Abstract
Human T cell leukemia viruses (HTLVs) are complex human retroviruses of the Deltaretrovirus genus. Four types have been identified thus far, with HTLV-1 and HTLV-2 much more prevalent than HTLV-3 or HTLV-4. HTLV-1 and HTLV-2 possess strictly related genomic structures, but differ significantly in pathogenicity, as HTLV-1 is the causative agent of adult T cell leukemia and of HTLV-associated myelopathy/tropical spastic paraparesis, whereas HTLV-2 is not associated with neoplasia. HTLVs code for a protein named Tax that is responsible for enhancing viral expression and drives cell transformation. Much effort has been invested to dissect the impact of Tax on signal transduction pathways and to identify functional differences between the HTLV Tax proteins that may explain the distinct oncogenic potential of HTLV-1 and HTLV-2. This review summarizes our current knowledge of Tax-1 and Tax-2 with emphasis on their structure, role in activation of the NF-κB (nuclear factor kappa-B) pathway, and interactions with host factors.
Keywords: HTLV, Tax proteins, signal transduction, NF-κB
INTRODUCTION
The Human T cell leukemia viruses (HTLVs) are complex retroviruses, belonging to the primate T-lymphotropic virus (PTLV) family. HTLVs are classified as Deltaretroviruses, together with bovine leukemia virus (BLV) and simian T-lymphotropic viruses (STLVs). HTLV-1 was originally described in 1980 (Poiesz et al., 1980) and was the first oncogenic retrovirus discovered in humans (reviewed by Gallo, 2011; Currer et al., 2012). HTLVs originated in Africa around 30,000–40,000 years ago through cross-species transmission of STLVs from monkeys to man. The virus evolved to HTLV and spread to different geographic regions with human migration (Van Dooren et al., 2001). STLVs with high homology to HTLVs are still present in Africa (Hajj et al., 2012). HTLVs are transmitted both vertically and horizontally (reviewed in Watanabe, 2011; Yasunaga and Matsuoka, 2011; Lairmore et al., 2012) but cell-to-cell transmission is essential and occurs through direct contact through the formation of a virological synapse (Nejmeddine et al., 2005; Asquith et al., 2007; Majorovits et al., 2008).
HTLV-1 has received much scientific attention due to its ability to transform primary human T-lymphocytes in cell culture and its association with a neoplasia and a neuropathology (Matsuoka and Jeang, 2007). The most important HTLV-1-associated diseases are the adult T cell leukemia (ATL), a very aggressive form of leukemia, and the HTLV-associated myelopathy/tropical spastic paraparesis (HAM/TSP), a neurological demyelinating disease (Osame et al., 1986; Bangham and Osame, 2005; Yoshida, 2010; Gessain and Mahieux, 2012; Yamagishi and Watanabe, 2012). Three additional genotypes of HTLV, named HTLV-2, HTLV-3, and HTLV-4, have been isolated and characterized, with HTLV-2 the most common after HTLV-1 (Manns and Blattner, 1991; Mahieux and Gessain, 2009). Although HTLVs share a similar genomic structure, HTLV-2 is much less pathogenic than HTLV-1 since it does not cause neoplastic disorders and is sporadically associated with cases of subacute myelopathy (Feuer and Green, 2005). Understanding the molecular basis of the different pathogenicity of HTLV-1 and HTLV-2 may thus provide important clues to the molecular mechanisms of cancer. All HTLVs possess an open reading frame (ORF) encoding the Tax transactivator, which is essential for proviral gene expression from the viral long terminal repeat (LTR) promoter and also regulates the expression and function of a number of cellular genes and proteins (Feuer and Green, 2005; Wycuff and Marriott, 2005; Calattini et al., 2006; Chevalier et al., 2006). Tax alone is capable of modulating several pathways by activating the transcription factors nuclear factor kappa-B (NF-κB) and cyclic AMP responsive binding protein (CREB; Currer et al., 2012; Tang et al., 2013a). Tax also interacts with proteins controlling cell cycle checkpoints (Akagi et al., 1996; Chlichlia and Khazaie, 2010). HTLV-1 Tax (Tax-1) is necessary and sufficient for T cell immortalization (Akagi et al., 1995) and an ATL-like syndrome has been observed in transgenic mice expressing Tax in the T cell compartment (Ohsugi, 2013). It is noteworthy to mention that in ATL patients, Tax expression is silenced in about 50% of the patients. This observation, along with the fact that Tax is capable of transforming primary T lymphocytes in vitro, suggests that Tax might be important for establishing the leukemic phenotype of ATL, but may become dispensable for its maintenance. In addition, recent studies suggest that other viral gene products may play relevant roles in HTLV-1-mediated transformation and may be responsible of the different HTLV-1 and HTLV-2 pathogenicity, including the antisense HTLV-1 genome transcript HBZ (Matsuoka and Jeang, 2011; Lairmore et al., 2012; Satou and Matsuoka, 2012; Zhao and Matsuoka, 2012), the HTLV-1 accessory protein p12 (Kannian and Green, 2010) and p13 (Silic-Benussi et al., 2010a, b).
Tax-1 has been very extensively studied and its interactome includes more than 100 proteins (for a recent review see Currer et al., 2012). To further clarify this point and to better understand the reasons for the difference in pathogenicity between HTLV-1 and HTLV-2 as well as from other HTLVs, in this review we have focused on the structural and functional properties of the different Tax proteins and their relations with other viral and cellular factors. The following sections highlight recent advances in the comprehension of: (i) the transformation potential of Tax-1, Tax-2, Tax-3, and Tax-4 proteins; (ii) Tax’s role in the deregulation of signal transduction focusing on studies which describe novel interactions of Tax proteins with host factors and contribute to the understanding of the molecular mechanisms of cell response to viral infection; and (iii) Tax-mediated activation of the NF-κB pathway focusing on differences between Tax-1 and Tax-2 involvement in canonical and non-canonical pathways.
SPECIFIC FEATURES OF DIFFERENT HTLV GENOTYPES
Following the discovery of HTLV-1 in 1980, three additional HTLVs were found: HTLV-2 in 1982 (Kalyanaraman et al., 1982; Vandamme, 2000; Gallo, 2002) and HTLV-3 and HTLV-4 in 2005 (Calattini et al., 2005; Wolfe et al., 2005). These four genotypes show specific geographical areas of distribution. HTLV-1, which includes seven subtypes (HTLV-1A to -1G), is endemic in Japan, sub-Saharan Africa, South America, the Caribbean Islands, and Melanesia. About 5–10 million people worldwide are infected with HTLV-1, most of whom are expected to remain asymptomatic throughout their lifetime (Gessain and Cassar, 2012). An estimated 2–5% of infected people develop clinical complications including ATL, HAM/TSP, infective dermatitis, uveitis, arthritis, and infection by Strongyloides stercoralis (Gonçalves, 2010). HTLV-2, for which the four subtypes -2A to -2D are known, is endemic within the Amerindian and Pygmy populations, and was found to be epidemic in intravenous drug users (Feuer and Green, 2005). In contrast to HTLV-1, HTLV-2 does not cause proliferative blood diseases. However, HTLV-2 has been linked to neurological disorders, arthritis, pneumonia, and with increased mortality (Araujo and Hall, 2004; Roucoux and Murphy, 2004; Biswas et al., 2010). The two new genotypes, termed HTLV-3 and HTLV-4, were discovered in asymptomatic individuals from Cameroon (Calattini et al., 2005; Wolfe et al., 2005); the pathogenic potential of these viruses is still unknown. HTLV-3 is closely related to the simian virus STLV-3, whereas an STLV corresponding to HTLV-4 has not yet been found (Sintasath et al., 2009).
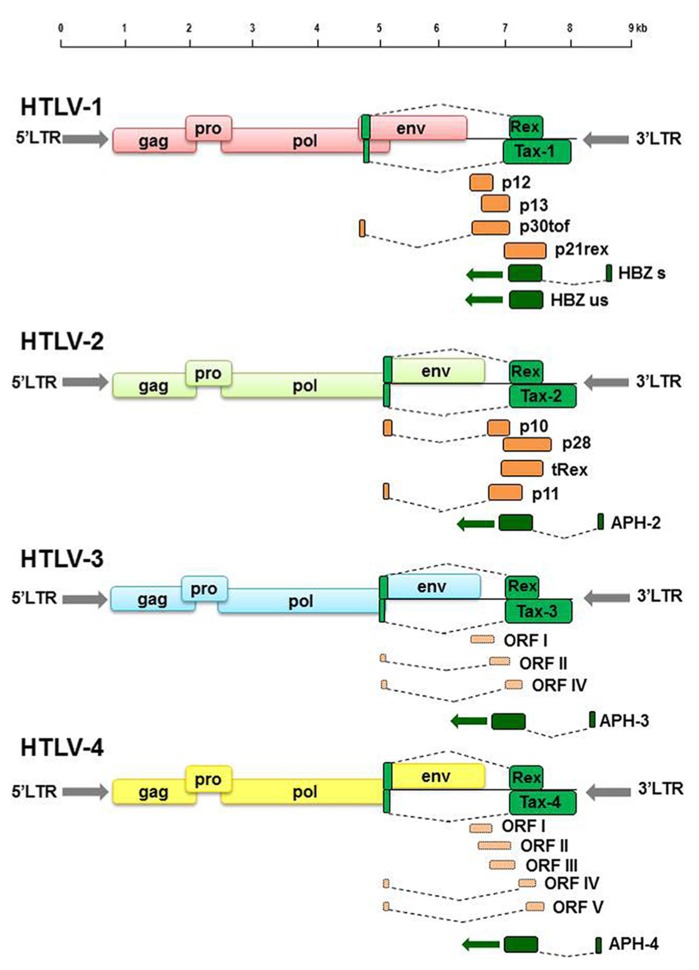
Comparative studies of the genomic sequences of all four HTLV genotypes have highlighted common as well as unique molecular features. HTLV-1 and HTLV-2 have a similar genomic structure and share approximately 70% nucleotide sequence homology (Feuer and Green, 2005). HTLV-3 and HTLV-4 have a genomic organization which is similar to that of HTLV-1 and HTLV-2 with the presence of gag, pro, pol, and env ORFs as well as of tax and rex, whereas ORFs for auxiliary proteins still need to be confirmed (Gessain et al., 2013; Figure 1). HTLV-3 shares about 62% identity with HTLV-1 and HTLV-4 shares 62–71% nucleotide similarity with HTLV-1, HTLV-2, and HTLV-3 (Switzer et al., 2006). HTLV-3 and HTLV-4 present LTRs that lack the distal 21 bp transcription regulatory repeat sequence (Switzer et al., 2006). Both HTLV-3 and HTLV-4 present on the antisense strand a potential ORF named APH-3 and APH-4, respectively (antisense protein of HTLV), analogous to the HBZ gene of HTLV-1 (Larocque et al., 2011) and APH-2 of HTLV-2 (Halin et al., 2009). Sequence alignment indicated that APH-3 and APH-4 are more closely related to APH-2 than to HBZ (Larocque et al., 2011). The proteins also present some differences, as APH-2, APH-3, and APH-4 do not contain a consensus bZIP (basic leucine zipper) domain present in HBZ, and differ in their subcellular localization as compared to HBZ (Halin et al., 2009; Larocque et al., 2011).
FIGURE 1.
Schematic representation of HTLV-1, HTLV-2, HTLV-3, and HTLV-4 genomic organization. Green colored boxes indicate ORF encoding regulatory proteins. Dark orange colored boxes indicate ORF encoding auxiliary proteins. Light orange colored boxes indicate putative ORF deduced by genomic sequences analyses.
