Abstract
Genital powder use has been associated with risk of epithelial ovarian cancer in some, but not all, epidemiologic investigations, possibly reflecting the carcinogenic effects of talc particles found in most of these products. Whether risk increases with number of genital-powder applications and for all histologic types of ovarian cancer also remains uncertain. Therefore, we estimated the association between self-reported genital powder use and epithelial ovarian cancer risk in eight population-based case-control studies. Individual data from each study was collected and harmonized. Lifetime number of genital-powder applications was estimated from duration and frequency of use. Pooled odds ratios were calculated using conditional logistic regression matched on study and age and adjusted for potential confounders. Subtype-specific risks were estimated according to tumor behavior and histology. 8,525 cases and 9,859 controls were included in the analyses. Genital powder use was associated with a modest increased risk of epithelial ovarian cancer (odds ratio 1.24, 95% confidence interval 1.15–1.33) relative to women who never used powder. Risk was elevated for invasive serous (1.20, 1.09–1.32), endometrioid (1.22, 1.04–1.43), and clear cell (1.24, 1.01–1.52) tumors, and for borderline serous tumors (1.46, 1.24–1.72). Among genital powder users, we observed no significant trend (p=0.17) in risk with increasing number of lifetime applications (assessed in quartiles). We noted no increase in risk among women who only reported non-genital powder use. In summary, genital powder use is a modifiable exposure associated with small-to-moderate increases in risk of most histologic subtypes of epithelial ovarian cancer.
Keywords: ovarian cancer, powder, talc, epidemiology
INTRODUCTION
Powders that are commonly applied either directly to the genital, perineal, or rectal area after bathing or indirectly to underwear, sanitary napkins, tampons, or stored contraceptive devices may contain talc because of its softness, absorbency, and lack of clumpiness (1). However, the presence of talc in commercially available powder formulations has varied over time, even within particular brands of products, limiting the ability of most epidemiologic studies to measure genital talc exposure accurately. Despite this, genital powder use, but not use on other parts of the body, has been linked to increased risk of ovarian cancer, suggesting that powder particles ascending the genital tract may predispose to ovarian cancer development (2–4). Meta-analyses of observational studies show 33–35% increased risk of ovarian cancer among women who have used genital powders (1, 4, 5), but evidence for a dose-response relationship has been inconsistent. Though dose response was not addressed in previous meta-analyses(1, 4, 5) some individual studies have reported significant dose-response (4, 6–10) while others have not (9, 11–15).
Epidemiologic and biologic studies show differences in risk-factor profiles and molecular characteristics between ovarian cancer subtypes defined by histology (serous, endometrioid, mucinous, clear cell) and behavior (borderline, invasive) (16). For instance, serous tumors are characterized by p53 mutations, while mucinous tumors have a high prevalence of KRAS mutations (17) and are not generally associated with reproductive risk factors (16, 18). Since most early studies of powder use and ovarian cancer did not include analysis by histologic subgroups (3, 6, 11, 19–21), histology-specific estimates were not available from these studies for meta-analysis. Most (2, 4, 8, 9, 22), but not all (10, 14, 15, 23), epidemiologic studies of genital powder use and risk of ovarian cancer that have evaluated histologic subgroups have found the association to be strongest for serous invasive tumors. Such tumors comprise the most common variety of ovarian cancer and few previous studies have had sufficient statistical power to evaluate the association between genital powder use and risk of other histologic subtypes. In the present study, we evaluated associations between genital powder use and risk of ovarian cancer overall, by invasiveness and by histologic type in a pooled analysis of eight population-based case-control studies with relevant data from the Ovarian Cancer Association Consortium (OCAC), a consortium founded in 2005 to validate promising genetic associations in epidemiologic studies of ovarian cancer.
MATERIALS AND METHODS
Participating studies
Studies participating in the OCAC consortium as of April 2010 that collected data on powder use were included. Each study was approved by an institutional ethics committee and all participants provided informed consent. Detailed description of the OCAC consortium is available elsewhere (24). Characteristics of the eight case-control studies contributing data to this analysis are presented in Table 1. Six studies were conducted in the USA (DOV (14), HAW (25), HOP (26), NCO (27), NEC (4), USC (28)) one study in Australia (AUS (7)) and one study in Canada (SON (15)). Overall, our analyses included 8,525 cases of ovarian, fallopian tube or peritoneal cancer and 9,859 controls. Five studies previously reported on powder use (AUS (7), DOV (14), NCO (27), NEC (4), SON (15),) three of which provided data for this analysis that had not been included in their previous powder-related publication (DOV, NEC, AUS). The remaining three studies have not previously published their genital powder-use data (HAW, HOP, USC).
Table 1.
Characteristics of eight studies included in the analysis of genital powder use and ovarian cancer
| Study* | Diagnosis Years | Controls | Cases | Histology† | Behavior‡ | Question used to define genital powder use | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Serous | Mucinous | Endometrioid | Clear cell | Invasive | Borderline | |||||
| AUS†† | 2002–2006 | 1449 | 1432 | 889 (62%) | 174 (12%) | 132 (9%) | 78 (5%) | 1158 (81%) | 274 (19%) | Have you ever used any sort of powder or talc on your genital area, in your underwear or on a sanitary pad or diaphragm? |
| DOV†† | 2002–2009 | 1841 | 1565 | 905 (58%) | 186 (12%) | 201 (13%) | 87 (6%) | 1153 (74%) | 412 (26%) | Before (reference date) did you ever use any of the following products routinely during one month or more? Powder on sanitary napkins or pads? Vaginal deodorant spray? Before (reference date) did you usually apply any powder to your genital (perineal) area after bathing? We are only interested in times when you did this for at least one year or longer.§ |
| HAW | 1993–2008 | 755 | 481 | 222 (46%) | 87 (18%) | 69 (14%) | 47 (10%) | 392 (82%) | 89 (19%) | Prior to (month/year of diagnosis||) did you ever use talc, baby, or deodorizing powder dusted or sprayed on your body? By regularly I mean at least once a month for 6 months or more. Did you ever use talc, baby or deodorizing powder as a dusting powder to the genital or rectal area? As a dusting powder to sanitary napkins? As a dusting powder to underwear? On a diaphragm or cervical cap? |
| HOP | 2003–2008 | 1489 | 735 | 433 (59%) | 53 (7%) | 75 (10%) | 47 (6%) | 568 (88%) | 80 (12%) | As an adult and prior to (reference month/year) did you ever use talc or baby powder or deodorizing powder with talc at least once a month for 6 months or more in any of the following ways: As a dusting powder or deodorizing spray more in any of the following ways: As a dusting powder or deodorizing spray to your genital or rectal areas? On your sanitary napkin? On your underwear? On your diaphragm or cervical cap? |
| NCO†† | 1999–2008 | 650 | 786 | 489 (62%) | 71 (9%) | 100 (13%) | 65 (8%) | 636 (81%) | 148 (19%) | Did you ever regularly use cornstarch, talc, baby or deodorizing powders (dusted or sprayed) at least 1 time per month for at least 6 months? If yes, please tell me if you used cornstarch, talc, baby or deodorizing powders in any of the following ways: directly to your genital or rectal areas? Applied to your sanitary napkins or tampons? Applied to birth control devices such as cervical cap or diaphragm? applied to your underwear? |
| NEC†† | 1992–2008 | 2329 | 2305 | 1234 (54%) | 281 (12%) | 352 (15%) | 276 (12%) | 1659 (77%) | 486 (23%) | Did you ever regularly use powder on your body or your underwear (at least once per month for any amount of time)? If yes, did you apply powder directly to your genital or rectal areas? To your sanitary napkins or tampons? To your underwear? |
| SON†† | 1989–1992 | 564 | 449 | 254 (57%) | 80 (18%) | 71 (16%) | 29 (6%) | 365 (81%) | 84 (19%) | Have you ever used sanitary napkins/tampons? If yes, could you tell me over what ages you’ve used them, for how many years, what percent of periods you’ve used them for, the usual number you’ve used for each period, whether they were deodorant pads/tampons, and if you used talcum powder or starch on them? Have you ever regularly used talcum powder or starch on your vaginal area after showering or bathing? |
| USC | 1993–1997 | 782 | 772 | 396 (52%) | 131 (17%) | 75 (10%) | 32 (4%) | 549 (73%) | 205 (27%) | Prior to (reference month/year), did you ever regularly use talc, baby, or deodorizing powder dusted or sprayed on your body? By regularly I mean at least once a month for 6 months or more. Did you ever use talc, baby, or deodorizing powder as a dusting powder to the genital or rectal area? as a dusting powder to sanitary napkins? as a dusting powder to underwear? on a diaphragm or cervical cap? |
AUS = Australia Ovarian Cancer Study and Australian Cancer Study (Ovarian Cancer), DOV = Diseases of the Ovary and their Evaluation, HAW = Hawaiian Ovarian Cancer Study, HOP = Hormones and Ovarian Cancer Prediction, NCO = North Carolina Ovarian Cancer Study, NEC = New England Case-Control Study of Ovarian Cancer, SON = Southern Ontario Ovarian Cancer Study, USC = University of Southern California Study of Lifestyle and Women’s Health
Cases listed by histology do not sum because mixed, other, undifferentiated, and unknown are not included.
