Abstract
BACKGROUND
Peginterferon–ribavirin therapy is the current standard of care for chronic infection with hepatitis C virus (HCV). The rate of sustained virologic response has been below 50% in cases of HCV genotype 1 infection. Boceprevir, a potent oral HCV-protease inhibitor, has been evaluated as an additional treatment in phase 1 and phase 2 studies.
METHODS
We conducted a double-blind study in which previously untreated adults with HCV genotype 1 infection were randomly assigned to one of three groups. In all three groups, peginterferon alfa-2b and ribavirin were administered for 4 weeks (the leadin period). Subsequently, group 1 (the control group) received placebo plus peginterferon–ribavirin for 44 weeks; group 2 received boceprevir plus peginterferon–ribavirin for 24 weeks, and those with a detectable HCV RNA level between weeks 8 and 24 received placebo plus peginterferon–ribavirin for an additional 20 weeks; and group 3 received boceprevir plus peginterferon–ribavirin for 44 weeks. Nonblack patients and black patients were enrolled and analyzed separately.
RESULTS
A total of 938 nonblack and 159 black patients were treated. In the nonblack cohort, a sustained virologic response was achieved in 125 of the 311 patients (40%) in group 1, in 211 of the 316 patients (67%) in group 2 (P<0.001), and in 213 of the 311 patients (68%) in group 3 (P<0.001). In the black cohort, a sustained virologic response was achieved in 12 of the 52 patients (23%) in group 1, in 22 of the 52 patients (42%) in group 2 (P = 0.04), and in 29 of the 55 patients (53%) in group 3 (P = 0.004). In group 2, a total of 44% of patients received peginterferon–ribavirin for 28 weeks. Anemia led to dose reductions in 13% of controls and 21% of boceprevir recipients, with discontinuations in 1% and 2%, respectively.
CONCLUSIONS
The addition of boceprevir to standard therapy with peginterferon–ribavirin, as compared with standard therapy alone, significantly increased the rates of sustained virologic response in previously untreated adults with chronic HCV genotype 1 infection. The rates were similar with 24 weeks and 44 weeks of boceprevir. (Funded by Schering-Plough [now Merck]; SPRINT-2 ClinicalTrials.gov number, NCT00705432.)
Chronic infection with the hepatitis C virus (HCV) affects more than 170 million people worldwide.1,2 Rates of sustained virologic response associated with peginterferon–ribavirin therapy remain below 50% and are often less than 30% among patients who have HCV genotype 1 infection and certain baseline characteristics, such as advanced fibrosis, diabetes, coinfection with the human immunodeficiency virus (HIV), or African heritage.3-9 Recent efforts to improve the rate of sustained virologic response have focused on oral direct-acting antiviral agents.10-13
Boceprevir is a linear peptidomimetic ketoamide serine protease inhibitor that binds reversibly to the HCV nonstructural 3 (NS3) active site.14 Like other protease inhibitors, boceprevir must be given with peginterferon–ribavirin to minimize the emergence of viral resistance.15,16 In the SPRINT-2 (Serine Protease Inhibitor Therapy 2) trial, we examined whether the addition of boceprevir to standard therapy could improve the rates of sustained virologic response in previously untreated patients infected with HCV genotype 1.
METHODS
STUDY DESIGN
A detailed description of the study methods is provided in the Supplementary Appendix (available with the full text of this article at NEJM.org). We conducted a phase 3, international, randomized, placebo-controlled study comparing the safety and efficacy of standard therapy with peginterferon alfa-2b and ribavirin (PegIntron and Rebetol, respectively; Merck) with the safety and efficacy of two treatment regimens in which boceprevir was added after a lead-in period of treatment with peginterferon–ribavirin alone (Fig. 1). Because of the marked difference in rates of sustained virologic response between blacks and nonblacks,7 self-identified blacks and nonblacks were enrolled separately into two cohorts.
Figure 1. Study Design.
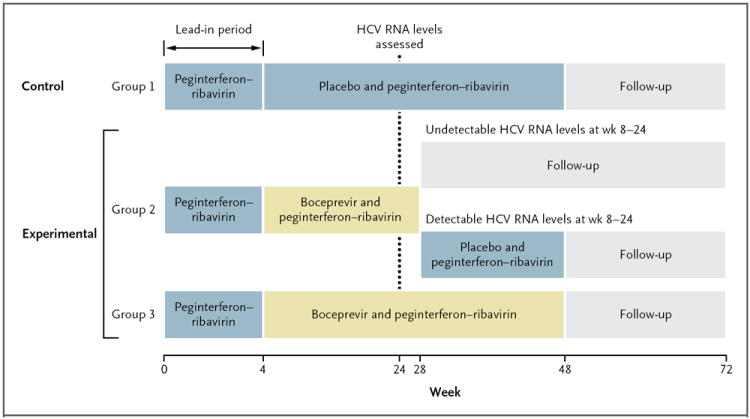
Patients in each of the two study cohorts were randomly assigned to a treatment group in a 1:1:1 ratio. All patients received peginterferon alfa-2b–ribavirin during the 4-week lead-in period. Subsequently, patients assigned to group 1 received 44 weeks of peginterferon alfa-2b–ribavirin as well as a placebo capsule; patients assigned to group 3 received peginterferon–ribavirin as well as boceprevir for 44 weeks; and patients assigned to group 2 received peginterferon–ribavirin as well as boceprevir for 24 weeks, and those with a detectable hepatitis C virus (HCV) RNA level at any visit between weeks 8 and 24 received peginterferon–ribavirin plus placebo from week 28 to week 48. Treatment was discontinued for reasons of futility if the HCV RNA level was detectable at the week 24 visit. Boceprevir was given for a total of 24 weeks in group 2 (irrespective of the rapidity of the decrease in the viral load) and, unless futility had been shown, for a total of 44 weeks in group 3. The x-axis numbers are not to scale.
The sponsor, patients, and study personnel were unaware of the assignment to the boceprevir or placebo group; the use of peginterferon and ribavirin was open label. The trial was conducted in accordance with the principles of Good Clinical Practice and the study protocol (including the data analysis plan; available at NEJM.org); the study design was approved by the appropriate institutional review boards and regulatory agencies. Each participant provided written informed consent before undergoing any study-related procedure. The trial was designed, managed, and analyzed by the industry authors in conjunction with the academic authors under the oversight of an independent data review advisory board. The academic authors had full access to all the data. The core writing team consisted of the principal academic author and all the industry authors, who were also responsible for the decision to submit the manuscript for publication, before which the sponsor reviewed a draft. Each author vouches for the fidelity of the trial conduct to the protocol and the completeness and accuracy of the results and data analyses.
