Abstract
Wnt and Sonic Hedgehog (Shh) signals are known to pattern the somite into dermomyotome, myotome and sclerotomal cell fates. By employing explants of presomitic mesoderm cultured with constant levels of Wnt3a conditioned medium and increasing levels of Shh, we found that differing levels of Shh signaling elicit differing responses from somitic cells: the lowest level of Shh signaling allows dermomyotomal gene expression, intermediate levels induce loss of dermomyotomal markers and activation of myogenic differentiation, and higher levels induce loss of myotomal markers and activation of sclerotomal gene expression. In addition, we have found that in the presence of high levels of Wnt signaling, instead of inducing sclerotomal markers, Shh signals act to maintain the expression of dermomyotomal and myotomal markers. One of the sclerotomal genes induced by high levels of Shh signaling is Nkx3.2. Forced expression of Nkx3.2 blocks somitic expression of the dermomyotomal marker Pax3 both in vitro and in vivo. Conversely, forced expression of Pax3 in somites can block Shh-mediated induction of sclerotomal gene expression and chondrocyte differentiation in vitro. Thus we propose that varying levels of Shh signals act in a morphogen-like manner to elicit differing responses from somitic cells, and that Pax3 and Nkx3.2 set up mutually repressing cell fates that promote either dermomyotome/myotome or sclerotome differentiation, respectively.
Keywords: Shh, Nkx3.2, Pax3, somite
Introduction
In the vertebrate embryo, somites give rise to multiple tissues including cartilage, bone and muscle of the vertebral column. Somites are pairs of spherical structures located adjacent to the neural tube of the developing embryo. They are formed by periodic budding from the presomitic mesoderm, the timing of which is a highly controlled event (Hirsinger et al., 2000; Pourquie, 2004; Stockdale et al., 2000). A newly formed somite consists of epithelial cells at the periphery, with a small number of mesenchymal cells in the center (somitocoele) (Christ et al., 2000). As a somite matures, the cells within the somite are separated into ventral and dorsal domains. The sclerotome is formed from cells located in the ventral domain of the somite, where the originally epithelial cells become mesenchymal. Cartilage and bone of the vertebrae and ribs are derived from the sclerotome. The dermomyotome is formed in the dorsal domain of the somite, where the cells remain epithelial. As somite maturation proceeds, the dermomyotome will give rise to both the dermis and an interstitial layer of differentiated skeletal muscle, the myotome (Stockdale et al., 2000).
The initial specification of the dorsal and ventral somitic cell fates is vital because it determines the number of precursors that will give rise to either cartilage, muscle or dermal cells. When a somite first forms, all the epithelial cells in the immature somite have the potential of adopting a sclerotomal or a dermomyotomal cell fate. In somite rotation experiments, when a newly formed somite was rotated 180 degrees, the cells were reprogrammed to become either sclerotome or dermomyotome based upon their new location (Aoyama and Asamoto, 1988; Dockter and Ordahl, 2000), indicating that cell fate had not yet been determined in these newly formed somites and that local environmental signals induce the appropriate somitic cell fate. Multiple extracellular signals expressed in the surrounding tissues determine somite cell fates (Stockdale et al., 2000). Wnt proteins secreted from the dorsal neural tube (Wnt1 and Wnt3a) and from the surface ectoderm (Wnt4, Wnt6 and Wnt7a) promote the formation of the dermomyotome and myotome (Christ et al., 1992; Fan et al., 1997; Fan and Tessier-Lavigne, 1994; Geetha-Loganathan et al., 2006; Geetha-Loganathan et al., 2005; Münsterberg et al., 1995; Schmidt et al., 2004). Ectopic expression of Wnt proteins has been noted to induce the expression of both Pax3 and Pax7, which are expressed throughout the dermomyotome and in proliferative myogenic precursors that migrate into the myotome (Fan et al., 1997; Fan and Tessier-Lavigne, 1994; Geetha-Loganathan et al., 2006; Otto et al., 2006; Relaix et al., 2006; Wagner et al., 2000). Furthermore, Wnt signals promote the expression of myotome-specific markers, including MyoD, Myf5 and Myosin Heavy Chain, in a PKA or PKC-dependent manner (Brunelli et al., 2007; Chen et al., 2005; Münsterberg et al., 1995). In addition to inducing the dorsal somite fate, Wnt proteins have also been documented to inhibit the expression of the sclerotomal marker Pax1 (Capdevila et al., 1998; Fan et al., 1997; Fan and Tessier-Lavigne, 1994).
Sonic Hedgehog (Shh), a signaling molecule secreted by both the notochord and floor plate of the neural tube, is essential to induce formation of the sclerotome and cartilage differentiation of ventral somitic cells (Chiang et al., 1996; Fan et al., 1995; Fan and Tessier-Lavigne, 1994; Johnson et al., 1994). In the Shh knock-out mouse, sclerotome formation is compromised, and no vertebral column is formed (Chiang et al., 1996). Similarly, mice lacking all hedgehog signaling due to a loss of the hedgehog signal transducer smoothened, fail to express the sclerotomal markers Pax1 and Nkx3.2 (Zhang et al., 2001). Moreover, gain-of-function studies have demonstrated that ectopic expression of Shh induced the expression of Pax1 while inhibiting the expression of the dermomyotomal marker Pax3 (Borycki et al., 1998; Johnson et al., 1994).
Shh also promotes the myotome cell fate (Borycki et al., 1999; Buttitta et al., 2003; Gustafsson et al., 2002; McDermott et al., 2005; Münsterberg et al., 1995). Mouse embryos lacking Shh or its receptor smoothened display markedly decreased Myf5 expression, suggesting that Shh is essential for muscle formation (Chiang et al., 1996; Zhang et al., 2001). Several lines of evidence support the idea that myogenesis in the epaxial (i.e., medial) domain of the myotome requires the combination of both Shh and Wnt signaling (reviewed in (Bailey et al., 2001)). A molecular understanding of how such signals can synergistically activate Myf5 expression can be explained by the dual requirement for both TCF and GLI binding sites in the enhancer element that drives Myf5 expression in the epaxial myotome (Borello et al., 2006).
Shh signals may promote myotome formation by promoting the maturation of dermomyotomal cells to become MyoD/Myf5 expressing myotomal cells (Feng et al., 2006; Hammond et al., 2007). In zebrafish, Shh signaling is necessary for Pax3/7-expressing cells in the dermomyotome to transition into MyoD-expressing cells; in the absence of Shh signaling, Pax3 and Pax7-expressing cells in the somite increase in number but fail to activate the myogenic program (Feng et al., 2006; Hammond et al., 2007). Therefore, Shh is required for the differentiation of Pax3/7-expressing dermomyotomal cells into myotomal cells and for the subsequent loss of Pax3/7 gene expression in these differentiated myocytes. These findings in zebrafish are consistent with earlier findings in chicken embryos indicating that ectopic expression of Shh in the somite induced loss of Pax3 expression while promoting premature myogenic differentiation (Amthor et al., 1999). Thus Shh acts in the somite to drive sclerotome formation, repress the expression of dermomyotomal gene expression, and induce the maturation of the dermomyotome into differentiated myotomal cells.
Prior studies have indicated that the dorsal-ventral identity of somitic cells is established by both antagonistic and synergistic activities of Wnt and Shh signals (Fan et al., 1995; Fan and Tessier-Lavigne, 1994; Münsterberg et al., 1995). Fan and Tessier-Levigne demonstrated that signaling by cells expressing either Wnt1 or Shh, when positioned adjacent to somites, is able to travel through the entire distance of a young somite and that these signaling pathways can induce mutually exclusive patterns of dermomyotomal or sclerotomal gene expression, respectively (Fan et al., 1995; Fan and Tessier-Lavigne, 1994). Thus cells within the somite must interpret Wnt signals, secreted by both the neural tube and surface ectoderm, and Shh, secreted by the notochord and floor plate, to establish dorsal and ventral somitic fields with clear borders. However, it is not clear from these prior studies whether Shh and Wnt act as morphogens to set up these different cell types, with distinct fates being designated by differing levels of signaling by these factors. To examine this issue more thoroughly, we have examined the response of somitic cells to varying levels of Shh in the presence of constant Wnt signaling. We find that differing levels of Shh elicit differing responses from somitic cells, with very low levels of Shh working in combination with Wnt signals to maintain dermomyotomal gene expression, slightly higher levels inducing both loss of dermomyotomal markers and activation of myogenic differentiation, and yet higher levels inducing the loss of myotomal markers and the activation of sclerotomal gene expression. In addition, we have found that in the presence of high levels of Wnt signaling, instead of inducing sclerotomal markers, Shh acts to maintain the expression of dermomyotomal and myotomal markers.
Two of the sclerotomal genes induced by high levels of Shh signaling are Nkx3.2 and Sox9, which are both known to promote cartilage formation (Lefebvre and Smits, 2005; Murtaugh et al., 2001; Zeng et al., 2002). Ectopic expression of either Nkx3.2 or Sox9 can induce ectopic cartilage formation in the chick embryo (Murtaugh et al., 2001; Zeng et al., 2002). Mice lacking Nkx3.2 (a.k.a., Bapx1) display a severe reduction of somitic Sox9 expression and defects in axial cartilage formation, suggesting that Nkx3.2 promotes chondrogenesis by inducing Sox9 expression (Akazawa et al., 2000; Lettice et al., 1999; Tribioli and Lufkin, 1999). Sox9 in turn activates the chondrogenic program by directly activating expression of cartilage-specific genes such as collagen II and aggrecan, and Sox9-deficient cells are incapable of chondrogenic differentiation (Akazawa et al., 2000; Bi et al., 2001; Lefebvre et al., 1997; Lettice et al., 1999; Sekiya et al., 2000; Tribioli and Lufkin, 1999). However because both Nkx3.2 and Sox9 are expressed during early phases of somite patterning prior to cartilage differentiation, it is not clear whether Nkx3.2 and Sox9 act only on a pre-existing ventral somite population to promote chondrogenesis or possibly also play an earlier role in demarcating the ventral somite field. Consistent with this latter notion, we have found that ectopic expression of Nkx3.2 blocks the expression of both dermomyotomal and myotomal markers in vitro and in vivo. Pax3 is a transcription factor expressed in the dermomyotome and subsequently in proliferating cells within the myotome (Tajbakhsh, 2003) (Bajard et al., 2006) Relaix, 2005 #689; Relaix, 2006 #687} (Gros et al., 2005) and plays an important role in promoting muscle differentiation (Maroto et al., 1997; Relaix et al., 2006; Relaix et al., 2005). We have found that forced expression of Pax3 in somites can block Shh-mediated induction of sclerotomal gene expression and chondrocyte differentiation in vitro. Thus we propose that varying levels of Shh signals act as a morphogen to elicit differing responses from somitic cells, and that Pax3 and Nkx3.2 set up mutually repressing cell states that promote either dermomyotome/myotome or sclerotome differentiation, respectively.
Materials and Methods
Materials
White leghorn chicken eggs where obtained from Charles River Laboratory (Willmington, MA) and Hy-Line International (Elizabethtown, PA). Mouse anti-Pax3, anti-Pax7 and anti-Myosin antibodies were purchased from Developmental Studies Hybridoma Bank. Rabbit anti-HA and rabbit anti-V5 antibodies were purchased from Sigma. Rabbit anti-GFP antibody was purchased from Abcam. N-Shh was produced as described in (Zeng et al., 2002). Human BMP4 protein was purchased from R&D. Wnt3a conditioned medium was produced from Wnt3a-secreting cells (ATCC) as instructed. RatB1a control and RatB1a-Wnt1 cells were gifts from Dr. Jan Kitajewski (Columbia University). Nkx3.2 virus was generated using RCAS (B)-Nkx3.2HA plasmid as described (Murtaugh et al., 2001). Alkaline phosphatase virus (RCAS (A)-AP) and Pax3 virus (RCAS (A)-AP) were generated as described in (Maroto et al., 1997). Plasmids for making in situ hybridization probes have been described before (Reshef et al., 1998; Zeng et al., 2002). For the construction of RCAS (B)-Sox9V5 plasmid, chicken Sox9 sequence (from PCR) was cloned into pBluescript-V5 vector (kindly provided by Dr. Tony Ip, UMass) so that V5 was tagged at the C-terminus of Sox9. Then pBluscript-Sox9V5 was cut with EcoRI and XbaI, and blunt ended, and subsequently cloned into the RCAS-B viral vector. Electroporation vector pMES-GFP was kindly provided by Dr. Tom Schulteiss (Beth Israel Deaconess Medical Center). pMES-Nkx3.2 was generated by PCR cloning of chicken Nkx3.2 into BamHI cut pMES vector. pMES-Sox9V5 was generated by cloning EcoRI and XbaI cut fragment from pBluescript-Sox9V5 into SmaI cut pMES-GFP vector. Viruses were produced according to the standard protocol (Johnson et al., 1994), and were titered by direct visualizing GFP expression (in the case of RCAS-GFP production) or indirect immunocytochemistry using anti-HA or anti-V5 antibodies. All viruses used have reached a titer of 108 particles/ml.
