Abstract
Cyanobacteria (blue-green algae) play profound roles in ecology and biogeochemistry. One model cyanobacterial species is the unicellular cyanobacterium Synechocystis sp. PCC 6803. This species is highly amenable to genetic modification. Its genome has been sequenced and many systems biology and molecular biology tools are available to study this bacterium. Recently, researchers have put significant efforts into understanding and engineering this bacterium to produce chemicals and biofuels from sunlight and CO2. To demonstrate our perspective on the application of this cyanobacterium as a photosynthesis-based chassis, we summarize the recent research on Synechocystis 6803 by focusing on five topics: rate-limiting factors for cell cultivation; molecular tools for genetic modifications; high-throughput system biology for genome wide analysis; metabolic modeling for physiological prediction and rational metabolic engineering; and applications in producing diverse chemicals. We also discuss the particular challenges for systems analysis and engineering applications of this microorganism, including precise characterization of versatile cell metabolism, improvement of product rates and titers, bioprocess scale-up, and product recovery. Although much progress has been achieved in the development of Synechocystis 6803 as a phototrophic cell factory, the biotechnology for “Compounds from Synechocystis” is still significantly lagging behind those for heterotrophic microbes (e.g., Escherichia coli).
Keywords: algae, biofuel, bioprocess scale-up, metabolism, systems biology
1. Introduction
Cyanobacteria grow in many different regions throughout the world. They have contributed significantly to both the food chains and the atmospheric oxygen levels, promoting ecological biodiversity. Worldwide, cyanobacteria convert solar energy into biomass-based chemical energy at a rate of ~450 Terawatts, which is >25 times higher than the total power used by humans [1]. Their powerful phototrophic metabolism has sparked research into utilization of cyanobacteria for generating renewable biofuels and chemicals using sunlight and the greenhouse gas CO2 [2,3]. Among all cyanobacterial species, Synechocystis sp. PCC 6803 (hereafter Synechocystis 6803) is one of the most extensively studied species since it was initially isolated from a freshwater lake in 1968. The entire genome, including four endogenous plasmids, was sequenced, and over 3000 genes have been annotated to date [4,5]. Synechocystis 6803 demonstrates versatile carbon metabolisms, growing under photoautotrophic, mixotrophic and heterotrophic conditions [6]. Additionally, biochemical similarities between the plant chloroplasts and Synechocystis 6803 make the latter an ideal system for studying the molecular mechanisms underlying stress responses and stress adaptation in higher plants [7]. More importantly, this species is naturally competent (homologous recombination at high frequency) [8]. The recent developments in synthetic biology have provided plenty of molecular biology tools to engineer Synechocystis 6803 as a photosynthetic host for the production of diverse types of chemicals. In this review paper, we mainly focus on advances in the application of Synechocystis 6803 for biosynthesis as well as the development of tools for rational genetic modification and bioprocess scale-up. This overview of Synechocystis 6803 presents our perspectives on the challenges in cyanobacterial research related to functional characterization, rational metabolic engineering, and bioprocess engineering.
2. Influential Factors for Synechocystis 6803 Cultivation
Synechocystis 6803 bio-productions require robust biomass growth. Optimal cyanobacterial cultivation depends on both nutrient availability (CO2, nitrogen, and phosphorus) and cultivation conditions (light irradiance, temperature, pH, etc.) [9]. Many studies have been performed to reveal Synechocystis physiology under nutrient “replete” versus “deplete” conditions, as well as its metabolic responses to environmental factors.
2.1. CO2 Fixation
Synechocystis 6803 is able to fulfill oxygenic photosynthesis and CO2 fixation. Ribulose-1,5-bisphosphate carboxylase (RuBisCO) catalyzes the first step of the Calvin Cycle for CO2 fixation. However, this enzyme is sensitive to O2. To solve this problem, Synechocystis has evolved a unique organelle, carboxysome, which encapsulates RuBisCO and concentrates inorganic carbon for biomass growth. In detail, the carboxysome membrane is rather impermeable to O2, but HCO− 3 in the cytosol is able to diffuse into the carboxysome via active transport [10]. Such subcellular localization of RuBisCO provides a useful barrier to O2 diffusion so that Synechocystis photorespiration (oxidation of Ribulose-1,5-bisphosphate by O2 that competes with carbon fixation) can be maintained at a very low level during autotrophic growth (<1% of total carbon fixation) [11].
Under CO2 limitation conditions, studies using the shotgun Liquid Chromatography-Mass Spectrometry (LC-MS/MS) method have revealed significant proteomic changes mediated by multiple transcriptional regulators in Synechocystis 6803, including proteins participating in inorganic carbon fixation, nitrogen transport and assimilation, as well as in the protection of the photosynthetic machinery from excess light [12]. Low inorganic carbonate concentration also activates the Synechocystis 6803 CO2-concentrating mechanism [13] via the induction of a highly efficient bicarbonate transporter (bicarbonate-binding protein CmpA) [14]. Therefore, Synechocystis 6803 has the capability to grow under very low CO2 concentrations [12,15].
2.2. Organic Carbon Utilization
Synechocystis 6803 can grow either photoautotrophically via the Calvin Cycle (maximum doubling time 7~10 h under optimal light conditions) or photoheterotrophically on glucose via the glycolysis pathway and the oxidative pentose phosphate pathway (doubling time ~3.5 days). Some sub-strains of Synechocystis 6803 cannot utilize glucose as the main carbon source [16,17,18,19]. Glucose-tolerant Synechocystis 6803 sub-strains will not grow on glucose under complete darkness unless given a daily pulse of white light [20]. Moreover, our recent study indicated that glucose tolerant Synechocystis 6803 may co-metabolize different organic acids (including pyruvate, acetate, and succinate) when inorganic carbon (CO2 or HCO− 3) is insufficient in the medium [21]. Although the use of organic acids as cyanobacterial feedstock is not cost-effective for scale-up biomass production, the organic substrate utilization capability may provide insight into the evolutionary transition from autotrophic to heterotrophic metabolisms in early life.
2.3. Light Harvesting
Previous studies have revealed that under sufficient irradiance and CO2 concentrations, RuBisCO is not the rate-limiting factor for Synechocystis 6803 photosynthetic growth. Rather, autotrophic growth is constrained by phosphoglycerate reduction due to ATP/NADPH limitation from photo-reactions [22]. Cyanobacteria cannot absorb all incoming sunlight due to light reflection, dissipation, shading effect, and the limited absorption spectrum (blue and red) of the photosynthetic antenna. Furthermore, photons absorbed by antennae cannot be fully used for energy conversion. Therefore, antenna truncation has been proposed to increase the efficiency of the light harvesting system [23]. Unfortunately, antenna modification has not produced any advantages in biomass production in Synechocystis 6803 [24]. With electron microscopy and hyper spectral confocal fluorescence microscopy, Collins et al. revealed that phycobilisome truncation mutants exhibit decreased concentrations of both photosystem I and photosystem II [25]. Currently, light harvesting is still the rate-limiting step for high-efficiency Synechocystis biomass growth.
2.4. Nitrogen and Phosphorus Uptake
Most cyanobacteria are able to use nitrate and ammonium as nitrogen sources [26,27]. Synechocystis 6803 cannot fix N2 naturally (absence of nitrogenase), but it is able to store ammonium nitrogen inside of the cell by producing cyanophycin, a polypeptide containing multiple arginine and aspartate residues. This polymer can be degraded by a hydrolytic enzyme cyanophycinase under nitrogen limitation condition [28]. Such nitrogen-storage capability of Synechocystis 6803 can be potentially used for removal of nitrogen from wastewater, and the resulting cyanophycin can be used as a bio-fertilizer. On the other hand, Krasikov and coworkers monitored the transcriptome of Synechocystis 6803 after nitrogen starvation [26], and observed three nitrogen starvation responses: (1) nitrogen assimilation is up-regulated; (2) cells exhibit chlorosis (the pigment degradation process of cyanobacteria) with a transcriptional repression of the phycobilisome genes; and (3) cells stop most of their enzymatic activities. In addition, photosynthetic carbon fixation is limited under nitrogen depletion conditions (i.e., RuBisCO encoding genes are strongly down-regulated) [29].
Synechocystis 6803 is capable of accumulating inorganic polyphosphate (polyP) inside the cell. Deletion of its phosphate regulator genes (phoU and sphU) significantly increases the intracellular polyP concentration; thus, such mutants can be used for Pi removal from wastewater [30]. Pi starvation enhances cyanobacterial phosphorus uptake. However, phosphorus deprivation harms photosynthetic machinery and slows down both energy and nucleotide sugar generation [31]. In general, both Pi and N depletion are coupled with the down-regulation of photosynthesis, and trigger the metabolic responses to maintain homeostasis. Chlorosis is observed under nitrogen or phosphorus deprivation [32]. Therefore, N and Pi supplements are crucial for Synechocystis 6803 cultivation, which accounts for the main cost in the scale-up of Synechocystis cultivation.
2.5. Cultivation Conditions
Synechocystis 6803 is commonly cultivated at 30 °C, using BG11 medium (pH = 7~8). Cells can maintain their growth in oxic conditions (or photo-bioreactors) and alkaline medium (pH = 10). Elemental analysis of nutrient requirements showed the Synechocystis 6803 dry biomass formula is CH1.62O0.40N0.22P0.01. In ideal photo-bioreactors, Synechocystis 6803 achieves a specific growth rate of 1.7~2.5 day−1 and a nitrate uptake rate of 0.46 g N/g dry cell weight day−1 [9]. Moreover, Synechocystis metabolism has unique responses to environmental stresses (e.g., cold-stress, hyperosmotic stress and salt stress). In unfavorable environments, Synechocystis 6803 shows activation of alternate pathways for the acquisition of carbon and nitrogen (e.g., breakdown of cyanophycin into arginine and aspartic acid) and for the reduction of photosynthesis efficiencies. Under such conditions, the specific growth rate could be less than 1 day−1. Thereby, environmental conditions are important considerations for algal bioprocess scale-up, especially for open pond systems.