Enrolled patients in each cohort were randomly assigned, in a 1:1:1 ratio and by means of an interactive voice-response system, to one of the three treatment groups, after stratification on the basis of the baseline HCV RNA level (≤400,000 vs. >400,000 IU per milliliter) and HCV genotype 1 subtype (1a vs. 1b). Patients in whom HCV could not be subtyped were randomly assigned to a treatment group within their HCV RNA stratum.
SELECTION OF PATIENTS
Eligibility criteria were a history of no previous treatment for HCV infection, age of 18 years or older, weight of 40 to 125 kg, chronic infection with HCV genotype 1, and plasma HCV RNA level of 10,000 IU per milliliter or greater. Exclusion criteria were liver disease of other cause, decompensated cirrhosis, renal insufficiency, HIV or hepatitis B infection, pregnancy or current breast-feeding, and active cancer. Liver-biopsy specimens were assigned Metavir fibrosis scores and steatosis scores by a single academic author who is a pathologist and was unaware of the assignment to the boceprevir or placebo group. The HCV genotype 1 subtype was determined with the use of the Trugene assay (Bayer Diagnostics) for purposes of randomization and by sequencing of the nonstructural 5B (NS5B) region (Virco) for subsequent analyses.
STUDY REGIMENS
Peginterferon alfa-2b was administered subcutaneously at a dose of 1.5 μg per kilogram of body weight once weekly; and weight-based oral ribavirin was administered at a total dose of 600 to 1400 mg per day in divided doses, given in the morning and evening. Treatment with boceprevir consisted of oral administration at a dose of 800 mg three times daily (to be taken with food and at an interval of 7 to 9 hours between doses) in four capsules of 200 mg each. Placebo was matched to boceprevir.
All patients received peginterferon–ribavirin during the 4-week lead-in period (Fig. 1). Patients randomly assigned to group 1 (the standard of care) received peginterferon–ribavirin treatment for 44 weeks after the lead-in period, as well as thrice-daily placebo beginning at week 5. Patients randomly assigned to group 2 (response-guided therapy) received peginterferon–ribavirin plus boceprevir for a total of 24 weeks after the lead-in period; if HCV RNA levels were undetectable from week 8 through week 24, treatment was considered complete, but if HCV RNA levels were detectable at any visit from week 8 up to but not including week 24, peginterferon–ribavirin was continued, and placebo was administered, at week 28 through week 48. Patients randomly assigned to group 3 (fixed-duration therapy) received peginterferon–ribavirin plus oral boceprevir for 44 weeks after the lead-in period.
In all three groups, the study treatment was discontinued for all patients with a detectable HCV RNA level at week 24, according to a standard futility rule. Boceprevir was given for 24 weeks in group 2 and 44 weeks in group 3. All patients were followed through week 72.
Viral breakthrough was defined as achievement of an undetectable HCV RNA level and subsequent occurrence of an HCV RNA level greater than 1000 IU per milliliter. Incomplete virologic response and rebound was defined as an increase of 1 log10 IU per milliliter in the HCV RNA level from the nadir, with an HCV RNA level greater than 1000 IU per milliliter (if both samples being compared were collected the same number of days after the last peginterferon injection). In cases in which the timing between the peginterferon injection and the HCV RNA sample collection was different for the two samples, an increase of 2 log10 IU per milliliter was required to meet this criterion. If a patient had virologic breakthrough or an incomplete virologic response and rebound while receiving therapy, boceprevir treatment could be discontinued, but peginterferon–ribavirin could be continued for up to 48 weeks with appropriate clinical follow-up.
EFFICACY ASSESSMENT
Plasma HCV RNA levels were measured with the use of the TaqMan 2.0 assay (Roche Diagnostics), which has lower limits of quantification and detection of 25 and 9.3 IU per milliliter, respectively; the lower limit of detection was used for decision making at various points throughout the study. HCV RNA testing was performed at the screening visit, at baseline, every 2 weeks through week 12, and at weeks 16, 20, 24, 28, 34, 40, 48, 52, 60, and 72 (depending on the treatment duration). Patients in whom study therapy was stopped because of futility were considered to have had treatment failure.
SAFETY ASSESSMENT
Adverse events were graded by investigators according to a modified World Health Organization grading system. Non–life-threatening hematologic adverse events were managed by means of dose reduction or administration of hematopoietic growth factors (or both). Reduction of the ribavirin dose or administration of erythropoietin was recommended when the hemoglobin level dropped to less than 10 g per deciliter, but these decisions were made at the discretion of the investigators; erythropoietin was to be stopped if the hemoglobin level rebounded to 12 g per deciliter or greater.
STATISTICAL ANALYSIS
The trial was designed as a superiority study to detect differences in the rates of sustained virologic response with either of the two boceprevir regimens (group 2 or group 3) as compared with standard therapy alone (group 1). The primary analyses involved all patients who had received at least one dose of any study medication; key secondary efficacy analyses were conducted for the modified intention-to-treat population, consisting of patients who completed the lead-in period of treatment and received at least one dose of boceprevir or placebo. Rates of response were determined separately (per protocol) for the non-black cohort and the black cohort.
The protocol-specified primary efficacy end point was a sustained virologic response, defined as undetectable HCV RNA levels for 24 weeks after the completion of therapy. If HCV RNA measurements for this time point or later were missing, the 12-week post-treatment measurement was used. Relapse was defined as the occurrence of an undetectable HCV RNA level at the end of treatment but a detectable HCV RNA level at some point during the follow-up period.
Within-cohort comparisons were performed with the use of the two-sided Cochran–Mantel–Haenszel chi-square test (after adjustment for baseline stratification factors). A step-down approach was applied to hypothesis testing. Group 3 was first compared with group 1. If the resultant P value was 0.05 or less, the superiority of fixed-duration therapy including boceprevir over standard therapy would be supported, and group 2 would then be compared with group 1. If this P value was also 0.05 or less, the superiority of response-guided therapy including boceprevir over standard therapy would likewise be established.
Secondary analyses were to be conducted only if the primary comparisons showed significant differences. Formal hypothesis testing comparing the two boceprevir groups was not specified in the protocol. A multivariate logistic-regression model that included baseline characteristics and treatment group was used to identify predictors of sustained virologic response. A stepwise procedure was used to identify independent covariates, with an alpha level of 0.05 as the threshold level for variables to be entered into, and retained in, the model.