Embryo explant culture and viral infection
Somite explants from chicken embryos of HH stage 10 were excised as described (Zeng et al., 2002). Briefly, chicken embryos were pinned onto an agar dish with the ventral side up. The embryos were treated with dispase (Roche, 2mg/ml) to dissociate the germ layers. Tungsten needles were used to dissociate the endoderm layer and to slice out the mesodermal somite tissues. When culturing the somites with the surface ectoderm, no dispase was used. The explants were incubated in 5μl concentrated virus stock (titer of 108/ml) for 30min-1hr on ice before being transferred onto the collagen gel (Zeng et al., 2002). The collagen gel was constructed as a lower collagen layer and an upper collagen layer, with the somite explants in the middle. The composition of the collagen layers is: 30% rat tail collagen I (BD biosciences) and 1X DMEM. A total of 25ul of collagen gel mixture was used for each explant. After the collagen solidified at room temperature, explants in collagen gel were cultured in 400 μl of α-MEM somite culture medium (with 10% FBS, 1% penstrep, 5% chick embryo extract, 1ng/ml bFGF) in 4-well dishes (Nunc) at 37°C for 1-6 days before harvesting for either RT-PCR analysis or immunocytochemistry (Holowacz et al., 2006). Results from Fig.1 were obtained using only embryos of 11 somites. Co-cultures with control Rat1B cells or Rat1B-Wnt1 cells were performed according to (Münsterberg et al., 1995). Briefly, the Rat1B cells were scraped off tissue culture grade plastic and subsequently plated onto an agar dish containing medium for 4hrs to overnight. Aggregates of Rat1B cells (which form on agar dishes) were cut into small pieces and co-cultured together with the presomitic mesoderm explants in the collagen gel.
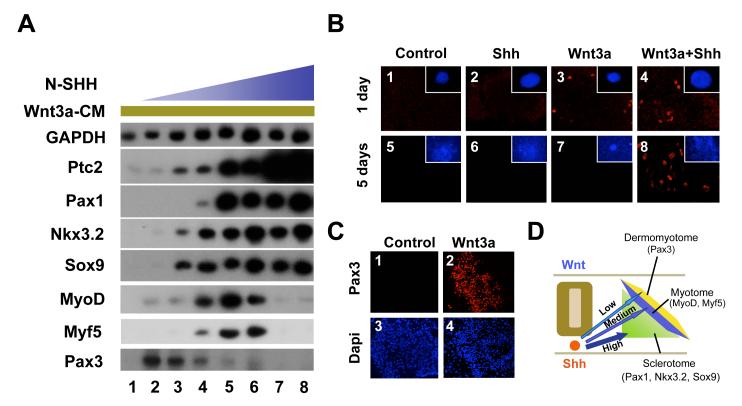
Figure 1. Shh concentration gradient patterns dorsal-ventral fates of the somite.
A. In the presence of constant levels of Wnt3a signals, differing levels of Shh are necessary to induce either dorsal or ventral somite markers. Presomitic mesoderm explants were cultured for five days in Wnt3a-conditioned medium and increasing amounts of N-SHH. Lane 1: no N-SHH. Lane 2: 34ng/ml N-SHH. Lane 3: 67ng/ml N-SHH. Lane 4: 125ng/ml N-SHH. Lane 5: 250ng/ml N-SHH. Lane 6: 500ng/ml N-SHH. Lane 7: 1μg/ml N-SHH. Lane 8: 2μg/ml N-SHH. Gene expression was assayed by RT-PCR. B. Shh is required to maintain but not initiate the expression of Pax3 in presomitic mesoderm exposed to Wnt signals. Presomitic mesoderm explants from stage 10 chicken embryos were cultured for either one day (panels 1-4) or five days (panels 5-8) in either control conditioned medium (panels 1 and 5), control conditioned medium containing 125ng/ml N-SHH (panels 2 and 6), Wnt3a-conditioned medium (panels 3 and 7), or the combination of Wnt3a-conditioned medium plus 125ng/ml N-SHH (panels 4 and 8). Pax3 expression was analyzed by immunocytochemistry and confocal microscopy. The inset in each panel represent a whole mount Dapi staining of the explant. The Dapi images were taken at the same magnification. Few somitic cells migrated out of the explants at day 1. By day 5, the explants were much larger due to cell proliferation and migration. It is evident that more cells were present in Shh-treated explants. C. Wnt3a alone is sufficient to induce Pax3 expression in somite IV-VI. Somites IV-VI explants from stage 10 chicken embryos were cultured for five days in either control conditioned medium (panels 1 and 3) or Wnt3a-conditioned medium (panels 2 and 4). Confocal images of Pax3 expression (panels 1 and 2) and DAPI staining (panels 3 and 4) are displayed. D. Model showing differing levels of Shh signal leads to differing cell fates in the somite.
RT-PCR analysis
Explants were processed by the RNeasy mini kit from Qiagen (Zeng et al., 2002). Primers sequences have been described before (Reshef et al., 1998; Zeng et al., 2002). All PCR analyses were normalized based on GAPDH expression quantified by a phospho-imager (BioRad).
Immunocytochemistry
Embryo explants were fixed by 4% paraformaldehyde and incubated with primary antibodies overnight (Holowacz et al., 2006). After washing with PBST, they were incubated with secondary antibodies (conjugated with Alexa 488 (green) or 594 (red) from Invitrogen) overnight, followed by repeated washing the day after. Electroporated embryos were serial-sectioned first on the cryostat (10μm thick, Microm HM560) before immunostaining. The sections on slides were incubated overnight with the primary antibodies, and for 4hrs with the secondary antibodies.
In situ hybridization
Whole mount in situ hybridization was performed as described (Murtaugh et al., 2001). After whole mount in situ, the embryos were sectioned on the cryostat (Microm HM560) at 10μm.
Electroporation
For in ovo electroporation, plasmid DNA of 1μg/ml concentration was injected into the lumen of the newly formed somites using a micromanipulator (Parker Picospritzer II), and immediately electroporated using the electroporator (BTX ECM830) on chicken HH Stage 14-16 embryos. The electrodes are Genetrodes by Genetronics, model 514. Electroporation conditions are: 30V, 20msec pulse length, 5 pulses with 600msec intervals. After the operation, the window of the shell was sealed with scotch tape, and allowed to incubate at 37°C for 2 days before the embryos were beheaded and fixed for immunocytochemistry.
Imaging
In situ hybridization and immunocytochemistry were visualized and photographed on the following microscopes: Olympus IX71 inverted microscope, Nikon Eclipse 800 compound microscope, Leica MZ16F stereo microscope and Leica TCS SP2 AOBS confocal microscope.
Results
Shh gradient determines dorsal-ventral somitic cell fates
To determine the effect of varying levels of Shh signaling on the specification of somitic cell fates, we cultured chicken presomitic mesoderm explants (isolated from stage 10 chicken embryos) with a constant amount of Wnt3a-conditioned medium and increasing levels of the N-terminal fragment of Sonic Hedgehog (N-Shh), which is soluble and a potent Shh agonist (Marti et al., 1995). We employed Wnt3a-conditioned medium (Willert et al., 2003) in these cultures, to ensure that each of the explants would receive the same amount of Wnt3a signal. Following incubation of the explants in increasing concentrations of N-Shh, we assayed the expression of both dorsal and ventral somite markers by RT-PCR analysis. We varied the N-Shh concentration in the culture medium from 34ng/ml-2μg/ml. We believe this level of Shh signaling reflects a physiological level of Shh signal transduction, as downstream markers induced by N-Shh were expressed at similar levels as in somite explants cultured in the presence of notochord (a source of endogenous Shh; data not shown). Our RT-PCR analysis indicated that Patched 2 (Ptc2), a previously described Hedgehog responsive gene (Pearse et al., 2001), displayed a gradual increase in expression in response to increasing levels of N-Shh (Fig. 1A, lanes 1-8). In contrast, the expression of dermomyotomal and myotomal markers responded to increasing levels of N-Shh in a complicated and biphasic pattern. While a low level of N-Shh (34ng/ml) induced the expression of Pax3 in somitic explants (Fig.1A, lane 2), further increasing levels of N-Shh (67ng/ml-250ng/ml) led to a steady decrease of Pax3 expression (Fig. 1A, lanes 3-6). Consistent with these results, an earlier analysis of mouse somite explants showed that a low level of Shh at 2.5nM (which is equivalent to 35ng/ml) did not inhibit Pax3 expression, while higher levels of Shh at 25nM (equivalent to 350ng/ml) inhibited expression of murine Pax3 (Fan et al., 1997). Interestingly, the loss of Pax3 gene expression induced by increasing levels of N-Shh correlated with the activation of MyoD and Myf5 at these intermediate concentrations of N-Shh (67-250ng/ml) (Fig. 1A, lanes 3-6). While intermediate levels of N-Shh induced MyoD and Myf5 expression (Fig 1A, lanes 4-6), concentrations of N-Shh at or above 500ng/ml led to a precipitous loss of both MyoD and Myf5 expression (Fig. 1A, lanes 7-8).
Expression of the sclerotomal markers, Nkx3.2, Sox9 and Pax1, were induced to maximal levels by a threshold intermediate concentration of N-Shh (125-500ng/ml) (Fig. 1A, lanes 3-5). Induction of Pax1 required greater levels of N-Shh than did either Nkx3.2 or Sox9 (Fig. 1A, lanes 3-5). These findings suggest that sclerotome gene expression requires a threshold level of Shh signaling, and that the induction of Pax1 requires a higher concentration of Shh, as compared to that of Nkx3.2 and Sox9. These in vitro findings are consistent with the fact that compared to other sclerotomal markers, Pax1 is only highly expressed in the ventral-medial domain of the sclerotome, next to the notochord that secretes Shh, and therefore in the region of the somite exposed to the highest level of Shh.
We were puzzled by the fact that low levels of N-Shh (when combined with Wnt3A) induced the expression of Pax3 in somite explants, as the dermomyotome expression domain of Pax3 is expanded in mutant mice embryos lacking Shh (Chiang et al., 1996; Martinelli and Fan, 2007). We considered the possibility that N-Shh might induce the expansion of a small population of Pax3-positive cells within the somite, because Shh is known to both induce somitic cell proliferation and block apoptosis (Borycki et al., 1999; Kruger et al., 2001; Teillet et al., 1998). To explore this possibility, we cultured the presomitic mesoderm explants for either one day or five days in Wnt3a conditioned medium either in the presence or in the absence of Shh (125ng/ml). After only one day of culture, explants treated with either control conditioned medium or with medium containing only Shh failed to express Pax3 (Fig.1B, panels 1 and 2). In striking contrast, after one day of culture Pax3 protein was easily detected in explants treated with Wnt3a alone (Fig.1B, panel 3). Addition of N-Shh (125ng/ml) did not significantly alter the expression level of Pax3 induced by Wnt3a conditioned medium, possibly because this level of Shh was not very high (Fig.1B, panel 4). This finding is consistent with prior reports indicating Wnt signaling alone is sufficient to induce Pax3 expression in explants of murine presomitic mesoderm cultured for 24 hours (Fan et al., 1997). Furthermore, work in zebrafish employing mutants in the hedgehog pathway or the hedgehog antagonist, cyclopamine, indicate that the induction of Pax3 or Pax7 expression in the dermomyotome of this species does not require hedgehog signaling (Feng et al., 2006; Hammond et al., 2007). In marked contrast to explants cultured for only one day, presomitic mesoderm explants cultured for five days did not express detectable Pax3 protein when cultured in Wnt3a conditioned medium alone (Fig.1B, panel 7). However, like the one-day cultures, the explants cultured for five days in the combination of Wnt3a-conditioned medium and N-Shh (125ng/ml) expressed significant levels of Pax3 (Fig.1B, panel 8). This finding is consistent with our RT-PCR data shown in Fig.1A, where the explants were analyzed after five days of culture. Thus our analysis suggests that Shh may be required to maintain Pax3 expression in explants of presomitic mesoderm cultured for five days in Wnt3a conditioned medium, although it may not be required to initiate Pax3 expression in explants cultured for only one day in Wnt3a conditioned medium. To investigate whether more mature somites also require exogenous Shh to express Pax3, we cultured somites IV-VI for five days in either the absence or presence of Wnt3a conditioned medium. In contrast to explants of presomitic mesoderm, high levels of Pax3 were detectable in explants of somite IV to VI that had been cultured for five days in Wnt3a conditioned medium (Fig.1C). Interestingly, these more mature somites behave in vitro as if they have already been exposed to Shh signals in vivo (Münsterberg et al., 1995). Therefore, our analyses suggest that lower levels of Shh signaling act in combination with Wnt signals to promote the maintenance of Pax3 expression in somites cultured in vitro.
