Abstract
Global warming has various effects on human health. The main indirect effects are on infectious diseases. Although the effects on infectious diseases will be detected worldwide, the degree and types of the effect are different, depending on the location of the respective countries and socioeconomical situations.
Among infectious diseases, water- and foodborne infectious diseases and vector-borne infectious diseases are two main categories that are forecasted to be most affected. The effect on vector-borne infectious diseases such as malaria and dengue fever is mainly because of the expansion of the infested areas of vector mosquitoes and increase in the number and feeding activity of infected mosquitoes. There will be increase in the number of cases with water- and foodborne diarrhoeal diseases.
Even with the strongest mitigation procedures, global warming cannot be avoided for decades. Therefore, implementation of adaptation measures to the effect of global warming is the most practical action we can take. It is generally accepted that the impacts of global warming on infectious diseases have not been apparent at this point yet in East Asia. However, these impacts will appear in one form or another if global warming continues to progress in future. Further research on the impacts of global warming on infectious diseases and on future prospects should be conducted.
Key words: dengue fever, global warming, Japanese encephalitis, vector-borne infectious diseases, water- and foodborne infectious diseases
1. Introduction
Global warming is an unequivocal phenomenon today. Global warming is one of the components of climate change, and it induces considerable impacts on human health. The emerging evidence of the effect of global warming on human health has been summarized in the fourth report of Intergovernmental Panel on Climate Change (IPCC).1 The effect of global warming on human health is divided into two categories: direct effect on the illness such as heat shock and increased mortality in population with other diseases and indirect effect on diseases such as infectious diseases and allergy (Table 1). The IPCC report states that climate change has altered the distribution of some infectious disease vectors, the seasonal distribution of some allergenic pollen species, and increased heat wave-related deaths. In the present review, the effect of global warming on infectious diseases is addressed. Furthermore, current research on the effect of global warming on infectious diseases is introduced.
Table 1.
Emerging and forecasted effects of climate change/global warming on infectious diseases and other human health conditions in the world
| Direct effect on other health conditions |
| Heat waves: Short-term increase in mortality, especially among those with cardiovascular and/or respiratory diseases, and increase in heat shock patients |
| Co-effect with air pollution: Increase in asthma and allergy patients |
| Storms and floods: Increase in morbidity and accidental death |
| Indirect effect on infectious diseases |
| Expansion of mosquito- and tick-infested areas, and increase in mosquito activity: Increase in the number of patients with mosquito-borne infectious diseases (i.e. dengue and malaria) and expansion of epidemic areas |
| Contamination of water and foods with bacteria: Increase in the number of patients with water- and foodborne infectious diseases |
| Deterioration of environmental and social conditions: Increased risk of infectious diseases |
2. Effect of Global Warming on Infectious Diseases
It has been assumed that global warming has profound effects on infectious diseases (Figure 1). The effect of global warming on infectious diseases is indirect. Although the effects have been detected worldwide, the degree and types of the effect are different, depending on the location of the respective countries and socioeconomical situations. Among infectious diseases, water- and foodborne infectious diseases and vector-borne infectious diseases are two main categories that are forecasted to be most affected.
Figure 1.
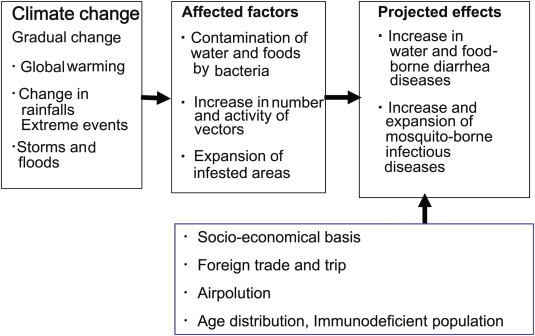
Simplified schematic diagram of the effect of climate change/global warming on infectious diseases. Modified from Ref. 2.