Cases listed by behavior do not sum to the total number of cases because 267 cases are missing behavior information.
In a separate series of questions, participants were asked about powder use with diaphragm storage. Duration was calculated from ages of use. Information on duration, frequency, and timing of use was only collected on genital/perinal powder use after bathing.
Controls were asked “Have you ever regularly used…”
NEC question varied slightly between the three study phases. Between 1992–1997 participants were asked, “As an adult and prior to (reference month/year), did you regularly use talc, baby, or deodorizing powders dusted or sprayed to your body in any of the following ways:”. Between 1998–2003, women were asked “Did you regularly apply cornstartch, talc, baby, or deodorizing body powder at least one time per month for six months or longer? If yes, please tell me if you regularly applied cornstarch, talc, baby or deodorizing body powders in any of the following ways:” Between 2003–2008 participants were asked the question listed above.
These studies previously published on genital powder use and ovarian cancer risk. AUS, DOV, and NEC provided new data to the pooled analyses presented here that were not included in previous publications.
Exposure and covariate data
Data collected from participants regarding genital powder use varied between studies. Harmonized analytic exposure variables were developed by comparing questionnaires between the eight participating studies. The majority of the studies have obtained information on duration and frequency of powder use, age at first powder use, use by sexual partners, and non-genital use (Table 1). We defined genital powder use as any type of powder (talc, baby, deodorizing, cornstarch, or unspecified/unknown) applied directly or indirectly (by application to sanitary pads, tampons, underwear) to the genital, perineal, or rectal area. Since study specific powder questions included varying degrees of detail regarding type and method of application, genital powder definitions differ between studies. Criteria for regular genital powder use varied between studies from “ever use” (AUS) to “one year or longer” (DOV); the specific wording for this question is provided in Table 1. Use of body powders on sites other than the genital area was defined as non-genital powder use. Women who reported both genital and non-genital powder use were classified as genital users. Two studies (DOV, SON) did not collect data on non-genital use and therefore women assigned to “no powder use” for these studies could have a history of non-genital powder exposure. Extensive information on known and suspected risk factors for ovarian cancer was collected in each study, including oral contraceptive (OC) use, parity, tubal ligation history, body mass index (BMI), race, and ethnicity.
Statistical methods
Participants missing case/control status (n=17) or tumor histology (n=19) were excluded from the analysis. We also excluded 1,119 participants who answered “do not know” or were missing data on genital powder use; most of these were from the NCO study which did not include genital powder questions for the first 720 participants. Furthermore, we excluded participants missing tubal ligation (n=55), OC duration (n=100), parity (n=3), or height or weight (BMI) (n=179). To examine differences in characteristics between cases and controls, we evaluated two-sample t-statistics (age, BMI) and chi square statistics (OC use, nulliparity, tubal ligation, race/ethnicity, powder use).
Study-specific odds ratios (ORs) and 95% confidence intervals (CI) were estimated using unconditional logistic regression and were summarized by forest plots, including study heterogeneity based on Cochran’s Q statistic. As no significant heterogeneity was observed between studies, we calculated pooled ORs and 95% CIs across the studies using conditional logistic regression matched on 5-year age groups and study. All analyses were adjusted for potential confounders: age (continuous), duration of oral contraceptive (OC) use (never use, use <2yrs, 2–<5yrs, 5–<10yrs, ≥10yrs), parity (0, 1, 2, 3, ≥4 children), tubal ligation history, BMI (quartiles based on distribution in controls), and race/ethnicity (non-Hispanic white, Hispanic white, black, Asian, other). Family history of breast or ovarian cancer were also considered as covariates but were not included in the final model.
Subtype-specific estimates were calculated for subgroups of ovarian cancer defined by behavior (invasive, borderline) and histology (serous, mucinous, endometrioid, clear cell) by comparing each case group to all controls. As borderline endometrioid and clear cell tumors are rare, we did not have sufficient numbers to evaluate those types separately.
In order to measure cumulative dose of genital-powder use, we estimated lifetime number of powder applications by multiplying total months of use by frequency of use per month, for all direct and indirect genital-powder applications. Women who reported multiple types of genital powder exposure (on underwear, on sanitary napkins or pads, directly to genital area) during the same time period were assigned the number of genital powder applications equal to the most commonly used type rather than the sum of applications across all types of genital powder exposure. We reasoned that contemporaneous powder applications were unlikely to be independent events and therefore should not be treated cumulatively.. Analyses of estimated lifetime number of applications excluded participants in the HOP study as data on age and frequency of use were not collected (n=2,224); genital powder users missing information on duration or frequency of use were omitted in the remaining studies (n=394). Never regular users of genital powders and women who only reported non-genital use were coded as having zero lifetime genital powder applications and comprised the reference group for this analysis. Categories were determined based on age-specific quartile cutpoints in controls (25th, 50th and 75th percentile cutpoints are 612, 1,872, and 5,400 for participants < 40 years old; 612, 2,160, and 7,200 for 41–50 years; 720, 3,600, and 10,800 for 51–60 years; 1,440, 5,760, and 14,440 for 61–70; 840, 7,200, and 18,000 for > 70 years). Trends were evaluated based on the median lifetime number of genital-powder applications for controls in each age-specific quartile using the Wald statistic and were performed both including and excluding never users of genital powders.