We propose that a gradient of Shh patterns somitic cells to adopt different cell fates (Fig.1D). Working in combination with Wnt signaling, the lowest level of Shh signal helps to maintain the expression of the dermomyotomal marker, Pax3, in vitro, while higher levels of Shh signaling both repress the expression of Pax3 and induce the expression of MyoD and Myf5. Greater levels of Shh signaling repress the expression of myotomal markers while allowing the continued expression of sclerotomal makers, which are apparently activated by a threshold level of Shh signaling. These in vitro findings are consistent with the in vivo expression patterns for Pax3, Myf5, MyoD, Nkx3.2, Sox9 and Pax1, which are expressed in progressively more ventralized somitic domains, respectively (Fig.1D).
Comparison of somitic expression of Pax3, Nkx3.2 and Sox9
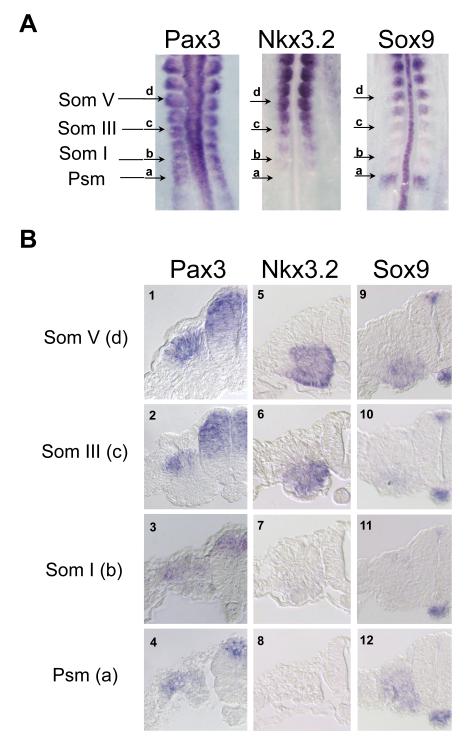
Our RT-PCR results led us to hypothesize that a relatively low concentration of N-Shh (as low as 67ng/ml) can induce factors that antagonize Pax3 expression. As Sox9 and Nkx3.2 were induced by intermediate concentrations of N-Shh that repressed the expression of Pax3 (Fig.1A, lanes 3 and 4), we considered the possibility that either Sox9 or Nkx3.2 may act to repress the expression of Pax3. To determine if Sox9 and Nkx3.2 are inhibitors of Pax3 expression, we first directly compared their expression with that of Pax3 during the period when the dorsal-ventral somite domains are determined. Whole mount in situ hybridization analysis of stage 10 chicken embryos revealed that Pax3 is expressed in the dorsal neural tube, throughout the rostral presomitic (-I somite) mesoderm and in dorsal regions of more mature somites (Figs. 2A and B). In the first somite, Pax3 is expressed throughout the somite, with higher levels of expression in the dorsal domain; as the somite matures, Pax3 expression is gradually restricted to the dorsal domain (Otto et al., 2006; Schubert et al., 2001; Williams and Ordahl, 1994). In contrast to Pax3, Nkx3.2 expression is absent from the presomitic mesoderm, but is strongly expressed in the ventral region of somite III as well as in the somitocoele (Fig 2A and 2B, panels 5-8). Interestingly, the loss of Pax3 expression from the ventral region of somite III correlates with the induction of Nkx3.2 expression in this somitic domain (Fig. 2B, compare panels 2 and 6). Unlike Pax3 and Nkx3.2, Sox9 exhibits a more dynamic expression pattern during somite maturation. Sox9 is initially expressed throughout the presomitic mesoderm before the budding of the first somite; its expression largely diminishes in the next two somites and only begins to reappear starting from the third somite in the anterior ventral domain (Fig 2A and 2B, panels 9-12). Sox9 is strongly expressed in the ventral region of more mature somites (somite V-X) (Fig.2A, and Fig.2B, panel 9). Our expression analyses indicate that dermomyotomal and sclerotomal gene expression is segregated into dorsal and ventral domains well before the morphological changes indicative of a differentiated dermomyotome and myotome. The restriction of Pax3 expression to the dorsal somite correlates with the induction of Nkx3.2 in the ventral region of the newly formed somite. In contrast, Sox9 expression is not yet expressed at high levels in the ventral domain of the somite until well after Pax3 expression is already restricted to the dorsal somite and Nkx3.2 is strongly expressed in the ventral somitic domain. These dynamic expression patterns raise the possibility that the induction of Nkx3.2 in the ventral somite may act to both inhibit Pax3 expression and augment that of Sox9 in this region of the somite.
Figure 2. In situ hybridization analysis of Pax3, Nkx3.2 and Sox9.
A. Whole mount in situ hybridization analysis of Pax3, Nkx3.2 and Sox9 on 12 somite (stage 10) chick embryos. Arrows mark the caudal to rostral levels of the somitic mesoderm. a, presomitic mesoderm (Psm). b, somite I. c, somite III. d, somite V. B. Sections of Pax3, Nkx3.2 and Sox9 ISH embryos at various axial levels. Panels 1-4, Pax3 expression. Panels 5-8, Nkx3.2 expression. Panels 9-12, Sox9 expression. Sections at the presomitic mesoderm level are shown in panels 4, 8 and 12. Sections at somite I level are shown in panels 3, 7 and 11. Sections at somite III level are shown in panels 2, 6 and 7. Sections at somite V level are shown in panels 1, 5 and 9.
Nkx3.2 and Sox9 inhibit Wnt3a-induced Pax3 expression
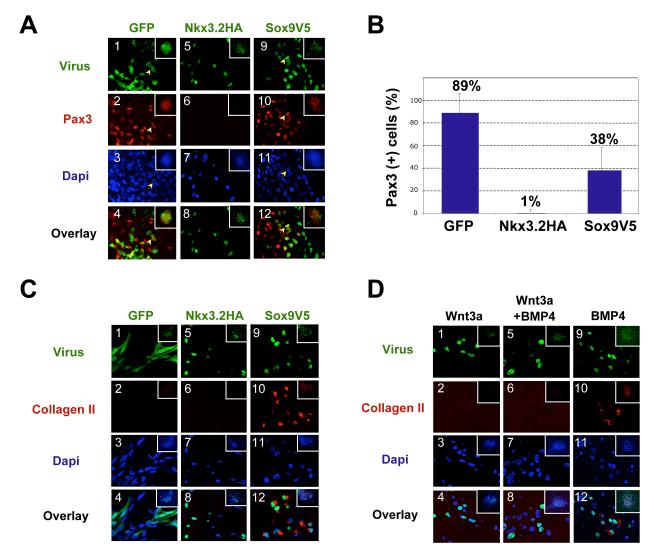
To explore whether Nkx3.2 or Sox9 have the potential to directly or indirectly repress the expression of dermomyotomal genes in the ventral somite, we examined the effect of retrovirally-expressed Nkx3.2 and Sox9 on the expression of a dorsal marker, Pax3, in isolated somites. In the embryo, Pax3 expression is induced by Wnt proteins secreted by either the neural tube (Wnt1, Wnt3a) or the surface ectoderm (Wnt4, Wnt6 and Wnt7a) (Fan et al., 1997; Otto et al., 2006; Reshef et al., 1998; Wagner et al., 2000). Consistent with these prior observations, culture of somites IV-VI from stage 10 chick embryos, which behave as if they have already seen a Shh signal in vivo (Münsterberg et al., 1995), expressed robust levels of Pax3 in response to Wnt3a conditioned medium alone (Fig. 1C). Thus, we investigated whether the expression of retroviral encoded Nkx3.2 or Sox9 would blunt the ability of Wnt3a conditioned medium to induce the expression of Pax3 in such cultured somites. Robust expression of Pax3 (red) was induced by Wnt3a conditioned medium in somite explants infected with a control virus encoding GFP (RCAS-GFP) (Fig.3A, panels 1-4). Extensive overlap was found between GFP and Pax3 expression, indicating that ectopic GFP expression does not inhibit Pax3 expression. In contrast, similarly cultured somites infected with a retrovirus encoding HA-tagged Nkx3.2 (RCAS-Nkx3.2HA) displayed markedly reduced Pax3 expression (Fig.3A, panels 5-8). Indeed, almost no Nkx3.2HA-expressing cells (green) expressed Pax3 (red), suggesting that forced Nkx3.2 expression strongly inhibits Pax3 expression in somitic cells (Fig.3A, panels 5-8). While somites infected with a retrovirus encoding V5-tagged Sox9 (RCAS-Sox9V5) still expressed Pax3, the majority of cells that expressed viral Sox9V5 did not express Pax3 (Fig.3A, panels 9-12). Some of the Sox9V5-expressing cells appear to form clusters that are devoid of Pax3 expression (Fig.3A, panels 9-12). It is possible that ectopic Sox9 expression has converted these cells into proliferating cartilage cells (see below) (Zeng et al., 2002).
Figure 3. Nkx3.2 and Sox9 inhibit Wnt3a-induced Pax3 expression in somite explants.
A. Somite explants immunostained for Pax3 expression following infection with retroviral encoded Nkx3.2HA, Sox9V5 or GFP. Somite IV-VI explants of stage 10 chicken embryos were cultured in Wnt3a conditioned medium for 5 days. Panels 1-4, RCAS-B-GFP infected explant, arrows indicating a cell that expressed GFP as well as Pax3. Panels 5-8, RCAS-B-Nkx3.2HA infected explant. Panels 9-12, RCAS-B-Sox9V5 infected explant, arrows indicating a cell that expressed Sox9V5 as well as Pax3. The inset within each panel shows a low powered view of each explant. Green: GFP, Nkx3.2HA and Sox9V5. Red, Pax3. Overlay, merged view of virus-expression (GFP, Nkx3.2 and Sox9) and Pax3. Dapi images are not overlaid with virus and Pax3 images so that yellow overlapping expression is more evident. No significant changes in cell numbers in these explants were observed. B. Quantification of the results of Fig. 3A. Percentage of virus-infected cells that express Pax3 was quantified. For each virus-infected sample, a total number of 500-1000 virus-infected cells from at least 5 different views were analyzed under the microscope. Standard deviations are shown. C. Somite explants immunostained for Collagen II expression following infection with retroviral encoded Nkx3.2HA, Sox9V5 or GFP. Somite explants were cultured under the same condition as described in Fig. 3A. Panels 1-4, RCAS-B-GFP infected explant. Panels 5-8, RCAS-B-Nkx3.2HA infected explant. Panels 9-12, RCAS-B-Sox9V5 infected explant. Green: GFP, Nkx3.2HA and Sox9V5. Red, Collagen II. Overlay, merged view of virus-expression (GFP, Nkx3.2 and Sox9), Collagen II and Dapi. The inset within each panel shows a low powered view of each explant. D. Nkx3.2 virus-infected cells do not adopt a cartilage fate in the presence of Wnt3a, but do express collagen II in the presence of exogenous BMP4. Somites IV-VI of stage 10 chicken embryos were cultured in either Wnt3a-conditioned medium (panels 1-4), Wnt3a-conditioned medium plus 100ng/ml BMP4 protein (panels 5-8), or control L-cell conditioned medium plus 100ng/ml BMP4 protein (panels 9-12). The explants were cultured altogether for 6 days before immunostaining. Green: GFP, Nkx3.2HA and Sox9V5. Red, Collagen II. Overlay, merged view of virus-expression (GFP, Nkx3.2 and Sox9), Collagen II and Dapi. The inset within each panel shows a low powered view of each explant.
We quantified this immunostaining result by calculating the percentage of virus-expressing cells that were Pax3-positive (Fig.3B). In GFP-infected explants, 89% of the GFP-expressing cells were Pax3-positive. In contrast, only 1% of the Nkx3.2-expressing cells were Pax3-positive, indicating that ectopic Nkx3.2 strongly inhibits the expression of Pax3 induced by Wnt3a. In RCAS-Sox9V5 infected explants, only 38% of the Sox9-expressing cells were Pax3-positive, suggesting that ectopic Sox9 represses, but does not completely inhibit, Wnt3a-induced Pax3 expression (Fig.3B). Because virally encoded Sox9 is known to activate expression of endogenous Nkx3.2 in infected somites (Zeng et al., 2002), it is possible that the reduced level of Pax3 expression observed in RCAS-Sox9V5 infected cells reflects the induction of Nkx3.2 in these cultures.