2.1. Effect of global warming on water- and foodborne infectious diseases
It has been predicted that the number of the patients with water- and foodborne infectious diseases is heavily affected by global warming. The number of cholera cases was increased by both high and low rainfalls in Bangladesh.3 The number of non-cholera diarrhoeal disease cases is also increased by high and low rainfalls and by higher temperature in Bangladesh.4 However, the degree of the effects on water- and foodborne infectious diseases depends on the levels of the social infrastructure. In the countries where water and food supply systems and sewage system are well established, the effect on water- and foodborne infectious diseases is expected to be less affected. Thus, the effect is assumed to be greater in developing countries but less in developed countries.
2.2. Effect of global warming on vector-borne infectious diseases
Vector-borne infectious diseases are caused by the pathogens transmitted by arthropods. Mosquitoes and ticks are the main vectors. The effect of global warming on vector-borne infectious diseases is indirect. Global warming affects geographical distribution and activity of the vectors. Thus, the levels of the influence depend on the kind of vectors.
The major mosquito-borne infectious diseases that have been reported to be affected by global warming include malaria, dengue fever, Japanese encephalitis (JE), and tick-borne encephalitis.
2.2.1. Malaria
Malaria has been considered to be the most important vector-borne infectious disease in the world. It has been reported that global warming changes the distribution, intensity of transmission, and seasonality of malaria in sub-Saharan Africa.5,6 Association between inter-annual variability in temperature and malaria transmission was detected in Africa. In Kenya, the number of malaria cases was associated with rainfall and high maximum temperature in preceding 3–4 months.7 In Ethiopia, malaria epidemics were associated with high minimum temperatures in the preceding months.8 On the other hand, there have also been reports that suggest no evident association between climate change and malaria in South America1 or in the Russian Federation.9 Thus, it is possible that the potential impact of global warming on malaria varies at local levels.1
2.2.2. Dengue fever
Dengue fever is an important vector-borne viral infectious disease in the world. There have been reports that suggest an association between global warming and epidemics of dengue fever.10–13 However, there also have been reports that suggest the absence of an association between global warming and epidemics of dengue fever. This is probably because of the presence of multiple other factors in addition to climate factors or the effect of global warming on dengue fever epidemics vary in the study regions. The model of vector abundance demonstrated a good agreement with the distribution of reported dengue cases in some areas in the world.14 Thus, it is predicted that the positive effect of global warming on the abundance and distribution of vector mosquitoes eventually leads to increase in the number of dengue patients and expansion of dengue virus endemic areas.
2.2.3. Other vector-borne infectious diseases
There have been reports on climate-related shifts in the distribution of ticks that may transmit tick-borne encephalitis virus in the regions. Northern or altitudinal shifts in tick distribution have been reported in Sweden and Canada15–17 and also altitudinal shifts in the Czech Republic.18
Severe outbreak of Murray Valley encephalitis caused by a mosquito-borne virus has been reported to occur after heavy rainfall and flooding in southern Australia.19 Heavy rain or flood can cause outbreak of Ross River fever caused by another mosquito-borne virus because of increased breeding of mosquitoes.20 It has been reported that the number of chikungunya patients increased after draught rather than heavy rainfall. There also have been reports on the effect of climate change on JE.21,22 The effects of climate change on vector-borne infectious diseases are complex. The available data until today in general support the conclusion that global warming increases the number of patients with vector-borne viruses. This effect is caused by expanded distribution or increase in the number and activity of the responsible vectors.
2.3. Current studies on the effect of global warming on the infectious diseases
2.3.1. Research on the northern border of Aedes albopictus-infested areas in Japan
The effect of global warming on infectious diseases has not become apparent as an increase in the number of patients with vector-borne infectious diseases or diarrhoeal diseases in Japan. However, there has been an expansion of the infested areas of an important vector mosquito, A albopictus. A albopictus is a major vector of dengue and chikungunya fever. The distribution of A albopictus in northern Japan has been long studied.23 The northern border of the habitat of A albopictus was at northern Kanto district, according to the research by U.S. occupational force after the World War II. The northern border has been moving northward and is at the northern Tohoku district in 2006.24 The northern border of the habitat of A albopictus is well accordant with the area with annual average temperature of 11°C and higher. This does not, however, directly suggest that epidemic of vector-borne infectious diseases such as dengue fever and chikungunya fever will occur in northern Japan but suggest that the area with the risk is expanding northward.