Assuming a rate of sustained virologic response of 45% in group 1 of the nonblack cohort, we calculated that 310 subjects per group would need to be enrolled for the study to have a statistical power of 90% to detect an absolute increase of 13 percentage points in the rate of sustained virologic response in group 3 as compared with group 1, with the use of a two-sided chi-square test and an alpha level of 0.05. Assuming a rate of sustained virologic response of 50% in the black cohort overall, 50 patients per group, and the use of a two-sided 95% confidence interval, we estimated that the true rate of a sustained virologic response in the black population could be estimated, within ±14%, for each of the three treatment groups.
Safety analyses included all patients who had been randomly assigned to a study group and had received at least one dose of any study medication.
RESULTS
STUDY PATIENTS
A total of 1246 and 226 patients were screened for the nonblack cohort and the black cohort, respectively, of whom 940 nonblack patients and 159 black patients were randomly assigned to a treatment group from August 2008 through January 2009 (Fig. S1 in the Supplementary Appendix). Two patients in the nonblack cohort did not receive any study drug and were not included in the analyses. All the other randomly assigned patients received at least 1 dose of study medication. Baseline characteristics are shown in Table 1.
Table 1.
Selected Baseline Characteristics of Patients Who Received at Least One Dose of Study Medication, According to Cohort and Treatment Group.*
| Characteristic | Nonblack Cohort | Black Cohort | Both Cohorts | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Group 1 (N = 311) | Group 2 (N = 316) | Group 3 (N = 311) | Group 1 (N = 52) | Group 2 (N = 52) | Group 3 (N = 55) | Group 1 (N = 363) | Group 2 (N = 368) | Group 3 (N = 366) | |
| Age — yr | 48±10 | 49±9 | 49±9 | 51±9 | 52±8 | 51±7 | 49±10 | 50±9 | 49±9 |
| Male sex — no. (%) | 171 (55) | 200 (63) | 188 (60) | 35 (67) | 29 (56) | 33 (60) | 206 (57) | 229 (62) | 221 (60) |
| Race — no. (%)† | |||||||||
| White | 296 (95) | 304 (96) | 295 (95) | 296 (82) | 304 (83) | 295 (81) | |||
| Black | 52 (100) | 52 (100) | 55 (100) | 52 (14) | 52 (14) | 55 (15) | |||
| Asian | 9 (3) | 4 (1) | 8 (3) | 9 (2) | 4 (1) | 8 (2) | |||
| Other | 6 (2) | 8 (3) | 8 (3) | 0 | 0 | 0 | 6 (2) | 8 (2) | 8 (2) |
| Region — no. (%) | |||||||||
| North America | 203 (65) | 226 (72) | 218 (70) | 51 (98) | 51 (98) | 52 (95) | 254 (70) | 277 (75) | 270 (74) |
| Europe | 98 (32) | 78 (25) | 83 (27) | 1 (2) | 1 (2) | 3 (5) | 99 (27) | 79 (21) | 86 (23) |
| Latin America | 10 (3) | 12 (4) | 10 (3) | 0 | 0 | 0 | 10 (3) | 12 (3) | 10 (3) |
| Weight — kg | 79±16 | 82±17 | 80±17 | 87±14 | 86±15 | 91±18 | 80±16 | 82±17 | 82±17 |
| HCV subtype — no. (%)‡ | |||||||||
| 1a | 186 (60) | 195 (62) | 197 (63) | 41 (79) | 39 (75) | 40 (73) | 227 (63) | 234 (64) | 237 (65) |
| 1b | 112 (36) | 111 (35) | 104 (33) | 9 (17) | 13 (25) | 13 (24) | 121 (33) | 124 (34) | 117 (32) |
| Missing data | 13 (4) | 10 (3) | 10 (3) | 2 (4) | 0 | 2 (4) | 15 (4) | 10 (3) | 12 (3) |
| HCV RNA level — no. (%) | |||||||||
| >400,000 IU/ml | 285 (92) | 287 (91) | 288 (93) | 52 (100) | 49 (94) | 53 (96) | 337 (93) | 336 (91) | 341 (93) |
| >800,000 IU/ml | 258 (83) | 268 (85) | 262 (84) | 50 (96) | 46 (88) | 51 (93) | 308 (85) | 314 (85) | 313 (86) |
| Metavir fibrosis score — no. (%)§ | |||||||||
| 0, 1, or 2 | 277 (89) | 279 (88) | 265 (85) | 51 (98) | 40 (77) | 48 (87) | 328 (90) | 319 (87) | 313 (86) |
| 3 or 4 | 23 (7) | 26 (8) | 36 (12) | 1 (2) | 8 (15) | 6 (11) | 24 (7) | 34 (9) | 42 (11) |
| Missing data | 11 (4) | 11 (3) | 10 (3) | 0 | 4 (8) | 1 (1) | 11 (3) | 15 (4) | 11 (3) |
| Steatosis — no. (%)§ | 192 (62) | 213 (67) | 208 (67) | 32 (62) | 33 (63) | 39 (71) | 224 (62) | 246 (67) | 247 (67) |
Plus–minus values are means ±SD. Boceprevir was given for 24 weeks in group 2 and for 44 weeks in group 3, irrespective of the rapidity of achievement of an undetectable HCV RNA level. See Table S1 in the Supplementary Appendix for a complete list of baseline characteristics.
Race was self-reported. Hispanic or Latino was given as a second self-identification by 8 to 13% of the patients in each treatment group in the nonblack cohort and in one patient in the black cohort.
The HCV subtype was ascertained by sequencing of the nonstructural 5B region.
Metavir scores and steatosis were determined on the basis of assessment of liver-biopsy specimens by a single pathologist who was unaware of the assignment to the boceprevir or placebo group. Possible fibrosis scores are as follows: 0 (indicating no fibrosis), 1 (indicating portal fibrosis without septa), 2 (indicating portal fibrosis with few septa), 3 (indicating numerous septa without cirrhosis), and 4 (indicating cirrhosis). Steatosis was analyzed as being present or absent.
A total of 49 patients discontinued the peginterferon–ribavirin therapy during the lead-in period and did not receive boceprevir or placebo. Discontinuation for reasons of futility at week 24 occurred in 84 of 311 patients (27%), 24 of 316 patients (8%), and 28 of 311 patients (9%) in the non-black cohort and in 24 of 52 patients (46%), 9 of 52 patients (17%), and 8 of 55 patients (15%) in the black cohort in groups 1, 2, and 3, respectively.
