Abstract
Children raised in poverty are prone to physical health problems late in life. To understand these findings and address the scientific challenge they represent, we must formulate integrative conceptual frameworks at the crossroads of behavioral and biomedical science, with a strong developmental emphasis. In this article, we outline such a framework and discuss research bearing on its validity. We address how childhood poverty gets under the skin, at the level of tissues and organs, in a manner that affects later disease risks. We also tackle questions about resilience; Even with lengthy exposure to childhood poverty, why do only a subset of people acquire diseases? Why are some individuals protected while others remain vulnerable? Maternal nurturance might be a source of resilience, buffering children from the long-term health consequences of poverty. We conclude with research priorities.
Scientists and physicians have changed how they think about late-life diseases. Long viewed as manifestations of aging, a number of common medical problems—including heart disease, stroke, and certain cancers–are now understood to be life course conditions that begin developing as early as childhood (Coe & Lubach, 2003; Gluckman et al., 2008; Matthews, 2005; Shonkoff et al., 2009). Some of the most provocative evidence for this perspective comes from studies of childhood socioeconomic status (SES). In dozens of large prospective studies, low childhood SES has been linked with adult health problems, including premature mortality and vulnerability to respiratory illness, cardiovascular disease, and other conditions (Galobardes et al., 2008; Hertzman & Boyce, 2010). Importantly, these associations are generally independent of traditional risk factors such as family history, racial/ethnic background, and cigarette smoking.
Like any epidemiologic findings, those on childhood SES can be interpreted in different ways (Cohen et al., 2010). First, risky genes may have aggregated in some families, predisposing them to both poverty and disease. Second, childhood illness may be an underlying confounder, with sick children both imperiling their families’ ability to save and also being prone to later health problems. Finally, childhood SES could simply act as a proxy for a lifetime of poverty (Matthews & Gallo, 2011). SES is fairly stable across the lifespan and has strong concurrent links with health (Braveman et al., 2010), so we could be observing the health effects of cumulative, rather than childhood, SES.
Each of these mechanisms explains some of the long-term health consequences attributed to low childhood SES. However, these pathways do not explain the phenomenon completely, according to studies using exceptional samples (e.g., Kittleson et al., 2006) and careful statistical controls (e.g., Melchior et al., 2007). Moreover, quasi-experimental studies of newborns adopted into higher-SES families and truly experimental studies of animals randomized to early-life deprivation (Avitsur et al., 2006; Kruschinski et al., 2008; Osler et al., 2006), together with the observational work, show that low childhood SES is causally related to later disease risks, at least in some categories. We also know that childhood SES affects later health in a manner that goes beyond shaping future socioeconomic attainment (e.g., Kittleson et al., 2006).
These findings have important practical implications. Since the recession that began in 2008, childhood poverty rates in the United States have climbed steadily. If low childhood SES exerts a lasting health toll, the recession’s biomedical repercussions could be felt well into the middle of the 21st century. These patterns also pose an important scientific challenge. To understand them, we need to formulate integrative conceptual frameworks at the crossroads of the behavioral and biomedical sciences, with a strong developmental emphasis (Matthews & Gallo, 2011; Taylor, 2010). These frameworks should address questions such as: How does childhood disadvantage get under the skin, at the level of tissues and organs, to affect risk for later medical problems? How does it remain there across multiple decades, slowly pushing forward the processes that give rise to disease? Questions about resilience are also relevant. Even with lengthy exposure to childhood poverty, only some people go on to develop any particular disease (Chen et al., 2012). Why are some individuals protected while others remain vulnerable?