2.3.2. Research on the relationship between summertime temperature and seroconversion rate to JE virus in pigs
JE virus is maintained in nature between vector mosquitoes and domestic pigs in endemic regions.25 The principal vector of JE virus is Culex tritaeniorhynchus in the East Asia. Pigs are also considered as an amplifier for JE virus and a natural host in the temperate zone. In the temperate zone, the vector mosquitoes started to be detected in May, then seroconversion of pigs occurs, and occurrence of human cases follows.26 In the countries where JE vaccination has been strongly implemented and most of the people have protective immunity, threat of JE is not reflected by the number of patients. Pigs are usually slaughtered to be shipped to the market before 6 months of age in Japan. Most pigs present in summer were born after the previous JE season had been over. Thus, they are naive to JE virus before the epidemic season starts. Naive pigs are highly susceptible to JE virus and develop high levels of viraemia and specific antibody after infection with JE virus. The seroconversion rate among sentinel sero-negative pigs reflects the prevalence and activity of JE virus.
Seroconversion rates to JE virus in sentinel pigs were assessed by measuring specific haemagglutination inhibition antibody to JE virus two to three times a month. Seroconversion rates of sentinel pigs from 1983 to 2003 were obtained from the Reports of the National Epidemiological Surveillance of Vaccine Preventable Diseases by the Ministry of Health, Labour and Welfare, Japan. The highest seroconversion rate in each of the prefectures was used for the analyses. The meteorologic data were obtained from Japan Meteorological Agency. The data at the same or the closest cities to those where the pig farms or slaughterhouses were located within the same prefecture were used for analyses along with the highest seroconversion rate in the year.
The relationship was analysed between the seroconversion rates among sentinel pigs and three meteorologic parameters in June, July, and August; (1) the number of days with the average temperature equal to or higher than 25°C, (2) the number of days with the highest temperature equal to or higher than 25°C, and (3) the number of days with the lowest temperature equal to or higher than 20°C (Figure 2). The results indicate that the seroconversion rates among sentinel pigs were correlated with each of the three meteorologic parameters and suggest that the prevalence and activity of JE virus is positively affected by summertime temperature.
Figure 2.
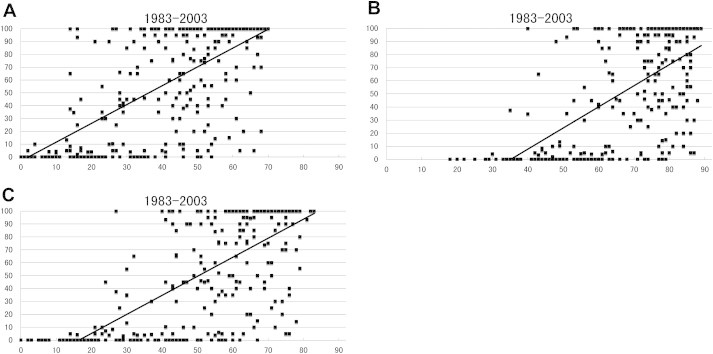
The relationship between seroconversion rate in sentinel pigs and the number of days with high temperature during June, July, and August in Japan; analyses using the data from 1983 to 2003. (A) The relationship between seroconversion rate in sentinel pigs and the number of days with the average temperature equal to or higher than 25°C. There was a significant correlation between these two parameters (r = 0.6738). (B) The relationship between seroconversion rate in sentinel pigs and the number of days with the highest temperature equal to or higher than 25°C. There was a significant correlation between these two parameters (r = 0.6031). (C) The relationship between seroconversion rate in sentinel pigs and the number of days with the lowest temperature equal to or higher than 20°C. There was a significant correlation between these two parameters (r = 0.6996). X-axis: days and y-axis: seroconversion rate (%).
Based on the analysis, seroconversion in sentinel pigs is calculated to occur when there are at least 3 days with the average temperature equal to or higher than 25°C, at least 35 days with the highest temperature equal to or higher than 25°C, and at least 17 days with the lowest temperature equal to or higher than 20°C during 92 days in June, July, and August. Furthermore, 50% seroconversion in sentinel pigs is calculated to occur when there are at least 37 days with the average temperature equal to or higher than 25°C, at least 65 days with the highest temperature equal to or higher than 25°C, and at least 50 days with the lowest temperature equal to or higher than 20°C.
