Abstract
Objectives
To compare the prognostic efficacy of 6MW and CPX tests in stable outpatients with chronic HF.
Background
Cardiopulmonary exercise (CPX) and 6 minute walk (6MW) tests are commonly applied as prognostic gauges for systolic heart failure (HF) patients, but few direct comparisons have been conducted.
Methods
Stable NYHA class II and III systolic HF patients (ejection fraction ≤35%) from Heart Failure: A Controlled Trial Investigating Outcomes of Exercise TraiNing (HF-ACTION) were studied. 6MW distance (6MWD) and CPX indices (peak oxygen consumption [VO2] and minute ventilation-carbon dioxide production [VE/VCO2] slope) were compared as predictors of all-cause mortality/hospitalization and all-cause mortality over 2.5 years mean follow-up.
Results
2,054 HF-ACTION participants underwent both CPX and 6MW tests at baseline (median age 59 years; 71% male; 64% NYHA class II and 36% NYHA class III). In unadjusted models and in models that included key clinical and demographic covariates, C-indices of 6MWD were 0.58 and 0.65 (unadjusted) and 0.62 and 0.72 (adjusted) in predicting all-cause mortality/ hospitalization and all-cause mortality, respectively. C-indices for peak VO2 were 0.61 and 0.68 (unadjusted) and 0.63 and 0.73 (adjusted). C-indices for VE/VCO2 slope were C=0.56 and 0.65 (unadjusted) and 0.61 and 0.71 (adjusted); combining peak VO2 and VE/VCO2 slope did not improve C-indices. Overlapping 95% confidence intervals and modest integrated discrimination improvement values confirmed similar prognostic discrimination by 6MWD and CPX indices within adjusted models.
Conclusion
In systolic HF outpatients 6MWD and CPX indices demonstrated similar utility as univariate predictors for all-cause hospitalization/mortality and all-cause mortality. However, 6MWD or CPX indices added only modest prognostic discrimination to models that included important demographic and clinical covariates.
Keywords: heart failure, prognosis, cardiopulmonary exercise testing, walking test
Introduction
Cardiopulmonary exercise (CPX) testing is generally regarded as the “gold standard” of aerobic assessment (1) with capacity to reliably discriminate differences along the continuum of low to high exercise performance. This CPX attribute has been incorporated into well-established applications to track performance (e.g., in relation to training or therapy) and as means to distinguish mechanisms underlying dyspnea and/or exercise limitation (1). CPX is also routinely applied as a prognostic tool (1). Peak oxygen uptake (VO2) and the ventilatory equivalent for carbon dioxide (VE/VCO2) slope are two CPX indices that have been extensively validated as function-based prognostic assessment (1-5), both independently and in combination (2,3).
The distance walked over 6 minutes is an alternative measure of function that has also been applied as the basis of function-based prognostic assessment (6,7). In comparison to the nontrivial costs and logistical challenges of CPX testing, a 6 minute walk (6MW) test is significantly less expensive and more convenient (6,7). Proponents of the 6MW test also emphasize its distinctive value as a measure of routine activity that may be more clinically relevant than a bicycle- or treadmill-based (7,8,9) maximal functional evaluation .
We compared the prognostic utility of 6MW and CPX testing using baseline data from the Heart Failure: A Controlled Trial Investigating Outcomes of Exercise TraiNing (HF-ACTION) study (10), a randomized controlled trial of an exercise training intervention for systolic HF patients. The HF-ACTION protocol entailed 6MW and CPX testing on the same day as part of the baseline assessment.
We hypothesized that CPX indices would more accurately discriminate all-cause hospitalization and mortality as well as all-cause mortality over the trial 2.5 year mean follow-up based on the assumption that gas exchange assessment is more informative than simple distance walked. We also expected that using CPX indices in combination would add to CPX prognostic discrimination.
METHODS
Details of the HF-ACTION protocol have been published elsewhere (10). The study enrolled ambulatory systolic HF patients identified by clinical and echocardiographic criteria (Left ventricular ejection fraction [LVEF] ≤35%), who were randomized between an aerobic exercise training arm with usual care vs. usual care alone. 6MW and CPX were completed prior to randomization. Exercise training entailed 36 supervised outpatient sessions plus home training that was initially combined with the supervised sessions, but which then continued independently for the duration of follow-up. The ultimate goal was home training, 5 days a week, using a treadmill or stationary cycle. Patients were followed over the course of the trial for hospitalizations and mortality. The clinical endpoint committee that monitored these assessments remained blinded to the patients’ assignments.
6MW tests were conducted in a standardized format, with explicit instructions provided in the HF-ACTION manual of operations, modeled after prior studies (11-13). Each of the 82 HF-ACTION sites was instructed to measure a 20-25 meter indoor course and to position a chair at either end, providing subjects a place to rest if necessary. L-shaped hallways were prohibited.
Consistent 6MW test methodology was specified in the HF-ACTION manual of operations, including standardized phrasing (e.g., “cover as much ground as possible… keep going… don’t worry if you have to sit down or stop to rest…”) and consistent timing of encouragement (1-minute intervals).
The HF-ACTION protocol was similarly uniform and rigorous in regard to CPX methodology. Symptom-limited exercise testing was completed using commercially available metabolic carts and motor driven treadmills, employing a modified Naughton protocol (14). The respiratory exchange ratio (RER) was used to gauge exercise effort; (RER) >1.1 was targeted as a high effort standard (1).
Peak VO2 was determined in the CPX Core Laboratory as the highest oxygen consumption normalized to body mass (VO2, mL/kg/min) for a given 15- or 20-second interval within the last 90 seconds of exercise or the first 30 seconds of recovery, whichever was higher. Mean VE/VCO2 slope was calculated based on VE/VCO2 slope data across the entire duration of exercise using the 15- or 20-second averaged data for VCO2 (L/min) and VE (L/min); this method has previously been demonstrated to maximize VE/VCO2 prognostic potential (15,16).
