Abstract
Event-related oscillations play a key role in understanding the brain dynamics and human information processing. In the present study, the Go/No-Go paradigm has been used to examine whether alcoholics have poor inhibitory control as compared to control subjects in terms of different oscillatory brain responses. The Matching Pursuits algorithm was used to decompose the event-related EEG into oscillations of different frequencies. It was found that alcoholics (n = 58) showed significant reduction in Delta (1.0 Hz – 3.0 Hz) and Theta (3.5 Hz – 7.0 Hz) power during No-Go trials as compared to controls (n = 29). This reduction was prominent at the frontal region. The decreased delta and theta power associated with No-Go processing perhaps suggests a deficient inhibitory control and information processing mechanism. A neuro-cognitive model has been provided to explain the findings. It is suggested that the oscillatory correlates during cognitive processing can be an endophenotypic marker in alcoholism.
Keywords: Event-related oscillations, Go/No-Go, alcoholism, inhibitory control, delta, theta, P300
1. Introduction
Frontal lobe pathology in alcoholism has been well documented and studied at the neurophysiological, morphological and neuropsychological levels (Moselhy et al., 2001). There has been compelling evidence for brain abnormalities, especially frontal dysfunction, in “nonamnesic” chronic alcoholic patients in terms of electrophysiological (e.g., Begleiter and Platz, 1972; Begleiter et al., 1980; Porjesz and Begleiter, 1987), neuroanatomical (e.g., Harper and Kril, 1985; Pfefferbaum et al., 1997), cerebral blood flow (e.g., Risberg and Berglund, 1987; Nicolas et al., 1993), glucose metabolism (e.g., Sachs et al., 1987; Adams et al., 1993), and a wide range of neuropsychological deficits (Jones and Parsons, 1971, 1972; Parsons, 1977; Miller, 1985; Beatty et al., 1996; Tzambazis and Stough, 2000). The frontal lobes play a major role in cognitive functions such as attention, working memory, creative and critical thinking, planning, decision making, inhibitory control, and emotional regulation (e.g., Shallice and Evans, 1978; Stuss and Benson, 1984; Rueckert and Grafman, 1996; Miotto et al., 1996). Frontal dysfunction, especially of prefrontal cortex, can lead to decreased will and energy, a tendency to engage in repetitive behaviors, difficulty in shifting response set, poor inhibitory control, abnormalities of affect and emotions, impulsivity, disinhibition and poor motivation (Nauta, 1971, 1972; Drewe, 1975; Fuster, 1989; Kraus and Maki, 1997).
Lack of inhibitory control has many manifestations including a tendency to distractibility, hyperreactivity, impulsivity, and symptoms that can be ascribed to poor control of external or internal influences (Fuster, 1989), and these specific deficits are implicated in many behavioral disorders including conduct disorder, antisocial personality disorder (ASPD), obsessive-compulsive disorder (OCD), attention deficit hyperactivity disorder (ADHD), and substance abuse disorders, which include alcoholism (Barkley, 1997; Rubia et al., 2001; Vogel-Sprott et al., 2001). Various studies have consistently identified disinhibitory psychopathology as a robust symptom constellation in alcoholics as well as in persons at risk for alcoholism (Gorenstein, 1979, 1987; Alterman and Tarter, 1983, 1986; Tarter and Alterman, 1984; Sher and Trull, 1994; Giancola et al., 1996a, 1996b; Fillmore and Vogel-Sprott, 1999, 2000; Vogel-Sprott et al., 2001).
According to Brutkowski (1965), there are two forms of inhibition that can be ascribed to prefrontal cortex: drive inhibition and response inhibition. It is speculated that motor/response inhibition is effected by the lateral cortex through the caudate nucleus and that drive inhibition is effected by the orbital cortex through the hypothalamus and the amygdala (cf. Fuster, 1989). The response inhibition, as assessed through Go/No-Go tasks, can be defined as the act of withholding or terminating a behavioral response and is considered to be governed by a cognitive inhibitory process (Logan et al., 1984). The neuroimaging studies showed activation in the inferior prefrontal area, middle and inferior frontal gyri, and anterior cingulate cortex, with the heightened activity at the right prefrontal areas during response inhibition (Konishi et al., 1999; Garavan et al., 1999; Braver et al., 2001).
As a valid measure for response inhibition, the Go/No-Go paradigm has been widely used in both animal and human experiments (Watanabe, 1986; Brown et al., 1989; Schroger, 1993). The Go/No-Go tasks generally involve making a response to a given stimulus (Go condition), while withholding the response to another stimulus (No-Go condition). Many studies have employed psychophysiological methods, especially event-related potentials (ERPs), in order to identify the neural correlates of response production and inhibition (Pfefferbaum et al., 1985; Pfefferbaum and Ford, 1988; Shibata et al., 1997, 1998; Filipovic et al., 1999; van Boxtel et al., 2001). These methods provide a safe, noninvasive approach to study neural correlates of mental processes. The ERP studies which employed Go/No-Go paradigms have yielded two significant electrophysiological signatures of response inhibition: first, the enlarged N2 component for the No-Go condition, a negative-going peak between, 200 and 300 msec over the frontocentral scalp location, and second, what is referred to as “No-Go P3”, an augmented positive-going peak usually peaking between 300 and 600 msec with maximum over frontocentral sites (Pfefferbaum et al., 1985; Elimer, 1993; Jodo and Kayama, 1992; Kopp et al., 1996). This anteriorly distributed No-Go (P300) potential had a markedly reduced amplitude in alcoholic patients as well as persons who are at high risk to develop alcoholism, indicating impaired inhibitory control (Pfefferbaum et al., 1991; Cohen et al., 1997a, 1997b; Fallgatter et al., 1998).
Although event-related brain oscillations have been intensively studied during cognitive processing (Basar-Eroglu et al., 1996a, 1996b; Basar et al., 1997, 1999, 2000, 2001a, 2001b, 2001c, 2001d; Klimesch, 1997, 1999; Klimesch et al., 1997a, 2001a, 2001b; Schurmann et al., 1997;, 2001; Schurmann and Basar, 1999, 2001; Doppelmayr et al., 2000; Sakowitz et al., 2000; Röhm et al., 2001; Kolev et al., 2001; Sauseng et al., 2002), only a few studies have attempted to investigate oscillatory changes related to alcohol or alcoholism (Laukka et al., 1997; Krause et al., 2002; Suresh et al. (a, b) in preparation). There is also a paucity of research examining oscillatory neural correlates of response inhibition using Go/No-Go paradigms (Shibata et al., 1997, 1998). Event-related oscillations are considered to be different from the ongoing “idling rhythms”, wherein a task- or process-related “partial phase resetting” occurs in different EEG frequency bands in response to sensory or cognitive stimulation (e.g., Basar et al., 1980; Makeig et al., 2002). Evidence suggests that ERP features arise from oscillatory changes due to sensory and cognitive processes in the dynamics of ongoing EEG rhythms of different frequency bands (Basar-Eroglu and Basar, 1991; Basar-Eroglu et al., 1992; Schurmann et al., 1995; Yordanova and Kolev, 1996, 1998; Karakas et al., 2000a, 2000b; Basar-Eroglu et al., 2001; Demiralp et al., 2001; Schurmann et al., 2001). Various cognitive processes have been attributed to different frequency rhythms of oscillatory responses. For example, delta responses are assumed to mediate signal detection and decision making (e.g., Basar et al., 1999, 2001a; Schurmann et al., 2001), while theta rhythms are attributed to different cognitive processes such as conscious awareness, episodic retrieval, and recognition memory (e.g., Klimesch et al., 1994, 2001a, 2001b; Doppelmayr et al., 1998; Basar et al., 2001d). The slow alpha rhythm (8–10 Hz) has been reported to be modulated as a function of attentional demands (e.g., Basar et al., 1997; Klimesch, 1997; Klimesch et al., 1998), and fast alpha activity (10–12 Hz) has been found to mediate semantic memory processes as well as stimulus-related aspects (e.g., Klimesch, 1996; Klimesch et al., 1994, 1997a, 1997b). Further, oscillatory gamma responses were shown to be involved in visual perception and cognitive integrative function (e.g., Basar-Eroglu et al., 1996a, 1996b; Schurmann et al., 1997; Basar et al., 2001a, 2001b).
The effects of acute alcohol administration on event-related oscillations were studied by Laukka et al. (1997). They found that alcohol significantly increased theta activity while the subjects were performing an attentional motor task of simulated automobile driving. Using the Event-related Desynchronization/Synchronization (ERD/ERS), a methodology first described by Pfurtscheller and Aranibar (1977), Krause et al. (2002) examined the effects of alcohol on ERD/ERS during an auditory memory task, and found that the administration of alcohol decreased the early-appearing ERS responses during auditory encoding and increased the later-appearing ERD responses during retrieval. Alcohol had significant effects on brain electric oscillatory systems in the theta frequency range during both memory encoding and retrieval as well as in the lower alpha frequency range during cognitive processing.
A more recent method, known as event-related coherence (ERCoh) which can reveal functional coupling of different brain areas (Rappelsberger et al., 1994), has been used to study the intra- and inter-hemispheric coherence during Go/No-Go tasks. Shibata et al. (1997) measured ERCoh for subjects performing a Go/No-Go task, and found that coherence in the No-Go condition was significantly higher than in the Go condition between F3 and F4 channels. It was suggested that synchronization of activity between bilateral dorsolateral frontal areas might therefore play an important role in the motor inhibition process in humans. In another similar study, they identified two different effects that were manifested during the No-Go condition in the event-related coherence: (i) alpha band synchronization between bilateral frontal areas that was related to the decision not to move, and (ii) theta band synchronization among bilateral frontal, central and parietal areas, which was presumably related to the motor inhibition process (Shibata et al., 1998). Nevertheless, it is to be noted that there have been no consistent attempts to investigate these event-related oscillatory responses during response inhibition in diagnosed or abstinent alcoholic subjects, despite the fact that alcoholics have been shown to have deficient inhibitory control.
It has been argued that P300 responses were primarily the outcome of oscillatory changes in delta and theta rhythms during stimulus processing (McCarthy and Donchin, 1981; Stampfer and Basar, 1985; Basar-Eroglu and Basar, 1991; Yordanova and Kolev, 1996; Demiralp et al., 1999). Since numerous studies demonstrated that alcoholics consistently showed deficits in P300 responses (e.g., Porjesz et al., 1980; Porjesz and Begleiter, 1996; Cohen et al., 2002) including the No-Go potential (e.g., Pfefferbaum et al., 1991; Cohen et al., 1997a, 1997b; Fallgatter et al., 1998), it was expected that alcoholics in the present experiment would show deficits primarily in delta and theta oscillatory responses that give rise to the P3 component.
The purpose of the present study was to examine the neural correlates of inhibitory control in alcoholics and in control subjects in terms of oscillatory brain activity in different frequency bands during the performance of a Go/No-Go task. We recorded behavioral responses and electroencephalogram (EEG) while the participants were performing this task. We hypothesized that alcoholics would show abnormalities in response inhibition as elicited by a Go/No-Go paradigm in terms of different brain oscillatory responses, primarily of delta and theta rhythms that might account for the deficient P300 component in alcoholics. It was expected that a comparison of magnitude, spatial and temporal characteristics of these oscillatory responses in alcoholic patients and healthy control subjects would allow us to specify the abnormalities of neural processes related to response inhibition in alcoholics.
2. Materials and Methods
2.1. Subjects
The experimental subjects were 58 alcoholics (33 males, 15 females) with age-range of 19–49 years, while 29 normal volunteers (15 males, 14 females) aged between 18 and 35 years served as controls. The demographic and clinical characteristics of the sample are presented in Table 1. Alcoholics were diagnosed according to DSM IV criteria for alcohol dependence and were recruited from Kings County Hospital, New York. Prior to testing, they had been detoxified in a 30-day treatment program, and none of the subjects was in the withdrawal phase. Controls were recruited either through notices posted in the SUNY Health Science Center, New York, or through newspaper advertisements. Only healthy volunteers without any personal and/or family history of major medical or psychiatric disorders and substance-related addictive illnesses were selected as control subjects.
Table 1.
Demographic and clinical characteristics of the sample.
| VARIABLES | ALCOHOLICS (N=58) | CONTROL (N=29) |
|---|---|---|
|
| ||
| Age (yr) | ||
| Mean | 38.27 | 24.05 |
| SD | 5.77 | 5.56 |
| Range | 19–49 | 18–35 |
|
| ||
| Education (yr) | ||
| Mean | 12.45 | 14.71 |
| SD | 2.18 | 3.16 |
| Range | 5–16 | 4–20 |
|
| ||
| Age of onset of drinking (yr) | ||
| Mean | 14.79 | NA |
| SD | 3.47 | NA |
| Range | 12–26 | NA |
|
| ||
| No. of drinking days per month* | ||
| Mean | 19.54 | 2.10 |
| SD | 10.51 | 2.69 |
| Range | 0–30 | 0–10 |
|
| ||
| No. of drinks† per drinking day* | ||
| Mean | 9.32 | 1.59 |
| SD | 7.14 | 1.78 |
| Range | 0–36 | 0–6 |
Data are for the 6 months prior to the treatment in alcoholic group.
One drink = 1 shot glass of hard liquor; 1 glass of wine; 1 bottle of beer.
NA = Not Applicable.
As an initial screening procedure, all the participants filled out a questionnaire containing details of personal and family history for medical, psychiatric, and addictive disorders. However, alcoholic subjects with past history of psychiatric disorders and with comorbid diagnoses of substance use were also included in this study. The clinical data were obtained using Bard/Porjesz Adult Alcoholism Battery (BAAB), a semi-structured clinical assessment schedule based on DSM IV criteria for the evaluation of clinical details of alcohol dependence and alcohol-related medical problems. Subjects were requested to abstain from alcohol and other CNS-acting substances for 5 days prior to testing. A questionnaire, documenting the drug use (alcohol, marijuana, cocaine, hallucinogens, methadone, tranquilizer, antidepressants, neuroleptics, other prescribed medications, nicotine, and caffeine) over the previous 5 days and the several hours prior to testing, was administered on the day of testing. Further, on the day of testing, all the subjects underwent both urine screen and Breathalyzer test for the purpose of screening for recent drug use. Positive findings on these tests would exclude the subject’s EEG data from any analyses. The Mini Mental State Examination (MMSE) (Folstein et al., 1975) was used to screen the participants for organicity. The subjects who had a history of major medical and neurological conditions including head injury, which would account for organicity, were also excluded from the study. All subjects had normal or corrected normal vision, and none reported hearing loss or impairment. An informed consent explaining the scope and methods of the study was also obtained before conducting the experiment. Experimental procedures and ethical guidelines were in accordance with approval from the Institutional Review Board (IRB).
