Abstract
Renal lipid accumulation exhibits slowly developing chronic kidney disease and is associated with increased oxidative stress. The impact of exercise on the obese- and oxidative stress-related renal disease is not well understood. The purpose of this study was to investigate whether a high-fat diet (HFD) would accelerate d-galactose-induced aging process in rat kidney and to examine the preventive effect of regular exercise on the obese- and oxidative stress-related renal disease. Oxidative stress was induced by an administration of d-galactose (100 mg/kg intraperitoneally injected) for 9 weeks, and d-galactose-treated rats were also fed with a high-fat diet (60% kcal as fat) for 9 weeks to induce obesity. We investigated the efficacy of regular exercise in reducing renal injury by analyzing Nε-carboxymethyllysine (CML), 8-hydroxygluanine (8-OHdG) and apoptosis. When rats were fed with a HFD for 9 weeks in d-galactose-treated rats, an increased CML accumulation, oxidative DNA damage and renal podocyte loss were observed in renal glomerular cells and tubular epithelial cells. However, the regular exercise restored all these renal changes in HFD plus d-galactose-treated rats. Our data suggested that long-term HFD may accelerate the deposition of lipoxidation adducts and oxidative renal injury in d-galactose-treated rats. The regular exercise protects against obese- and oxidative stress-related renal injury by inhibiting this lipoxidation burden.
Keywords: exercise, d-galactose, high-fat diet, oxidative stress, renal injury
I. Introduction
Obesity-related diseases are global epidemics impacting the health of adults. Obese individuals are more prone to cardiovascular disease, dyslipidemia, sleep apnea, hypertension and renal disease [24, 29, 43]. Renal lipid accumulation may enhance oxidative stress and inflammation in the kidney and contribute to the development of renal injury [31, 38]. Reactive oxygen species (ROS) play critical roles in the development and progression of kidney damage [10, 27]. Obesity-related nephropathy is associated with the increase of proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), due to increased ROS production [8].
Advanced glycation end products (AGEs) are stable end products of a non-enzymatic glycation reaction [37]. AGEs formation is accelerated under hyperglycemic condition as well as under oxidative stress. The advanced lipoxidation end products (ALEs) are similar adducts but are formed from oxidatively damaged lipids [40]. Hyperlipidemia leads to intracellular accumulation of fatty acids and cholesteryl esters [42] and chemical modification, particularly oxidation, of both proteins and lipids [30]. Reactive carbonyl species (RCS), such as aldehydes acrolein, malondialdehyde and glyoxal, react with proteins to generate these stable adducts [1]. Glyoxal is a dicarbonyl compound deriving also from autoxidation of Amadori products (glycoxidation) and reacting with lysine residues with formation of Nε-carboxymethyllysine (CML), which is therefore both an ALE and an AGE [39]. AGE/ALE accumulation is associated with aging and several age-associated diseases such as Alzheimer’s disease and renal insufficiency [25, 36]. The receptor for AGEs (RAGE) was shown to be predominantly involved in mediating AGE/ALE-induced tissue injury [47]. The interaction between AGE/ALE and RAGE trigger signaling pathways leading to a chronic inflammation and cell damage [22]. Moreover, an increased lipid accumulation in renal tissues causes mesangial cell activation resulting in glomerular hypertrophy and matrix deposition, podocyte injury and proteinuria [13]. A high-fat diet (HFD) was found to be a significant risk factor for kidney disease [17, 34]. Although a large body of evidence suggests that both enhanced intracellular lipid accumulation and oxidative stress were significantly associated with AGE/ALE formation and accumulation in renal tissues, the relationship between HFD and oxidative stress in AGEs/ALE-induced renal injury still remain unknown.
Exercise is reported to reduce oxidative stress in rats and mice [3, 26]. Moderate exercise by inducing the expression of antioxidant enzymes reduces oxidative stress [11]. In addition, regular moderate exercise reduces renal CML contents in obese Zucker rats [6]. However, the mechanism as to how exercise reduces oxidative stress and AGE/ALE formation is not known.
This study aimed to investigate the pathogenic role of oxidative stress and HFD mechanism underlying ALEs-induced renal injury, by assessing whether HFD accelerates AGE/ALE renal accumulation and promote AGE/ALE-induced renal injury in d-galactose-injected oxidative stressed rats, a well-known animal model of oxidative stress. In addition, we evaluate the inhibitory effect of AGE/ALE renal accumulation and the renopreventive effect of long-term regular exercise in this animal model.
