Abstract
The anterior pituitary gland comprises 5 types of hormone-producing cells and non-endocrine cells, such as folliculostellate (FS) cells. The cells form a lobular structure surrounded by extracellular matrix (ECM) but are not randomly distributed in each lobule; hormone-producing cells have affinities for specific cell types (topographic affinity), and FS cells form a homotypic meshwork. To determine whether this cell and ECM organization can be reproduced in vitro, we developed a 3-dimensional (3D) model that utilizes hanging drop cell culture. We found that the topographic affinities of hormone-producing cells were indeed maintained (ie, GH to ACTH cells, GH to TSH cells, PRL to LH/FSH cells). Fine structures in hormone-producing cells retained their normal appearance. In addition, FS cells displayed well-developed cytoplasmic protrusions, which interconnected with adjacent FS cells to form a 3D meshwork. In addition, reassembly of gap junctions and pseudofollicles among FS cells was observed in cell aggregates. Major ECM components—collagens and laminin—were deposited and distributed around the cells. In sum, the dissociated anterior pituitary cells largely maintained their in vivo anterior pituitary architectures. This culture system appears to be a powerful experimental tool for detailed analysis of anterior pituitary cell organization.
Keywords: anterior pituitary, 3D cell culture, hanging drop method, hormone-producing cells, folliculostellate cells
I. Introduction
The anterior pituitary gland consists of 5 types of hormone-producing cells, non-endocrine folliculostellate (FS) cells, and vascular cells. In the anterior pituitary gland, these cells form a functional unit (the so-called lobular structure), which is surrounded by extracellular matrix (ECM) [17, 19]. In the lobule, hormone-producing cells are organized with specific topographical affinities (eg GH–ACTH cells, GH–TSH cells, and PRL–LH/FSH cells) [1, 13, 14]. FS cells are located in the center of the lobule, and their cytoplasmic protrusions envelop hormone-producing cells [17, 19]. They also form pseudofollicles and meshworks by means of homotypical interconnection within the gland [17]. The meshwork was hypothesized to be an excitable network that transmits signals through gap junctions [4]. In addition, ECM components such as laminin and collagens were found to regulate morphology and proliferation of hormone-producing cells and FS cells [6, 12, 20]. Such local cell–cell and cell–ECM interactions may have pivotal roles in the functional organization of anterior pituitary (for a comprehensive review, see [3]).
In this study, we applied a hanging drop 3-dimensional (3D) technique to conventional primary monolayer cell culture and showed that this in vitro 3D model of the anterior pituitary successfully displayed the in vivo characteristics of the interior of the lobular structure.
II. Materials and Methods
Animals
Male Wistar rats were purchased from Japan SLC (Shizuoka, Japan). For morphological analysis of FS cells, we used transgenic S100b-GFP rats, which express GFP in FS cells under the control of exogenous S100b protein gene promoter [9]. The animals were given conventional food and water ad libitum and were kept under a 12 hr light/12 hr dark cycle. All animal experiments were performed after receiving approval from the Institutional Animal Experiment Committee of the Jichi Medical University and were conducted in accordance with the Institutional Regulations for Animal Experiments and Fundamental Guideline for Proper Conduct of Animal Experiments and Related Activities in Academic Research Institutions, under the jurisdiction of the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Hanging drop 3D cell culture
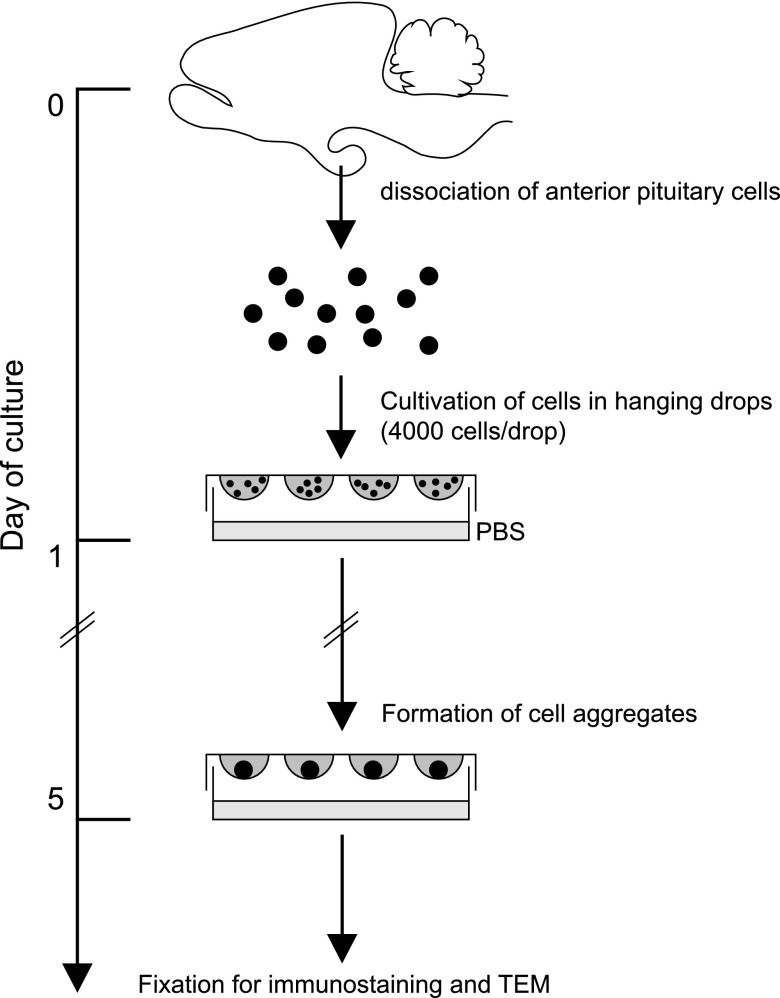