2.2. Experimental Paradigm
The subjects were seated one meter away from the computer screen, on which the visual stimuli for the task were presented. The visual stimuli subtended a visual angle of approximately 1°, and consisted of: (i) a cross (fixation stimulus), (ii) a circle (Go or No-Go stimulus), and (iii) a dollar sign (reinforcement sign). The circles appeared at any of the four corners of the screen, while the cross and dollar sign appeared at the center of the screen. The circles at the top right and bottom left corners served as Go stimuli, for which the subjects had to respond by pressing a button as quickly as possible, whereas the circles that appeared at the top left and bottom right corners served as No-Go stimuli.
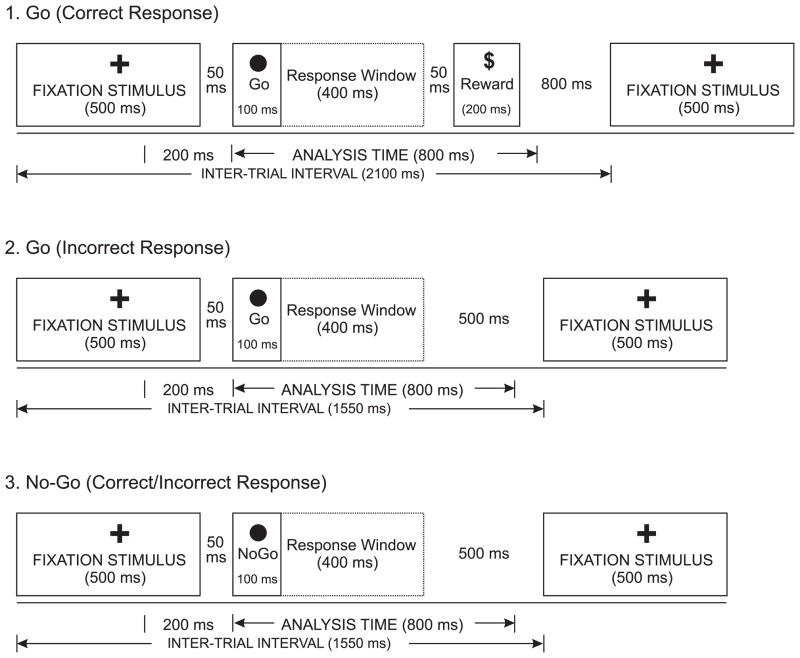
The experimental phase consisted of 100 trials, having 25 circles in each of the four corners of the computer screen presented in a random order. The sequence, exposure time, and interstimulus intervals of the task are illustrated in Figure 1. The subjects were instructed to press a button whenever they saw a circle in either the top right or bottom left corner. The subjects would gain 25 cents for a correct button press and would lose the same amount for an incorrect button press. If the subject pressed correctly, he/she would see a dollar sign to indicate a gain of 25 cents. However, there was no feedback given for incorrect responses. The inter-trial intervals (ITI) for the Go trials with and without Dollar sign (i.e., correct and incorrect Go trials) were 2100 msec and 1550 msec respectively, while the ITI for the No-go trials was 1550 msec (as the Dollar sign never appeared in the No-go trials). The probabilities of occurrence of Go and No-go stimuli were kept equal (50/50), and the order of these stimuli was randomized.
Figure 1.
The illustration of Go/No-Go task, showing (1) Correct response for the Go trials, (2) Incorrect response for the Go trials, and (3) Correct/incorrect responses for the No-Go trials.
2.3. Electrophysiological Data Acquisition
EEG activity was recorded using a 61-lead electrode cap (Electro-cap International, Inc., Eaton, OH) that included 19 channels of the 10–20 International System and 42 additional electrode sites as follows: FPZ, AFZ, AF1, AF2, AF7, AF8, F1, F2, F5, F6, FCZ, FC1, FC2, FC3, FC4, FC5, FC6, FT7, FT8, C1, C2, C5, C6, CPZ, CP1, CP2, CP3, CP4, CP5, CP6, TP7, TP8, P1, P2, P5, P6, POZ, OZ, PO1, PO2, PO7 and PO8 (Electrode Position Nomenclature, American Electroencephalographic Association, 1991). The electrodes were referenced to the nose and the ground electrode was placed on the forehead. The electrooculogram (EOG) was recorded with horizontal and vertical leads placed at the outer canthus and supraorbitally on the left eye. The impedance was maintained below 5 KOhms. The signals were amplified with a gain of 10,000 by a set of amplifiers (Sensorium, Charlotte, VT) with bandpass of 0.02–100 Hz. The data were recorded on a Neuroscan system (Version 4.1) (Neurosoft, Inc., El Paso, TX) with a sampling rate of 512 Hz.
The subjects were seated in a comfortable, reclining chair located in a dim-lit sound-attenuated RF-shielded room (IAC, Industrial Acoustics, Bronx, NY) and were instructed about the task requirements and response pattern. All the subjects were given practice trials in order to learn the task before starting the experimental phase. The practice phase consisted of 20 Go and No-Go trials respectively, and the stimulus presentation was identical to that of the experimental phase as explained in the experimental paradigm. However, during the practice phase, a feedback signal (i.e., a beep) was given whenever the subject’s button-press response was incorrect, and no reward was accrued. Recording was done only during the experimental phase. The subjects were asked to concentrate on the Go and No-Go stimuli rather than on the dollar sign. The total amount gained as reward was not displayed during stimulus presentation. However, the subjects received the full amount at the end of the experiment without deductions for errors, although they were not informed about this while performing the experiment. The behavioral data such as correct and incorrect responses and response time were also recorded.
2.4. Signal Analysis
Detailed investigation of evoked response electroencephalogram data, which generally have a non-stationary character and contain both time-locked and non-time locked events, requires a time-frequency analytical approach. A number of methods exist to calculate time-frequency energy distributions of temporal data, and these include: the short-time Fourier transform; the continuous wavelet transform; and matching pursuit decomposition. Durka et al. (2001a, 2001b) discuss the relative merits of each method. In particular, they point out the tradeoff between time and frequency intrinsic to the short-time Fourier transform method, and the varying time-frequency resolution inherent in the continuous wavelet transform method (e.g., good time resolution and poor frequency resolution in the high frequency region). The matching pursuits (MP) method of Mallat and Zhang (1993) provides good time and frequency resolutions at both high and low frequencies. This is achieved by choosing separable parameters that optimize the representation of structures present in the signal.
The matching pursuit algorithm described by Mallat and Zhang (1993) is an iterative procedure in which a linear expansion of the signal is computed via successive approximations with elements of a highly redundant atom dictionary composed of modulated and translated discrete Gaussians (Gabor functions). After m iterations, the matching pursuit algorithm decomposes the signal f into:
where gn denotes a selected atom from the dictionary and Rm f is the residual data after m iterations. During each iteration the algorithm selects an atom from the dictionary for which the inner product of the atom with the signal, 〈Rm f,gn〉, is largest, and then passes the residual to the next iteration. The algorithm continues until a specified percentage of the signal’s energy is accounted for (99% for the analyses presented here). The energy-frequency-time distribution of the signal can then be calculated through:
in which the term Wgn (t,ω) is the Wigner distribution of the atom gn (t,ω), t denotes time and ω frequency.
Durka et al. (2001a) describe modifications to the Mallat and Zhang (1993) algorithm, which removes possible bias caused by fixed a priori parameterization of the atoms in the dictionary, through the use of stochastic dictionaries. In this method the parameters (time, frequency and scale) of the subset of atoms to be used (selected from the infinite waveform dictionary) are randomized prior to each decomposition. This negates the requirement for a fixed subsampling of the parameter space and possible statistical bias. This method was implemented here to study event related brain oscillations using code made available by Durka et al. (2001b) at http://brain.fuw.edu.pl/~mp.
Following the methods outlined in Tallon-Baudry and Bertrand (1999), energy-frequency-time distributions (calculated via matching pursuits) of the event-related responses have been examined. The total energy response is acquired by calculation of the average of individual trial time-frequency-energy distributions – this average enhances structures that occur in a similar time-frequency region, as related to the stimulus onset and irrespective of their phase relations. For comparison purposes, event-related desynchronization and synchronization (ERD/ERS) time courses, following Pfurtscheller and Lopes da Silva (1999), were calculated for eight predetermined frequency bands: Delta (1.0–3.0 Hz), Theta (3.5–7.0 Hz), Alpha 1 (7.5–9.0 Hz), Alpha 2 (9.5–12.0 Hz), Beta 1 (12.5– 16.0 Hz), Beta 2 (16.5–20.0 Hz), Beta 3 (20.5–28.0 Hz), and Gamma (28.5–50.0 Hz). An epoch length of 800 msec following the stimulus onset along with 200 msec prestimulus EEG segment was used for the matching pursuits analysis (Figures 3 & 5). The maximum threshold amplitude to remove artifacts was 100 3V and the minimum number of artifact-free trials was kept at 15 for the analysis. The trials with incorrect responses (i.e., button-press responses to No-Go trials and omissions of responses to Go trials) were removed from the analyses. The area under each of the energy curves was calculated via summation for the time course of 100 ms post-stimulus between 300–400 ms for Delta, Theta, and Alpha frequencies, and between 550–650 ms for Beta and Gamma frequencies, based on the peak values in the distributions.
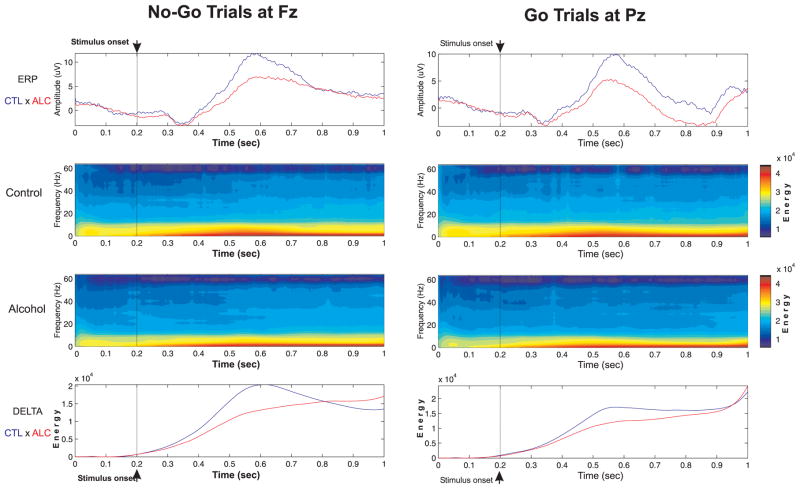
Figure 3.
The ERP waveforms, frequency-time-amplitude plots, and energy curves of Delta power during No-Go and Go trials in controls and alcoholics.
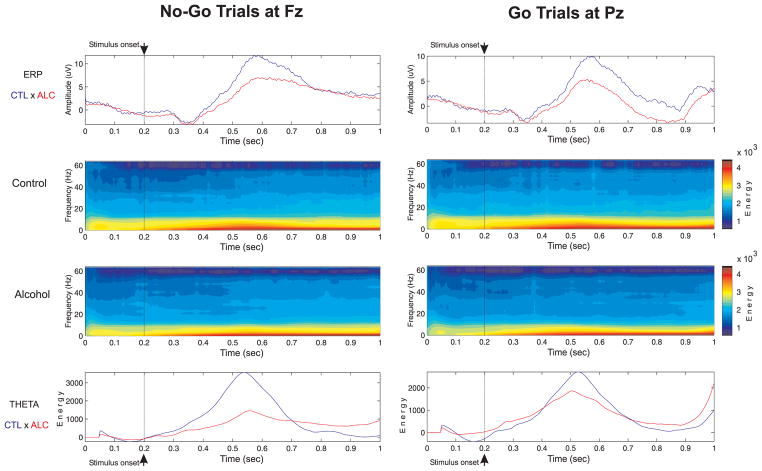
Figure 5.
The ERP waveforms, frequency-time-amplitude plots, and energy curves of Theta power during No-Go and Go trials in controls and alcoholics.
2.5. Statistical Analysis
The objective was to compare the total band power (obtained from the area under the energy curve) of each of the eight different frequency bands between alcoholic and control subjects for No-Go as well as Go trials. The study design included 2 groups (alcoholics, n = 58; controls, n = 29), 2 trial conditions (No-go; Go), and 8 frequency bands (Delta, Theta, Alpha 1, Alpha 2, Beta 1, Beta 2, Beta 3, Gamma).
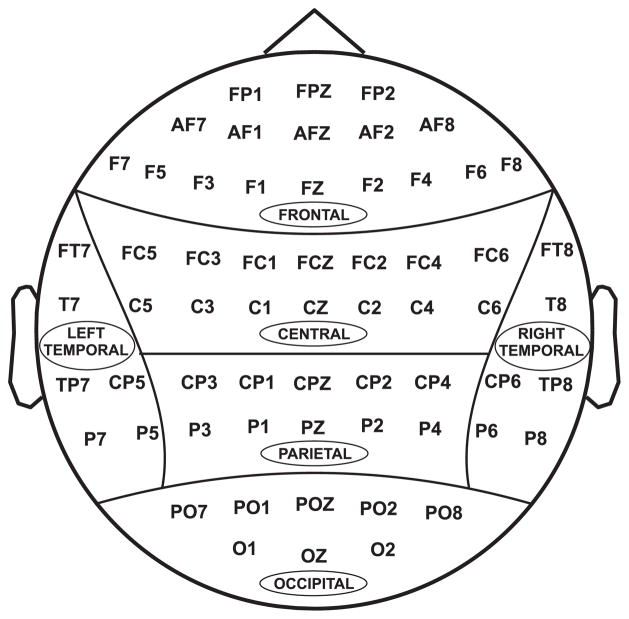
All 61 electrodes were grouped into six scalp regions, as shown in Figure 2. Initially, the Multivariate Analysis of Variance (MANOVA) of each band power on group, age, gender, and their interactions was computed. Since there was no significant gender effect on band power, the Multivariate Analysis of Covariance (MANCOVA), having age as a covariance, was performed for comparing each band power between alcoholic and control group on each scalp region separately. The demographic and behavioral data were analyzed using t-test and Chi-Square test.
Figure 2.
Regional grouping of electrodes: (1) Frontal, (2) Central, (3) Parietal, (4) Occipital, (5) Left-temporal, and (6) Right-temporal.
3. Results
The focus of this study is the comparison between control and alcoholic subjects. Therefore, we only report main effects and those interactions that involve group as a factor. The comparison between trial conditions is beyond the scope of this study.