In summary, when compared to Escherichia coli or Saccharomyces cerevisiae (specific growth rates >10 day−1), one of the major limitations in large-scale cultivation of Synechocystis 6803 is the slow growth. Although optimization of cultivation conditions in photoreactors could resolve this problem to some extent, high cost associated with photoreactor manufacturing and operation limits the large-scale cyanobacterial production. Alternatively, there might be an answer with the development of synthetic biology and systems biology. Hopefully, bottlenecks for cyanobacterial bioprocess will be solved via systems metabolic engineering in the future.
3. Genetic and Molecular Tools
To produce chemicals in a heterologous host, it is essential to manipulate its genetic codes and control gene expression levels so that carbon can flow through the desired pathways. Synthetic biology has advanced the development of numerous genetic and molecular biology tools for model microbial hosts (such as E. coli and S. cerevisiae). Similar tools are being developed for Synechocystis 6803, allowing for genetic manipulation at transcriptional, translational, and posttranslational levels.
3.1. Plasmid Vectors
Expression of heterologous genes requires introduction of DNAs to the host, either on plasmids or in the chromosome. The two types of plasmid vectors that are typically used are integrative and replicative plasmids. Integrative plasmids integrate a gene into the genomic DNA of Synechocystis 6803 by homologous recombination [33]. Such plasmids do not survive long inside the expression host and are designed to insert a desired gene into specific sites of the genomic DNAs. The most representative integrative vector is pTCP2031V, which integrates the target gene to the slr2031 site of the Synechocystis chromosome [34]. Besides slr2031, other neutral sites, such as the psbA1 gene locus, were also used to construct high-efficiency integrative vectors [35]. In contrast, replicative plasmids allow fast introduction of heterologous genes into the host. RSF1010 is an E. coli-derived shuttle vector and is used extensively in Synechocystis 6803 as a replicative plasmid [2,36]. Other shuttle vectors that have been developed in Synechocystis 6803 include pFC1 [37], pFF11 [38], and pFCLV7 [39]. However, replicative vectors are genetically less stable, and antibiotic pressure is usually required for continuous proliferation of the vector in the host [40].
3.2. Transformation and Segregation
Synechocystis 6803 during the exponential growth phase has high transformation efficiencies through natural transformation, ultrasonic transformation, or electroporation [8]. For natural transformation, a pretreatment with EDTA for two days increased efficiency by 23%. Transformation efficiencies for circular DNA have been shown to be ~30% higher than linear DNA [8]. Successful transformation can be increased by two orders of magnitude via deletion of sll1354 (the gene encoding the exonuclease RecJ) [41]. However, it should be noted that among sub-strains that bear the common name Synechocystis 6803, the Kazusa strain is non-competent for transformation [18,42]. Because Synechocystis 6803 contains more than one genome copy per cell, only one chromosome is involved in the recombination event once the integrative vector is introduced [6]. To obtain a genetically homogeneous recombinant strain, segregation with selection pressure is necessary, by continuously streaking the colonies onto plates containing increasing level of antibiotics [6]. Therefore, the choice of the integration site in Synechocystis 6803 is very important because complete segregation is impossible if the inactivation of the gene locus confers significant disadvantages to the host growth.
3.3. Transcriptional Control Tools
Gene expression can be controlled by proper promoters. Several Synechocystis 6803 native promoters are used to overexpress heterologous proteins. PrbcLS and PpsbA2 are strong native promoters involved in the expression of RuBisCO and photosystem II, respectively [2]. They have been used to control pdc and adh genes for ethanol synthesis [43], or to overexpress the fatty acid pathway(accBCDA/tesA/fatB2 genes) [44]. Some native promoters have the ability to control gene expression according to environmental signals (e.g., light cycles/intensity, nitrogen-stress, salt-stress, and temperature), allowing expression levels of the controlled genes to respond to environmental changes. For example, promoters associated with genes clpP, slr1634, and rbpP showed distinctive circadian patterns [45], which could express heterologous genes with a diurnal rhythm. To express a pathway with multiple enzymes, inducible promoters that reduce stress from early gene expression are advantageous. The Synechocystis 6803 nickel-sensing system, NrsR and NrsS (encoded by nrsR and nrsS), detects extracellular nickel concentrations and triggers gene expression from the nrsBACD promoter. Placing heterologous genes under the control of PnrsBACD would allow the gene expression to be nickel-inducible [46]. Recently, Huang et al. characterized a series of inducible promoters commonly used in E. coli and found most of them lost their inducibility when used in Synechocystis 6803 [47]. For Synechocystis, Ptrc led to high expression levels in the absence of its inducer (leaky), while Plac, Ptet, and PR have low expression levels even with high inducer concentrations. It is still not clear why these promoters behaved differently in E. coli and Synechocystis 6803. Several factors could cause such differences including the specificity and concentration of sigma factors, inducer membrane permeability, and unknown regulators that may interact with these promoters. Currently, Synechocystis 6803 still lacks non-leaky, inducible promoters with a large inducible range.
As a part of the RNA polymerase holo-enzyme complex, sigma factors play central roles in transcription initiation. Synechocystis 6803 has evolved specialized sigma factors that can be divided in three groups: sigma factors with consistent expression throughout growth, sigma factors that respond to specific environmental conditions, and sigma factors related to motility [48]. For example, engineering of sigma factor SigE increased the level of many sugar catabolic enzymes in Synechocystis 6803 [49]. Furthermore, transcription factors (both repressors and activators) together with their cognate promoters can be potentially used to create biosensors for intracellular signals in Synechocystis 6803 [50,51].
3.4. Translational Control Tools
Gene expression can be controlled at the level of translation. The ribosomal binding site (RBS) is an important genetic element involved in mRNA translation initiation. In prokaryotes, there is a correlation between the RBS sequence and the protein level of its downstream gene [52]. Prediction and optimization of the RBS strength could be guided by thermodynamic models (to predict interaction of mRNA with synthetic RBS) [33,53]. Other post-transcriptional control tools include RNA processing and RNA silencing and antisense RNAs (asRNAs), which play crucial roles in gene translational regulation in eukaryotic cells [54]. AsRNAs have been extensively found in prokaryotes [55], including Synechocystis 6803 [56]. For example, IsrR is a transcript from the noncoding strand of isiA, which is an iron stress-induced gene. The IsrR/isiA mRNA duplex is degraded in normal conditions. Under continuous iron deficiency, transcription of isiA will exceed that of IsrR and accumulated IsiA influences photosynthesis apparatus reorganization. Thus, IsrR acts as a photosynthesis-related RNA silencer in Synechocystis 6803. In other studies, several asRNAs are found to suppress the translation of genes involved in Synechocystis photosystem II. So far, there are ~73 asRNAs in Synechocystis 6803 and ~10% of annotated genes are associated with asRNA [55,57]. Moreover, clustered regularly interspaced short palindromic repeats (CRISPR) is a type of small RNAs-based foreign nucleic acid silencing system analogous to RNAi in eukaryotic organisms [58], which can be used for RNA-guided translation engineering. There are several CRISPR-associated genes that have been discovered in Synechocystis 6803 [59,60], offering a new tool to control Synechocystis gene expression.
3.5. Posttranslational Control Tools
Protein degradation system plays significant roles in cell metabolism via elimination of misfolded and damaged proteins. Degradation tags are short peptide sequences that mark a protein for degradation. They have been used to control protein expression in many microbial systems including Synechocystis 6803 [47,61]. For example, engineering the amino acid sequence of the SsrA tag leads to a series of protein degradation tags with various strengths in Synechocystis 6803 [62]. Therefore, it is possible to control cellular enzyme levels that affect the flux of metabolic pathways.
In summary, the advances of synthetic biology in Synechocystis 6803 are still far behind that in E. coli. Many standard biological parts that are widely used in E. coli did not work properly in Synechocystis 6803 [33]. Taken into account the additional challenges, such as the thylakoid membrane and the multiple-copy chromosomes, it might take extra efforts to develop synthetic biology tools for Synechocystis 6803. Due to the importance for genetic engineering, a new set of synthetic biology tools standardized for Synechocystis 6803 should be created in the near future.
4. High Throughput System Biology for Synechocystis 6803
Rational engineering of biosynthetic pathways requires understanding of cell-wide metabolism. High-throughput “omics” (transcriptomics, proteomics, and metabolomics) tools have been applied widely to analyze Synechocystis 6803’s dynamic process under various physiological conditions [63].
4.1. Transcriptomics
Transcriptomics can provide detailed transcriptional profiles of metabolic pathways under diverse physiological situations. Quantitative reverse-transcript PCR is a traditional method to reveal the metabolic responses in Synechocystis 6803. However, high throughput microarray surpasses PCR-based transcriptional analysis. For example, microarray analyses of 163 transcriptome data sets, previously generated in Synechocystis 6803, are used to identify coordination and interaction between each cellular process [64]. The study found that many genes associated with photosynthesis, energy metabolism, and translation are commonly regulated together to help cells adapt under stressed conditions [64]. RNA-seq (Transcriptome Sequencing) has offered an accurate and comprehensive view of global cellular responses in Synechocystis 6803. This new method has been used to search for potential genes that could improve ethanol tolerance in Synechocystis 6803 [65]. Moreover, Yoshikawa et al. applied transcriptomics to analyze central cellular metabolism of Synechocystis 6803 under different trophic conditions [19]. The results showed that the expression level of most genes related to central metabolism was unchanged under auto- and mixotrophic conditions. However, several key genes involved in the glycolytic and pentose phosphate pathways (such as gap1, gnd and rpiA) exhibited a higher transcriptional level under mixotrophic conditions than autotrophic conditions.