Statistics
Statistical analyses were performed by the Data Coordinating Center (Duke Clinical Research Institute, Durham, North Carolina) using SAS software version 9.2 (SAS Institute Inc, Cary, North Carolina). The relationship of 6MW distance (6MWD) to baseline patient characteristics was summarized using medians with interquartile range of 6MWD across categories of various baseline attributes. Pearson correlation coefficients between baseline characteristics and 6MWD were also calculated for continuous variables.
Unadjusted Pearson correlation coefficients and adjusted partial correlation coefficients were used to assess the association between 6MWD and CPX parameters (peak VO2 and VE/VCO2 slope). The same set of covariates was used for both peak VO2 and VE/VCO2 to adjust the correlations of the given CPX variable with 6MWD. Covariates used for adjustment comprised all identified predictors from previously developed multivariable linear models of each exercise measurement (6MWD and CPX measures) that were objectively selected using backward elimination methods (17).
As a measure of the degree to which a model accurately discriminates events from non-events, C-index estimates with associated 95% confidence intervals (CI) from unadjusted and adjusted Cox proportional hazards models were used to compare the individual roles of 6MWD and CPX measures (peak VO2 and VE/VCO2 slope) with respect to the primary endpoint of all-cause hospitalization or mortality, and the secondary endpoint of all-cause mortality. Peak VO2 and VE/VCO2 slope were assessed independently and in combination within each prognostic model.
The 95% CI for the C-Index in the various models served as a surrogate for hypothesis tests to compare model discrimination. As a general rule, if two models of the same endpoint produce 95% CIs for the C-index that shared no common values, they were regarded as significantly different in terms of discrimination, whereas C-indices with widely overlapping CIs were interpreted as lacking significant differences between the two models.
6MWD and CPX indices were assessed within unadjusted models (i.e., 6MWD and CPX indices as univariate predictors) as well as in models adjusted for demographic and clinical covariates. Baseline covariates used for the adjustment were based on Cox proportional hazards models which were previously developed for these endpoints. They were selected using a stepwise method based on a bootstrap-backward selection process (17). Relative risks associated with normalized 6MWD and CPX measures were expressed as hazard ratios (HRs) with 95% CI.
In order to ensure comparability while optimizing sample size, Cox models were applied to complete-case data for patients who had non-missing values for 6MWD, peak VO2, and VE/VCO2 slope. All parameters were converted to standard normal z-scores prior to their inclusion in the Cox models, and the model assumption of linearity was assessed with respect to each standardized measure. Examination of cubic splines revealed that the relationship of 6MWD to the mortality/hospitalization endpoint was constant beyond 1 standard deviation from the mean value; for this reason, the 6MWD relationship was truncated, and the HR for values of 6MWD>1 SD beyond the mean was set to 1 (i.e., no additional relationship of the measure with death/hospitalization beyond that point).
The integrated discrimination improvement (IDI) statistic was calculated to assess the relative impact of introducing each exercise measure to the models adjusted for demographic and clinical variables (18). The IDI examines models in terms of degree of discrimination, as measured by the separation between mean predicted probabilities among patients with and without endpoints in each model. Continuous variables are expressed as median (25, 75th percentiles) and discrete variables as percent. For all analyses, a two-tailed p<0.05 was required to reject the null hypothesis.
Results
Of the 2,331 patients enrolled in HF-ACTION, 211 subjects underwent CPX testing on cycle ergometers and were excluded from the analysis. In 20 other subjects, it was unclear whether a cycle or treadmill had been utilized during the CPX test, so they also were excluded. Of the 2,100 that remained, 2,054 had both 6MW and CPX tests. These patients (N=2,054, 88% of the original HF-ACTION population) represent the cohort for this analysis. Within this group, 2,030 patients had both 6MWD and peak VO2 measurements, and 2,013 had 6MWD, peak VO2 and VE/VCO2 slope measurements.
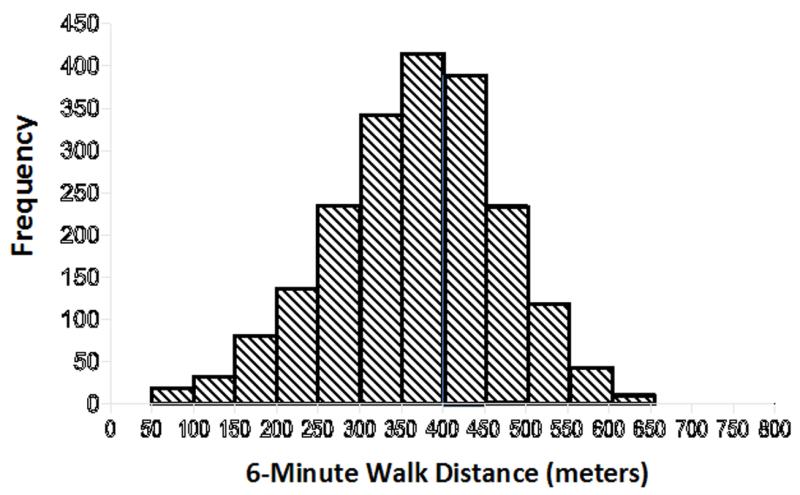
Table 1 shows the distribution of baseline patient characteristics in the study population, generally indicative of a middle-aged cohort with mild to moderate functional impairment. Figure 1 illustrates the distribution of 6MWD data, highlighting a wide range of walking capacities and nearly symmetric distribution of 6MWD in the HF-ACTION study population.