Toward a Mechanistic Understanding
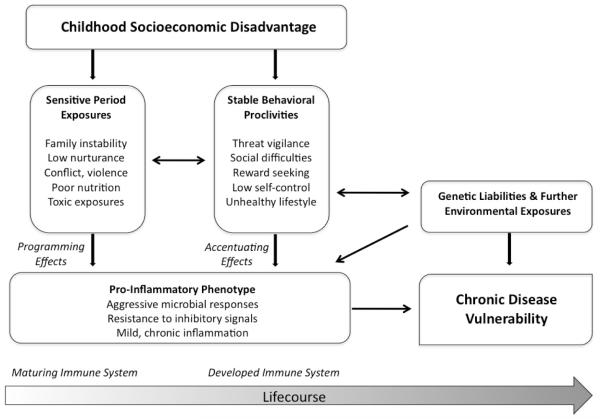
Our research team is tackling these questions, guided by the Biological Embedding Model in Figure 1 (Miller et al., 2011a). This framework draws on insights from research on fetal programming, stress physiology, and emotional development. It begins by articulating the social and physical conditions to which low-SES children are disproportionately exposed. (We acknowledge that our work has focused solely on the experiences of children in high-income Western countries.) Socially, these conditions can include unstable family structures, caregivers who are unresponsive and use harsh discipline, and neighborhood violence (Conger & Donnellan, 2007; Repetti et al., 2002). Physically, low-SES children may experience household crowding, inadequate nutrition, and more exposure to second-hand smoke, infectious micro-organisms, and industrial pollutants (Evans, 2004; Wright & Subramanian, 2007).
Figure 1.
Model depicting how early disadvantage brings about pro-inflammatory phenotype and subsequent vulnerability to health problems.
The model articulates how these conditions enable disadvantage to become embedded in the function of immune cells. As others suggest (Coe & Lubach, 2008; Wright, 2007), social and physical “pollutants” can get programmed into cells of the immune system called monocytes, whose main function is to regulate inflammation. As a result of this programming, monocytes are endowed with a pro-inflammatory phenotype (as are the cells they eventually mature into, called macrophages). Because this process unfolds during sensitive periods of immunologic development, when the system is maximally plastic, the phenotype gets embedded in a manner that persists across the lifespan. As a result, when monocytes and macrophages subsequently encounter microbial invaders, they mount especially aggressive inflammatory responses and are relatively insensitive to signals that normally terminate those responses. In the context of acute health threats, this stance might be adaptive, facilitating recovery from injuries and infections. But it would come at a long-term cost (McDade, 2005): By mounting aggressive responses that are difficult to contain, the child’s immune system would be in a perpetual state of mild inflammation. Over the lifespan, this kind of persistent inflammation is known to foster pathogenic changes that lead to diseases of aging.
According to the model, over the life course, these pro-inflammatory tendencies are accentuated through behavioral proclivities that are also brought on by childhood disadvantage. Because low-SES children are frequently exposed to life events that are uncontrollable and unpredictable, they come to view the world as a place that requires constant vigilance against threats and mistrust of others (Chen et al., 2004). These traits are the starting points for a self-promoting cycle of social difficulties, marked by conflictual, unsupportive interactions (Repetti et al., 2011). In low-SES settings, children also may develop poor self-regulation skills (Blair & Raver, 2012; Repetti et al., 2002) because meeting basic needs requires them to discount the future in favor of accessing immediate resources that could soon disappear. These traits can result in unhealthy behaviors like cigarette smoking, or a poor diet and sedentary lifestyle, culminating in obesity. As children mature, these social difficulties and unhealthy lifestyles persist, further accentuating pro-inflammatory tendencies (Miller et al., 2011a; Repetti et al., 2011; Taylor, 2010). Through these pathways, behavioral proclivities from childhood experience continue to reverberate in the immune system.
Why do pro-inflammatory tendencies matter for health? When the body is injured or infected with a pathogen, the immune system launches an inflammatory response, causing white blood cells to accumulate at the afflicted site. Macrophages, along with dendritic cells, attempt to eliminate the pathogen, rid the body of infected tissue, and repair any damage. All this is orchestrated by molecules called pro-inflammatory cytokines, such as interleukin-1β (Ia–1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-a). These molecules have wide-ranging functions that include directing cells to sites of injury and infection, signaling them to proliferate and differentiate, and activating functions related to killing and repair.