We estimated the association between genital powder use and ovarian cancer risk within strata to evaluate potential modification of effect defined using a cutpoint BMI of 30 based on the World Health Organization’s definition of obesity, endometriosis, parity, tubal ligation/hysterectomy, and menopausal status. We used likelihood-ratio statistics comparing models with and without interaction terms to determine statistically significant interactions. To estimate calendar year of first use, we subtracted the years since first use (age at study entry minus age at first genital powder use) from median calendar year of the participant’s study. All analyses were performed in SAS v9.2 (SAS, Cary, NC) and Stata v9.2 (StataCorp, College Station, TX). All p-values are two-sided. Analyses have been independently verified by two separate study groups (HAW and NCO).
RESULTS
This pooled analysis of eight case-control studies included 9,859 controls and 8,525 ovarian cancer cases. Genital powder use was reported by 2,511 (25%) of the controls and 2,600 (31%) of the cases, while powder use only on other (non-genital) parts of the body was reported by 1,533 (16%) of the controls and 1,282 (15%) of the cases (Table 2). The prevalence of genital powder use in controls varied widely between study sites, highest in AUS (45%) and lowest in HAW (15%, Table 3).
Table 2.
Characteristics of cases and controls included in the pooled analysis*
| Controls (N=9,859) | Cases (N=8,525) | |
|---|---|---|
| Mean (std) or N (%) | Mean (std) or N (%) | |
| Age | 55 (12) | 55 (12) |
| OC use | ||
| Never | 2995 (30) | 3411 (40) |
| Ever | 6864 (70) | 5114 (60) |
| Parous | ||
| No | 1468 (15) | 2196 (26) |
| Yes | 8391 (85) | 6329 (74) |
| Tubal Ligation | ||
| No | 7359 (75) | 6994 (82) |
| Yes | 2500 (25) | 1531 (18) |
| Body Mass Index | 26.5 (6.1) | 27.0 (6.6) |
| Race/Ethnicity | ||
| Non-Hispanic White | 8629 (88) | 7433 (87) |
| Hispanic White | 197 (2) | 214 (3) |
| Black | 273 (3) | 268 (3) |
| Asian | 350 (4) | 313 (4) |
| Other † | 407 (4) | 291 (4) |
| Powder use ‡ | ||
| Never use | 5815 (59) | 4643 (54) |
| Non-genital use only | 1533 (16) | 1282 (15) |
| Genital use | 2511 (25) | 2600 (31) |
All characteristics listed except age differed significantly (<0.01) between cases and controls. Cases include both borderline and invasive ovarian cancers.
There are six cases and three controls missing race/ethnicity information.
Categories for non-genital and genital powder use are mutually exclusive.
Table 3.
Association between powder use and risk of ovarian cancer (borderline and invasive combined) by study site
| Site | Controls (%)(N= 9,859) | Cases (%)(N= 8,525) | Age-adjusted OR (95% CI)* | Multivariate OR (95% CI)* |
|---|---|---|---|---|
| AUS | ||||
| No powder use | 305 (21) | 300 (21) | 1.00 | 1.00 |
| Non-genital use only | 486 (34) | 427 (30) | 0.85 (0.69–1.05) | 0.92 (0.74–1.14) |
| Genital use | 658 (45) | 705 (49) | 1.04 (0.85–1.26) | 1.13 (0.92–1.38) |
| DOV† | ||||
| No powder use | 1544 (83) | 1293 (83) | 1.00 | 1.00 |
| Genital use | 297 (16) | 272 (17) | 1.14 (0.95–1.37) | 1.13 (0.93–1.36) |
| HAW | ||||
| No powder use | 489 (65) | 326 (68) | 1.00 | 1.00 |
| Non-genital use only | 154 (20) | 81 (17) | 0.79 (0.58–1.07) | 0.69 (0.50–0.96) |
| Genital use | 112 (15) | 74 (15) | 0.99 (0.72–1.37) | 0.99 (0.70–1.41) |
| HOP | ||||
| No powder use | 989 (66) | 439 (60) | 1.00 | 1.00 |
| Non-genital use only | 184 (13) | 102 (14) | 1.23 (0.94–1.61) | 1.23 (0.93–1.62) |
| Genital use | 316 (21) | 194 (26) | 1.37 (1.11–1.69) | 1.34 (1.07–1.67) |
| NCO | ||||
| No powder use | 391 (60) | 469 (60) | 1.00 | 1.00 |
| Non-genital use only | 137 (21) | 122 (16) | 0.75 (0.57–0.99) | 0.74 (0.56–0.99) |
| Genital use | 122 (19) | 195 (25) | 1.33 (1.03–1.74) | 1.37 (1.05–1.80) |
| NEC | ||||
| No powder use | 1239 (53) | 1129 (49) | 1.00 | 1.00 |
| Non-genital use only | 454 (19) | 421 (18) | 1.02 (0.87–1.19) | 1.04 (0.88–1.22) |
| Genital use | 636 (27) | 755 (33) | 1.30 (1.14–1.49) | 1.28 (1.12–1.47) |
| SON† | ||||
| No powder use | 364 (65) | 252 (56) | 1.00 | 1.00 |
| Genital use | 200 (35) | 197 (44) | 1.43 (1.11–1.85) | 1.35 (1.03–1.76) |
| USC | ||||
| No powder use | 494 (63) | 435 (56) | 1.00 | 1.00 |
| Non-genital use only | 118 (15) | 129 (17) | 1.25 (0.94–1.66) | 1.14 (0.85–1.52) |
| Genital use | 170 (22) | 208 (27) | 1.39 (1.10–1.77) | 1.36 (1.06–1.74) |
| Pooled‡ | ||||
| No powder use | 5815 (59) | 4643 (54) | 1.00 | 1.00 |
| Non-genital use only | 1533 (16) | 1282 (15) | 0.98 (0.90–1.07) | 0.98 (0.89–1.07) |
| Genital use | 2511 (25) | 2600 (31) | 1.25 (1.16–1.34) | 1.24 (1.15–1.33) |
Study-specific estimates were determined using unconditional logistic regression and pooled ORs were estimated using conditional logistic regression conditioned on 5yr age groups and study. Multivariate models are adjusted for age (continuous), oral contraceptive duration (never use, <2yrs, 2–<5yrs, 5–<10yrs, >=10yrs), parity (0,1,2,3,4+ children), tubal ligation history (no, yes), BMI (quartiles), race/ethnicity (non-Hispanic white, Hispanic white, black, Asian, other).