Table 1. Baseline Characteristics.
| Parameter | N | Median | 25th, 75th IQR |
|---|---|---|---|
| Age (years) | 2054 | 59 | 51, 68 |
| Body Mass Index (kg/m2) | 2049 | 30.1 | 26.3, 35.4 |
| Height (cm) | 2049 | 173 | 166, 180 |
| 6MWD (meters) | 2054 | 372 | 300, 434 |
| Peak VO2 (ml/kg/min) | 2030 | 14.6 | 11.7, 17.7 |
| VE/VCO2 slope | 2030 | 32.4 | 28.1, 38.3 |
| Sex (Male/Female) | 1459 / 595 | 71% / 29% | |
|
NYHA Class (Class II/Class
III/IV) |
1317 / 737 | 64% / 36% |
IQR- interquartile range; 6MWD -6 minute walk distance; VO2-oxygen consumption; VE/VCO2- minute ventilation-carbon dioxide production (VE/VCO2); NYHA – New York Heart Association
Figure 1. Distribution of 6 minute walk test distances in the HF-ACTION study population.
Six minute walk distance varied widely among HF-ACTION participants, affording an excellent opportunity to assess its prognostic efficacy.
Table 2 shows the distribution of 6MWD values according to various key clinical characteristics, with Pearson correlation coefficients for continuous attributes. Older age (r=−0.23) and higher BMI (r=−0.13) correlated with shorter 6MWD among the continuous variables. Gender, race, NYHA Class, and other categorical variables also demonstrated significant relationships with 6MWD.
Table 2. Distribution of 6MWD by Baseline Characteristics.
| Variables | Category | N | 6MWD Median (25th,75th IQR) |
Pearson Correlation (for continuous variables) |
|---|---|---|---|---|
| Age | <40 years | 153 | 407 (346, 457) | −0.23 |
| 40-59 | 933 | 385 (307, 450) | ||
| 60-69 | 558 | 366 (305, 425) | ||
| ≥70 years | 410 | 332 (262, 391) | ||
| BMI | <27.6 | 676 | 376 (307, 439) | −0.13 |
| 27.6-33.1 | 677 | 383 (307, 442) | ||
| ≥33.1 | 696 | 358 (290, 424) | ||
| LVEF | <21.5 | 676 | 363 (287, 427) | 0.06 |
| 21.5-28.2 | 673 | 379 (304, 439) | ||
| ≥28.2 | 695 | 373 (310, 434) | ||
|
Carvedilol
equivalents (mg/day) |
Low dose (<30) | 1021 | 366 (296, 430) | 0.04 |
| High dose (≥30) | 1015 | 375 (302, 439) | ||
| BDI II | <6 | 656 | 387 (322, 442) | −0.12 |
| 6-11 | 729 | 373 (301, 439) | ||
| ≥12 | 664 | 355 (274, 421) |
| Sex | Male | 1459 | 380 (304, 441) |
| Female | 595 | 354 (290, 415) | |
| Race | White | 1216 | 384 (313, 445) |
| African American | 697 | 349 (280, 416) | |
| Other | 111 | 385 (320, 439) | |
| Country | USA | 1874 | 371 (300, 435) |
| Canada | 180 | 375 (300, 424) | |
| NYHA Class | II | 1317 | 396 (335, 454) |
| III/IV | 737 | 319 (252, 386) | |
| CCS Angina Class | No Angina | 1695 | 371 (300, 433) |
| Class I | 186 | 387 (326, 449) | |
| Class II-IV | 171 | 356 (282, 410) | |
| HF Etiology | Ischemic | 1043 | 366 (293, 429) |
| Non-Ischemic | 1011 | 380 (307, 442) | |
| Mitral Regurgitation | Low (none-moderate) | 1667 | 376 (305, 440) |
| High (severe) | 227 | 366 (274, 420) | |
|
ECG Vent Cond Prior
to Baseline CPX |
Normal | 868 | 378 (305, 442) |
| LBBB | 319 | 385 (315, 449) | |
| RBBB | 76 | 366 (305, 427) | |
| IVCD | 273 | 366 (296, 420) | |
| Paced | 469 | 356 (287, 420) | |
| Diabetes | No | 1384 | 384 (314, 442) |
| Yes | 670 | 348 (274, 411) | |
| PAD | No | 1920 | 374 (304, 436) |
| Yes | 124 | 321 (238, 404) | |
| COPD | No | 1819 | 376 (305, 428) |
| Yes | 218 | 327 (262, 405) |
BMI-body mass index; LVEF-Left ventricular ejection fraction; BDI-Beck depression index; NYHA-New York Heart Association; CCS-Canadian Cardiovascular Society; HF-heart failure; ECG Vent Cond-electrocardiogram ventricular conduction abnormality; PAD-peripheral arterial disease; COPD-chronic obstructive lung disease
Table 3 shows unadjusted and adjusted correlations between 6MWD and CPX parameters. Significant covariates used in the adjusted model were height, weight, number of hospitalizations during 6 months prior to baseline, geographic region, New York Heart Association (NYHA) Class (II vs. III/IV), age, race, peripheral vascular disease, electrocardiogram (ECG) ventricular conduction abnormality, body mass index (BMI), sex, LVEF, and diabetes mellitus. While 6MWD correlated significantly with both peak VO2 and VE/VCO2 slope, with or without adjustment for covariates, correlations were slightly stronger with peak VO2 in each case. After adjusting for covariates, correlations of both CPX indices with 6MWD were substantially weaker, indicating the degree to which covariates may have accounted for the unadjusted correlations.
Table 3. Correlations of 6MWD to CPX Indices.
| Parameter | N | Unadjusted vs. Adjusted* |
Correlation with 6MWD |
P value |
|---|---|---|---|---|
| Peak VO2 (ml/kg/min) |
2030 | Unadjusted R | 0.54 | P<.0001 |
| 1920 | Adjusted R* | 0.33 | P<.0001 | |
| VE/VCO2 slope |
2014 | Unadjusted R | −0.26 | P<.0001 |
| 1905 | Adjusted R* | −0.17 | P<.0001 |
6MWD-6 minute walk distance; VO2-oxygen consumption; VE/VCO2-minute ventilation-carbon dioxide production
“Adjusted Correlations” are the partial correlations from models including covariates in the final adjusted model of 6MW or any CPX Parameter
Tables 4a and 4b demonstrate the respective contributions of 6MWD, peak VO2, and VE/VCO2 slope to unadjusted and adjusted models of all-cause hospitalization/mortality (Table 4a) and mortality (Table 4b). The HRs are closer to one for the given exercise parameter in the adjusted model, as compared with the HR in the unadjusted model. However, the c-index is higher in the adjusted model than the unadjusted model; i.e., with more variables in the adjusted model, the overall discrimination improves.
