Abstract
Background
Ulipristal acetate (UPA) is a new effective option to prevent unintended pregnancies up to 5 days after unprotected intercourse. We used pooled data from two Phase III studies to refine our understanding of the efficacy of UPA by time from unprotected intercourse and the effects of other factors on pregnancy rates.
Study Design
Data from two Phase III studies were pooled to create a larger analysis population. Analyses were performed on the first participation of 2183 women.
Results
A total of 41 women became pregnant despite the use of UPA, yielding an overall proportion pregnant of 1.9% (1.3%–2.5%). Proportions of pregnant women were higher among those with further acts of unprotected intercourse in the same cycle and among obese women. These varied from 1.3% (0.9%–2.0%) among nonobese women who had no further acts of unprotected intercourse (n=1704) to 8.3% (0.2%–38.5%) among obese women who had subsequent unprotected intercourse (n=12).
Conclusions
UPA is effective and safe in preventing pregnancy after unprotected intercourse. Its effectiveness is lower among women who have subsequent unprotected intercourse and among obese women.
Keywords: Emergency contraception, Efficacy, Obesity, Selective progesterone receptor modulator
1. Introduction
Emergency contraception (EC) is used as a contraceptive backup option to reduce the risk of pregnancy following unprotected intercourse (UPI). Combined treatment with ethinyl estradiol and levonorgestrel (LNG) has been replaced by treatment with LNG alone because it is more effective [1]. However, clinical trials have shown that the efficacy of LNG has very limited, if any, effectiveness if taken beyond 96 h after sexual intercourse [2], falling short of covering the 5-day lifespan of sperm in the female genital tract. Until recently, the only available method consistently effective 5 days after intercourse was the insertion of a copper intrauterine device (IUD) [3], although its use has been very limited by the need for insertion by a skilled health care professional and misperceptions about IUDs among women. Recent development in EC pharmacology in the form of a progesterone receptor modulator pill [ulipristal acetate (UPA)] provides a new effective option to prevent unintended pregnancies, encompassing the 5-day period that sperm can survive in the female genital tract. Based on the results of two large-scale Phase III studies designed to provide statistical evidence that UPA is effective for EC up to 120 h after intercourse [4,5], UPA was approved by the European Medicines Agency (EMA) in 2009 (brand name ellaOne) and by the Food and Drug Administration in 2010 (brand name ella) as a safe and effective method of EC for use up to 5 days after unprotected sexual intercourse. A meta-analysis consisting of two pooled randomized efficacy trials that included a LNG comparison group also showed that in comparison with LNG, UPA significantly reduced the risk of becoming pregnant, whether used within 72 h [odds ratio (OR), 0.58; 95% confidence interval (CI), 0.33–0.99) or 24 h (OR, 0.35; 95% CI, 0.11–0.93) or 120 h (OR, 0.55; 95% CI, 0.32–0.93) after unprotected sexual intercourse [5]. Based on these results, the EMA changed the product license to indicate superior efficacy of UPA over LNG EC. The same study showed that UPA seemed to be as well tolerated as LNG and was associated with no greater risk of menstrual disturbance.
To refine our estimates of the efficacy of UPA by time from UPI and explore the effects of other factors on the probability of pregnancy, we use pooled data from the two Phase III studies conducted to study UPA 30 mg for EC, which provide better generalizability of results by diversifying the sociodemographic composition of the sample including women from three countries and increases statistical power of the analysis.
2. Population
Data are drawn from the two Phase III studies designed to estimate and provide evidence of the efficacy of 30 mg UPA for EC up to 120 h after UPI. The first study, conducted in the United States between November 2006 and May 2008, was a single-arm open-label study that evaluated the efficacy of UPA in 1241 women who took it for EC 48 to 120 h after intercourse [3]. The second study, conducted in the United States, the UK and Ireland between April 2007 and April 2009, was a randomized, controlled, single-blind, study that evaluated the efficacy of UPA in comparison with LNG 1.5 mg in 1899 women [5]. In this trial, women who presented up to 120 h after UPI were eligible for enrollment. In both studies, eligibility criteria were kept as broad as possible in order to favor generalizability of study results at least for Europe and North America. In particular, both studies included teenagers and women who were obese. A summary of each trial is provided in Table 1. Full descriptions of each study have been provided elsewhere [4,5]. All data from both studies were provided to the principal author in order to conduct a full independent reanalysis of the data sets, with no reference to the prior analyses performed by the manufacturer.