As prior work has established that forced expression of either Nkx3.2 or Sox9 can activate chondrogenesis in somite explants cultured in medium containing BMPs (Murtaugh et al., 2001), we investigated whether somites cultured in Wnt3a conditioned medium and infected with either RCAS-Nkx3.2HA or RCAS-Sox9V5 have similarly adopted a cartilage cell fate. To assay for chondrocyte differentiation, we evaluated collagen II expression in these explants via immunocytochemistry. While neither RCAS-GFP infected cells nor RCAS-Nkx3.2HA infected cells expressed any collagen II (Fig.3C, panels 1-8), RCAS-Sox9V5-infected cells were all collagen II-positive (Fig.3C, panels 9-12). This finding suggests that ectopic expression of Nkx3.2 is not sufficient to activate the chondrocyte differentiation program in somites cultured in Wnt conditioned medium, while expression of ectopic Sox9 is able to activate this differentiation program in similarly cultured cells.
It is possible that RCAS-Nkx3.2-infected cells did not adopt a cartilage fate due to an insufficient amount of BMP in the medium, as prior work has indicated that Nkx3.2 requires a higher amount of BMP4 than does Sox9 to induce chondrogenesis (Zeng et al., 2002). To examine if Nkx3.2 failed to induce collagen II expression due to an insufficient amount of BMP in the culture medium, we administered exogenous BMP4 to RCAS-Nkx3.2HA-infected somites exposed to Wnt3a conditioned medium. In the presence of both exogenous BMP4 and Wnt3a conditioned medium, RCAS-Nkx3.2HA infected cells still failed to express any detectable collagen II (Fig.3D, panels 5-8). In contrast, RCAS-Nkx3.2 infected somites cultured in the presence of exogenous BMP4 and in the absence of Wnt3a conditioned medium expressed detectable levels of collagen II (Fig.3D, panels 9-12). These findings indicate that Wnt3a conditioned medium blocks the ability of Nkx3.2 to induce cartilage formation even in the presence of exogenous BMP4.
Nkx3.2, but not Sox9, inhibits surface ectoderm-induced Pax3 expression
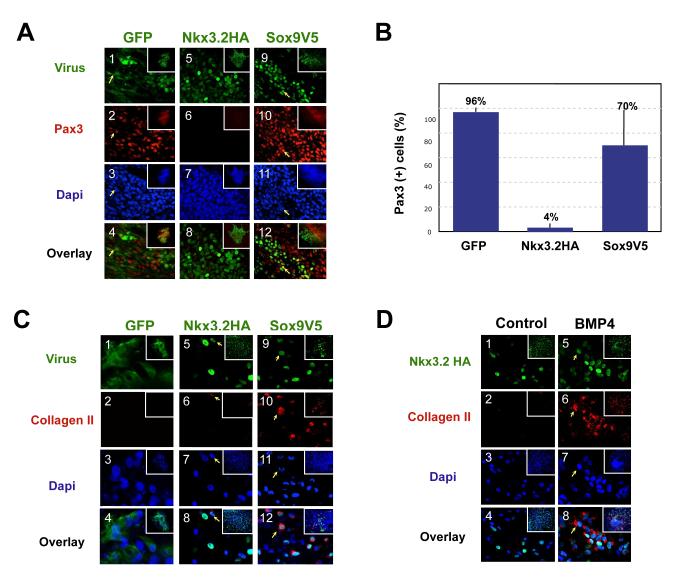
As Pax3 expression in the somite is strongly induced by signals from the surface ectoderm, we examined the effects of exogenous Nkx3.2 and Sox9 on the induction of Pax3 by signals from this tissue (Brunelli et al., 2007; Reshef et al., 1998). Culture of somites IV-VI (but not somites I-III) with the adjacent surface ectoderm induces the expression of Pax3 in such explants (Reshef et al., 1998). We thus infected explants of somites IV-VI cultured with the adjacent surface ectoderm with either RCAS-GFP, RCAS-Nkx3.2HA or RCAS-Sox9V5. While we observed extensive overlapping expression of GFP (green) and Pax3 (red) (Fig. 4A, panels 1-4) in the RCAS-GFP infected explants, very few of the RCAS-Nkx3.2HA infected explants expressed Pax3 (Fig.4A, panels 5-8). Unlike RCAS-Nkx3.2HA infected explants, explants infected with RCAS-Sox9V5 exhibited robust Pax3 expression, and many of the Sox9V5 positive cells were also Pax3 positive (Fig.4A, panels 9-12), indicating that ectopic Sox9 does not inhibit the expression of Pax3 induced by the surface ectoderm. Quantification of the immunostaining result described above indicated that 96% of the GFP-infected cells expressed Pax3, while only 4% of the Nkx3.2HA-expressing cells were Pax3-positive, and 70% of cells that expressed Sox9V5 were Pax3-positive (Fig.4B). Therefore, our results indicate that ectopic Nkx3.2 expression efficiently inhibits Pax3 expression induced by signals from the surface ectoderm as compared to ectopic Sox9 expression, which only slightly dampens Pax3 expression in these same cultures.
Figure 4. Effects of Nkx3.2 and Sox9 on surface-ectoderm induced Pax3 expression.
A. Pax3 expression in somite/ectoderm explants infected with GFP, Nkx3.2HA and Sox9V5. Stage 10 chicken embryo explants of somite IV-VI with surface ectoderm attached were cultured for 5 days. Panels 1-4, RCAS-B-GFP infected explant. Panels 5-8, RCAS-B-Nkx3.2HA infected explant. Panels 9-12, RCAS-B-Sox9V5 infected explant. The inset within each panel shows a low powered view of each explant. Arrows indicating cells that expressed GFP or Sox9V5 as well as Pax3. The inset within each panel shows a low powered view of each explant. Green: GFP, Nkx3.2HA and Sox9V5. Red, Pax3. Overlay, merged view of virus-expression (GFP, Nkx3.2 and Sox9) and Pax3. Dapi images are not overlaid with virus and Pax3 images so that yellow overlapping expression is more evident. No significant changes in cell numbers in these explants were observed. B. Quantification of the results of Fig.4A. Percentage of virus infected cells that express Pax3 was quantified. For each virus-infected sample, a total number of 500-1000 virus-infected cells from 5 different views were analyzed under the microscope. C. Somite/ectoderm explants immunostained for Collagen II expression following infection with retroviral encoded Nkx3.2HA, Sox9V5 or GFP. Somite explants were cultured under the same condition as described in Fig. 4A. Panels 1-4, RCAS-B-GFP infected explant. Panels 5-8, RCAS-B-Nkx3.2HA infected explant. Arrow, an Nkx3.2HA-expressing cell that also expresses a low level of Collagen II. Panels 9-12, RCAS-B-Sox9V5 infected explant. Green: GFP, Nkx3.2HA and Sox9V5. Red, Collagen II. Overlay, merged view of virus-expression (GFP, Nkx3.2 and Sox9), Collagen II and Dapi. The inset within each panel shows a low powered view of each explant. D. Nkx3.2 virus-infected cells adopt a cartilage fate in the presence of exogenous BMP4. Stage 10 chicken embryo explants of somite IV-VI with surface ectoderm attached were cultured for 6 days in either control medium (panels 1-4) or in medium supplemented with 100ng/ml BMP4 protein (panels 5-8). The inset within each panel shows a low powered view of each explant. Nkx3.2HA, green. Collagen II, red. Dapi, blue. Overlay, merged image of Nkx3.2HA, Collagen II and Dapi.
We determined whether ectopic expression of either Nkx3.2 or Sox9 was able to drive cartilage differentiation of somites cultured with adjacent surface ectoderm. While we were unable to detect expression of the cartilage-specific marker collagen II in somite/ectoderm explants infected with RCAS-GFP (Fig4C, panels 1-4), explants infected with RCAS-Nkx3.2HA occasionally exhibited low levels of collagen II expression (in less than 1% of the infected cells) (arrows, Fig.4C, panels 5-8). In contrast, explants infected with RCAS-Sox9V5 robustly expressed collagen II (Fig.4C, panels 9-12). In the presence of exogenous BMP4, however, RCAS-Nkx3.2HA induced significant amount of collagen II expression in somite/ectoderm explants (Fig. 4D, panels 5-8), confirming again that Nkx3.2 requires BMP signaling to induce chondrogenesis (Zeng et al., 2002). Taken together, our findings indicate that ectopic Nkx3.2 blocks the induction of Pax3 by either soluble Wnt3A or signals from the surface ectoderm without inducing chondrocyte differentiation. Nkx3.2-mediated repression of Pax3 expression is therefore similar to the loss of Pax3 expression in the ventral domain of the newly formed somite, which occurs well before chondrogenesis takes place in these same cells. In contrast, Sox9, which efficiently induces Collagen II expression does not block Pax3 expression.
Ectopic Nkx3.2 inhibits the expression of dermomyotomal markers in vivo
Our in vitro findings with somite explants indicate that Nkx3.2 can efficiently inhibit the expression of Pax3 in response to either Wnt3a or signals from the dorsal ectoderm; in contrast, Sox9 can decrease but not block the expression of this dorsal somite marker. To evaluate whether ectopic expression of Nkx3.2 can similarly block expression of dorsal somitic markers in vivo, we electroporated Nkx3.2 into the dorsal somite cells of chick embryos via in ovo electroporation. Nkx3.2 was cloned into the electroporation vector pMES, which includes an IRES-GFP module that enables GFP to be co-expressed in the same cells as Nkx3.2 (James and Schultheiss, 2003; Krull, 2004). pMES-Nkx3.2 was subsequently electroporated into the dorsal cells of newly formed somites in stage 13 to 15 chicken embryos. To confirm ectopic Nkx3.2 expression in dorsal somite cells following electroporation of pMES-Nkx3.2, we performed whole mount in situ hybridization (ISH) analysis using an Nkx3.2 RNA probe. Transverse sections of these electroporated embryos revealed that endogenous Nkx3.2 was expressed in ventral somite cells and that exogenous Nkx3.2 was expressed in dorsal somite cells (Fig.5A). For comparison, we also electroporated a Sox9-encoding plasmid (pMES-Sox9V5) into the newly formed somites. To confirm the ectopic expression of Sox9 in the dorsal somite cells, we performed immunocytochemistry employing an anti-V5 antibody. Indeed, the epithelial cells in the dorsal lateral domain of the somite expressed ectopic Sox9V5 (red), and were also GFP-positive (green) (Fig.5B). Therefore, both Nkx3.2 and Sox9 were targeted to the dorsal somite cells, and our immunocytochemistry result reassured us that we could use GFP expression to identify cells co-expressing either ectopic Nkx3.2 or Sox9 plus GFP.
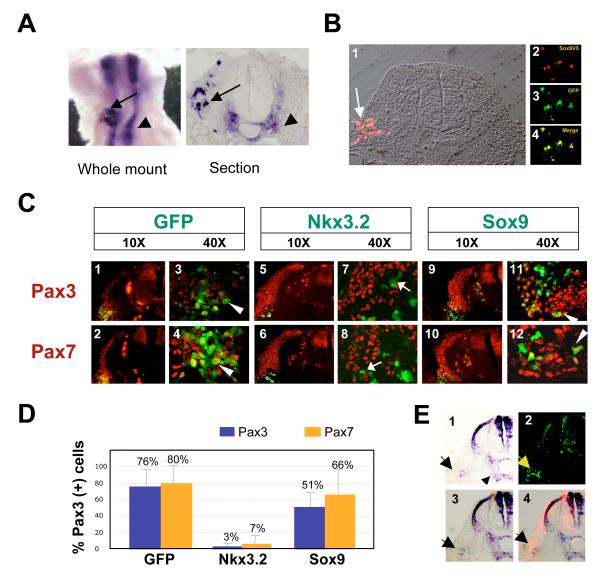
Figure 5. Nkx3.2, but not Sox9, inhibits the expression of Pax3 and Pax7 in ovo.