COMPARISON OF Tax PROTEINS STRUCTURES
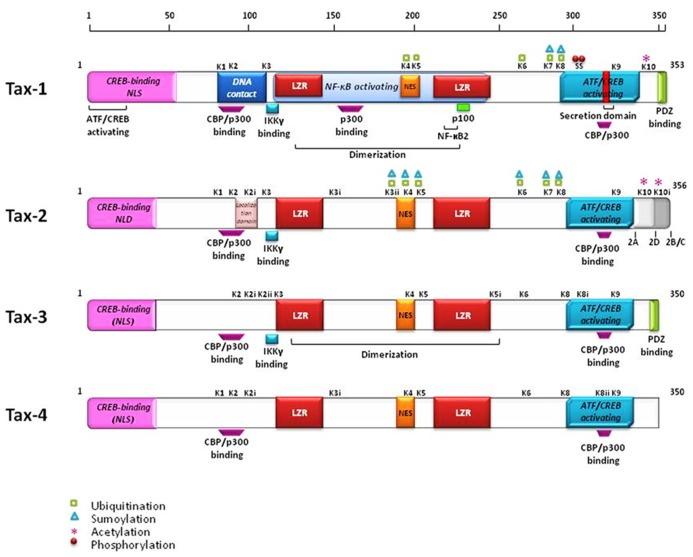
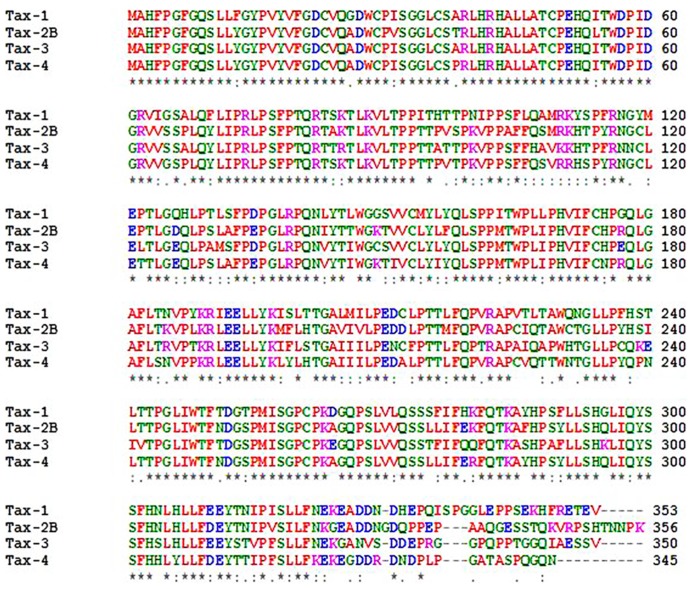
The tax gene is encoded within the pX region of HTLV, located between env and the 3′-LTR, and is highly conserved among all four genotypes and different subtypes of the virus (Currer et al., 2012). The Tax protein is common to all primate members of the PTLVfamily. The primary amino acids (aa) sequence of Tax-1 is composed of 353 residues, and is organized into functional domains that have been extensively investigated (Bertazzoni et al., 2011; Currer et al., 2012). The structural and functional domains of Tax-1, Tax-2, Tax-3, and Tax-4 are shown in Figure 2. The N-terminal region of Tax-1 contains a CREB-binding region (Yin et al., 1995) that spans aa 1–60. This region is involved in the interaction with ATF/CREB transcription factors and represents a binding domain required for interaction with proteins involved in transcription, cell cycle progression, and cell signaling regulation (Suzuki et al., 1993a; Goren et al., 1995). All Tax proteins contain the CREB-binding domain within their N-terminus. A nuclear localization signal (NLS) is located within the first 60 aa in Tax-1 (Smith and Greene, 1992), Tax-3 and Tax-4 (Calattini et al., 2006; Switzer et al., 2009). A nuclear localization determinant (NLD) is present within the first 42 aa in Tax-2 (Sheehy et al., 2006; Turci et al., 2006a). An additional localization domain is attributed to Tax-2 at aa position 90–100, which confers to the protein a more abundant accumulation into the cytoplasm as compared to Tax-1 (Meertens et al., 2004a). All four Tax proteins contain a conserved region representing a nuclear export sequence (NES) that has been functionally characterized in both Tax-1 and Tax-2 and is located at aa position 189–202 in Tax-1 (Alefantis et al., 2003; Chevalier et al., 2005). Two leucine zipper-like motif regions (LZRs) are present at aa 116–145 and 213–248 in Tax-1 and conserved in Tax-2, Tax-3, and Tax-4 as well. These regions are required for protein dimerization and binding of cellular factors (Jin and Jeang, 1997; Basbous et al., 2003). Tax-1 and Tax-3 are characterized by the presence of a PDZ-binding motif (PBM) at the C-terminal region (Chevalier et al., 2006) whereas this is missing in Tax-2 and Tax-4. This domain is required for interactions between Tax-1 and cellular factors such as the tumor suppressors hDlg, MAGI-1, and Scribble and the synapse-associated protein DlgI (Rousset et al., 1998; Suzuki et al., 1999; Okajima et al., 2008; Yoshida et al., 2008; Makokha et al., 2013). The absence of the PDZ domain renders Tax-2 unable to interact with these factors. The four subtypes of HTLV-2 (-2A to -2D) code for similar but not identical Tax proteins. Tax-2A is composed of 331 aa and is shorter than Tax-2B, Tax-2C, and Tax-2D (356, 356, and 344 aa, respectively; Feuer and Green, 2005), Tax-2B is the variant that has been studied in greatest detail (Bertazzoni et al., 2011). Tax-1 and Tax-2B share 85% aa similarity, whereas Tax-3 displays 26 and 30% divergence with respect to Tax-1 and Tax-2, respectively. The comparison of Tax-4 with Tax-1, Tax-2, and Tax-3 shows 83, 91, and 85% aa similarity, respectively (Switzer et al., 2009), as outlined in Figure 3. The main characteristic that distinguishes Tax-1 from Tax-2 is the presence only in Tax-1 of a motif spanning aa 225–232 that activates the non-canonical NF-κB pathway through interaction with the p100 factor (Shoji et al., 2009). A second relevant difference between Tax-1 and Tax-2 is the presence in Tax-1, but not in Tax-2, of the PBM at the C-terminus (Higuchi and Fujii, 2009; Bertazzoni et al., 2011; Rende et al., 2012). Based on sequence homology, all the Tax proteins possess two functional regions involved in CBP (CREB-binding protein)/p300 binding: a KID-like domain between residues 81 and 95 and a second domain named C-terminal transcriptional activating CR2 domain, between aa 312 and 319. These domains present some differences between Tax-1, Tax-2, Tax-3, and Tax-4. One of the major differences is related to the lysine residue at position 85, which is necessary for Tax-1 to bind CBP/p300 (Hiramatsu and Yoshikura, 1986); this residue is substituted by an arginine in Tax-2, Tax-3, and Tax-4 (Calattini et al., 2006; Chevalier et al., 2006).
FIGURE 2.
Structural and functional domains of the Tax proteins. The position of the lysines and their ubiquitination, sumoylation, and acetylation are indicated. Red dots identify phosphorylated serines.
FIGURE 3.
ClustalW alignments of Tax-1, Tax-2B, Tax-3, and Tax-4 proteins.
Post-translational modifications of Tax-1 and Tax-2 such as phosphorylation, acetylation, ubiquitination, and sumoylation have been extensively described in recent reviews (Bertazzoni et al., 2011; Lodewick et al., 2011; Kfoury et al., 2012). Recent comparative analyses of Tax-1 and Tax-2 post-translational modifications centered on the contribution of ubiquitination and sumoylation to the intracellular localization of Tax and its ability to activate NF-κB (Lamsoul et al., 2005; Kfoury et al., 2006; Nasr et al., 2006; Turci et al., 2009, 2012; Bonnet et al., 2012; Xiao, 2012; Zane and Jeang, 2012; Journo et al., 2013). These studies highlighted the role of specific lysines as targets for sumoylation and ubiquitination in Tax-1 and Tax-2 (lysines K1 to K10 in Tax-1, see Figure 2). Lysines K6 and K8 are critical for NF-κB activation and are highly conserved in all Tax proteins. Additional lysines, indicated by additional roman numbers, are present in Tax-2 (K2i, K3i, K3ii, and K10i, Figure 2) whereas Tax-3 and Tax-4 contain 11 and 10 lysines, respectively, but not all are conserved at the same position. Alignment of the predicted Tax-4 sequence shows the absence of K10, which is a target of acetylation in Tax-1 and possibly in Tax-2 (Lodewick et al., 2009; Journo et al., 2013).
Tax is generally defined as a multifunctional protein, since it is able to activate both viral and host gene transcription and to act as mediator of several cellular pathways. Of particular importance was the demonstration that Tax-1 is able to immortalize and transform human primary CD4+ T cells (Grassmann et al., 1992; Franchini, 1995; Yoshida, 2001) and the finding that Tax-1-transgenic mice develop ATL (Hasegawa et al., 2006; Ohsugi, 2013). It is well-established that Tax-1 plays a relevant role in the oncogenesis induced by HTLV infection. Tax-1 acts as a pleiotropic protein conferring proliferative and survival properties to HTLV-1 – infected cells by modulating regulatory factors that induce cell proliferation, cell cycle progression, inhibition of apoptosis, and interference with DNA repair. The main factors include CREB, CBP/p300, NF-κB, cyclin-dependent kinases (CDKs), and Akt (reviewed by Kannian and Green, 2010; Currer et al., 2012).
TRANSFORMATION POTENTIAL OF Tax PROTEINS
Tax-1 is able to transform T-lymphocytes and fibroblasts and to induce tumors in transgenic mice (Azran et al., 2004; Matsuoka and Jeang, 2007; Kannian and Green, 2010; Ohsugi, 2013). The ability to transform primary human T cells was also demonstrated for Tax-2 (Ross et al., 1996; Feuer and Green, 2005). Both proteins are able to inhibit the function of the tumor suppressor p53. A comparative study between Tax-1 and Tax-2 subtypes demonstrated that Tax-2A inhibits p53 function less efficiently than Tax-1 or Tax-2B (Mahieux et al., 2000). Although several in vitro studies have investigated the ability of Tax to inactivate p53 (Tabakin-Fix et al., 2006) by acting on the CREB or NF-κB pathway, the mechanism of this inhibition has not yet been completely clarified. A recent study by Zane et al. (2012) identified a cooperative role for the cellular factor Wip1 (wild-type p53-induced phosphatase 1) in Tax-1-mediated inactivation of p53; a subsequent study demonstrated interactions between Tax-1 and Wip1 (Dayaram et al., 2013). In a study that employed transgenic mice expressing Tax-1 which develop mature T cell leukemia and lymphoma, Ohsugi et al. (2013) demonstrated that Tax-1 alters p53 function and that this effect precedes NF-κB activation.
Tax-2 transforms rat fibroblasts less efficiently than Tax-1 (Endo et al., 2002). On the other hand, both viruses immortalizes primary human T cells at a comparable efficiency (Feuer and Green, 2005). Higuchi et al. (2007) demonstrated that the non-canonical NF-κB factor p100 and the PBM present in Tax-1, but not in Tax-2, are essential for the transformation of a T cell. Tsubata et al. (2005) had already shown that the PBM domain is critical for the ability of Tax-1 to induce interleukin-2 (IL-2)-independent growth of the IL-2-dependent T cell line CTLL-2, and that Tax-2 lacks this ability. Xie et al. (2006) showed that the deletion of the PBM in a recombinant HTLV-1 molecular clone (HTLV-1/ΔPBM) alters the requirement for the establishment and maintenance of persistent infection in rabbits. A motif responsible for the distinct transforming activity of Tax-1 and Tax-2 was identified by using a series of Tax-1/Tax-2 chimeric proteins. A region corresponding to aa 225–232 of Tax-1 was shown to play a crucial role in Tax-1’s transforming activity, involving stimulation of the non-canonical NF-κB/p100 pathway (Shoji et al., 2009). Imai et al. (2013) recently demonstrated that Tax-2B can immortalize human CD4+ T cells. By infecting peripheral blood mononuclear cells (PBMCs) with lentiviruses encoding Tax-1 or Tax-2B they observed a higher immortalization activity of Tax-2B as compared to Tax-1.