The inflammatory response is essential for survival—without it, minor injuries or infections would become lethal. However, if it becomes persistent, the response can lead to various diseases. Indeed, researchers increasingly view mild, chronic inflammation as an important contributor to common illnesses associated with aging, including heart disease, stroke, autoimmune disorders, and some cancers (Nathan and Ding, 2010).
The body has natural mechanisms to slow down inflammation, one of which involves the hormone cortisol. Cortisol acts by binding to glucocorticoid receptors inside monocytes and macrophages, and then sequestering the molecular machinery that normally orchestrates inflammation (Sternberg, 2006). These actions explain why synthetic versions of cortisol, like prednisone, are such effective anti-inflammatory medications.
Early Disadvantage and Later Inflammation
Our model hypothesizes that early disadvantage programs a pro-inflammatory phenotype and gives rise to behavioral proclivities that continually accentuate this tendency. The phenotype has two manifestations: First, when monocytes and macrophages encounter microbial invaders, they mount especially aggressive inflammatory responses. Second, these cells become relatively insensitive to signals, like cortisol, that normally terminate the inflammatory response.
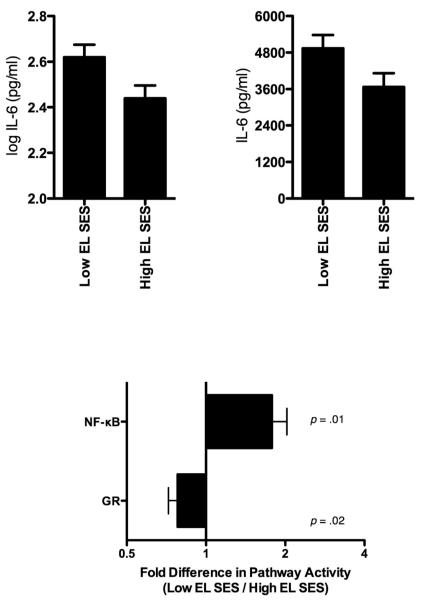
In a first test of these ideas, we recruited healthy young adults (Miller et al., 2009). Half had been raised in low-SES families, as indicated by their parents’ occupations, and half came from high-SES families. The groups were matched on current occupational prestige, which minimized the potential for confounding by adult SES. To examine the hypothesis that early disadvantage lays the foundation for a pro-inflammatory phenotype, we drew blood and cultured participants’ white blood cells in vitro with a series of microbial products. We measured how aggressively their cells responded to these products by looking at production of IL-6, a cytokine that regulates inflammation. Participants raised in low-SES families had more pronounced IL-6 responses to flagellin, a bacterial product, and to poly I:C, a viral analogue, than participants from high-SES families (see Figure 2). These associations persisted after adjustment for various demographic and biobehavioral confounders. Thus, early life disadvantage had prompted monocytes—the cells that generate most of the IL-6 in the culture system we used—to mount relatively aggressive responses to some microbial stimuli.
Figure 2.
The peripheral blood mononuclear cells of adults reared in low-SES families have more pronounced IL-6 responses to poly I:C (a viral analogue, upper left) and flagellin (a bacterial product, upper right), compared with adults from high-SES families. Those raised in low-SES families also display upregulation of genes with response elements for NF-κB, the molecular lynchpin of inflammation, and downregulation of genes with response elements for the anti-inflammatory glucocrticoid receptor (bottom panel).
The model suggests that in addition to prompting aggressive microbial responses, early disadvantage renders monocytes resistant to anti-inflammatory signals like cortisol. To test this idea, we extracted RNA from participants’ white blood cells and performed genome-wide transcriptional profiling. This technique quantified the activity of 18,000+ different genes–nearly all those used by humans–and revealed how “loudly” participants’ cells were “hearing” various molecular signals (Miller & Cole, 2010). Among participants raised in low-SES families, genes with response elements for nuclear factor-kappa B (NF-kB), the molecular lynchpin of inflammation (see Figure 2b), were more active. At the same time, genes with response elements for the glucocorticoid receptor, were less active; the glucocorticoid receptor binds cortisol and enables it to exert anti-inflammatory effects, like blocking the activities of NF-kB. Notably, there were no SES differences in cortisol. Early disadvantage may have tuned participants’ monocytes to be partially resistant to anti-inflammatory signals transmitted via cortisol.