Table 4a. Prognostic Utility of 6MWD vs. CPX Indices in Predicting All-Cause Hospitalization/Mortality.
| Model | Parameter | Chi Squire statistic |
P value | Hazard Ratio* ( 95% confidence interval) |
C-Index (95% confidence interval) |
IDI**** |
|---|---|---|---|---|---|---|
| Unadjusted Univariate predictors |
6MWD*** (Z<1) |
99 | <.0001 | 0.75 (0.70,0.79) | 0.58 (0.57, 0.60) | |
| Peak VO2 | 158 | <.0001 | 0.69 (0.65,0.73) | 0.61 (0.59, 0.62) | ||
| VE/VCO2 Slope |
85 | <.0001 | 1.27 (1.21, 1.33) | 0.56 (0.55, 0.58) | ||
| Adjusted** | 6MWD*** (Z<1) |
48 | <.0001 | 0.78 (0.73, 0.84) | 0.62 (0.60, 0.64) | 0.019 |
| Peak VO2 | 80 | <.0001 | 0.72 (0.67, 0.77) | 0.63 (0.61, 0.65) | 0.043 | |
| VE/VCO2 Slope |
19 | <.0001 | 1.15 (1.08, 1.22) | 0.61 (0.59, 0.62) | 0.009 |
6MWD-6 minute walk distance; VO2-oxygen consumption; VE/VCO2-minute ventilation-carbon dioxide production; IDI-Integrated Discrimination Improvement
Hazard Ratio based on Z score
--All-Cause Hospitalization/Mortality Model adjusted for Gender, Region (US vs. Non-US), Mitral Regurgitation, ECG Ventricular Conduction Abnormality, Blood Urea Nitrogen (BUN), Left Ventricular Ejection Fraction (LVEF), Carvedilol Equivalent Dose, and Kansas City Cardiomyopathy Questionnaire Symptom Stability Score --All-Cause Mortality Model adjusted for Gender, BMI, Loop Diuretic Dose, Angina Class, ECG Ventricular Conduction Abnormality, LVEF, and Creatinine
--6MWD (normalized) is truncated at 1 standard deviation in the model of Hospitalization/Mortality because of its lack of relationship with this endpoint beyond that point. Truncation in this case implies that the Hazard Ratio for values of 6MWD>1 is set to 1.
--Other truncated covariates are carvedilol equivalent dose-truncated above 50 mg/day; BMI-body mass index-truncated above 25 kg/m2; Cr-truncated above 2.3 mg/dl.
IDI Model includes N=2013 patients with non-missing values for 6MW, Peak VO2, and VE/VCO2
Table 4b. Prognostic Utility of 6MWD vs. CPX Indices in Predicting All-Cause Mortality.
| Model | Parameter | Chi Squire statistic |
P value | Hazard Ratio* ( 95% confidence interval) |
C-Index (95% confidence interval) |
IDI |
|---|---|---|---|---|---|---|
| Unadjusted Univariate predictors |
6MWD | 94 | <.0001 | 0.61 (0.55, 0.67) | 0.65 (0.62, 0.68) | |
| Peak VO2 | 123 | <.0001 | 0.48 (0.42, 0.55) | 0.68 (0.65, 0.71) | ||
| VE/Vco2 Slope |
130 | <.0001 | 1.58 (1.46, 1.71) | 0.65 (0.61, 0.68) | ||
| Adjusted** | 6MWD | 55 | <.0001 | 0.65 (0.57, 0.73) | 0.72 (0.69, 0.75) | 0.005 |
| Peak VO2 | 77 | <.0001 | 0.51 (0.44, 0.59) | 0.73 (0.71, 0.76) | 0.010 | |
| VE/VCO2 Slope |
45 | <.0001 | 1.37 (1.25, 1.51) | 0.71 (0.68, 0.74) | 0.004 |
6MWD-6 minute walk distance; VO2-oxygen consumption; VE/VCO2-minute ventilation-carbon dioxide production; IDI-Integrated Discrimination Improvement
Hazard Ratio based on Z score
--All-Cause Hospitalization/Mortality Model adjusted for Gender, Region (US vs. Non-US), Mitral Regurgitation, ECG Ventricular Conduction Abnormality, Blood Urea Nitrogen (BUN), Left Ventricular Ejection Fraction (LVEF), Carvedilol Equivalent Dose, and Kansas City Cardiomyopathy Questionnaire Symptom Stability Score --All-Cause Mortality Model adjusted for Gender, BMI, Loop Diuretic Dose, Angina Class, ECG Ventricular Conduction Abnormality, LVEF, and Creatinine
--6MWD (normalized) is truncated at 1 standard deviation in the model of Hospitalization/Mortality because of its lack of relationship with this endpoint beyond that point. Truncation in this case implies that the Hazard Ratio for values of 6MWD>1 is set to 1.
--Other truncated covariates are carvedilol equivalent dose-truncated above 50 mg/day; BMI-body mass index-truncated above 25 kg/m2; Cr-truncated above 2.3 mg/dl.