II. Materials and Methods
Animals and experimental design
Thirty two male SD rats (8-weeks old) were used in this study. They were all individually housed in a temperature-controlled room with a 12-hr light/dark cycle and had free access to drinking water. After acclimation for 2 weeks, the rats were divided into four groups: (1) normal control rats (CON, n=8), (2) d-galactose-induced oxidative stressed rats (GAL, n=8), (3) HFD+d-galactose-induced oxidative stressed and obese rats (HFD+GAL, n=8) and (4) HFD+d-galactose-induced oxidative stressed and obese rats exercised regularly on a treadmill (EXE, n=8). The rats in the CON and GAL groups were fed a standard laboratory chow (3.34 kcal/g, PMI Nutrition International, MO, USA). To induce obesity, the rats in the HFD+GAL and EXE groups were fed a high-fat diet containing 60% kcal fat (5.24 kcal/g, D12492, Research Diets, NJ, USA) for 9 weeks. To induce oxidative stress, the rats in the GAL, HFD+GAL, EXE groups were injected intraperitoneally with d-galactose (100 mg/kg/day), while the rats in the CON group were injected with an equal volume of the vehicle (0.9% saline). The rats in the EXE groups were subjected to a treadmill exercise for 8 weeks (12 meter·/min–1·60 min–1, 15 degree grade, 5 days/week) according to a published protocol [2], and the rats in other sedentary groups were not exercised. The exercise training was begun 1 week after the onset of HFD feeding. All experimental procedures were performed under the supervision of our Institutional Animal Care and Use Committee.
Analysis of metabolic data
The body weight was monitored consecutively. One day before autopsy, the animals were fasted for 15 hr at least and immediately anesthetized and killed. Blood samples were collected from the tail vein, and total cholesterol, triglyceride (TG), HDL cholesterol (HDL) and LDL cholesterol (LDL) were measured using an automated analyzer (Wako, Tokyo, Japan).
Immunohistochemical staining
Immunohistochemistry was performed as previously described [33]. Antibodies were a mouse anti-CML (TransGenic, Kobe, Japan), a mouse anti-8-hydroxygluanine (8-OHdG) antibody (Santa Cruz Biotechnology, CA, USA) and a rabbit anti-synaptopodin (Santa Cruz Biotechnology, CA, USA). For detection of CML, 8-OHdG and synaptopodin, the sections were incubated with the Envision kit (DAKO, CA, USA) and visualized by 3,3'-diaminobenzidine tetrahydrochloride. Negative controls for immunohistochemistry were run by incubating the sections with nonimmune serum instead of the primary antibody. The intensity of immunohistochemical staining of CML and 8-OHdG was analyzed in eight randomly selected mm2 areas of renal cortex, and the positive signal areas of synaptopodin was determined per one glomerulus in a total of 40 glomeruli using Image J software (NIH, MD, USA).
Double staining for TUNEL and Wilms tumor antigen-1
In order to confirm the apoptotic cell death in renal podocytes, a sequential immunostaining was performed. The first staining of TUNEL was performed with a kit (DeadEnd apoptosis detection system, Promega, WI, USA) according to the manufacturer’s instructions. Apoptotic cells were detected with FITC-conjugated streptavidin (Santa Cruz Biotechnology). The second sequence of staining using rabbit anti-Wilms tumor antigen-1 (WT-1, Santa Cruz Biotechnology) was performed on the same section with tetramethyl rhodamine isothiocyanate (TRITC)-conjugated goat anti-rabbit IgG antibody (Santa Cruz Biotechnology). To prevent cross-reactivity between two staining sequences the slides were incubated with normal mouse serum (Dako) after the first staining. For morphometric analysis, the positive cell numbers of WT-1 per one glomerulus in a total of 40 glomeruli was determined using Image J software.
Apoptosis analysis
To evaluate apoptosis in renal tissues, the TUNEL assay was performed with a kit (DeadEnd apoptosis detection system, Promega) according to the manufacturer’s instructions. Apoptotic cells were detected with peroxidase conjugated streptavidin. The number of TUNEL-positive cells per unit area (mm2) was then determined in counted in a total of 5 fields.
Statistical analysis
Comparisons between groups were performed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test using GraphPad Prism 4.0 software (Graph pad, CA, USA).
III. Results
Body weight and blood lipid profile
Body weights and blood lipid levels at the end of the experiment are shown in Fig. 1. The body weight in the GAL group was slightly increased compared to the control group. In rats received both the d-galactose and HFD, body weight was significantly increased compared to the control rats and reduced by exercise training. Total cholesterol TG and LDL levels were significantly increased in the HFD+GAL group (P<0.01 vs. GAL group). EXE group showed significantly reduced in total cholesterol, TG and LDL levels as compared to the HFD+GAL group. However, no differences in lipid levels were noted between the CON group and the GAL group.
Fig. 1.
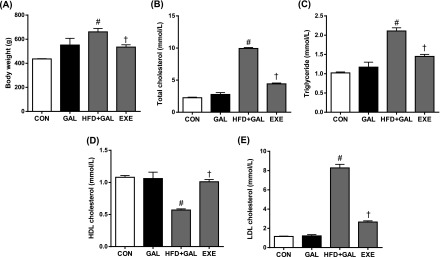
Body weight (A) and blood lipid levels (B to E). CON, normal control rats; GAL, d-galactose-induced oxidative stressed rats; HFD+GAL, high fat diet plus d-galactose-induced oxidative stressed and obese rats, EXE, high-fat diet plus d-galctose-induced oxidative stressed and obese rats exercised regularly. Values in the bar graphs represent means±SE, n=8. #P<0.01 compared with GAL group; †P<0.01 compared with HFD+GAL group.