Figure 1 shows a schematic representation of the general experimental protocol for hanging drop 3D cell culture of rat anterior pituitary. Animals aged 8 to 10 weeks (weight, 250–300 g) were deeply anesthetized intraperitoneally with pentobarbital sodium (Kyoritsu Seiyaku, Tokyo, Japan), and ice-cold Ca2+- and Mg2+-free (CMF) Hanks’ solution was perfused through the left ventricle. Excised anterior pituitaries were minced into pieces and then incubated in CMF Hanks’ solution containing 1% trypsin (Life Technologies, Carlsbad, CA, USA) and 0.2% collagenase L (Nitta Gelatin, Osaka, Japan) for 15 min at 37°C, followed by incubation in the same solution containing 5 µg/ml of DNase I (Roche, Basel, Switzerland) for 5 min at 37°C and incubation in CMF Hanks’ solution containing 5 mM ethylenediaminetetraacetic acid (Wako Pure Chemical Industries, Osaka, Japan) for 5 min at 37°C. After these sequential digestions, the cells were dispersed in CMF Hanks’ solution by gentle pipetting and filtered through nylon mesh (BD Biosciences, San Jose, CA, USA) to remove undigested tissue. The cells were then resuspended in M199 (Life Technologies) supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA), 100 units/ml penicillin, and 100 µg/ml of streptomycin (Life Technologies). Until this point, the protocol was identical to that for 2D cell culture [11]. For 3D culture, a 25 µl-drop containing 4000 cells was placed on the undersurface of plastic Petri dish lids (90 mm; Asahi Glass, Tokyo, Japan), which were then cultured over the sterile PBS (hanging drop) for 5 days at 37°C in a humidified incubator with 5% CO2 (Fig. 1). Resulting cell aggregates were analyzed on an IX71 inverted fluorescence microscope (Olympus, Tokyo, Japan).
Fig. 1.
Schematic of the experimental protocol for hanging drop 3D cell culture of rat anterior pituitary cells. Note that the model requires only common cell cultureware.
Immunofluorescence microscopy
Twenty to 30 cell aggregates were mounted on an MAS-coated glass slide (Matsunami Glass, Osaka, Japan) and immediately fixed with ice-cold 4% paraformaldehyde (PFA) in 50 mM phosphate buffer (PB; pH 7.4) for 3 hr. The fixed cells were washed and stored in phosphate buffer saline (PBS) at 4°C until staining. For immunocytochemistry, the cells were permeabilized in PBS containing 0.2% Triton X-100 (Sigma-Aldrich) for 20 min at room temperature and then incubated in blocking solution (2% normal goat serum or normal donkey serum in PBS) for 30 min at room temperature, after which they were incubated with primary antibodies for 90 min at 30°C. The primary antibodies included rabbit polyclonal anti-rat GH (1 : 6400; gift from Prof. K. Wakabayashi, Gunma University, Japan), anti-rat PRL (1 : 5000; gift from Prof. K. Wakabayashi), anti-ovine LHβ (1 : 3200; Advance, Tokyo, Japan), anti-mouse laminin (1 : 1600, LSL-LB-1013: Cosmo Bio, Tokyo, Japan), anti-rat type I and anti-mouse type III collagen (1 : 1000 and 1 : 1500, respectively; gifts from Prof. T. Nakamura of Hokkaido University) antibodies, mouse monoclonal anti-human 17-39-ACTH (1 : 3200; EMD Millipore, Billerica, MA, USA), anti-human TSHβ (1 : 400; Merck KGaA, Darmstadt, Germany) antibodies and goat polyclonal anti-human LHβ antibody (1 : 200; Santa Cruz Biotechnology, Santa Cruz, CA, USA). The cells were then incubated with secondary antibodies for 30 min at 30°C. The secondary antibodies included Alexa Fluor 488-conjugated goat anti-rabbit IgG, Alexa Fluor 488-conjugated goat anti-mouse IgG and Alexa Fluor 568-conjugated donkey anti-goat IgG (all 1 : 200; Life Technologies). Coverslips were mounted onto the cells using Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA). Stained cells or GFP-expressing FS cells were subsequently analyzed on a FV1000 confocal laser microscope (Olympus). Images were processed for presentation using Photoshop CS5 software (Adobe Systems, San Jose, CA, USA). Volocity version 3.0 (PerkinElmer, Waltham, MA, USA) was used to reconstruct 3D images.