IDI Model includes N=2013 patients with non-missing values for 6MW, Peak VO2, and VE/VCO2
Although chi square tests confirm the significant association of 6MWD, peak VO2, and VE/VCO2 slope with both endpoints even after inclusion of common clinical and laboratory covariates, the small IDI estimates associated with inclusion of these exercise test variables in adjusted models suggest that they contribute only a modest degree of added discrimination. The addition of peak VO2 to the adjusted model of the primary endpoint (all cause hospitalization/mortality) produced the highest IDI (0.04), with 6MWD producing an IDI of 0.02 in that model. The IDI was 0.01 or less for the addition of each of these 3 measures to the adjusted model of mortality. The widely overlapping 95% CIs for the C-Index estimates of models containing each of the three exercise measures, as well as similar IDI values in the adjusted models, suggest that 6MWD and CPX measures do not differ significantly from one another in their prognostic discrimination of these endpoints.
Table 5 shows the C-indices pertaining to normalized 6MWD and CPX measures in models of all-cause hospitalization/mortality and all-cause mortality, respectively. In an unadjusted model of all-cause hospitalization/mortality, the C-index (0.58) associated with 6MWD (truncated at 1 SD above the mean as described above) is numerically lower than the C-index of peak VO2 (0.61) and greater than the C-index associated with VE/VCO2 (0.56). The 6MWD and CPX measures were also assessed relative to an adjusted model with the covariates: gender, region [US/Non-US], mitral regurgitation, ECG ventricular conduction abnormality, blood urea nitrogen (BUN), LVEF, beta-blocker dose, and Kansas City Cardiomyopathy Questionnaire Symptom Stability Score. Without peak VO2, VE/VCO2 or 6MWD, the model predicted all-cause hospitalization/mortality with a C-index of 0.60. Adding 6MWD to the model increased the C-index to 0.62. Adding peak VO2 (instead of 6MWD) increased the C-index to 0.63. Adding VE/VCO2 slope (instead of 6MWD or peak VO2) increased the C-index to 0.61. When peak VO2 and VE/VCO2 slope were used in combination within the model, C-index increased to 0.63, no better than the same model minus VE/VCO2 slope. Combining 6MWD and peak VO2 within the model increased the C-index to 0.64. However, when all three functional indices (6MWD, peak VO2, and VE/VCO2 slope) were used in the model together, the C-index remained at 0.64. Notably, when peak VO2 and VE/VCO2 slope were entered into the model together, peak VO2 had a larger influence on prognosis (p<0.001) while the impact of VE/VCO2 slope was non-significant (p=0.57).
Table 5. C-Index of 6MWD vs. CPX Indices in Unadjusted and Adjusted Models of All-Cause Hospitalization/Mortality and All-Cause Mortality.
| All-cause Hospitalization/Mortality |
All-cause Mortality | |||
|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |
| Model without 6MWD, peak VO2 or VE/VCO2 slope |
NA | 0.60 | NA | 0.69 |
| Model with 6MWD * | 0.58 | 0.62 | 0.65 | 0.72 |
| Model with Peak VO2 | 0.61 | 0.63 | 0.68 | 0.73 |
| Model with VE/VCO2 Slope | 0.56 | 0.61 | 0.65 | 0.71 |
| Model with Peak VO2 and VE/VCO2 Slope |
0.61 | 0.63 | 0.70 | 0.74 |
| Model with Peak VO2 and 6MWD* |
0.61 | 0.64 | 0.69 | 0.74 |
| Model with Peak VO2 and VE/VCO2 Slope and 6MWD * |
0.61 | 0.64 | 0.71 | 0.74 |
| Model parameters are gender, region, mitral regurgitation, ECG conduct abnl, BUN, LVEF, carvedilol equivalent dose, KCCQ symptom stability score |
Model parameters are gender, BMI, loop diuretic dose, angina class, ECG conduct abnl, LVEF, Cr |
|||
6MWD-6 minute walk distance; VO2-oxygen consumption; VE/VCO2-minute ventilation-carbon dioxide production
Region-US vs. Non-US; ECG conduct abnl –ECG ventricular conduction abnormality (prior to the CPX); BUN- blood urea nitrogen, LVEF-left ventricular ejection fraction; KCCQ- Kansas City Cardiomyopathy Questionnaire; BMI-body mass index; Cr-creatinine
6MWD (normalized) is truncated at 1 standard deviation in the model of Hospitalization/Mortality because of its lack of relationship with this endpoint beyond that point. Truncation in this case implies that the Hazard Ratio for values of 6MWD>1 is set to 1. Other truncated covariates are carvedilol equivalent dose-truncated above 50 mg/day; BMI-body mass index-truncated above 25 kg/m2; Cr-truncated above 2.3 mg/dl.
Unadjusted model contains only the stated exercise variable(s). Adjusted model includes the given exercise variable(s) plus the model covariates listed.
Table 5 also displays the C-statistics relating 6MWD and CPX measures to all-cause mortality. In unadjusted models, the C-index associated with peak VO2 (0.68) was slightly higher than the C-index associated with 6MWD (0.65). However, C-indices of 6MWD and VE/VCO2 slope (0.65) were equivalent. In a model for all-cause mortality with the covariates gender, BMI, loop diuretic dose, Canadian angina class, ECG ventricular conduction abnormalities, LVEF, and serum creatinine, the C-index was 0.69. Adding 6MWD, peak VO2, and VE/VCO2 slope individually to the model increased the C-indices to 0.72, 0.73, and 0.71, respectively. Combining the functional indices modestly increased prognostic discrimination; the C-index increased to 0.74 with any combination of the functional indices (C-index = 0.74 in relation to 6MWD and peak VO2 or to peak VO2 and VE/VCO2 slope or to 6MWD, peak VO2, and VE/VCO2 slope).
Discussion
In this secondary analysis from HF-ACTION, we showed that a 6MW test provides useful prognostic information for both the composite outcome of all-cause hospitalization/mortality as well as the outcome of all-cause mortality in NYHA class II and III HF outpatients receiving state-of-the art therapy for systolic HF. In both unadjusted and adjusted models, the prognostic information provided by 6MWD, as estimated by the C-index, was similar to that for peak VO2 and VE/VCO2 slope attained using CPX even when peak VO2 and VE/VCO2 slope were assessed in combination. While the C-statistic to predict all-cause hospitalization/mortality and all-cause mortality for CPX was numerically larger than that for 6MWD, the difference was too small to be clinically meaningful. Individually, 6MWD and peak VO2 provided similar levels of discrimination as univariate predictors; unadjusted models of either exercise parameter predicting hospitalization/mortality or all-cause mortality had discrimination that approached that of models with known clinical and demographic covariates without exercise parameters. However, there was little augmentation in discrimination resulting from the addition of either exercise measure to the adjusted models.