Oxidative status and CML accumulation in renal tissues
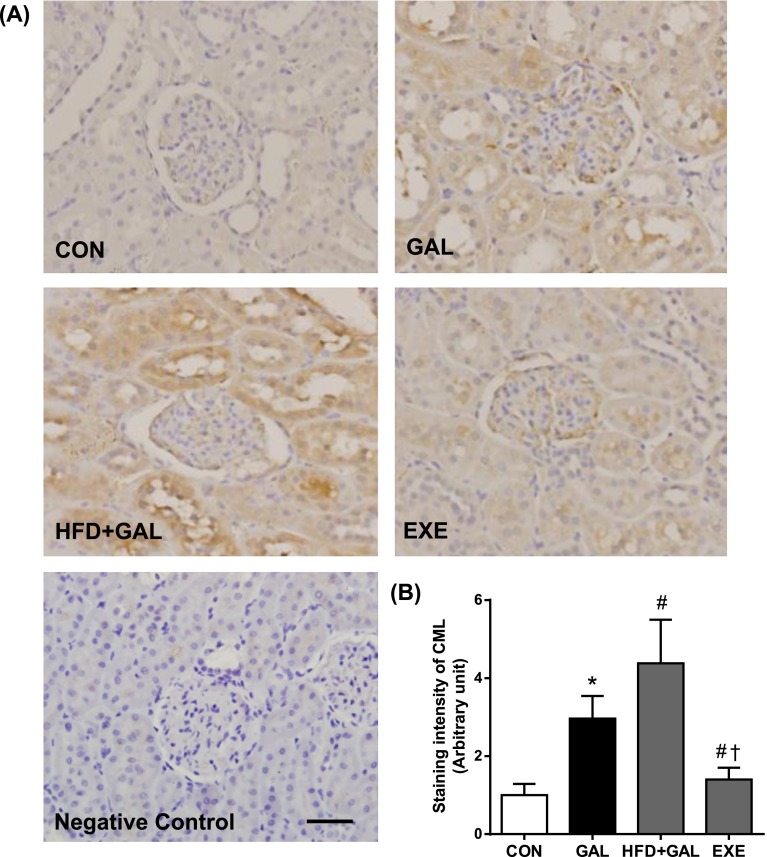
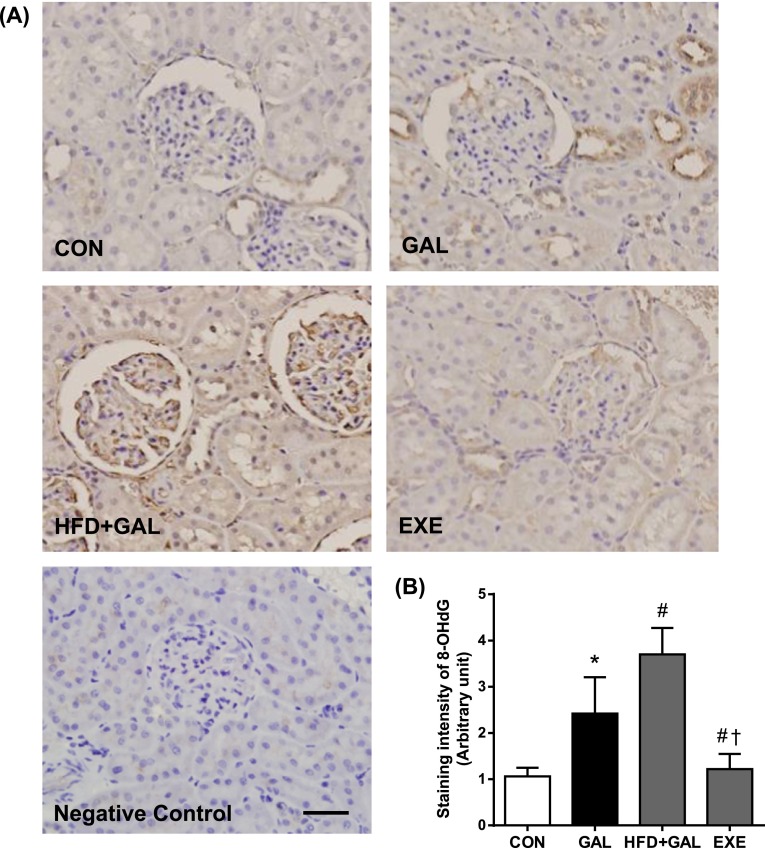
To determine whether HFD accelerates the renal accumulation of CML and oxidative renal injury, we examine the immunohistochemical staining for CML and 8-OHdG. The oxidation of guanine to form 8-OHdG acts as a marker of oxidative DNA damage [4]. As shown in Figs. 2 and 3, the immunoreactivities of CML and 8-OHdG in the GAL and HFD+GAL groups were significantly higher compared to the CON group. The expressions of CML and 8-OHdG were mainly distributed in the regions of the glomeruli and renal tubules. Especially, high expressions of CML and 8-OHdG were observed in the proximal tubular epithelial cells. Moreover, the staining intensities of the HFD+GAL group were significantly higher than the GAL group, which indicated that the HFD had an accelerating effect on the renal accumulation of AGE/ALE and oxidative renal injury induced by d-galactose. The regular exercise suppressed the expressions of CML and 8-OHdG compared to the HFD+GAL group (Figs. 2B and 3B).