Electron microscopy
For transmission electron microscopy, 50 to 100 cell aggregates were collected in a 1.5-ml tube and centrifuged at 300×g for 5 min at 20°C. The resulting pellets were immediately fixed with 2.5% glutaraldehyde (Merck KGaA) in 10 mM PB for 2 hr. After washing in PB, the samples were postfixed in ice-cold 1% OsO4 in PB for 90 min. The samples were then dehydrated in an ethanol series and embedded in Quetol 812 epoxy resin (Nissin EM, Tokyo, Japan). Ultrathin sections were prepared, stained with 2% uranyl acetate and lead citrate, and then observed using an H7600 transmission electron microscope (Hitachi, Tokyo, Japan). Cells in the male rat that produced anterior pituitary hormone were identified according to criteria proposed in previous immunohistochemical studies [8, 15, 21–23].
III. Results
Hanging drop 3D culture of anterior pituitary cells
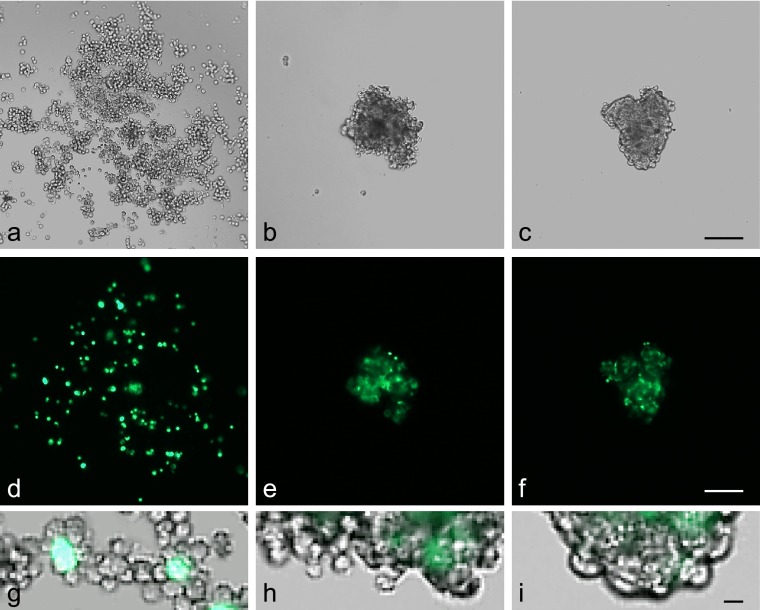
During 5-day culture, anterior pituitary cells settled at the center of the drop under gravity and formed a spherical cell aggregate (diameter, <200 µm; Fig. 2a–c). After 3 days of culture, the shape of the aggregate remained irregular and individual cells were identifiable (Fig. 2b, h). After 5 days, the aggregates were smaller and had a round/oval shape with a smooth outer layer (Fig. 2c, i). Regarding FS cell morphology, FS cells were round and had limited contact with other FS cells after 1 day of culture (Fig. 2d). However, within 5 days they had become stellate, with cytoplasmic protrusions, and gradually formed a meshwork (Fig. 2e, f). We also tested the effect of cell number on aggregate formation (1000, 2000, 4000 and 6000 cells/drop). Aggregate size varied by number of cells cultured. However, there was no noticeable difference in aggregate formation by size: they all formed compact cell aggregates with a developed FS cell meshwork within 5 days (data not shown).
Fig. 2.
Formation of cell aggregates in 3D cell culture. Anterior pituitary cells from S100b-GFP transgenic rat were cultured in a hanging drop (25 µl of medium). The top panel shows phase-contrast images of anterior pituitary cells in a drop (a–c). The middle panel shows FS cells expressing GFP (d–f). The bottom panel shows merged images at high magnification (g–i). Images were obtained 1 day (a, d, g), 3 days (b, e, h) and 5 days (c, f, i) after plating. After 5 days of culture, the cells formed tightly compacted aggregates with a smooth outer layer. Bars=100 µm (a–f), 10 µm (g–i).