4.2. Proteomics
Proteomics mainly focuses on the determination of protein expression levels, protein-protein interactions, and proteins’ roles in cellular processes. Proteomics can provide functional information on metabolic regulation. It has been used to probe the autotrophic metabolism in Synechocystis 6803 under different CO2 concentrations [12]. Via shotgun LC-MS/MS and Western blot approaches, Synechocystis 6803 proteomic responses to CO2 limitation and excess light have been revealed [12]. Moreover, the response of Synechocystis 6803 to toxic chemicals (hexane, ethanol, and butanol) has been investigated by iTRAQ-LC-MS/MS [66,67,68]. When treated with these chemicals, the cell induces a large number of proteins involved in photosynthesis, molecular transport, sulfur relay system, cell membrane/envelope modification, heat-shock, and cell mobility, implying that Synechocystis 6803 can employ multiple pathways to overcome chemical toxicity. The discovered stress-resistant proteins can be potentially overexpressed in the engineered Synechocystis 6803 host to improve their tolerance to the toxic products.
4.3. Metabolomics
Metabolomics focuses on low-molecular-weight metabolites. Metabolomics is the consequence of various enzymatic reactions and provides the most straightforward characterization of metabolic responses to genetic modifications or environmental changes. Compared to other omics studies, few metabolomic research studies have been performed in Synechocystis 6803. Krall et al. have compared three sampling strategies including quenching, filtering, and centrifugation, using a gas chromatography-mass spectrometry (GC-MS)-based metabolomics analysis in Synechocystis 6803 [69]. This study found that sampling is very important in metabolomic analyses because many metabolites turn over quickly. Both fast-filtering and centrifugation are better than cold methanol-water quenching for maintaining the integrity of the metabolite pool produced in Synechocystis 6803. In a recent study, transcriptomics and metabolomics were integrated to analyze Synechocystis 6803 under either autotrophic or mixotrophic conditions [19]. Metabolomics analysis revealed that the oxidative pentose phosphate pathway and glycolysis in cyanobacteria are active under mixotrophic conditions rather than autotrophic conditions. The results also showed that the obtained transcriptomic datasets have low connection with metabolomic datasets, indicating omics data at different levels do not necessarily correlate. This study suggests a crucial need for integrative studies utilizing multiple omics strategies to accurately describe cellular regulations and functions [19].
In summary, omics applications in Synechocystis 6803 are still in their infancy. Although omics technologies could provide “big data” on the levels of transcripts, proteins, and metabolites, elucidation of detailed molecular mechanisms still requires large quantities of subsequent in vivo and in vitro experiments. Ultimately, omics analysis will be used to systematically guide metabolic engineering to improve the efficiency of the engineered Synchocystis 6803 cell factory.
5. Metabolic Modeling for Synechocystis 6803
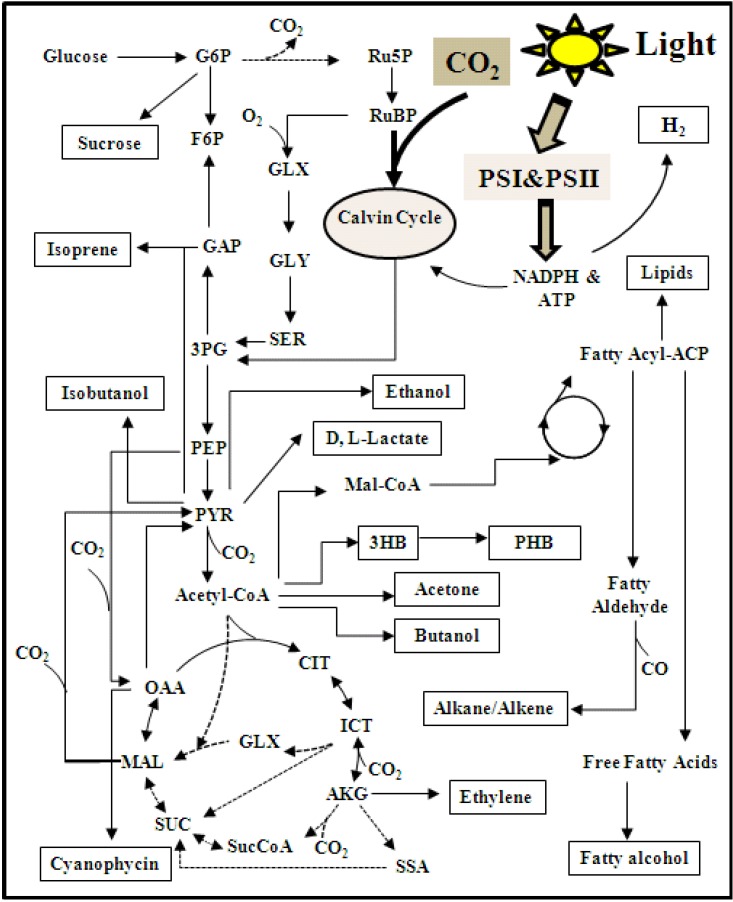
Our knowledge regarding Synechocystis 6803 metabolism is still not complete (Figure 1). For example, it is well-accepted that Synechocystis 6803 has a broken TCA cycle [70]. However, a recent research study has discovered that two new enzymes, which are widely present in cyanobacterial species, can close the TCA cycle [71,72]. Therefore, the function of the TCA cycle in Synechocystis 6803 is still under debate. Moreover, the presence of the oxidative pentose phosphate (OPP) pathway under light conditions in Synechocystis 6803 is controversial [73,74,75,76]. To provide a clear picture for Synechocystis 6803 metabolism, model-based analyses have been coupled with experimental data and bio-informatics to characterize Synechocystis physiology.
Figure 1.
Central metabolic pathways and products from Synechocystis 6803. The functions of several pathways (marked as dot-lines) are still not verified. Abbreviation: 3PG: 3-phosphoglycerate; 3HB: 3-hydroxybutyrate; AKG: α-ketoglutarate; CIT: citrate; F6P: fructose 6-phosphate; G6P: glucose 6-phosphate; GAP: glyceraldehyde 3-phosphate; GLX: glyoxylate; GLY: glycine; ICT: isocitrate; MAL: malate; Mal-CoA: Malonyl-CoA; OAA: oxoacetate; PEP: phosphoenolpyruvate; PHB: polyhydroxybutyrate; PSI&PSII: photosystem I & photosystem II; PYR: pyruvate; Ru5P: ribulose-5-phosphate; RuBP: ribulose-1,5-diphosphate; SER: serine; SUC: succinate; SucCoA: succinyl-CoA; SSA: succinic semialdehyde.
5.1. Flux Balance Analysis (FBA)
FBA can quantitatively characterize the metabolic properties of genome-scale networks [77,78]. Based on the mass balance of all the metabolic intermediates in the metabolic network, a steady-state stoichiometric model is constructed including a set of linear equations for all metabolic reactions. External input and output measurements are employed as constraints. The model is then determined by satisfying a given objective function, e.g., maximization of biomass or a certain product. Shastri and Morgan were the first to reconstruct the metabolic network of Synechocystis 6803 using a flux balance approach [79]. The hetero-, auto-, and mixotrophic metabolisms under optimal growth conditions were evaluated and compared. Subsequently, a high-quality genome-scale metabolic network was applied to identify two photosynthetic apparatus and highlight the high photosynthetic robustness in Synechocystis 6803 [80]. However, the objective function required in FBA may not always represent the real cell physiology [81]. Besides, due to poorly determined stoichiometric systems, the metabolic model may have multiple solutions that meet the object function equally well [82,83]. Additional constraints are needed for the accurate description of cell suboptimal metabolism [84].
5.2. Flux Coupling Finder (FCF)
The FCF framework elucidates the topological and flux connectivity features of genome-scale metabolic networks [85]. Through efficient assessing and comparing of the outcomes of each deletion, FCF identifies the optimal location for equivalent knockouts among multiple targets. FCF investigates the autotrophy, mixotrophy, heterotrophy, and light-activated heterotrophy in Synechocystis 6803, and identifies bottlenecks for hydrogen and ethanol production (e.g., cofactor balances and CO2 fixation). Integration of FCF with transcriptomic data also reveals new insight into metabolic shifts triggered by the availability of light [86]. Such a framework shows the ability to identify rate-limiting enzymes during strain engineering.
5.3. 13C Metabolic Flux Analysis (13C-MFA)
13C-MFA can provide precise information of intracellular fluxes [87]. This method is based on the isotopic fingerprints of metabolic products under a defined 13C-substrate [88]. The labeling information not only highlights the functional pathways and fills the gaps in the genome map [88,89], but also determines absolute carbon fluxes through the metabolic network. 13C-MFA has been efficiently applied to analyze photomixotrophic and heterotrophic metabolism [90]. The photoautotrophic metabolism depends on a non-steady-state 13C-pulse to capture the isotopic dynamics in free metabolites. Recently, a non-steady state 13C pulse method [91] and isotopically non-stationary metabolic flux analysis (INST-MFA) have been developed to investigate the fluxes through photoautotrophic metabolisms [11]. INST-MFA still faces two difficulties. First, isotopomer analysis of low abundant metabolites by GC-MS or LC-MS is technically difficult. Second, the presence of enzyme channeling in the Calvin cycle and glycolysis pathway provides alternative carbon routes in Synechocystis 6803 (i.e., channeling may cause more 13C-accumulation in the downstream metabolites than their precursors during dynamic labeling experiments) and thus complicates model calculations [11,91].
6. Applications of Synechocystis 6803 as a Cell Factory
Synechocystis 6803 has been used as the host strain for the production of biofuels, commodity chemicals, biomaterials, and health-related compounds (Table 1).