Table 1. Study design of the two Phase III trials comprising the pooled data for the current analysis.
| Study | Design | Study population | Primary end point | Treatment | N |
|---|---|---|---|---|---|
| Fine et al [4] | Prospective, multicenter, open label | • Women ≥18 years old | Proportion pregnant | UPA 30 mg | 1533 |
| • Regular cycle length | |||||
| • 48–120 h after UP | |||||
| Glasier et al [5] | Prospective, multicenter, randomized, single blind, parallel group | • Women ≥16 years old | Proportion pregnant | UPA 30 mg | 1104 |
| • Regular cycle length | |||||
| • 0–120 h after UPIa | LNG 1.5 mga | 1117 |
These women were excluded from our study but were part of the initial Glasier study.
2.1. Study population
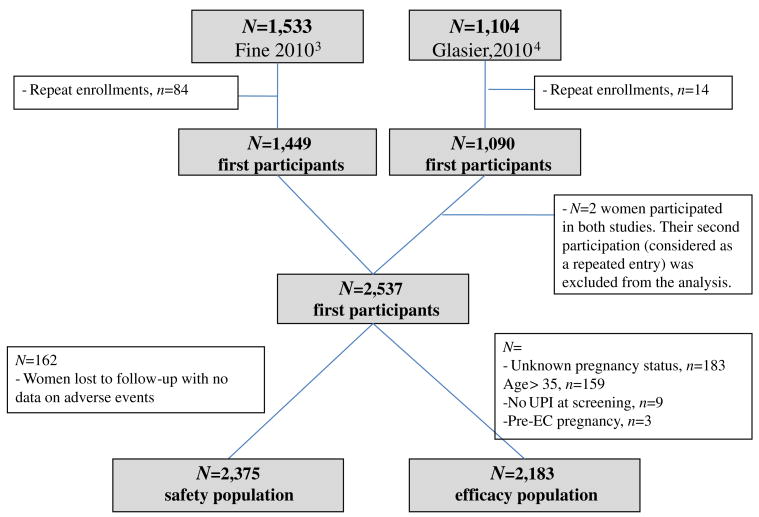
Data from the two Phase III trials were pooled to create a larger analysis population, comprising 2537 women treated with 30 mg of UPA (contributing a total of 2637 enrollments), in order to assess efficacy by time from UPI and other factors contributing to differences in becoming pregnant. Among these women, 100 enrolled more than once in the study (98 multiple enrollments within studies and 2 women participated in both studies). We excluded enrollments beyond the first one from this analysis because of lack of interdependence between observations belonging to the same woman and the limited number of multiple enrollments, which renders difficult the estimation of random effects.
Demographics are presented based on the first participation of 2537 women who received treatment. Data on adverse events were missing for 162 of these women, all of whom were lost to follow-up (LFU); thus, the safety population consisted of 2375 participants. The efficacy analysis is based on the first participation of 2183 women who were under the age of 36 years, had known pregnancy status after EC intake and did not have an identified pre-UPA treatment pregnancy, based on independent Data Safety Monitoring Board (DSMB) evaluation (three pregnancies were judged to have started before EC intake based on β-human chorionic gonadotropin at enrollment) (Fig. 1). Three women became pregnant after treatment, but their pregnancies were not considered compatible with a treatment failure by the DSMB (the pregnancies were judged to have resulted from acts of intercourse subsequent to treatment). Therefore, these women were included in the efficacy analysis but were not considered as failures (they contribute to the denominator but not to the numerator of pregnancy rates). All 183 women (n=176) younger than 36 years with unknown pregnancy status were lost to follow-up.
Fig. 1.
Study populations of EC with UPA.
2.2. Analysis among women receiving UPA treatment
We performed a subgroup analysis to compare proportions becoming pregnant across women's demographic and medical characteristics. We also stratified the analysis by reasons for UPA intake (contraceptive failure vs. nonuse of contraception) and delay in treatment administration up to 120 h after UPI. We used Fisher's Exact Test and the Fisher's Halton Freeman Exact Test to test for differences in proportions becoming pregnant. Further analysis of the effects of demographic characteristics (age, parity, race/ethnicity), medical factors [smoking status, body mass index (BMI), or weight] and behavioral factors (multiple acts of UPI before EC intake, subsequent acts of UPI in the same cycle after EC intake and treatment delay) on the probability of pregnancy was performed using multivariate logistic regression models in which we retained only those factors that were statistically significantly associated with treatment failure. Obesity was defined in this study as having a BMI greater than 30 kg/m2. Because some pregnancies may have resulted from further acts of UPI following the use of UPA (beyond the three identified by the DSMB), we performed a subanalysis of factors associated with pregnancy after excluding the 131 subjects who reported subsequent UPI after EC intake.