Resilience to Disadvantage
Not all low-SES children develop medical problems as adults. In fact, a sizeable minority stay in good health across the lifespan, as was illustrated in a study in which adults were intentionally exposed to a rhinovirus and monitored for developing the common cold (Cohen et al., 2004). To the extent they were raised in low-SES families, participants were more likely to develop colds. But even among those from the lowest SES category, fewer than half actually became sick. Findings like these raise questions about differential vulnerability. Why do some individuals succumb to the health effects of early disadvantage while others are protected? Research on resilience suggests that parental nurturance plays a role. Poor families face a multitude of stressors that require emotional resources that parents might otherwise devote to nurturing (Conger & Donnellan, 2007). Nonetheless, when children grapple with major stressors, a nurturing adult can be a powerful buffer, reducing the risk of social, academic, and psychiatric difficulties (Luthar, 2006). These benefits may extend to health, as seen in a study of rural youths that found that maternal nurturance buffered disadvantaged children from increases in allostatic load, a composite indicator of risk in various physiological systems (Evans et al., 2007).
When we analyzed our dataset further, using participants’ retrospective accounts of maternal warmth (Chen et al., 2011), mothers’ warmth may have acted as a buffer against the pro-inflammatory tendencies previously observed. Specifically, among participants who were raised in low-SES families, maternal warmth was associated with smaller IL-6 responses to certain microbial stimuli and reduced activity of genomic signaling pathways that support inflammation. Importantly, these associations were independent of depressive symptoms, socially desirable responding, and other traits that might bias participants’ retrospective accounts of maternal warmth.
To determine the robustness of these patterns, we analyzed a national sample of adults, the Midlife in the United States (MIDUS) study (Miller et al., 2011b). In addition to replicating the findings regarding maternal nurturance, we hoped to examine their implications for midlife health by assessing the metabolic syndrome (MetS), a cluster of signs that includes high blood pressure, impaired glucose control, abdominal adiposity, and lipid dysregulation. MetS, a precursor and contributor to diabetes, heart disease, stroke, and other conditions, is highly prevalent in the United States, with rates estimated at 25 to 39 percent and rising. As expected, low childhood SES was associated with higher MetS prevalence at midlife, independent of traditional risk factors. But almost half of participants raised in low-SES households were free of MetS at midlife, suggesting that resilience was common.
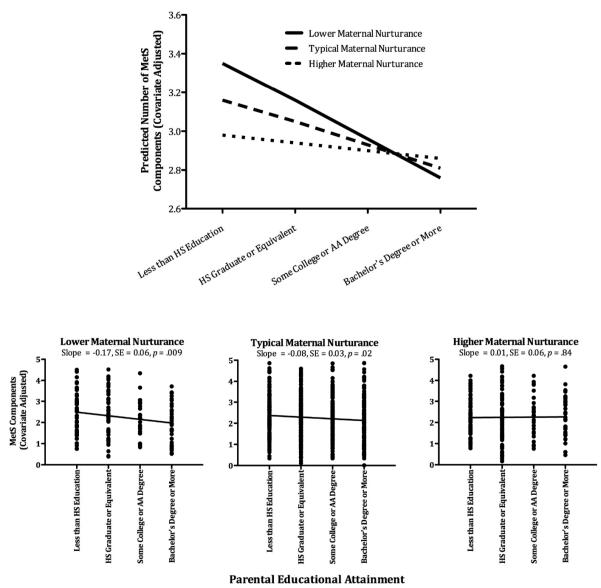
To identify potential sources of resilience, we analyzed participants’ retrospective accounts of nurturance. Once again, maternal nurturance offset the metabolic consequences of childhood disadvantage (see Figure 3), suggesting buffering. Indeed, childhood disadvantage was robustly associated with MetS among participants who recalled low maternal nurturance. But this association became progressively weaker as participants recalled more nurturance. In fact, among those who recalled maternal nurturance one standard deviation above the sample average, there was no association between childhood SES and MetS.
