Abstract
Objectives
Laboratory tests are now being used to identify seropositive cases in patients suspected of having a Lyme borreliosis (LB) infection. From 2005 to 2009, we analyzed the serological and epidemiological characteristics of 53 LB positive cases in Korea using immunoblot assay.
Methods
During the five-year study period, a total of 1897 serum samples from suspected LB cases were referred to us for further laboratory diagnosis. The bacterial strains Borrelia afzeli pKo, Borrelia garinii 935T and Borrelia burgdorferi B31 were used for indirect immunofluorescent antibody assay. Immunoblot assay was performed using the recomBlot Borrelia.
Results
Based on the information from the clinicians, the main symptoms of LB infection were rash and fever (66.0%), neurological symptoms (30.2%), and arthritis (5.7%). Of the 53 cases, 16 (30.2%) were infected abroad and the remaining 37 cases (69.8%) were suspected to have been infected in Korea. Immunoblot assays detected high levels of the antigens p41 (FlaB) of B. burgdorferi and OspC of B. garinii in infected samples.
Conclusions
The causative bacteria of LB were not isolated from humans yet but from vector ticks and rodents in Korea, and a few cases were reported with serological diagnosis. Our results suggest that LB is present in all areas of Korea and indicate that B. garinii and B. burgdorferi may be the predominant bacteria in patients with LB. However, further studies are needed to isolate and identify the causative bacteria for LB in patients.
Keywords: Borrelia, Borreliosis, immunoblot, serology
1. Introduction
Lyme borreliosis (LB) is an infectious disease transmitted by Ixodes spp. ticks and caused by the spirochete bacteria Borrelia burgdorferi sensu lato. These bacteria are classified into three human pathogens, B. burgdorferi sensu stricto, Borrelia afzelii and Borrelia garinii. It has been reported that the antigenicity and pathogenicity of LB varies depending on the region [1,2].
In 1993, B. burgdorferi and B. garinii were isolated from Ixodes persulcatus and Apodemus agarius captured in the Chungcheongbuk and Gangwon provinces in Korea [3]. B. burgdorferi and B. afzelii were also isolated from I. persulcatus, I. nipponesis, I. granulatus and A. agarius captured in Gangwon and Jeollanam provinces [3]. The causative bacteria have not yet been isolated from humans in Korea; however, clinical cases for which a diagnosis was made based on serological and molecular findings have recently been reported [4].
An initial symptom of LB is erythema chronicum migrans, a rash formed at the site of the tick bite. Other symptoms, such as fever, headache, myalgia and lethargy, are persistently present for several weeks. In the absence of prompt antibiotic treatment, these symptoms can lead to chronic myalgia, arthritis and impaired visual acuity [1,2].
LB is characterized by the variability of clinical symptoms and incidences. For a confirmatory diagnosis, both clinical diagnosis and laboratory tests are required. Laboratory tests for LB include indirect immunofluorescent antibody assay (IFA) and immunoblot assay. Immunoblot assay is also used to make a confirmatory diagnosis in areas where LB is not an endemic disease or where its incidence is relatively low [1,5]. In this study, we used immunoblot assay to analyze the serological and epidemiological characteristics of 53 positive LB cases reported in Korea between 2005 and 2009.
2. Materials and Methods
2.1. Sera
During the period from 2005 to 2009, when a LB case was suspected, serum samples were referred to us for further laboratory diagnosis. Detailed information on an important clinical marker for LB and on other characteristic manifestations was obtained from the attending clinicians.
2.2. Bacterial strains and culture media
The bacterial strains B. afzeli pKo, B. garinii 935T, and B. burgdorferi B31 were cultured in BSK (Barbour-Stoenner-Kelly) -II medium (Sigma, USA) for 7–14 days. The bacteria were harvested by centrifugation and washed three times in PBS (pH 7.2). The bacteria were suspended at a concentration of 2 × 108 cell/mL and used to prepare slides for IFA.
2.3. Indirect immunofluorescent antibody assay
The concentration of the cultured bacterial strains was adjusted to approximately 2 × 106 cell/10 μL. These bacterial strains were evenly distributed to an 18-well spot slide glass and then dried. Following a 10-minute fixation using acetone, the slides were preserved at −70°C until required.
The test serum was diluted by serial 2-fold dilutions from a titer of 1:16 to 1:1024. 20 μL of the diluted serum was placed in each well of an 18-well spot slide glass and the slides were incubated in a humidified chamber at 37°C for 30 minutes. The wells were then washed twice with PBS (pH 7.2) and once with distilled water. FITC-conjugated anti-human immunoglobulin (MP Biomedicals, Ohio, USA) was added and the slides were incubated in a humidified chamber at 37°C for 30 minutes. Using the same methods, the slides were washed and then dried. The slides were treated with a mounting solution to prepare them for microscopy. Positive samples were defined as those in which the IgM titer was ≥1:16 or the IgG titer was ≥1:256. Samples with positive readings were subjected to immunoblot assay.