3.1. Demographic and Behavioral data
The average age of alcoholics was significantly higher than that of controls (t = −10.97; p = 0.000). The proportion of male subjects were higher in alcohol group (χ2 = 4.371; p = 0.037). The alcoholics were found relatively less educated than controls (t = 4.00; p = 0.000). The MMSE scores of alcoholics (mean = 27.41; SD = 2.33) were significantly lesser (t = 3.22; p = 0.002) than that of controls (mean = 28.87; SD = 1.20). However, there was no significant difference in the reaction time (t = 0.970; p = 0.335) between controls (mean = 312.80 msec; SD = 41.11) and alcoholics (mean = 305.47 msec; SD = 29.36). Although the error rates of button press responses in Go and No-Go trials were higher for alcoholics [mean = 6.76, SD = 5.68 (Go); mean = 2.81, SD = 5.60 (No-Go)] than for controls [mean = 4.97, SD = 3.60 (Go); mean = 1.10, SD = 1.37 (No-Go)], this difference was not statistically significant [t = 1.549, p = 0.125 (Go); t = 1.613, p = 0.110 (No-Go)]. However, the total response error was significantly (t = 2.062, p = 0.042) higher in alcoholics (mean = 9.57, SD = 8.66) than in controls (mean = 6.07, SD = 4.06).
3.2. Band Power: Between-Group Comparisons
Significant group differences were observed only in the Delta and Theta bands. The control and alcoholic subjects showed no significant differences in alpha, beta, and gamma band power. Therefore the results obtained in delta and theta power have been summarized.
3.2.1. Delta Responses
A significant reduction in the delta responses of alcoholics during No-Go and Go trials is illustrated in Figure 3. The results of the delta power between the two groups (by MANCOVA) on delta power are summarized in Table 2. In the No-Go trials, the alcoholics showed significantly less mean delta power in frontal, central, parietal, left-temporal and right-temporal regions as compared to control subjects. In the Go trials, the significant reduction in delta power was observed at frontal, parietal, occipital, and right-temporal regions. The post-hoc comparisons of mean delta power between controls and alcoholics in each of the electrodes (after being adjusted for multiple comparisons) during No-Go and Go trials are shown in Figure 4. Alcoholics manifested significantly lower delta power in several electrodes of different regions during No-Go as well as Go trials.
Table 2.
The comparison of Delta power (μV2) between control and alcohol group for the No-Go and Go trials (using MANCOVA).
| REGION | NO-GO | GO | ||
|---|---|---|---|---|
| F-value | p-value | F-value | p-value | |
| Frontal | 2.837 | 0.000*** | 1.798 | 0.010* |
| Central | 1.744 | 0.019* | 1.518 | 0.060 |
| Parietal | 2.271 | 0.003** | 1.909 | 0.015* |
| Occipital | 1.281 | 0.216 | 1.746 | 0.044* |
| Left-Temporal | 2.427 | 0.006** | 1.652 | 0.083 |
| Right-Temporal | 2.605 | 0.003** | 2.333 | 0.009** |
p < 0.05
p < 0.01
p < 0.001
Figure 4.
The mean Delta power (μV2) during No-Go and Go trials in controls and alcoholics.
3.2.2 Theta Responses
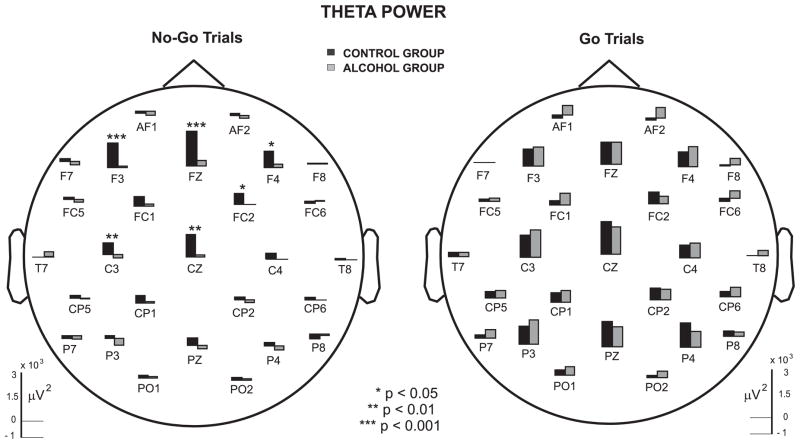
Figure 5 illustrates that there is a marked reduction in theta activity of alcoholics as compared to controls. The statistical comparison of theta power between controls and alcoholics, based on MANCOVA, is shown in Table 3. During the No-Go trials, theta power was significantly reduced in alcoholics at the frontal region. While alcoholics showed reduction in the average values of theta power in central and other regions, this was not statistically significant due to larger variance in band power values. In contrast, in the Go trials, there was no significant difference observed between controls and alcoholics. However, although there was a trend toward significance in frontal and central regions during Go trials, the post-hoc analysis showed that none of the individual electrodes were significant, as shown in Figure 6. It can be observed that alcoholics manifest significantly lower theta power than controls in specific electrodes in frontal and central regions during No-Go trials.
Table 3.
The comparison of Theta power (μV2) between control and alcohol group for the No-Go and Go trials (using MANCOVA).
| REGION | NO-GO | GO | ||
|---|---|---|---|---|
| F-value | p-value | F-value | p-value | |
| Frontal | 1.746 | 0.014* | 1.472 | 0.063 |
| Central | 1.375 | 0.117 | 1.441 | 0.087 |
| Parietal | 1.436 | 0.114 | 1.003 | 0.463 |
| Occipital | 0.843 | 0.635 | 0.561 | 0.909 |
| Left-Temporal | 0.423 | 0.953 | 0.767 | 0.684 |
| Right-Temporal | 1.291 | 0.228 | 1.235 | 0.263 |
p < 0.05
Figure 6.
The mean Theta power (μV2) during No-Go and Go trials in controls and alcoholics.
4. Discussion
The present study was designed to examine the oscillatory neural responses in a Go/No-Go paradigm in alcoholics and nonalcoholic control subjects. The decomposition of event-related EEG into different frequencies of oscillatory responses using Matching Pursuits (MP) produced distinct energy curves for both control and alcohol groups. The energy curves for the delta and theta bands are illustrated in Figures 3 & 5. The results yielded three major findings: 1) the alcoholic subjects showed a significantly lower band power in delta as well as theta oscillations, 2) the reduction in band power activity of alcoholics was more robust in No-Go trials as compared to Go trials, and 3) the reduction in fronto-centrally distributed theta activity during No-Go responses in alcoholics was prominent only at the frontal region. Although these three findings are inter-related, each emphasizes a different aspect of impairment in diagnosed alcoholic subjects. The first finding focuses mainly on the deficient oscillatory responses in alcoholic individuals during the Go/No-Go task, the second indicates the impairment of inhibitory control in alcoholics, whereas the third finding is suggestive of frontal deficits in alcoholics as compared to nonalcoholic control subjects. In general, at the level of information processing, the deficient oscillatory responses in the No-Go condition would indicate a deficit in frontal lobe functions that involve response inhibition and executive control.
4.1. Functional and neural correlates of Delta and Theta oscillations
The findings in the present study of deficient responses in delta and theta activity in alcoholics, particularly in the No-Go condition, imply a dysfunction in the cognitive as well as neural correlates that mediate and/or cause these oscillations. According to Karakas et al. (2000a), cognitive functions are represented by the integrative activity of neuroelectric oscillations that occur during the parallel processing of neural networks. They hypothesized that the delta response represents degrees of consciousness while the theta oscillation represents different amounts and forms of attention. Studies on normal individuals indicated that the delta response is mainly related to signal detection and decision making (Basar-Eroglu et al., 1992; Basar, 1999; Basar et al., 2001a). It is reported that delta activity is generated by cortico-cortical interactions (Devrim et al., 1999), and is a product of the distributed network system of the brain (Basar-Eroglu et al., 1992; Basar, 1999). On the other hand, event-related theta oscillations are related to cortico-hippocampal (Basar, 1999; Miller, 1991) or fronto-limbic interactions (Karakas et al., 2000b), and are associated with a complex set of cognitive processes including alertness, arousal or readiness (Basar et al., 1999), episodic encoding and retrieval processes (Klimesch et al., 1994, 1997a), and selective attention and short-term memory (Basar-Eroglu et al., 1992; Demilrap and Basar, 1992; Karakas, 1997; Klimesch, 1999). Therefore, the alcoholic individuals who have suppressed delta as well as theta responses are likely to show deficits in cognitive functions that are mediated by these oscillatory processes. There is a body of neuropsychological evidence that supports this view, by showing a wide range of cognitive impairments including attention, working memory, encoding and retrieval processes, and other deficits of executive functions in alcohol dependent individuals (Tarter, 1976, 1980; Miller, 1985; Nixon and Bowlby, 1996; Ihara et al., 2000; Noel et al., 2001; Moselhy et al., 2001; Ratti et al., 2002).
According to Karakas et al. (2000a, 2000b), the basic phenomenon of brain neuroelectric processes is not the ERP but the event-related oscillations. Thus, the P300 is considered to be the outcome of the ‘interplay’ between the theta and the delta oscillations. An increase of 600% of delta response and, 200% of theta response has been evoked by oddball experiments (Basar, 1999). Like P300, delta and theta oscillations are observed across different modalities (Schurman et al., 2001; Yordanova et al., 2002). The theta oscillations have been rather well studied in behaving rats (Bland, 1986; O’Keefe and Recce, 1993; Skaggs et al., 1996; Wang, 2002) and in humans (Klimesch et al., 2000, 2001b; Kahana et al., 1999, 2001; Tesche and Karhu, 2000; Basar et al., 2001d). The studies that examined frontal midline theta (Fm θ) reported that complex mental tasks and bimodal stimulations produce this activation in human subjects (Mizuki et al., 1980, 1983; Lang et al., 1987; Westphal et al., 1990, Demiralp and Basar, 1992; Basar et al., 2001d). Miller (1991) theorized that the major role of theta rhythm is in associative and integrative brain function, and that theta activity in frontal regions is associated with hippocampal theta rhythm. It is worth mentioning that theta activity is also involved in controlling the reactivity of human frontal lobes (Basar, 1998, 1999). Studies done in our laboratory have recently demonstrated a marked attenuation in frontal theta in alcoholic subjects as well as in young nonalcoholic offspring of alcoholic fathers during a complex task involving mental calculation [Suresh et al. (a, b) in preparation]. The finding of the present study that theta activity was markedly suppressed in alcoholics at more anterior regions (i.e., frontal and central electrodes) during No-Go trials, reinforces the notion that that the alcoholic subjects have deficient cognitive functions as well as dysfunctional neural substrates that mediate these functions.
Hippocampal inhibitory interneurons play a central role in theta activity by rhythmically inhibiting pyramidal cells (Chapman and Lacaille, 1999). During each theta cycle, GABAergic afferents may disinhibit pyramidal cells by inhibiting tonically active interneurons (Fox, 1989, Ylinen et al., 1995, Toth et al., 1997). The theta activity may also involve the cholinergic excitation of interneurons (Pitler and Alger, 1992; Behrends and ten Bruggencate, 1993; William and Kauer, 1997; McMahon et al., 1998; Wang, 2002). Moreover, the importance of cholinergic mechanisms in cognitive functioning has long been recognized. Lesions of the fimbria-fornix, which conveys septohippocampal cholinergic and GABAergic fibers to the hippocampus interfere with both learning and memory and also with the generation of the theta rhythm (Brito and Brito, 1990; Givens and Sarter, 1997; Wu et al., 2000). However, there are only few studies on the neurochemical substrates of delta oscillations. Joho et al. (1999) reported that cortical interneurons with Kv3.1 (a voltage-gated, fast activating/deactivating potassium (K+) channel) are involved in the generation and maintenance of cortical fast gamma as well as slow delta oscillations. It is also believed that the activity of Kv3.1 channels may also influence the amount of GABA released from fast-spiking GABAergic interneurons.
The role of the GABAA receptor system in modulating the behavioral and pharmacological effects of ethanol has been well studied (Ticku, 1990; Korpi, 1994; Mihic and Harris, 1996; Grobin et al., 1998; Chester and Cunningham, 2002). Linford-Hughes et al. (1998) reported that abstinent alcohol-dependent subjects had decreased levels of GABA-benzodiazepine receptors as compared to non-alcohol-dependent subjects within the frontal, parietal, and temporal cortices. The association between dysfunction in the cholinergic system and cognitive impairment in chronic alcoholism has also been reported (Hodges et al., 1990; Freund and Ballinger, 1991; Arendt, 1994; Grunberger et al., 1998). These findings reinforce the notion that alcohol dependent individuals are characterized by deficient neurobiological and cognitive systems that can be elicited through electrophysiological, neurobiological, and neuropsychological procedures.
4.2. Disinhibition and Alcoholism
Our finding that the oscillatory responses associated with the No-Go condition are compromised in alcoholics is supportive of the view that alcoholism is frequently associated with poor inhibitory mechanisms at the neural, cognitive, and behavioral levels. The theta activity at the cellular level is produced by the inhibitory interneurons of the hippocampus (Chapman and Lacaille, 1999). It has also been demonstrated that frontal midline theta serves a response controlling function (cf. Basar et al., 2001d). Further, it was also reported that the reduced theta and low alpha support the failure of the inhibitory control at the cognitive level (Klimesch et al., 2000), a notion that supports the disinhibition hypothesis. According to sensory-inhibition theory, theta is associated with generalized inhibition of non-relevant sensory systems during perceptual processing (Sainsbury, 1998). Many studies have demonstrated that alcoholics perform poorly in inhibition-related psychophysiological as well as neuropsychological tasks such as Go/No-Go, Stop-Signal, Stroop paradigms, and Continuous Performance Test (Cohen et al., 1997a; Fallgatter et al., 1998; Finn et al., 1999). Moreover, a number of studies have reported the presence of disinhibitory behaviors and externalizing psychopathology in alcoholics as well as in individuals at risk to develop alcohol dependence (e.g., Pihl et al., 1990; Finn et al., 1994; McGue et al., 1997; Conrod et al., 1997). Since P300 has been considered as an index of Central Nervous System (CNS) inhibition (Begleiter and Porjesz, 1999), the P300 amplitude reduction in alcohol dependent individuals perhaps reflects CNS hyperexcitability and disinhibition.