Table 1.
Comparing three cyanobacterial species for chemical synthesis (DCW: dry cell weight).
| Strains | Chemicals | Genetic modification | Productivity | Growth conditions | References |
|---|---|---|---|---|---|
| Synechocystis 6803 | Ethanol | pdc and slr1192; ΔphaAB | 5.50 g/L | photoautotrophic; Sparging with 5% CO2-air | [92] |
| Promoter rbc | |||||
| Fatty acids | tesA, tesA137 (codon optimized), accBCDA, fatB1, fatB1, fatB2; ΔphaAB, Δsll1951, Δslr2001-slr2002, Δslr1710, Δslr2132 | 197 ± 14 mg/L | photoautotrophic; Bubbled with 1% CO2 | [44] | |
| Promoter psbA2, rbc, cpc, trc | |||||
| Isoprene | ispS (codon optimized) | 50 μg/g DCW/day | photoautotrophic | [93] | |
| Promoter psbA2 | |||||
| Alk(a/e)nes | sll0208 & sll0209 | 2.3 mg/L/OD730 | photoautotrophic | [94] | |
| Promoter rbc | |||||
| Fatty alcohols | far, at3g11980 Δagp, ΔphaAB | 761 ± 216 µg/g DCW | photoautotrophic | [95] | |
| Promoter psbD13, rbcL12 | |||||
| Sucrose | sps, spp, ugp ΔggpS | 35 mg/L/OD730 | photoautotrophic with 600 mM NaCl | [96] | |
| Promoter petE | |||||
| Hydrogen | ΔnarB, ΔnirA | 186 nmol/mg chl a/h | nitrogen-limiting in the dark | [97] | |
| Synechococcus 7002 | Hydrogen | ΔldhA | 14.1 mol per day per 1017 cells | Anaerobic in the dark | [98] |
| Sucrose | ΔglgA-1, ΔglgA-II | 71 ± 3 mol per 1017 cells | Under hypersaline condition | [99] | |
| Synechococcus 7942 | Ethanol | pdc and adhII | 0.23 g/L | photoautotrophic | [43] |
| Promoter rbcLS | |||||
| Isobutyraldehyde | kivd, alsS, ilvC, ilvD and rbclS | 1.1 g/L | photoautotrophic with NaHCO3 | [100] | |
| Promoter LlacO1, trc, tac | |||||
| Isobutanol | kivd, alsS, ilvCD and yqhD | 0.45 g/L | photoautotrophic with NaHCO3 | [100] | |
| Promoter LlacO1, trc | |||||
| Fatty acids | tesA and Δaas | 80 ± 10 mg/DCW | photoautotrophic; Bubbled with CO2 | [101] | |
| Promoter trc | |||||
| Hydrogen | hydEF, hydG, hydA | 2.8 µmol/h/mg Chl-a | Anaerobic in the dark | [102] | |
| Promoter psbA1, lac |
6.1. Biofuels
Several biofuels have been recently biosynthesized in engineered cyanobacteria, including ethanol, butanol, isobutanol, fatty acids, and fatty alcohols [93,95,100,103,104]. Among them, ethanol, isobutanol, fatty acids, and fatty alcohols were produced in Synechocystis 6803 [44,92,95,105,106]. Synechocystis 6803 has a native transhydrogenase (slr1434 and slr1239, [107]), thus, it is suitable for alcohol production since transhydrogenase can supply NADH from the dephosphorylation of NADPH. Taking isobutanol as an example, Varman et al. engineered a Synechocystis 6803 strain that can produce isobutanol [105]. In this case, two genes from the Lactococcus lactis Ehrlich pathway, kivD and adhA, which are involved in converting 2-ketoisovalerate into isobutanol, are heterologously expressed in Synechocystis 6803 under the control of Ptac. This strain produced 298 and 114 mg/L of isobutanol under mixotrophic and autotrophic conditions, respectively. For fatty alcohols production in Synechocystis 6803, Qi et al. constructed a mutant strain by integrating multiple copies of fatty acyl-CoA reductase gene into the chromosome and disrupting the native glycogen/poly-β-hydroxybutyrate biosynthetic pathways (generated 761 µg fatty alcohols/g dry cell weight) [95]. Moreover, fatty acids (precursors for diesel fuels) were produced in Synechocystis 6803 after several rounds of genetic modification and optimization, achieving 197 mg/L [44]. Finally, H2 is a clean biofuel, which is also widely used for upgrading fossil fuels and for chemical synthesis in the petroleum and chemical industries. Synechocystis 6803 can use its oxygen sensitive and bidirectional hydrogenase to synthesize H2. Current Synechocystis H2 production requires an anaerobic atmosphere and the inactivation of photosystems II (such as a dark condition). Synechocystis H2 evolution can be enhanced by supplying glucose into culture medium [108]. To improve cyanobacterial H2 production, redirecting the electron supply (e.g., disrupt nitrate assimilation pathway) and heterologous expression of hydrogenase are two effective strategies of metabolic engineering (Table 1).
6.2. Commodity Chemical
A variety of commodity chemicals have been recently biosynthesized in Synechocystis 6803. Acetone is a common solvent, and biosynthesis of acetone in Clostridia and recombinant E. coli has been achieved [109]. Zhou et al. have recently designed a novel acetone biosynthetic pathway in Synechocystis 6803 to synthesize acetone from CO2 [110]. In this pathway, two C. acetobutylicum genes encoding an acetoacetate decarboxylase and a coenzyme A transferase are integrated into Synechocystis 6803 chromosome [110]. In addition, the native polyhydroxybutyrate (PHB) synthase gene phaCE and the phosphotransacetylase encoding gene pta were both deleted to reduce the competitive consumption of acetyl-CoA and acetoacetyl-CoA. The acetone titer of the final engineered strain reached 36 mg/L.
Moreover, ethylene and isoprene are important precursors for the production of synthetic rubbers and polymer. Ethylene has been biosynthesized in Synechocystis 6803 by the expression of codon-optimized ethylene-forming-enzyme encoding gene (efe) from Pseudomonas syringae [111,112]. The ethylene production rate of the engineered Synechocystis 6803 can reach to 171 mg/L per day after the optimization of efe expression levels, light intensity, and nutrient status [112]. Isoprene was produced in Synechocystis 6803 by the expression of a codon optimized isoprene synthase from Pueraria montana, which converted the dimethylallyl diphosphate from the native methyl-erythritol-4-phosphate (MEP) pathway to isoprene [93]. In addition, lactic acid is used in the food and pharmaceutical industries [113]. Recently, Angermayr et al. have accomplished the photosynthetic production of l-lactate in Synechocystis 6803 by integrating the Bacillus subtilis ldh gene, which encodes an l-lactate dehydrogenase, into the host’s genome [114]. Coexpression of a transhydrogenase led to another 5-fold increase in l-lactate production (up to 288 mg/L), making its titer higher than that from other cyanobacteria, such as Synechococcus elongates PCC 7942 (~56 mg/L).
6.3. Polyesters
Biopolymer polyhydroxyalkanoate (PHA) can be biosynthesized in Synechocystis 6803 [115]. The PHA biosynthetic operon from Ralstonia eutropha was introduced into Synechocystis 6803, leading to a two-fold enhancement in PHA synthase activity [116]. (S)- and (R)-3-hydroxybutyrate (3HB) serves as the building blocks for PHA biosynthesis. An engineered Synechocystis 6803 strain (overexpress thioesterase and acetoacetyl-CoA reductases, and knockout polyhydroxybutyrate polymerase) was developed to produce up to 533 mg/L 3HB [117]. Polyhyroxybutyrate (PHB), the most common type of PHA, is naturally accumulated in Synechocystis 6803 under nitrogen-starved or phosphorus-starved conditions (granules up to 0.8 micron, 27 mg/L) [118]. Studies by rational gene disruptions have led to high levels of PHB accumulation in Synechocystis 6803. Transposon insertion was also used to probe the PHB biosynthetic pathway. It is found that disruption of sll0461, a gene encoding the gamma-glutamyl phosphate reductase (proA), and sll0565, a gene encoding an unknown protein, improved PHB accumulation [119].
In summary, although genetic modification of Synechocystis 6803 is more difficult than that of E. coli, various products can be successfully produced by engineered Synechocystis 6803.
7. Challenges and Perspectives
While large-scale production of value-added products using heterotrophic engineered microbes (e.g., E. coli and S. cerevisiae) have been demonstrated [120], there are several roadblocks in using autotrophic Synechocystis 6803 as a cell factory.
7.1. Low Product Titer and Rate
At the current stage, production titers and rates from engineered Synechocystis 6803 cannot compete with that from heterotrophic fermentation. For example, fatty acids were produced in an engineered E. coli at 5.2 g/L with 73% of the theoretical yield in three days [121]. However, when a similar pathway was introduced to Synechocystis 6803, the highest fatty acid titer was below 0.2 g/L after 2-week cultivation [44]. Similarly, when an engineered fatty alcohol pathway containing a thioesterase, an acyl-CoA synthase, and a fatty acyl-CoA reductase was expressed in E. coli, fatty alcohols were produced at ~600 mg/L [122]. The titer in Synechocystis 6803 from the same pathway was 0.2 mg/L, 3000-fold lower than E. coli production [106]. The lower titer and synthesis efficiency may be due to many reasons: lower protein expression level, inefficient NADH/NADPH-generating pathways, and slower cell metabolisms. In general, for most products synthesized by cyanobacterial species, titers are below 1 g/L (Table 1). Moreover, most successfully engineered heterologous pathways are short in cyanobacteria, often containing ~2 to 5 heterologous enzymes. As heterologous pathways become larger and more complicated, metabolic optimizations of cyanobacterial species become increasingly difficult. Therefore, Synechocystis 6803 may not be an ideal platform for the biosynthesis of products from complicated heterologous pathways, at least at the current stage.