Adverse events were collected by open-ended questioning from the time of enrollment to the end of the study. Each adverse effect was evaluated for intensity and association with the study medication. Association with the study medication was based on the evaluation of the lead investigator at each site. Cycle length of the treatment cycle was analyzed among women who did not become pregnant after treatment and completed the menstrual calendar data (n=2318). Menstrual cycle length was defined as the number of days from the first day of bleeding up to and including the day before the next menses. Princeton University's Institutional Review Board approved this study.
3. Results
A majority of women in the study were in their 20s, and half of them had ever been pregnant. A sizable fraction of women qualified as being obese (16.6%), and a third were current smokers. The efficacy population [defined as women who had a known pregnancy status and who were not pregnant before taking EC (Fig. 1)] was slightly older than the group of women (younger than 36 years) LFU [23.4 years (95% CI, 23.3–23.6), 22.7 years (95% CI, 22.1–23.3); p=.03). Women in the efficacy population were less likely to be white than women in the LFU group (66.1% vs. 72.7%, p=.01) (Table 2). Most women had used EC after an act of UPI where no contraception was used (70.9%); 28% had used it after a condom failure. A small minority (5.7%) reported further acts of UPI after EC intake.
Table 2. Characteristics of the study population: all women (N=2537) and women who contributed to the efficacy analysis (N=2342).
| All women (N=2537) | Efficacy population (N=2183) | LFU less than36 years(N=176) | p | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| n | % | n | % | n | % | |||
| Age, years | <20 | 521 | 20.5 | 473 | 21.7 | 47 | 26.7 | .09 |
| 20–24 | 1017 | 40.1 | 926 | 42.4 | 82 | 46.7 | ||
| 25–29 | 551 | 21.7 | 520 | 23.8 | 30 | 17.1 | ||
| 30–35 | 282 | 11.1 | ||||||
| >35 | 166 | 6.5 | 264 | 12.1 | 17 | 9.7 | ||
| BMI, kg/m2 | <18.5 | 92 | 3.6 | 81 | 3.7 | 6 | 3.4 | .46 |
| 18.5–<25 | 1423 | 56.1 | 1243 | 56.9 | 110 | 62.5 | ||
| 25–<30 | 595 | 23.5 | 506 | 23.2 | 37 | 21.0 | ||
| 30 or more | 422 | 16.6 | 351 | 16.1 | 22 | 12.5 | ||
| Missing | 5 | 0.2 | 2 | 0.1 | 1 | 0.6 | ||
| Weight, kg | ≤85 | 2178 | 85.8 | 1883 | 86.3 | 158 | 89.8 | .14 |
| >85 | 354 | 13.9 | 298 | 13.6 | 17 | 9.7 | ||
| Missing | 5 | 0.2 | 2 | 0.1 | 1 | 0.6 | ||
| Race/ethnicity | White | 1684 | 66.4 | 1453 | 66.6 | 128 | 72.7 | .01 |
| Black/African American | 498 | 19.6 | 432 | 19.8 | 19 | 10.8 | ||
| Asian | 47 | 1.9 | 41 | 1.9 | 1 | 0.6 | ||
| Other | 303 | 11.9 | 253 | 11.6 | 27 | 15.3 | ||
| Unknown | 5 | 0.2 | 4 | 0.2 | 1 | 0.6 | ||
| Ever pregnant | Yes | 1275 | 50.3 | 1032 | 47.3 | 83 | 47.2 | .98 |
| No | 1262 | 49.7 | 1151 | 52.7 | 93 | 52.8 | ||
| Smoking | Current | 867 | 34.2 | 732 | 33.5 | 70 | 39.8 | .09 |