Table 6 lists many of the landmark studies (19-27) that validated 6MW test as a prognostic measure for systolic HF patients and those which compared it to CPX testing. These and related studies (28-31) were quite small, enrolled patients with different etiologies and severities of heart failure, and used variable protocols to administer the tests. Heterogeneity of results is thus not surprising (31).
Table 6. MW test for HF: 6MW to predict outcomes, and studies comparing 6MW and CPX.
| Prior Study | Study Population | Results |
|---|---|---|
| 6MW test as a prognostic marker | ||
| Bittner, et al. (19) | 833 patients
|
<300 m quartile: Significantly greater chance of death (10.23% vs. 2.99%; p=0.01), hospitalization (40.91% vs. 19.90%; p=0.002), and HF hospitalization (22.16% vs. 1.99%; p<0.0001). |
| Bettencourt et al. (20) | 139 patients
|
<350 m independently predicted all-cause mortality |
| Ingle et al (21) | 1,592 HF patients
|
6MWD independently predicted mortality among patients with >mild left ventricular systolic dysfunction |
| 6MW test for prognostication in comparison to CPX | ||
| Cahalin et al (22) | 45 patients
|
|
| Roul et al (23) | 121 patients
|
|
| Zugck et al (24) | 113 patients
|
|
| Lucas et al (25) | 307 patients
|
|
| Opasich et al (26) | 315 HF patients
|
|
| Guazzi et al (2) | 253 HF patients
|
|
| Rostagno, et al (27) | 214 patients
|
|
In comparison to prior studies, HF-ACTION stands out for its larger study population, comprehensive assessments, and emphasis on contemporary evidence-based therapy. Our data are noteworthy in showing efficacy of 6MWD as a continuous prognostic marker among a large HF population with a wide range of performance capacities, nearly all of whom were receiving beta-blockers, ACE inhibitors or angiotensin receptor blockers. Whereas prior literature demonstrated greatest 6MWD prognostic discrimination for patients with very low performance, in this study 6MWD was predictive across a wide spectrum of performance capacities and essentially matched the efficacy of CPX as a prognostic tool across the full range of patients.
A notable attribute of the HF-ACTION protocol was that it provided explicit instructions on how to implement the 6MW test. While proponents of the 6MW test often emphasize the ease and convenience of its application, inconsistencies in its administration may inadvertently diminish the reliability of the results (32). In HF-ACTION, significant efforts were undertaken to standardize optimal technique for both 6MW and CPX testing, providing a robust comparison between these two performance assessments.
Baseline 6MWD correlated more strongly with peak VO2 than with VE/VCO2 slope, suggesting that 6MWD and peak VO2 share more physiological underpinnings (33). Cardiac output, peripheral perfusion capacity, and skeletal muscle health are integral to each of these performance measures, and differ from the physiological determinants underlying VE/VCO2 slope (e.g., ventilation-perfusion abnormalities, chemoreceptor responses, intrinsic respiratory capacity, and cardiopulmonary coupling) (3). Although we therefore expected that VE/VCO2 slope would add independent value to the prognostic model that included peak VO2, this was not the case. VE/VCO2 slope added only minor prognostic enhancement.
The prognostic efficacy of 6MWD demonstrated in this analysis resonates with a multitude of recent literature highlighting the prognostic utility of other walking assessments such as gait speed and the 400 meter corridor walk (34,35). The physiological principles underlying these different assessments of walking capacity appear similar, and reinforce the value of the 6MW test as a valid, sensitive, and clinically meaningful prognostic tool.
Strengths and Limitations
As the largest randomized controlled trial of exercise training ever conducted in HF patients, HF-ACTION provided an unparalleled opportunity to compare the prognostic utility of 6MW and CPX testing in this common clinical setting. Thus, the large sample size and rigorous protocol for performing both tests in a contemporary HF population receiving evidence-based drug and device therapy represent major strengths of the current study.
Certain limitations should also be recognized. Since HF-ACTION is an exercise training trial, the exercise intervention may have affected the relationship between functional assessments and outcomes. However, this treatment effect has similar bearing on 6MW and CPX assessments and does not confound the analysis.
Although CPX tests were repeated on approximately 400 HF-ACTION subjects to exclude familiarization (36), similar assessment of possible familiarization effects were never tested in relation to 6MW tests in HF-ACTION. Other studies have suggested this may have bearing on 6MWD assessments (37). Therefore, it cannot be assumed that 6MWD assessments will consistently provide equivalent prognostic discrimination when used for serial evaluations. Nonetheless, the fact that the initial 6MWD assessments yielded prognostic information similar to that of CPX suggests that the predictive implications of walking distance are robust.
Although HRs associated with standardized values of 6MWD and the CPX measures are provided in the tables, comparisons of these ratios should be made with caution. Given the fundamental differences in the nature of the various exercise measures, the risk associated with one SD difference in a given measure may not be directly comparable to the risk associated with an equivalent difference in another measure. The HF-ACTION protocol entailed completing 6MW and CPX testing at the same baseline visit, eliminating fluctuations in mood, health, or other clinical dynamics that would have been more likely if tests were performed on separate days. However, the protocol did not randomize the order in which the 6MW and CPX tests were conducted; since the 6MW test generally preceded the CPX test, this may have biased the results.