Information on non-genital powder use was not collected in the SON and DOV study
p-value for heterogeneity between multivariate study specific ORs equal to 0.61; calculated using Conchran’s Q statistic test
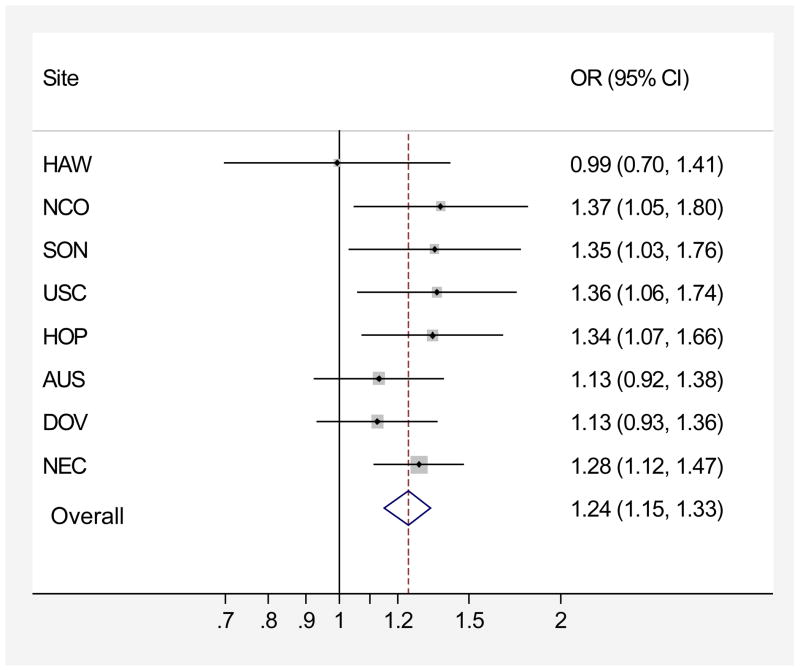
In the pooled analysis, ever regular use of genital powder was associated with a modest increase in risk of ovarian cancer (OR=1.24, 95% CI=1.15–1.33, Table 3) relative to women who reported no powder use (AUS, HAW, HOP, NCO, NEC, USC) or no genital powder use (DOV, SON). We observed no heterogeneity in the risk associated with genital powder use between studies regardless of the reference group (p=0.61, Figure 1). Results were similar for genital powder users compared to a combined reference group including never users and women whose use of powder was exclusively non-genital (covariate-adjusted OR=1.25, 95% CI= 1.16–1.34; data not shown), reflecting the absence of an association between powder use on other parts of the body with ovarian cancer risk (Table 3).
Figure 1.
Association between genital powder use and ovarian cancer risk in eight studies, p-heterogeneity=0.61. Adjusted for age (continuous), oral contraceptive duration (never use, <2yrs, 2–<5yrs, 5–<10yrs, >=10yrs), parity (0, 1, 2, 3, 4+ children), tubal ligation history, BMI (quartiles), race/ethnicity (non-Hispanic white, Hispanic white, black, Asian, other). Studies listed in decreasing order of effect size standard error (funnel plot). No evidence of heterogeneity based on Conchran’s Q statistic (p=0.61). AUS=Australian Cancer Study, DOV=Diseases of the Ovary and their Evaluation Study, HAW=Hawaii Ovarian Cancer Study, HOP=Hormones and Ovarian Cancer Prediction Study, NCO=North Carolina Ovarian Cancer Study, NEC=New England Case-Control Study of Ovarian Cancer, SON=Southern Ontario Ovarian Cancer Study
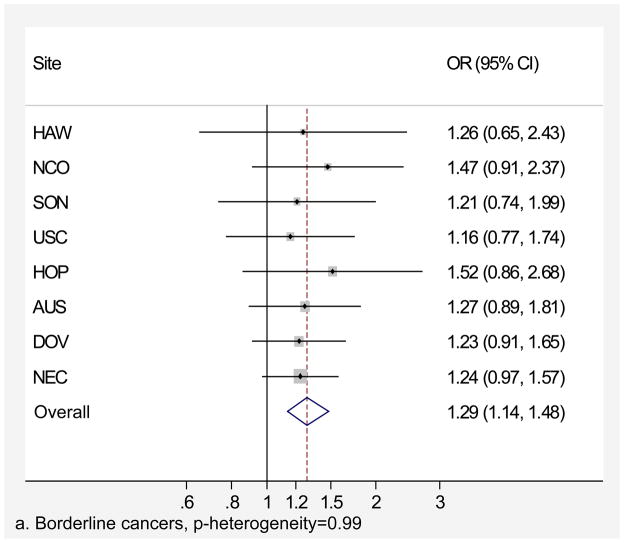
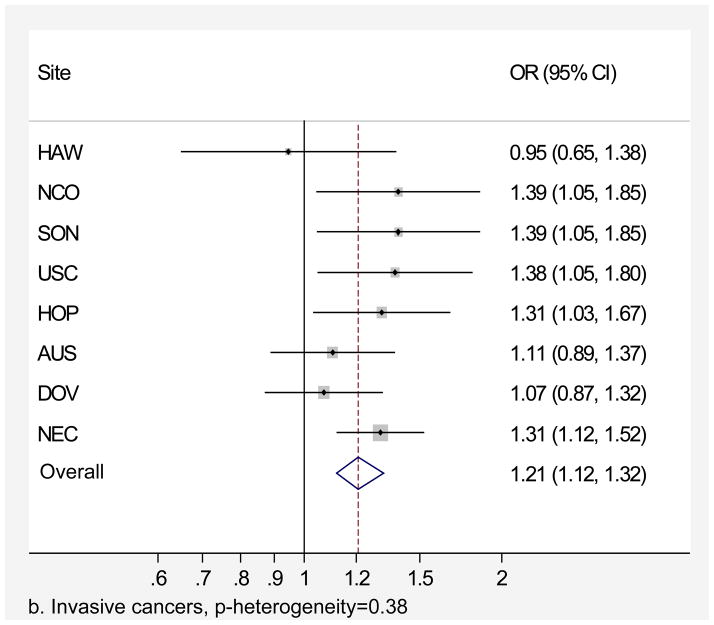
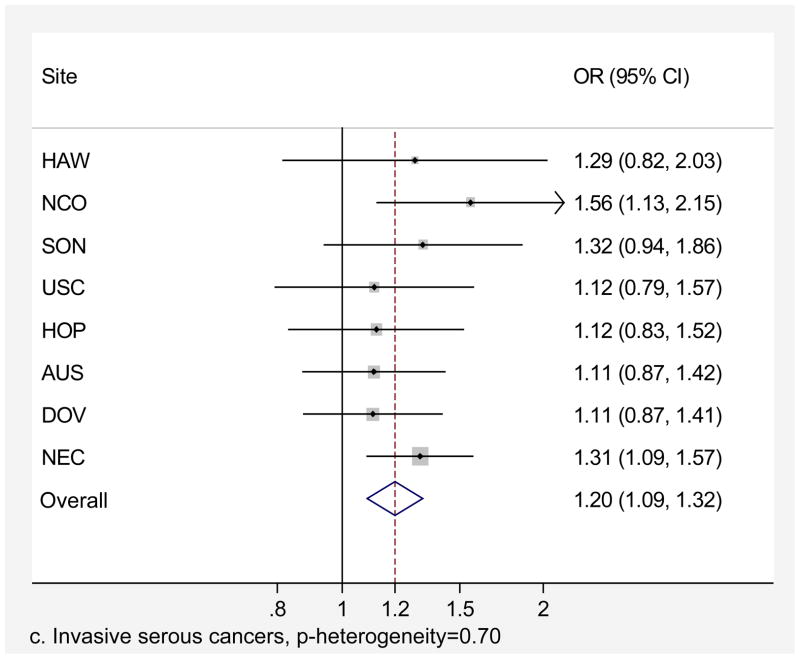
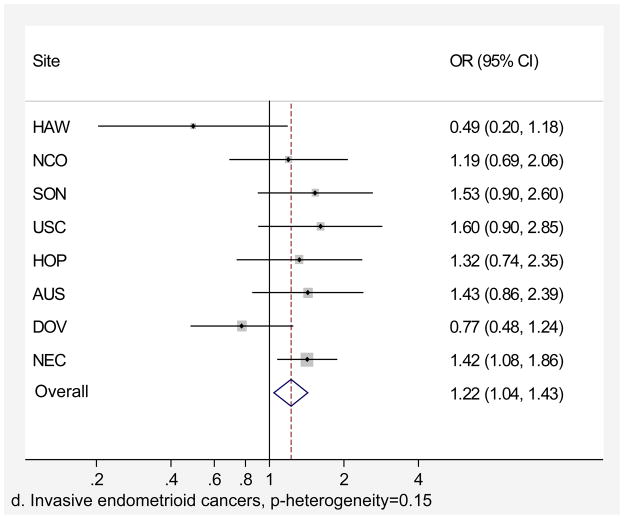
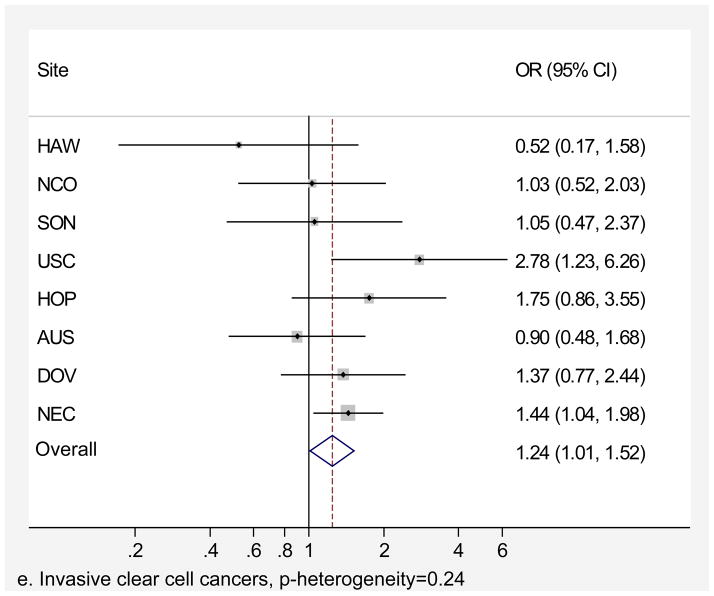
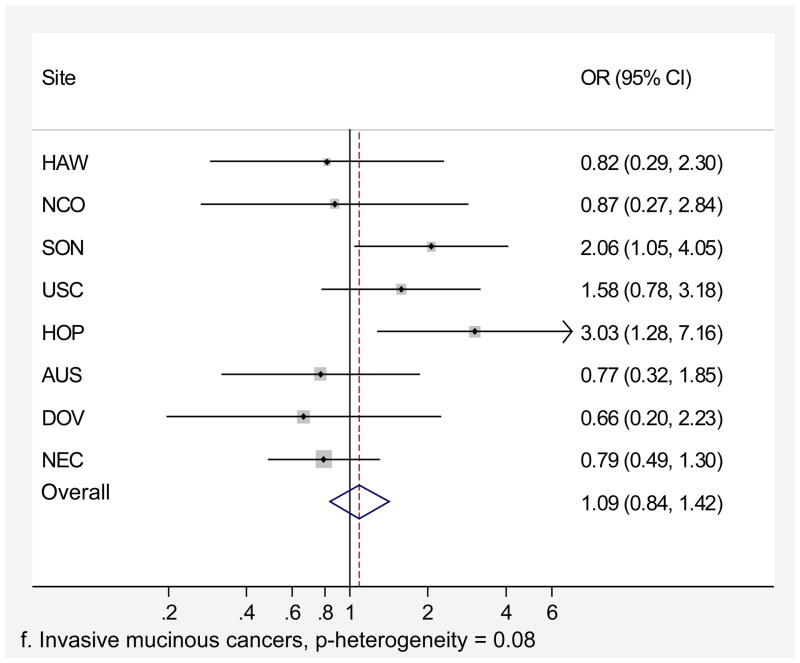
Genital powder use was associated with a similar increased risk of borderline and invasive ovarian cancer overall (Table 4). For borderline tumors, the association was stronger for the serous subtype (OR=1.46, 95% CI=1.24–1.72; Table 4) and non-significant for the mucinous subtype. For invasive ovarian cancer, we observed small increases in risk of serous (OR=1.20, 95% CI=1.09–1.32), endometrioid (OR=1.22, 95% CI=1.04–1.43), and clear cell (OR=1.24, 95% CI=1.01–1.52) cancer but no significant increase in risk of mucinous cancer (OR=1.09, 95% CI= 0.84–1.42). Similarly, we observed no significant increase in risk when borderline