| None or former | 1670 | 65.8 | 1451 | 66.5 | 106 | 60.2 | ||
| Other acts of UPI in the same | No | 2203 | 86.8 | 1884 | 86.3 | 157 | 89.2 | .28 |
| cycle, before EC intake | Yes | 334 | 13.2 | 299 | 13.7 | 19 | 10.8 | |
| Reasons for requesting EC | No contraception | 1798 | 70.9 | 1557 | 71.3 | 118 | 67.1 | .23 |
| Condom failure or other error in contraceptive use | 739 | 29.1 | 626 | 28.7 | 58 | 32.9 | ||
| Time from UPI to treatment, h | 0–24 | 364 | 14.3 | 313 | 14.3 | 22 | 12.5 | .23 |
| >24–48 | 388 | 15.3 | 338 | 15.5 | 24 | 13.6 | ||
| >48–72 | 1031 | 40.6 | 883 | 40.4 | 86 | 48.9 | ||
| >72–96 | 523 | 20.6 | 455 | 20.8 | 34 | 19.3 | ||
| >96 | 231 | 9.1 | 194 | 8.9 | 10 | 5.7 | ||
| Unprotected sexual intercourse | Yes | 144 | 5.7 | 131 | 6.0 | 17 | NA | NA |
| after EC intakea | No | 2222 | 87.6 | 2044 | 93.6 | 4 | ||
| Missing | 171 | 6.7 | 8 | 0.4 | 0 | |||
Twenty-one women reported whether they had unprotected sexual intercourse after EC intake at their first follow-up visit when their pregnancy status could not be ascertained and then were subsequently LFU. This information is not known for the other 155 women who were LFU.
A total of 41 women became pregnant despite the use of UPA for EC (excluding the three pregnancies that were not considered compatible with EC failure), yielding an overall proportion pregnant of 1.9% (95% CI, 1.3–2.5). The efficacy results by 24-h interval from UPI to treatment are summarized in Fig. 2 and Table 2. There was no statistically significant effect of treatment delay on pregnancy (p=.91).
Fig. 2.
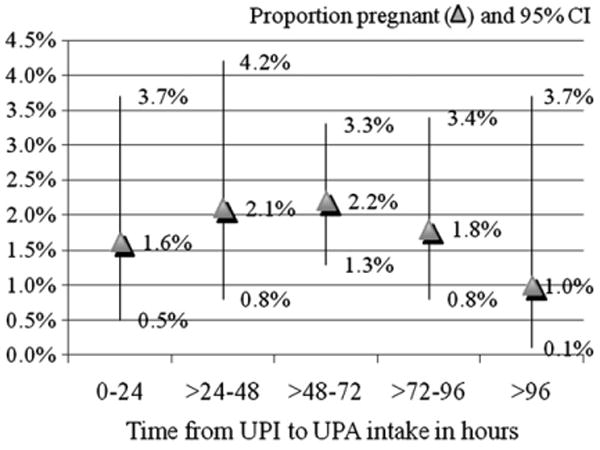
Percentage pregnant (▲) and 95% CI (vertical lines) by 24-h intervals — UPI to UPA treatment.
The most significant contributor to pregnancies following UPA intake was subsequent UPI, as the odds of experiencing a pregnancy were four times (OR, 4.3; 95% CI, 1.9–9.5; p<.001) as high in women who reported having further acts of UPI in the same cycle they used EC, compared with those who had either protected intercourse or no intercourse until the next menstrual cycle (Table 3). Obese women were twice as likely to experience an EC failure compared with nonobese women (OR, 2.1; 95% CI, 1.0–4.3; p=.04]. When considering women's weight instead of BMI status, our results also show more than a twofold increase in the odds of pregnancy among women who weighed more than 85 kg (187 lbs) [OR, 2.2 (1.1–4.6]); p=.03] as compared to those weighing less (Table 3). Proportions becoming pregnant did not vary by women's age, race, parity, smoking status, reason for EC intake or multiple/single acts of UPI before EC intake. Proportions becoming pregnant varied from 1.3% (95% CI, 0.9–2.0) among nonobese women who did not report further acts of UPI (n=1704) to 8.3% (95% CI, 0.2–38.5) for obese women who reported further acts of UPI after EC intake (n=12) (Table 4).