EFFICACY
Response rates were significantly higher among patients receiving a boceprevir-containing regimen than among controls (Table 2). Among nonblacks, the rate of a sustained virologic response was 40% with the standard of care and was significantly higher (P<0.001) in both boceprevir groups — 67% in group 2 and 68% in group 3 — for relative increases of 68% and 70%, respectively, over control rates. Among blacks, the rate of a sustained virologic response was 23% in group 1, 42% in group 2 (P = 0.04, vs. group 1), and 53% in group 3 (P = 0.004, vs. group 1). In a modified intention-to-treat analysis that included all non-blacks receiving at least one dose of boceprevir or placebo, the respective rates of sustained virologic response in groups 1, 2, and 3 were 42%, 70% (P<0.001, vs. group 1), and 71% (P<0.001, vs. group 1), the corresponding rates among blacks were 26%, 47% (P = 0.04, vs. group 1), and 53% (P = 0.01, vs. group 1). In the nonblack cohort, viral breakthrough occurred in 1 to 2% of patients in each treatment group, whereas rates of relapse were lower in the two boceprevir groups than in the standard-therapy group. The numbers of events in the smaller black cohort were too few to permit comparison between treatment groups.
Table 2.
Rates of Virologic Responses among Patients Who Received at Least One Dose of Any Study Medication, According to Cohort and Treatment Group.*
| Characteristic | Nonblack Cohort | Black Cohort | Both Cohorts | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 (N = 311) | Group 2 (N = 316) | P Value for Group 2 vs. Group 1 | Group 3 (N = 311) | P Value for Group 3 vs. Group 1 | Group 1 (N = 52) | Group 2 (N = 52) | P Value for Group 2 vs. Group 1 | Group 3 (N = 55) | P Value for Group 3 vs. Group 1 | Group 1 (N = 363) | Group 2 (N = 368) | P Value for Group 2 vs. Group 1 | Group 3 (N = 366) | P Value for Group 3 vs. Group 1 | |
| no. of patients/total no. (%) | no. of patients/total no. (%) | no. of patients/total no. (%) | no. of patients/ total no. (%) | no. of patients/total no. (%) | no. of patients/ total no. (%) | ||||||||||
| Response at end of therapy† | 176/311 (57) | 235/316 (74) | <0.001 | 241/311 (77) | <0.001 | 15/52 (29) | 26/52 (50) | 0.04 | 36/55 (65) | <0.001 | 191/363 (53) | 261/368 (71) | <0.001 | 277/366 (76) | <0.001 |
| Rate of relapse‡ | 37/162 (23) | 21/232 (9) | <0.001 | 18/230 (8) | <0.001 | 2/14 (14) | 3/25 (12) | 1.00 | 6/35 (17) | 1.00 | 39/176 (22) | 24/257 (9) | <0.001 | 24/265 (9) | <0.001 |
| Sustained virologic response§ | |||||||||||||||
| All patients who received treatment | 125/311 (40) | 211/316 (67) | <0.001 | 213/311 (68) | <0.001 | 12/52 (23) | 22/52 (42) | 0.04 | 29/55 (53) | 0.004 | 137/363 (38) | 233/368 (63) | <0.001 | 242/366 (66) | <0.001 |
| Modified ITT population | 125/297 (42) | 211/303 (70) | <0.001 | 213/299 (71) | <0.001 | 12/47 (26) | 22/47 (47) | 0.04 | 29/55 (53) | 0.001 | 137/344 (40) | 233/350 (67) | <0.001 | 242/354 (68) | <0.001 |
| HCV RNA level at wk 4 | |||||||||||||||
| Undetectable or decreased by ≥1 log10 IU/ml | 121/234 (52) | 187/228 (82) | <0.001 | 178/218 (82) | <0.001 | 12/26 (46) | 16/24 (67) | 0.17 | 22/36 (61) | 0.30 | 133/260 (51) | 203/252 (81) | <0.001 | 200/254 (79) | <0.001 |
| Decreased by <1 log10 IU/ml | 3/62 (5) | 21/73 (29) | <0.001 | 31/79 (39) | <0.001 | 0/21 | 6/24 (25) | 0.02 | 5/16 (31) | 0.01 | 3/83 (4) | 27/97 (28) | <0.001 | 36/95 (38) | <0.001 |
| HCV RNA detectability at wk 4 | |||||||||||||||
| Undetectable¶ | 27/28 (96) | 16/18 (89) | 0.55 | 18/20 (90) | 0.56 | 2/2 (100) | 1/1 (100) | 0/0 | 29/30 (97) | 17/19 (89) | 0.55 | 18/20 (90) | 0.56 | ||
| Detectable∥ | 97/268 (36) | 192/283 (68) | <0.001 | 191/277 (69) | <0.001 | 10/45 (22) | 21/47 (45) | 0.03 | 27/52 (52) | 0.003 | 107/313 (34) | 213/330 (65) | <0.001 | 218/329 (66) | <0.001 |
| HCV RNA detectability at wk 8 | |||||||||||||||
| Undetectable | 48/56 (86) | 170/190 (89) | 0.47 | 166/182 (91) | 0.31 | 3/4 (75) | 14/18 (78) | 1.00 | 18/22 (82) | 0.57 | 51/60 (85) | 184/208 (88) | 0.50 | 184/204 (90) | 0.25 |
| Detectable∥ | 73/233 (31) | 38/104 (37) | 0.38 | 44/102 (43) | 0.046 | 8/38 (21) | 8/25 (32) | 0.38 | 8/29 (28) | 0.57 | 81/271 (30) | 46/129 (36) | 0.25 | 52/131 (40) | 0.06 |
| HCV RNA detectability wk 8 through wk 24 | |||||||||||||||
| Undetectable | 37/40 (93) | 143/147 (97) | 0.17 | 137/142 (96) | 0.38 | 3/3 (100) | 13/15 (87) | 0.99 | 18/19 (95) | 1.00 | 40/43 (93) | 156/162 (96) | 0.40 | 155/161 (96) | 0.40 |
| Detectable** | 78/118 (66) | 52/70 (74) | 0.26 | 48/65 (74) | 0.32 | 8/13 (62) | 7/12 (58) | 1.00 | 7/8 (88) | 0.36 | 86/131 (66) | 59/82 (72) | 0.37 | 55/73 (75) | 0.16 |
| Baseline Metavir fibrosis score†† | |||||||||||||||
| 0, 1, or 2 | 111/277 (40) | 194/279 (70) | <0.001 | 186/265 (70) | <0.001 | 12/51 (24) | 19/40 (48) | 0.03 | 25/48 (52) | 0.004 | 123/328 (38) | 213/319 (67) | <0.001 | 211/313 (67) | <0.001 |
| 3 or 4 | 9/23 (39) | 13/26 (50) | 0.57 | 18/36 (50) | 0.44 | 0/1 | 1/8 (12) | 1.00 | 4/6 (67) | 0.43 | 9/24 (38) | 14/34 (41) | 1.00 | 22/42 (52) | 0.31 |
Boceprevir was given for 24 weeks in group 2 and for 44 weeks in group 3, irrespective of the rapidity of achievement of an undetectable HCV RNA level. P values were calculated for the rates of sustained virologic response with the use of the Cochran–Mantel–Haenszel chi-square test, with adjustment for prespecified stratification factors; P values for other response data were calculated by means of Fisher’s exact test. ITT denotes intention to treat.