and invasive mucinous tumors were considered together (data not shown). Risk associated with genital powder use was consistent across studies for borderline and invasive tumors as well as invasive serous, endometrioid, and clear cell subtypes (p for heterogeneity >0.1; Figures 2 a,b,c,d,e), but not for mucinous tumors (p=0.08; Figure 2f). Genital powder use was associated with increased risk of invasive mucinous tumors in SON, HOP (significantly), and USC (non-significantly) while in the remaining studies (HAW, NCO, AUS, DOV, and NEC) genital powder use was non-significantly associated with reduced risk.
Table 4.
Association between powder use and risk of ovarian cancer by behavior and histology
| Model 1* | OR (95% CI)† | Model 2* | OR (95% CI)† | |||
|---|---|---|---|---|---|---|
| No powder use | Genital powder use | No genital powder use | Genital powder use | |||
| n (%) | n (%) | n (%) | n (%) | |||
| Controls | 5815 (59) | 2511 (25) | 7348 (75) | 2511 (25) | ||
| All borderline cases | 1035 (58) | 504 (28) | 1.29 (1.14–1.48) | 1247 (72) | 504 (28) | 1.30 (1.15–1.47) |
| Serous | 567 (57) | 300 (30) | 1.46 (1.24–1.72) | 700 (70) | 300 (30) | 1.45 (1.24–1.69) |
| Mucinous | 409 (60) | 184 (27) | 1.17 (0.96–1.42) | 502 (73) | 184 (27) | 1.19 (0.98–1.43) |
| All invasive cases | 3470 (54) | 2009 (31) | 1.21 (1.12–1.32) | 4471 (69) | 2009 (31) | 1.23 (1.14–1.32) |
| Serous | 1952 (53) | 1197 (32) | 1.20 (1.09–1.32) | 2519 (68) | 1197 (32) | 1.24 (1.13–1.35) |
| Mucinous | 206 (57) | 94 (26) | 1.09 (0.84–1.42) | 269 (74) | 94 (26) | 1.06 (0.82–1.36) |
| Endometrioid | 568 (55) | 304 (30) | 1.22 (1.04–1.43) | 723 (70) | 304 (30) | 1.20 (1.03–1.40) |
| Clear Cell | 327 (54) | 187 (31) | 1.24 (1.01–1.52) | 420 (69) | 187 (31) | 1.26 (1.04–1.52) |
In model 1, the reference group is restricted to women with no powder use except for the DOV and SON studies as these did not collect data on non-genital powder use. The number of cases who reported non-genital powder use was 212 (13%) of all borderline cases, 133 (13%) serous borderline, 93 (14%) mucinous borderline, 1001 (15%) of all invasive, 567 (15%) serous invasive, 63 (17%) mucinous invasive, 155 (15%) endometrioid invasive, 93 (15%) clear cell invasive. In model 2, the reference group includes all women who did not use genital powders (non-users and non-genital users combined).
ORs were estimated using conditional logistic regression conditioned on 5yr age groups and adjusted for age (continuous), oral contraceptive duration (never use, <2yrs, 2–<5yrs, 5–<10yrs, >=10yrs), parity (0,1,2,3,4+ children), tubal ligation history (no, yes), BMI (quartiles), race/ethnicity (non-Hispanic white, Hispanic white, black, Asian, other).