Figure 3.
Maternal nurturance buffers against higher counts of MetS components in those from low-SES backgrounds. The top panel displays counts of MetS components as a function of childhood SES and maternal nurturance, which is depicted at lower (−1 SD), typical (sample mean), and higher (+1 SD) levels of the sample distribution. The bottom panel displays counts of MetS components by childhood SES. For illustration, the sample has been stratified into tertiles on maternal nurturance.
While provocative, both of these studies on resilience had important limitations, particularly their cross-sectional designs and reliance on retrospective accounts. These features make it difficult to draw firm conclusions about directionality and pinpoint what processes actually functioned as buffers. Clearly, more research using prospective designs and better measurement is needed to elucidate these issues.
Family Emotional Climate
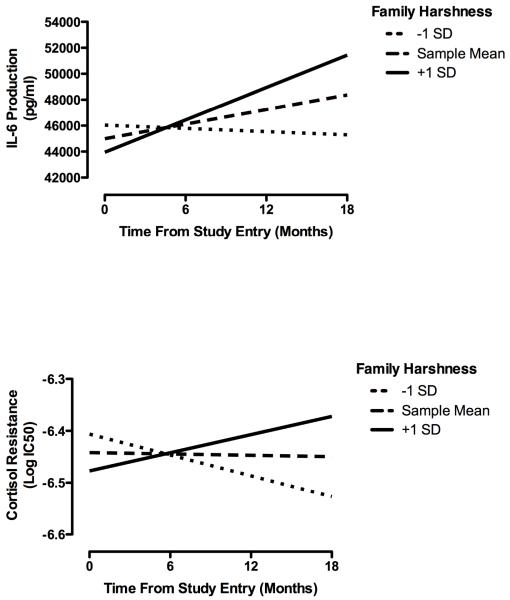
The findings on nurturance may help us understand the mechanisms of disadvantage. In particular, they suggest that the health consequences of early disadvantage are conferred, at least partly, through the emotional climate in an individual’s family of origin. In a final study, we tested this possibility, using a more methodologically rigorous and developmentally sensitive design in which female adolescents were assessed on four occasions over 1.5 years (Miller & Chen, 2010). At baseline, participants completed the Risky Families Questionnaire, which assessed the harshness of their early family climate. At all visits, we collected blood to measure the emergence of pro-inflammatory tendencies. To the extent that they were raised in harsh families, participants manifested an increasingly pro-inflammatory phenotype over the 1.5-year follow-up (see Figure 4) that was marked by increasingly aggressive IL-6 responses to an in vitro bacterial stimulus as well as increasing resistance to cortisol’s anti-inflammatory properties. In participants from harsher families, cortisol became progressively less effective at blocking cytokine production.
Figure 4.
Early-life family climate and inflammatory trajectory in adolescence. To the extent that they were raised in a harsh family climate,, participants had increasing stimulated IL-6 production over the followup (upper panel), and became less sensitive (more resistant) to cortisol’s anti-inflammatory properties (lower panel). Family harshness depicted at lower (−1 SD), typical (sample mean), and higher (+1 SD) levels of the sample distribution. IL-6 was measured in whole blood cultures incubated with the bacterial product lipopolysaccharide for 6 hours.
Looking Ahead
Childhood disadvantage leaves an immunologic residue that manifests in aggressive pro-inflammatory responses to microbial stimuli and resistance to cortisol-propagated anti-inflammatory signals. Research should elucidate the pathways through which the pro-inflammatory phenotype is instantiated. At least two scenarios are plausible and they are not mutually exclusive. The first is a biological programming scenario in which durable functional changes get embedded in cells during sensitive periods of immunologic maturation. To evaluate this possibility, research will need to delve further into molecular biology, where scientists are identifying epigenetic and transcriptional mechanisms that allow cells to “remember” past events (Robison & Nestler, 2011).