7.2. Product Loss and Process Failure during Long-Term Cultivation
Cyanobacterial-based biosynthesis is often slower than heterotrophic bacterial fermentation. It often takes cyanobacteria weeks to synthesize a chemical to reasonable titers, increasing the cost for operation and maintenance. Long-period cultivation may increase the risk of microbial contaminations by bacteria or viruses. During this long phototrophic cultivation process, unwanted photo-chemical reactions may occur, leading to product degradation. For example, isobutanol can be slowly lost under light conditions [105]. Furthermore, algal photo-bioreactors often suffer from bio-film fouling during long-term incubation, leading to reduction of light penetration and phototrophic efficiency. Additional efforts for photo-bioreactor cleaning are required.
7.3. Production Costs and Scale up
Commercial large-scale microalgae facilities have been established to produce food supplements, such as β-carotene, astaxanthin, and polyunsaturated fatty acid since 1960s [123]. Previous industrial studies have shown that cyanobacterial bioprocesses face several challenges. For example, large-scale cyanobacterial cultivation can be performed in either open ponds or closed bioreactors [123]. Open pond cyanobacterial cultivation requires adequate sunlight and warm temperatures all year round, therefore placing strong geographic limitations on their production. Open pond cultivation not only has the disadvantage of loss of water due to evaporation, but also has contamination risks by waterfowl, insects, and fungi. More importantly, environmental issues will be raised if genetically modified organisms are used in outdoor ponds. On the other hand, closed bioreactors can be easily controlled (temperature, light and CO2 supply conditions) and are less susceptible to contamination [124]. However, they are more expensive to operate.
Furthermore, producing gaseous products by photosynthetic hosts is challenging due to the use of CO2 and product harvesting. For example, Algenol has developed a process to produce ethanol directly from cyanobacteria [125]. A flexible plastic film photobioreactor has to be built to facilitate the product collection. In addition, cyanobacterial processes use large amounts of water, and thus require energy intensive product harvesting processes. Thereby, production of molecules with low margins (such as commodity chemicals and fuels) often needs extensive process design to maximize both yield and productivity. It might be more feasible to couple a cyanobacterial-based bioprocess with the wastewater treatment to improve its economical margins. Under all circumstances, rigorous cradle-to-gate life cycle analysis and economic analysis are necessary to evaluate the applicability and environmental benefits of the cyanobacterial bioprocess.
8. Conclusions
Systems analysis and metabolic modification of Synechocystis 6803 are now mainly studied in the laboratory. Although Synechocystis 6803 has become a potential photosynthetic host, the productivity of engineered Synechocystis 6803 strains are still low as compared to that from E. coli and S. cerevisiae. In order to take the photosynthetic bio-production to industrial scales, low efficiency of cell metabolisms and limitations in cyanobacterial bioprocesses need to be solved by microbiologists, synthetic biologists, and chemical engineers. Meanwhile, both economic benefits and environmental risks for the use of genetically modified Synechocystis 6803 should be rigorously investigated for the cyanobacterial industry.
Acknowledgments
We are thankful for Charlie Macintosh and Arul Varman for help with the manuscript preparation. The cyanobacterial research is supported by US National Science Foundation. YY is funded by the National Basic Research Program of China (973 Project, Grant #2012CB721006) and the Specialized Research Fund for the Doctoral Program of Higher Education of China (Grant #20100146120021). FZ is funded by I-CARES at Washington University.
References
- 1.Pisciotta J.M., Zou Y., Baskakov I.V. Light-Dependent Electrogenic Activity of Cyanobacteria. PLoS One. 2010;5:e10821. doi: 10.1371/journal.pone.0010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang B., Wang J., Zhang W., Meldrum D.R. Application of synthetic biology in cyanobacteria and algae. Front.Microbiol. 2012;3:344. doi: 10.3389/fmicb.2012.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruffing A.M. Engineered cyanobacteria: Teaching an old bug new tricks. Bioeng. Bugs. 2011;2:136–149. doi: 10.4161/bbug.2.3.15285. [DOI] [PubMed] [Google Scholar]
- 4.Kaneko T., Nakamura Y., Sasamoto S., Watanabe A., Kohara M., Matsumoto M., Shimpo S., Yamada M., Tabata S. Structural analysis of four large plasmids harboring in a unicellular cyanobacterium, Synechocystis sp. PCC 6803. DNA Res. 2003;10:221–228. doi: 10.1093/dnares/10.5.221. [DOI] [PubMed] [Google Scholar]
- 5.Kaneko T., Sato S., Kotani H., Tanaka A., Asamizu E., Nakamura Y., Miyajima N., Hirosawa M., Sugiura M., Sasamoto S., et al. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 6.Vermaas W. Molecular genetics of the cyanobacterium Synechocystis sp. PCC 6803: Principles and possible biotechnology applications. J. Appl. Phycol. 1996;8:263–273. doi: 10.1007/BF02178569. [DOI] [Google Scholar]
- 7.Los D.A., Zorina A., Sinetova M., Kryazhov S., Mironov K., Zinchenko V.V. Stress Sensors and Signal Transducers in Cyanobacteria. Sensors. 2010;10:2386–2415. doi: 10.3390/s100302386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zang X.N., Liu B., Liu S.M., Arunakumara K.K.I.U., Zhang X.C. Optimum conditions for transformation of Synechocystis sp PCC 6803. J. Microbiol. 2007;45:241–245. [PubMed] [Google Scholar]
- 9.Kim H.W., Vannela R., Zhou C., Rittmann B.E. Nutrient acquisition and limitation for the photoautotrophic growth of Synechocystis sp. PCC6803 as a renewable biomass source. Biotechnol. Bioeng. 2011;108:277–285. doi: 10.1002/bit.22928. [DOI] [PubMed] [Google Scholar]
- 10.Pierce J., Carlson T.J., Williams J.G.K. A cyanobacterial mutant requiring the expression of ribulose bisphosphate carboxylase from a photosynthetic anaerobe. Proc. Natl. Acad. Sci. USA. 1989;86:5753–5757. doi: 10.1073/pnas.86.15.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young J.D., Shastri A.A., Stephanopoulos G., Morgan J.A. Mapping photoautotrophic metabolism with isotopically nonstationary 13C flux analysis. Metab. Eng. 2011;13:656–665. doi: 10.1016/j.ymben.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Battchikova N., Vainonen J.P., Vorontsova N., Keranen M., Carmel D., Aro E.M. Dynamic Changes in the Proteome of Synechocystis 6803 in Response to CO2 Limitation Revealed by Quantitative Proteomics. J. Proteome Res. 2010;9:5896–5912. doi: 10.1021/pr100651w. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan A., Schwarz R., Lieman-Hurwitz J., Reinhold L. Physiological and molecular aspects of the inorganic carbon-concentrating mechanism in cyanobacteria. Plant Physiol. 1991;97:851–855. doi: 10.1104/pp.97.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maeda S., Price G.D., Badger M.R., Enomoto C., Omata T. Bicarbonate binding activity of the CmpA protein of the cyanobacterium Synechococcus sp. strain PCC 7942 involved in active transport of bicarbonate. J. Biol. Chem. 2000;275:20551–20555. doi: 10.1074/jbc.M003034200. [DOI] [PubMed] [Google Scholar]
- 15.Xu M., Bernat G., Singh A., Mi H.L., Rogner M., Pakrasi H.B., Ogawa T. Properties of mutants of Synechocystis sp. Strain PCC 6803 lacking inorganic carbon sequestration systems. Plant Cell Physiol. 2008;49:1672–1677. doi: 10.1093/pcp/pcn139. [DOI] [PubMed] [Google Scholar]
- 16.Williams J.G.K. Construction of specific mutations in photosystem-II photosynthetic reaction center by genetic-engineering methods in Synechocystis-6803. Methods Enzymol. 1988;167:766–778. doi: 10.1016/0076-6879(88)67088-1. [DOI] [Google Scholar]
- 17.Bartsevich V.V., Pakrasi H.B. Molecular-identification of an abc transporter complex for manganese—Analysis of a cyanobacterial mutant strain impaired in the photosynthetic oxygen evolution process. EMBO J. 1995;14:1845–1853. doi: 10.1002/j.1460-2075.1995.tb07176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikeuchi M., Tabata S. Synechocystis sp. PCC 6803—A useful tool in the study of the genetics of cyanobacteria. Photosynth. Res. 2001;70:73–83. doi: 10.1023/A:1013887908680. [DOI] [PubMed] [Google Scholar]
- 19.Yoshikawa K., Hirasawa T., Ogawa K., Hidaka Y., Nakajima T., Furusawa C., Shimizu H. Integrated transcriptomic and metabolomic analysis of the central metabolism of Synechocystis sp. PCC 6803 under different trophic conditions. Biotechnol. J. 2013;8:571–580. doi: 10.1002/biot.201200235. [DOI] [PubMed] [Google Scholar]