Response at the end of therapy was defined as an undetectable HCV RNA level at the time that the study therapy was discontinued. The end of therapy was the actual (not assigned) end of treatment.
Rate of relapse was defined as the proportion of patients with a detectable HCV RNA level at the end of the follow-up period, as calculated among the patients with an undetectable level at the end of the treatment period who did not have missing follow-up data.
Sustained virologic response was defined as an undetectable HCV RNA level at the end of the follow-up period. The 12-week post-treatment HCV RNA level was used (as specified in the protocol) if the 24-week post-treatment level was not available. A sensitivity analysis was performed on data from only patients with an undetectable HCV RNA level documented at 24 weeks after treatment: the rates of sustained virologic response for groups 1, 2, and 3 in the nonblack cohort were 39%, 66%, and 68%, respectively, and the rates in the black cohort were 21%, 42%, and 51%, respectively.
In the black cohort, the sample was too small to calculate the P value for the comparison between group 2 and group 1.
Detectable HCV RNA was defined as an HCV RNA level above the limit of detection (9.3 IU per milliliter) at the specified week in patients for whom HCV RNA values were available.
HCV RNA detectability at weeks 8 through 24 was ascertained only in patients receiving more than 28 weeks of therapy. Only 22% of patients (82 of 368) in group 2 were assigned a 48-week fixed treatment duration.
The Metavir fibrosis scores were determined on the basis of assessment of liver-biopsy specimens by a single pathologist who was unaware of the assignment to the boceprevir or placebo group. Possible fibrosis scores are as follows: 0 (indicating no fibrosis), 1 (indicating portal fibrosis without septa), 2 (indicating portal fibrosis with few septa), 3 (indicating numerous septa without cirrhosis), and 4 (indicating cirrhosis).
The 4-week lead-in period of peginterferon–ribavirin treatment allowed for the assessment of interferon responsiveness and its relationship to sustained virologic response. At week 4, 23% of nonblacks and 38% of blacks had a decrease of less than 1 log10 IU per milliliter in the HCV RNA level from baseline, which was associated with lower rates of sustained virologic response (Table 2) and higher rates of boceprevir-resistance–associated variants (genotypic mutations of the protease conferring reduced sensitivity to boceprevir) (Table 3) than was a decrease of 1 log10 IU per milliliter or more in the HCV RNA level, regardless of the treatment group. However, whether the decrease in the HCV RNA level at week 4 was more or less than 1 log10 IU per milliliter, rates of sustained virologic response were consistently higher in the boceprevir groups than in the control group.
Table 3.
Common Clinical Adverse Events, Resistance-Associated HCV Variants, and Hematologic Abnormalities, According to Treatment Group.*
| Adverse Event | Group 1 (N = 363) | Group 2 (N = 368) | P Value for Group 2 vs. Group 1 | Group 3 (N = 366) | P Value for Group 3 vs. Group 1 |
|---|---|---|---|---|---|
| Investigator-reported clinical adverse events — no. (%) | |||||
| Fatigue | 217 (60) | 196 (53) | 0.09 | 209 (57) | 0.50 |
| Headache | 153 (42) | 168 (46) | 0.37 | 167 (46) | 0.37 |
| Nausea | 153 (42) | 175 (48) | 0.16 | 159 (43) | 0.76 |
| Anemia | 107 (29) | 182 (49) | <0.001 | 179 (49) | <0.001 |
| Pyrexia | 121 (33) | 123 (33) | 0.99 | 118 (32) | 0.81 |
| Chills | 102 (28) | 134 (36) | 0.02 | 121 (33) | 0.15 |
| Dysgeusia | 64 (18) | 137 (37) | <0.001 | 156 (43) | <0.001 |
| Insomnia | 118 (33) | 117 (32) | 0.87 | 122 (33) | 0.81 |
| Boceprevir-resistance–associated variants — no. (%) | |||||
| Overall | 59/350 (17) | 52/354 (15) | |||
| HCV RNA level at wk 4 | |||||
| Decrease of ≥1 log10 IU/ml from baseline | 10/232 (4) | 13/231 (6) | |||
| Decrease of <1 log10 IU/ml from baseline | 49/95 (52) | 38/94 (40) | |||
| Hematologic variables — no. (%) | |||||
| Decreased absolute neutrophil count | |||||
| Grade 3: 500 to <750 per mm3 | 50 (14) | 87 (24) | <0.001 | 90 (25) | <0.001 |
| Grade 4: <500 per mm3 | 16 (4) | 21 (6) | 0.50 | 29 (8) | 0.06 |
| Use of granulocyte-stimulating agent | 21 (6) | 43 (12) | 0.006 | 31 (8) | 0.20 |
| Decreased platelet count | |||||
| Grade 3: 25,000 to <50,000 per mm3 | 5 (1) | 11 (3) | 0.21 | 13 (4) | 0.09 |
| Grade 4: <25,000 per mm3 | 0 | 1 (<1) | 0.99 | 1 (<1) | 0.99 |
| Decreased hemoglobin concentration† | |||||
| Grade 3: 6.5 to <8.0 g/dl | 6 (2) | 7 (2) | 0.99 | 12 (3) | 0.23 |
| Grade 4: <6.5 g/dl | 0 | 2 (1) | 0.50 | 1 (<1) | 0.99 |
| Red-cell transfusion | 2 (1) | 11 (3) | 0.02 | 9 (2) | 0.06 |
| Erythropoietin use | |||||
| Patients | 87 (24) | 159 (43) | <0.001 | 159 (43) | <0.001 |
| Days | 0.01 | 0.005 | |||
| Mean | 121 | 94 | 156 | ||
| Median | 109 | 85 | 149 |
The table lists (in decreasing order of overall frequency) all specified adverse events occurring during the treatment period or within 30 days after the end of treatment, regardless of the cause of the event, that were reported in 30% or more of patients in any of the three treatment groups. Table S2B in the Supplementary Appendix lists the adverse events reported in 15% or more of patients in any treatment group, as well as laboratory abnormalities of grade 0, 1, or 2. Terms are from the Medical Dictionary for Regulatory Activities (version 13.0). The nominal P values were calculated with the use of Fisher’s exact test for categorical variables and the t-test for continuous variables; they were not corrected for multiple comparisons.