Fig. 2.
Renal CML accumulation. (A) Representative CML immunohistochemistry in renal tissues. Negative control section incubated with nonimmune mouse IgG was unstained. Bar=50 µm. (B) Quantitative analysis of CML immunostaining signal intensity. The intensity of immunohistochemical staining of CML was analyzed in eight randomly selected mm2 areas of renal cortex. Values in the bar graphs represent means±SE, n=8. *P<0.01 compared with CON group; #P<0.01 compared with GAL group; †P<0.01 compared with HFD+GAL group.
Fig. 3.
Detection of oxidatively modified DNA. (A) Representative 8-OHdG immunohistochemitry in renal tissues. Bar=50 µm. (B) Quantitative analysis of 8-OHdG immunostaining signal intensity. The intensity of immunohistochemical staining of 8-OHdG was analyzed in eight randomly selected mm2 areas of renal cortex. Values in the bar graphs represent means±SE, n=8. *P<0.01 compared with CON group; #P<0.01 compared with GAL group; †P<0.01 compared with HFD+GAL group.
Glomerular podocyte loss
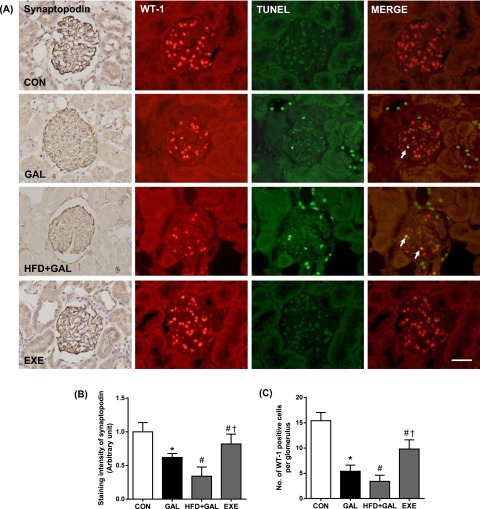
The loss of glomerular podocytes precedes and predicts the onset of clinical nephropathy [20]. To evaluate the loss of podocytes in renal tissues, we performed imuunohistochemical staining for synaptopodin and WT-1, well-known podocyte markers [23, 32]. Average numbers of podocytes per glomerular section were determined by counting cells and measuring areas that were positively labeled with two podocyte markers. In the GAL group, synaptopodin and WT-1 positive cell counts tended to decrease compared with the CON group. The staining areas of synaptopodin and the number of WT-1-positive cells in the HFD+GAL group were significantly lower than the GAL group. In double staining, some TUNEL-positive cells were localized within the region where WT-1 was positively stained. This mixed color staining (TUNEL; green, WT-1; red) was obtained in podocytes, confirming the apoptosis of podocytes (Fig. 4). The regular exercise visibly increased the positive cells and areas in the kidney glomeruli (Fig. 4B and 4C).
Fig. 4.
Glomerular podocyte loss. (A) Representative photomicrographs of synaptopodin staining and double-staining for TUNEL (green) and WT-1 (red). Mixed yellow-colored cells in the merged images (arrows) indicate that apoptotic cell death may have occurred in podocyte. Bar=50 µm. Quantitative analyses of (B) positive areas of synaptopodin and (C) positive cells of WT-1. Values in the bar graphs represent means±SE (n=8). *P<0.01 compared with CON group; #P<0.01 compared with GAL group; †P<0.01 compared with HFD+GAL group.
Apoptosis assay in renal tissues
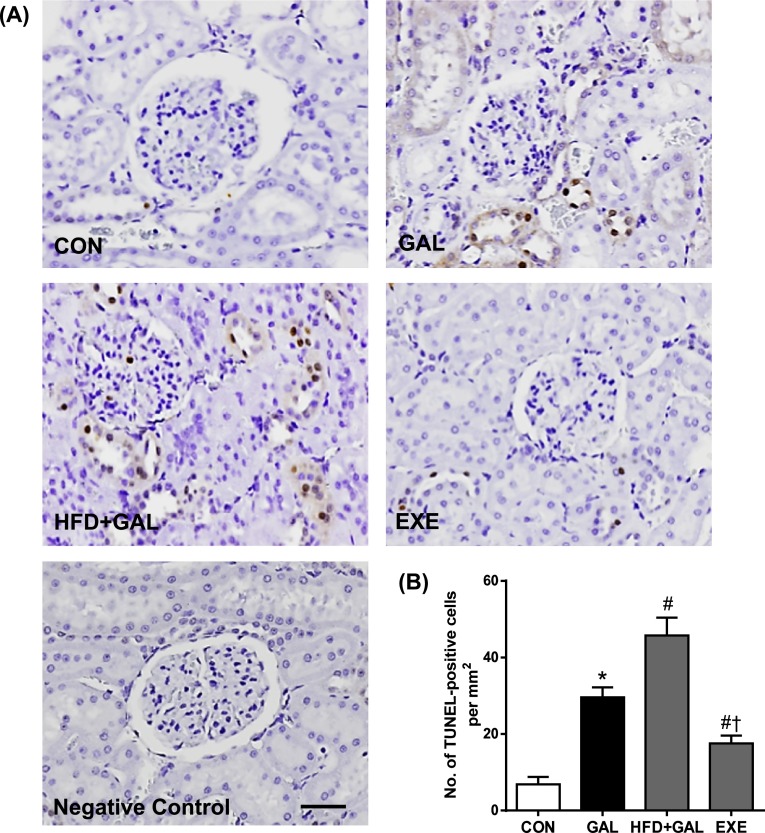
TUNEL staining used widely as a marker for apoptosis [7]. In the CON group, a TUNEL-positive nucleus was barely detected. In the GAL and HFD+GAL groups, many TUNEL-positive cells were observed in renal glomeruli and tubular epithelial cells. The numbers of TUNEL-positive cells in the HFD+GAL group were significantly higher than the GAL group. However, the regular exercise prevented the increase in the positive cells that was seen in the GAL and HFD+GAL groups (Fig. 5).