Figure 2.
Association between genital powder use and subgroups of ovarian cancer defined by behavior and histology. Estimates are adjusted for the same covariates as in the model presented in figure 1.
We evaluated cumulative genital-powder exposure as a composite variable of frequency and duration of use. We observed similar increased risks of all non-mucinous subtypes of epithelial ovarian cancer combined across quartiles of genital powder compared to non-use: ORQ1=1.18, 95% CI=1.02–1.36, ORQ2=1.22, 95% CI=1.06–1.41, ORQ3=1.22, 95% CI=1.06–1.40, ORQ4= 1.37, 95% CI=1.19–1.58 (Table 5). Although a significant increase in risk with an increasing number of genital powder applications was found for non-mucinous epithelial ovarian cancer when non-users were included in the analysis (p-trend<0.0001), no trend in cumulative use was evident in analyses restricted to ever-users of genital powder (p-trend=0.17; Table 5). Taken together, these observations suggest that the significant trend test largely reflects the comparison of ever regular use to never use. Since tubal ligation or hysterectomy would block the transport of powder through the genital tract to the ovaries, we performed a sensitivity analysis excluding women who started genital powder use after these procedures. We observed similar associations when we excluded the 65 cases and 79 controls who started genital powder use for the first time after surgery (ORQ1=1.19, 95% CI=1.03–1.38, ORQ2=1.19, 95% CI=1.03–1.38, ORQ3=1.21, 95% CI=1.04–1.39, ORQ4= 1.36, 95% CI=1.18–1.57). For studies that collected data on timing of powder use and tubal ligation/hysterectomy, we were able to identify timing of genital powder exposure in relation to surgery based on age of powder use and age at surgery. Restricting our exposure to genital powder applications that occurred before tubal ligation or hysterectomy made no substantive difference in the results.
Table 5.
Association between estimated lifetime applications of genital powder and risk of ovarian cancer (borderline and invasive combined)
| Lifetime number of applications* | All Cases (N= 7,587) | Non-mucinous cases (N= 6,361) | |||
|---|---|---|---|---|---|
| Controls (%) | Cases (%) | OR† (95 % CI) | Cases (%) | OR† (95 % CI) | |
| Never users | 6175 (76) | 5384 (71) | 1.00 | 4472 (70) | 1.00 |
| Quartile 1 | 509 (6) | 534 (7) | 1.14 (1.00–1.31) | 467 (7) | 1.18 (1.02–1.36) |
| Quartile 2 | 512 (6) | 541 (7) | 1.23 (1.08–1.41) | 456 (7) | 1.22 ( 1.06–1.41) |
| Quartile 3 | 497 (6) | 542 (7) | 1.22 (1.07–1.40) | 457 (7) | 1.22 (1.06–1.40) |
| Quartile 4 | 486 (6) | 586 (8) | 1.32 (1.16–1.52) | 509 (8) | 1.37 (1.19–1.58) |
| p-trend‡ | 0.17 | 0.17 | |||
Age specific 25th, 50th and 75th percentile cutpoints are 612, 1,872, and 5,400 for participants < 40 years old; 612, 2,160, and 7,200 for 41–50 years; 720, 3,600, and 10,800 for 51–60 years; 1,440, 5,760, and 14,440 for 61–70; 840, 7,200, and 18,000 for > 70 years.
ORs were estimated using conditional logistic regression conditioned on 5yr age groups and adjusted for age (continuous), oral contraceptive duration (never use, <2yrs, 2–<5yrs, 5–<10yrs, >=10yrs), parity (0,1,2,3,4+ children), tubal ligation history (no, yes), BMI (quartiles), race/ethnicity (non-Hispanic white, Hispanic white, black, Asian, other).
Trend excludes never users.
The association between any genital-powder use and ovarian cancer risk was stronger among women with BMI < 30 kg/m2 (OR=1.28, 95% CI=1.17–1.39) than for women with BMI > 30 (OR=1.14, 95% CI=0.98–1.32, p-interaction=0.01). We observed no significant interactions between genital powder use and parity, reported history of endometriosis, tubal ligation/hysterectomy, or menopausal status (all p-interaction > 0.1). The association between genital powder use and ovarian cancer risk was similar for women who started use between 1952 and 1961 (OR=1.36, 95% CI=1.19–1.56), between 1962 and 1972 (OR=1.27, 95% CI=1.11–1.46), and after 1972 (OR=1.31, 95% CI=1.15–1.51). However, we observed an attenuated association for women who started genital powder use before 1952 (OR=1.08, 95% CI=0.93–1.25).
DISCUSSION
This pooled analysis of eight case-control studies suggests that genital powder use is associated with a modest 20–30% increase in risk of developing epithelial ovarian cancer, including serous, endometrioid, and clear cell tumors, but is less relevant to invasive mucinous tumors. Our findings are consistent with and extend the findings of three meta-analyses that have reported an increased risk of epithelial ovarian cancer with genital-powder use (1, 4, 5) by including dose response and histology specific analyses. Our estimate of the overall association between genital powder use and ovarian cancer risk was slightly attenuated compared to previous estimates from meta-analyses. Possible reasons for the difference include the lack of restriction to published results, data harmonization between studies that allowed similar definitions for the exposure and covariates, and chance. Based on the consistency in the epidemiologic literature on talc-based powder and ovarian cancer risk, the International Agency for Research on Cancer (IARC) classified talc-based body powder as a class 2b carcinogen “possibly carcinogenic to human beings” (29).
The biologic plausibility for the observed association between genital-powder use and ovarian cancer risk has been challenged because evidence for dose-response has been inconsistent (2, 4, 5, 9, 10, 15, 22). The lack of significant dose-response may reflect the difficulty inherent in accurate recollection of specific details of frequency and duration of genital-powder use. Also, because not all powder products contain talc, various products may differ in their potential carcinogenic effects. Alternatively, the association between genital-powder exposure and ovarian cancer risk may not be linear and a modest exposure may be sufficient to increase cancer risk. Talc-containing powders are hypothesized to promote cancer development by ascending the female genital tract and interacting directly with the ovarian surface epithelium, leading to local inflammation characterized by increased rates of cell division, DNA repair, oxidative stress, and elevated inflammatory cytokines (13). Particles in solution easily ascend the genital tract (30, 31). Our finding of slightly attenuated associations following exclusion of women with powder exposure after tubal ligation or hysterectomy are not supportive of this hypothesis, but risk estimates in this subgroup analysis may have randomly differed from those including all women because of the reduction in sample size. Talc particles have been observed in the ovaries of humans (32) and in rodent models (33, 34), but little is known about the biologic effects of genital powder use.
In the current analyses of the various histological subtypes of ovarian cancer, we confirmed previous reports of increased risk of serous invasive tumors with genital-powder use (2, 4, 8, 9, 22). We also observed significantly increased risk of both endometrioid and clear cell invasive ovarian tumors with use of genital powder, and this finding was consistent across studies. It has been suggested that both endometrioid and clear cell ovarian tumors may originate from ectopic uterine endometrium (endometriosis) implanted on the ovary (17). In contrast, we observed no significant associations between genital powder use and either borderline or invasive mucinous ovarian cancer. The lack of a significant association for mucinous tumors may be due to the relatively small number of these tumors or could be an indication that powder exposure is not relevant to the pathogenesis of this histologic type. Studies have noted that ovarian cancer risk factors and molecular characteristics differ for mucinous tumors (16–18, 23, 35–39).