The results suggest that seroconversion rate in sentinel pigs are higher in the years when the temperature is higher during summertime. If global warming continues, it is possible that activity of JE virus becomes constantly higher in northern area of East Asia.8
3. Direct Effect of Global Warming on Human Health Other Than Infectious Diseases
It has been reported that global warming has direct effects on various aspects of human health including infectious diseases. They include heat-related diseases caused by heat waves, injuries, and deaths caused by extreme geological events.
Heat shock is a health problem that is most directly affected by the ambient temperature. In the studies in Japan, there has been a positive relationship between the temperature and the number of heat shock cases in most of the major cities in Japan. The number of heat shock cases increases sharply when the temperature becomes 32°C and higher. Based on these observations, it is assumed that global warming will increase the number of heat shock patients. However, adaptation measures such as introduction of air-conditioning system are expected to ease the effect.
It has also been reported that global warming increases the mortality rate, especially among those with cardiovascular and/or respiratory diseases. Many studies have demonstrated that there is a temperature at which the mortality rate is at the lowest level. This temperature is called the optimum temperature. The mortality rate is higher at both extremes of temperature, that is, high and low sides. Thus, the temperature-mortality relation is usually “V” shaped. The 80–85 percentile value of the daily maximum temperature is the best index of the optimum temperature.27,28
4. Projected Trends in the Effect of Global Warming on Human Health in the World
The projected trends of the effect of global warming on human health were also summarized in the fourth report of IPCC.1 The projected trends in climate change-related effects include (1) increase in malnutrition and consequent disorders; (2) increase in the number of people suffering from death, disease, and injury from heat waves, floods, storms, fires, and droughts; (3) change of habitants of some infectious disease vectors; (4) mixed effects on malaria (the geographical range will contract in some areas, whereas it will expand and the transmission season may be changed); (5) increase in the burden of diarrhoeal diseases; (6) increase in cardiorespiratory morbidity and mortality associated with ground-level ozone; and (7) increase in the number of population at risk of dengue. On the other hand, there will be some benefits to health, including fewer deaths from cold. The benefits will be, however, outweighed by the negative effects of rising temperatures worldwide in developing countries.
The projected trends in the global warming effect in the East Asian countries may be different from those in other regions of the world. There will be increase in the number of heat shock cases and in increase in mortality rate among those who have cardiovascular and respiratory disorders, unless appropriate adaptation measures are taken. As stated above, there has not been apparent profound effect on infectious diseases in East Asia yet. It is predicted that the impacts will appear in one form or another if global warming continues to progress in future. Different from the projected trends in the developing counties, it is unlikely that global warming induce a great increase in the number of patients with diarrhoeal diseases in East Asian countries where social infrastructures are well established.
Furthermore, it is possible that activity of vector mosquitoes will become constantly high even in Northeast Asia. These trends, however, do not directly mean the increase in the number of patients with vector-borne infectious diseases, if appropriate countermeasures are taken.
5. Adaptation to the Global Warming
Even with the strongest mitigation measures, global warming cannot be avoided for decades. Therefore, implementation of adaptation procedures to the global warming is the most practical action we can take. There are multiple adaptation measures to the effect on infectious diseases. These include (1) vector control, (2) development of vaccines and implementation of vaccination, (3) development of new drugs, (4) establishment of surveillance and control programs, and (5) forecast of epidemic and development of preventive measures.
The impacts of global warming on infectious diseases have not been apparent at this point yet in East Asia. Research on the impacts of global warming on infectious diseases and future prospects in East Asia should be conducted in a wide range of research subjects.
In Conclusions, many studies have suggested that climate change has various negative effects on human health including infectious diseases. However, it should be noted that the levels of the impacts of climate change on human health will differ among regions, depending on various factors, such as social infrastructures, and establishment of countermeasures.