Toxicity grades for decreased hemoglobin levels are modified World Health Organization grades based on nadir hemoglobin levels during the treatment period.
The percentages of patients with undetectable HCV RNA levels at week 8 who had a sustained virologic response were high, irrespective of the treatment regimen, but this response at week 8 occurred approximately three times as often in the boceprevir groups as in the control group. At this time point, the patients had received boceprevir or placebo for 4 weeks and peginterferon–ribavirin for 8 weeks.
The rates of sustained virologic response among nonblacks were similar in group 2 (67%) and in group 3 (68%), whereas among blacks they were 42% and 53%, respectively. Among nonblack boceprevir recipients whose HCV RNA levels became undetectable by week 8 (60%) and those with undetectable HCV RNA levels through week 24 (47%), the rate of sustained virologic response was 97% in group 2 (which had received 24 weeks of boceprevir and a total of 28 weeks of therapy) and 96% in group 3 (which had received 44 weeks of boceprevir and a total of 48 weeks of therapy) (Table 2).
In group 2, a total of 22% of the patients with a detectable HCV RNA level between week 8 and week 24 received therapy for more than 28 weeks. Among patients in whom HCV RNA levels were still detectable at week 8, rates of sustained virologic response were 74% in group 2 (after receiving 24 weeks of boceprevir) as well as in group 3 (after receiving 44 weeks of boceprevir).
Predictors of sustained virologic response were identical in models for each cohort. Rates of sustained virologic response in patients with advanced fibrosis were lower than in those with mild fibrosis, although the numbers of patients with a Metavir fibrosis score of 3 or 4 (indicating bridging fibrosis or cirrhosis) were small, particularly in the black cohort (Table 4). In an expanded model that included virologic responses during the treatment period, a decrease in the HCV RNA level by 1 log10 IU per milliliter or more at the end of the 4-week lead-in period was strongly predictive of a sustained virologic response (odds ratio vs. a decrease of <1 log10 IU per milliliter, 9.0; 95% confidence interval, 6.3 to 12.8; P<0.001). In general, subgroup analyses across a range of baseline factors favored group 2 and group 3 over group 1, with no consistent differences between groups 2 and 3 (Fig. S2 in the Supplementary Appendix). For groups 1, 2, and 3 in the nonblack cohort, rates of sustained virologic response were 33%, 64%, and 59%, respectively, among patients with a hemoglobin level of 10 g per deciliter or higher during the treatment period, as compared with 60%, 72%, and 79%, respectively, among patients with a nadir hemoglobin level of less than 10 g per deciliter during the treatment period.
Table 4.
Odds Ratios for a Sustained Virologic Response, According to Predictor Variables.*
| Variable† | Odds Ratio (95% CI) | P Value‡ |
|---|---|---|
| Treatment group | ||
| Group 3 vs. group 1 | 3.5 (2.6–4.9) | <0.001 |
| Group 2 vs. group 1 | 3.1 (2.3–4.3) | <0.001 |
| Black cohort vs. nonblack cohort§ | 0.5 (0.3–0.7) | <0.001 |
| Baseline HCV RNA level ≤400,000 vs. >400,000 IU/ml | 3.9 (2.1–7.1) | <0.001 |
| Age ≤40 vs. >40 yr | 1.5 (1.0–2.1) | 0.03 |
| No cirrhosis vs. cirrhosis | 2.5 (1.4–4.6) | 0.003 |
| Statin use vs. no statin use | 3.4 (1.1–10.7) | 0.04 |
The odds ratios were estimated in a multivariate stepwise logistic-regression model that included baseline predictors of sustained virologic response in all treated patients (in the black and nonblack cohorts combined). CI denotes confidence interval.
Only covariates remaining significant at an alpha level of 0.05 after adjustment for the other variables were retained in the model and are listed in the table. Factors entered but not retained in the model were region, sex, age, weight, body-mass index, hepatitis C virus (HCV) genotype 1 subtype as ascertained by means of the Trugene assay, hepatic steatosis, platelet count, alanine aminotransferase level, and Metavir fibrosis score at baseline. In an expanded model that included response data during the treatment period, an HCV RNA level at week 4 that was undetectable or that had decreased by 1 log10 IU per milliliter or more from baseline (vs. a decrease of <1 log10 IU per milliliter) had the highest odds ratio: 9.0 (95% CI, 6.3 to 12.8; P<0.001).
P values were calculated with the use of the chi-square test.
Race was self-reported.
SAFETY
Adverse events occurred in more than 98% of the study patients, with serious adverse events in 9%, 11%, and 12% of patients in groups 1, 2, and 3, respectively (Table S2 in the Supplementary Appendix). There were six deaths during the study: four patients in the control group died, as did two patients in the boceprevir groups. Two suicides (one in group 1 and one in group 2) were judged to have possibly been related to peginterferon. No other deaths were considered to be drug-related.
Fatigue, headache, and nausea were the most common clinical adverse events in all treatment groups (Table 3). Dysgeusia occurred more than twice as often in boceprevir recipients than in controls. Anemia was reported as an adverse event in 29% of controls and 49% of boceprevir recipients. Anemia was classified as grade 1 in 36% of controls, grade 2 in 17%, grade 3 in 2%, and grade 4 in 0%; the respective percentages among boceprevir recipients were 43%, 31%, 3%, and 1%. Four patients in group 1 discontinued the study owing to anemia, as compared with six patients in group 2 and seven patients in group 3. Overall, 13% of controls and 21% of boceprevir recipients required dose reductions because of anemia. Erythropoietin was administered in 24% of controls and 43% of boceprevir recipients. A total of 85% and 86% of patients in group 2 and group 3, respectively, had neutropenia of grade 1 to 4, as compared with 77% of those in the control group; 28% and 33% of patients in groups 1 and 2, respectively, had grade 1 to 4 thrombocytopenia, as compared with 13% of controls.
DISCUSSION
SPRINT-2 compared two regimens of boceprevir added to peginterferon alfa-2b–ribavirin therapy (the standard of care) and the standard of care alone. Two distinct cohorts were enrolled on the basis of self-identified race (nonblack patients and black patients) to allow for an independent estimate of rates of response among black patients, a group historically underrepresented in HCV-treatment trials.