Immunofluorescence of hormone-producing cells
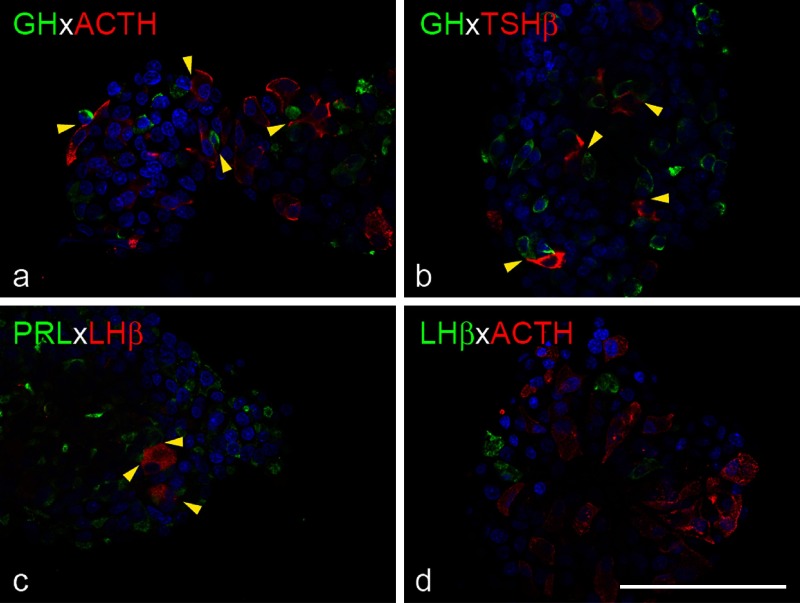
Immunocytochemistry was performed to evaluate the shape and distribution of hormone-producing cells. GH cells were round or oval (Fig. 3a, b). ACTH and TSH cells were elongated and polygonal, respectively (Fig. 3a, b, d). They frequently attached to GH cells (Fig. 3a, b, yellow arrowheads). PRL cells were irregularly shaped and often attached to large, oval LH cells (Fig. 3c, yellow arrowheads). LH and ACTH cells, which have limited affinity in vivo, were located separately in cell aggregates (Fig. 3d).
Fig. 3.
Topographic affinity of hormone-producing cells in 3D cell culture. Cell aggregates from Wistar rat were fixed 5 days after plating and stained for hormones. Immunofluorescence