Fig. 5.
In situ detection of fragmented DNA. (A) Representative TUNEL staining in renal tissues. Negative control was obtained by omitting terminal transferase from the labeling procedure. Bar=50 µm. (B) Quantitative analysis of TUNEL-positive cells. Values in the bar graphs represent means±SE, n=8. *P<0.01 compared with CON group; #P<0.01 compared with GAL group; †P<0.01 compared with HFD+GAL group.
IV. Discussion
The present study provides novel evidence that a HFD accelerated the renal accumulation of AGE/ALE and podocyte loss in d-galactose-induced oxidative stressed rats. We showed that rats, when fed with a HFD for 9 weeks, become obese, have increased plasma total cholesterol, TG and LDL levels and have an enhanced oxidative DNA damage in the renal tissues of the d-galactose-induced oxidative stressed rats. Our results described the link between a HFD and oxidative stress in the renal tissues. In addition, we showed that the regular exercise has the renopreventive effect in this animal model.
d-Galactose, a reducing sugar, metabolized at normal concentration, but the excessive levels of d-galactose can be converted into aldose and hydroperoxide under the catalysis of galactose oxidase, resulting in the generation of a superoxide anion and oxygen-derived free radicals [44]. When d-galactose is injected to rats for 6–9 weeks, free radical production is increased in renal tissues [18]. Mice and rats injected with d-galactose have been used as an animal model of oxidative stress [23, 32, 36].
Extensive renal accumulation of lipids associated with increased renal lipid synthesis via a SREBP-1c-dependent pathway has been demonstrated in animals fed HFD (40–60% kcal fat) [16, 41]. In our study, we demonstrated that the elevated levels of circulating lipids and the enhanced AGE/ALE accumulation. Moreover, d-galactose-injected rats fed a HFD showed accelerated renal injury associated with more pronounced renal ALE/AGE content and oxidative DNA damage. Our data indicate that a HFD plus d-galactose induced an extensive formation and renal deposition of AGE/ALE, such as CML. This product is known to produce carbonyl and oxidative stress which may cause injury both directly and via receptor-mediated mechanisms [12]. The accelerated renal injury observed in rats in the HFD+GAL group compared with the GAL group indicates that a HFD plays a pivotal role in d-galactose-induced oxidative renal injury. The hyperlipidemia induced by a HFD was associated with the enhanced deposition of ALE/AGE.
AGE/ALE has been reported to induce apoptosis in various cell lines, such as mesangial cells and endothelial cells [14, 45]. Renal cell apoptosis or necrosis can inevitably affect glomerular filtration rate and endothelial function, resulting in renal failure [5]. In this study, the HFD can amplify the d-galactose-induced apoptotic renal cell death. Oxidative stress or pro-apoptotic cytokine through interaction between AGE/ALE and RAGE were involved in podocyte apoptosis [46]. Among the three intrinsic cells in glomerulus, podocyte is one of the important ingredients of filtration barrier which has special cytobiological trait and physiological function. The injury of podocyte can inevitably lead to the occurrence of proteinuria [48]. Podocyte apoptosis has been demonstrated to correlate with worsening renal function [35]. Consistent with this interpretation, the result of the present study demonstrates that the increased renal levels of CML and 8-OHdG in HFD plus d-galactose-treated rat are involved, at least in part, in the injury of podocytes. Although there was disappearance of podocyte markers from glomeruli, apoptosis was observed by TUNEL staining predominantly in tubular epithelial cells. Liu et al. also showed that d-galactose treatment in mice induced extensive tubular damage by presence of necrotic tubular epithelial cells [19]. These results suggest that d-galactose-induced renal injury was occurred predominantly in both proximal and distal tubular epithelial cells.