As compared with peginterferon alfa-2b–ribavirin therapy alone, the addition of boceprevir significantly increased the rate of a sustained virologic response among previously untreated black and nonblack patients infected with HCV genotype 1, including those with a decrease of less than 1 log10 IU per milliliter in the HCV RNA level at week 4. Among nonblack patients, the combination therapy with boceprevir was associated with a relative increase of approximately 70% in the rates of sustained virologic response over standard therapy. Although lower among black patients than among nonblack patients, the rates of sustained virologic response with the boceprevir regimens were nearly double those with the standard of care. Patients with an undetectable HCV RNA level at week 8 had a higher rate of sustained virologic response than patients with a detectable level at week 8, irrespective of treatment regimen. Given the relatively small numbers of patients with cirrhosis in the trial, further study is warranted to define optimal therapy in this population.
Our study evaluated a response-guided treatment strategy with individualized treatment duration on the basis of the HCV RNA level between weeks 8 and 24. Patients in whom the HCV RNA level became undetectable by week 8 and remained so up to week 24 were given boceprevir plus peginterferon–ribavirin for 24 weeks. The rates of sustained virologic response among both black and nonblack patients were significantly higher with response-guided therapy than with standard treatment. Regardless of the virologic response at week 4 or week 8, response-guided therapy that included 24 weeks of boceprevir administration resulted in overall rates of sustained virologic response that were similar to those after 44 weeks of triple therapy. Patients who had undetectable HCV RNA levels by week 8 had very high rates of sustained virologic response as compared with patients who had detectable levels between weeks 8 and 24. There were too few black patients in whom the HCV RNA level was detectable between weeks 8 and 24 to conclusively define the optimal treatment for this population.
This trial featured the use of peginterferon–ribavirin for 4 weeks (the lead-in period) before boceprevir was added. Theoretically, a lead-in phase would serve to lower HCV RNA levels before exposure to a protease inhibitor, thereby reducing the risk of viral breakthrough or resistance to the direct-acting antiviral agent, as noted in a phase 2 study in which boceprevir with lead-in therapy was compared with boceprevir without lead-in therapy.10 However, lead-in therapy has other benefits, such as allowing for assessment of the relationship between interferon responsiveness and subsequent sustained virologic response in patients receiving boceprevir. Patients with a poor response to interferon, defined as a reduction in the HCV RNA level of less than 1 log10 IU per milliliter after 4 weeks of peginterferon–ribavirin therapy, had sufficiently high rates of sustained virologic response, as compared with the control group, to dispel concern that the addition of a protease inhibitor to the treatment regimen would be equivalent to functional monotherapy. However, these patients were less likely than patients with a robust response to interferon to have a sustained virologic response after boceprevir was added.17-19 Thus, patients who have a poor response to interferon may need to be monitored closely to determine who may benefit from better therapies, once they are available. Conversely, in patients with undetectable HCV RNA levels after the lead-in period, boceprevir administration may not result in a higher rate of sustained virologic response than that achieved with the use of peginterferon and ribavirin alone. The lead-in period can further serve to test both compliance and tolerability before exposure to a class of drugs to which resistance can develop.15,16
The regimens that included boceprevir were associated with increased rates of anemia, and nearly twice as many boceprevir recipients as controls had a hemoglobin level of less than 9.5 g per deciliter or received erythropoietin (43% vs. 24%). Among patients receiving erythropoietin, the average duration of use was shortest in group 2. Neither the incidence of serious adverse events nor the frequency of discontinuation owing to an adverse event differed significantly between patients receiving boceprevir and those receiving standard therapy. The rate of a sustained virologic response was significantly greater with boceprevir plus peginterferon–ribavirin than with peginterferon–ribavirin alone among both black and nonblack patients.
Supplementary Material
Acknowledgments
The opinions expressed in this report represent the consensus of the coauthors and do not necessarily reflect the formal position of Merck or the other institutions listed as authors’ affiliations.
Supported by Schering-Plough (now Merck).
Dr. Poordad reports receiving consulting fees from Merck, Vertex, Abbott, Gilead, Achillion, Genentech, and Tibotec; grant support from Merck; and payment for development of educational presentations from Merck. Dr. McCone reports receiving lecture fees and speaker’s fees from Schering-Plough (now part of Merck). Dr. Bacon reports receiving consulting fees from Gilead, Three Rivers Pharmaceuticals, Valeant, Vertex, and Human Ge-nome Sciences; grant support from Roche, Gilead, Bristol-Myers Squibb, Three Rivers Pharmaceuticals, Valeant, Vertex, Human Genome Sciences, Wyeth, and Romark Laboratories; and lecture or speaker’s fees from Three Rivers Pharmaceuticals, Gilead, and Schering-Plough (now part of Merck); as well as having served on data and safety monitoring boards for Novartis, Isis, Vertex, and Gilead. Dr. Manns reports receiving consulting fees from Schering-Plough (now part of Merck), Roche, Bristol-Myers Squibb, Gilead, Valeant, Boehringer Ingelheim, Novartis, Idenix, Tibotec, Vertex, GlaxoSmithKline, and Merck; grant support from Schering-Plough (now part of Merck), Roche, Gilead, Novartis, Boehringer Ingelheim, and Bristol-Myers Squibb; and payment for development of educational presentations from Schering-Plough (now part of Merck), Roche, Bristol-Myers Squibb, GlaxoSmithKline, and Gilead. Dr. Sulkowski reports receiving consulting fees from Vertex, Boehringer Ingelheim, Pfizer, Bristol-Myers Squibb, GlaxoSmithKline, Pharmasset, Abbott, Tibotec, Roche, and Gilead; fees for expert testimony from Abbott; and grant support from Vertex, Abbott, Boehringer Ingelheim, Tibotic, Gilead, Pharmasset, Zymogenetics, Bristol-Myers Squibb, Orasure, Achillion, and Roche. Dr. Jacobson reports receiving consulting fees from Bristol-Myers Squibb, Novartis, Gilead, Schering-Plough (now part of Merck), Pfizer, Vertex, GlobeImmune, Human Genome Sciences, Boehringer Ingelheim, Pharmasset, Zymogenetics, Tibotec, Abbott, Roche–Genentech, Anadys, Sanofi-Aventis, Achillion, GlaxoSmithKline, and Biolex; grant support from Schering-Plough (now part of Merck), Tibotec, Roche–Genentech, Pharmasset, Anadys, Boehringer Ingelheim, Novartis, Gilead, Vertex, GlobeImmune, Human Genome Sciences, Pfizer, Bristol-Myers Squibb, and Zymogenetics; lecture or speaker’s fees from Schering-Plough (now part of Merck), Gilead, Bristol-Myers Squibb, and Roche–Genentech; and payment for development of educational presentations from Bristol-Myers Squibb, Gilead, and Vertex. Dr. Reddy reports receiving consulting fees from Roche, Merck, Salix, Vertex, Tibotec, and Human Genome Sciences; grant support from Roche, Vertex, Tibotec, Bristol-Myers Squibb, and Gilead; and payment for development of educational presentations from ViralEd. Dr. Good-man reports receiving consulting fees from Merck and Gilead Sciences; grant support from Schering-Plough (now part of Merck), GlaxoSmithKline, Bristol-Myers Squibb, Novartis, Globe-Immune, Gilead Sciences; and lecture or speaker’s fees from Bristol-Myers Squibb. Ms. Boparai and Drs. DiNubile, Sniukiene, Brass, and Albrecht report being employees of Merck and owning stock or stock options in the company. Dr. Bronowicki reports receiving fees for board membership from Schering-Plough (now part of Merck), Roche, Gilead, Bristol-Myers Squibb, Janssen, and Bayer; consulting fees from Merck, Boeh-ringer Ingelheim, and Novartis; lecture or speaker’s fees from Schering-Plough (now part of Merck), Roche, Bayer and Bristol-Myers Squibb; and reimbursement for travel expenses from Roche.