Studies of Tax-2-immortalized T cells demonstrated that Tax-2 causes a dysregulation of autophagy; this may represent a novel survival mechanism in Tax-2-immortalized T cells (Ren et al., 2012). A similar action was attributed to Tax-1, thus suggesting that autophagy may play an important role in the HTLV life cycle (Tang et al., 2013b). Tax-3 was shown to be able to activate the NF-κB pathway and bind CBP in the T cell line CEM, thus suggesting that Tax-3 has in vitro transforming activity (Chevalier et al., 2006). The transforming properties of Tax-4 remain to be investigated.
Tax AND SIGNAL TRANSDUCTION DEREGULATION
The role of Tax in HTLV-1-induced oncogenesis has been investigated in large part by analyzing the capacity of Tax-1 to interact with selected cellular factors that play a crucial role in signaling pathways. A list of Tax-interacting proteins is presented in Table 1. Tax-1 expression deregulates several signaling pathways involved in the cell cycle, cell proliferation, and cell survival, primarily through the deregulation of two major cellular transcription factor pathways: CREB/ATF and NF-κB (Sun and Yamaoka, 2005; Nyborg et al., 2010). Tax-1 constitutively activates NF-κB by causing a deregulated expression of a variety of cellular genes. Tax-dependent NF-κB activation has been extensively studied and the current state of knowledge will be described in the next section. Tax-1 activation through the cellular transcription factor CREB has been well-characterized at the level of the HTLV-1 promoter located in the LTR region. Within the HTLV-1 promoter three conserved 21 bp repeat enhancer elements called viral CRE elements (vCRE) are present that can be recognized within a complex containing Tax-1 and a phosphorylated form of CREB. The Tax/CREB/vCREB complexes can be associated to other host factors. The best characterized are the cellular coactivators CBP and p300 (Kashanchi and Brady, 2005), which stimulate Tax-mediated transactivation by chromatin remodeling (Nyborg et al., 2010). In addition, Tax-1 interacts with the SWI/SNF chromatin remodeling complexes (Easley et al., 2010) and may be involved in the nucleosome eviction activity mediated by the nucleosome assembly protein 1 (NAP1; Sharma and Nyborg, 2008). Additional host factors that directly interact with Tax-1 and act in the Tax-mediated transactivation are the transducer of regulated CREB (TORC) proteins. TORC-1 and TORC-2 are required for Tax activation whereas TORC-3 enhances Tax-dependent transcription (Koga et al., 2004; Siu et al., 2006). Several cellular factors that interact with Tax and participate to HTLV-1 promoter activation have been identified. The transcriptional activator CIITA affects the functional interaction of the transcription factors CREB, ATF1, and PCAF with Tax-1 (Tosi et al., 2011) and Tax-2 activation of HTLV-2 LTR is strongly inhibited by CIITA (Orlandi et al., 2011). Recently, Tang et al. (2013a) demonstrated that the LKB1 tumor suppressor and the salt inducible kinases (SIKs) act as negative regulatory factors in the activation of HTLV-1 LTR by Tax. They showed that LKB1 and SIK interact with Tax and that this association enables LTR activation by TORCs, CREB, and Tax-1 (Tang et al., 2013a). Additional cellular mediators of Tax-induced activation of HTLV-1 LTR belong to the group I p21-activated kinases (Paks) which physically interact with Tax and CREB-regulating transcriptional coactivators to facilitate HTLV-1 transcription (Chan et al., 2013).
Table 1.
Tax-1 interacting proteins and deregulated pathways.
The role of Tax-1 in the expression of cellular genes containing CRE elements was demonstrated for several genes involved in cell cycle and proliferation. Recently Kim et al. (2010) demonstrated that Tax-1 deregulates cyclin D1 gene expression thus determining its overexpression. The mechanism requires an enhanced binding between p300 and phosphorylated CREB and TORC-2. The interaction of Tax-1 with CREB/ATF factors also represses the expression of several genes, including cyclin A (Kibler and Jeang, 2001), p53 (Mulloy et al., 1998), c-myc (Semmes et al., 1996a), and the ZNF268 gene, whichplays a role in the differentiation of blood cells during development and in the pathogenesis of leukemia (Wang et al., 2008).
The main functional and structural differences between Tax-1, Tax-2, and Tax-3 are presented in Table 2. It is evident that the Tax proteins differ not only in their transformation abilities, structural properties, and protein interactions, as described in the previous sections, but also in additional aspects of cellular interactions. Tax-2 is distributed both in the nucleus and in the cytoplasm, showing a more diffuse distribution in the cytoplasm compared to Tax-1 (Turci et al., 2009). We have recently demonstrated that Tax-1 and Tax-2 colocalize with TAB2-containing cytoplasmic structures that include RelA and calreticulin (Avesani et al., 2010). Compared to Tax-1, Tax-2 differs in post-transcriptional modification (Lodewick et al., 2011) and we have shown that, in transfected cells, lysine usage for sumoylation differs between Tax-1 and Tax-2 (Turci et al., 2012). When compared to Tax-1, Tax-2 is less efficient in the induction of micronuclei formation (Semmes et al., 1996b), is unable to suppress multilineage hematopoiesis from CD34+ cells in vitro (Tripp et al., 2003) and to direct the lipid raft translocation of IκB kinase alpha (IKKα) and IKKβ in transfected cells and in Tax-2-immortalized primary T cells (Huang et al., 2009).
Table 2.
Summary of main functional and structural differences between Tax-1, Tax-2, and Tax-3.
| Tax-1 | Tax-2a | Tax-3 | Reference | |
|---|---|---|---|---|
| Transactivating activity | Highera | Lowerb | n.d.c | Semmes etal. (1996a) |
| Transformation capacity | Higher | Lower | n.d. | Endo etal. (2002) |
| Micronuclei formation | + | - | n.d. | Semmes etal. (1996b) |
| Cell cycle arrest | + | - | n.d. | Tripp etal. (2005) |
| Hematopoiesis suppression | + | - | n.d. | Tripp etal. (2003) |
| Reduction of histone gene expression | + | - | n.d. | Harrod et al. (2000);Ego et al. (2002) |
| Inhibition of p53 functions | Higher | Lower | + | Mahieux et al. (2000);Meertens et al. (2004b),Jeong et al. (2005);Calattini et al. (2006) |
| Total viral mRNA expression | Higher | Lower | n.d. | Li and Green (2007) |
| Proinflammatory cytokine expression | Higher | Lower | n.d. | Banerjee etal. (2007) |
| Presence of PDZ motif | + | - | n.d. | Feuer and Green (2005) |
| Interaction with PDZ-binding proteins | + | - | + | Higuchi and Fujii (2009) |
| Interaction with p100 | + | - | n.d. | Shoji etal. (2009) |
| Preferential cellular localization | Nucleus | Cytoplasm | n.d. | Turci etal. (2009) |
| NF-κB transactivation | + | + | + | Chevalier etal. (2012) |
| NF-κB transactivation (lipid raft translocation of IKK) | + | - | n.d. | Huang etal. (2009) |
| In vitro CK2 phosphorylation | + | - | n.d. | Bidoia etal. (2010) |
| Oligo-sumoylation | + | - | n.d. | Turci etal. (2009) |
| Nuclear bodies | Larger | Smaller | n.d. | Turci etal. (2009) |
| Ubiquitination and sumoylation | + | + | n.d. | Turci etal. (2012);Zane etal. (2012) |
| Nuclear localization | + | + | + | Calattini etal. (2006) |
| T cell immortalization | + | + | + | Chevalier et al. (2006); Imai et al. (2013) |
The properties of Tax-2 include those described for Tax-2A and/or Tax-2B reported in the literature.
Higher and lower refers to a comparison between Tax-1 and Tax-2.
n.d.: not determined.
Recent reports have investigated the different role of Tax-1 and Tax-2 in innate immunity. We have previously demonstrated that HIV-1/HTLV-2 coinfection in drug users is associated to a delayed progression of AIDS (Turci et al., 2006b). CC-chemokines are produced spontaneously by T lymphocytes of HIV-1/HTLV-2 coinfected subjects (Lewis et al., 2000). Recently, it has been demonstrated that Tax-1 and Tax-2 induce the expression of the CC-chemokines MIP1-α/CCL 3 MIP-1β/CCL4, and RANTES/CCL5 and that downregulate CCR5 in monocytes and PBMCs (Barrios et al., 2011; Balistrieri et al., 2013). Furthermore, a significant decrease of HIV-1 replication has been reported in cultures of PMBCs infected by HIV-1 and treated with Tax-1 or Tax-2 (Barrios et al., 2013). In this in vitro cell system the effect of Tax-2 on HIV-1 replication is higher than that of Tax-1 and the authors suggest that Tax-2 may act as an immunomodulatory protein during HTLV-2 infection.
An emerging role in HTLV-1 pathogenesis is attributed to the antisense protein HBZ which contains a bZIP motif, required to form heterodimers with cellular transcription factors. HBZ inhibits viral and cellular expression by interacting with CREB and additional transcription factors and, in contrast to Tax-1, is consistently expressed in ATL cells (Matsuoka and Green, 2009). Compared to HTLV-1, HTLV-2 expresses an antisense protein named APH-2, which is structurally different from HBZ, lacking the classical bZIP domain. APH-2 is able to interact with CREB and to repress the activation of HTLV-2 gene expression mediated by Tax-2 (Halin et al., 2009). A recent study has shown that APH-2 may interact with Tax-2 and when co-expressed with Tax-2, impairs the ability of Tax-2 to activate AP-1 transcription (Marban et al., 2012). AP-1 pathway involves several factors, including Jun, Fos, Maf, and ATF that act on cell proliferation, apoptosis, and oncogenic transformation (Shaulian and Karin, 2001). The distinct structural and functional diversities of HBZ and APH-2 and their interactions with Tax proteins may be relevant for the different pathogenicity of HTLV-1 and HTLV-2 and the mechanisms need to be further investigated.