Table 3. Sociodemographic and medical factors associated with pregnancy rates among all women in the efficacy population and among women who reported no further acts of UPI.
| All women in the efficacy population (n=2173)a | Women who did not report further acts of UPI (n=2044) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Observed PR, % (95% CI) | p | Adjusted ORb | p | Observed PR, % (95% CI) | p | Adjusted ORb | p | ||
| Age (years) | <25 | 1.9% (1.3–2.8) | .87 | 1.8% (1.1–2.6) | .59 | 1 | |||
| 25–34 | 1.8% (1.0–3.0) | 1.4% (0.7–2.5) | |||||||
| BMI (kg/m2) | <30 | 1.6% (1.1–2.3) | .08 | 1 | .04 | 1.3% (0.9–2.0) | .05 | 1 | .04 |
| >30 | 3.1% (1.6–5.5) | 2.1 (1.0–4.3) | 3.0% (1.4–5.4) | 2.2 (1.1–4.7) | |||||
| Weight | ≤85 kg (187 lbs) | 1.6% (1.1–2.3) | .06 | 1 | .03 | 1.4% (0.9–2.0) | .04 | 1 | .03 |
| >85 kg (187 lbs) | 3.4% (1.6–6.1) | 2.2 (1.1–4.6) | 3.1% (1.4–5.9) | 2.3 (1.1–5.1) | |||||
| Race | White | 2.0% (1.3–2.9) | .19 | 1.9% (1.3–2.8) | .39 | 1 | |||
| Black/African American | 2.3% (1.1–4.2) | 1.5% (0.5–3.2) | |||||||
| Asian | 2.4% (0.1–12.9) | 0% (0.0–1.0) | |||||||
| Other | 0.4% (0.0–2.2) | 0.4% (0.0–2.3) | |||||||
| Ever pregnant | Yes No | 2.4% (1.6–3.6) 1.4% (0.8–2.2) | .08 | 2.1% (1.3–3.2) 1.2% (0.6–2.0) | .11 | ||||
| Smoking | Current | 1.9% (1.2–2.7) | 1 | 1.6% (1.0–2.4) | 1.0 | ||||
| None or former | 1.9% (1.0–3.2) | 1.6% (0.8–2.9) | |||||||
| Other acts of unprotected sex in the cycle before EC intake | Yes | 1.3% (0.4–3.4) | .65 | 1.1% (0.2–3.2) | .61 | ||||
| No | 2.0% (1.4 –2.7) | 1.7% (1.1–2.4) | |||||||
| Further UPI after EC intake | Yes | 6.2% (2.7–11.7) | 4.3 (1.9–9.5) | <.001 | |||||
| No | 1.6% (1.1–2.3) | .002 | 1 | ||||||
| Reasons for requesting EC | No method | 1.8% (1.2–2.6) | .73 | 1.9% (0.9–3.3) | .56 | ||||
| Condom failure or other error in contraceptive use | 2.1% (1.1–3.5) | 1.5% (0.9–2.3) | |||||||
| Hours since UPI | 0–24 | 1.6% (0.5–3.7) | .91 | 1.7 % (0.5–3.8) | .81 | ||||
| >24–48 | 2.1% (0.8–4.2) | 1.3% (0.4–3.3) | |||||||
| >48–72 | 2.2% (1.3–3.3) | 2.1% (1.2–3.3) | |||||||
| >72–96 | 1.8% (0.8–3.4) | 1.2% (0.4–2.7) | |||||||
| >96–120 | 1.0% (0.1–3.7) | 1.1% (0.1–3.9) | |||||||
PR, pregnancy rates.
Excludes 10 women who had missing data on weight (n=2) or UPI after UPA intake (n=8).
Adjusted OR; results from logistic regression models. These multivariate models include either the variable BMI or the variable weight. The adjusted OR for further acts of intercourse is 4.2 (1.9–9.4) (not shown) when controlling for weight and 4.3 (1.9–9.5) (shown) when controlling for BMI.
Table 4. Proportions becoming pregnant by BMI status and whether further acts of UPI took place after EC intake.
| Further acts of intercourse | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| No | Yes | Total | ||||
|
|
|
|
||||
| n | % and 95% CI | n | % and 95% CI | n | % and 95% CI | |
| Nonobese | 1704 | 1.3% (0.9–2.0) | 119 | 5.9% (2.4–11.7) | 1823 | 1.6% (1.1–2.3) |
| Obese | 338 | 3.0% (1.4–5.4) | 12 | 8.3% (0.2–38.5) | 350 | 3.1% (1.6–5.5) |
| Total | 2042 | 1.6% (1.1–2.3) | 131 | 6.1% (2.7–11.7) | 2173 | |
Note: 10 women had missing data on weight or UPI after UPA intake.