for GH (green) and ACTH (red), GH (green) and TSHβ (red), PRL (green) and LHβ (red), and LHβ (green) and ACTH (red) are shown in a, b, c and d, respectively. DAPI was used to stain nuclei. Topographic affinities between hormone-producing cells were replicated in 3D culture (yellow arrowheads). Bar=100 µm.
FS cell morphology
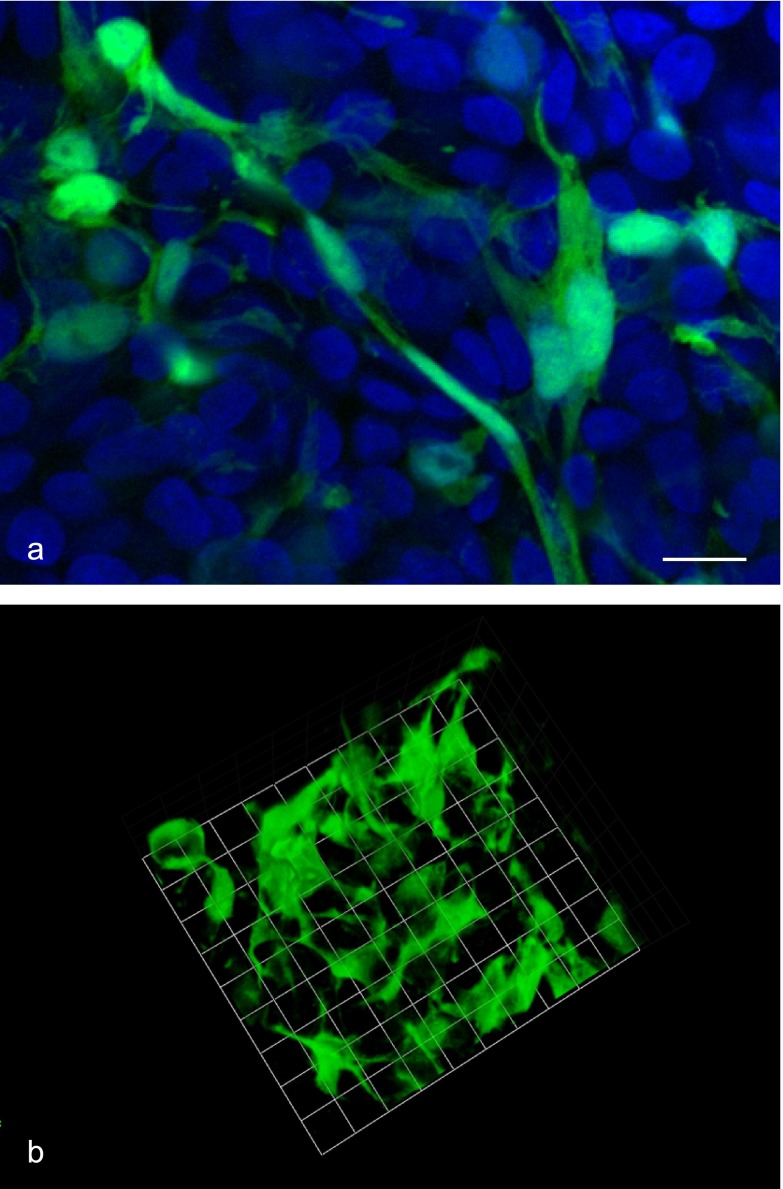
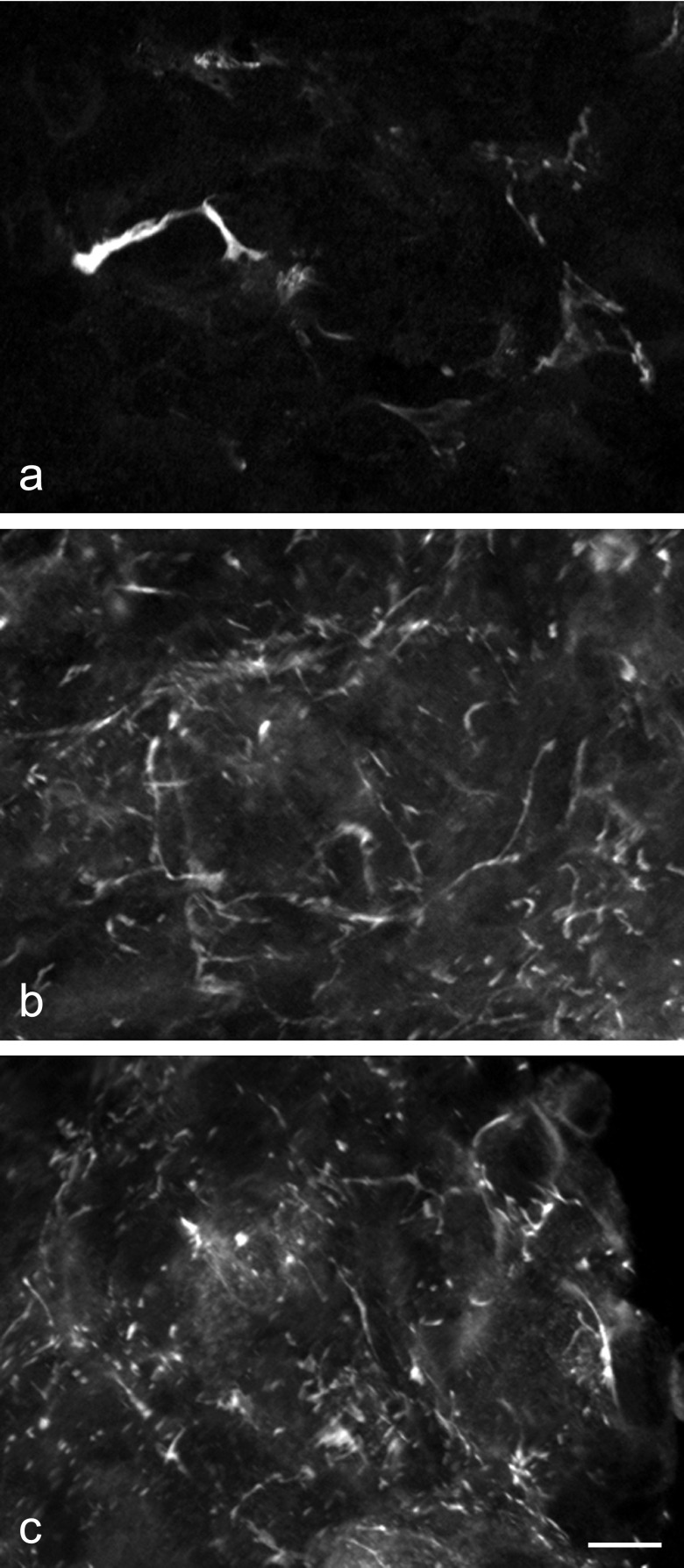
A confocal laser microscope was used to observe FS cell morphology. FS cells had well-developed filopodia extensions, which interacted with neighboring FS cells (Fig. 4a). A 3D image reconstruction showed that FS cells formed a ‘sinusoid-like’ 3D meshwork with their extended cytoplasmic protrusions, similar to in vivo anterior pituitary (Fig. 4b).
Fig. 4.