The second scenario emphasizes the ways that childhood disadvantage continues to reverberate in people’s behavior, relationships, and personality over the life course (Caspi, 2000). Multiwave prospective studies should be done that map trajectories of behavior, biology, and disease across development. Some teams have already begun this process. Using structural equation modeling, one study found that low SES fosters a harsh family-of-origin environment, which leads to diminished psychosocial functioning in adulthood, and then to low-grade inflammation (Taylor et al., 2006). Another team used multiwave data from a birth cohort to show how early disadvantage gives rise to lifestyle practices, social difficulties, and mood problems, with ramifications for health in early adulthood (Danese et al., 2009; Melchior et al., 2007).
Besides detailing the pathways to a pro-inflammatory phenotype, research must take a broader mechanistic and geopolitical view. Inflammation is a central biological pathway to many diseases, but other behavioral and biological pathways, involving the cardiac, vascular, neural, and metabolic systems, may also contribute (e.g., Blair & Raver, 2011; Matthews, 2005; Taylor, 2011). Finally, our research has focused on children in high-income Western countries; it is unclear how generalizable the findings are to developing countries, where poverty is even more common and extreme, and the scarcity of basic resources may create entirely different pathways of vulnerability.
Some people are buffered from the health consequences of early disadvantage, and maternal nurturance could be a source of this resilience. Given the methodologic limitations of the studies presented, a top priority is to substantiate them using more rigorous prospective designs and better measurement tools. Also important are studies aimed at delineating the mechanisms of buffering. Does parental nurturance offset children’s biological responses to stress in real time? Does it imbue children with psychological resources–trust of others, a sense of meaning, or self-regulation skills–that help them navigate challenges later in development? Or does it lead to different exposure profiles altogether? Children’s experiences of poverty vary markedly. Maternal nurturance could operate by limiting children’s exposures to the social and physical pollutants discussed earlier, with downstream implications for immunologic development. Of course, any of these mechanisms could be operative, and so could a multitude of others, working at the individual, family, neighborhood, or institutional levels. If research can pinpoint the specific mechanisms that confer resilience, that knowledge might serve as the basis for interventions that improve health through the life course by building stronger families.
Acknowledgments
Preparation of this article was supported by grant R01 HD058502 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
Neither author reports a scientific or financial conflict of interest.
References
- Avitsur R, Hunzeker J, Sheridan JF. Role of early stress in the individual differences in host response to viral infection. Brain, Behavior, and immunity. 2006;20(4):339–348. doi: 10.1016/j.bbi.2005.09.006. doi:10.1016/j.bbi.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Blair C, Raver CC. Child development in the context of adversity: experiential canalization of brain and behavior. American Psychologist. 2012;67(4):309–318. doi: 10.1037/a0027493. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=22390355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconomic disparities in health in the United States: what the patterns tell us. American Journal of Public Health. 2010;100(Suppl 1):S186–96. doi: 10.2105/AJPH.2009.166082. doi:10.2105/AJPH.2009.166082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A. The child is father of the man: Personality continuities from childhood to adulthood. Journal of Personality and Social Psychlogy. 2000;78:158–172. doi: 10.1037//0022-3514.78.1.158. doi:10.1037/0022-3514.78.1.158. [DOI] [PubMed] [Google Scholar]
- Chen E, Langer DA, Raphaelson YE, Matthews KA. Socioeconomic status and health in adolescents: the role of stress interpretations. Child Development. 2004;75(4):1039–1052. doi: 10.1111/j.1467-8624.2004.00724.x. doi:10.1111/j.1467-8624.2004.00724.x. [DOI] [PubMed] [Google Scholar]
- Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Molecular Psychiatry. 2011;16(7):729–737. doi: 10.1038/mp.2010.53. doi:10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Miller GE, Lachman ME, Gruenewald TL, Seeman TE. Protective factors for adults from low-childhood socioeconomic circumstances: the benefits of shift-and-persist for allostatic load. Psychosomatic Medicine. 2012;74(2):178–186. doi: 10.1097/PSY.0b013e31824206fd. doi:10.1097/PSY.0b013e31824206fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe CL, Lubach GR. Fetal programming: Prenatal origins of health and disease. Current Directions in Psychological Science. 2008;17:36–41. doi:10.1111/j.1467-8721.2008.00544.x. [Google Scholar]
- Coe CL, Lubach GR. Critical periods of special health relevance for psychoneuroimmunology. Brain Behavior and Immunity. 2003;17:3–12. doi: 10.1016/s0889-1591(02)00099-5. doi:10.1016/S0889-1591(02)00099-5. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Chen E, Matthews KA. Childhood socioeconomic status and adult health. Annals of the New York Academy of Sciences. 2010;1186:37–55. doi: 10.1111/j.1749-6632.2009.05334.x. doi:10.1111/j.1749-6632.2009.05334.x. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Turner RB, Alper CM, Skoner DP. Childhood socioeconomic status and host resistance to infectious illness in adulthood. Psychosomatic Medicine. 2004;66(1534-7796):553–558. doi: 10.1097/01.psy.0000126200.05189.d3. doi:10.1097/01.psy.0000126200.05189.d3. [DOI] [PubMed] [Google Scholar]
- Conger RD, Donnellan MB. An interactionist perspective on the socioeconomic context of human development. Annual Review of Psychology. 2007;58:175–199. doi: 10.1146/annurev.psych.58.110405.085551. doi:10.1146/annurev.psych.58.110405.085551. [DOI] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, et al. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Archives of Pediatric and Adolescent Medicine. 2009;163(12):1135–1143. doi: 10.1001/archpediatrics.2009.214. doi:10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW. The environment of childhood poverty. American Psychologist. 2004;59(2):77–92. doi: 10.1037/0003-066X.59.2.77. doi:10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- Evans GW, Kim P, Ting AH, Tesher HB, Shannis D. Cumulative risk, maternal responsiveness, and allostatic load among young adolescents. Developmental Psychology. 2007;43(2):341–351. doi: 10.1037/0012-1649.43.2.341. doi:10.1037/0012-1649.43.2.341. [DOI] [PubMed] [Google Scholar]
- Galobardes B, Lynch JW, smith GD. Is the association between childhood socioeconomic circumstances and cause-specific mortality established? Update of a systematic review. Journal of Epidemiology and Community Health. 2008;62(5):387–390. doi: 10.1136/jech.2007.065508. doi:10.1136/jech.2007.065508. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. New England Journal of Medicine. 2008;359(1):61–73. doi: 10.1056/NEJMra0708473. doi:10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzman C, Boyce T. How experience gets under the skin to create gradients in developmental health. Annual Review of Public Health. 2010;31:329–347. doi: 10.1146/annurev.publhealth.012809.103538. doi:10.1146/annurev.publhealth.012809.103538. [DOI] [PubMed] [Google Scholar]
- Kittleson MM, Meoni LA, Wang NY, Chu AY, Ford DE, Klag MJ. Association of childhood socioeconomic status with subsequent coronary heart disease in physicians. Archives of Internal Medicine. 2006;166(21):2356–2361. doi: 10.1001/archinte.166.21.2356. doi:10.1001/archinte.166.21.2356. [DOI] [PubMed] [Google Scholar]
- Kruschinski C, Skripuletz T, Bedoui S, Raber K, Straub RH, Hoffmann T, et al. Postnatal life events affect the severity of asthmatic airway inflammation in the adult rat. Journal of Immunology. 2008;180(6):3919–3925. doi: 10.4049/jimmunol.180.6.3919. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18322200. [DOI] [PubMed] [Google Scholar]
- Luthar SS. Resilience in development: A synthesis of research across five decades. Developmental psychopathology. In: Cicchetti D, Cohen DJ, editors. Risk, disorder, and adaptation. 2nd ed. Vol. 3. John Wiley & Sons; New York: 2006. pp. 739–795. [Google Scholar]