We thank all the patients, health care providers, and investigators involved in the study; Dr. Lisa Pedicone (of Merck) for her helpful advice and invaluable support; and Joann DiLullo, Bernard Akyena, Jill Williams, and Karyn Davis (of Merck) for their technical assistance in the preparation of the manuscript.
Footnotes
No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Global surveillance and control of hepatitis C: report of a WHO Consultation organized in collaboration with the Viral Hepatitis Prevention Board, Antwerp, Belgium. J Viral Hepat. 1999;6:35–47. [PubMed] [Google Scholar]
- 2.Wasley A, Alter MJ. Epidemiology of hepatitis C: geographic differences and temporal trends. Semin Liver Dis. 2000;20:1–16. doi: 10.1055/s-2000-9506. [DOI] [PubMed] [Google Scholar]
- 3.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–65. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 4.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–82. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 5.McHutchison JG, Lawitz EJ, Shiffman ML, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;361:580–93. doi: 10.1056/NEJMoa0808010. Erratum, N Engl J Med 2009;361:1027. [DOI] [PubMed] [Google Scholar]
- 6.Chung RT, Andersen J, Volberding P, et al. Peginterferon alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451–9. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muir AJ, Bornstein JD, Killenberg PG. Peginterferon alfa-2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-Hispanic whites. N Engl J Med. 2004;350:2265–71. doi: 10.1056/NEJMoa032502. Erratum, N Engl J Med 2004;351:1268. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Torres M, Jeffers LJ, Sheikh MY, et al. Peginterferon alfa-2a and ribavirin in Latino and non-Latino whites with hepatitis C. N Engl J Med. 2009;360:257–67. doi: 10.1056/NEJMoa0805062. Erratum, N Engl J Med 2010;363:2474. [DOI] [PubMed] [Google Scholar]
- 9.Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon Alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–50. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- 10.Kwo PY, Lawitz EJ, McCone J, et al. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 2010;376:705–16. doi: 10.1016/S0140-6736(10)60934-8. [DOI] [PubMed] [Google Scholar]
- 11.McHutchison JG, Everson GT, Gordon SC, et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009;360:1827–38. doi: 10.1056/NEJMoa0806104. Erratum, N Engl J Med 2009;361:1516. [DOI] [PubMed] [Google Scholar]
- 12.McHutchison JG, Manns MP, Muir AJ, et al. Telaprevir for previously treated chronic HCV infection. N Engl J Med. 2010;362:1292–303. doi: 10.1056/NEJMoa0908014. Erratum, N Engl J Med 2010;362:1647. [DOI] [PubMed] [Google Scholar]
- 13.Hézode C, Forestier N, Dusheiko G, et al. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360:1839–50. doi: 10.1056/NEJMoa0807650. [DOI] [PubMed] [Google Scholar]
- 14.Venkatraman S, Bogen SL, Arasappan A, et al. Discovery of (1R,5S)-N-[3-amino-1-(cyclobutylmethyl)-2,3-dioxopropyl]-3-[2(S)-[[[(1,1-dimethylethyl)amino] carbonyl]amino]-3,3-dimethyl-1-oxobutyl]-6,6-dimethyl-3-azabicyclo [3.1.0]hexan-2(S)-carboxamide (SCH 503034), a selective, potent, orally bioavailable hepatitis C virus NS3 protease inhibitor: a potential therapeutic agent for the treatment of hepatitis C infection. J Med Chem. 2006;49:6074–86. doi: 10.1021/jm060325b. [DOI] [PubMed] [Google Scholar]
- 15.Kieffer TL, Sarrazin C, Miller JS, et al. Telaprevir and pegylated interferon-alpha-2a inhibit wild-type and resistant genotype 1 hepatitis C virus replication in patients. Hepatology. 2007;46:631–9. doi: 10.1002/hep.21781. [DOI] [PubMed] [Google Scholar]
- 16.Sarrazin C, Rouzier R, Wagner F, et al. SCH 503034, a novel hepatitis C virus protease inhibitor, plus pegylated interferon alpha-2b for genotype 1 nonresponders. Gastroenterology. 2007;132:1270–8. doi: 10.1053/j.gastro.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 17.Buti M, Lurie Y, Zakharova NG, et al. Randomized trial of peginterferon alfa-2b and ribavirin for 48 or 72 weeks in patients with hepatitis C virus genotype 1 and slow virologic response. Hepatology. 2010;52:1201–7. doi: 10.1002/hep.23816. [DOI] [PubMed] [Google Scholar]
- 18.Jessner W, Gschwantler M, Steindl-Munda P, et al. Primary interferon resistance and treatment response in chronic hepatitis C infection: a pilot study. Lancet. 2001;358:1241–2. doi: 10.1016/S0140-6736(01)06356-5. [DOI] [PubMed] [Google Scholar]
- 19.Thompson AJ, Muir AJ, Sulkowski MS, et al. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology. 2010;139:120–9. doi: 10.1053/j.gastro.2010.04.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