A. Ectopic expression of Nkx3.2 in the dorsal somite cells. In situ hybridization (ISH) analysis of chicken embryos electroporated with pMES-Nkx3.2-GFP into the lumen of the newly formed somites two days after electroporation, using an Nkx3.2 RNA probe. 5 embryos were analyzed and all exhibited ectopic Nkx3.2 in the dorsal somitic cells. Left panel: whole mount ISH; right panel: a cross section of the whole mount ISH embryo. Arrows: exogenous Nkx3.2 expression. Arrowheads: endogenous Nkx3.2 expression. B. Ectopic expression of Sox9 in the dorsal somite cells. Immunocytochemistry analysis on sectioned embryos electroporated with pMES-Sox9-GFP two days after electroporation. 5 sections were analyzed and all cells that expressed GFP also expressed Sox9V5. GFP-positive Panel 1, V5 staining showing Sox9V5 expression in dorsal somite cells. A bright field view of the section was overlaid on the fluorescent image. Panel 2, magnified view of Sox9V5-positive cells. Panel 3, magnified view of GFP-positive cells. Panel 4, merged image (yellow) of Sox9V5 and GFP. C. Nkx3.2, but not Sox9, inhibited Pax3 and Pax7 expression. Embryos electroporated with either pMES-GFP (vector), pMES-Nkx3.2-GFP or pMES-Sox9V5-GFP into the lumen of the newly formed somites were whole mount fixed and serial sectioned. The sections were immunostained with antibodies against Pax3 and Pax7. Cells harboring the introduced DNA are GFP-positive, and thus are green. Only overlaid images are shown. Most of the cells that expressed the introduced plasmids were laterally located. However, the few medially-targeted cells exhibit the same phenotype as these laterally located cells. Panels 1-4, GFP electroporated embryos (Panels 1-2 are 10X views, and Panels 3-4 are 40X views). Panels 5-8, Nkx3.2 electroporated embryos (Panels 5-6 are 10X views, and Panels 7-8 are 40X views). Panels 9-12, Sox9V5 electroporated embryos (Panels 9-10 are 10X views, panels 11-12 are 40X views). Arrows: cells that express introduced DNA (GFP-positive), but are not Pax3 or Pax7 -positive (red). Arrowheads: cells that express introduced DNA (GFP-positive), and are also Pax3 or Pax7-positive (red), and are thus yellow. D. Quantification of the results of Fig.5C. Percentage of electroporated cells that express Pax3 and Pax7 was quantified. For each electroporated sample, a total number of 100-200 targeted cells from at least 3 embryos were analyzed under the confocal microscope. Only the target cells (GFP-positive) that were located in the normal Pax3 and Pax7 expression domains were used for counting. Pax3, blue. Pax7, orange. E. Nkx3.2 induced the expression of Sox9 in the dorsal somite cells. Embryos electroporated with pMES-Nkx3.2-GFP into the lumen of the newly formed somites were subjected to whole mount in situ hybridization and sectioning, using a Sox9 RNA probe. 5 embryos were analyzed. Panel 1, in situ hybridization of Sox9. Arrowhead: exogenous Sox9 expression. Arrow: endogenous Sox9 expression. A bright field view of the section was overlaid on the fluorescent image. Panel 2, Anti-GFP staining indicating the ectopic expression of the introduced DNA pMES-Nkx3.2-GFP (arrow). Panel 3, Overlay of the bright field ISH image with the GFP immunostaining image (arrow). Panel 4, merged bright field image of ISH and immunofluorescent staining of endogenous Pax3, showing ectopic Sox9 expression within the Pax3-expressing domain.
To determine the effects of ectopic Nkx3.2 on dorsal somitic gene expression, we evaluated the expression of dermomyotomal markers Pax3 and Pax7 (Relaix et al., 2006). We observed disrupted expression of Pax3 and Pax7 in the dermomyotome following ectopic expression of Nkx3.2 (Fig.5C, panels 5-6). We are confident that ectopic Nkx3.2 was targeted into the dorsal Pax3 and Pax7-positive cell population, because Nkx3.2-expressing cells lie dorsal to the myosin-positive myotome cells (data not shown). Examination at higher magnification indicated that dorsal cells expressing Nkx3.2 (green) no longer expressed Pax3 and Pax7 (red) (Fig.5C, panels 7-8). In contrast, Pax3 and Pax7 expression were not perturbed in the dermomyotome of embryos electroporated with either GFP or Sox9 expression vehicles (Fig.5C, panels 1-4 and 9-12). Thus ectopic expression of Nkx3.2 was able to inhibit the expression of Pax3 and Pax7 in vivo, suggesting that Nkx3.2 prevents the adoption of dorsal somitic cell fates in vivo. Quantification of the immunostaining result following in ovo electroporation indicated that in cells electroporated with GFP alone, 76% expressed Pax3 and 80% expressed Pax7 (Fig. 5D). In contrast, in cells electroporated with Nkx3.2, only 3% expressed Pax3 and only 7% expressed Pax7. In cells electroporated wth Sox9V5, 51% expressed Pax3 and 66% expressed Pax7 (Fig.5D). Therefore, these in vivo results are consistent with our in vitro studies and together indicate that ectopic expression of Nkx3.2, but not Sox9, efficiently inhibits expression of both Pax3 and Pax7.
In addition, we evaluated whether ectopic expression of Nkx3.2 in the dorsal somite promotes these cells to adopt a ventral cell fate by examining the expression of the sclerotomal marker, Sox9. Following electroporation of Nkx3.2 into the dorsal somite, we observed expression of endogenous Sox9 around the notochord and in the neural tube and in addition, noted ectopic expression of Sox9 in dorsal somitic cells, indicating that Nkx3.2 had reprogrammed these dorsal cells to adopt a ventral cell fate (Fig.5E, arrow). In summary, we have found that in vivo expression of Nkx3.2 in dorsal somitic cells strongly inhibited expression of Pax3 and Pax7, and induced ectopic Sox9 expression.
High level of Wnt signaling inhibits Hedgehog-induced Nkx3.2 expression
Our findings indicate that forced expression of Nkx3.2 can repress the expression of the dermomyotomal markers Pax3 and Pax7 in vitro and in vivo. Therefore, in order for the somite to differentiate normally, Nkx3.2 expression has to be excluded from the dorsal domain. Since prior reports have indicated that Wnt proteins could inhibit the expression of the sclerotome marker Pax1 (Capdevila et al., 1998; Fan et al., 1997; Fan and Tessier-Lavigne, 1994), we investigated whether Wnt signals would similarly inhibit Nkx3.2 expression. Because in the presence of Wnt3a conditioned medium, Shh is still able to induce Nkx3.2 expression in cultured presomitic mesoderm (Fig.1A), we suspected that the Wnt signals in such conditioned medium may not be long-lived enough or of sufficient intensity to block sclerotomal gene expression. Therefore, to examine whether high levels of Wnt signaling could inhibit Nkx3.2 expression induced by Shh, we surrounded explants of presomitic mesoderm with aggregates of Wnt1-expressing cells and added soluble N-Shh (125ng/ml) to the cultures simultaneously. The explants were harvested after five days of culturing. As observed previously, Wnt signals alone failed to induce the expression of either dermomyotomal (i.e., Pax3) or myotomal (i.e., MyoD and Myf5) markers in presomitic mesoderm explants cultured for five days (Fig. 6A, lane 2). Intermediate level of Shh signal alone (125ng/ml) also failed to induce Pax3, Myf5 and MyoD, but strongly induced the expression of Nkx3.2 (Fig. 6A, lanes 3). In striking contrast, the combination of Shh and high levels of Wnt signals led to robust expression of Pax3, Myf5 and MyoD, and simultaneously inhibited the expression of Nkx3.2 (Fig. 6A, lane 4), demonstrating that Wnt signals inhibit the expression of Nkx3.2. Interestingly, we observed that co-culture of presomitic mesoderm with Wnt1-expressing cells did not blunt the induction of Patched2, indicating that Wnt signals did not attenuate the expression of all Shh responsive genes in these cultures (Fig. 6A, lane 4).
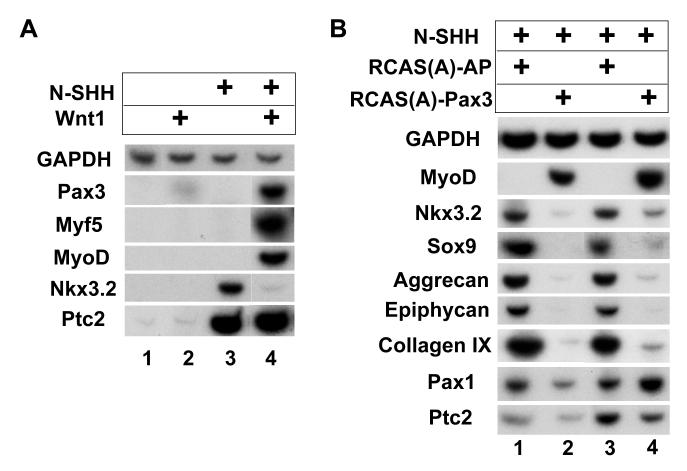
Figure 6. High level of Wnt signaling and Pax3 expression inhibit Nkx3.2 expression.
A. High level of Wnt signaling inhibits Nkx3.2 expression and promotes dorsal somite cell fates. RatB1a cells secreting Wnt1 were co-cultured with presomitic mesoderm explants of stage 10 chicken embryos in the presence or absence of N-SHH. Explants were harvested after 5 days culture for RT-PCR analysis of indicated genes. Lane 1, control RatB1a cells co-cultured with presomitic mesoderm explants. Lane 2, Wnt1-secreting RatB1a cells co-cultured with presomitic mesoderm explants. Lane 3, control RatB1a cells co-cultured with presomitic mesoderm explants in the presence of 250ng/ml N-SHH. Lane 4, Wnt1-secreting RatB1a cells co-cultured with presomitic mesoderm explants in the presence of 250ng/ml N-SHH. B. Forced expression of Pax3 inhibits the expression of Nkx3.2 and chondrogenic markers induced by N-SHH. Presomitic mesoderm explants were infected with avian retroviruses encoding either Pax3 or Alkaline phosphatase (AP, control). 500ng/ml N-SHH was present in all cultures. Explants were harvested after 5 days culture for RT-PCR analysis of indicated genes. Lanes 1 and 3, RCAS-A-AP infected explants. Lanes 2 and 4, RCAS-A-Pax3 infected explants.
We speculate that the co-expression of Pax3, MyoD, and Myf5 in these cultures (Fig. 6A, lane 4, as well as Fig.1A, lane 4) likely reflects a differential exposure of the somitic cells on the surface of the somite versus those in the interior of the somite to either Wnt, Shh, or both signals which manifests itself as a combination of cell fate responses to different levels of these signals. While this experimental set up does not allow for the equal exposure of all somitic cells to the same levels of Wnt/Shh signaling, it does mimic the in vivo situation, in which different somitic domains are differentially exposed to different levels of Wnt and Shh signals, and therefore adopt different fates. In summary, these results suggest that a high level of Wnt signals (from both dorsal neural tube and surface ectoderm) promote Shh-mediated expression of both dermomyotomal and myotomal markers at the expense of the sclerotomal marker Nkx3.2, and would thus act to restrict Nkx3.2 expression to the ventral domain of the somite. These findings are consistent with and extend prior work which has indicated that Wnt and Shh signals can act antagonistically to induce dermomyotomal (Pax3) versus sclerotomal (Pax1) gene expression, respectively (Borycki et al., 1998; Fan et al., 1995; Fan and Tessier-Lavigne, 1994; Johnson et al., 1994).
Forced expression of Pax3 blocks Shh-mediated Nkx3.2 expression and chondrogenesis
As discussed above, surrounding presomitic mesoderm with aggregates of Wnt1-expressing cells biases the response of the presomitic cells to Shh signals to promote Pax3 expression at the expense of the sclerotomal marker Nkx3.2. Because Pax3 is sufficient to induce myogenesis in somites (Maroto et al., 1997), we wondered whether the continuous expression of Pax3 was acting to simultaneously repress Shh-mediated induction of Nkx3.2 and other sclerotomal markers. To examine this issue, we infected presomitic mesoderm with retroviruses encoding either Pax3 (RCAS-Pax3) or alkaline phosphatase (RCAS-AP) as a control. We subsequently challenged these infected explants to activate sclerotomal/chondrocyte differentiation markers by the addition of N-Shh (250ng/ml). As noted previously (Maroto et al., 1997), infection of presomitic mesoderm with RCAS-Pax3 efficiently activated the expression of MyoD (Fig. 6B, lanes 2 and 4). While N-Shh induced the expression of both sclerotomal markers (i.e., Nkx3.2 and Sox9) and cartilage differentiation markers (i.e., aggrecan, epiphycan and collagen IX) in RCAS-AP infected cultures (Fig. 6B, lanes 1 and 3), the expression of both sclerotomal and cartilage markers were markedly repressed in the RCAS-Pax3 infected explants (Fig. 6B, lanes 2 and 4). In contrast, the expression of Patched2 and Pax1 in these cultures were either not affected or only slightly dampened by the expression of exogenous Pax3 (Fig. 6B, compare lanes 1 and 2, and lanes 3 and 4), suggesting that the effects of Pax3 were not due to attenuated Hedgehog signal transduction per se. These findings indicate that forced expression of Pax3 can both repress N-Shh mediated induction of a subset of sclerotomal markers (Nkx3.2 and Sox9) and block cartilage differentiation of presomitic mesoderm. The ability of forced Pax3 expression to block both sclerotome induction and cartilage differentiation is consistent with our findings that high levels of Wnt signaling (plus N-Shh) can both promote stable expression of Pax3 and similarly repress the induction of the sclerotomal marker Nkx3.2 (Fig. 6A).