There are two major, empirically based theories on the development of inhibitory processes at the cognitive and behavioral level: (1) The inefficient-inhibition theory proposed by Bjorklund and Harnishfeger (1990, 1995), and (2) The susceptibility-to-interference theory held by Dempster (1992, 1993). According to the inefficient-inhibition theory, inhibitory processes block the spread of activation that would have otherwise been executed, and are linked to the maturation of the nervous system. These inhibitory processes become more evident during the course of child development, resulting in less irrelevant information entering working memory, and thus increasing its functional capacity. On the other hand, the susceptibility-to-interference theory, adopting a neuropsychological perspective, argues that inhibition has a variety of operating characteristics that may vary on temporal (e.g., proactive, coactive, and retroactive), formal (motoric, perceptual, and linguistic), and spatial (internal and external) dimensions. These dimensions have different developmental trajectories thereby producing a stage-like quality to the development of a child’s sensitivity to interference. However, both theories assume that the development of inhibitory capacity is closely associated to maturational changes of the frontal lobes.
According to Vogel-Sprott et al. (2001), the lack of behavioral inhibition under the effect of alcohol can be due to (1) decreased relative salience of No-Go versus Go signals, (2) decreased working memory capacity, or (3) decreased inhibition system activity. Relating disinhibition and alcoholism, Begleiter and Porjesz (1999) proposed a model that the predisposition to develop alcoholism involves an initial, general state of CNS disinhibition/hyperexcitability that is characterized as a homeostatic imbalance between excitatory and inhibitory neural mechanisms. Finn et al. (1999) proposed another model in which the executive processes of working memory and conditional associative learning are involved in behavioral inhibition. They showed that individuals with low working memory capacity were more susceptible to alcohol’s effect of increasing impulsive behavior suggesting that alcohol reduces the capacity of working memory to modulate response inhibition. These models and findings strongly support our view that disinhibition plays a central role in the genesis and maintenance of alcoholism and comorbid symptoms.
4.3. Frontal lobe and Alcoholism
We hypothesize that the inhibitory deficits observed in alcoholics may be due to a dysfunctional frontal network system. This hypothesis is based on the following observations: Firstly, since response inhibition is a function of frontal lobes (Fuster, 1989), the deficient oscillatory activity during No-Go condition would imply a frontal lobe dysfunction in terms of information processing. This observation can be supported by the ERP findings that the fronto-central maximum No-Go potentials (No-Go P300) were suppressed in alcoholic subjects as compared to controls (Pfefferbaum et al., 1991; Cohen et al., 1997a; Fallgatter et al., 1998). Secondly, as our findings show, No-Go responses associated with theta oscillations in alcoholic subjects were found to be significantly attenuated in the frontal region. The ‘frontal theta’, which is a major oscillation of the frontal cortex and is involved in response controlling function (Basar et al., 2001d), has been reported to be suppressed in alcohol dependent individuals (Suresh et al. (a), in preparation). Thirdly, a number of studies provide strong evidence for frontal lobe dysfunction in alcoholics at neuropsychological, neurophysiological, neurochemical, and neuroradiological levels (for a review, Moselhy et al., 2001). And lastly, in recent years, the view that executive functions of the prefrontal cortex are dysfunctional in alcoholic patients is gaining prominence (Giancola and Moss, 1998; Finn et al., 1999; Ihara et al., 2000; Noel et al., 2001; Ratti et al., 2002).
4.3.1. Neuro-cognitive Models on Alcoholism
There have been three main hypotheses on the association between the effects of alcohol on brain structures (Noel et al., 2001; Ratti et al., 2002): (i) Right-hemisphere abnormality, (ii) Diffused/global brain dysfunction, and (iii) Frontal lobe dysfunction. According to the right-hemisphere hypothesis, the nondominant hemisphere is more affected by the effects of alcohol (Bertera and Parsons, 1978; Nicolas et al., 1993; Beatty et al., 1996). On the other hand, the diffused brain dysfunction hypothesis postulates that alcohol causes generalized deficits that involve many structures of the brain and the dysfunction resembles that of premature aging (Chelune and Parker, 1981; Parsons, 1994; Tivis et al., 1995). Lastly, the frontal deficit hypothesis maintains that the anterior regions of the brain (i.e., frontal lobe structures) are mainly involved in the effects of alcohol (Tuck and Jackson, 1991; Adams et al., 1993; Cieslieski et al., 1995; Giancola and Moss, 1998; Noel et al., 2001).
The frontal dysfunction hypothesis in alcoholism has been frequently discussed (e.g., Ihara et al., 2000; Ratti et al., 2002) and supported by many neuropsychological findings (e.g., Tarter, 1975; Krill et al., 1997; Dao-Castellana et al., 1998; Noel et al., 2001). It may be interesting to note that the frontal deficit hypothesis can explain the basic premises of the other two hypotheses (i.e., the right-hemisphere hypothesis and the diffused dysfunction hypothesis) by the virtue of two established facts: 1) frontal lobes have rich reciprocal connections with other cortical and subcortical areas of the brain (Fuster, 1989; Mesulam, 2000), and 2) prefrontal (executive) functions are involved in all the controlled processes of willed actions associated with any of the cognitive functions (Godefroy et al., 1999; Badgaiyan, 2000; Collette and Van der Linden, 2002). Therefore, it is possible that the deficits observed in cognitive tests that were meant to elicit functions of other areas (e.g., visuospatial functions of parietal lobes) could have been directly or indirectly associated with frontal network systems, and thus would have influenced the profile of impairments in alcoholics. For example, Sullivan et al. (1992) reported that while copying Rey-Osterrieth complex figure, alcoholics displayed abnormality in both organizational strategy and accuracy, whereas schizophrenics showed impairments only in strategy formation. They report that the anatomical basis for such dysfunction may stem from the regions of frontal lobes, or of fronto-striatal circuitry, or of corticocortical circuitry of the frontal and parietal lobes.
Giancola and Moss (1998) postulated a cognitive-neurobehavioral model of alcoholism implicating executive cognitive functioning and the fronto-striatal system as the important determinants in the etiology of alcoholism and its comorbid disorders. Considering the findings that alcoholics showed impairments in different executive functions such as planning (e.g., Pishkin et al., 1985), abstraction (e.g., Parker et al., 1991), attention (e.g., Smith and Oscar-Berman, 1992), shifting of attention or set-shifting (e.g., Sullivan et al., 1993), cognitive flexibility (e.g., Glenn et al., 1993), and concept generation or fluency (e.g., Beatty et al., 1993), it is plausible that the cognitive, motor and behavioral disinhibition observed in alcoholics might be caused by poor executive control of frontal lobes. Noel et al. (2001) substantiates this claim by suggesting that the deficits in alcoholic subjects are mainly observed in the controlled processes where the inhibition of dominant response occurs and executive functions are invoked (Godefroy et al., 1999).
However, it is to be noted that a compromised executive cognitive functioning is not specific to alcoholism alone but also observed in diverse psychopathological conditions such as schizophrenia (Evans et al., 1997; Mahurin et al., 1998; Hutton et al., 1998; Krabbendam et al., 1999; Velligan and Bow-Thomas, 1999), depression (Fossatti et al., 2002); obsessive compulsive disorder (Purcell et al., 1998), attention-deficit hyperactivity disorder (Kempton et al., 1999; Shallice et al., 2002) and autism (Pennington and Ozonoff, 1996). On the other hand, the components of executive functions that contribute to a pathology might vary for different disorders (Pennington and Ozonoff, 1996). Besides, there has been no consensus in the definition and categories of executive functions (Stuss and Alexander, 2000; Tirapu-Ustarroz et al., 2002). Therefore, the models that emphasized only the executive functions in explaining alcoholism and comorbid disorders have been discouraged (Bates, 2000). However, a neuro-cognitive model of alcoholism that integrates diverse aspects of cognitive dysfunction and comorbidity has not yet been proposed. A hypothetical neurocognitive model has been thus attempted in the current study to explain and integrate the frontal deficits in alcoholism.
4.3.2. Prefrontal Network Systems Model of Alcoholism
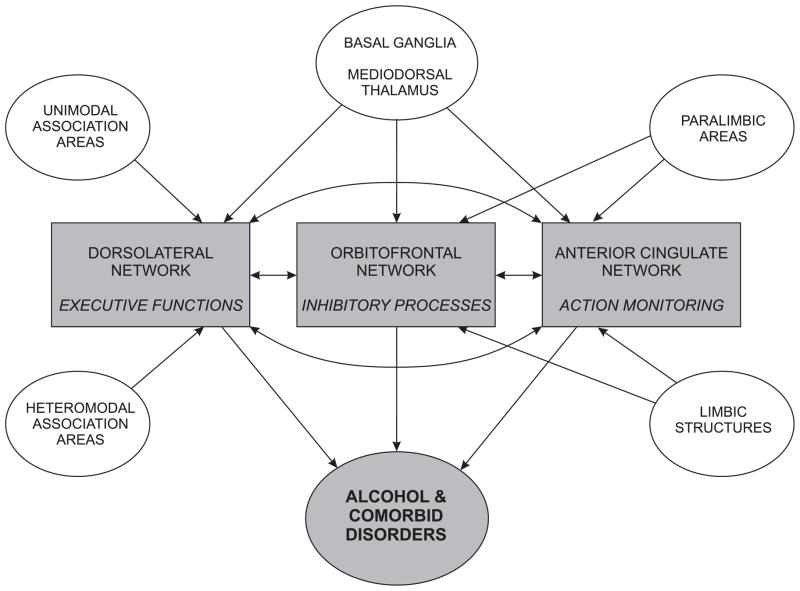
We propose that the attenuated oscillatory responses associated with inhibitory processes (No-Go responses) in alcoholics may have been caused by three major network systems of the prefrontal cortex: (i) the dorsolateral executive network system, responsible for executive functions, (ii) the orbitofrontal inhibitory network system that mediates inhibitory processes, and (iii) the anterior-cingulate action-monitoring network system that is involved in action monitoring and error processing (Cummings, 1993; Masterman and Cummings, 1997; Tekin and Cummings, 2002). This model is an outcome of three important observations. First, the functional fractionation of the Go/No-Go task involves executive functions, inhibitory processes, and action-monitoring components. Secondly, all three prefrontal networks are highly interactive and mutually contributory, and the dysfunction in one system would disrupt the effective functioning of the other systems. Lastly, alcoholics are known to have deficits in functions involving all three prefrontal systems. It is hypothesized that any dysfunction within the network system would affect the functional integrity of one or more of the systems, and thus would manifest an abnormality in information processing and behavior. In alcoholics, the diverse symptomatology and comorbidity may differentially involve the neural structures to display a particular clinical picture.
The schematic diagram of this model is shown in Figure 7. The main connections and interactions of these circuits among themselves and with other regions of the brain have been outlined. The dorsolateral network system which receives input from unimodal and multimodal association areas of the cortex, basal ganglia and mediodorsal thalamus has reciprocal connections with the other two network systems, and predominantly mediates executive cognitive functions. The limbic structures, paralimbic areas, basal ganglia and mediodorsal thalamus give input to orbitofrontal as well as anterior cingulate network systems. The dysfunction in the orbitofrontal network results in disinhibitory behavior, emotional lability and impulsivity, while abnormality in the anterior cingulate network would cause deficits in error correction, self-regulation, and apathy. As explained earlier, alcoholics have deficits in executive functions, inhibitory processes, and action monitoring, thus implicating all of these prefrontal networks. It can be suggested that the orbitofrontal circuit that is often implicated in disinhibition and addictive behavior is also predominantly influenced by the dorsolateral prefrontal circuit (Hoaken et al., 1998), and by the anterior cingulate circuit (Ridderinkhof et al., 2002; van Veen and Carter, 2002; Luu et al., 2003). The executive functions are involved in the ongoing regulation of behavioral inhibitory system (Finn et al., 1999), and some authors include inhibitory control as part of the executive functions (e.g., Norman and Shallice, 1986; Baddeley, 1996; Collette and Van der Linden, 2002). In monkey studies using the Go/No-Go task, damage to the dorsolateral prefrontal cortex has been shown to impair the response inhibition function (Iversen and Mishkin, 1970; Butters et al., 1973; Sasaki et al., 1989). In humans, the imaging studies showed activation in different brain regions including dorsolateral prefrontal cortex during the Go/No-Go task (Kawashima et al., 1996; Casey et al., 1997). However, different aspects of this model have to be explored and validated by further studies.
Figure 7.
The schematic diagram showing interactions within and among three major prefrontal network systems in producing alcoholism and comorbid disorders.
It may be postulated that each of the prefrontal network systems are differentially involved in producing a typical clinical picture as well as comorbid symptoms in alcoholics. On the background of the theory of oscillatory neural assemblies that involves parallel distributed processing (Basar, 1998), oscillatory responses might reveal the network mechanisms involved in specific cognitive functions as well as in different disorders. Hence, the experimental paradigms involving electrophysiological (ERPs and oscillations) as well as imaging studies on tasks accounting for fractionated components of these major cognitive functions in normals as well as in alcoholics would help elucidate the neural mechanisms involved in alcoholism and comorbid disorders. Such studies on a wide range of behavioral and addictive disorders might determine the specificity of these markers. On the other hand, the studies of individuals at high risk to develop alcoholism might ascertain the role of heredity in cognitive functions as well as in the causation of alcoholism.
4.4. State or Trait?
The state versus trait controversy is common to any behavioral disorder wherein the etiology and pathogenesis is polymorphic. A meta-analysis on the twin and family studies of the human EEG and ERPs concluded that genomic variation contributes significantly to individual differences in these measures (van Beijsterveldt and van Baal, 2002). It is also reported that human brain oscillations are highly heritable, and the average heritability of delta, theta, alpha, beta was found to be 76, 89, 89, and 86% respectively (Van Beijsterveldt, 1996; Porjesz et al., 2002a, 2002b). Anokhin et al. (2001) showed a strong heritability of slow EEG rhythms that contribute to P300. Further, a number of authors suggest that the predisposition to develop alcoholism is largely inherited (e.g., Cloninger, 1987; Hesselbrock, 1995; Porjesz and Begleiter, 1998; Begleiter and Porjesz, 1999). The studies on individuals at high risk for developing alcoholism as well as the findings from linkage analysis strengthen this notion (e.g., Begleiter et al., 1984, 1998; Polich et al., 1994; Porjesz et al., 2002a, b). Moreover, the high-risk individuals are reported to have neuropsychological impairments (e.g., Peterson et al., 1992; Knop et al., 1993), and a dysfunctional GABA-benzodiazepam receptor system (e.g., Volkow et al., 1995). Recently, it has been reported that chronic alcoholism in humans alters the expression of GABAA genes (Lewohl et al., 1997), mitochondrial genes (Fan et al., 1999), and myelin related genes (Lewohl et al., 2000).