FS cell morphology in 3D cell culture. Cell aggregates from S100b-GFP transgenic rat were fixed 5 days after plating, and GFP-expressing FS cells were observed with a confocal laser microscope. A focal plane image (green: FS cells, blue: DAPI) and the 3D reconstructed image of FS cells are shown in a and b, respectively. FS cells had highly elongated cell protrusions (filopodia formation), which interacted with neighboring FS cells to form a 3D meshwork. Bar=10 µm (a), 9.6 µm/unit (b).
Electron micrograph of cell aggregates
A transmission electron microscope was used for subcellular analysis of cell aggregates. With the exception of PRL cells, the fine structural and cytological profiles—including the distribution and size of secretory granules—of hormone-producing cells (ie LH/FSH cells: Fig. 5a) were almost the same as those observed in normal rat anterior pituitary. PRL cells had fewer secretory granules than did in vivo PRL cells, and well-developed rough endoplasmic reticulum and Golgi apparatus were observed (Fig. 5a). We also noted gap junctions between FS cells (Fig. 5b, inset). Several agranular cells (FS cells) formed pseudofollicles, and microvilli were observed in the follicular lumen (Fig. 5c, arrow).
Fig. 5.

Electron micrographs of cell aggregates. Cell aggregates from S100b-GFP transgenic rat were fixed 5 days after plating and observed with a transmission electron microscope. (a) Hormone-producing cells in 3D cell culture. Note that the LH/FSH cell, which has many large and small secretory granules and well-developed cell organelles, is surrounded by several PRL cells. In general, due to the disruption of the hypothalamic regulatory system [16], PRL cells had well-developed cell organelles and contained few irregularly shaped secretory granules in cytoplasm (*: LH/FSH cell, **: PRL cells). (b) A FS cell surrounding granular cells. An FS cell directly adheres to an adjacent agranular cell with gap junction (black arrowhead) (inset). (c) FS cells form a pseudofollicle (arrow). The microvilli were noted in the follicular lumen. Bars=1 µm.
Immunofluorescence of extracellular matrix components
Immunocytochemistry was performed to evaluate the presence of extracellular matrix components (Fig. 6a–c). Type I and III collagens (the primary fibrillar collagens expressed in the anterior pituitary) were seen in the cell aggregates. Type I collagen staining showed spot-like pattern in contrast to string-like ramifying structure of type III collagen, and type III collagen was predominant in type I collagen (Fig. 6a, b). These collagen fibers were distributed around anterior pituitary cells. Laminin, which is an important component of basement membrane, was also seen in the aggregates (Fig. 6c). The staining pattern and the distribution were similar to those of type III collagen (Fig. 6b, c).
Fig. 6.
Immunofluorescence for type I and III collagen and laminin. Cell aggregates from Wistar rat were fixed 5 days after plating and stained for extracellular matrix components. (a) Type I collagen deposition was noted, but expression levels were not high. (b) Type III collagen and (c) laminin were distributed 3-dimensionally in cell aggregates and formed reticular fibers. Bar=10 µm.
IV. Discussion
The study showed that dispersed cells formed a compact cell aggregate with a smooth outer layer within 5 days (Fig. 2). The aggregates displayed (1) topographical affinities between hormone-producing cells, (2) well-preserved fine structures of all types of hormone-producing cells and FS cells, (3) meshwork, gap junction, and pseudofollicle formation by reassembled FS cells, and (4) ECM deposition, all of which are observed in anterior pituitary in vivo.
Immunocytochemical analysis showed that the morphological characteristics of all types of hormone-producing cells were essentially identical to those of anterior pituitary cells in vivo (Fig. 3). The fine structure of hormone-producing cells (eg, the shape, size, and distribution of secretory granules, endoplasmic reticulum, and mitochondria) appeared normal, except for PRL cells (Fig. 5a). The results suggest that anterior pituitary cells retained their morphological characters in the 3D cell culture. The 5 types of hormone-producing cells in the gland are not distributed randomly; rather, specific cell types have topographic affinities [13]. The present study showed that these topographical affinities were conspicuously reconstructed in this 3D cell culture (GH to ACTH cells, GH to TSH cells, PRL to LH/FSH cells). The regulatory mechanisms of cell–cell affinity have not yet been determined, and the 3D cell culture system may allow us to identify these mechanisms.
Soji and colleagues have extensively studied the morphological and functional characteristics of FS cells [5, 17–19]. According to their reports, FS cells are interconnected via gap junctions and form meshworks, and, in addition to the established hypophyseal–portal vein system, signals transmitted through gap junctions regulate hormone release from hormone-producing cells. The present study showed that dispersed FS cells formed a 3D meshwork with neighboring FS cells (Fig. 4a, b). In cell aggregates, FS cells reassembled gap junctions, formed pseudofollicles, and encircled hormone-producing cells with their cytoplasmic protrusions (Fig. 5b, c). This is the first report showing that FS cells reconstructed 3D FS cell organization in vitro. Recently, our group found that FS cells secrete CXCL12, a chemokine, to evoke other FS cells [7]. Similar mechanisms are likely involved in reconstructing the FS cell meshwork in 3D cell culture.