Tax AND THE NF-κB PATHWAY
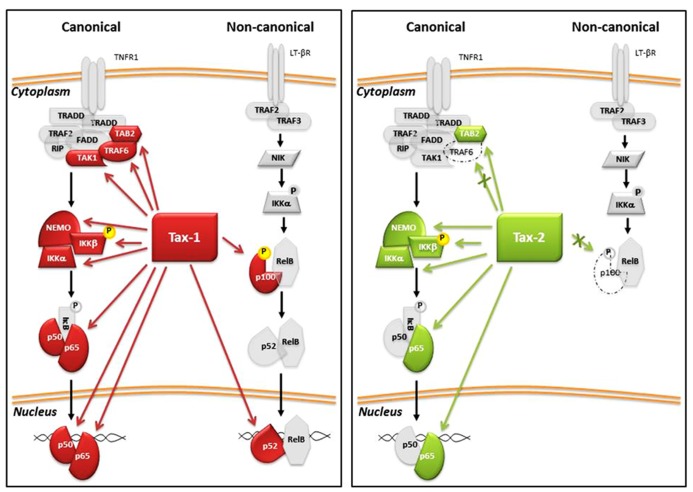
Enhanced NF-κB activation is one of the principal consequences of the expression of Tax in the infected cells. The NF-κB family of inducible transcription factors regulate diverse biological processes, including the growth and survival of both T cells and non-lymphoid cells. Activation of NF-κB transcription factors occurs through two tightly controlled signaling processes known as the canonical and non-canonical NF-κB pathways. The principal activators and regulators of these two pathways are illustrated in Figure 4. The canonical pathway is activated by different receptor signals including inflammatory cytokines, genotoxic stress, antigens, and toll like receptors (TLRs), whereas the activation of the non-canonical pathway involves signaling molecules that are recognized only by a specific subset of tumor necrosis factor receptors (TNFRs), such as lymphotoxin-β, BAFF, RANKL, and TWEAK (Sun, 2011). The NF-κB transcription factors family includes five members: RelA/p65, c-Rel, RelB, p50, and p52. The p50 and p52 proteins are expressed as precursor proteins named p105 and p100, respectively. The processing of these precursors to mature forms requires proteasome activity. The five members form dimers with one another and can bind to a variety of target DNA sequences called κB sites to modulate gene expression. p50 and p52 can activate transcription by forming heterodimers with RelA/p65, c-Rel, or RelB. In the cytoplasm, the NF-κB complexes are inactive since they are bound to inhibitory IκB proteins (IκBα, IκBβ, IκB∊, etc.). Activation of the pathway requires IκB protein degradation and translocation of NF-κB dimers to the nucleus. The common step of activation is mediated by the IKK complex, which phosphorylates IκB and targets it to proteosomal degradation. The IKK complex consists of two active kinases, IKKα and IKKβ, and the regulatory scaffolding protein NEMO (IKKγ). Tax directly interacts with these factors, leading to a persistent activation of NF-κB-mediated transcription. Tax-1 stimulates the activation of both the canonical and non-canonical NF-κB pathway thought the interaction with the IKK factors; the Tax/IKKγ interaction is required for recruiting Tax to the IKK catalytic subunits and for Tax-mediated IKK activation (Sun and Yamaoka, 2005).
FIGURE 4.
Tax-1 and Tax-2 interactions with factors of NF-κB pathway. Red arrows indicate Tax-1 interaction and the green arrows indicate Tax-2 interactions. Dashed proteins indicate the absence of interaction with Tax-2.
Other cellular proteins are important for Tax-mediated NF-κB activation. Tax-1 interacts with NRP/optineurin and TAX1BP1 (Journo et al., 2009; Shembade et al., 2011), and with the ubiquitin-specific peptidase USP20 (Yasunaga et al., 2011). A recent study demonstrated that Tax-1 promotes Bcl-3 expression and nuclear translocation of RelA/p65 (Gao et al., 2013). Kim et al. (2008) provided evidence that Tax-1 induces Bcl-3 expression primarily through activation of the NF-κB pathway. Another recent study has demonstrated that Tax-1 transactivates CD69, a marker of early activation of lymphocytes, through both NF-κB and CREB signaling pathways (Ishikawa et al., 2013).
Tax-1-mediated activation of the non-canonical NF-κB pathway is important in virus-induced tumorigenesis. A region of Tax-1 spanning aa 225–232 is essential for activation of the non-canonical pathway. In contrast to Tax-1, Tax-2 is not able to activate the non-canonical pathway and does not interact with or induce processing of p100 into p52 (Higuchi et al., 2007; Shoji et al., 2009). Furthermore, Tax-2 has been demonstrated to not be able to interact with TRAF6, a protein with E3 ligase activity that, in the presence of Tax-1, positively regulates the activation of NF-κB pathway (Journo et al., 2013).
Ubiquitination and sumoylation of Tax-1 and Tax-2 are involved in NF-κB activation (Lamsoul et al., 2005; Kfoury et al., 2006; Nasr et al., 2006; Turci et al., 2009, 2012; Journo et al., 2013). Both Tax proteins co-immunoprecipitate and colocalize with IKKγ/NEMO, TAB2, and RelA/p65 in transfected cells (Meertens et al., 2004a; Sun and Yamaoka, 2005; Avesani et al., 2010). Expression of Tax proteins induces IKKα and RelA/p65 nuclear translocation (Higuchi et al., 2007; Ho et al., 2012). An overactivation of NF-κB by Tax induces cellular senescence (Zhi et al., 2011). By knockdown experiments, Ho et al. (2012) demonstrated that chronic activation of NF-κB by Tax-1 results in rapid senescence (Tax-induced rapid senescence, Tax-IRS) that is dependent on IKKα and p65/RelA activation. The Tax-IRS phenomenon constitutes a host checkpoint response to the overactivation of NF-κB that prevents cellular transformation (Zhi et al., 2011; Ho et al., 2012) and represents an interesting mechanism of host cell protection from the deregulating activities of viral proteins.
CONCLUSION
HTLV-1 and HTLV-2 can efficiently transform T-lymphocytes, but only HTLV-1 causes ATL. Although additional viral products play important roles in the HTLV pathogenesis, Tax represents a key factor in the early stage of T cell oncogenesis. In this review we have dissected the structural and functional features of the HTLV Tax proteins, focusing mainly on Tax-1 and Tax-2. These two proteins share many common properties including the capacity of transforming and immortalizing T cells, of transactivating NF-κB pathway and being modified by both ubiquitination and sumoylation. They significantly differ for the presence of a PDZ motif, which is missing in Tax-2, and for the activation of non-canonical NF-κB which is attributed only to Tax-1. The knowledge derived by studying Tax’s interactions with cellular factors and their effects on the induction of altered responses in cell pathway regulation confirms the complexity of HTLV oncogenesis. An interesting issue that needs to be explored in the future is the frequent downregulation of the expression of viral genes coded by the plus-strand (including Tax) in circulating leukemic cells from ATL patients. This phenomenon is likely due to epigenetic silencing of the plus-strand promoter, e.g., by methylation and/or expression of repressors of the Polycomb family (Satou and Matsuoka, 2012, 2013; Yamagishi and Watanabe, 2012). A pivotal mechanism that involves a circuit controlled signaling by miRNA has been recently demonstrated by Yamagishi et al. (2012) showing that miR-31 loss, in ATL primary cells, mediated by Polycomb-dependent epigenetic gene silencing, is associated to the overexpression of the NF-κB inducing kinase NIK and leads to constitutive activation of NF-κB oncogenic signaling.
It is likely that further studies aimed at dissecting the functional differences between the Tax proteins will reveal novel functions of host factors that are involved in the signal pathways altered in ATL and may become potential targets for effective therapies against leukemia.
The studies of the differences between Tax-1, Tax-2, Tax-3, and Tax-4 interacting cell factors and transactivating activities will provide useful information to the understating of Tax-1 structural transformation that may open a new approach on HTLV studies based on Tax-1 peculiarities and interactions with addition viral products, that are not present in HTLV-2, HTLV-3, and HTLV-4 Tax proteins.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Donna D’Agostino for comments on the manuscript. This work was supported by AIRC (Associazione Italiana per la Ricerca sul Cancro) through a 2008 AIRC-Cariverona Regional grant (Maria G. Romanelli and Vincenzo Ciminale) and an AIRC Investigator Grant (Vincenzo Ciminale), by the University of Verona Joint project and by research funds to Maria G. Romanelli. Erica Diani was supported in part by an AIRC-Cariverona Regional fellowship.
REFERENCES
- Akagi T., Ono H., Shimotohno K. (1995). Characterization of T cells immortalized by Tax1 of human T-cell leukemia virus type 1. Blood 86 4243–4249 [PubMed] [Google Scholar]
- Akagi T., Ono H., Shimotohno K. (1996). Expression of cell-cycle regulatory genes in HTLV-I infected T-cell lines: possible involvement of Tax1 in the altered expression of cyclin D2, p18Ink4 and p21Waf1/Cip1/Sdi1. Oncogene 12 1645–1652 [PubMed] [Google Scholar]
- Alefantis T., Barmak K., Harhaj E. W., Grant C., Wigdahl B. (2003). Characterization of a nuclear export signal within the human T cell leukemia virus type I transactivator protein Tax. J. Biol. Chem. 278 21814–21822 10.1074/jbc.M211576200 [DOI] [PubMed] [Google Scholar]
- Araujo A., Hall W. W. (2004). Human T-lymphotropic virus type II and neurological disease. Ann. Neurol. 56 10–19 10.1002/ana.20126 [DOI] [PubMed] [Google Scholar]
- Asquith B., Zhang Y., Mosley A. J., de Lara C. M., Wallace D. L., Worth A. et al. (2007). In vivo T lymphocyte dynamics in humans and the impact of human T-lymphotropic virus 1 infection. Proc. Natl. Acad. Sci. U.S.A. 104 8035–8040 10.1073/pnas.0608832104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avesani F., Romanelli M. G., Turci M., Di Gennaro G., Sampaio C., Bidoia C. et al. (2010). Association of HTLV Tax proteins with TAK1-binding protein 2 and RelA in calreticulin-containing cytoplasmic structures participates in Tax-mediated NF-κB activation. Virology 408 39–48 10.1016/j.virol.2010.08.023 [DOI] [PubMed] [Google Scholar]
- Azran I., Schavinsky-Khrapunsky Y., Aboud M. (2004). Role of Tax protein in human T-cell leukemia virus type-I leukemogenicity. Retrovirology 1 20 10.1186/1742-4690-1-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangham C. R., Osame M. (2005). Cellular immune response to HTLV-1. Oncogene 24 6035–6046 10.1038/sj.onc.1208970 [DOI] [PubMed] [Google Scholar]