Overall, 1434 (60.4%) of the 2375 subjects having received 30 mg UPA who completed information on adverse events reported a total of 3674 adverse events. Conversely, 941 individuals reported no adverse events at follow-up. Among the 3674 reported adverse events, 509 (13.9%) were considered by the lead investigator at each study site as certainly or probably related to the study medication, 1232 as possibly related (33.5%) and 1410 (38.4%) as not or probably not related to the medication. The remaining 523 (14.2%) were of unknown or unspecified relationship.
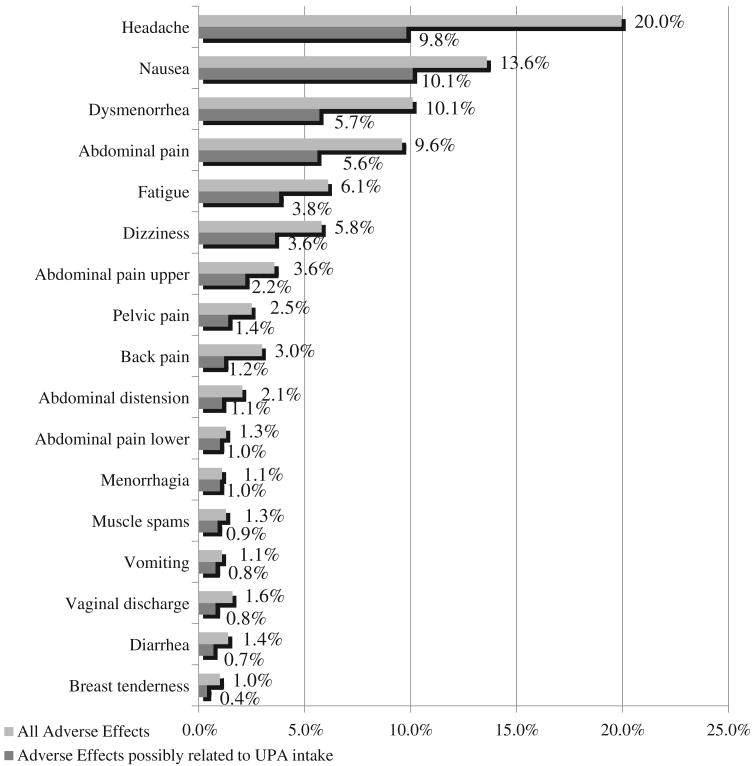
The most frequently reported adverse effects included headaches (20.0%), nausea (13.6%), dysmenorrhea (10.1%) and abdominal pain (9.6%) (Fig. 3). Headaches (9.8%) and nausea (10%) remained the most frequently cited symptoms when selecting symptoms that were considered as certainly, probably or possibly related to UPA intake.
Fig. 3.
Frequency of reported symptoms following UPA intake.
Four serious adverse events were reported (0.17% of all women). These involved a case of seizure, a case of urinary tract infection, a case of right contact lens-related corneal ulcer and a case of dizziness. Only the last event (dizziness) was considered to be possibly related to the intake of UPA.
Women who did not become pregnant after treatment reported a mean increase of 2.4 days (95% CI, 2.1–2.8) from cycle length reported at screening and a median increase of 1 day from cycle length reported at screening. Less than 3% (2.7%; n=63) were still in amenorrhea at the end of follow-up. Among those who reported posttreatment menses, the mean duration of cycle was 31.3 days (95% CI, 30.9– 31.6); 21.8% (n=487) had an increase in cycle length greater than 7 days and 7.1% (n=159) had a decrease in cycle length greater than 7 days (Fig. 4). Menses following UPA intake were normal in duration and volume in the majority of the women.
Fig. 4.
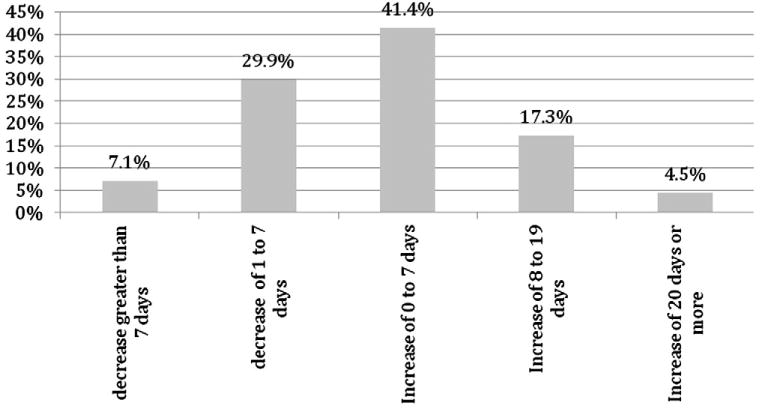
Distribution of change in cycle length from cycle length reported at screening: median change +1 day.