Limitations of our pooled analysis include differences in the wording of questions about genital powder use between studies and the retrospective nature of the exposure ascertainment. Women who were classified as genital-powder users varied from “ever” use (AUS) or “ever regular” use (SON) to powder use for at least six months (HAW, HOP, NCO, NEC, USC) or at least one year (DOV). Differences in genital powder questions result in varying levels of misclassification of true genital powder exposure. However, since exposure definitions are the same for cases and controls within each study, misclassification genital powder exposure due to the question wording would be non-differential, leading to an underestimate of the true association for any given study. These studies were retrospective in nature and therefore potentially susceptible to bias if cases were more likely to report genital-powder use than controls. Although non-genital powder use was not associated with ovarian cancer risk, it is nevertheless possible that any overreporting of powder use by cases might have been limited to reporting of genital powder. Our analyses were also limited by missing data on genital powder use; however, missingness was not associated with the distribution of any of the ovarian cancer risk factors examined and was thus not likely to bias our results. Strengths of our analysis include a large sample size and pooled analysis of individual data, allowing evaluation of the association of genital powder use with less common histologic subgroups of ovarian cancer, careful harmonization of the data based on comparison of study questionnaires, the use of a composite variable combining duration and frequency to assess dose-response relationships.
In conclusion, our large pooled analysis of case-control studies shows a small-to-moderate (20–30%) increased risk of ovarian cancer with genital-powder use, most clearly pertaining to non-mucinous epithelial ovarian tumors. More work is needed to understand how genital powders may exert a carcinogenic effect, and which constituents (e.g. talc) may be involved. Since there are few modifiable risk factors for ovarian cancer, avoidance of genital powders may be a possible strategy to reduce ovarian cancer incidence.
Acknowledgments
Support for the Ovarian Cancer Association Consortium was provided by donations from family and friends of the Kathryn Sladek Smith to the Ovarian Cancer Research fund. In addition, these studies were supported by National Institutes of Health (R01-CA54419, R01-CA112523, R01-CA87538, R01-CA95023, RO1-CA76016, R01-CA58598, R01-CA17054, R01-CA14089, R01-CA61132, R03-CA113148, R03-CA115195, N01-CN25403, N01-PC-67010, N01-CN55424, N01-PC67001, P50-CA105009, P01-CA17054), the U.S. Department of Defense (DAMD17-01-1-0729, DAMD17-02-1-0669, DAMD17-02-1-0666), National Health & Medical Research Council of Australia (199600), Cancer Council of Tasmania, Cancer Foundation of Western Australia; California Cancer Research Program (00-01389V-20170, 2II0200), and National Health Research and Development Program, Health and Welfare Canada 6613-1415-53. Individual investigators are supported by National Institutes of Health (K07-CA143047 (WS), R25-CA098566 (MAM)), Department of Defense (W81XWH-10-1-02802 (KLT)), and the National Health and Medical Research Council of Australia (PMW, CMN). No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank all the individuals who participated in these studies as well as the researchers, clinicians, and support staff who contributed to this work. The Australian Ovarian Cancer Study Management Group (D. Bowtell, G. Chenevix-Trench, A. deFazio, D. Gertig, A. Green, P. Webb) and Australian Cancer Study Investigators (A. Green, P. Parsons, N. Hayward, P. Webb, D. Whiteman) thank all the clinical and scientific collaborators (see http://www.aocstudy.org/) and the women for their contribution.
Footnotes
The authors have no conflict of interest to disclose.
References
- 1.Langseth H, Hankinson SE, Siemiatycki J, Weiderpass E. Perineal use of talc and risk of ovarian cancer. J Epidemiol Community Health. 2008 Apr;62(4):358–60. doi: 10.1136/jech.2006.047894. [DOI] [PubMed] [Google Scholar]
- 2.Merritt MA, Green AC, Nagle CM, Webb PM. Talcum powder, chronic pelvic inflammation and NSAIDs in relation to risk of epithelial ovarian cancer. Int J Cancer. 2007 Jan 1;122(1):170–6. doi: 10.1002/ijc.23017. [DOI] [PubMed] [Google Scholar]
- 3.Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 1999 Sep 1;91(17):1459–67. doi: 10.1093/jnci/91.17.1459. [DOI] [PubMed] [Google Scholar]
- 4.Cramer DW, Liberman RF, Titus-Ernstoff L, Welch WR, Greenberg ER, Baron JA, et al. Genital talc exposure and risk of ovarian cancer. Int J Cancer. 1999 May 5;81(3):351–6. doi: 10.1002/(sici)1097-0215(19990505)81:3<351::aid-ijc7>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 5.Huncharek M, Geschwind JF, Kupelnick B. Perineal application of cosmetic talc and risk of invasive epithelial ovarian cancer: a meta-analysis of 11,933 subjects from sixteen observational studies. Anticancer Res. 2003 Mar-Apr;23(2C):1955–60. [PubMed] [Google Scholar]
- 6.Booth M, Beral V, Smith P. Risk factors for ovarian cancer: a case-control study. Br J Cancer. 1989 Oct;60(4):592–8. doi: 10.1038/bjc.1989.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merritt M, Green A, Nagle C, Webb P Group ACSaAOCS . Talcum powder, chronic pelvic inflammation and NSAIDs in relation to risk of epithelial ovarian cancer. International Journal of Cancer. 2008;122(1):170–6. doi: 10.1002/ijc.23017. [DOI] [PubMed] [Google Scholar]
- 8.Wu AH, Pearce CL, Tseng CC, Templeman C, Pike MC. Markers of inflammation and risk of ovarian cancer in Los Angeles County. Int J Cancer. 2009 Mar 15;124(6):1409–15. doi: 10.1002/ijc.24091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook LS, Kamb ML, Weiss NS. Perineal powder exposure and the risk of ovarian cancer. Am J Epidemiol. 1997 Mar 1;145(5):459–65. doi: 10.1093/oxfordjournals.aje.a009128. [DOI] [PubMed] [Google Scholar]
- 10.Mills PK, Riordan DG, Cress RD, Young HA. Perineal talc exposure and epithelial ovarian cancer risk in the Central Valley of California. Int J Cancer. 2004 Nov 10;112(3):458–64. doi: 10.1002/ijc.20434. [DOI] [PubMed] [Google Scholar]
- 11.Whittemore AS, Wu ML, Paffenbarger RS, Jr, Sarles DL, Kampert JB, Grosser S, et al. Personal and environmental characteristics related to epithelial ovarian cancer. II. Exposures to talcum powder, tobacco, alcohol, and coffee. Am J Epidemiol. 1988 Dec;128(6):1228–40. doi: 10.1093/oxfordjournals.aje.a115077. [DOI] [PubMed] [Google Scholar]