This makes the interpretation of the results of the studies quite difficult. Understanding of the effect of climate change on human health has been progressed very much in recent years; however, it is true that much more studies and data are needed to further understand in detail the effect of climate change.
There are other important issues to be considered especially in the study on the effect of infectious diseases. The number of patients with infectious diseases is affected by multiple factors. There are differences in virulence among strains in each pathogen. Thus, the number of symptomatic infection can vary depending on the level of the virulence of dominant strains of the pathogen. Furthermore, the changes in the number of cases depend heavily on the accuracy of surveillance and reporting systems, which are not still established in many developing countries. Thus, in the studies of the effect of global warming on infectious diseases, multiple biological, sociological, and economical factors should be taken into account.
Acknowledgements
This study was partially supported by the grants from Global Environment Research Fund by the Ministry of Environment, Japan (S-8) and from Research on Emerging and Re-emerging Infectious Diseases by the Ministry of Health, Labour and Welfare, Japan (H20-Shinkou-ippan-013).
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Confalonieri U., Menne B., Akhtar P. Human health. Climate change 2007: impacts, adaptation and vulnerability. In: Parry M.L., Canziani O.F., Palutikof J.P., editors. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; Cambridge, UK: 2007. pp. 391–431. [Google Scholar]
- 2.Kurane I. The emerging and forecasted effect of climate change on human health. J Health Sci. 2009;55:865–869. [Google Scholar]
- 3.Hashizume M., Armstrong B., Hajat S. The effect of rainfall on the incidence of cholera in Bangladesh. Epidemiology. 2008;19:103–110. doi: 10.1097/EDE.0b013e31815c09ea. [DOI] [PubMed] [Google Scholar]
- 4.Hashizume M., Armstrong B., Hajat S. Association between climate variability and hospital visits for non-cholera diarrhea in Bangladesh: effects and vulnerable groups. Int J Epidemiol. 2007;36:1030–1037. doi: 10.1093/ije/dym148. [DOI] [PubMed] [Google Scholar]
- 5.Hay S.I., Rogers D.J., Randolph S.E. Hot topic or hot air? Climate change and malaria resurgence in East African highlands. Trends Parasitol. 2002;18:530–534. doi: 10.1016/s1471-4922(02)02374-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craig M.H., Kleinschmidt I., Nawn J.B. Exploring 30 years of malaria case data in KwaZulu-Natal, South Africa. Part I. The impact of climatic factors. Trop Med Int Health. 2004;9:1247–1257. doi: 10.1111/j.1365-3156.2004.01340.x. [DOI] [PubMed] [Google Scholar]
- 7.Githeko A.K., Ndegwa W. Predicting malaria epidemics in the Kenyan highlands using climate data: a tool for decision makers. Glob Change Hum Health. 2001;2:54–63. [Google Scholar]
- 8.Abeku T., van Oortmarssen G., Borsboom G. Spatial and temporal variations of malaria epidemic risk in Ethiopia: factors involved and implications. Acta Trop. 2003;87:331–340. doi: 10.1016/s0001-706x(03)00123-2. [DOI] [PubMed] [Google Scholar]
- 9.Semenov S.M., Gelver E.S., Yasyukevich V.V. Temperature conditions for development of two species of malaria pathogens in Russia in 20th century. Dokl Akad Nauk. 2002;387:131–136. doi: 10.1023/a:1021781222614. [DOI] [PubMed] [Google Scholar]
- 10.Hales S., Wienstein P., Souares Y., Woodward A. El Nino and the dynamics of vectorborne disease transmission. Environ Health Perspect. 1999;107:99–102. doi: 10.1289/ehp.9910799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hales S., de Wet N., Maindonald J., Woodward A. Potential effect of population and climate changes on global distribution of dengue fever: an empirical model. Lancet. 2002;360:830–834. doi: 10.1016/S0140-6736(02)09964-6. [DOI] [PubMed] [Google Scholar]