Discussion
Shh acts in a morphogen-like manner to pattern dorsal-ventral cell fates in the somite
Our work suggests that a Shh gradient determines the dorsal-ventral pattern of cell fates within the somite (Fig.7). In the presence of a constant amount of Wnt signals, increasing levels of Shh induce a graded response in somitic cells; low levels of Shh promote the expression of the dermomyotomal marker Pax3, intermediate levels of Shh induce Myf5 and MyoD expression with a concomitant loss of Pax3 expression, and higher levels of Shh inhibit the expression of dermomyotomal/myotomal markers while promoting the expression of sclerotomal markers. Superimposed on this graded response to Shh signals, our findings also suggest that the relative level of Wnt signaling can modify the response of somitic cells to Shh signals; with high levels of Wnt signaling acting to both repress the induction of sclerotomal genes while promoting the induction of both dermomyotomal and myotomal gene expression. Our work suggests that a Shh concentration gradient works in a morphogen-like fashion to differentially pattern dorsal-ventral somitic fates. A morphogen is defined as a signaling molecule that acts directly on a cohort of equivalent cells to elicit specific responses depending on its concentration (Gurdon and Bourillot, 2001). Our in vitro analysis of somites cultured for several days in constant levels of soluble Wnt3a plus increasing levels of Shh has demonstrated that increasing thresholds of Shh sequentially promote dermomyotomal, myotomal and sclerotomal gene expression. Because our analysis of gene expression was performed after 5 days in culture it is possible that differing levels of Shh can modulate either the induction/maintenance of differential gene expression in these cultures or the differential survival/proliferation of distinct cell types. Indeed we noted that low levels of Shh are apparently not necessary for Wnt-mediated induction of Pax3, but only for the maintenance of Pax3 expression in these cultures. Prior work has indicated that a gradient of Shh protein is deposited within the developing somite (Gritli-Linde et al., 2001), and that mouse embryos that lack Smoothened, the receptor for hedgehog signaling, failed to express the sclerotomal markers Pax1 and Nkx3.2 and exhibited much reduced expression of the myotomal marker Myf5, suggesting that Shh acts directly on somitic cells to specify their cell fates (Zhang et al., 2001). Since Shh diffuses throughout the somite (Fan et al., 1995; Fan and Tessier-Lavigne, 1994) and acts directly on these cells, we propose that Shh acts in a morphogen-like fashion to specify different somitic cell fates by either inducing or maintaining the expression of dermomyotomal, myotomal and sclerotomal genes at relatively greater levels of Shh signaling, respectively. Indeed this is similar to the established role for Shh in patterning cell fates in the ventral neural tube (Ericson et al., 1996; Roelink et al., 1995; Tanabe et al., 1995).
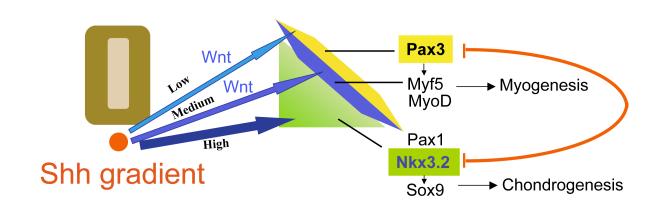
Figure 7. A gradient of Shh patterns the somite into mutually repressing muscle and cartilage cell fates.
Low levels of Shh promote the maintenance of Pax3 expression induced by Wnt signals. Medium levels of Shh, along with Wnt signals, promote the expression of the myotomal factors Myf5 and MyoD. High levels of Shh promote the expression of the sclerotomal factors Nkx3.2, Sox9 and Pax1, and simultaneously repress expression of both dermomyotomal and myotomal markers. Mutual repression by Pax3 and Nkx3.2 ensures that cells adopt either a dermomyotomal or sclerotomal cell fate. While Pax3 induces the expression of MyoD/Myf5 and promotes somitic myogenesis, Nkx3.2 and Sox9 promote somitic chondrogenesis.
We were surprised to find that low levels (as low as 34ng/ml) of Shh promoted Pax3 expression in the presomitic mesoderm in our long-term cultures (five days), even though Wnt signals alone are sufficient to induce the expression of Pax3 in somites cultured for only one day. In contrast to this apparent in vitro requirement for Shh signals to maintain somitic Pax3 expression, both Shh null mice embryos or chicken embryos with early notochord ablation display an expansion of Pax3 expression (Borycki et al., 1999; Chiang et al., 1996; Dietrich et al., 1997), suggesting that Shh negatively regulates Pax3 expression in the dermomyotome. In addition, loss of Hedgehog signaling in zebrafish embryos resulted in an overproduction of Pax3/Pax7-positive cells in the dermomyotome of the somite (Feng et al., 2006; Hammond et al., 2007), again suggesting that hedgehog signals are required to restrict the expression of these dermomyotomal Pax genes. In light of these in vivo findings, we suggest that the signals required to initiate versus maintain the expression of Pax3 may be distinct and that Shh signals may only be necessary to maintain either the expression of Pax3 or the survival and expansion of Pax3 expressing cells in explants of presomitic mesoderm that lack other signals that perform such functions in vivo. Indeed it is possible that Shh signals act to maintain the viability of Pax3 expressing presomitic mesodermal cells cultured in vitro, as is apparently the case for both myotomal cells (Borycki et al., 1999) and hypaxial limb muscle (Kruger et al., 2001) in vivo.
In the presence of Wnt3a conditioned medium, intermediate levels of Shh (67-250ng/ml), induced the loss of Pax3 expression concomitant with the induction of MyoD and Myf5. This finding is very reminiscent of the requirement for hedgehog signals to induce the differentiation of Pax3/Pax7-positive dermomyotomal cells in zebrafish (Feng et al., 2006; Hammond et al., 2007). Indeed, morpholino-based knockdown of MyoD and Myf5 in zebrafish is sufficient to enhance the expression of Pax3/7 (Hammond et al., 2007), indicating that these myogenic regulatory factors (MRFs) are necessary to repress the expression of these dermomyotomal Pax genes. Our finding that intermediate levels of Shh simultaneously promote the induction of MRFs and the loss of Pax3 expression is completely consistent with both these recent reports in zebrafish and with earlier findings in the chick indicating that ectopic Shh signals promote premature skeletal muscle differentiation of dermomyotomal cells (Amthor et al., 1999). A clear focus for the future will be to clarify the details of how hedgehog signaling leads to MRF induction in Pax3/7 expressing dermomyotomal cells and how the induction of MRFs leads to a reciprocal down-regulation of Pax3/7 expression. Intermediate levels of N-Shh, in addition to inducing the activation of MRF expression in somites cultured in Wnt3a conditioned medium, also induced the expression of sclerotomal markers in these cultures. While we were unable to address whether the same or different cells express sclerotomal versus myotomal markers in these cultures (due to the absence of good antibodies against chick sclerotomal markers), we think it is most likely that somitic cells in these cultures express only one program of gene expression and not both simultaneously (discussed below).
Finally, high levels of Shh (greater than 500ng/ml of N-Shh) led to the loss of all dermomyotomal and myotomal markers in presomitic mesoderm cultured in the presence of Wnt3a conditioned medium. In concert with the loss of all dermomyotomal and myotomal marker expression at these relatively high concentrations of Shh, expression of the sclerotomal markers Pax1, Nkx3.2 and Sox9 were maintained at these levels of Shh signaling. Prior to this work, Münsterberg et al demonstrated that increasing N-Shh concentrations up to 200ng/ml promoted the expression of both MyoD and Pax1 in cultured chick somites (Münsterberg et al., 1995). Our work is consistent with that report, and we have observed that even higher concentrations of Shh can act to repress MyoD gene expression while continuing to induce that of sclerotomal markers. Our finding is also consistent with in ovo notochord implantation in chick embryos (Dietrich et al., 1997). In such a study, heterotopic grafting of a notochord between the neural tube and the dorsal somite only induced MyoD expression in cells that were located a short distance away from the implant (Dietrich et al., 1997). In addition to noting a graded response of dermomyotomal, myotomal and sclerotomal markers to differing levels of Shh, we also observed that expression of various sclerotomal markers displayed a requirement for differing threshold levels of Shh. Expression of Pax1 was induced by a higher concentration of Shh as compared to that of Nkx3.2 and Sox9. Indeed, prior work has indicated that induction of the sclerotomal markers Pax1 and Twist1 in cultured mouse somites required different levels of Shh (Fan et al., 1995). A requirement for higher levels of Shh to induce Pax1 expression (compared to Nkx3.2 and Sox9) is consistent with the localized expression of Pax1 to the medial-ventral domain within the sclerotome, as compared to other sclerotome markers which are expressed more laterally (Christ et al., 2004; Johnson et al., 1994).
Nkx3.2 and Pax3 induce mutually repressive somitic cell fates
Somites are divided into dorsal and ventral domains with clear borders marked by differential gene expression, even before the morphological distinction of dermomyotome and sclerotome (See Fig. 2). Importantly, the expression of the dermomyotomal marker Pax3 is initially expressed throughout the dorsal-ventral extent of the presomitic mesoderm and its expression is extinguished in the ventral somite in response to Shh (Borycki et al., 1999; Chiang et al., 1996) concomitant with the activation of Nkx3.2 in this somitic domain (this work). In contrast, Sox9 and Pax3 are transiently co-expressed in the presomitic mesoderm throughout the dorsal-ventral axis. We have found that forced expression of the Shh-induced transcriptional repressor, Nkx3.2, can block the expression of Pax3 induced by either Wnt3a or signals from the surface ectoderm in explanted chicken somites without inducing cartilage differentiation in such cells. Furthermore, we have found that ectopic expression of Nkx3.2 in the dorsal somite was capable of inhibiting Pax3 and Pax7 expression in vivo. In contrast, forced expression of Sox9, which induced robust chondrocyte differentiation of somites, attenuated but did not efficiently inhibit Pax3 gene expression induced by either Wnt3a conditioned medium or signals from the surface ectoderm. These findings are consistent with the expression profile of Sox9 and Nkx3.2 in the embryo, and suggest that Shh restricts the expression of dermomyotomal genes such as Pax3 and Pax7 in part by inducing the expression of Nkx3.2 in the ventral somite (Murtaugh et al., 2001).
Forced Nkx3.2 expression can block the induction of dermomyotomal markers by Wnt signals without inducing cartilage differentiation in somite. The absence of cartilage differentiation in such explants may be a consequence of a Wnt-mediated repression of Sox9 gene expression (Day et al., 2005; Hill et al., 2005; Long, 2008; Rodda and McMahon, 2006). In prior work, we demonstrated that ectopic Nkx3.2 induces the expression of Sox9 in somites cultured in the absence of exogenous Wnt signals (Zeng et al., 2002). Apparently, Wnt3a prevents Nkx3.2 from inducing collagen II expression (this work) perhaps by promoting ß-catenin-mediated Sox9 degradation (Akiyama et al., 2004). In contrast, Wnt3a did not block chondrogenesis in RCAS-Sox9 infected explants, as exogenous Sox9 may be present at high enough levels to overcome this degradation. Furthermore, we showed that Nkx3.2 requires a higher level of BMP4 to promote chondrogenesis (this work and Zeng, 2002 #408). Thus, our findings suggest that induction of a ventral somitic cell fate by Nkx3.2 consists of two steps: (1) in the presence of high levels of Wnt signals, and a low level of BMP4, Nkx3.2 represses dorsal somite gene expression (i.e. Pax3) in the absence of inducing cartilage differentiation, and (2) in the absence of Wnt signaling and the presence of a higher level of BMP4, Nkx3.2 induces the chondrocyte differentiation program.
We and others have reported that Sox9 expression in the somite is induced by Sonic hedgehog (Shh) (Buttitta et al., 2003; Zeng et al., 2002). Although it is known that Shh acts through Gli proteins to induce Sox9 expression, it was not clear how this takes place. Our past work indicated that Nkx3.2, which is downstream of Pax1 and likely Gli proteins (Buttitta et al., 2003; Rodrigo et al., 2003), induced Sox9 expression in whole somite explants. However, our RT-PCR analysis did not exclude the possibility that Nkx3.2 selectively amplifies a population of ventral somitic sclerotomal precursor cells (Zeng et al., 2002). In our current work, we found that ectopic Nkx3.2 expression in the dorsal somite cells was able to induce Sox9 expression while repressing Pax3 and Pax7 expression. Therefore, our findings strongly suggest a role for Nkx3.2 in the initiation of Sox9 gene expression in cartilage progenitors in the embryo. This notion is consistent with a study in 10T1/2 cells, which suggested that Nkx3.2 promotes the expression of Sox9 during the chondrogenic differentiation of these cells (Lengner et al., 2005), and is also consistent with observations in Nkx3.2 null mouse embryos, where reduced Sox9 expression was observed (Tribioli and Lufkin, 1999).