Related to Go/No-Go paradigm, Cohen et al. (1997b) reported that the individuals at high-risk to develop alcoholism showed decreased P300 amplitude, thus suggesting a genetic influence of the No-Go potential. On the issue of whether inhibitory process measured in this paradigm is a trait or state variable in alcoholism, it should be noted that developmental theories of inhibition suggest that inhibitory ability is a trait variable that can be identifiable in different developmental stages (van der Molen, 2000). Another line of evidence for this argument comes from the presence of externalizing or disinhibitory psychopathology in alcoholics and offspring at high risk to develop alcoholism (Zucker and Gomberg, 1986; Regier et al., 1990; Sher and Trull, 1994; Pihl and Bruce, 1995; McGue et al., 1997). Recently, Begleiter and Porjesz (1999) provided neurophysiological, neurochemical, and genetic evidence to theorize that the genetic predisposition to develop alcoholism involves disinhibition/hyperexcitability, a state of homeostatic imbalance of CNS caused by a disequilibrium in the homeostatic mechanisms that control the critical balance between excitation and inhibition.
Although the results of the present study allow us only to draw a moderate inference related to the nature of alcoholism, based on the above mentioned views and findings, we propose that, like P300 amplitude, the attenuated delta and theta responses in alcoholics found during No-Go trials might serve as endophenotypic markers. However, replication studies in different subgroups of alcoholic and high-risk subjects are suggested in order to confirm the findings and implications of the present study. Moreover, the longitudinal studies in children at high-risk to develop alcoholism would help solve the question as to whether these dysfunctions are due to chronic use of alcoholism or due to inherent predisposition. In conclusion, the results of the present study suggest that the deficient oscillatory responses found in alcoholics during No-Go trials is associated with impairments in frontal inhibitory control and information processing mechanisms. The proposed neuro-cognitive model explains the involvement of frontal network systems in the development of alcoholism. The task-specific brain oscillations, considered to be the functional correlates of cognitive systems, may also prove to be an endophenotypic marker in alcoholism.
Acknowledgments
The authors are grateful to the valuable assistance of Edward Babington, Aquanette Sass, Carlene Haynes, Joyce Alonzia, Aleksey Dumer, and Alyson Wahl. This study was supported by the NIH grant # 5 RO1 AA002686 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA).
References
- Adams KM, Gilman S, Koeppe RA, Kluin KJ, Brunberg JA, Dede D, Berent S, Kroll PD. Neuropsychological deficits are correlated with frontal hypometabolism in positron emission tomography studies of older alcoholic patients. Alcohol Clin Exp Res. 1993;17:205–210. doi: 10.1111/j.1530-0277.1993.tb00750.x. [DOI] [PubMed] [Google Scholar]
- Alterman AI, Tarter RE. The transmission of psychological vulnerability. Implications for alcoholism etiology. J Nerv Ment Dis. 1983;171:147–154. doi: 10.1097/00005053-198303000-00003. [DOI] [PubMed] [Google Scholar]
- Alterman AI, Tarter RE. An examination of selected typologies. Hyperactivity, familial, and antisocial alcoholism. Recent Dev Alcohol. 1986;4:169–189. [PubMed] [Google Scholar]
- Anokhin AP, van Baal GC, van Beijsterveldt CE, de Geus EJ, Grant J, Boomsma DI. Genetic correlation between the P300 event-related brain potential and the EEG power spectrum. Behav Genet. 2001;31:545–554. doi: 10.1023/a:1013341310865. [DOI] [PubMed] [Google Scholar]
- Arendt T. Impairment in memory function and neurodegenerative changes in the cholinergic basal forebrain system induced by chronic intake of ethanol. J Neural Transm (Suppl) 1994;44:173–187. doi: 10.1007/978-3-7091-9350-1_13. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Exploring the central executive. Q J Exp Psychol. 1996;49A:5–28. [Google Scholar]
- Badgaiyan RD. Executive control, willed actions, and nonconscious processing. Hum Brain Mapp. 2000;9:38–41. doi: 10.1002/(SICI)1097-0193(2000)9:1<38::AID-HBM4>3.0.CO;2-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Basar E. EEG-Brain Dynamics: Relation between EEG and Brain Evoked Potentials. Elsevier; New York: 1980. [Google Scholar]
- Basar E. Principles and Approaches. Springer; Berlin: 1998. Brain Function and Oscillations. I. Brain Oscillations. [Google Scholar]
- Basar E. Neurophysiology and Cognitive Processes. Springer; Berlin: 1999. Brain Function and Oscillations. II. Integrative Brain Function. [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Are cognitive processes manifested in event-related gamma, alpha, theta and delta oscillations in the EEG? Neurosci Lett. 1999;259:165–168. doi: 10.1016/s0304-3940(98)00934-3. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Brain oscillations in perception and memory. Int J Psychophysiol. 2000;35:95–124. doi: 10.1016/s0167-8760(99)00047-1. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol. 2001a;39:241–248. doi: 10.1016/s0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Basar E, Schurmann M, Basar-Eroglu C, Demiralp T. Selectively distributed gamma band system of the brain. Int J Psychophysiol. 2001b;39:129–135. doi: 10.1016/s0167-8760(00)00136-7. [DOI] [PubMed] [Google Scholar]
- Basar E, Schurmann M, Basar-Eroglu C, Karakas S. Alpha oscillations in brain functioning: an integrative theory. Int J Psychophysiol. 1997;26:5–29. doi: 10.1016/s0167-8760(97)00753-8. [DOI] [PubMed] [Google Scholar]
- Basar E, Schurmann M, Demiralp T, Basar-Eroglu C, Ademoglu A. Event-related oscillations are ‘real brain responses’--wavelet analysis and new strategies. Int J Psychophysiol. 2001c;39:91–127. doi: 10.1016/s0167-8760(00)00135-5. [DOI] [PubMed] [Google Scholar]
- Basar E, Schurmann M, Sakowitz O. The selectively distributed theta system: functions. Int J Psychophysiol. 2001d;39:197–212. doi: 10.1016/s0167-8760(00)00141-0. [DOI] [PubMed] [Google Scholar]
- Basar-Eroglu C, Basar E. A compound P300-40 Hz response of the cat hippocampus. Int J Neurosci. 1991;60:227–237. doi: 10.3109/00207459109167035. [DOI] [PubMed] [Google Scholar]
- Basar-Eroglu C, Basar E, Demiralp T, Schurmann M. P300-response: possible psychophysiological correlates in delta and theta frequency channels. A review. Int J Psychophysiol. 1992;13:161–179. doi: 10.1016/0167-8760(92)90055-g. [DOI] [PubMed] [Google Scholar]
- Basar-Eroglu C, Demiralp T, Schurmann M, Basar E. Topological distribution of oddball ‘P300’ responses. Int J Psychophysiol, 2001. 2001;39:213–220. doi: 10.1016/s0167-8760(00)00142-2. [DOI] [PubMed] [Google Scholar]
- Basar-Eroglu C, Struber D, Kruse P, Basar E, Stadler M. Frontal gamma-band enhancement during multistable visual perception. Int J Psychophysiol. 1996a;24:113–125. doi: 10.1016/s0167-8760(96)00055-4. [DOI] [PubMed] [Google Scholar]
- Basar-Eroglu C, Struber D, Schurmann M, Stadler M, Basar E. Gamma-band responses in the brain: a short review of psychophysiological correlates and functional significance. Int J Psychophysiol. 1996b;24:101–112. doi: 10.1016/s0167-8760(96)00051-7. [DOI] [PubMed] [Google Scholar]
- Bates ME. Utility of component-process approaches for understanding complex alcohol-related behavior within an executive functioning framework: comment on Giancola (2000) Exp Clin Psychopharmacol. 2000;8:598–600. doi: 10.1037//1064-1297.8.4.598. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Hames KA, Blanco CR, Nixon SJ, Tivis LJ. Visuospatial perception, construction and memory of alcoholism. J Stud Alcohol. 1996;57:136–143. doi: 10.15288/jsa.1996.57.136. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Katzung VM, Nixon SJ, Moreland VJ. Problem-solving deficits in alcoholics: evidence from the California Card Sorting Test. J Stud Alcohol. 1993;54:687–692. doi: 10.15288/jsa.1993.54.687. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Platz A. The effects of alcohol on the central nervous system in humans. In: Kissin R, Begleiter H, editors. The Biology of Alcoholism: Physiology and Behavior. Vol. 2. Plenum Press; New York: 1972. pp. 293–343. [Google Scholar]
- Begleiter H, Porjesz B. What is inherited in the predisposition toward alcoholism? A proposed model. Alcohol Clin Exp Res. 1999;23:1125–1135. doi: 10.1111/j.1530-0277.1999.tb04269.x. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225:1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Reich T, Edenberg HJ, Goate A, van Eerdewegh P, Polich J, Rohrbaugh J, Kuperman S, Bauer LO, O’Connor SJ, Chorlian DB, Li TK, Conneally PM, Hesselbrock V, Rice JP, Schuckit MA, Cloninger R, Nurnberger J, Jr, Crowe R, Bloom FE. Quantitative trait loci analysis of human event-related brain potentials: P3 voltage. Electroencephalogr Clin Neurophysiol. 1998;108:244–250. doi: 10.1016/s0168-5597(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Tenner M. Neuroradiological and neurophysiological evidence of brain deficits in chronic alcoholics. Acta Psychiatr Scand (suppl 286) 1980;62:3–13. doi: 10.1111/j.1600-0447.1980.tb08050.x. [DOI] [PubMed] [Google Scholar]
- Behrends JC, ten Bruggencate G. Cholinergic modulation of synaptic inhibition in the guinea pig hippocampus in vitro: excitation of GABAergic interneurons and inhibition of GABA-release. J Neurophysiol. 1993;69:626–629. doi: 10.1152/jn.1993.69.2.626. [DOI] [PubMed] [Google Scholar]
- Bertera JH, Parsons OA. Impaired visual search in alcoholics. Alcohol Clin Exp Res. 1978;2:9–14. doi: 10.1111/j.1530-0277.1978.tb04685.x. [DOI] [PubMed] [Google Scholar]
- Bjorklund DF, Harnishfeger KK. The resources construct in cognitive development: diverse sources of evidence and a theory of inefficient inhibition. Dev Rev. 1990;10:48–71. [Google Scholar]
- Bjorklund DF, Harnishfeger KK. The evolution of inhibition mechanisms and their role in human cognition and behavior. In: Dempster FN, Brainerd CJ, editors. Interference and Inhibition in Cognition. Academic Press; San Diego: 1995. pp. 141–173. [Google Scholar]
- Bland BH. The physiology and pharmacology of hippocampal formation theta rhythms. Prog Neurobiol. 1986;26:1–54. doi: 10.1016/0301-0082(86)90019-5. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Brito GN, Brito LS. Septohippocampal system and the prelimbic sector of frontal cortex: a neuropsychological battery analysis in the rat. Behav Brain Res. 1990;36:127–146. doi: 10.1016/0166-4328(90)90167-d. [DOI] [PubMed] [Google Scholar]
- Brown D, Fenwick P, Howard R. The contingent negative variation in a Go/No Go avoidance task: Relationships with personality and subjective state. Int J Psychophysiol. 1989;7:35–45. doi: 10.1016/0167-8760(89)90029-9. [DOI] [PubMed] [Google Scholar]
- Brutkowski S. Functions of prefrontal cortex in animals. Physiol Rev. 1965;45:721–746. doi: 10.1152/physrev.1965.45.4.721. [DOI] [PubMed] [Google Scholar]
- Butters N, Butter CM, Rosen J, Stein D. Behavioral effects of sequential and one-stage ablations of orbital prefrontal cortex in the monkey. Exp Neurol. 1973;39:204–214. doi: 10.1016/0014-4886(73)90223-9. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, Castellanos FX, Haxby JV, Noll DC, Cohen JD, Forman SD, Dahl RE, Rapoport JL. A developmental functional MRI study of prefrontal activation during performance of a go–no-go task. J Cogn Neurosci. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Chapman A, Lacaille JC. Cholinergic Induction of Theta-Frequency Oscillations in Hippocampal Inhibitory Interneurons and Pacing of Pyramidal Cell Firing. J Neurosci. 1999;19:8637–8645. doi: 10.1523/JNEUROSCI.19-19-08637.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelune GJ, Parker JB. Neuropsychological deficits associated with chronic alcohol abuse. Clin Psychol Rev. 1981;1:181–195. [Google Scholar]
- Chester JA, Cunningham CL. GABAA receptor modulation of the rewarding and aversive effects of ethanol. Alcohol. 2002;26:131–143. doi: 10.1016/s0741-8329(02)00199-4. [DOI] [PubMed] [Google Scholar]
- Cieslieski KT, Waldorf AV, Jung RE. Anterior brain deficits in chronic alcoholism. Cause or effect? J Nerv Ment Dis. 1995;183:756–761. doi: 10.1097/00005053-199512000-00005. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–416. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- Cohen HL, Ji J, Chorlian DB, Begleiter H, Porjesz B. Alcohol-related ERP changes recorded from different modalities: a topographic analysis. Alcohol Clin Exp Res. 2002;26:303–317. [PubMed] [Google Scholar]
- Cohen HL, Porjesz B, Begleiter H, Wang W. Neurophysiological correlates of response production and inhibition in alcoholics. Alcohol Clin Exp Res. 1997a;21:1398–1406. [PubMed] [Google Scholar]
- Cohen HL, Porjesz B, Begleiter H, Wang W. Neuroelectric correlates of response production and inhibition in individuals at risk to develop alcoholism. Biol Psychiatry. 1997b;42:57–67. doi: 10.1016/S0006-3223(96)00221-1. [DOI] [PubMed] [Google Scholar]
- Collette F, Van der Linden M. Brain imaging of the central executive component of working memory. Neurosci Biobehav Rev. 2002;26:105–125. doi: 10.1016/s0149-7634(01)00063-x. [DOI] [PubMed] [Google Scholar]