4. Discussion
Using pooled data from the two large-scale Phase III clinical trials of 30 mg UPA for EC, we found that 1.9% became pregnant, with no increase in the risk of pregnancy over time up to 5 days following UPI. These findings contrast with those of a recent meta-analysis combining the results of four WHO trials, which shows sustained efficacy of LNG in the first 4 days after UPI, followed by a pronounced decline in efficacy on the fifth day [2]. The authors of this meta-analysis, however, do not exclude the possibility of a gradual loss in efficacy of LNG EC in the first 4 days in certain settings, pointing out moderate heterogeneity among studies [2]. While our study provides no insights on the mechanism of action of UPA, earlier work suggests that UPA is more effective than LNG EC pills in delaying ovulation when ovulation is imminent (when the leading follicle reaches 15–20 mm), which could explain the difference in effectiveness patterns over time [6,7]. UPA, when taken after ovulation, has been found to decrease endometrium thickness and alter l-selectin ligands, but whether this change would inhibit implantation is unknown [8]. LNG EC taken before the luteinizing hormone surge has also been reported in one study to alter the luteal phase secretory pattern of glycodelin in serum and the endometrium [9], although two later studies explicitly designed to assess endometrial glycodelin expression did not confirm these findings [10,11].
Pooling the data increases statistical power and allows a more in-depth analysis of factors associated with UPA failures, generalizable to the population living in Europe and North America. Our results indicate that the overall probability of pregnancy hides significant differentials among two subgroups. Probabilities were much higher among those with further acts of UPI after treatment in the same cycle and among obese women. These varied from a low of 1.3% among nonobese women who had no further acts of UPI to 8.3% among obese women who had further acts of UPI. This later group of 12 women represented less than 1% of our efficacy population. Probabilities of becoming pregnant did not vary by treatment delay, women's age, race/ethnicity, parity, smoking status or reason for EC intake in the multivariate context.
Our results indicate a significant twofold increase in the risk of pregnancy among obese women. A recent meta-analysis reported an even greater risk of EC failure for obese women taking LNG EC pills (OR, 4.41; p<.05) [12]. These results suggest alternate strategies for contraceptive backup for obese women or women weighing over 85 kg that are more effective than LNG EC pills, such as providing advance supplies of UPA or offering IUD insertion [12]. In addition, more research is needed to assess the value of increasing the dosage of EC pills whether LNG or UPA among obese women.
In the study of Brache et al. [7] examining the effect of immediate preovulatory administration of UPA, the authors conclude that UPA intake delays follicular rupture by 4 to 10 days, but that hormonal production resumes in most cycles, resulting in ovulation occurring later. This result suggests that further unprotected acts of intercourse after UPA intake would entail an increased risk of pregnancy. Our results show that one (20%) in five pregnancies following UPA-EC intake occurred among the minority of women (6%) who admitted having at least one act of unprotected sex after EC intake. Available only by prescription, UPA provides an opportunity for physicians to counsel women about the fourfold increase in the risk of failure due to subsequent UPI. This information is particularly critical for obese women for whom UPA seems to be less effective. Our results, however, need further confirmation as only 12 women had a BMI greater than 30 kg/m2 and reported further acts of UPI, limiting the precision of the risk of pregnancy in this very small subgroup of our study population.
Only one serious adverse event (dizziness) was considered by the investigator to be possibly related to treatment. The most frequent adverse events were headache (20.0%), nausea (13.6%), abdominal pain (10.1%), dysmenorrhea (9.6%), fatigue (6.1%) and dizziness (5.8%). These rates were only half as high when considering only symptoms that were possibly related to EC intake.