In the present study, we detected deposition of ECM components in cell aggregates (Fig. 6). In 3D cell culture, type III collagen was more plentiful than type I collagen, although they are present in similar amounts around capillaries in vivo [10]. Type III collagen production generally begins during the early phase of wound healing, while type I collagen production starts during the late phase [2]. Dissociated anterior pituitary cells in 3D cell culture might produce collagens in the same way. Type III collagen and laminin formed ramifying fibers and were distributed around anterior pituitary cells. ECMs are often localized around capillaries, but not around glandular cells, in vivo [10]. The random distribution of ECMs may be due to the lack of a vascular system in 3D cell culture.
In conclusion, cell aggregates closely resembled the cellular and subcellular organization of the rat anterior pituitary in vivo. This culture system has the potential to become a powerful experimental tool in pituitary research, as it allows researchers to analyze the mechanism of anterior pituitary cell organization under physiologically relevant conditions.
V. Acknowledgments
We thank Prof. K. Inoue (Saitama University, Japan) for supplying the transgenic rats, Prof. K. Wakabayashi (Gunma University, Japan) for providing the anti-rat GH and rat PRL antibodies, Prof. T Nakamura (Hokkaido University, Japan) for supplying type I and type III collagen antibodies, and David Kipler, ELS (Supernatant Communications) for revising the language of the manuscript. This work was partly supported by a Grant-in-Aid for Scientific Research (C) (22590192) and by a Grant-in-Aid for Young Scientists (B) (23790233) (25860147) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, by promotional funds for the Keirin Race of the Japan Keirin Association, and by the Jichi Medical University Young Investigator Award from Jichi Medical University School of Medicine.
VI. References
- 1.Allaerts W., Mignon A., Denef C. Selectivity of juxtaposition between cup-shaped lactotrophs and gonadotrophs from rat anterior pituitary in culture. Cell Tissue Res. 1991;263:217–225. doi: 10.1007/BF00318763. [DOI] [PubMed] [Google Scholar]
- 2.Betz P. Immunohistochemical parameters for the age estimation of human skin wounds. A review. Am. J. Forensic Med. Pathol. 1995;16:203–209. doi: 10.1097/00000433-199509000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Denef C. Paracrinicity: the story of 30 years of cellular pituitary crosstalk. J. Neuroendocrinol. 2008;20:1–70. doi: 10.1111/j.1365-2826.2007.01616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fauquier T., Guérineau N. C., McKinney R. A., Bauer K., Mollard P. Folliculostellate cell network: a route for long-distance communication in the anterior pituitary. Proc. Natl. Acad. Sci. U S A. 2001;98:8891–8896. doi: 10.1073/pnas.151339598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hattori K., Shirasawa N., Suzuki H., Otsuka T., Wada I., Yashiro T., Herbert D. C., Soji T., Hashitani H. Intercellular communication within the rat anterior pituitary gland. XV. Properties of spontaneous and LHRH-induced Ca2+ transients in the transitional zone of the rat anterior pituitary in situ. Endocrinology. 2013;154:400–409. doi: 10.1210/en.2012-1501. [DOI] [PubMed] [Google Scholar]
- 6.Horiguchi K., Kikuchi M., Kusumoto K., Fujiwara K., Kouki T., Kawanishi K., Yashiro T. Living-cell imaging of transgenic rat anterior pituitary cells in primary culture reveals novel characteristics of folliculo-stellate cells. J. Endocrinol. 2010;204:115–123. doi: 10.1677/JOE-09-0333. [DOI] [PubMed] [Google Scholar]
- 7.Horiguchi K., Ilmiawati C., Fujiwara K., Tsukada T., Kikuchi M., Yashiro T. Expression of chemokine CXCL12 and its receptor CXCR4 in folliculostellate (FS) cells of the rat anterior pituitary gland: the CXCL12/CXCR4 axis induces interconnection of FS cells. Endocrinology. 2012;153:1717–1724. doi: 10.1210/en.2011-1937. [DOI] [PubMed] [Google Scholar]
- 8.Inoue K., Kurosumi K. Ultrastructural immunocytochemical localization of LH and FSH in the pituitary of the untreated male rat. Cell Tissue Res. 1984;235:77–83. doi: 10.1007/BF00213726. [DOI] [PubMed] [Google Scholar]