- 20.Anderson S.L., Mcintosh L. Light-activated heterotrophic growth of the cyanobacterium Synechocystis sp. strain PCC 6803: A blue-light-requiring process. J. Bact. 1991;173:2761–2767. doi: 10.1128/jb.173.9.2761-2767.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.You L., He L., Tang Y.J. 13C-metabolism Analysis of Photoheterotrophic Cyanobacterium Synechocystis sp. Strain PCC 6803. Appl. Environ. Microbiol. 2013 submitted for publication. [Google Scholar]
- 22.Marcus Y., Altman-Gueta H., Wolff Y., Gurevitz M. Rubisco mutagenesis provides new insight into limitations on photosynthesis and growth in Synechocystis PCC6803. J. Exp. Bot. 2011;62:4173–4182. doi: 10.1093/jxb/err116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beckmann J., Lehr F., Finazzi G., Hankamer B., Posten C., Wobbe L., Kruse O. Improvement of light to biomass conversion by de-regulation of light-harvesting protein translation in Chlamydomonas reinhardtii. J. Biotechnol. 2009;142:70–77. doi: 10.1016/j.jbiotec.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Page L.E., Liberton M., Pakrasi H.B. Reduction of photoautotrophic productivity in the cyanobacterium Synechocystis sp. strain PCC 6803 by phycobilisome antenna truncation. Appl. Environ. Microbiol. 2012;78:6349–6351. doi: 10.1128/AEM.00499-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins A.M., Liberton M., Jones H.D.T., Garcia O.F., Pakrasi H.B., Timlin J.A. Photosynthetic pigment localization and thylakoid membrane morphology are altered in Synechocystis 6803 phycobilisome mutants. Plant Physiol. 2012;158:1600–1609. doi: 10.1104/pp.111.192849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krasikov V., Aguirre von Wobeser E., Dekker H.L., Huisman J., Matthijs H.C. Time-series resolution of gradual nitrogen starvation and its impact on photosynthesis in the cyanobacterium Synechocystis PCC 6803. Physiol. Plant. 2012;145:426–439. doi: 10.1111/j.1399-3054.2012.01585.x. [DOI] [PubMed] [Google Scholar]
- 27.Flores E., Herrero A. Nitrogen assimilation and nitrogen control in cyanobacteria. Biochem. Soc. Trans. 2005;33:164–167. doi: 10.1042/BST0330164. [DOI] [PubMed] [Google Scholar]
- 28.Richter R., Hejazi M., Kraft R., Ziegler K., Lockau W. Cyanophycinase, a peptidase degrading the cyanobacterial reserve material multi-l-arginyl-poly-l-aspartic acid (cyanophycin) Eur. J. Biochem. 1999;263:163–169. doi: 10.1046/j.1432-1327.1999.00479.x. [DOI] [PubMed] [Google Scholar]
- 29.Osanai T., Imamura S., Asayama M., Shirai M., Suzuki I., Murata N., Tanaka K. Nitrogen induction of sugar catabolic gene expression in Synechocystis sp PCC 6803. DNA Res. 2006;13:185–195. doi: 10.1093/dnares/dsl010. [DOI] [PubMed] [Google Scholar]
- 30.Burut-Archanai S., Eaton-Rye J.J., Incharoensakdi A., Powtongsook S. Phosphorus removal in a closed recirculating aquaculture system using the cyanobacterium Synechocystis sp. PCC 6803 strain lacking the SphU regulator of the Pho regulon. Biochem. Eng. J. 2013;74:69–75. doi: 10.1016/j.bej.2013.03.004. [DOI] [Google Scholar]
- 31.Fuszard M.A., Ow S.Y., Gan C.S., Noirel J., Ternan N.G., McMullan G., Biggs C.A., Reardon K.F., Wright P.C. The quantitative proteomic response of Synechocystis sp. PCC6803 to phosphate acclimation. Aquat. Biosyst. 2013;9:5. doi: 10.1186/2046-9063-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collier J.L., Grossman A.R. Chlorosis induced by nutrient deprivation in Synechococcus sp. strain PCC 7942: Not all bleaching is the same. J. Bact. 1992;174:4718–4726. doi: 10.1128/jb.174.14.4718-4726.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heidorn T., Camsund D., Huang H.H., Lindberg P., Oliveira P., Stensjo K., Lindblad P. Synthetic biology in cyanobacteria: Engineering and analyzing novel functions. Method Enzymol. 2011;497:539–579. doi: 10.1016/B978-0-12-385075-1.00024-X. [DOI] [PubMed] [Google Scholar]
- 34.Muramatsu M., Sonoike K., Hihara Y. Mechanism of downregulation of photosystem I content under high-light conditions in the cyanobacterium Synechocystis sp. PCC 6803. Microbiology (SGM) 2009;155:989–996. doi: 10.1099/mic.0.024018-0. [DOI] [PubMed] [Google Scholar]
- 35.Mohamed A., Jansson C. Influence of light on accumulation of photosynthesis-specific transcripts in the cyanobacterium Synechocystis-6803. Plant Mol. Biol. 1989;13:693–700. doi: 10.1007/BF00016024. [DOI] [PubMed] [Google Scholar]
- 36.Mermet-bouvier P., Cassier-chauvat C., Marraccini P., Chauvat F. Transfer and replication of Rsf1010-derived plasmids in several cyanobacteria of the general Synechocystis and Synechococcus. Curr. Microbiol. 1993;27:323–327. [Google Scholar]
- 37.Mermet-bouvier P., Chauvat F. A Conditional expression vector for the cyanobacteria Synechocystis sp. strains PCC6803 and PCC6714 or Synechococcus sp. strains PCC7942 and PCC6301. Curr. Microbiol. 1994;28:145–148. doi: 10.1007/BF01571055. [DOI] [PubMed] [Google Scholar]
- 38.Ferino F., Chauvat F. A Promoter-probe vector-host system for the cyanobacterium, Synechocystis PCC6803. Gene. 1989;84:257–266. doi: 10.1016/0378-1119(89)90499-X. [DOI] [PubMed] [Google Scholar]
- 39.Chauvat F., Devries L., Vanderende A., Vanarkel G.A. A host-vector system for gene cloning in the cyanobacterium Synechocystis PCC6803. Mol. Gen. Genet. 1986;204:185–191. doi: 10.1007/BF00330208. [DOI] [Google Scholar]
- 40.Becker E.C., Meyer R.J. Acquisition of resista nce genes by the IncQ plasmid R1162 is limited by its high copy number and lack of a partitioning mechanism. J. Bact. 1997;179:5947–5950. doi: 10.1128/jb.179.18.5947-5950.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kufryk G.I., Sachet M., Schmetterer G., Vermaas W.F. Transformation of the cyanobacterium Synechocystis sp. PCC6803 as a tool for genetic mapping: Optimization of efficiency. FEMS Microbiol. Lett. 2002;206:215–219. doi: 10.1111/j.1574-6968.2002.tb11012.x. [DOI] [PubMed] [Google Scholar]
- 42.Tajima N., Sato S., Maruyama F., Kaneko T., Sasaki N.V., Kurokawa K., Ohta H., Kanesaki Y., Yoshikawa H., Tabata S., et al. Genomic structure of the cyanobacterium Synechocystis sp. PCC6803 strain GT-S. DNA Res. 2011;18:393–399. doi: 10.1093/dnares/dsr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deng M.D., Coleman J.R. Ethanol synthesis by genetic engineering in cyanobacteria. Appl. Environ. Microbiol. 1999;65:523–528. doi: 10.1128/aem.65.2.523-528.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu X., Sheng J., Curtiss R., III. Fatty acid production in genetically modified cyanobacteria. Proc. Natl. Acad. Sci. USA. 2011;108:6899–6904. doi: 10.1073/pnas.1103014108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aoki S., Kondo T., Ishiura M. A promoter-trap vector for clock-controlled genes in the cyanobacterium Synechocystis sp. PCC6803. J. Microbiol. Methods. 2002;49:265–274. doi: 10.1016/S0167-7012(01)00376-1. [DOI] [PubMed] [Google Scholar]
- 46.Liu X., Curtiss R., III. Nickel-inducible lysis system in Synechocystis sp. PCC 6803. Proc. Natl. Acad. Sci. USA. 2009;106:21550–21554. doi: 10.1073/pnas.0911953106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang H.H., Camsund D., Lindblad P., Heidorn T. Design and characterization of molecular tools for a synthetic biology approach towards developing cyanobacterial biotechnology. Nucleic Acids Res. 2010;38:2577–2593. doi: 10.1093/nar/gkq164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Imamura S., Asayama M. Sigma factors for cyanobacterial transcription. Gene Regul. Syst. Biol. 2009;3:65–87. doi: 10.4137/grsb.s2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Osanai T., Oikawa A., Azuma M., Tanaka K., Saito K., Hirai M.Y., Ikeuchi M. Genetic engineering of group 2 sigma factor SigE widely activates expressions of sugar catabolic genes in Synechocystis species PCC 6803. J. Biol. Chem. 2011;286:30962–30971. doi: 10.1074/jbc.M111.231183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang F., Keasling J. Biosensors and their applications in microbial metabolic engineering. Trends Microbiol. 2011;19:323–329. doi: 10.1016/j.tim.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 51.Zhang F., Carothers J.M., Keasling J.D. Design of a dynamic sensor-regulator system for production of chemicals and fuels derived from fatty acids. Nat. Biotechnol. 2012;30:354–359. doi: 10.1038/nbt.2149. [DOI] [PubMed] [Google Scholar]