- Conrod PJ, Petersen JB, Pihl RO. Disinhibited personality and sensitivity to alcohol reinforcement: Independent correlates of drinking behavior in sons of alcoholics. Alcohol Clin Exp Res. 1997;21:1320–1332. [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- Dao-Castellana MH, Samson Y, Legault F, Martinot JL, Aubin HJ, Crouzel C, Feldman L, Barrucand D, Rancurel G, Feline A, Syrota A. Frontal dysfunction in neurologically normal chronic alcoholic subjects: metabolic and neuropsychological findings. Psychol Med. 1998;28:1039–1048. doi: 10.1017/s0033291798006849. [DOI] [PubMed] [Google Scholar]
- Demilrap T, Basar E. Theta rhymicities following expected visual and auditory targets. Int J Psychophysiol. 1992;13:147–160. doi: 10.1016/0167-8760(92)90054-f. [DOI] [PubMed] [Google Scholar]
- Demiralp T, Ademoglu A, Istefanopulos Y, Basar-Eroglu C, Basar E. Wavelet analysis of oddball P300. Int J Psychophysiol. 2001;39:221–227. doi: 10.1016/s0167-8760(00)00143-4. [DOI] [PubMed] [Google Scholar]
- Demiralp T, Ademoglu A, Schürmann M, Basar-Eroglu C, Basar E. Detection of P300 waves in single trials by the wavelet transform (WT) Brain Lang. 1999;66:108–128. doi: 10.1006/brln.1998.2027. [DOI] [PubMed] [Google Scholar]
- Dempster FN. The rise and fall of the inhibitory mechanism: toward a unified theory of cognitive development and aging. Dev Rev. 1992;12:45–75. [Google Scholar]
- Dempster FN. Resistance to interference: developmental changes in a basic processing mechanism. In: Howe ML, Pasnak R, editors. Emerging Themes in Cognitive Development: Foundations. I. Springer; New York: 1993. pp. 3–27. [Google Scholar]
- Devrim M, Demiralp T, Ademoglu A, Kurt A. A model for P300 generation based on responses to near-threshold visual stimuli. Brain Res Cogn Brain Res. 1999;8:37–43. doi: 10.1016/s0926-6410(99)00007-5. [DOI] [PubMed] [Google Scholar]
- Doppelmayr M, Klimesch W, Schwaiger J, Auinger P, Winkler T. Theta synchronization in the human EEG and episodic retrieval. Neurosci Lett. 1998;257:41–44. doi: 10.1016/s0304-3940(98)00805-2. [DOI] [PubMed] [Google Scholar]
- Doppelmayr M, Klimesch W, Schwaiger J, Stadler W, Rohm D. The time locked theta response reflects interindividual differences in human memory performance. Neurosci Lett. 2000;278:141–144. doi: 10.1016/s0304-3940(99)00925-8. [DOI] [PubMed] [Google Scholar]
- Drewe EA. Go-no go learning after frontal lobe lesions in humans. Cortex. 1975;11:8–16. doi: 10.1016/s0010-9452(75)80015-3. [DOI] [PubMed] [Google Scholar]
- Durka PJ, Ircha D, Blinowska KJ. Stochastic time-frequency dictionaries for Matching Pursuit. IEEE Trans Sig Proc. 2001a;49:507–510. [Google Scholar]
- Durka PJ, Ircha D, Neuper C, Pfurtscheller G. Time-frequency microstructure of event-related EEG desynchronization (ERD) and synchronization (ERS) Med Biol Eng Computing. 2001b;39:315–321. doi: 10.1007/BF02345286. [DOI] [PubMed] [Google Scholar]
- Elimer M. Effects of attention and stimulus probability on ERPs in a go/nogo task. Biol Psychol. 1993;35:123–138. doi: 10.1016/0301-0511(93)90009-w. [DOI] [PubMed] [Google Scholar]
- Evans JJ, Chua SE, McKenna PJ, Wilson BA. Assessment of the dysexecutive syndrome in schizophrenia. Psychol Med. 1997;27:635–646. doi: 10.1017/s0033291797004790. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Wiesbeck GA, Weijers HG, Boening J, Strik WK. Event-related correlates of response suppression as indicators of novelty seeking in alcoholics. Alcohol Alcohol. 1998;33:475–481. doi: 10.1093/alcalc/33.5.475. [DOI] [PubMed] [Google Scholar]
- Fan L, van der Brug M, Chen WB, Dodd PR, Matsumoto I, Niwa S, Wilce PA. Increased expression of a mitochondrial gene in human alcoholic brain revealed by differential display. Alcohol Clin Exp Res. 1999;23:408–413. [PubMed] [Google Scholar]
- Filipovic SR, Jahanshahi M, Rothwell JC. Cortical potentials related to decision-making: comparison of two types of go/no-go decision. Neuroreport. 1999;10:3583–3587. doi: 10.1097/00001756-199911260-00022. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. An alcohol model of impaired inhibitory control and its treatment in humans. Exp Clin Psychopharmacol. 1999;7:49–55. doi: 10.1037//1064-1297.7.1.49. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. Response inhibition under alcohol: Effects of cognitive and motivational conflict. J Stud Alcohol. 2000;61:239–246. doi: 10.15288/jsa.2000.61.239. [DOI] [PubMed] [Google Scholar]
- Finn PR, Justus A, Mazas C, Steinmetz JE. Working memory, executive processes and the effects of alcohol on Go/No-Go learning: testing a model of behavioral regulation and impulsivity. Psychopharmacology (Berl) 1999;146:465–472. doi: 10.1007/pl00005492. [DOI] [PubMed] [Google Scholar]
- Finn PR, Kessler DN, Hussong AM. Risk for alcoholism and classical conditioning to signals for punishment: evidence for a weak behavioral inhibition system? J Abnorm Psychol. 1994;103:293–301. doi: 10.1037//0021-843x.103.2.293. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fossati P, Ergis AM, Allilaire JF. Executive functioning in unipolar depression: a review. Encephale. 2002;28:97–107. [PubMed] [Google Scholar]
- Fox SE. Membrane potential and impedance changes in hippocampal pyramidal cells during theta rhythm. Exp Brain Res. 1989;77:283–294. doi: 10.1007/BF00274985. [DOI] [PubMed] [Google Scholar]
- Freund G, Ballinger WE. Loss of synaptic receptors can precede morphologic changes induced by alcoholism. Alcohol Alcohol (Suppl) 1991;1:385–391. [PubMed] [Google Scholar]
- Fuster JM. The Prefrontal Cortex. Raven Press; New York: 1989. [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci USA. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancola PR, Martin CS, Tarter RE, Pelham WE, Moss HB. Executive cognitive functioning and aggressive behavior in preadolescent boys at high risk for substance abuse/dependence. J Stud Alcohol. 1996a;57:352–359. doi: 10.15288/jsa.1996.57.352. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Moss HB. Executive cognitive functioning in alcohol use disorders. Recent Dev Alcohol. 1998;14:227–251. doi: 10.1007/0-306-47148-5_10. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Moss HB, Martin CS, Kirisci L, Tarter RE. Executive cognitive functioning predicts reactive aggression in boys at high risk for substance abuse: a prospective study. Alcohol Clin Exp Res. 1996b;20:740–744. doi: 10.1111/j.1530-0277.1996.tb01680.x. [DOI] [PubMed] [Google Scholar]
- Givens B, Sarter M. Modulation of cognitive processes by transsynaptic activation of the basal forebrain. Behav Brain Res. 1997;84:1–22. doi: 10.1016/s0166-4328(96)00146-5. [DOI] [PubMed] [Google Scholar]
- Glenn SW, Errico AL, Parsons OA, King AC, Nixon SJ. The role of antisocial, affective, and childhood behavioral characteristics in alcoholics’ neuropsychological performance. Alcohol Clin Exp Res. 1993;17:162–169. doi: 10.1111/j.1530-0277.1993.tb00742.x. [DOI] [PubMed] [Google Scholar]
- Godefroy O, Cabaret M, Petit-Chenal V, Pruvo JP, Rousseaux M. Control functions of the frontal lobes. Modularity of the central-supervisory system? Cortex. 1999;35:1–20. doi: 10.1016/s0010-9452(08)70782-2. [DOI] [PubMed] [Google Scholar]
- Gorenstein EE. Relationships of subclinical depression, psychopathy and hysteria to patterns of alcohol consumption and abuse in males and females. Curr Alcohol. 1979;7:207–217. [PubMed] [Google Scholar]
- Gorenstein EE. Cognitive-perceptual deficit in an alcoholism spectrum disorder. J Stud Alcohol. 1987;48:310–318. doi: 10.15288/jsa.1987.48.310. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABAA receptors in the acute and chronic effects of ethanol. Psychopharmacology. 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Grunberger J, Linzmayer L, Walter H, Stohr H, Saletu-Zyhlarz GM, Grunberger M, Lesch OM. Psychophysiological diagnostics in alcohol dependency: Fourier analysis of pupillary oscillations and the receptor test for determination of cholinergic deficiency. Alcohol Alcohol. 1998;33:541–548. doi: 10.1093/alcalc/33.5.541. [DOI] [PubMed] [Google Scholar]
- Harper C, Kril J. Brain atrophy in chronic alcoholic patients: a quantitative pathological study. J Neurol Neurosurg Psychiatry. 1985;48:211–217. doi: 10.1136/jnnp.48.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselbrock VM. The genetic epidemiology of alcoholism. In: Begleiter H, Kissin B, editors. Alcohol and Alcoholism, Volume 1, The Genetics of Alcoholism. Oxford University Press; New York: 1995. pp. 17–39. [Google Scholar]
- Hoaken PN, Giancola PR, Pihl RO. Executive cognitive functions as mediators of alcohol-related aggression. Alcohol Alcohol. 1998;33:47–54. doi: 10.1093/oxfordjournals.alcalc.a008347. [DOI] [PubMed] [Google Scholar]
- Hodges H, Ribeiro AM, Gray JA, Marchbanks RM. Low dose tetrahydroaminoacridine (THA) improves cognitive function but does not affect brain acetylcholine in rats. Pharmacol Biochem Behav. 1990;36:291–298. doi: 10.1016/0091-3057(90)90406-8. [DOI] [PubMed] [Google Scholar]
- Hutton SB, Puri BK, Duncan LJ, Robbins TW, Barnes TR, Joyce EM. Executive function in first-episode schizophrenia. Psychol Med. 1998;28:463–473. doi: 10.1017/s0033291797006041. [DOI] [PubMed] [Google Scholar]
- Ihara H, Berrios GE, London M. Group and case study of the dysexecutive syndrome in alcoholism without amnesia. J Neurol Neurosurg Psychiatry. 2000;68:731–737. doi: 10.1136/jnnp.68.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen SD, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Exp Brain Res. 1970;11:376–386. doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- Jodo E, Kayama Y. Relation of a negative ERP component to response inhibition in a Go/No-go task. Electroencephalogr Clin Neurophysiol. 1992;82:477–482. doi: 10.1016/0013-4694(92)90054-l. [DOI] [PubMed] [Google Scholar]
- Joho RH, Ho CS, Marks GA. Increased gamma- and decreased delta-oscillations in a mouse deficient for a potassium channel expressed in fast-spiking interneurons. J Neurophysiol. 1999;82:1855–1864. doi: 10.1152/jn.1999.82.4.1855. [DOI] [PubMed] [Google Scholar]
- Jones B, Parsons OA. Impaired abstract ability in chronic alcoholics. Arch Gen Psychiatry. 1971;24:71–75. doi: 10.1001/archpsyc.1971.01750070073010. [DOI] [PubMed] [Google Scholar]
- Jones B, Parsons OA. Specific vs generalized deficits of abstracting ability in chronic alcoholics. Arch Gen Psychiatry. 1972;26:380–384. doi: 10.1001/archpsyc.1972.01750220090017. [DOI] [PubMed] [Google Scholar]
- Kahana MJ, Seelig D, Madsen JR. Theta returns. Curr Opin Neurobiol. 2001;11:739–744. doi: 10.1016/s0959-4388(01)00278-1. [DOI] [PubMed] [Google Scholar]
- Kahana MJ, Sekuler R, Caplan JB, Kirschen M, Madsen JR. Human theta oscillations exhibit task dependence during virtual maze navigation. Nature. 1999;399:781–784. doi: 10.1038/21645. [DOI] [PubMed] [Google Scholar]
- Karakas S. A descriptive framework for information processing: an integrative approach. Int J Psychophysiol. 1997;26:353–368. doi: 10.1016/s0167-8760(97)00775-7. [DOI] [PubMed] [Google Scholar]
- Karakas S, Erzengin OU, Basar E. A new strategy involving multiple cognitive paradigms demonstrates that ERP components are determined by the superposition of oscillatory responses. Clin Neurophysiol. 2000a;111:1719–1732. doi: 10.1016/s1388-2457(00)00418-1. [DOI] [PubMed] [Google Scholar]
- Karakas S, Erzengin OU, Basar E. The genesis of human event-related responses explained through the theory of oscillatory neural assemblies. Neurosci Lett. 2000b;285:45–48. doi: 10.1016/s0304-3940(00)01022-3. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Satoh K, Itoh H, Ono S, Furumoto S, Gotoh R, Koyama M, Yoshioka S, Takahashi T, Takahashi K, Yanagisawa T, Fukuda H. Functional anatomy of GO/NO-GO discrimination and response selection–a PET study in man. Brain Res. 1996;728:79–89. [PubMed] [Google Scholar]
- Kempton S, Vance A, Maruff P, Luk E, Costin J, Pantelis C. Executive function and attention deficit hyperactivity disorder: stimulant medication and better executive function performance in children. Psychol Med. 1999;29:527–538. doi: 10.1017/s0033291799008338. [DOI] [PubMed] [Google Scholar]
- Klimesch W. Memory processes, brain oscillations and EEG synchronization. Int J Psychophysiol. 1996;24:61–100. doi: 10.1016/s0167-8760(96)00057-8. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG-alpha rhythms and memory processes. Int J Psychophysiol. 1997;26:319–340. doi: 10.1016/s0167-8760(97)00773-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Pachinger T, Ripper B. Brain oscillations and human memory performance: EEG correlates in the upper alpha and theta band. Neurosci Lett. 1997a;238:9–12. doi: 10.1016/s0304-3940(97)00771-4. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Russegger H, Pachinger T, Chwaiger J. Induced alpha band power changes in the human EEG and attention. Neurosci Lett. 1998;244:73–76. doi: 10.1016/s0304-3940(98)00122-0. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schimke H, Ripper B. Theta synchronization and alpha desynchronization in a memory task. Psychophysiology. 1997b;34:169–176. doi: 10.1111/j.1469-8986.1997.tb02128.x. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schwaiger J, Winkler T, Gruber W. Theta oscillations and the ERP old/new effect: independent phenomena? Clin Neurophys. 2000;111:781–793. doi: 10.1016/s1388-2457(00)00254-6. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Stadler W, Pollhuber D, Sauseng P, Rohm D. Episodic retrieval is reflected by a process specific increase in human electroencephalographic theta activity. Neurosci Lett. 2001a;302:49–52. doi: 10.1016/s0304-3940(01)01656-1. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Yonelinas A, Kroll N, Lazzara M, Rohm D, Gruber W. Theta sychronisation during episodic retrieval: neural correlates of conscious awareness. Cognit Brain Res. 2001b;12:33–38. doi: 10.1016/s0926-6410(01)00024-6. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schimke H, Schwaiger J. Episodic and semantic memory: an analysis in the EEG theta and alpha band. Electroencephalogr Clin Neurophysiol. 1994;91:428–441. doi: 10.1016/0013-4694(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Knop J, Goodwin DW, Jensen P, Penick E, Pollock V, Gabrielli W, Teasdale TW, Mednick SA. A 30-year follow-up study of the sons of alcoholic men. Acta Psychiatr Scand (Suppl) 1993;370:48–53. doi: 10.1111/j.1600-0447.1993.tb05360.x. [DOI] [PubMed] [Google Scholar]