- 9.Itakura E., Odaira K., Yokoyama K., Osuna M., Hara T., Inoue K. Generation of transgenic rats expressing green fluorescent protein in S-100beta-producing pituitary folliculo-stellate cells and brain astrocytes. Endocrinology. 2007;148:1518–1523. doi: 10.1210/en.2006-1390. [DOI] [PubMed] [Google Scholar]
- 10.Kaidzu S., Noda T., Tane N., Yashiro T. Collagen synthesis by rat anterior pituitary cells in vivo and in vitro. Acta Histochem. Cytochem. 2000;33:81–87. [Google Scholar]
- 11.Kikuchi M., Kusumoto K., Fujiwara K., Takahashi K., Tando Y., Yashiro T. Live staining and isolation of specific hormone-producing cells from rat anterior pituitary by cytochemistry with lectins and cholera toxin B subunit. Acta Histochem. Cytochem. 2011;44:159–164. doi: 10.1267/ahc.11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuchenbauer F., Hopfner U., Stalla J., Arzt E., Stalla G. K., Páez-Pereda M. Extracellular matrix components regulate ACTH production and proliferation in corticotroph tumor cells. Mol. Cell. Endocrinol. 2001;175:141–148. doi: 10.1016/s0303-7207(01)00390-2. [DOI] [PubMed] [Google Scholar]
- 13.Noda T., Kaidzu S., Kikuchi M., Yashiro T. Topographic affinities of hormone-producing cells in the rat anterior pituitary gland. Acta Histochem. Cytochem. 2001;34:313–319. [Google Scholar]
- 14.Noda T., Kikuchi M., Kaidzu S., Yashiro T. Rat anterior pituitary cells in vitro can partly reconstruct in vivo topographic affinities. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2003;272:548–555. doi: 10.1002/ar.a.10065. [DOI] [PubMed] [Google Scholar]
- 15.Nogami H., Yoshimura F. Fine structural criteria of prolactin cells identified immunohistochemically in the male rat. Anat. Rec. 1982;202:261–274. doi: 10.1002/ar.1092020211. [DOI] [PubMed] [Google Scholar]
- 16.Orgnero de Gaisán E., Maldonado C., Diaz Gavier M. F., Aoki A. Diversity of pituitary cells in primary cell culture. An immunocytochemical study. Ann. Anat. 1997;179:453–460. doi: 10.1016/s0940-9602(97)80049-0. [DOI] [PubMed] [Google Scholar]
- 17.Shirasawa N., Mabuchi Y., Sakuma E., Horiuchi O., Yashiro T., Kikuchi M., Hashimoto Y., Tsuruo Y., Herbert D. C., Soji T. Intercellular communication within the rat anterior pituitary gland: X. Immunohistocytochemistry of S-100 and connexin 43 of folliculo-stellate cells in the rat anterior pituitary gland. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2004;278:462–473. doi: 10.1002/ar.a.20040. [DOI] [PubMed] [Google Scholar]
- 18.Shirasawa N., Sakuma E., Wada I., Naito A., Horiuchi O., Mabuchi Y., Kanai M., Herbert D. C., Soji T. Intercellular communication within the rat anterior pituitary: XIV electron microscopic and immunohistochemical study on the relationship between the agranular cells and GnRH neurons in the dorsal pars tuberalis of the pituitary gland. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2007;290:1388–1398. doi: 10.1002/ar.20596. [DOI] [PubMed] [Google Scholar]
- 19.Soji T., Herbert D. C. Intercellular communication between rat anterior pituitary cells. Anat. Rec. 1989;224:523–533. doi: 10.1002/ar.1092240410. [DOI] [PubMed] [Google Scholar]
- 20.Toral C., Solano-Agama C., Reyes-Márquez B., Sabanero M., Talamás P., González del Pliego M., Mendoza-Garrido M. E. Role of extracellular matrix-cell interaction and epidermal growth factor (EGF) on EGF-receptors and actin cytoskeleton arrangement in infantile pituitary cells. Cell Tissue Res. 2007;327:143–153. doi: 10.1007/s00441-006-0248-7. [DOI] [PubMed] [Google Scholar]
- 21.Tougard C., Picart R., Tixier-Vidal A. Immunocytochemical localization of glycoprotein hormones in the rat anterior pituitary. A light and electron microscope study using antisera against rat bet subunits: a comparison between preembedding and postembeeding methods. J. Histochem. Cytochem. 1980;28:101–114. doi: 10.1177/28.2.6986430. [DOI] [PubMed] [Google Scholar]
- 22.Yashiro T., Nogami H., Yoshimura F. Immunohistochemical study of the postnatal development of pituitary thyrotrophs in the rat, with special reference to cluster formation. Cell Tissue Res. 1981;216:39–46. doi: 10.1007/BF00234543. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimura F., Nogami H. Fine structural criteria for identifying rat corticotrophs. Cell Tissue Res. 1981;219:221–228. doi: 10.1007/BF00210144. [DOI] [PubMed] [Google Scholar]