- 52.Ma J., Campbell A., Karlin S. Correlations between Shine-Dalgarno sequences and gene features such as predicted expression levels and operon structures. J. Bact. 2002;184:5733–5745. doi: 10.1128/JB.184.20.5733-5745.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salis H.M., Mirsky E.A., Voigt C.A. Automated design of synthetic ribosome binding sites to control protein expression. Nat. Biotechnol. 2009;27:946–950. doi: 10.1038/nbt.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beiter T., Reich E., Williams R.W., Simon P. Antisense transcription: A critical look in both directions. Cell Mol. Life Sci. 2009;66:94–112. doi: 10.1007/s00018-008-8381-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Georg J., Hess W.R. cis-Antisense RNA, another level of gene regulation in bacteria. Microbiol. Mol. Biol. Rev. 2011;75:286–300. doi: 10.1128/MMBR.00032-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duhring U., Axmann I.M., Hess W.R., Wilde A. An internal antisense RNA regulates expression of the photosynthesis gene isiA. Proc. Natl. Acad. Sci. USA. 2006;103:7054–7058. doi: 10.1073/pnas.0600927103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Georg J., Voss B., Scholz I., Mitschke J., Wilde A., Hess W.R. Evidence for a major role of antisense RNAs in cyanobacterial gene regulation. Mol. Syst. Biol. 2009;5:305. doi: 10.1038/msb.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Horvath P., Barrangou R. CRISPR/Cas, the immune system of bacteria and archaea. Science. 2010;327:167–170. doi: 10.1126/science.1179555. [DOI] [PubMed] [Google Scholar]
- 59.Makarova K.S., Haft D.H., Barrangou R., Brouns S.J., Charpentier E., Horvath P., Moineau S., Mojica F.J., Wolf Y.I., Yakunin A.F., et al. Evolution and classification of the CRISPR-Cas systems. Nat. Rev. Microbiol. 2011;9:467–477. doi: 10.1038/nrmicro2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scholz I., Lange S.J., Hein S., Hess W.R., Backofen R. CRISPR-Cas systems in the cyanobacterium Synechocystis sp. PCC6803 exhibit distinct processing pathways involving at least two Cas6 and a Cmr2 protein. PLoS One. 2013;8:e56470. doi: 10.1371/journal.pone.0056470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Kasteren S. Synthesis of post-translationally modified proteins. Biochem. Soc. Trans. 2012;40:929–944. doi: 10.1042/BST20120144. [DOI] [PubMed] [Google Scholar]
- 62.Landry B.P., Stockel J., Pakrasi H.B. Use of degradation tags to control protein levels in the cyanobacterium Synechocystis sp. Strain PCC6803. Appl. Environ. Microbiol. 2013;79:2833–2835. doi: 10.1128/AEM.03741-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hagemann M. Molecular biology of cyanobacterial salt acclimation. FEMS Microbiol. Rev. 2011;35:87–123. doi: 10.1111/j.1574-6976.2010.00234.x. [DOI] [PubMed] [Google Scholar]
- 64.Singh A.K., Elvitigala T., Cameron J.C., Ghosh B.K., Bhattacharyya-Pakrasi M., Pakrasi H.B. Integrative analysis of large scale expression profiles reveals core transcriptional response and coordination between multiple cellular processes in a cyanobacterium. Bmc. Syst. Biol. 2010;4:105. doi: 10.1186/1752-0509-4-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang J., Chen L., Huang S., Liu J., Ren X., Tian X., Qiao J., Zhang W. RNA-seq based identification and mutant validation of gene targets related to ethanol resistance in cyanobacterial Synechocystis sp. PCC6803. Biotechnol. Biofuels. 2012;5:89. doi: 10.1186/1754-6834-5-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tian X., Chen L., Wang J., Qiao J., Zhang W. Quantitative proteomics reveals dynamic responses of Synechocystis sp. PCC6803 to next-generation biofuel butanol. J. Proteomics. 2013;78:326–345. doi: 10.1016/j.jprot.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 67.Qiao J., Wang J., Chen L., Tian X., Huang S., Ren X., Zhang W. Quantitative iTRAQ LC-MS/MS proteomics reveals metabolic responses to biofuel ethanol in cyanobacterial Synechocystis sp. PCC6803. J. Proteome Res. 2012;11:5286–5300. doi: 10.1021/pr300504w. [DOI] [PubMed] [Google Scholar]
- 68.Liu J., Chen L., Wang J., Qiao J., Zhang W. Proteomic analysis reveals resistance mechanism against biofuel hexane in Synechocystis sp. PCC6803. Biotechnol. Biofuels. 2012;5:68. doi: 10.1186/1754-6834-5-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krall L., Huege J., Catchpole G., Steinhauser D., Willmitzer L. Assessment of sampling strategies for gas chromatography-mass spectrometry (GC-MS) based metabolomics of cyanobacteria. J. Chromatogr. B. 2009;877:2952–2960. doi: 10.1016/j.jchromb.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 70.Pearce J., Leach C.K., Carr N.G. The incomplete tricarboxylic acid cycle in the blue-green alga Anabaena variabilis. J. Gen. Microbiol. 1969;55:371–378. doi: 10.1099/00221287-55-3-371. [DOI] [PubMed] [Google Scholar]
- 71.Zhang S., Bryant D.A. The tricarboxylic acid cycle in Cyanobacteria. Science. 2011;334:1551–1553. doi: 10.1126/science.1210858. [DOI] [PubMed] [Google Scholar]
- 72.Steinhauser D., Fernie A.R., Araújo W.L. Unusual cyanobacterial TCA cycles: Not broken just different. Trends Plant Sci. 2012;17:503–509. doi: 10.1016/j.tplants.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 73.Pelroy R.A., Rippka R., Stanier R.Y. Metabolism of glucose by unicellular blue-green algae. Arch. Microbiol. 1972;87:303–322. doi: 10.1007/BF00409131. [DOI] [PubMed] [Google Scholar]
- 74.Yang C.Y., Hua Q.H., Shimizu K.S. Integration of the information from gene expression and metabolic fluxes for the analysis of the regulatory mechanisms in Synechocystis. Appl. Microbiol. Biotechnol. 2002;58:813–822. doi: 10.1007/s00253-002-0949-0. [DOI] [PubMed] [Google Scholar]
- 75.Kahlon S., Beeri K., Ohkawa H., Hihara Y., Murik O., Suzuki I., Ogawa T., Kaplan A. A putative sensor kinase, Hik31, is involved in the response of Synechocystis sp. strain PCC6803 to the presence of glucose. Microbiology. 2006;152:647–655. doi: 10.1099/mic.0.28510-0. [DOI] [PubMed] [Google Scholar]
- 76.Herranen M., Battchikova N., Zhang P., Graf A., Sirpiö S., Paakkarinen V., Aro E.-M. Towards Functional Proteomics of Membrane Protein Complexes in Synechocystis sp. PCC6803. Plant Physiol. 2004;134:470–481. doi: 10.1104/pp.103.032326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Orth J.D., Thiele I., Palsson B.O. What is flux balance analysis? Nat. Biotechnol. 2010;28:245–248. doi: 10.1038/nbt.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Varma A., Palsson B.O. Metabolic Flux Balancing: Basic Concepts, Scientific and Practical Use. Nat. Biotechnol. 1994;12:994–998. doi: 10.1038/nbt1094-994. [DOI] [Google Scholar]
- 79.Shastri A.A., Morgan J.A. Flux balance analysis of photoautotrophic metabolism. Biotechnol. Prog. 2005;21:1617–1626. doi: 10.1021/bp050246d. [DOI] [PubMed] [Google Scholar]
- 80.Nogales J., Gudmundsson S., Knight E.M., Palsson B.O., Thiele I. Detailing the optimality of photosynthesis in cyanobacteria through systems biology analysis. Proc. Natl. Acad. Sci. USA. 2012;109:2678–2683. doi: 10.1073/pnas.1117907109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fischer E., Sauer U. Large-scale in vivo flux analysis shows rigidity and suboptimal performance of Bacillus subtilis metabolism. Nat. Genet. 2005;37:636–640. doi: 10.1038/ng1555. [DOI] [PubMed] [Google Scholar]
- 82.Mahadevan R., Schilling C.H. The effects of alternate optimal solutions in constraint-based genome-scale metabolic models. Metab. Eng. 2003;5:264–276. doi: 10.1016/j.ymben.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 83.Price N.D., Reed J.L., Palsson B.O. Genome-scale models of microbial cells: Evaluating the consequences of constraints. Nat. Rev. Microbiol. 2004;2:886–897. doi: 10.1038/nrmicro1023. [DOI] [PubMed] [Google Scholar]
- 84.Chen X., Alonso A.P., Allen D.K., Reed J.L., Shachar-Hill Y. Synergy between 13C-metabolic flux analysis and flux balance analysis for understanding metabolic adaption to anaerobiosis in E. coli. Metab. Eng. 2011;13:38–48. doi: 10.1016/j.ymben.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 85.Burgard A.P., Nikolaev E.V., Schilling C.H., Maranas C.D. Flux coupling analysis of genome-scale metabolic network reconstructions. Genome Res. 2004;14:301–312. doi: 10.1101/gr.1926504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Montagud A., Zelezniak A., Navarro E., de Córdoba P.F., Urchueguía J.F., Patil K.R. Flux coupling and transcriptional regulation within the metabolic network of the photosynthetic bacterium Synechocystis sp. PCC6803. Biotechnol. J. 2011;6:330–342. doi: 10.1002/biot.201000109. [DOI] [PubMed] [Google Scholar]
- 87.Wiechert W. 13C metabolic flux analysis. Metab. Eng. 2001;3:195–206. doi: 10.1006/mben.2001.0187. [DOI] [PubMed] [Google Scholar]
- 88.You L., Page L., Feng X., Berla B., Pakrasi H.B., Tang Y.J. Metabolic pathway confirmation and discovery through 13C-labeling of proteinogenic amino acids. J. Vis. Exp. 2012;59:e3583. doi: 10.3791/3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tang J.K.-H., You L., Blankenship R.E., Tang Y.J. Recent advances in mapping environmental microbial metabolisms through 13C isotopic fingerprints. J. Royal Soc. Interface. 2012;9:2767–2780. doi: 10.1098/rsif.2012.0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang C., Hua Q., Shimizu K. Metabolic flux analysis in Synechocystis using isotope distribution from 13C-labeled glucose. Metab. Eng. 2002;4:202–216. doi: 10.1006/mben.2002.0226. [DOI] [PubMed] [Google Scholar]