This study presents several limitations. A regrettably large fraction of women (7%) were LFU, which may have affected our estimates of the risk of pregnancy, although race/ethnicity and age, the two characteristics by which women LFU differed from the efficacy population, were not associated with pregnancy risk in our analysis. Nevertheless, the true proportion of women becoming pregnant could be as large as 9.2% (8.1–10.4) if all women LFU became pregnant or as low as 1.7% (1.3–2.4) if none did; in our analyses, we made the standard assumption that the risk of pregnancy in women LFU was the same as in those with follow-up. While pooling data from two study results in a more diverse population, our study was confined only to populations living in Europe and North America; therefore, only a small number of Asian women were included. Further investigation of UPA efficacy in this population is warranted.
Acknowledgments
Both clinical trials were funded by HRA pharma. Caroline Moreau received funding from HRA to conduct the pooled analysis. James Trussell was the chair of the independent DSMB of both studies; all members of the DSMB received a small honorarium for each Data Safety Monitoring Board meeting. He received no funding from the manufacturer for this study. This work was supported, in part, by the Eunice Kennedy Shriver National Institute of Child Health and Human Development grant for Infrastructure for Population Research at Princeton University (Grant No. R24HD047879) (J. Trussell).
References
- 1.Raymond E, Taylor D, Trussell J, Steiner MJ. Minimum effectiveness of the levonorgestrel regimen of emergency contraception. Contraception. 2004;69:79–81. doi: 10.1016/j.contraception.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Piaggio G, Kapp N, von Hertzen H. Effect on pregnancy rates of the delay in the administration of levonorgestrel for emergency contraception: a combined analysis of four WHO trials. Contraception. 2011;84:35–9. doi: 10.1016/j.contraception.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Cleland K, Zhu H, Goldstuck N, Cheng L, Trussell J. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012 doi: 10.1093/humrep/des140. http://dx.doi.org/10.1093/humrep/des140. [DOI] [PMC free article] [PubMed]
- 4.Fine P, Mathé H, Ginde S, Cullins V, Morfesis J, Gainer E. Ulipristal acetate taken 48–120 hours after intercourse for emergency contraception. Obstet Gynecol. 2010;115:257–63. doi: 10.1097/AOG.0b013e3181c8e2aa. [DOI] [PubMed] [Google Scholar]
- 5.Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375:555–62. doi: 10.1016/S0140-6736(10)60101-8. [DOI] [PubMed] [Google Scholar]
- 6.Croxatto HB, Brache V, Pavez M, et al. Pituitary-ovarian function following the standard levonorgestrel emergency contraceptive dose or a single 0.75-mg dose given on the days preceding ovulation. Contraception. 2004;70:442–50. doi: 10.1016/j.contraception.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Brache V, Cochon L, Jesam C, Maldonado R, Salvatierra AM, Levy DP, et al. Immediate pre-ovulatory administration of 30 mg ulipristal acetate significantly delays follicular rupture. Hum Reprod. 2010;25:2256–63. doi: 10.1093/humrep/deq157. [DOI] [PubMed] [Google Scholar]
- 8.Stratton P, Levens ED, Hartog B, Piquion J, Wei Q, Merino M, Nieman LK. Endometrial effects of a single early luteal dose of the selective progesterone receptor modulator CDB-2914. Fertil Steril. 2010;93:2035–41. doi: 10.1016/j.fertnstert.2008.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durand M, Sépala M, del Carmen Cravioto M, et al. Late follicular phase administration of levonorgestrel as an emergency contraceptive changes the secretory pattern of glycodelin in serum and endometrium during the luteal phase of the menstrual cycle. Contraception. 2005;71:451–7. doi: 10.1016/j.contraception.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 10.do Nascimento JA, Seppala M, Perdigao A, et al. In vivo assessment of the human sperm acrosome reaction and the expression of glycodelin-A in human endometrium after levonorgestrel-emergency contraceptive pill administration. Hum Reprod. 2007;22:2190–5. doi: 10.1093/humrep/dem119. [DOI] [PubMed] [Google Scholar]
- 11.Palomino WA, Kohen P, Devoto L. A single midcycle dose of levonorgestrel similar to emergency contraceptive does not alter the expression of the l-selectin ligand or molecular markers of endometrial receptivity. Fertil Steril. 2010;94:1589–94. doi: 10.1016/j.fertnstert.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Glasier AF, Cameron ST, Blithe DL, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel Contraception. 2011;84:363–7. doi: 10.1016/j.contraception.2011.02.009. [DOI] [PubMed] [Google Scholar]