- Béraud C., Sun S. C., Ganchi P., Ballard D. W., Greene W. C. (1994). Human T-cell leukemia virus type I Tax associates with and is negatively regulated by the NF-kappa B2 p100 gene product: implications for viral latency. Mol. Cell. Biol. 14 1374–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balistrieri G., Barrios C., Castillo L., Umunakwe T. C., Giam C. Z., Zhi H. et al. (2013). Induction of CC-chemokines with antiviral function in macrophages by the human T lymphotropic virus type 2 transactivating protein, Tax2. Viral Immunol. 26 3–12 10.1089/vim.2012.0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee P., Rochford R., Antel J., Canute G., Wrzesinski S., Sieburg M. et al. (2007). Proinflammatory cytokine gene induction by human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2 Tax in primary human glial cells. J. Virol. 81 1690–1700 10.1128/JVI.01513-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrios C. S., Abuerreish M., Lairmore M. D., Castillo L., Giam C. Z., Beilke M. A. (2011). Recombinant human T-cell leukemia virus types 1 and 2 Tax proteins induce high levels of CC-chemokines and downregulate CBR5 in human peripheral blood mononuclear cells. Viral Immunol. 24 429–439 10.1089/vim.2011.0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrios C. S., Castillo L., Giam C. Z., Wu L., Beilke M. A. (2013). Inhibition of HIV type 1 replication by human T lymphotropic virus types 1 and 2 Tax proteins in vitro. AIDS Res. Hum. Retroviruses 29 1061–1067 10.1089/aid.2013.0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbous J., Bazarbachi A., Granier C., Devaux C., Mesnard J. M. (2003). The central region of human T-cell leukemia virus type 1 Tax protein contains distinct domains involved in subunit dimerization. J. Virol. 77 13028–13035 10.1128/JVI.77.24.13028-13035.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertazzoni U., Turci M., Avesani F., Di Gennaro G., Bidoia C., Romanelli M. G. (2011). Intracellular localization and cellular factors interaction of HTLV-1 and HTLV-2 Tax proteins: similarities and functional differences. Viruses 3 541–560 10.3390/v3050541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidoia C., Mazzorana M., Pagano M. A., Arrigoni G., Meggio F., Pinna L. A. et al. (2010). The pleiotropic protein kinase CK2 phosphorylates HTLV-1 Tax protein in vitro, targeting its PDZ-binding motif. Virus Genes 41 149–157 10.1007/s11262-010-0494-3 [DOI] [PubMed] [Google Scholar]
- Biswas H. H., Kaidarova Z., Garratty G., Gibble J. W., Newman B. H., Smith J. W., et al. (2010). HTLV Outcomes Study. Increased all-cause and cancer mortality in HTLV-II infection. J. Acquir. Immune Defic. Syndr. 54 290–296 10.1097/QAI.0b013e3181cc5481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet A., Randrianarison-Huetz V., Nzounza P., Nedelec M., Chazal M., Waast L., et al. (2012). Low nuclear body formation and tax SUMOylation do not prevent NF-kappaB promoter activation. Retrovirology 9 77 10.1186/1742-4690-9-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calattini S., Chevalier S. A., Duprez R., Afonso P., Froment A., Gessain A., et al. (2006). Human T-cell lymphotropic virus type 3: complete nucleotide sequence and characterization of the human tax3 protein. J. Virol. 80 9876–9888 10.1128/JVI.00799-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calattini S., Chevalier S. A., Duprez R., Bassot S., Froment A., Mahieux R., et al. (2005). Discovery of a new human T-cell lymphotropic virus (HTLV-3) in Central Africa. Retrovirology 2 30 10.1186/1742-4690-2-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. P., Siu Y. T., Kok K. H., Ching Y. P., Tang H. M., Jin D. Y. (2013). Group I p21-activated kinases facilitate Tax-mediated transcriptional activation of the human T-cell leukemia virus type 1 long terminal repeats. Retrovirology 10 47 10.1186/1742-4690-10-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Ren T., Sun S. C. (2012). New insight into the oncogenic mechanism of the retroviral oncoprotein Tax. Protein Cell 3 581–589 10.1007/s13238-012-2047-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier S. A., Durand S., Dasgupta A., Radonovich M., Cimarelli A., Brady J. N., et al. (2012). The transcription profile of Tax-3 is more similar to Tax-1 than Tax-2: insights into HTLV-3 potential leukemogenic properties. PLoS ONE 7:e41003 10.1371/journal.pone.0041003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier S. A., Meertens L., Calattini S., Gessain A., Kiemer L., Mahieux R. (2005). Presence of a functional but dispensable nuclear export signal in the HTLV-2 Tax protein. Retrovirology 2 70 10.1186/1742-4690-2-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier S. A., Meertens L., Pise-Masison C., Calattini S., Park H., Alhaj A. A., et al. (2006). The tax protein from the primate T-cell lymphotropic virus type 3 is expressed in vivo and is functionally related to HTLV-1 Tax rather than HTLV-2 Tax. Oncogene 25 4470–4482 10.1038/sj.onc.1209472 [DOI] [PubMed] [Google Scholar]
- Ching Y. P., Chan S. F., Jeang K. T., Jin D. Y. (2006). The retroviral oncoprotein Tax targets the coiled-coil centrosomal protein TAX1BP2 to induce centrosome overduplication. Nat. Cell Biol. 8 717–724 10.1038/ncb1432 [DOI] [PubMed] [Google Scholar]
- Chlichlia K., Khazaie K. (2010). HTLV-1 Tax: linking transformation, DNA damage and apoptotic T-cell death. Chem. Biol. Interact. 188 359–365 10.1016/j.cbi.2010.06.005 [DOI] [PubMed] [Google Scholar]
- Chu Z. L., DiDonato J. A., Hawiger J., Ballard D. W. (1998). The tax oncoprotein of human T-cell leukemia virus type 1 associates with and persistently activates IkB kinases containing IKKα and IKKβ. J. Biol. Chem. 273 15891–15894 10.1074/jbc.273.26.15891 [DOI] [PubMed] [Google Scholar]
- Currer R., Van Duyne R., Jaworski E., Guendel I., Sampey G., Das R., et al. (2012). HTLV tax: a fascinating multifunctional co-regulator of viral and cellular pathways. Front. Microbiol. 3:406. 10.3389/fmicb.2012.00406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayaram T., Lemoine F. J., Donehower L. A., Marriott S. J. (2013). Activation of WIP1 phosphatase by HTLV-1 Tax mitigates the cellular response to DNA damage. PLoS ONE 8:e55989. 10.1371/journal.pone.0055989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkin S. S., Guo X., Fryrear K. A., Mihaylova V. T., Gupta S. K., Belgnaoui S. M., et al. (2008). HTLV-1 Tax oncoprotein subverts the cellular DNA damage response via binding to DNA-dependent protein kinase. J. Biol. Chem. 283 36311–36320 10.1074/jbc.M804931200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easley R., Carpio L., Guendel I., Klase Z., Choi S., Kehn-Hall K., et al. (2010). Human T-lymphotropic virus type 1 transcription and chromatin-remodeling complexes. J. Virol. 84 4755–4768 10.1128/JVI.00851-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ego T., Ariumi Y., Shimotohno K. (2002). The interaction of HTLV-1 Tax with HDAC1 negatively regulates the viral gene expression. Oncogene 21 7241–7246 10.1038/sj.onc.1205701 [DOI] [PubMed] [Google Scholar]
- Endo K., Hirata A., Iwai K., Sakurai M., Fukushi M., Oie M., et al. (2002). Human T-cell leukemia virus type 2 (HTLV-2) Tax protein transforms a rat fibroblast cell line but less efficiently than HTLV-1 Tax. J. Virol. 76 2648–2653 10.1128/JVI.76.6.2648-2653.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuer G., Green P. L. (2005). Comparative biology of human T-cell lymphotropic virus type 1 (HTLV-1) and HTLV-2. Oncogene 24 5996–6004 10.1038/sj.onc.1208971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini G. (1995). Molecular mechanisms of human T-cell leukemia/lymphotropic virus type I infection. Blood 86 3619–3639 [PubMed] [Google Scholar]
- Fujii M., Tsuchiya H., Chuhjo T., Akizawa T., Seiki M. (1992). Interaction of HTLV-1 Tax1 with p67SRF causes the aberrant induction of cellular immediate early genes through CArG boxes. Genes Dev. 6 2066–2076 10.1101/gad.6.11.2066 [DOI] [PubMed] [Google Scholar]
- Gallo R. C. (2002). Human retroviruses after 20 years: a perspective from the past and prospects for their future control. Immunol. Rev. 185 236–265 10.1034/j.1600-065X.2002.18520.x [DOI] [PubMed] [Google Scholar]
- Gallo R. C. (2011). Research and discovery of the first human cancer virus, HTLV-1. Best Pract. Res. Clin. Haematol. 24 559–565 10.1016/j.beha.2011.09.012 [DOI] [PubMed] [Google Scholar]
- Gao C., Wang X., Chen L., Wang J. H., Gao Z. T., Wang H. (2013). Knockdown of Bcl-3 inhibits cell growth and induces DNA damage in HTLV-1-infected cells. Asian Pac. J. Cancer Prev. 14 405–408 10.7314/APJCP.2013.14.1.405 [DOI] [PubMed] [Google Scholar]
- Gessain A., Cassar O. (2012). Epidemiological aspects and world distribution of HTLV-1 infection. Front. Microbiol. 3:388 10.3389/fmicb.2012.00388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessain A., Mahieux R. (2012). Tropical spastic paraparesis and HTLV-1 associated myelopathy: clinical, epidemiological, virological and therapeutic aspects. Rev. Neurol. (Paris) 168 257–269 10.1016/j.neurol.2011.12.006 [DOI] [PubMed] [Google Scholar]
- Gessain A., Rua R., Betsem E., Turpin J., Mahieux R. (2013). HTLV-3/4 and simian foamy retroviruses in humans: discovery, epidemiology, cross-species transmission and molecular virology. Virology 435 187–199 10.1016/j.virol.2012.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves D. U., Proietti F. A., Ribas J. G., Araújo M. G., Pinheiro S. R., Guedes A. C., et al. (2010). Epidemiology, treatment, and prevention of human T-cell leukemia virus type 1-associated diseases. Clin. Microbiol. Rev. 23 577–589 10.1128/CMR.00063-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren I., Semmes O. J., Jeang K. T., Moelling K. (1995). The amino terminus of Tax is required for interaction with the cyclic AMP response element binding protein. J. Virol. 69 5806–5811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassmann R., Berchtold S., Radant I., Alt M., Fleckenstein B., Sodroski J. G., et al. (1992). Role of human T-cell leukemia virus type 1 X region proteins in immortalization of primary human lymphocytes in culture. J. Virol. 66 4570–4575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajj H. E., Nasr R., Kfoury Y., Dassouki Z., Nasser R., Kchour G., et al. (2012). Animal models on HTLV-1 and related viruses: what did we learn? Front. Microbiol. 3:333 10.3389/fmicb.2012.00333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halin M., Douceron E., Clerc I., Journo C., Ko N. L., Landry S., et al. (2009). Human T-cell leukemia virus type 2 produces a spliced antisense transcript encoding a protein that lacks a classic bZIP domain but still inhibits Tax2-mediated transcription. Blood 114 2427–2438 10.1182/blood-2008-09-179879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller K., Wu Y., Derow E., Schmitt I., Jeang K. T., Grassmann R. (2002). Physical interaction of human T-cell leukemia virus type 1 Tax with cyclin-dependent kinase 4 stimulates the phosphorylation of retinoblastoma protein. Mol. Cell. Biol. 22 3327–3338 10.1128/MCB.22.10.3327-3338.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harhaj E. W., Sun S. C. (1999). IKKgamma serves as a docking subunit of the IkappaB kinase (IKK) and mediates interaction of IKK with the human T-cell leukemia virus Tax protein. J. Biol. Chem. 274 22911–22914 10.1074/jbc.274.33.22911 [DOI] [PubMed] [Google Scholar]
- Harrod R., Kuo Y. L., Tang Y., Yao Y., Vassilev A., Nakatani Y., et al. (2000). P300 and p300/camp-responsive element-binding protein associated factor interact with human T-cell lymphotropic virus type-1 Tax in a multi-histone acetyltransferase/activator-enhancer complex. J. Biol. Chem. 275 11852–11857 10.1074/jbc.275.16.11852 [DOI] [PubMed] [Google Scholar]