In addition, we examined the effect of intervention by the regular exercise on the increases in AGE/ALE accumulation, oxidative DNA damage and podocyte loss in kidney tissues. The intervention by the regular exercise inhibited increases in AGE/ALE deposition, renal 8-OHdG levels and podocyte loss in HFD plus d-galactose-treated rats. A substantial body of evidence showed the renoprotective effects of the physical exercise in diabetic nephropathy [15] and chronic kidney disease [9]. Physical exercise has a positive influence on physical capacity, hypertension, left ventricular function, lipid metabolism and oxidative status [21, 28]. Coelho et al. showed that physical training performed before the onset of chronic kidney disease is capable of improving oxidative stress parameters, possibly by reducing oxidant production [9]. In addition, the moderate regular exercise reduced the burden of AGEs in diabetes- and obesity-associated nephropathy rats. Exercise-induced higher energy demands might decrease the pool of reactive intermediates for glycation or lipoxidation [6]. Our results indicated that the reduction of AGE/ALE accumulation by the regular exercise might have been a major mechanism of exercise-associated renoprotection in HFD plus d-galactose-induced oxidative stressed and obese rats.
In conclusion, our data indicate that a HFD may promote d-galactose-induced oxidative renal injury and AGE/ALE renal accumulation in rats. However, the underline mechanisms of renal cell damage in HFD plus d-galactose-tread rats need further investigation. We also demonstrated that the regular exercise protects against renal injury. These effects could be attributed, at least in a part, to reduced AGE/ALE deposition and oxidative stress. We show here that the regular exercise might be an easy and effective strategy to reduce oxidative stress-related renal disease in obese people.
V. Acknowledgments
This work was supported by National Research Foundation of Korea Grant funded by the Korean Government (NRF-2012-332-2012A029).
VI. References
- 1.Ahmed N. Advanced glycation endproducts—role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005;67:3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Asghar M., Hussain T., Lokhandwala M. F. Higher basal serine phosphorylation of D1A receptors in proximal tubules of old Fischer 344 rats. Am. J. Physiol. Renal Physiol. 2002;283:F350–355. doi: 10.1152/ajprenal.00361.2001. [DOI] [PubMed] [Google Scholar]
- 3.Asghar M., George L., Lokhandwala M. F. Exercise decreases oxidative stress and inflammation and restores renal dopamine D1 receptor function in old rats. Am. J. Physiol. Renal Physiol. 2007;293:F914–919. doi: 10.1152/ajprenal.00272.2007. [DOI] [PubMed] [Google Scholar]
- 4.Beckman K. B., Ames B. N. Oxidative decay of DNA. J. Biol. Chem. 1997;272:19633–19636. doi: 10.1074/jbc.272.32.19633. [DOI] [PubMed] [Google Scholar]
- 5.Bonegio R., Lieberthal W. Role of apoptosis in the pathogenesis of acute renal failure. Curr. Opin. Nephrol. Hypertens. 2002;11:301–308. doi: 10.1097/00041552-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Boor P., Celec P., Behuliak M., Grancic P., Kebis A., Kukan M., Pronayova N., Liptaj T., Ostendorf T., Sebekova K. Regular moderate exercise reduces advanced glycation and ameliorates early diabetic nephropathy in obese Zucker rats. Metabolism. 2009;58:1669–1677. doi: 10.1016/j.metabol.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 7.Charriaut-Marlangue C., Ben-Ari Y. A cautionary note on the use of the TUNEL stain to determine apoptosis. Neuroreport. 1995;7:61–64. [PubMed] [Google Scholar]
- 8.Chow F. Y., Nikolic-Paterson D. J., Ma F. Y., Ozols E., Rollins B. J., Tesch G. H. Monocyte chemoattractant protein-1-induced tissue inflammation is critical for the development of renal injury but not type 2 diabetes in obese db/db mice. Diabetologia. 2007;50:471–480. doi: 10.1007/s00125-006-0497-8. [DOI] [PubMed] [Google Scholar]
- 9.Coelho B. L., Rocha L. G., Scarabelot K. S., Scheffer D. L., Ronsani M. M., Silveira P. C., Silva L. A., Souza C. T., Pinho R. A. Physical exercise prevents the exacerbation of oxidative stress parameters in chronic kidney disease. J. Ren. Nutr. 2010;20:169–175. doi: 10.1053/j.jrn.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Forbes J. M., Coughlan M. T., Cooper M. E. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57:1446–1454. doi: 10.2337/db08-0057. [DOI] [PubMed] [Google Scholar]