While Nkx3.2 knockout mice embryos displayed reduced expression of Sox9 in the sclerotome, it is not known whether expression of dorsal somite markers such as Pax3 or Pax7 is found in more ventral positions in such animals (Akazawa et al., 2000; Lettice et al., 1999; Tribioli and Lufkin, 1999). Gene expression analysis in mice embryos lacking both Nkx3.2 and the closely related gene, Nkx3.1 (which is also transiently expressed in the sclerotome (Herbrand et al., 2002; Tanaka et al., 1999)), revealed that the expression of Myf5 is still restricted to the myotomal region in the developing somites of these mutant embryos and is not expressed in the ventral-most somitic domain in the combined absence of Nkx3.2 and Nkx3.1 (data not shown, L.Z., A. B. L., and Hans Arnold). Thus, it seems likely that other Shh-induced factors in addition to Nkx3.2 may also restrict expression of dermomyotomal and myotomal markers to the dorsal somite. Consistent with this idea, it has been reported that the Wnt-antagonist, Sfrp2, is also induced by Shh in the sclerotome and can prevent Wnt1 or Wnt4 from inducing Pax3 expression in mouse somites (Lee et al., 2000). In the chick, Sfrp2 has been reported to be expressed in the dermomyotome (Ladher et al., 2000), in the sclerotome (Lin et al., 2007), or in different somite compartments in different developmental stages (Terry et al., 2000), while Sfrp1 is expressed in the ventral somite (Terry et al., 2000). Thus, it is possible that Shh-mediated induction of Nkx3.2 and Sfrps in the sclerotome may act in parallel to block Wnt-mediated induction of dermomyotomal and myotomal markers in ventral regions of the somite.
Co-culture of somites with Wnt1-producing cells alters the response of somitic cells to Shh signals, promoting expression of the dermomyotomal/myotomal genes Pax3 and Myf5, and repressing the induction of Nkx3.2 and other sclerotomal markers (see also (Capdevila et al., 1998; Fan et al., 1997; Fan and Tessier-Lavigne, 1994)). Pax3 plays an essential role in somitic myogenesis, where it activates the expression of myogenic markers MyoD and Myf5 as well as FGF signaling (Brunelli et al., 2007; Buckingham and Relaix, 2007; Lagha et al., 2008; Maroto et al., 1997). Pax3 mutant embryos exhibit loss of muscle progenitors in the epaxial and hypaxial dermomyotome and fail to form limb muscle (Relaix et al., 2006; Relaix et al., 2005). We found that forced expression of the dermomyotomal marker Pax3 both promoted myogenesis and blocked Shh-induced expression of both Nkx3.2 and other markers of cartilage differentiation. While RT-PCR analysis of such cultures did not exclude the possibility that ectopic Pax3 induced a clonal expansion of muscle precursors in such somite explants, genetic evidence has indicated that the combined loss of both Pax3 and its homolog Pax7 resulted in either the apoptosis of dermomyotomal cells or the re-specification of these cells into collagen-II-expressing cartilage cells (Relaix et al., 2006). Thus, we propose that the stable expression of Pax3 or Pax7 results in the commitment of somitic cells to the dermomyotomal/myotomal cell fate and precludes the induction of both sclerotomal markers (Sox9 and Nkx3.2) and cartilage differentiation of these cells in response to Shh signals. Conversely, our results indicate that forced expression of Nkx3.2 induces a cell state that is incompatible with dermomyotomal/myotomal gene expression. Thus the stable induction of either Pax3/Pax7 or Nkx3.2 induce mutually repressing cell states that exclude the co-expression of both dermomyotomal/myotomal and sclerotomal markers in the same cell, and thereby ensures that somitic cells eventually become committed to give rise to either dermomyotomal/myotomal or sclerotomal derivatives but not both (Fig.7). A similar logic has apparently been employed to pattern the neural tube, where dorsally restricted Pax genes and ventrally restricted Nkx family members both mutually inhibit one another’s expression and promote either distinct dorsal or ventral neural tube cell fates, respectively (Briscoe et al., 2000).
Acknowledgements
We thank Dr. Tom Linsenmayer for an antibody against chicken type II Collagen. This work has been supported by grants to ABL from the NIH (AR048524; GM054879), and grants to LZ from the Arthritis National Research Foundation and from the NIH (AR054611).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akazawa H, Komuro I, Sugitani Y, Yazaki Y, Nagai R, Noda T. Targeted disruption of the homeobox transcription factor bapx1 results in lethal skeletal dysplasia with asplenia and gastroduodenal malformation [In Process Citation] Genes Cells. 2000;5:499–513. doi: 10.1046/j.1365-2443.2000.00339.x. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, Deng JM, Taketo MM, Nakamura T, Behringer RR, McCrea PD, de Crombrugghe B. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004;18:1072–87. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amthor H, Christ B, Patel K. A molecular mechanism enabling continuous embryonic muscle growth - a balance between proliferation and differentiation. Development. 1999;126:1041–53. doi: 10.1242/dev.126.5.1041. [DOI] [PubMed] [Google Scholar]
- Aoyama H, Asamoto K. Determination of somite cells: independence of cell differentiation and morphogenesis. Development. 1988;104:15–28. doi: 10.1242/dev.104.1.15. [DOI] [PubMed] [Google Scholar]
- Bailey P, Holowacz T, Lassar AB. The origin of skeletal muscle stem cells in the embryo and the adult. Curr Opin Cell Biol. 2001;13:679–89. doi: 10.1016/s0955-0674(00)00271-4. [DOI] [PubMed] [Google Scholar]
- Bajard L, Relaix F, Lagha M, Rocancourt D, Daubas P, Buckingham ME. A novel genetic hierarchy functions during hypaxial myogenesis: Pax3 directly activates Myf5 in muscle progenitor cells in the limb. Genes Dev. 2006;20:2450–64. doi: 10.1101/gad.382806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi W, Huang W, Whitworth DJ, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc Natl Acad Sci U S A. 2001;98:6698–703. doi: 10.1073/pnas.111092198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borello U, Berarducci B, Murphy P, Bajard L, Buffa V, Piccolo S, Buckingham M, Cossu G. The Wnt/beta-catenin pathway regulates Gli-mediated Myf5 expression during somitogenesis. Development. 2006;133:3723–32. doi: 10.1242/dev.02517. [DOI] [PubMed] [Google Scholar]
- Borycki AG, Brunk B, Tajbakhsh S, Buckingham M, Chiang C, Emerson CP., Jr. Sonic hedgehog controls epaxial muscle determination through Myf5 activation. Development. 1999;126:4053–63. doi: 10.1242/dev.126.18.4053. [DOI] [PubMed] [Google Scholar]
- Borycki AG, Mendham L, Emerson CP., Jr. Control of somite patterning by Sonic hedgehog and its downstream signal response genes. Development. 1998;125:777–90. doi: 10.1242/dev.125.4.777. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Pierani A, Jessell TM, Ericson J. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell. 2000;101:435–45. doi: 10.1016/s0092-8674(00)80853-3. [DOI] [PubMed] [Google Scholar]
- Brunelli S, Relaix F, Baesso S, Buckingham M, Cossu G. Beta catenin-independent activation of MyoD in presomitic mesoderm requires PKC and depends on Pax3 transcriptional activity. Dev Biol. 2007;304:604–14. doi: 10.1016/j.ydbio.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Buckingham M, Relaix F. The role of Pax genes in the development of tissues and organs: Pax3 and Pax7 regulate muscle progenitor cell functions. Annu Rev Cell Dev Biol. 2007;23:645–73. doi: 10.1146/annurev.cellbio.23.090506.123438. [DOI] [PubMed] [Google Scholar]
- Buttitta L, Mo R, Hui CC, Fan CM. Interplays of Gli2 and Gli3 and their requirement in mediating Shh-dependent sclerotome induction. Development. 2003;130:6233–43. doi: 10.1242/dev.00851. [DOI] [PubMed] [Google Scholar]
- Capdevila J, Tabin C, Johnson RL. Control of dorsoventral somite patterning by Wnt-1 and beta-catenin. Dev Biol. 1998;193:182–94. doi: 10.1006/dbio.1997.8806. [DOI] [PubMed] [Google Scholar]
- Chen AE, Ginty DD, Fan CM. Protein kinase A signalling via CREB controls myogenesis induced by Wnt proteins. Nature. 2005;433:317–22. doi: 10.1038/nature03126. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–13. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Christ B, Brand-Saberi B, Grim M, Wilting J. Local signalling in dermomyotomal cell type specification. Anat Embryol (Berl) 1992;186:505–10. doi: 10.1007/BF00185464. [DOI] [PubMed] [Google Scholar]
- Christ B, Huang R, Scaal M. Formation and differentiation of the avian sclerotome. Anat Embryol (Berl) 2004;208:333–50. doi: 10.1007/s00429-004-0408-z. [DOI] [PubMed] [Google Scholar]
- Christ B, Huang R, Wilting J. The development of the avian vertebral column. Anat Embryol (Berl) 2000;202:179–94. doi: 10.1007/s004290000114. [DOI] [PubMed] [Google Scholar]
- Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–50. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Dietrich S, Schubert FR, Lumsden A. Control of dorsoventral pattern in the chick paraxial mesoderm. Development. 1997;124:3895–908. doi: 10.1242/dev.124.19.3895. [DOI] [PubMed] [Google Scholar]
- Dockter J, Ordahl CP. Dorsoventral axis determination in the somite: a re-examination. Development. 2000;127:2201–6. doi: 10.1242/dev.127.10.2201. [DOI] [PubMed] [Google Scholar]
- Ericson J, Morton S, Kawakami A, Roelink H, Jessell TM. Two critical periods of Sonic Hedgehog signaling required for the specification of motor neuron identity. Cell. 1996;87:661–73. doi: 10.1016/s0092-8674(00)81386-0. [DOI] [PubMed] [Google Scholar]
- Fan CM, Lee CS, Tessier-Lavigne M. A role for WNT proteins in induction of dermomyotome. Dev Biol. 1997;191:160–5. doi: 10.1006/dbio.1997.8713. [DOI] [PubMed] [Google Scholar]
- Fan CM, Porter JA, Chiang C, Chang DT, Beachy PA, Tessier-Lavigne M. Long-range sclerotome induction by sonic hedgehog: direct role of the amino-terminal cleavage product and modulation by the cyclic AMP signaling pathway. Cell. 1995;81:457–65. doi: 10.1016/0092-8674(95)90398-4. [DOI] [PubMed] [Google Scholar]
- Fan CM, Tessier-Lavigne M. Patterning of mammalian somites by surface ectoderm and notochord: evidence for sclerotome induction by a hedgehog homolog. Cell. 1994;79:1175–86. doi: 10.1016/0092-8674(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Feng X, Adiarte EG, Devoto SH. Hedgehog acts directly on the zebrafish dermomyotome to promote myogenic differentiation. Dev Biol. 2006;300:736–46. doi: 10.1016/j.ydbio.2006.08.056. [DOI] [PubMed] [Google Scholar]
- Geetha-Loganathan P, Nimmagadda S, Huang R, Christ B, Scaal M. Regulation of ectodermal Wnt6 expression by the neural tube is transduced by dermomyotomal Wnt11: a mechanism of dermomyotomal lip sustainment. Development. 2006;133:2897–904. doi: 10.1242/dev.02464. [DOI] [PubMed] [Google Scholar]
- Geetha-Loganathan P, Nimmagadda S, Prols F, Patel K, Scaal M, Huang R, Christ B. Ectodermal Wnt-6 promotes Myf5-dependent avian limb myogenesis. Dev Biol. 2005;288:221–33. doi: 10.1016/j.ydbio.2005.09.035. [DOI] [PubMed] [Google Scholar]
- Gritli-Linde A, Lewis P, McMahon AP, Linde A. The whereabouts of a morphogen: direct evidence for short- and graded long-range activity of hedgehog signaling peptides. Dev Biol. 2001;236:364–86. doi: 10.1006/dbio.2001.0336. [DOI] [PubMed] [Google Scholar]