- Hasegawa H., Sawa H., Lewis M. J., Orba Y., Sheehy N., Yamamoto Y., et al. (2006). Thymus-derived leukemia-lymphoma in mice transgenic for the Tax gene of human T-lymphotropic virus type I. Nat. Med. 12 466–472 10.1038/nm1389 [DOI] [PubMed] [Google Scholar]
- Higuchi M., Fujii M. (2009). Distinct functions of HTLV-1 Tax1 from HTLV-2 tax2 contribute key roles to viral pathogenesis. Retrovirology 6 117 10.1186/1742-4690-6-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M., Tsubata C., Kondo R., Yoshida S., Takahashi M., Oie M., et al. (2007). Cooperation of NF-kappaB2/p100 activation and the PDZ domain binding motif signal in human T-cell leukemia virus type 1 (HTLV-1) Tax1 but not HTLV-2 Tax2 is crucial for interleukin-2-independent growth transformation of a T-cell line. J. Virol. 81 11900–11907 10.1128/JVI.00532-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H., Suzuki T., Fujisawa J., Inoue J., Yoshida M. (1994). Tax protein of human T-cell leukemia virus type I binds to the ankyrin motifs of inhibitory factor kappa B and induces nuclear translocation of transcription factor NF-κB proteins for transcriptional activation. Proc. Natl. Acad. Sci. U.S.A. 91 3584–3588 10.1073/pnas.91.9.3584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu K., Yoshikura H. (1986). Frequent partial deletion of human adult T-cell leukemia virus type I proviruses in experimental transmission: pattern and possible implication. J. Virol. 58 508–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y. K., Zhi H., DeBiaso D., Philip S., Shih H. M., Giam C. Z. (2012). HTLV-1 tax-induced rapid senescence is driven by the transcriptional activity of NF-κB and depends on chronically activated IKKα and p65/RelA. J. Virol. 86 9474–9483 10.1128/JVI.00158-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Ren T., Guan H., Jiang Y., Cheng H. (2009). HTLV-1 Tax is a critical lipid raft modulator that hijacks IkappaB kinases to the microdomains for persistent activation of NF-kappaB. J. Biol. Chem. 284 6208–6217 10.1074/jbc.M806390200 [DOI] [PubMed] [Google Scholar]
- Imai M., Higuchi M., Kawamura H., Yoshita M., Takahashi M., Oie M., et al. (2013). Human T cell leukemia virus type 2 (HTLV-2) Tax2 has a dominant activity over HTLV-1 Tax1 to immortalize human CD4+ T cells. Virus Genes 46 39–46 10.1007/s11262-012-0831-9 [DOI] [PubMed] [Google Scholar]
- Ishikawa C., Kawakami H., Uchihara J. N., Senba M., Mori N. (2013). CD69 overexpression by human T-cell leukemia virus type 1 Tax transactivation. Biochim. Biophys. Acta 1833 1542–1552 10.1016/j.bbamcr.2013.03.006 [DOI] [PubMed] [Google Scholar]
- Jeong S. J., Pise-Masison C. A., Radonovich M. F., Park H. U., Brady J. N. (2005). Activated AKT regulates NF-kB activation, p53 inhibition and cell survival in HTLV-1-transformed cells. Oncogene 24 6719–6728 10.1038/sj.onc.1208825 [DOI] [PubMed] [Google Scholar]
- Jin D. Y., Jeang K. T. (1997). Transcriptional activation and self-association in yeast: protein-protein dimerization as a pleiotropic mechanism of HTLV-I Tax function. Leukemia 11(Suppl. 3) 3–6 [PubMed] [Google Scholar]
- Jin D. Y., Spencer F., Jeang K. T. (1998). Human T cell leukemia virus type 1 oncoprotein Tax targets the human mitotic checkpoint protein MAD1. Cell 93 81–91 10.1016/S0092-8674(00)81148-4 [DOI] [PubMed] [Google Scholar]
- Jin D. Y., Teramoto H., Giam C. Z., Chun R. F., Gutkind J. S., Jeang K. T. (1997). A human suppressor of c-Jun N-terminal kinase 1 activation by tumor necrosis factor alpha. J. Biol. Chem. 272 25816–25823 10.1074/jbc.272.41.25816 [DOI] [PubMed] [Google Scholar]
- Journo C., Bonnet A., Favre-Bonvin A., Turpin J., Vinera J., Côté E., et al. (2013). Human T cell leukemia virus type 2 tax-mediated NF-κB activation involves a mechanism independent of Tax conjugation to ubiquitin and SUMO. J. Virol. 87 1123–1136 10.1128/JVI.01792-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Journo C., Filipe J., About F., Chevalier S. A., Afonso P. V., Brady J. N., et al. (2009). NRP/optineurin Cooperates with TAX1BP1 to potentiate the activation of NF-kappaB by human T-lymphotropic virus type 1 tax protein. PLoS Pathog. 5:e1000521 10.1371/journal.ppat.1000521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman V. S., Sarngadharan M. G., Robert-Guroff M., Miyoshi I., Golde D., Gallo R. C. (1982). A new subtype of human T-cell leukemia virus (HTLV-II) associated with a T-cell variant of hairy cell leukemia. Science 218 571–573 10.1126/science.6981847 [DOI] [PubMed] [Google Scholar]
- Kannian P., Green P. L. (2010). Human T Lymphotropic Virus Type 1 (HTLV-1): Molecular Biology and Oncogenesis. Viruses 2 2037–2077 10.3390/v2092037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashanchi F., Brady J. N. (2005). Transcriptional and post-transcriptional gene regulation of HTLV-1. Oncogene 24 5938–5951 10.1038/sj.onc.1208973 [DOI] [PubMed] [Google Scholar]
- Kfoury A. G., Stehlik J., Renlund D. G., Snow G., Seaman J. T., Gilbert E. M., et al. (2006). Impact of repetitive episodes of antibody-mediated or cellular rejection on cardiovascular mortality in cardiac transplant recipients: defining rejection patterns. J. Heart Lung Transplant. 25 1277–1282 10.1016/j.healun.2006.08.009 [DOI] [PubMed] [Google Scholar]
- Kfoury Y., Nasr R., Journo C., Mahieux R., Pique C., Bazarbachi A. (2012). The multifaceted oncoprotein Tax: subcellular localization, posttranslational modifications, and NF-κB activation. Adv. Cancer Res. 113 85–120 10.1016/B978-0-12-394280-7.00003-8 [DOI] [PubMed] [Google Scholar]
- Kibler K. V., Jeang K. T. (2001). CREB/ATF-dependent repression of cyclin A by human T-cell leukemia virus type 1 Tax protein. J. Virol. 75 2161–2173 10.1128/JVI.75.5.2161-2173.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. M., Geiger T. R., Egan D. I., Sharma N., Nyborg J. K. (2010). The HTLV-1 tax protein cooperates with phosphorylated CREB, TORC2 and p300 to activate CRE-dependent cyclin D1 transcription. Oncogene 29 2142–2152 10.1038/onc.2009.498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. M., Sharma N., Nyborg J. K. (2008). The proto-oncogene Bcl3, induced by Tax, represses Tax-mediated transcription via p300 disment from the human T-cell leukemia virus type 1 promoter. J. Virol. 82 11939–11947 10.1128/JVI.01356-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga H., Ohshima T., Shimotohno K. (2004). Enhanced activation of tax-dependent transcription of human T-cell leukemia virus type I (HTLV-I) long terminal repeat by TORC3. J. Biol. Chem. 279 52978–52983 10.1074/jbc.M409021200 [DOI] [PubMed] [Google Scholar]
- Ku S. C., Lee J., Lau J., Gurumurthy M., Ng R., Lwa S. H., et al. (2008). XBP-1, a novel human T-lymphotropic virus type 1 (HTLV-1) tax binding protein, activates HTLV-1 basal and tax-activated transcription. J. Virol. 82 4343–4353 10.1128/JVI.02054-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok R. P., Laurance M. E., Lundblad J. R., Goldman P. S., Shih H., Connor L. M., et al. (1996). Control of cAMP-regulated enhancers by the viral transactivator Tax through CREB and the co-activator CBP. Nature 380 642–646 10.1038/380642a0 [DOI] [PubMed] [Google Scholar]
- Lairmore M. D., Haines R., Anupam R. (2012). Mechanisms of human T-lymphotropic virus type 1 transmission and disease. Curr. Opin. Virol. 2 474–481 10.1016/j.coviro.2012.06.007 [DOI] [PubMed] [Google Scholar]
- Lamsoul I., Lodewick J., Lebrun S., Brasseur R., Burny A., Gaynor R. B., et al. (2005). Exclusive ubiquitination and sumoylation on overlapping lysine residues mediate NF-kappaB activation by the human T-cell leukemia virus tax oncoprotein. Mol. Cell. Biol. 25 10391–10406 10.1128/MCB.25.23.10391-10406.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larocque é., Halin M., Landry S., Marriott S. J., Switzer W. M., Barbeau B. (2011). Human T-cell lymphotropic virus type 3 (HTLV-3)- and HTLV-4-derived antisense transcripts encode proteins with similar Tax-inhibiting functions but distinct subcellular localization. J. Virol. 85 12673–12685 10.1128/JVI.05296-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M. J., Gautier V. W., Wang X. P., Kaplan M. H., Hall W. W. (2000). Spontaneous production of C-C chemokines by individuals infected with human T lymphotropic virus type II (HTLV-II) alone and HTLV-II/HIV-1 coinfected individuals. J. Immunol. 165 4127–4132 [DOI] [PubMed] [Google Scholar]
- Li M., Green P. L. (2007). Detection and quantitation of HTLV-1 and HTLV-2 mRNA species by real-time RT-PCR. J. Virol. Methods 142 159–168 10.1016/j.jviromet.2007.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodewick J., Lamsoul I., Bex F. (2011). Move or die: the fate of the Tax oncoprotein of HTLV-1. Viruses 3 829–857 10.3390/v3060829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodewick J., Lamsoul I., Polania A., Lebrun S., Burny A., Ratner L., et al. (2009). Acetylation of the human T-cell leukemia virus type 1 Tax oncoprotein by p300 promotes activation of the NF-kappaB pathway. Virology 386 68–78 10.1016/j.virol.2008.12.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahieux R., Gessain A. (2009). The human HTLV-3 and HTLV-4 retroviruses: new members of the HTLV family. Pathol. Biol. (Paris) 57 161–166 10.1016/j.patbio.2008.02.015 [DOI] [PubMed] [Google Scholar]
- Mahieux R., Pise-Masison C. A., Lambert P. F., Nicot C., De Marchis L., Gessain A., et al. (2000). Differences in the ability of human T-cell lymphotropic virus type 1 (HTLV-1) and HTLV-2 tax to inhibit p53 function. J. Virol. 74 6866–6874 10.1128/JVI.74.15.6866-6874.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majorovits E., Nejmeddine M., Tanaka Y., Taylor G. P., Fuller S. D., Bangham C. R. (2008). Human T-lymphotropic virus-1 visualized at the virological synapse by electron tomography. PLoS ONE 3:e2251 10.1371/journal.pone.0002251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makokha G. N., Takahashi M., Higuchi M., Saito S., Tanaka Y., Fujii M. (2013). Human T-cell leukemia virus type 1 Tax protein interacts with and mislocalizes the PDZ domain protein MAGI-1. Cancer Sci. 104 313–320 10.1111/cas.12087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns A., Blattner W. A. (1991). The epidemiology of the human T-cell lymphotrophic virus type I and II: etiologic role in human diseases. Transfusion 31 67–75 10.1046/j.1537-2995.1991.31191096189.x [DOI] [PubMed] [Google Scholar]
- Marban C., McCabe A., Bukong T. N., Hall W. W., Sheehy N. (2012). Interplay between the HTLV-2 Tax and APH-2 proteins in the regulation of the AP-1 pathway. Retrovirology 9 98 10.1186/1742-4690-9-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka M., Green P. L. (2009). The HBZ gene, a key player in HTLV-1 pathogenesis. Retrovirology 6 71 10.1186/1742-4690-6-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka M., Jeang K. T. (2007). Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat. Rev. Cancer 7 270–280 10.1038/nrc2111 [DOI] [PubMed] [Google Scholar]