- 12.Corwin A.L., Larasati R.P., Bangs M.J. Epidemic dengue transmission in southern Sumatra, Indonesia. Trans R Soc Trop Med Hyg. 2001;95:257–265. doi: 10.1016/s0035-9203(01)90229-9. [DOI] [PubMed] [Google Scholar]
- 13.Cazelles B., Chavez M., McMichael A.J., Hales S. Nonstationary influence of El Nino on the synchronous dengue epidemics in Thailand. PLoSMed. 2005;2:e106. doi: 10.1371/journal.pmed.0020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hopp M.J., Foley J.A. Worldwide fluctuations in dengue fever cases related to climate variability. Clim Res. 2003;25:85–94. [Google Scholar]
- 15.Lindgren E., Gustafson R. Tick-borne encephalitis in Sweden and climate change. Lancet. 2001;358:16–18. doi: 10.1016/S0140-6736(00)05250-8. [DOI] [PubMed] [Google Scholar]
- 16.Lindgren E., Talleklint L., Polfeldt T. Impact of climatic change on the northern latitude limit and population density of the disease-transmitting European tick Ixodes ricinus. Environ Health Perspect. 2000;108:119–123. doi: 10.1289/ehp.00108119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barker I.K., Lindsay L.R. Lyme borreliosis in Ontario: determining the risks. CMAJ. 2000;162:1573–1574. [PMC free article] [PubMed] [Google Scholar]
- 18.Daniel M., Danielova V., Kriz B., Kott I. An attempt to elucidate the increased incidence of tick-borne encephalitis and its spread to higher altitudes in the Czech Republic. Int J Med Microbiol. 2004;293:55–62. doi: 10.1016/s1433-1128(04)80009-3. [DOI] [PubMed] [Google Scholar]
- 19.Nicholls N. A method for predicting Murray Valley encephalitis in southern Australia using Southern Oscillation. Aust J Exp Biol Med Sci. 1986;64:587–594. doi: 10.1038/icb.1986.62. [DOI] [PubMed] [Google Scholar]
- 20.Mackenzie J., Lindsay M., Daniels P. The effect of climate on the incidence of vector borne viral diseases in Australia: the potential value of seasonal sorecasting. In: Hammer L.G., Nichooles N., Mitchell C., editors. Applications of Seasonal Climate Forecasting in Agricultural and Natural Ecosystem. The Australian Experience. Kluwer Academic Publishers; Dordrecht, The Netherland: 2000. pp. 429–452. [Google Scholar]
- 21.Bi P., Zhang Y., Parton K.A. Weather variables and Japanese encephalitis in the metropolitan area of Jinan city, China. J Infect. 2007;55:551–556. doi: 10.1016/j.jinf.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Hsu S.M., Yen A.M., Chen T.M. The impact of climate on Japanese encephalitis. Epidemiol Infect. 2008;16:980–987. doi: 10.1017/S0950268807009454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi M., Nihei N., Kurihara M. Analysis of northern expansion of Aedes albopictus (Diptera: culicidae) in Japan by geographical information system. J Med Entomol. 2002;39:4–11. doi: 10.1603/0022-2585-39.1.4. [DOI] [PubMed] [Google Scholar]
- 24.Kabayashi M., Komagata O., Nihei N. Global warming and vector-borne infectious diseases. J Disaster Res. 2008;3:105–112. [Google Scholar]
- 25.Oya A., Kurane I. Japanese encephalitis for a reference to international travelers. J Travel Med. 2007;14:259–268. doi: 10.1111/j.1708-8305.2007.00134.x. [DOI] [PubMed] [Google Scholar]
- 26.Arai S., Matsunaga Y., Takasaki T., Vaccine Preventable Diseases Surveillance Program of Japan Japanese encephalitis: surveillance and elimination effort in Japan from 1982 to 2004. Jpn J Infect Dis. 2008;61:333–338. [PubMed] [Google Scholar]
- 27.Honda Y., Ona M. Issues in health risk assessment of current and future heat extremes. Glob Health Action. 2009;2:1–6. doi: 10.3402/gha.v2i0.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honda Y., Kabuto M., Ono M., Uchiyama I. Determination of optimum daily maximum temperature using climate data. Environ Health Prev Med. 2007;12:209–216. doi: 10.1265/ehpm.12.209. [DOI] [PMC free article] [PubMed] [Google Scholar]