While our data indicate that 6MWD or CPX indices peak VO2 and VE/VCO2 slope add only modest prognostic value to models that already include demographic and clinical covariates that could be gathered as part of a comprehensive clinical assessment, both tests provide useful assessments of a patient’s aerobic capacity. In addition, CPX testing may be more likely to detect exercise-related hemodynamic instability, ischemia, arrhythmias, and symptoms that are clinically important (1), but which were outside the focus of this investigation.
Finally, in addition to peak VO2 and VE/VCO2 slope, CPX provides the potential to assess several additional indices that may increase prognostic information. Oscillatory expiratory breathing, end-tidal PCO2, VE/VO2 ratios, recovery gas exchange dynamics and heart rate and blood pressure responses are among an extensive array of CPX assessments that can be used to enhance prognostic assessment (1,2,38). While this study highlights the utility of 6MWD relative to the two most commonly reported indices of CPX testing, it does not address the utility of a comprehensive CPX evaluation.
Conclusion
The 6MW test provides useful prognostic information for all-cause hospitalization and mortality among stable NYHA class II and III HF patients receiving state-of-the-art therapy. Although CPX testing is often assumed to provide superior function-based prognostic assessment in HF patients, we demonstrated that 6MWD provided prognostic value that was similar to peak VO2, VE/VCO2 slope, and their combination in a relatively stable HF population. These data suggest that a 6MW test may substitute for CPX testing as an inexpensive, practical clinical tool to help gauge prognosis in the large and growing HF population. Although 6MWD and peak VO2 both demonstrated utility as univariate predictors in unadjusted prognostic models for all-cause hospitalization/mortality and all-cause mortality, both measures added only modest prognostic discrimination to models that included important demographic and clinical covariates.
Abbreviations
- HF
Heart Failure
- NYHA
New York Heart Association
- CPX
Cardiopulmonary Exercise
- 6MW
6 Minute Walk
- 6MWD
6 Minute Walk Distance
- VO2
Oxygen consumption
- VE/VCO2
Ventilation Equivalent for Exhaled Carbon Dioxide
- RER
Respiratory Exchange Ratio
- LVEF
Left Ventricular Ejection Fraction
- MOO
Manuel of Operations
- HF-ACTION
Heart Failure: A Controlled Trial Investigating Outcomes of Exercise TraiNing
- PVD
Peripheral Vascular Disease
- BMI
Body Mass Index
- ECG
Electrocardiogram
- CI
Confidence Interval
- IDI
Integrated Discrimination Improvement
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Balady GJ, Arena R, Sietsema K, et al. Clinician’s Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122(2):191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 2.Guazzi M, Dickstein K, Vicenzi M, Arena R. Six-minute walk test and cardiopulmonary exercise testing in patients with chronic heart failure: a comparative analysis on clinical and prognostic insights. Circ Heart Fail. 2009;2(6):549–55. doi: 10.1161/CIRCHEARTFAILURE.109.881326. [DOI] [PubMed] [Google Scholar]
- 3.Arena R, Myers J, Guazzi M. The clinical and research applications of aerobic capacity and ventilatory efficiency in heart failure: an evidence-based review. Heart Fail Rev. 2008;13(2):245–69. doi: 10.1007/s10741-007-9067-5. [DOI] [PubMed] [Google Scholar]
- 4.Arena R, Myers J, Guazzi M. Cardiopulmonary exercise testing is a core assessment for patients with heart failure. Congest Heart Fail. 2011;17(3):115–9. doi: 10.1111/j.1751-7133.2011.00216.x. [DOI] [PubMed] [Google Scholar]
- 5.Forman DE, Myers J, Lavie CJ, et al. Cardiopulmonary exercise testing: relevant but underused. Postgrad Med. 2010;122(6):68–86. doi: 10.3810/pgm.2010.11.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faggiano P, D’Aloia A, Gualeni A, et al. The 6 minute walking test in chronic heart failure: indications, interpretation and limitations from a review of the literature. Eur J Heart Fail. 2004;6(6):687–91. doi: 10.1016/j.ejheart.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 7.ATS statement: guidelines for the six-minute walk test. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 8.Du H, Newton PJ, Salamonson Y, et al. A review of the six-minute walk test: its implication as a self-administered assessment tool. Eur J Cardiovasc Nurs. 2009;8(1):2–8. doi: 10.1016/j.ejcnurse.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Solway S, Brooks D, Lacasse Y, Thomas S. A qualitative systematic overview of the measurement properties of functional walk tests used in the cardiorespiratory domain. Chest. 2001;119(1):256–270. doi: 10.1378/chest.119.1.256. [DOI] [PubMed] [Google Scholar]
- 10.O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1439–50. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kavanaugh T, Meyers MG, Baigrie RS, et al. Quality of life and cardiorespiratory function in chronic heart failure: effects of 12 months’ aerobic training. Heart. 1996;76:42–9. doi: 10.1136/hrt.76.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer K, Schwaibold M, Westbrook S, et al. Effects of exercise training and activity restriction on 6-minute walking test performance in patients with chronic heart failure. Am Heart J. 1997;133(4):447–53. doi: 10.1016/s0002-8703(97)70187-x. [DOI] [PubMed] [Google Scholar]
- 13.Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132(8):919–23. [PMC free article] [PubMed] [Google Scholar]