- Matsuoka M., Jeang K. T. (2011). Human T-cell leukemia virus type 1 (HTLV-1) and leukemic transformation: viral infectivity, Tax, HBZ and therapy. Oncogene 30 1379–1389 10.1038/onc.2010.537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meertens L., Chevalier S., Weil R., Gessain A., Mahieux R. (2004a). A 10-amino acid domain within human T-cell leukemia virus type 1 and type 2 Tax protein sequences is responsible for their divergent subcellular distribution. J. Biol. Chem. 279 43307–43320 10.1074/jbc.M400497200 [DOI] [PubMed] [Google Scholar]
- Meertens L., Pise-Masison C., Quere N., Brady J., Gessain A., Mahieux R. (2004b). Utilization of the CBP but not the p300 co-activator by human T-lymphotropic virus type-2 Tax for p53 inhibition. Oncogene 23 5447–5458 10.1038/sj.onc.1207719 [DOI] [PubMed] [Google Scholar]
- Mori N., Morishita M., Tsukazaki T., Giam C. Z., Kumatori A., Tanaka Y., et al. (2001). Human T-cell leukemia virus type i oncoprotein Tax represses Smad-dependent transforming growth factor beta signaling through interaction with CREB-binding protein/p300. Blood 97 2137–2144 10.1182/blood.V97.7.2137 [DOI] [PubMed] [Google Scholar]
- Mulloy J. C., Kislyakova T., Cereseto A., Casareto L., LoMonico A., Fullen J., et al. (1998). Human T-cell lymphotropic/leukemia virus type 1 Tax abrogates p53-induced cell cycle arrest and apoptosis through its CREB/ATF functional domain. J. Virol. 72 8852–8860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasr R., Chiari E., El Sabban M., Mahieux R., Kfoury Y., Abdulhay M., et al. (2006). Tax ubiquitylation and sumoylation control critical cytoplasmic and nuclear steps of NF-kappa B activation. Blood 107 4021–4029 10.1182/blood-2005-09-3572 [DOI] [PubMed] [Google Scholar]
- Nejmeddine M., Barnard A. L., Tanaka Y., Taylor G. P, Bangham C. R. (2005). Human T-lymphotropic virus, type 1, tax protein triggers microtubule reorientation in the virological synapse. J. Biol. Chem. 280 29653–29660 10.1074/jbc.M502639200 [DOI] [PubMed] [Google Scholar]
- Nyborg J. K., Egan D., Sharma N. (2010). The HTLV-1 Tax protein: revealing mechanisms of transcriptional activation through histone acetylation and nucleosome disassembly. Biochim. Biophys. Acta 1799 266–274 10.1016/j.bbagrm.2009.09.002 [DOI] [PubMed] [Google Scholar]
- Ohsugi T. (2013). A transgenic mouse model of human T cell leukemia virus type 1-associated diseases. Front. Microbiol. 4:49. 10.3389/fmicb.2013.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsugi T., Ishida T., Shimasaki T., Okada S., Umezawa K. (2013). p53 dysfunction precedes the activation of nuclear factor-κB during disease progression in mice expressing Tax, a human T-cell leukemia virus type 1 oncoprotein. Carcinogenesis 10.1093/carcin/bgt144 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Okajima M., Takahashi M., Higuchi M., Ohsawa T., Yoshida S., Yoshida Y., et al. (2008). Human T-cell leukemia virus type 1 Tax induces an aberrant clustering of the tumor suppressor Scribble through the PDZ domain-binding motif dependent and independent interaction. Virus Genes 37 231–240 10.1007/s11262-008-0259-4 [DOI] [PubMed] [Google Scholar]
- Orlandi C., Forlani G., Tosi G., Accolla R. S. (2011). Molecular and cellular correlates of the CIITA-mediated inhibition of HTLV-2 Tax-2 transactivator function resulting in loss of viral replication. J. Transl. Med. 9 106 10.1186/1479-5876-9-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osame M., Usuku K., Izumo S., Ijichi N., Amitani H., Igata A., et al. (1986). HTLV-I associated myelopathy, a new clinical entity. Lancet 1 1031–1032 10.1016/S0140-6736(86)91298-5 [DOI] [PubMed] [Google Scholar]
- Peloponese J. M. Jr., Jeang K. T. (2006). Role for Akt/protein kinase B and activator protein-1 in cellular proliferation induced by the human T-cell leukemia virus type 1 tax oncoprotein. J. Biol. Chem. 281 8927–8938 10.1074/jbc.M510598200 [DOI] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. (1980). Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. U.S.A. 77 7415–7419 10.1073/pnas.77.12.7415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy T. R., Tang H., Li X., Wong-Staal F. (1997). Functional interaction of the HTLV-1 transactivator Tax with activating transcription factor-4 (ATF4). Oncogene 14 2785–2792 10.1038/sj.onc.1201119 [DOI] [PubMed] [Google Scholar]
- Ren T., Dong W., Takahashi Y., Xiang D., Yuan Y., Liu X., et al. (2012). HTLV-2 Tax immortalizes human CD4+ memory T lymphocytes by oncogenic activation and dysregulation of autophagy. J. Biol. Chem. 287 34683–34693 10.1074/jbc.M112.377143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rende F., Cavallari I., Romanelli M. G., Diani E., Bertazzoni U., Ciminale V. (2012). Comparison of the genetic organization, expression strategies and oncogenic potential of HTLV-1 and HTLV-2. Leuk. Res. Treatment. 2012 876153 10.1155/2012/876153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross T. M., Pettiford S. M., Green P. L. (1996). The tax gene of human T-cell leukemia virus type 2 is essential for transformation of human T lymphocytes. J. Virol. 70 5194–5202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roucoux D. F., Murphy E. L. (2004). The epidemiology and disease outcomes of human T-lymphotropic virus type II. AIDS Rev. 6 144–154 [PubMed] [Google Scholar]
- Rousset R., Fabre S., Desbois C., Bantignies F., Jalinot P. (1998). The C-terminus of the HTLV-1 Tax oncoprotein mediates interaction with the PDZ domain of cellular proteins. Oncogene 16 643–654 10.1038/sj.onc.1201567 [DOI] [PubMed] [Google Scholar]
- Satou Y., Matsuoka M. (2012). Molecular and cellular mechanism of leukemogenesis of ATL: emergent evidence of a significant role for HBZ in HTLV-1-induced pathogenesis. Leuk. Res. Treatment 2012 213653 10.1155/2012/213653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satou Y., Matsuoka M. (2013). Virological and immunological mechanisms in the pathogenesis of human T-cell leukemia virus type 1. Rev. Med. Virol. 23 269–280 10.1002/rmv.1745 [DOI] [PubMed] [Google Scholar]
- Semmes O. J., Barrett J. F., Dang C. V., Jeang K. T. (1996a). Human T-cell leukemia virus type I Tax masks c-myc function through a cAMP-dependent pathway. J. Biol. Chem. 271 9730–9738 10.1074/jbc.271.16.9730 [DOI] [PubMed] [Google Scholar]
- Semmes O. J., Majone F., Cantemir C., Turchetto L., Hjelle B., Jeang K. T. (1996b). HTLV-I and HTLV-II Tax: differences in induction of micronuclei in cells and transcriptional activation of viral LTRs. Virology 217 373–379 10.1006/viro.1996.0126 [DOI] [PubMed] [Google Scholar]
- Sharma N., Nyborg J. K. (2008). The coactivators CBP/p300 and the histone chaperone NAP1 promote transcription-independent nucleosome eviction at the HTLV-1 promoter. Proc. Natl. Acad. Sci. U.S.A. 105 7959–7963 10.1073/pnas.0800534105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaulian E., Karin M. (2001). AP-1 in cell proliferation and survival. Oncogene 20 2390–2400 [DOI] [PubMed] [Google Scholar]
- Sheehy N., Lillis L., Watters K., Lewis M., Gautier V., Hall W. (2006). Functional analysis of human T lymphotropic virus type 2 Tax proteins. Retrovirology 3 20 10.1186/1742-4690-3-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shembade N., Pujari R., Harhaj N. S., Abbott D. W., Harhaj E. W. (2011). The kinase IKKα inhibits activation of the transcription factor NF-κB by phosphorylating the regulatory molecule TAX1BP1. Nat. Immunol. 12 834–843 10.1038/ni.2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji T., Higuchi M., Kondo R., Takahashi M., Oie M., Tanaka Y., et al. (2009). Identification of a novel motif responsible for the distinctive transforming activity of human T-cell leukemia virus (HTLV) type 1 Tax1 protein from HTLV-2 Tax2. Retrovirology 6 83 10.1186/1742-4690-6-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuh M., Derse D. (2000). Ternary complex factors and cofactors are essential for human T-cell leukemia virus type 1 tax transactivation of the serum response element. J. Virol. 74 11394–11397 10.1128/JVI.74.23.11394-11397.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silic-Benussi M., Cavallari I., Vajente N., Vidali S., Chieco-Bianchi L., Di Lisa F., et al. (2010a). Redox regulation of T-cell turnover by the p13 protein of human T-cell leukemia virus type 1: distinct effects in primary versus transformed cells. Blood 116 54–62 10.1182/blood-2009-07-235861 [DOI] [PubMed] [Google Scholar]
- Silic-Benussi M., Biasiotto R., Andresen V., Franchini G., D’Agostino D. M., Ciminale V. (2010b). HTLV-1 p13, a small protein with a busy agenda. Mol. Aspects Med. 31 350–358 10.1016/j.mam.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sintasath D. M., Wolfe N. D., Lebreton M., Jia H., Garcia A. D., Le Doux-Diffo J., et al. (2009). Simian T-lymphotropic virus diversity among nonhuman primates, Cameroon. Emerg. Infect. Dis. 15 175–184 10.3201/eid1502.080584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu Y. T., Chin K. T., Siu K. L., Yee Wai Choy E., Jeang K. T., Jin D. Y. (2006). TORC1 and TORC2 coactivators are required for tax activation of the human T-cell leukemia virus type 1 long terminal repeats. J. Virol. 80 7052–7059 10.1128/JVI.00103-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. R., Greene W. C. (1992). Characterization of a novel nuclear localization signal in the HTLV-I tax transactivator protein. Virology 187 316–320 10.1016/0042-6822(92)90320-O [DOI] [PubMed] [Google Scholar]
- Sun S. (2011). Non-canonical NF-kB signalling pathway. Cell Res. 21 71–85 10.1038/cr.2010.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S. C., Yamaoka S. (2005). Activation of NF-kappaB by HTLV-I and implications for cell transformation. Oncogene 24 5952–5964 10.1038/sj.onc.1208969 [DOI] [PubMed] [Google Scholar]
- Suzuki T., Fujisawa J. I., Toita M., Yoshida M. (1993a). The transactivator Tax of human T-cell leukemia virus type I (HTLV-I) interacts with cAMP-responsive element (CRE) binding and CRE modulator proteins that bind to the 21-base-pair enhancer of HTLV-I. Proc. Natl. Acad. Sci. U.S.A. 90 610–614 10.1073/pnas.90.2.610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Hirai H., Fujisawa J., Fujita T., Yoshida M. (1993b). A trans-activator Tax of human T-cell leukemia virus type 1 binds to NF-kappa B p50 and serum response factor (SRF) and associates with enhancer DNAs of the NF-kappa B site and CArG box. Oncogene 8 2391–2397 [PubMed] [Google Scholar]
- Suzuki T., Hirai H., Murakami T., Yoshida M. (1995). Tax protein of HTLV-1 destabilizes the complexes of NF-κB and IκB-alpha and induces nuclear translocation of NF-κB for transcriptional activation. Oncogene 10 1199–1207 [PubMed] [Google Scholar]
