Martínez-Vilalta J, Vanderklein D, Mencuccini M. Tree height and age-related decline in growth in Scots pine (Pinus sylvestris L.) Oecologia. 2007;150:529–544. doi: 10.1007/s00442-006-0552-7. [DOI] [PubMed] [Google Scholar]
- Mencuccini M, Martínez-Vilalta J, Vanderklein D, Hamid HA, Korakaki E, Lee S, Michiels B. Size-mediated ageing reduces vigour in trees. Ecol. Lett. 2005;8:1183–1190. doi: 10.1111/j.1461-0248.2005.00819.x. [DOI] [PubMed] [Google Scholar]
- Mérian P, Lebourgeois F. Size-mediated climate-growth relationships in temperate forests: A multi-species analysis. For. Ecol. Manage. 2011;261:1382–1391. [Google Scholar]
- Moser B, Temperli C, Schneiter G, Wohlgemuth T. Potential shift in tree species composition after interaction of fire and drought in the Central Alps. Eur. J. For. Res. 2010;129:625–633. [Google Scholar]
- Oberhuber W, Stumböck M, Kofler W. Climate-tree-growth relationships of Scots pine stands (Pinus sylvestris L.) exposed to soil dryness. Trees. 1998;13:19–27. [Google Scholar]
- Oberhuber W. The role of climate in the mortality of Scots pine (Pinus sylvestris L.) exposed to soil dryness. Dendrochronologia. 2001;19(1):45–55. [Google Scholar]
- Pallardy SG. Physiology of woody plants. Academic Press, Elsevier; Amsterdam: 2008. [Google Scholar]
- Pichler P, Oberhuber W. Radial growth response of coniferous forest trees in an inner Alpine environment to heat-wave in 2003. For. Ecol. Manage. 2007;242:688–699. [Google Scholar]
- R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. ISBN 3-900051-07-0, URL http://www.R-project.org/ [Google Scholar]
- Repo T, Lehto T, Finér L. Delayed soil thawing affects root and shoot functioning and growth in Scots pine. Tree Physiol. 2008;28:1583–1591. doi: 10.1093/treephys/28.10.1583. [DOI] [PubMed] [Google Scholar]
- Rigling A, Bigler C, Eilmann B, Feldmeyer-Christe E, Gimmi U, Ginzler C, Graf U, Mayer P, Vacciano G, Weber P, Wohlgemuth T, Zweifel R, Dobbertin M. Driving factors of a vegetation shift from Scots pine to pubescent oak in dry Alpine forests. Glob. Ch. Biol. 2012 doi: 10.1111/gcb.12038. doi:10.1111/gcb.12038. [DOI] [PubMed] [Google Scholar]
- Rossi S, Deslauriers A, Anfodillo T, Carrer M. Age-dependent xylogenesis in timberline conifers. New Phytol. 2008;177:199–208. doi: 10.1111/j.1469-8137.2007.02235.x. [DOI] [PubMed] [Google Scholar]
- Schmid I,, Kazda M. Root distribution of Norway spruce in monospecific and mixed stands on different soils. For. Ecol. Manage. 2002;159:37–47. [Google Scholar]
- Schuster R, Oberhuber W. Drought sensitivity of three co-occurring conifers within a dry inner Alpine environment. Trees. 2012 doi: 10.1007/s00468-012-0768-6. doi:10.1007/s00468-012-0768-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard SW, Sachs DL. Assessment of interspecific competition using relative height and distance indices in an age sequence of seral interior cedar-hemlock forests in British Columbia. Can. J. For. Res. 2004;34:1228–1240. [Google Scholar]
- Suarez ML, Kitzberger T. Recruitment patterns following a severe drought: long-term compositional shifts in Patagonian forests. Can. J. For. Res. 2008;38:3002–2010. [Google Scholar]
- Szeicz JM, MacDonald GM. Age dependent tree ring growth responses of subarctic white spruce to climate. Can. J. For. Res. 1994;24:120–132. [Google Scholar]
- Szeicz JM, MacDonald GM. Dendroclimatic reconstruction of summer temperatures in northwestern Canada since AD 1638 based on age-dependent modelling. Quat. Res. 1995;44:257–266. [Google Scholar]
- Wigley TM, Briffa KR, Jones PD. On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J. Clim. Appl. Met. 1984;23:201–213. [Google Scholar]
- Zeide B. Analysis of growth equations. For. Sci. 1993;39:594–616. [Google Scholar]