- Kolev V, Yordanova J, Schurmann M, Basar E. Increased frontal phase-locking of event-related alpha oscillations during task processing. Int J Psychophysiol. 2001;39:159–165. doi: 10.1016/s0167-8760(00)00139-2. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain. 1999;122:981–991. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- Kopp B, Mattler U, Goertz R, Rist F. N2, P3 and the lateralized readiness potential in a nogo task involving selective response priming. Electroencephalogr Clin Neurophysiol. 1996;99:19–27. doi: 10.1016/0921-884x(96)95617-9. [DOI] [PubMed] [Google Scholar]
- Korpi ER. Role of GABAA receptors in the actions of alcohol and in alcoholism: recent advances. Alcohol Alcohol. 1994;29:115–129. [PubMed] [Google Scholar]
- Krabbendam L, de Vugt ME, Derix MM, Jolles J. The behavioural assessment of the dysexecutive syndrome as a tool to assess executive functions in schizophrenia. Clin Neuropsychol. 1999;13:370–375. doi: 10.1076/clin.13.3.370.1739. [DOI] [PubMed] [Google Scholar]
- Kraus MF, Maki PM. Effects of amantidine hydrochloride on symptoms of frontal lobe dysfunction in brain injury: case studies and review. J Neuropsychiatry Clin Neurosci. 1997;9:222–230. doi: 10.1176/jnp.9.2.222. [DOI] [PubMed] [Google Scholar]
- Krause CM, Aromaki A, Sillanmaki L, Astrom T, Alanko K, Salonen E, Peltola O. Alcohol-induced alterations in ERD/ERS during an auditory memory task. Alcohol. 2002;26:145–153. doi: 10.1016/s0741-8329(01)00204-x. [DOI] [PubMed] [Google Scholar]
- Krill JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–998. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Lang M, Lang W, Diekmann V, Kornhuber HH. The frontal theta rhythm indicating motor and cognitive learning. In: Johnson R, Rohrbaugh JW, Parasuraman R, editors. Current Trends in Event-related Potential Research. EEG suppl. 40. Elsevier; Amsterdam: 1987. pp. 322–327. [PubMed] [Google Scholar]
- Laukka SJ, Järvilehto T, Alexandrov Y, Lindqvist J. Influence of alcohol on human frontal midline theta activity and task execution. Dev Brain Dysfunc. 1997;10:128–132. [Google Scholar]
- Lewohl JM, Crane DI, Dodd PR. Expression of the alpha 1, alpha 2 and alpha 3 isoforms of the GABAA receptor in human alcoholic brain. Brain Res. 1997;751:102–112. doi: 10.1016/s0006-8993(96)01396-0. [DOI] [PubMed] [Google Scholar]
- Lewohl JM, Wang L, Miles MF, Zhang L, Dodd PR, Harris RA. Gene expression in human alcoholism: microarray analysis of frontal cortex. Alcohol Clin Exp Res. 2000;24:1873–1882. [PubMed] [Google Scholar]
- Lingford-Hughes AR, Acton PD, Gacinovic S, Suckling J, Busatto GF, Boddington SJ, Bullmore E, Woodruff PW, Costa DC, Pilowsky LS, Ell PJ, Marshall EJ, Kerwin RW. Reduced levels of GABA-benzodiazepine receptor in alcohol dependency in the absence of gray matter atrophy. Br J Psychiatry. 1998;173:116–122. doi: 10.1192/bjp.173.2.116. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: A model and a method. J Exp Psychol Hum Percept Perform. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychol Sci. 2003;14:47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- Mahurin RK, Velligan DI, Miller AL. Executive-frontal lobe cognitive dysfunction in schizophrenia: a symptom subtype analysis. Psychiatry Res. 1998;79:139–149. doi: 10.1016/s0165-1781(98)00031-6. [DOI] [PubMed] [Google Scholar]
- Makeig S, Westerfield M, Jung TP, Enghoff S, Townsend J, Courchesne E, Sejnowski TJ. Dynamic brain sources of visual evoked responses. Science. 2002;295:690–694. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- Mallat S, Zhang Z. Matching pursuit with time-frequency dictionaries. IEEE Trans Sig Proc. 1993;41:3397–3415. [Google Scholar]
- Masterman DL, Cummings JL. Frontal-subcortical circuits: the anatomic basis of executive, social and motivated behaviors. J Psychopharmacol. 1997;11:107–114. doi: 10.1177/026988119701100203. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Donchin E. A metric for thought: a comparison of P300 latency and reaction time. Science. 1981;211:77–79. doi: 10.1126/science.7444452. [DOI] [PubMed] [Google Scholar]
- McGue M, Slutske W, Taylor J, Iacono WG. Personality and substance use disorders: I. Effects of gender and alcoholism subtype. Alcohol Clin Exp Res. 1997;21:513–520. [PubMed] [Google Scholar]
- McMahon LL, Williams JH, Kauer JA. Functionally distinct groups of interneurons identified during rhythmic carbachol oscillations in hippocampus in vitro. J Neurosci. 1998;18:5640–5651. doi: 10.1523/JNEUROSCI.18-15-05640.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. Principles of Behavioral and Cognitive Neurology. 2. F. A. Davis Company; Philadelphia: 2000. [Google Scholar]
- Mihic SJ, Harris RA. Alcohol actions at the GABAA receptor/chloride channel complex. In: Deitrich RA, Erwin VG, editors. Pharmacological Effects of Ethanol on the Nervous System. CRC Press; Boca Raton: 1996. pp. 51–72. [Google Scholar]
- Miller L. Neuropsychological assessment of substance abusers: review and recommendations. J Subst Abuse Treat. 1985;2:5–17. doi: 10.1016/0740-5472(85)90017-0. [DOI] [PubMed] [Google Scholar]
- Miller R. Cortico-hippocampal Interplay and the Representation of Contexts in the Brain. Springer; Berlin: 1991. [Google Scholar]
- Miotto EC, Bullock P, Polkey CE, Morris RG. Spatial working memory and strategy formation in patients with frontal lobe excisions. Cortex. 1996;32:613–630. doi: 10.1016/s0010-9452(96)80034-7. [DOI] [PubMed] [Google Scholar]
- Mizuki Y, Hashimoto M, Tanaka T, Inanaga K, Tanaka M. A new physiological tool for assessing anxiolytic effects in humans: frontal midline theta activity. Psychopharmacology. 1983;80:311–314. doi: 10.1007/BF00432111. [DOI] [PubMed] [Google Scholar]
- Mizuki Y, Tanaka M, Isozaki H, Nishijima H, Inanaga K. Periodic appearance of theta rhythm in the frontal midline area during performance of a mental task. Electroencephalogr Clin Neurophysiol. 1980;49:345–351. doi: 10.1016/0013-4694(80)90229-1. [DOI] [PubMed] [Google Scholar]
- Moselhy HF, Georgiou G, Kahn A. Frontal lobe changes in alcoholism: A review of literature. Alcohol Alcohol. 2001;36:357–368. doi: 10.1093/alcalc/36.5.357. [DOI] [PubMed] [Google Scholar]
- Nauta WJH. The problem of the frontal lobes: a reinterpretation. J Psychiatr Res. 1971;8:167–187. doi: 10.1016/0022-3956(71)90017-3. [DOI] [PubMed] [Google Scholar]
- Nauta WJH. Neural association of the frontal cortex. Acta Neurobiol Exp. 1972;32:125–140. [PubMed] [Google Scholar]
- Nicolas JM, Catafau AM, Estruch R, Lomena FJ, Salamero M, Herranz R, Monforte R, Cardenal C, Urbano-Marquez A. Regional cerebral blood flow-SPECT in chronic alcoholism: relation to neuropsychological testing. J Nucl Med. 1993;34:1452–1459. [PubMed] [Google Scholar]
- Nixon SJ, Bowlby D. Evidence of alcohol-related efficiency deficits in an episodic learning task. Alcohol Clin Exp Res. 1996;20:21–24. doi: 10.1111/j.1530-0277.1996.tb01037.x. [DOI] [PubMed] [Google Scholar]
- Noel X, Van der Linden M, Schmidt N, Sferrazza R, Hanak C, Le Bon O, De Mol J, Kornreich C, Pelc I, Verbanck P. Supervisory attentional system in nonamnesic alcoholic men. Arch Gen Psychiatry. 2001;58:1152–1158. doi: 10.1001/archpsyc.58.12.1152. [DOI] [PubMed] [Google Scholar]
- Norman DA, Shallice T. Attention to action: willed and automatic control of behavior. In: Davidson RJ, Schwartz GE, Shapiro D, editors. Consciousness and Self regulation. Advances in Research and Theory. Plenum Press; New York: 1986. pp. 1–18. [Google Scholar]
- O’Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- Parker ES, Parker DA, Harford TC. Specifying the relationship between alcohol use and cognitive loss: the effects of consumption and psychological distress. J Stud Alcohol. 1991;52:366–373. doi: 10.15288/jsa.1991.52.366. [DOI] [PubMed] [Google Scholar]
- Parsons OA. Psychological deficits in alcoholics: facts and fancies. Alcohol Clin Exp Res. 1977;1:51–56. doi: 10.1111/j.1530-0277.1977.tb05767.x. [DOI] [PubMed] [Google Scholar]
- Parsons OA. Neuropsychological measures and event-related potentials in alcoholics: interrelations, long-term reliabilities, and prediction of resumption of drinking. J Clin Psychol. 1994;50:37–46. doi: 10.1002/1097-4679(199401)50:1<37::aid-jclp2270500105>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. J Child Psychol Psychiatry. 1996;37:51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Peterson JB, Finn PR, Pihl RO. Cognitive dysfunction and the inherited predisposition to alcoholism. J Stud Alcohol. 1992;53:154–160. doi: 10.15288/jsa.1992.53.154. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM. ERPs to stimuli requiring response production and inhibition: Effects of age, probability and visual noise. Electroencephalogr Clin Neurophysiol. 1988;71:55–63. doi: 10.1016/0168-5597(88)90019-6. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, Weller BJ, Kopel BS. ERPs to response production and inhibition. Electroencephalogr Clin Neurophysiol. 1985;60:423–434. doi: 10.1016/0013-4694(85)91017-x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, White PM, Mathalon D. Event-related potentials in alcoholic men: P3 amplitude reflects family history but not alcohol consumption. Alcohol Clin Exp Res. 1991;15:839–850. doi: 10.1111/j.1530-0277.1991.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res. 1997;21:521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Aranibar A. Event-related cortical desynchronization detected by power measurements of scalp EEG. Electroenceph Clin Neurophysiol. 1977;42:817–826. doi: 10.1016/0013-4694(77)90235-8. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Pihl RO, Bruce KR. Cognitive impairments in children of alcoholics. Alcohol Health Res World. 1995;19:142–147. [PMC free article] [PubMed] [Google Scholar]
- Pihl RO, Peterson J, Finn PR. Inherited predisposition to alcoholism: Characteristics of sons of male alcoholics. J Abnorm Psychol. 1990;99:291–301. doi: 10.1037//0021-843x.99.3.291. [DOI] [PubMed] [Google Scholar]
- Pishkin V, Lovallo WR, Bourne LE., Jr Chronic alcoholism in males: Cognitive deficit as a function of age of onset, age and duration. Alcohol Clin Exp Res. 1985;9:400–406. doi: 10.1111/j.1530-0277.1985.tb05571.x. [DOI] [PubMed] [Google Scholar]
- Pitler TA, Alger BE. Cholinergic excitation of GABAergic interneurons in the rat hippocampal slice. J Physiol. 1992;450:127–142. doi: 10.1113/jphysiol.1992.sp019119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Pollock VE, Bloom FE. Meta-analysis of P300 amplitude from males at risk for alcoholism. Psychol Bull. 1994;115:55–73. doi: 10.1037/0033-2909.115.1.55. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Almasy L, Edenberg HJ, Wang K, Chorlian DB, Foroud T, Goate A, Rice JP, O’Connor SJ, Rohrbaugh J, Kuperman S, Bauer LO, Crowe RR, Schuckit MA, Hesselbrock V, Conneally PM, Tischfield JA, Li TK, Reich T, Begleiter H. Linkage disequilibrium between the beta frequency of the human EEG and a GABAA receptor gene locus. Proc Natl Acad Sci USA. 2002a;99:3729–3733. doi: 10.1073/pnas.052716399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Evoked brain potentials and alcoholism. In: Parsons OA, Butter N, Nathan P, editors. Neuropsychology of Alcoholism: Implications for Diagnosis and Treatment. Guilford press; New York: 1987. pp. 45–63. [Google Scholar]
- Porjesz B, Begleiter H. Effects of alcohol on electrophysiological activity of the brain. In: Begleiter H, Kissin B, editors. Alcohol and Alcoholism: Volume 2. The Pharmacology of Alcohol and Alcohol Dependence. Oxford University Press; New York: 1996. pp. 207–247. [Google Scholar]
- Porjesz B, Begleiter H. Genetic basis of event-related potentials and their relationship to alcoholism and alcohol use. J Clin Neurophysiol. 1998;15:44–57. doi: 10.1097/00004691-199801000-00006. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Garozzo R. Visual evoked potential correlates of information processing deficits in chronic alcoholics. Adv Exp Med Biol. 1980;126:603–623. doi: 10.1007/978-1-4684-3632-7_46. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Wang K, Almasy L, Chorlian DB, Stimus AT, Kuperman S, O’Connor SJ, Rohrbaugh J, Bauer LO, Edenberg HJ, Goate A, Rice JP, Reich T. Linkage and linkage disequilibrium mapping of ERP and EEG phenotypes. Biol Psychol. 2002b;61:229–248. doi: 10.1016/s0301-0511(02)00060-1. [DOI] [PubMed] [Google Scholar]
- Purcell R, Maruff P, Kyrios M, Pantelis C. Cognitive deficits in obsessive-compulsive disorder on tests of frontal-striatal function. Biol Psychiatry. 1998;43:348–357. doi: 10.1016/s0006-3223(97)00201-1. [DOI] [PubMed] [Google Scholar]
- Rappelsberger P, Pfurtscheller G, Filz O. Calculation of event-related coherence--a new method to study short-lasting coupling between brain areas. Brain Topogr. 1994;7:121–127. doi: 10.1007/BF01186770. [DOI] [PubMed] [Google Scholar]