- Suzuki T., Hirai H., Yoshida M. (1994). Tax protein of HTLV-1 interacts with the Rel homology domain of NF-kappa B p65 and c-Rel proteins bound to the NF-kappa B binding site and activates transcription. Oncogene 9 3099–3105 [PubMed] [Google Scholar]
- Suzuki T., Ohsugi Y., Uchida-Toita M., Akiyama T., Yoshida M. (1999). Tax oncoprotein of HTLV-1 binds to the human homologue of Drosophila discs large tumor suppressor protein, hDLG, and perturbs its function in cell growth control. Oncogene 18 5967–5972 10.1038/sj.onc.1203008 [DOI] [PubMed] [Google Scholar]
- Switzer W. M., Qari S. H., Wolfe N. D., Burke D. S., Folks T. M., Heneine W. (2006). Ancient origin and molecular features of the novel human T-lymphotropic virus type 3 revealed by complete genome analysis. J. Virol. 80 7427–7438 10.1128/JVI.00690-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer W. M., Salemi M., Qari S. H., Jia H., Gray R. R., Katzourakis A., et al. (2009). Ancient, independent evolution and distinct molecular features of the novel human T-lymphotropic virus type 4. Retrovirology 6 9 10.1186/1742-4690-6-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabakin-Fix Y., Azran I., Schavinky-Khrapunsky Y., Levy O., Aboud M. (2006). Functional inactivation of p53 by human T-cell leukemia virus type 1 Tax protein: mechanisms and clinical implications. Carcinogenesis 27 673–681 10.1093/carcin/bgi274 [DOI] [PubMed] [Google Scholar]
- Tang H. M., Gao W. W., Chan C. P., Siu Y. T., Wong C. M., Kok K. H., et al. (2013a). LKB1 tumor suppressor and salt-inducible kinases negatively regulate human T-cell leukemia virus type 1 transcription. Retrovirology 10 40 10.1186/1742-4690-10-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S. W., Chen C. Y., Klase Z., Zane L., Jeang K. T. (2013b). The cellular autophagy pathway modulates human T-cell leukemia virus type 1 replication. J. Virol. 87 1699–1707 10.1128/JVI.02147-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi G., Forlani G., Andresen V., Turci M., Bertazzoni U., Franchini G., et al. (2011). Major histocompatibility complex class II transactivator CIITA is a viral restriction factor that targets human T-cell lymphotropic virus type 1 Tax-1 function and inhibits viral replication. J. Virol. 85 10719–10729 10.1128/JVI.00813-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp A., Banerjee P., Sieburg M., Planelles V., Li F., Feuer G. (2005). Induction of cell cycle arrest by human T-cell lymphotropic virus type 1 Tax in hematopoietic progenitor (CD34+) cells: modulation of p21cip1/waf1 and p27kip1 expression. J. Virol. 79 14069–14078 10.1128/JVI.79.22.14069-14078.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp A., Liu Y., Sieburg M., Montalbano J., Wrzesinski S., Feuer G. (2003). Human T-cell leukemia virus type 1 tax oncoprotein suppression of multilineage hematopoiesis of CD34+ cells in vitro. J. Virol. 77 12152–12164 10.1128/JVI.77.22.12152-12164.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubata C., Higuchi M., Takahashi M., Oie M., Tanaka Y., Gejyo F., et al. (2005). PDZ domain-binding motif of human T-cell leukemia virus type 1 Tax oncoprotein is essential for the interleukin 2 independent growth induction of a T-cell line. Retrovirology 23 2–46 10.1186/1742-4690-2-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turci M., Lodewick J., Di Gennaro G., Rinaldi A. S., Marin O., Diani E., et al. (2012). Ubiquitination and sumoylation of the HTLV-2 Tax-2B protein regulate its NF-κB activity: a comparative study with the HTLV-1 Tax-1 protein. Retrovirology 9 102 10.1186/1742-4690-9-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turci M., Lodewick J., Righi P., Polania A., Romanelli M. G., Bex F., et al. (2009). HTLV-2B Tax oncoprotein is modified by ubiquitination and sumoylation and displays intracellular localization similar to its homologue HTLV-1 Tax. Virology 386 6–11 10.1016/j.virol.2009.01.003 [DOI] [PubMed] [Google Scholar]
- Turci M., Romanelli M. G., Lorenzi P., Righi P., Bertazzoni U. (2006a). Localization of human T-cell lymphotropic virus type II Tax protein is dependent upon a nuclear localization determinant in the N-terminal region. Gene 365 119–124 10.1016/j.gene.2005.09.043 [DOI] [PubMed] [Google Scholar]
- Turci M., Pilotti E., Ronzi P., Magnani G., Boschini A., Parisi S. G., et al. (2006b). Coinfection with HIV-1 and human T-Cell lymphotropic virus type II in intravenous drug users is associated with delayed progression to AIDS. J. Acquir. Immune Defic. Syndr. 41 100–106 10.1097/01.qai.0000179426.04166.12 [DOI] [PubMed] [Google Scholar]
- Twizere J. C., Springael J. Y., Boxus M., Burny A., Dequiedt F., Dewulf J. F., et al. (2007). Human T-cell leukemia virus type-1 Tax oncoprotein regulates G-protein signaling. Blood 109 1051–1060 10.1182/blood-2006-06-026781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandamme A. M. (2000). The Fifth European Workshop on Virus Evolution and Molecular Epidemiology, Leuven, Belgium, 30 August to 4 September 1999. Evolving viruses and virologists. Trends Microbiol. 8 8–10 10.1016/S0966-842X(99)01664-9 [DOI] [PubMed] [Google Scholar]
- Van Dooren S., Salemi M., Vandamme A. M. (2001). Dating the origin of the African human T-cell lymphotropic virus type-i (HTLV-I) subtypes. Mol. Biol. Evol. 18 661–671 10.1093/oxfordjournals.molbev.a003846 [DOI] [PubMed] [Google Scholar]
- Wang D., Guo M. X., Hu H. M., Zhao Z. Z., Qiu H. L., Shao H. J., et al. (2008). Human T-cell leukemia virus type 1 oncoprotein tax represses ZNF268 expression through the cAMP-responsive element-binding protein/activating transcription factor pathway. J. Biol. Chem. 283 16299–16308 10.1074/jbc.M706426200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T. (2011). Current status of HTLV-1 infection. Int. J. Hematol. 94 430–434 10.1007/s12185-011-0934-4 [DOI] [PubMed] [Google Scholar]
- Wolfe N. D., Heneine W., Carr J. K., Garcia A. D., Shanmugam V., Tamoufe U., et al. (2005). Emergence of unique primate T-lymphotropic viruses among central African bushmeat hunters. Proc. Natl. Acad. Sci. U.S.A. 102 7994–7999 10.1073/pnas.0501734102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K., Bottazzi M. E., de la Fuente C., Deng L., Gitlin S. D., Maddukuri A., et al. (2004). Protein profile of Tax-associated complexes. J. Biol. Chem. 279 495–508 10.1074/jbc.M310069200 [DOI] [PubMed] [Google Scholar]
- Wu X., Sun S. C. (2007). Retroviral oncoprotein Tax deregulates NF-κB by activating Tak1 and mediating the physical association of Tak1-IKK. EMBO Rep. 8 510–515 10.1038/sj.embor.7400931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wycuff D. R., Marriott S. J. (2005). The HTLV-I Tax oncoprotein: hyper-tasking at the molecular level. Front. Biosci. 10:620–642. 10.2741/1558 [DOI] [PubMed] [Google Scholar]
- Xiao G. (2012). NF-kappaB activation: Tax sumoylation is out, but what about Tax ubiquitination? Retrovirology 9 78 10.1186/1742-4690-9-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L., Yamamoto B., Haoudi A., Semmes O. J., Green P. L. (2006). PDZ binding motif of HTLV-1 Tax promotes virus-mediated T-cell proliferation in vitro and persistence in vivo. Blood 107 1980–1988 10.1182/blood-2005-03-1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi M., Nakano K., Miyake A., Yamochi T., Kagami Y., Tsutsumi A., et al. (2012). Polycomb-mediated loss of miR-31 activates NIK-dependent StateNF-κB pathway in adult T cell leukemia and other cancers. Cancer Cell 21121-35 10.1016/j.ccr.2011.12.015 [DOI] [PubMed] [Google Scholar]
- Yamagishi M., Watanabe T. (2012). Molecular hallmarks of adult T cell leukemia. Front. Microbiol. 3:334. 10.3389/fmicb.2012.00334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Ishida T., Nakano K., Yamagishi M., Yamochi T., Tanaka Y., et al. (2011). SMYD3 interacts with HTLV-1 Tax and regulates subcellular localization of Tax. Cancer Sci. 102 260–266 10.1111/j.1349-7006.2010.01752.x [DOI] [PubMed] [Google Scholar]
- Yasunaga J., Lin F. C., Lu X., Jeang K. T. (2011). Ubiquitin-specific peptidase 20 targets TRAF6 and human T cell leukemia virus type 1 tax to negatively regulate NF-kappaB signaling. J. Virol. 85 6212–6219 10.1128/JVI.00079-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasunaga J., Matsuoka M. (2011). Molecular mechanisms of HTLV-1 infection and pathogenesis. Int. J. Hematol. 94 435–442 10.1007/s12185-011-0937-1 [DOI] [PubMed] [Google Scholar]
- Yin M. J., Christerson L. B., Yamamoto Y., Kwak Y. T., Xu S., Mercurio F., et al. (1998). HTLV-I Tax protein binds to MEKK1 to stimulate IkappaB kinase activity and NF-kappaB activation. Cell 93 875–884 10.1016/S0092-8674(00)81447-6 [DOI] [PubMed] [Google Scholar]
- Yin M. J., Paulssen E. J., Seeler J. S., Gaynor R. B. (1995). Protein domains involved in both in vivo and in vitro interactions between human T-cell leukemia virus type I tax and CREB. J. Virol. 69 3420–3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M. (2001). Multiple viral strategies of HTLV-1 for dysregulation of cell growth control. Annu. Rev. Immunol. 19 475–496 10.1146/annurev.immunol.19.1.475 [DOI] [PubMed] [Google Scholar]
- Yoshida M. (2010). Molecular approach to human leukemia: isolation and characterization of the first human retrovirus HTLV-1 and its impact on tumorigenesis in adult T-cell leukemia. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 86 117–130 10.2183/pjab.86.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Satou Y., Yasunaga J., Fujisawa J., Matsuoka M. (2008). Transcriptional control of spliced and unspliced human T-cell leukemia virus type 1 bZIP factor (HBZ) gene. J. Virol. 82 9359–9368 10.1128/JVI.00242-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Minoda Y., Yoshida R., Yoshida H., Iha H., Kobayashi T., et al. (2008). HTLV-1 Tax-mediated TAK1 activation involves TAB2 adapter protein. Biochem. Biophys. Res. Commun. 365 189–194 10.1016/j.bbrc.2007.10.172 [DOI] [PubMed] [Google Scholar]
- Zane L., Jeang K. T. (2012). The importance of ubiquitination and sumoylation on the transforming activity of HTLV Tax-1 and Tax-2. Retrovirology 9 103 10.1186/1742-4690-9-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zane L., Yasunaga J., Mitagami Y., Yedavalli V., Tang S. W., Chen C. Y., et al. (2012). Wip1 and p53 contribute to HTLV-1 Tax-induced tumorigenesis. Retrovirology 21 9–114 10.1186/1742-4690-9-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T., Matsuoka M. (2012). HBZ and its roles in HTLV-1 oncogenesis. Front. Microbiol. 3:247 10.3389/fmicb.2012.00247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi H., Yang L., Kuo Y. L., Ho Y. K., Shih H. M., Giam C. Z. (2011). NF-κB hyper-activation by HTLV-1 tax induces cellular senescence, but can be alleviated by the viral anti-sense protein HBZ. PLoS Pathog. 7:e1002025 10.1371/journal.ppat.1002025 [DOI] [PMC free article] [PubMed] [Google Scholar]