- 91.Huege J., Goetze J., Schwarz D., Bauwe H., Hagemann M., Kopka J. Modulation of the major paths of carbon in photorespiratory mutants of Synechocystis. PLoS One. 2011;6:e16278. doi: 10.1371/journal.pone.0016278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gao Z., Zhao H., Li Z., Tan X., Lu X. Photosynthetic production of ethanol from carbon dioxide in genetically engineered cyanobacteria. Energy Environ. Sci. 2012;5:9857–9865. doi: 10.1039/c2ee22675h. [DOI] [Google Scholar]
- 93.Lindberg P., Park S., Melis A. Engineering a platform for photosynthetic isoprene production in cyanobacteria, using Synechocystis as the model organism. Metab. Eng. 2010;12:70–79. doi: 10.1016/j.ymben.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 94.Wang W., Liu X., Lu X. Engineering cyanobacteria to improve photosynthetic production of alka(e)nes. Biotechnol. Biofuels. 2013;6:69. doi: 10.1186/1754-6834-6-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qi F., Yao L., Tan X., Lu X. Construction, characterization and application of molecular tools for metabolic engineering of Synechocystis sp. Biotechnol. Lett. 2013 doi: 10.1007/s10529-013-1252-0. in press. [DOI] [PubMed] [Google Scholar]
- 96.Du W., Liang F., Duan Y., Tan X., Lu X. Exploring the photosynthetic production capacity of sucrose by cyanobacteria. Metab. Eng. 2013;19:17–25. doi: 10.1016/j.ymben.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 97.Baebprasert W., Jantaro S., Khetkorn W., Lindblad P., Incharoensakdi A. Increased H2 production in the cyanobacterium Synechocystis sp. strain PCC6803 by redirecting the electron supply via genetic engineering of the nitrate assimilation pathway. Metab. Eng. 2011;13:610–616. doi: 10.1016/j.ymben.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 98.McNeely K., Xu Y., Bennette N., Bryant D.A., Dismukes G.C. Redirecting reductant flux into hydrogen production via metabolic engineering of fermentative carbon metabolism in a cyanobacterium. Appl. Environ. Microbiol. 2010;76:5032–5038. doi: 10.1128/AEM.00862-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu Y., Guerra L.T., Li Z., Ludwig M., Dismukes G.C., Bryant D.A. Altered carbohydrate metabolism in glycogen synthase mutants of Synechococcus sp. strain PCC7002: Cell factories for soluble sugars. Metab. Eng. 2013;16:56–67. doi: 10.1016/j.ymben.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 100.Atsumi S., Higashide W., Liao J.C. Direct photosynthetic recycling of carbon dioxide to isobutyraldehyde. Nat. Biotechnol. 2009;27:1177–1180. doi: 10.1038/nbt.1586. [DOI] [PubMed] [Google Scholar]
- 101.Ruffing A.M., Jones H.D.T. Physiological effects of free fatty acid production in genetically engineered Synechococcus elongatus PCC7942. Biotechnol. Bioeng. 2012;109:2190–2199. doi: 10.1002/bit.24509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ducat D.C., Sachdeva G., Silver P.A. Rewiring hydrogenase-dependent redox circuits in cyanobacteria. Proc. Natl. Acad. Sci. USA. 2011;108:3941–3946. doi: 10.1073/pnas.1016026108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chisti Y. Biodiesel from microalgae beats bioethanol. Trends Biotechnol. 2008;26:126–131. doi: 10.1016/j.tibtech.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 104.Lan E.I., Liao J.C. Metabolic engineering of cyanobacteria for 1-butanol production from carbon dioxide. Metab. Eng. 2011;13:353–363. doi: 10.1016/j.ymben.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 105.Varman A.M., Xiao Y., Pakrasi H.B., Tang Y.J. Metabolic engineering of Synechocystis sp. strain PCC6803 for isobutanol production. Appl. Environ. Microbiol. 2013;79:908–914. doi: 10.1128/AEM.02827-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tan X., Yao L., Gao Q., Wang W., Qi F., Lu X. Photosynthesis driven conversion of carbon dioxide to fatty alcohols and hydrocarbons in cyanobacteria. Metab. Eng. 2011;13:169–176. doi: 10.1016/j.ymben.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 107.Nakao M., Okamoto S., Kohara M., Fujishiro T., Fujisawa T., Sato S., Tabata S., Kaneko T., Nakamura Y. CyanoBase: The cyanobacteria genome database update 2010. Nucleic Acids Res. 2010;38:379–381. doi: 10.1093/nar/gkp915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cournac L., Guedeney G., Peltier G., Vignais P.M. Sustained photoevolution of molecular hydrogen in a mutant of Synechocystis sp. strain PCC6803 deficient in the type I NADPH-dehydrogenase complex. J. Bacteriol. 2004;186:1737–1746. doi: 10.1128/JB.186.6.1737-1746.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bermejo L.L., Welker N.E., Papoutsakis E.T. Expression of Clostridium acetobutylicum ATCC 824 genes in Escherichia coli for acetone production and acetate detoxification. Appl. Environ. Microbiol. 1998;64:1079–1085. doi: 10.1128/aem.64.3.1079-1085.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhou J., Zhang H.F., Zhang Y.P., Li Y., Ma Y.H. Designing and creating a modularized synthetic pathway in cyanobacterium Synechocystis enables production of acetone from carbon dioxide. Metab. Eng. 2012;14:394–400. doi: 10.1016/j.ymben.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 111.Guerrero F., Carbonell V., Cossu M., Correddu D., Jones P.R. Ethylene synthesis and regulated expression of recombinant protein in Synechocystis sp. PCC6803. PLoS One. 2012;7:e50470. doi: 10.1371/journal.pone.0050470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ungerer J., Tao L., Davis M., Ghirardi M., Maness P.C., Yu J.P. Sustained photosynthetic conversion of CO2 to ethylene in recombinant cyanobacterium Synechocystis 6803. Energy Environ. Sci. 2012;5:8998–9006. doi: 10.1039/c2ee22555g. [DOI] [Google Scholar]
- 113.Wee Y.J., Kim J.N., Ryu H.W. Biotechnological production of lactic acid and its recent applications. Food Technol. Biotechnol. 2006;44:163–172. [Google Scholar]
- 114.Angermayr S.A., Paszota M., Hellingwerf K.J. Engineering a Cyanobacterial Cell Factory for Production of Lactic Acid. Appl. Environ. Microbiol. 2012;78:7098–7106. doi: 10.1128/AEM.01587-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Luengo J.M., Garcia B., Sandoval A., Naharro G., Olivera E.R. Bioplastics from microorganisms. Curr. Opin. Microbiol. 2003;6:251–260. doi: 10.1016/s1369-5274(03)00040-7. [DOI] [PubMed] [Google Scholar]
- 116.Sudesh K., Taguchi K., Doi Y. Effect of increased PHA synthase activity on polyhydroxyalkanoates biosynthesis in Synechocystis sp. PCC6803. Int. J. Biol. Macromol. 2002;30:97–104. doi: 10.1016/S0141-8130(02)00010-7. [DOI] [PubMed] [Google Scholar]
- 117.Wang B., Pugh S., Nielsen D.R., Zhang W.W., Meldrum D.R. Engineering cyanobacteria for photosynthetic production of 3-hydroxybutyrate directly from CO2. Metab. Eng. 2013;16:68–77. doi: 10.1016/j.ymben.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 118.Wu G.F., Wu Q.Y., Shen Z.Y. Accumulation of poly-β-hydroxybutyrate in cyanobacterium Synechocystis sp. PCC6803. Bioresource Technol. 2001;76:85–90. doi: 10.1016/S0960-8524(00)00099-7. [DOI] [PubMed] [Google Scholar]
- 119.Tyo K.E.J., Jin Y.-S., Espinoza F.A., Stephanopoulos G. Identification of gene disruptions for increased poly-3-hydroxybutyrate accumulation in Synechocystis PCC6803. Biotechnol. Prog. 2009;25:1236–1243. doi: 10.1002/btpr.228. [DOI] [PubMed] [Google Scholar]
- 120.Shin J.H., Kim H.U., Kim D.I., Lee S.Y. Production of bulk chemicals via novel metabolic pathways in microorganisms. Biotechnol. Adv. 2012 doi: 10.1016/j.biotechadv.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 121.Zhang F., Ouellet M., Batth T.S., Adams P.D., Petzold C.J., Mukhopadhyay A., Keasling J.D. Enhancing fatty acid production by the expression of the regulatory transcription factor FadR. Metab. Eng. 2012;14:653–660. doi: 10.1016/j.ymben.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 122.Zheng Y.N., Li L.L., Liu Q., Yang J.M., Wang X.W., Liu W., Xu X., Liu H., Zhao G., Xian M. Optimization of fatty alcohol biosynthesis pathway for selectively enhanced production of C12/14 and C16/18 fatty alcohols in engineered E. coli. Microb. Cell Fact. 2012;11:65. doi: 10.1186/1475-2859-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wijffels R.H., Kruse O., Hellingwerf K.J. Potential of industrial biotechnology with cyanobacteria and eukaryotic microalgae. Curr. Opin. Biotechnol. 2013;24:405–413. doi: 10.1016/j.copbio.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 124.Oncel S., Sabankay M. Microalgal biohydrogen production considering light energy and mixing time as the two key features for scale-up. Bioresour. Technol. 2012;121:228–234. doi: 10.1016/j.biortech.2012.06.079. [DOI] [PubMed] [Google Scholar]
- 125.Chance R., McCool B., Coleman J. A Cyanobacteria-Based Photosynthetic Process for the Production of Ethanol; Presented at the National Research Council Committee on Sustainable Development of Algal Biofuels; Washington, DC, USA. 13 June 2011; [(accessed on 2 August 2011)]. Available online: http://khlaw.com/Files/10645_Chance_corrected.pdf. [Google Scholar]