- Ratti MT, Bo P, Giardini A, Soragna D. Chronic alcoholism and the frontal lobe: which executive functions are imparied? Acta Neurol Scand. 2002;105:276–281. doi: 10.1034/j.1600-0404.2002.0o315.x. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Ridderinkhof KR, de Vlugt Y, Bramlage A, Spaan M, Elton M, Snel J, Band GP. Alcohol consumption impairs detection of performance errors in mediofrontal cortex. Science. 2002;298:2209–2211. doi: 10.1126/science.1076929. [DOI] [PubMed] [Google Scholar]
- Risberg J, Berglund M. Evoked brain potentials and alcoholism. In: Parsons OA, Butter N, Nathan P, editors. Neuropsychology of Alcoholism: Implications for Diagnosis and Treatment. Guilford press; New York: 1987. pp. 64–75. [Google Scholar]
- Röhm D, Klimesch W, Haider H, Doppelmayr M. The role of theta and alpha oscillations for language comprehension in the human electroencephalogram. Neurosci Lett. 2001;310:137–140. doi: 10.1016/s0304-3940(01)02106-1. [DOI] [PubMed] [Google Scholar]
- Rubia K, Taylor E, Smith AB, Okksannen H, Overmeyer S, Newman S. Neuropsychological analyses of impulsiveness in childhood hyperactivity. Br J Psychiatry. 2001;179:138–143. doi: 10.1192/bjp.179.2.138. [DOI] [PubMed] [Google Scholar]
- Rueckert L, Grafman J. Sustained attention deficits in patients with right frontal lesions. Neuropsychologia. 1996;34:953–963. doi: 10.1016/0028-3932(96)00016-4. [DOI] [PubMed] [Google Scholar]
- Sachs H, Russell JAG, Christman DR, Cook B. Alteration of regional cerebral glucose metabolic rate in non-Korsakoff chronic alcoholism. Arch Neurol. 1987;44:1242–1251. doi: 10.1001/archneur.1987.00520240024007. [DOI] [PubMed] [Google Scholar]
- Sainsbury RS. Hippocampal theta: a sensory-inhibition theory of function. Neurosci Biobehav Rev. 1998;22:237–241. doi: 10.1016/s0149-7634(97)00011-0. [DOI] [PubMed] [Google Scholar]
- Sakowitz OW, Schurmann M, Basar E. Oscillatory frontal theta responses are increased upon bisensory stimulation. Clin Neurophysiol. 2000;111:884–893. doi: 10.1016/s1388-2457(99)00315-6. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Gemba H, Tsujimoto T. Suppression of visually initiated hand movement by stimulation of the prefrontal cortex in the monkey. Brain Res. 1989;495:100–107. doi: 10.1016/0006-8993(89)91222-5. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Gruber W, Doppelmayr M, Stadler W, Schabus M. The interplay between theta and alpha oscillations in the human electroencephalogram reflects the transfer of information between motor systems. Neurosci Lett. 2002;324:121–124. doi: 10.1016/s0304-3940(02)00225-2. [DOI] [PubMed] [Google Scholar]
- Schroger E. Event-related potentials to auditory stimuli following transient shifts of spatial attention in a Go/No go task. Biol Psychol. 1993;36:183–207. doi: 10.1016/0301-0511(93)90017-3. [DOI] [PubMed] [Google Scholar]
- Schurmann M, Basar E. Alpha oscillations shed new light on relation between EEG and single neurons. Neurosci Res. 1999;33:79–80. doi: 10.1016/s0168-0102(98)00116-3. [DOI] [PubMed] [Google Scholar]
- Schurmann M, Basar E. Functional aspects of alpha oscillations in the EEG. Int J Psychophysiol. 2001;39:151–158. doi: 10.1016/s0167-8760(00)00138-0. [DOI] [PubMed] [Google Scholar]
- Schurmann M, Basar-Eroglu C, Basar E. Gamma responses in the EEG: elementary signals with multiple functional correlates. Neuroreport. 1997;8:531–534. doi: 10.1097/00001756-199701200-00030. [DOI] [PubMed] [Google Scholar]
- Schurmann M, Basar-Eroglu C, Kolev V, Basar E. A new metric for analyzing single-trial event-related potentials (ERPs) application to human visual P300 delta response. Neurosci Lett. 1995;197:167–170. doi: 10.1016/0304-3940(95)11912-g. [DOI] [PubMed] [Google Scholar]
- Schurmann M, Basar-Eroglu C, Kolev V, Basar E. Delta responses and cognitive processing: single-trial evaluations of human visual P300. Int J Psychophysiol. 2001;39:229–239. doi: 10.1016/s0167-8760(00)00144-6. [DOI] [PubMed] [Google Scholar]
- Shallice T, Evans ME. The involvement of the frontal lobes in cognitive estimation. Cortex. 1978;14:294–303. doi: 10.1016/s0010-9452(78)80055-0. [DOI] [PubMed] [Google Scholar]
- Shallice T, Marzocchi GM, Coser S, Del Savio M, Meuter RF, Rumiati RI. Executive function profile of children with attention deficit hyperactivity disorder. Dev Neuropsychol. 2002;21:43–71. doi: 10.1207/S15326942DN2101_3. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Trull TJ. Personality and disinhibitory psychopathology: alcoholism and antisocial personality disorder. J Abnorm Psychol. 1994;103:92–102. doi: 10.1037//0021-843x.103.1.92. [DOI] [PubMed] [Google Scholar]
- Shibata T, Shimoyama I, Ito T, Abla D, Iwasa H, Koseki K, Yamanouchi N, Sato T, Nakajima Y. The synchronization between brain areas under motor inhibition process in humans estimated by event-related EEG coherence. Neurosci Res. 1998;31:265–271. doi: 10.1016/s0168-0102(98)00046-7. [DOI] [PubMed] [Google Scholar]
- Shibata T, Shimoyama I, Ito T, Abla D, Iwasa H, Koseki K, Yamanouchi N, Sato T, Nakajima Y. The time course of interhemispheric EEG coherence during a GO/NO-GO task in humans. Neurosci Lett. 1997;233:117–120. doi: 10.1016/s0304-3940(97)00652-6. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Wilson MA, Barnes C. Theta phase procession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus. 1996;6:149–172. doi: 10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Smith ME, Oscar-Berman M. Resource-limited information processing in alcoholism. J Stud Alcohol. 1992;53:514–518. doi: 10.15288/jsa.1992.53.514. [DOI] [PubMed] [Google Scholar]
- Stampfer HG, Basar E. Does frequency analysis lead to better understanding of human event-related potentials? Int J Neurosci. 1985;26:181–196. doi: 10.3109/00207458508985616. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP. Executive functions and the frontal lobes: a conceptual view. Psychol Res. 2000;63:289–298. doi: 10.1007/s004269900007. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Benson DF. Neuropsychological studies of the frontal lobes. Psychol Bull. 1984;95:3–28. [PubMed] [Google Scholar]
- Sullivan EV, Mathalon DH, Ha CN, Zipursky RB, Pfefferbaum A. The contribution of constructional accuracy and organizational strategy to nonverbal recall in schizophrenia and chronic alcoholism. Biol Psychiatry. 1992;32:312–333. doi: 10.1016/0006-3223(92)90036-y. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Mathalon DH, Zipursky RB, Kersteen-Tucker Z, Knight RT, Pfefferbaum A. Factors of the Wisconsin Card Sorting Test as measures of frontal-lobe function in schizophrenia and in chronic alcoholism. Psychiatry Res. 1993;46:175–199. doi: 10.1016/0165-1781(93)90019-d. [DOI] [PubMed] [Google Scholar]
- Suresh S, Porjesz B, Chorlian DB, Jones K, Wang K, Stimus A, Babington E, Begleiter H. Frontal midline theta activity during a mental task in alcoholics. (in preparation, a) [Google Scholar]
- Suresh S, Porjesz B, Chorlian DB, Jones K, Wang K, Stimus A, Babington E, Begleiter H. Frontal midline theta response during a mental task in offspring of male alcoholics. (in preparation, b) [Google Scholar]
- Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object recognition. Trends Cogn Sci. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- Tarter RE. Psychological deficit in chronic alcoholics: a review. Int J Addict. 1975;10:327–368. [Google Scholar]
- Tarter RE. Empirical investigations of psychological deficits. In: Tarter I, Sugarman AA, editors. Alcoholism: Interdisciplinary Approaches to an Enduring Problem. Addison-Wesley; Reading: 1976. pp. 359–394. [Google Scholar]
- Tarter RE. Brain damage in chronic alcoholics: a review of the psychological evidence. In: Richter D, editor. Addiction and Brain Damage. Croom Helm; London: 1980. pp. 267–297. [Google Scholar]
- Tarter RE, Alterman AI. Neuropsychological deficits in alcoholics: etiological considerations. J Stud Alcohol. 1984;45:1–9. doi: 10.15288/jsa.1984.45.1. [DOI] [PubMed] [Google Scholar]
- Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res. 2002;53:647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Tesche C, Karhu J. Theta oscillations index human hippocampal activation during a working memory task. Proc Natl Acad Sci USA. 2000;97:919–924. doi: 10.1073/pnas.97.2.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticku MK. Alcohol and GABA-benzodiazepine receptor function. Ann Med. 1990;22:241–246. doi: 10.3109/07853899009148934. [DOI] [PubMed] [Google Scholar]
- Tirapu-Ustarroz J, Munoz-Cespedes JM, Pelegrin-Valero C. Executive functions: the need for the integration of concepts. Rev Neurol. 2002;34:673–685. [PubMed] [Google Scholar]
- Tivis R, Beatty WW, Nixon SJ, Parsons OA. Patterns of cognitive impairment among alcoholics: are there subtypes? Alcohol Clin Exp Res. 1995;19:496–500. doi: 10.1111/j.1530-0277.1995.tb01537.x. [DOI] [PubMed] [Google Scholar]
- Toth K, Freund TF, Miles R. Disinhibition of rat hippocampal pyramidal cells by GABAergic afferents from the septum. J Physiol. 1997;500:463–474. doi: 10.1113/jphysiol.1997.sp022033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuck RR, Jackson M. Social, neurological and cognitive disorders in alcoholics. Med J Aust. 1991;155:225–229. doi: 10.5694/j.1326-5377.1991.tb142226.x. [DOI] [PubMed] [Google Scholar]
- Tzambazis K, Stough C. Alcohol impairs speed of information processing and simple and choice reaction time and differentially impairs higher-order cognitive abilities. Alcohol Alcohol. 2000;35:197–201. doi: 10.1093/alcalc/35.2.197. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CE, van Baal GC. Twin and family studies of the human electroencephalogram: a review and a meta-analysis. Biol Psychol. 2002;61:111–138. doi: 10.1016/s0301-0511(02)00055-8. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt T. The Genetics of Electrophysiological Indices of Brain Activity: An EEG study in Adolescent Twins. Universiteit Van Amsterdam; Amsterdam: 1996. [Google Scholar]
- van Boxtel GJ, van der Molen MW, Jennings JR, Brunia CH. A psychophysiological analysis of inhibitory motor control in the stop-signal paradigm. Biol Psychol. 2001;58:229–262. doi: 10.1016/s0301-0511(01)00117-x. [DOI] [PubMed] [Google Scholar]
- van der Molen MW. Developmental changes in inhibitory processing: evidence from psychophysiological measures. Biol Psychol. 2000;54:207–239. doi: 10.1016/s0301-0511(00)00057-0. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav. 2002;77:477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Velligan DI, Bow-Thomas CC. Executive function in schizophrenia. Semin Clin Neuropsychiatry. 1999;4:24–33. doi: 10.1053/SCNP00400024. [DOI] [PubMed] [Google Scholar]
- Vogel-Sprott M, Easdon C, Fillmore M, Finn P, Justus A. Alcohol and behavioral control: cognitive and neural mechanisms. Alcohol Clin Exp Res. 2001;25:117–121. [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Begleiter H, Hitzemann R, Pappas N, Burr G, Pascani K, Wong C, Fowler JS, Wolf AP. Regional brain metabolic response to lorazepam in subjects at risk for alcoholism. Alcohol Clin Exp Res. 1995;19:510–516. doi: 10.1111/j.1530-0277.1995.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Wang XJ. Pacemaker neurons for the theta rhythm and their synchronization in the septohippocampal reciprocal loop. J Neurophysiol. 2002;87:889–900. doi: 10.1152/jn.00135.2001. [DOI] [PubMed] [Google Scholar]
- Watanabe M. Prefrontal unit activity during delayed conditional Go/No Go discrimination in the monkey. II Relation to go and no-go responses. Brain Res. 1986;382:15–27. doi: 10.1016/0006-8993(86)90105-8. [DOI] [PubMed] [Google Scholar]
- Westphal KP, Grozinger B, Diekmann V, Scherb W, Reess J, Leibing U, Kornhuber HH. Slower theta activity over the midfrontal cortex in schizophrenic patients. Acta Psychiatr Scand. 1990;81:132–138. doi: 10.1111/j.1600-0447.1990.tb06465.x. [DOI] [PubMed] [Google Scholar]
- Williams JH, Kauer JA. Properties of carbachol-induced oscillatory activity in rat hippocampus. J Neurophysiol. 1997;78:2631–2640. doi: 10.1152/jn.1997.78.5.2631. [DOI] [PubMed] [Google Scholar]
- Wu M, Shanabrough M, Leranth C, Alreja M. Cholinergic Excitation of Septohippocampal GABA But Not Cholinergic Neurons: Implications for Learning and Memory. J Neurosci. 2000;20:3900–3908. doi: 10.1523/JNEUROSCI.20-10-03900.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylinen A, Soltesz I, Bragin A, Penttonen M, Sik A, Buzsaki G. Intracellular correlates of hippocampal theta rhythm in identified pyramidal cells, granule cells, and basket cells. Hippocampus. 1995;5:78–90. doi: 10.1002/hipo.450050110. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Kolev V. Event-related alpha oscillations are functionally associated with P300 during information processing. Neuroreport. 1998;9:3159–3164. doi: 10.1097/00001756-199810050-00007. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Kolev V. Brain theta response predicts P300 latency in children. Neuroreport. 1996;7:277–280. doi: 10.1097/00001756-199612200-00055. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Kolev V, Rosso OA, Schurmann M, Sakowitz OW, Ozgoren M, Basar E. Wavelet entropy analysis of event-related potentials indicates modality-independent theta dominance. J Neurosci Methods. 2002;117:99–109. doi: 10.1016/s0165-0270(02)00095-x. [DOI] [PubMed] [Google Scholar]
- Zucker RA, Gomberg ES. Etiology of alcoholism reconsidered. The case for a biopsychosocial process. Am Psychol. 1986;41:783–793. doi: 10.1037//0003-066x.41.7.783. [DOI] [PubMed] [Google Scholar]