- Gros J, Manceau M, Thome V, Marcelle C. A common somitic origin for embryonic muscle progenitors and satellite cells. Nature. 2005;435:954–8. doi: 10.1038/nature03572. [DOI] [PubMed] [Google Scholar]
- Gurdon JB, Bourillot PY. Morphogen gradient interpretation. Nature. 2001;413:797–803. doi: 10.1038/35101500. [DOI] [PubMed] [Google Scholar]
- Gustafsson MK, Pan H, Pinney DF, Liu Y, Lewandowski A, Epstein DJ, Emerson CP., Jr. Myf5 is a direct target of long-range Shh signaling and Gli regulation for muscle specification. Genes Dev. 2002;16:114–26. doi: 10.1101/gad.940702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond CL, Hinits Y, Osborn DP, Minchin JE, Tettamanti G, Hughes SM. Signals and myogenic regulatory factors restrict pax3 and pax7 expression to dermomyotome-like tissue in zebrafish. Dev Biol. 2007;302:504–21. doi: 10.1016/j.ydbio.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbrand H, Pabst O, Hill R, Arnold H-H. Transcription factors Nkx3.1 and Nkx3.2 (Bapx1) play an overlapping role in sclerotome development of the mouse. Mech. of Dev. 2002;117:217–224. doi: 10.1016/s0925-4773(02)00207-1. [DOI] [PubMed] [Google Scholar]
- Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8:727–38. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Hirsinger E, Jouve C, Dubrulle J, Pourquie O. Somite formation and patterning. Int Rev Cytol. 2000;198:1–65. doi: 10.1016/s0074-7696(00)98002-1. [DOI] [PubMed] [Google Scholar]
- Holowacz T, Zeng L, Lassar AB. Asymmetric localization of numb in the chick somite and the influence of myogenic signals. Dev Dyn. 2006;235:633–45. doi: 10.1002/dvdy.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James RG, Schultheiss TM. Patterning of the avian intermediate mesoderm by lateral plate and axial tissues. Dev Biol. 2003;253:109–24. doi: 10.1006/dbio.2002.0863. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Laufer E, Riddle RD, Tabin C. Ectopic expression of Sonic hedgehog alters dorsal-ventral patterning of somites. Cell. 1994;79:1165–73. doi: 10.1016/0092-8674(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Kruger M, Mennerich D, Fees S, Schafer R, Mundlos S, Braun T. Sonic hedgehog is a survival factor for hypaxial muscles during mouse development. Development. 2001;128:743–52. doi: 10.1242/dev.128.5.743. [DOI] [PubMed] [Google Scholar]
- Krull CE. A primer on using in ovo electroporation to analyze gene function. Dev Dyn. 2004;229:433–9. doi: 10.1002/dvdy.10473. [DOI] [PubMed] [Google Scholar]
- Ladher R, Church V, Allen S, Robson L, Abdelfattah A, Brown N, Hattersley G, Rosen V, Luyten F, Dale L, Francis-West PL. Cloning and expression of the Wnt antagonists Sfrp-2 and Frzb during chick development. PG - 183-98. Dev. Biol. 2000;218:183–198. doi: 10.1006/dbio.1999.9586. [DOI] [PubMed] [Google Scholar]
- Lagha M, Kormish JD, Rocancourt D, Manceau M, Epstein JA, Zaret KS, Relaix F, Buckingham ME. Pax3 regulation of FGF signaling affects the progression of embryonic progenitor cells into the myogenic program. Genes Dev. 2008;22:1828–37. doi: 10.1101/gad.477908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Buttitta LA, May NR, Kispert A, Fan CM. SHH-N upregulates Sfrp2 to mediate its competitive interaction with WNT1 and WNT4 in the somitic mesoderm. Development. 2000;127:109–18. doi: 10.1242/dev.127.1.109. [DOI] [PubMed] [Google Scholar]
- Lefebvre V, Huang W, Harley VR, Goodfellow PN, de Crombrugghe B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol Cell Biol. 1997;17:2336–46. doi: 10.1128/mcb.17.4.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V, Smits P. Transcriptional control of chondrocyte fate and differentiation. Birth Defects Res C Embryo Today. 2005;75:200–12. doi: 10.1002/bdrc.20048. [DOI] [PubMed] [Google Scholar]
- Lengner CJ, Hassan MQ, Serra RW, Lepper C, van Wijnen AJ, Stein JL, Lian JB, Stein GS. Nkx3.2-mediated repression of Runx2 promotes chondrogenic differentiation. J Biol Chem. 2005;280:15872–9. doi: 10.1074/jbc.M411144200. [DOI] [PubMed] [Google Scholar]
- Lettice LA, Purdie LA, Carlson GJ, Kilanowski F, Dorin J, Hill RE. The mouse bagpipe gene controls development of axial skeleton, skull, and spleen. Proc Natl Acad Sci USA. 1999;96:9695–700. doi: 10.1073/pnas.96.17.9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CT, Lin YT, Kuo TF. Investigation of mRNA expression for secreted frizzled-related protein 2 (sFRP2) in chick embryos. J Reprod Dev. 2007;53:801–10. doi: 10.1262/jrd.18081. [DOI] [PubMed] [Google Scholar]
- Long F. Targeting intercellular signals for bone regeneration from bone marrow mesenchymal progenitors. Cell Cycle. 2008;7:2106–11. doi: 10.4161/cc.7.14.6257. [DOI] [PubMed] [Google Scholar]
- Maroto M, Reshef R, Munsterberg AE, Koester S, Goulding M, Lassar AB. Ectopic Pax-3 activates MyoD and Myf-5 expression in embryonic mesoderm and neural tissue. Cell. 1997;89:139–48. doi: 10.1016/s0092-8674(00)80190-7. [DOI] [PubMed] [Google Scholar]
- Marti E, Bumcrot DA, Takada R, McMahon AP. Requirement of 19K form of Sonic hedgehog for induction of distinct ventral cell types in CNS explants. Nature. 1995;375:322–5. doi: 10.1038/375322a0. [DOI] [PubMed] [Google Scholar]
- Martinelli DC, Fan CM. Gas1 extends the range of Hedgehog action by facilitating its signaling. Genes Dev. 2007;21:1231–43. doi: 10.1101/gad.1546307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott A, Gustafsson M, Elsam T, Hui CC, Emerson CP, Jr., Borycki AG. Gli2 and Gli3 have redundant and context-dependent function in skeletal muscle formation. Development. 2005;132:345–57. doi: 10.1242/dev.01537. [DOI] [PubMed] [Google Scholar]
- Murtaugh LC, Zeng L, Chyung JH, Lassar AB. The chick transcriptional repressor Nkx3.2 acts downstream of Shh to promote BMP-dependent axial chondrogenesis. Developmental Cell. 2001;1:411–422. doi: 10.1016/s1534-5807(01)00039-9. [DOI] [PubMed] [Google Scholar]
- Münsterberg AE, Kitajewski J, Bumcrot DA, McMahon AP, Lassar AB. Combinatorial signaling by Sonic hedgehog and Wnt family members induces myogenic bHLH gene expression in the somite. Genes Dev. 1995;9:2911–22. doi: 10.1101/gad.9.23.2911. [DOI] [PubMed] [Google Scholar]
- Otto A, Schmidt C, Patel K. Pax3 and Pax7 expression and regulation in the avian embryo. Anat Embryol (Berl) 2006;211:293–310. doi: 10.1007/s00429-006-0083-3. [DOI] [PubMed] [Google Scholar]
- Pearse RV, 2nd, Vogan KJ, Tabin CJ. Ptc1 and Ptc2 transcripts provide distinct readouts of Hedgehog signaling activity during chick embryogenesis. Dev Biol. 2001;239:15–29. doi: 10.1006/dbio.2001.0430. [DOI] [PubMed] [Google Scholar]
- Pourquie O. The chick embryo: a leading model in somitogenesis studies. Mech Dev. 2004;121:1069–79. doi: 10.1016/j.mod.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Relaix F, Montarras D, Zaffran S, Gayraud-Morel B, Rocancourt D, Tajbakhsh S, Mansouri A, Cumano A, Buckingham M. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol. 2006;172:91–102. doi: 10.1083/jcb.200508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F, Rocancourt D, Mansouri A, Buckingham M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005;435:948–53. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- Reshef R, Maroto M, Lassar AB. Regulation of dorsal somitic cell fates: BMPs and Noggin control the timing and pattern of myogenic regulator expression. Genes Dev. 1998;12:290–303. doi: 10.1101/gad.12.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133:3231–44. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- Rodrigo I, Hill RE, Balling R, Munsterberg A, Imai K. Pax1 and Pax9 activate Bapx1 to induce chondrogenic differentiation in the sclerotome. Development. 2003;130:473–82. doi: 10.1242/dev.00240. [DOI] [PubMed] [Google Scholar]
- Roelink H, Porter JA, Chiang C, Tanabe Y, Chang DT, Beachy PA, Jessell TM. Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of sonic hedgehog autoproteolysis. Cell. 1995;81:445–55. doi: 10.1016/0092-8674(95)90397-6. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Stoeckelhuber M, McKinnell I, Putz R, Christ B, Patel K. Wnt 6 regulates the epithelialisation process of the segmental plate mesoderm leading to somite formation. Dev Biol. 2004;271:198–209. doi: 10.1016/j.ydbio.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Schubert FR, Tremblay P, Mansouri A, Faisst AM, Kammandel B, Lumsden A, Gruss P, Dietrich S. Early mesodermal phenotypes in splotch suggest a role for Pax3 in the formation of epithelial somites. Dev Dyn. 2001;222:506–21. doi: 10.1002/dvdy.1211. [DOI] [PubMed] [Google Scholar]
- Sekiya I, Tsuji K, Koopman P, Watanabe H, Yamada Y, Shinomiya K, Nifuji A, Noda M. SOX9 enhances aggrecan gene promoter/enhancer activity and is up-regulated by retinoic acid in a cartilage-derived cell line, TC6. J Biol Chem. 2000;275:10738–44. doi: 10.1074/jbc.275.15.10738. [DOI] [PubMed] [Google Scholar]
- Stockdale FE, Nikovits W, Jr., Christ B. Molecular and cellular biology of avian somite development. Dev Dyn. 2000;219:304–21. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1057>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S. Stem cells to tissue: molecular, cellular and anatomical heterogeneity in skeletal muscle. Curr Opin Genet Dev. 2003;13:413–22. doi: 10.1016/s0959-437x(03)00090-x. [DOI] [PubMed] [Google Scholar]
- Tanabe Y, Roelink H, Jessell TM. Induction of motor neurons by Sonic hedgehog is independent of floor plate differentiation. Curr Biol. 1995;5:651–8. doi: 10.1016/s0960-9822(95)00130-8. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Lyons GE, Izumo S. Expression of the Nkx3.1 homobox gene during pre and postnatal development. Mech Dev. 1999;85:179–82. doi: 10.1016/s0925-4773(99)00084-2. [DOI] [PubMed] [Google Scholar]
- Teillet M, Watanabe Y, Jeffs P, Duprez D, Lapointe F, Le Douarin NM. Sonic hedgehog is required for survival of both myogenic and chondrogenic somitic lineages. Development. 1998;125:2019–30. doi: 10.1242/dev.125.11.2019. [DOI] [PubMed] [Google Scholar]
- Terry K, Magan H, Baranski M, Burrus LW. Sfrp-1 and sfrp-2 are expressed in overlapping and distinct domains during chick development. Mech Dev. 2000;97:177–82. doi: 10.1016/s0925-4773(00)00407-x. [DOI] [PubMed] [Google Scholar]
- Tribioli C, Lufkin T. The murine Bapx1 homeobox gene plays a critical role in embryonic development of the axial skeleton and spleen. Development. 1999;126:5699–711. doi: 10.1242/dev.126.24.5699. [DOI] [PubMed] [Google Scholar]
- Wagner J, Schmidt C, Nikowits W, Jr., Christ B. Compartmentalization of the somite and myogenesis in chick embryos are influenced by wnt expression. Dev Biol. 2000;228:86–94. doi: 10.1006/dbio.2000.9921. [DOI] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–52. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Williams BA, Ordahl CP. Pax-3 expression in segmental mesoderm marks early stages in myogenic cell specification. Development. 1994;120:785–796. doi: 10.1242/dev.120.4.785. [DOI] [PubMed] [Google Scholar]
- Zeng L, Kempf H, Murtaugh LC, Sato ME, Lassar AB. Shh establishes an Nkx3.2/Sox9 autoregulatory loop that is maintained by BMP signals to induce somitic chondrogenesis. Genes Dev. 2002;16:1990–2005. doi: 10.1101/gad.1008002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XM, Ramalho-Santos M, McMahon AP. Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R symmetry by the mouse node. Cell. 2001;106:781–92. [PubMed] [Google Scholar]