- Matthews KA, Gallo LC. Psychological perspectives on pathways linking socioeconomic status and physical health. Annual Review of Psychology. 2011;62:501–530. doi: 10.1146/annurev.psych.031809.130711. doi:10.1146/annurev.psych.031809.130711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA. Psychological perspectives on the development of coronary heart disease. American Psychologist. 2005;60(8):783–796. doi: 10.1037/0003-066X.60.8.783. doi:10.1037/0003-066X.60.8.783. [DOI] [PubMed] [Google Scholar]
- McDade TW. Life history, maintenance, and the early origins of immune function. American Journal of Human Biology. 2005;17(1):81–94. doi: 10.1002/ajhb.20095. doi:10.1002/ajhb.20095. [DOI] [PubMed] [Google Scholar]
- Melchior M, Moffitt TE, Milne BJ, Poulton R, Caspi A. Why do children from socioeconomically disadvantaged families suffer from poor health when they reach adulthood? A life-course study. American Journal of Epidemiology. 2007;166(8):966–974. doi: 10.1093/aje/kwm155. doi:10.1093/aje/kwm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E. Harsh family climate in early life presages the emergence of pro-inflammatory phenotype in adolescence. Psychological Science. 2010;21:848–856. doi: 10.1177/0956797610370161. doi:10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok A, Walker H, Lim A, Nicholls EP, et al. Low early-life social class leaves a biological residue manifest by decreased glucocorticoid and increased pro-inflammatory signaling. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14716–14721. doi: 10.1073/pnas.0902971106. doi:10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cole SW. Functional genomic approaches in behavioral medicine research. In: Steptoe A, editor. Handbook of behavioral medicine: Methods and applications. Springer; London: 2010. pp. 443–454. [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin. 2011a;137:959–997. doi: 10.1037/a0024768. doi:10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Lachman ME, Chen E, Gruenewald TL, Karlamangla AS, Seeman TE. Pathways to resilience: maternal nurturance as a buffer against the effects of childhood poverty on metabolic syndrome at midlife. Psychol Sci. 2011b;22(12):1591–1599. doi: 10.1177/0956797611419170. doi:10.1177/0956797611419170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140(6):871–882. doi: 10.1016/j.cell.2010.02.029. doi:10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Osler M, Petersen L, Prescott E, Teasdale TW, Sorensen TI. Genetic and environmental influences on the relation between parental social class and mortality. International Journal of Epidemiology. 2006;35(5):1272–1277. doi: 10.1093/ije/dyl045. doi:10.1093/ije/dyl045. [DOI] [PubMed] [Google Scholar]
- Repetti RL, Robles TF, Reynolds B. Allostatic processes in the family. Development and Psychopathology. 2011;23(3):921–938. doi: 10.1017/S095457941100040X. doi:10.1017/S095457941100040X. [DOI] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128(2):330–366. doi:10.1037/0033-2909.128.2.330. [PubMed] [Google Scholar]
- Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nature Reviews Neuroscience. 2011;12(11):623–637. doi: 10.1038/nrn3111. doi:10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301(21):2252–2259. doi: 10.1001/jama.2009.754. doi:10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Sternberg EM. Neural regulation of innate immunity: A coordinated nonspecific host response to pathogens. Nature Reviews Immunology. 2006;6(4):318–328. doi: 10.1038/nri1810. doi:10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE. Mechanisms linking early life stress to adult health outcomes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(19):8507–8512. doi: 10.1073/pnas.1003890107. doi:10.1073/pnas.1003890107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biological Psychiatry. 2006;60(8):819–824. doi: 10.1016/j.biopsych.2006.03.016. doi:10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Wright RJ. Prenatal maternal stress and early caregiving experiences: implications for childhood asthma risk. Paediatric and Perinatal Epidemiology. 2007;21(suppl 3):8–14. doi: 10.1111/j.1365-3016.2007.00879.x. doi:10.1111/j.1365-3016.2007.00879.x. [DOI] [PubMed] [Google Scholar]
- Wright RJ, Subramanian SV. Advancing a multilevel framework for epidemiologic research on asthma disparities. Chest. 2007;132(5 Suppl):757S–769S. doi: 10.1378/chest.07-1904. doi:10.1378/chest.07-19. [DOI] [PubMed] [Google Scholar]