- 12.Wong C, Hempling RE, Piver MS, Natarajan N, Mettlin CJ. Perineal talc exposure and subsequent epithelial ovarian cancer: a case-control study. Obstet Gynecol. 1999 Mar;93(3):372–6. doi: 10.1016/s0029-7844(98)00439-6. [DOI] [PubMed] [Google Scholar]
- 13.Ness RB, Grisso JA, Cottreau C, Klapper J, Vergona R, Wheeler JE, et al. Factors related to inflammation of the ovarian epithelium and risk of ovarian cancer. Epidemiology. 2000 Mar;11(2):111–7. doi: 10.1097/00001648-200003000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Rosenblatt KA, Weiss NS, Cushing-Haugen KL, Wicklund KG, Rossing MA. Genital powder exposure and the risk of epithelial ovarian cancer. Cancer Causes Control. 2011 May;22(5):737–42. doi: 10.1007/s10552-011-9746-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang S, Risch HA. Perineal talc exposure and risk of ovarian carcinoma. Cancer. 1997 Jun 15;79(12):2396–401. doi: 10.1002/(sici)1097-0142(19970615)79:12<2396::aid-cncr15>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 16.Risch HA, Marrett LD, Jain M, Howe GR. Differences in risk factors for epithelial ovarian cancer by histologic type. Results of a case-control study. Am J Epidemiol. 1996 Aug 15;144(4):363–72. doi: 10.1093/oxfordjournals.aje.a008937. [DOI] [PubMed] [Google Scholar]
- 17.Gilks CB. Molecular abnormalities in ovarian cancer subtypes other than high-grade serous carcinoma. J Oncol. 2010:Article ID: 740968. doi: 10.1155/2010/740968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Purdie DM, Webb PM, Siskind V, Bain CJ, Green AC. The different etiologies of mucinous and nonmucinous epithelial ovarian cancers. Gynecol Oncol. 2003 Jan;88(1 Pt 2):S145–8. doi: 10.1006/gyno.2002.6706. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Wu PC, Lang JH, Ge WJ, Hartge P, Brinton LA. Risk factors for epithelial ovarian cancer in Beijing, China. Int J Epidemiol. 1992 Feb;21(1):23–9. doi: 10.1093/ije/21.1.23. [DOI] [PubMed] [Google Scholar]
- 20.Harlow BL, Weiss NS. A case-control study of borderline ovarian tumors: the influence of perineal exposure to talc. American journal of epidemiology. 1989 Aug;130(2):390–4. doi: 10.1093/oxfordjournals.aje.a115345. [DOI] [PubMed] [Google Scholar]
- 21.Tzonou A, Polychronopoulou A, Hsieh CC, Rebelakos A, Karakatsani A, Trichopoulos D. Hair dyes, analgesics, tranquilizers and perineal talc application as risk factors for ovarian cancer. International journal of cancer Journal international du cancer. 1993 Sep 30;55(3):408–10. doi: 10.1002/ijc.2910550313. [DOI] [PubMed] [Google Scholar]
- 22.Gertig DM, Hunter DJ, Cramer DW, Colditz GA, Speizer FE, Willett WC, et al. Prospective study of talc use and ovarian cancer. J Natl Cancer Inst. 2000 Feb 2;92(3):249–52. doi: 10.1093/jnci/92.3.249. [DOI] [PubMed] [Google Scholar]
- 23.Gates MA, Rosner BA, Hecht JL, Tworoger SS. Risk factors for epithelial ovarian cancer by histologic subtype. Am J Epidemiol. 2010 Jan 1;171(1):45–53. doi: 10.1093/aje/kwp314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berchuck A, Schildkraut JM, Pearce CL, Chenevix-Trench G, Pharoah PD. Role of genetic polymorphisms in ovarian cancer susceptibility: development of an international ovarian cancer association consortium. Adv Exp Med Biol. 2008;622:53–67. doi: 10.1007/978-0-387-68969-2_5. [DOI] [PubMed] [Google Scholar]
- 25.Goodman MT, Lurie G, Thompson PJ, McDuffie KE, Carney ME. Association of two common single-nucleotide polymorphisms in the CYP19A1 locus and ovarian cancer risk. Endocr Relat Cancer. 2008 Dec;15(4):1055–60. doi: 10.1677/ERC-08-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lo-Ciganic WH, Zgibor JC, Bunker CH, Moysich KB, Edwards RP, Ness RB. Aspirin, nonaspirin nonsteroidal anti-inflammatory drugs, or acetaminophen and risk of ovarian cancer. Epidemiology. 2012 Mar;23(2):311–9. doi: 10.1097/EDE.0b013e3182456ad3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moorman PG, Palmieri RT, Akushevich L, Berchuck A, Schildkraut JM. Ovarian cancer risk factors in African-American and white women. Am J Epidemiol. 2009 Sep 1;170(5):598–606. doi: 10.1093/aje/kwp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pike MC, Pearce CL, Peters R, Cozen W, Wan P, Wu AH. Hormonal factors and the risk of invasive ovarian cancer: a population-based case-control study. Fertil Steril. 2004 Jul;82(1):186–95. doi: 10.1016/j.fertnstert.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Cogliano V. Carcinogenicity of carbon black, titanium dioxide, and talc. Lancet Oncol. 2006 Apr;7(4):295–6. doi: 10.1016/s1470-2045(06)70651-9. [DOI] [PubMed] [Google Scholar]
- 30.Egli GE, Newton M. The transport of carbon particles in the human female reproductive tract. Fertil Steril. 1961 Mar-Apr;12:151–5. doi: 10.1016/s0015-0282(16)34084-5. [DOI] [PubMed] [Google Scholar]
- 31.deBoer CH. Transport of particulate matter through the human female genital tract. J Reprod Fert. 1972;28:295–7. doi: 10.1530/jrf.0.0280295. [DOI] [PubMed] [Google Scholar]
- 32.Heller DS, Gordon RE, Katz N. Correlation of asbestos fiber burdens in fallopian tubes and ovarian tissue. Am J Obstet Gynecol. 1999 Aug;181(2):346–7. doi: 10.1016/s0002-9378(99)70559-4. [DOI] [PubMed] [Google Scholar]
- 33.Fleming JS, Beaugie CR, Haviv I, Chenevix-Trench G, Tan OL. Incessant ovulation, inflammation and epithelial ovarian carcinogenesis: Revisiting old hypotheses. Mol Cell Endocrinol. 2006 Mar 9;247(1–2):4–21. doi: 10.1016/j.mce.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 34.Heller DS, Westhoff C, Gordon RE, Katz N. The relationship between perineal cosmetic talc usage and ovarian talc particle burden. Am J Obstet Gynecol. 1996 May;174(5):1507–10. doi: 10.1016/s0002-9378(96)70597-5. [DOI] [PubMed] [Google Scholar]
- 35.Chiaffarino F, Parazzini F, Bosetti C, Franceschi S, Talamini R, Canzonieri V, et al. Risk factors for ovarian cancer histotypes. Eur J Cancer. 2007 May;43(7):1208–13. doi: 10.1016/j.ejca.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 36.Heinzelmann-Schwarz VA, Gardiner-Garden M, Henshall SM, Scurry JP, Scolyer RA, Smith AN, et al. A distinct molecular profile associated with mucinous epithelial ovarian cancer. British journal of cancer. 2006 Mar 27;94(6):904–13. doi: 10.1038/sj.bjc.6603003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurian AW, Balise RR, McGuire V, Whittemore AS. Histologic types of epithelial ovarian cancer: have they different risk factors? Gynecol Oncol. 2005 Feb;96(2):520–30. doi: 10.1016/j.ygyno.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 38.Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010 Mar;34(3):433–43. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurman RJ, Shih Ie M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm. Hum Pathol. 2011 Jul;42(7):918–31. doi: 10.1016/j.humpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]