- 14.Patterson JA, Naughton J, Pietras RJ, Gunnar RM. Treadmill exercise in assessment of patients with cardiac disease. Am J Cardiol. 1972;30:757–762. doi: 10.1016/0002-9149(72)90151-8. [DOI] [PubMed] [Google Scholar]
- 15.Arena R, Myers J, Abella J, et al. Development of a ventilatory classification system in patients with heart failure. Circulation. 2007;115(18):2410–7. doi: 10.1161/CIRCULATIONAHA.107.686576. [DOI] [PubMed] [Google Scholar]
- 16.Arena R, Humphrey R, Peberdy MA. Prognostic ability of VE/VCO2 slope calculations using different exercise test time intervals in subjects with heart failure. Eur J Cardiovasc Prev Rehabil. 2003;10(6):463–8. doi: 10.1097/01.hjr.0000102817.74402.5b. [DOI] [PubMed] [Google Scholar]
- 17.O’Connor CM, Whellan DJ, Wojdyla D, et al. Factors related to morbidity and mortality in patients with chronic heart failure with systolic dysfunction: the HF-ACTION predictive risk score model. Circ Heart Fail. 2012;5(1):63–71. doi: 10.1161/CIRCHEARTFAILURE.111.963462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pencina MJ, D’Agostino RB, D’Agostino RB, Jr, Vasan RS. Statist Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 19.Bittner V, Weiner DH, Yusuf S, et al. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. SOLVD Investigators. JAMA. 1993;270:1702–1707. [PubMed] [Google Scholar]
- 20.Bettencourt P, Ferreira A, Dias P, et al. Predictors of prognosis in patients with stable mild to moderate heart failure. J Card Fail. 2000;6:306–13. doi: 10.1054/jcaf.2000.20558. [DOI] [PubMed] [Google Scholar]
- 21.Ingle L, Rigby AS, Carroll S, et al. Prognostic value of the 6 min walk test and self-perceived symptom severity in older patients with chronic heart failure. Eur H J. 2007;28:560–568. doi: 10.1093/eurheartj/ehl527. [DOI] [PubMed] [Google Scholar]
- 22.Cahalin LP, Mathier MA, Semigran MJ, et al. The Six-Minute Walk Test Predicts Peak Oxygen Uptake and Survival in Patients With Advanced Heart Failure. Chest. 1996;110:325–332. doi: 10.1378/chest.110.2.325. TG. [DOI] [PubMed] [Google Scholar]
- 23.Roul G, Germain P, Bareiss P. Does the 6-minute walk test predict the prognosis in patients with NYHA class II or III chronic heart failure? Am Heart J. 1998;136:449–57. doi: 10.1016/s0002-8703(98)70219-4. [DOI] [PubMed] [Google Scholar]
- 24.Zugck C, Krüger C, Dürr S, et al. Is the 6-minute walk test a reliable substitute for peak oxygen uptake in patients with dilated cardiomyopathy? Eur Heart J. 2000;21(7):540–9. doi: 10.1053/euhj.1999.1861. [DOI] [PubMed] [Google Scholar]
- 25.Lucas C, Stevenson LW, Johnson W, et al. The 6-min walk and peak oxygen consumption in advanced heart failure: aerobic capacity and survival. Am Heart J. 1999;138:618–24. doi: 10.1016/s0002-8703(99)70174-2. [DOI] [PubMed] [Google Scholar]
- 26.Opasich C, Pinna GD, Mazza A, et al. Six-minute walking performance in patients with moderate to severe heart failure. Eur Hrt J. 2001;22:488–96. doi: 10.1053/euhj.2000.2310. [DOI] [PubMed] [Google Scholar]
- 27.Rostagno C, Olivo G, Comeglio M, et al. Prognostic value of 6-minute walk corridor test in patients with mild to moderate heart failure: comparison with other methods of functional evaluation. Eur J Heart Fail. 2003;5(3):247–52. doi: 10.1016/s1388-9842(02)00244-1. [DOI] [PubMed] [Google Scholar]
- 28.Rubim VA, Neto CD, Romeo JLM, Montera MW. Prognostic value of the six-minute walk test in heart failure. Arq Bras Cardiol. 2006;86(2):120–5. doi: 10.1590/s0066-782x2006000200007. [DOI] [PubMed] [Google Scholar]
- 29.Arslan S, Erol MKE, Gundogdu F, et al. Prognostic value of the six-minute walk test in stable outpatients with heart failure. Tex Heart Inst J. 2007;34:166–9. [PMC free article] [PubMed] [Google Scholar]
- 30.Olsson LG, Swedberg K, Clark AL, et al. Six minute corridor walk test as an outcome measure for the assessment of treatment in randomized, blinded intervention trials of chronic heart failure: a systematic review. Eur Heart J. 2005;26(8):778–93. doi: 10.1093/eurheartj/ehi162. [DOI] [PubMed] [Google Scholar]
- 31.Casanova C, Celli BR, Barria P, et al. The 6-min walk distance in healthy subjects: reference standards from seven countries. Eur Respir J. 2011;37(1):150–6. doi: 10.1183/09031936.00194909. [DOI] [PubMed] [Google Scholar]
- 32.Guyatt GH, Pugsley SO, Sullivan MJ, et al. Effect of encouragement on walking test performance. Thorax. 1984;39:818–22. doi: 10.1136/thx.39.11.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carvalho EE, Costa DC, Crescêncio JC, et al. Heart failure: comparison between six-minute walk test and cardiopulmonary test. Arq Bras Cardiol. 2011;97(1):59–64. doi: 10.1590/s0066-782x2011005000056. [DOI] [PubMed] [Google Scholar]
- 34.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–8. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295(17):2018–26. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 36.Bensimhon DR, Leifer ES, Ellis SJ, et al. Reproducibility of peak oxygen uptake and other cardiopulmonary exercise testing parameters in patients with heart failure (from the Heart Failure and A Controlled Trial Investigating Outcomes of exercise traiNing) Am J Cardiol. 2008;102(6):712–7. doi: 10.1016/j.amjcard.2008.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu G, Sanderson B, Bittner V. The 6-minute walk test: how important is the learning effect? Am Heart J. 2003;146(1):129–33. doi: 10.1016/S0002-8703(03)00119-4. [DOI] [PubMed] [Google Scholar]
- 38.Sun XG, Hansen JE, Beshai JF, Wasserman K. Oscillatory breathing and exercise gas exchange abnormalities prognosticate early mortality and morbidity in heart failure. J Am Coll Cardiol. 2010;55(17):1814–23. doi: 10.1016/j.jacc.2009.10.075. [DOI] [PubMed] [Google Scholar]