- 11.Gomez-Cabrera M. C., Domenech E., Vina J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic. Biol. Med. 2008;44:126–131. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Iacobini C., Menini S., Ricci C., Scipioni A., Sansoni V., Mazzitelli G., Cordone S., Pesce C., Pugliese F., Pricci F., Pugliese G. Advanced lipoxidation end-products mediate lipid-induced glomerular injury: role of receptor-mediated mechanisms. J. Pathol. 2009;218:360–369. doi: 10.1002/path.2536. [DOI] [PubMed] [Google Scholar]
- 13.Joles J. A., Kunter U., Janssen U., Kriz W., Rabelink T. J., Koomans H. A., Floege J. Early mechanisms of renal injury in hypercholesterolemic or hypertriglyceridemic rats. J. Am. Soc. Nephrol. 2000;11:669–683. doi: 10.1681/ASN.V114669. [DOI] [PubMed] [Google Scholar]
- 14.Kaji Y., Amano S., Usui T., Oshika T., Yamashiro K., Ishida S., Suzuki K., Tanaka S., Adamis A. P., Nagai R., Horiuchi S. Expression and function of receptors for advanced glycation end products in bovine corneal endothelial cells. Invest. Ophthalmol. Vis. Sci. 2003;44:521–528. doi: 10.1167/iovs.02-0268. [DOI] [PubMed] [Google Scholar]
- 15.Koh K. H., Dayanath B., Doery J. C., Polkinghorne K. R., Teede H., Kerr P. G. Effect of exercise on albuminuria in people with diabetes. Nephrology. 2011;16:704–709. doi: 10.1111/j.1440-1797.2011.01508.x. [DOI] [PubMed] [Google Scholar]
- 16.Kume S., Uzu T., Araki S., Sugimoto T., Isshiki K., Chin-Kanasaki M., Sakaguchi M., Kubota N., Terauchi Y., Kadowaki T., Haneda M., Kashiwagi A., Koya D. Role of altered renal lipid metabolism in the development of renal injury induced by a high-fat diet. J. Am. Soc. Nephrol. 2007;18:2715–2723. doi: 10.1681/ASN.2007010089. [DOI] [PubMed] [Google Scholar]
- 17.Lai Y. H., Chen L. J., Cheng J. T. Role of TNF-alpha in renal damage in mice showing hepatic steatosis induced by high fat diet. Horm. Metab. Res. 2013;45:38–42. doi: 10.1055/s-0032-1321871. [DOI] [PubMed] [Google Scholar]
- 18.Lei M., Hua X., Xiao M., Ding J., Han Q., Hu G. Impairments of astrocytes are involved in the d-galactose-induced brain aging. Biochem. Biophys. Res. Commun. 2008;369:1082–1087. doi: 10.1016/j.bbrc.2008.02.151. [DOI] [PubMed] [Google Scholar]
- 19.Liu C. M., Ma J. Q., Lou Y. Chronic administration of troxerutin protects mouse kidney against d-galactose-induced oxidative DNA damage. Food Chem. Toxicol. 2010;48:2809–2817. doi: 10.1016/j.fct.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Meyer T. W., Bennett P. H., Nelson R. G. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia. 1999;42:1341–1344. doi: 10.1007/s001250051447. [DOI] [PubMed] [Google Scholar]
- 21.Moinuddin I., Leehey D. J. A comparison of aerobic exercise and resistance training in patients with and without chronic kidney disease. Adv. Chronic Kidney Dis. 2008;15:83–96. doi: 10.1053/j.ackd.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Moore K. J., Freeman M. W. Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscler. Thromb. Vasc. Biol. 2006;26:1702–1711. doi: 10.1161/01.ATV.0000229218.97976.43. [DOI] [PubMed] [Google Scholar]
- 23.Mundel P., Heid H. W., Mundel T. M., Kruger M., Reiser J., Kriz W. Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J. Cell. Biol. 1997;139:193–204. doi: 10.1083/jcb.139.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muoio D. M., Newgard C. B. Obesity-related derangements in metabolic regulation. Annu. Rev. Biochem. 2006;75:367–401. doi: 10.1146/annurev.biochem.75.103004.142512. [DOI] [PubMed] [Google Scholar]
- 25.Nass N., Bartling B., Navarrete Santos A., Scheubel R. J., Borgermann J., Silber R. E., Simm A. Advanced glycation end products, diabetes and ageing. Z. Gerontol. Geriatr. 2007;40:349–356. doi: 10.1007/s00391-007-0484-9. [DOI] [PubMed] [Google Scholar]
- 26.Navarro A., Gomez C., Lopez-Cepero J. M., Boveris A. Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R505–511. doi: 10.1152/ajpregu.00208.2003. [DOI] [PubMed] [Google Scholar]
- 27.Nistala R., Whaley-Connell A., Sowers J. R. Redox control of renal function and hypertension. Antioxid. Redox. Signal. 2008;10:2047–2089. doi: 10.1089/ars.2008.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pechter U., Maaroos J., Mesikepp S., Veraksits A., Ots M. Regular low-intensity aquatic exercise improves cardio-respiratory functional capacity and reduces proteinuria in chronic renal failure patients. Nephrol. Dial. Transplant. 2003;18:624–625. doi: 10.1093/ndt/18.3.624. [DOI] [PubMed] [Google Scholar]
- 29.Poirier P., Giles T. D., Bray G. A., Hong Y., Stern J. S., Pi-Sunyer F. X., Eckel R. H. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Arterioscler. Thromb. Vasc. Biol. 2006;26:968–976. doi: 10.1161/01.ATV.0000216787.85457.f3. [DOI] [PubMed] [Google Scholar]
- 30.Rice-Evans C., Leake D., Bruckdorfer K. R., Diplock A. T. Practical approaches to low density lipoprotein oxidation: whys, wherefores and pitfalls. Free Radic. Res. 1996;25:285–311. doi: 10.3109/10715769609149053. [DOI] [PubMed] [Google Scholar]
- 31.Saiki A., Nagayama D., Ohhira M., Endoh K., Ohtsuka M., Koide N., Oyama T., Miyashita Y., Shirai K. Effect of weight loss using formula diet on renal function in obese patients with diabetic nephropathy. Int. J. Obes. 2005;29:1115–1120. doi: 10.1038/sj.ijo.0803009. [DOI] [PubMed] [Google Scholar]
- 32.Schiffer M., Mundel P., Shaw A. S., Bottinger E. P. A novel role for the adaptor molecule CD2-associated protein in transforming growth factor-beta-induced apoptosis. J. Biol. Chem. 2004;279:37004–37012. doi: 10.1074/jbc.M403534200. [DOI] [PubMed] [Google Scholar]
- 33.Sohn E. J., Kim C. S., Kim Y. S., Jung D. H., Jang D. S., Lee Y. M., Kim J. S. Effects of magnolol (5,5'-diallyl-2,2'-dihydroxybiphenyl) on diabetic nephropathy in type 2 diabetic Goto-Kakizaki rats. Life Sci. 2007;80:468–475. doi: 10.1016/j.lfs.2006.09.037. [DOI] [PubMed] [Google Scholar]
- 34.Stemmer K., Perez-Tilve D., Ananthakrishnan G., Bort A., Seeley R. J., Tschop M. H., Dietrich D. R., Pfluger P. T. High-fat-diet-induced obesity causes an inflammatory and tumor-promoting microenvironment in the rat kidney. Dis. Model. Mech. 2012;5:627–635. doi: 10.1242/dmm.009407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Susztak K., Raff A. C., Schiffer M., Bottinger E. P. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. [PubMed] [Google Scholar]
- 36.Thornalley P. J. Protein and nucleotide damage by glyoxal and methylglyoxal in physiological systems—role in ageing and disease. Drug Metabol. Drug Interact. 2008;23:125–150. doi: 10.1515/dmdi.2008.23.1-2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thorpe S. R., Baynes J. W. Maillard reaction products in tissue proteins: new products and new perspectives. Amino Acids. 2003;25:275–281. doi: 10.1007/s00726-003-0017-9. [DOI] [PubMed] [Google Scholar]
- 38.Trovati M., Cavalot F. Optimization of hypolipidemic and antiplatelet treatment in the diabetic patient with renal disease. J. Am. Soc. Nephrol. 2004;15 Suppl 1:S12–20. doi: 10.1097/01.asn.0000093238.09114.40. [DOI] [PubMed] [Google Scholar]
- 39.Uchida K. Role of reactive aldehyde in cardiovascular diseases. Free Radic. Biol. Med. 2000;28:1685–1696. doi: 10.1016/s0891-5849(00)00226-4. [DOI] [PubMed] [Google Scholar]
- 40.Vlassara H. Advanced glycation in health and disease: role of the modern environment. Ann. N. Y. Acad. Sci. 2005;1043:452–460. doi: 10.1196/annals.1333.051. [DOI] [PubMed] [Google Scholar]
- 41.Wei P., Lane P. H., Lane J. T., Padanilam B. J., Sansom S. C. Glomerular structural and functional changes in a high-fat diet mouse model of early-stage Type 2 diabetes. Diabetologia. 2004;47:1541–1549. doi: 10.1007/s00125-004-1489-1. [DOI] [PubMed] [Google Scholar]
- 42.Weinberg J. M. Lipotoxicity. Kidney Int. 2006;70:1560–1566. doi: 10.1038/sj.ki.5001834. [DOI] [PubMed] [Google Scholar]
- 43.Wolk R., Somers V. K. Obesity-related cardiovascular disease: implications of obstructive sleep apnea. Diabetes Obes. Metab. 2006;8:250–260. doi: 10.1111/j.1463-1326.2005.00508.x. [DOI] [PubMed] [Google Scholar]
- 44.Wu D. M., Lu J., Zheng Y. L., Zhou Z., Shan Q., Ma D. F. Purple sweet potato color repairs d-galactose-induced spatial learning and memory impairment by regulating the expression of synaptic proteins. Neurobiol. Learn. Mem. 2008;90:19–27. doi: 10.1016/j.nlm.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 45.Yamagishi S., Inagaki Y., Okamoto T., Amano S., Koga K., Takeuchi M., Makita Z. Advanced glycation end product-induced apoptosis and overexpression of vascular endothelial growth factor and monocyte chemoattractant protein-1 in human-cultured mesangial cells. J. Biol. Chem. 2002;277:20309–20315. doi: 10.1074/jbc.M202634200. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto Y., Yamamoto H. Interaction of receptor for advanced glycation end products with advanced oxidation protein products induces podocyte injury. Kidney Int. 2012;82:733–735. doi: 10.1038/ki.2012.163. [DOI] [PubMed] [Google Scholar]
- 47.Yan S. F., Ramasamy R., Naka Y., Schmidt A. M. Glycation, inflammation, and RAGE: a scaffold for the macrovascular complications of diabetes and beyond. Circ. Res. 2003;93:1159–1169. doi: 10.1161/01.RES.0000103862.26506.3D. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y., Chen B., Hou X. H., Guan G. J., Liu G., Liu H. Y., Li X. G. Effects of mycophenolate mofetil, valsartan and their combined therapy on preventing podocyte loss in early stage of diabetic nephropathy in rats. Chin. Med. J. 2007;120:988–995. [PubMed] [Google Scholar]