2.4. Immunoblot assay
Immunoblot assay was performed using the recomBlot Borrelia test (Mikrogen, Neuried, Germany) according to the manufacturer’s instructions. The test serum was diluted using the reactant solution to a ratio of 1:200 for IgG and 1:10 for IgM. The reaction was performed at room temperature for 10–16 hours. The strips were washed four times and then treated with a peroxidase conjugate. When the color developed, the strip was examined and the results were determined. The recomBlot Borrelia immunoblot test can detect IgG and IgM antibodies directed against B. burgdorferi using recombinant antigens of B. burgdorferi, B. afzelii and B. garinii like p100, VlsE, p39, OspA, OspC, p41 and p18. Scores that depend on the presence of antibodies for each antigen are calculated. Scores of <4 points were defined as negative, scores between 5 and 6 points were border-line and scores >7 points were accepted as positive.
3. Results
Between 2005 and 2009, a total of 1897 serum samples from suspected LB cases were referred to us for further laboratory diagnosis. The number of tested serum samples and the number of seropositive cases are described in Figure 1. Between 2005 and 2009, the number of suspected cases of LB (212, 226, 360, 434, and 665) increased annually. The number of confirmed positive cases was 2, 3, 23, 9, and 16, over the five years studied. The accumulated monthly occurrence of confirmed LB cases for the five years from 2005 to 2009 is shown in Figure 2. The highest number of confirmed cases of LB was found in May, and no cases were seen in January. The age and gender distribution of LB positive cases is described in Table 1. Regarding these cases, 50.9% were male and the 30–39 years age group had the highest proportion.
Figure 1.
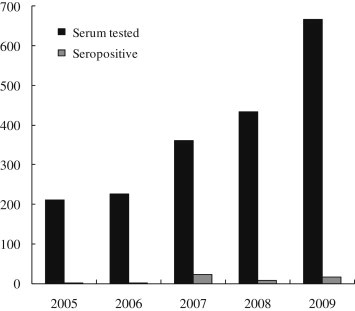
Annual distribution (from 2005 to 2009) of the number of serum samples from Lyme borreliosis suspected case and the number that tested seropositive for Lyme borreliosis.
Figure 2.
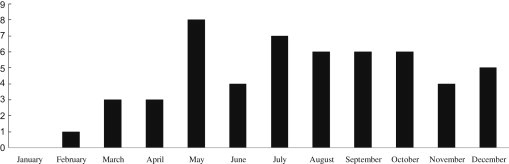
Accumulated monthly distribution of confirmed Lyme borreliosis positive cases for the five years from 2005 to 2009.
Table 1.
Distribution of Lyme borreliosis positive cases by gender and age, 2005–2009
| No. of positive | Percent (%) | |
|---|---|---|
| Gender | ||
| Male | 27 | 50.9 |
| Female | 26 | 49.1 |
| Age (in yr) | ||
| NAa | 6 | 11.3 |
| >20 | 2 | 3.8 |
| 20–29 | 7 | 13.2 |
| 30–39 | 15 | 28.3 |
| 40–49 | 9 | 17.0 |
| 50–59 | 7 | 13.2 |
| 60–69 | 5 | 9.4 |
| ≤70 | 2 | 3.8 |
NA = data not available.
Based on the clinicians’ information about cases, 16 of 53 cases (30.2%) were identified as having been infected outside Korea; seven of them (13.2%) were infected in America, more than in any other country. The remaining 37 cases (69.8%) were thought to have been infected in Korea, because there was no history of travel abroad. The regional distribution of the 37 domestic cases of LB is shown in Figure 3. With 12 cases, Seoul had the highest number of cases. The distribution in the provinces of Geyonggi, Gangwon, Jeollabuk, Chungcheongbuk, Chungcheongnam, Gyeongsangbuk and Gyeongsangnam, ranged from one to seven. Thus, it appears that LB is present in many areas of Korea. Twenty cases (37.3%) were known to have been bitten by a tick; 15 cases (75.0%) were infected abroad and five cases (25%) were bitten in Korea. The serologically diagnosed positive cases presented with various symptoms that included fever, fatigue, vasculitis, arthritis, neurological symptoms and meningitis; however, the main symptoms were rash and fever (66.0%), neurological involvement (30.2%), and arthritis (5.7%).
Figure 3.
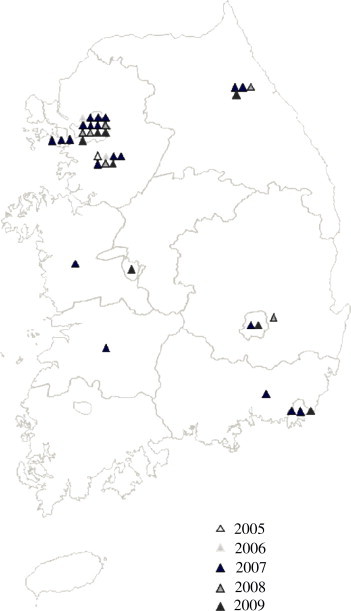
Regional distribution of the 37 domestic Lyme borreliosis positive cases, 2005–2009. The color of the triangle indicates the year.
We analyzed the characteristics of the LB positive sera using an immunoblot assay and compared them to the main symptoms (Table 2). An immunoblot assay of IgG and IgM showed that antigen p41 (FlaB) of B. burgdorferi was detected at the highest frequency, the next highest was antigen OspC of B. garinii and then p100. For IgM, in early infection cases with symptoms like rashes and fever, antigen p41 of B. burgdorferi was detected at the highest frequency, followed by OspC of B. garinii. Antigen p41 of B. burgdorferi was also detected at the highest frequency in cases with neurological symptoms. Thus, these cases were presumed to be infected with B. burgdorferi and B. garinii. For cases with chronic symptoms, a comparison of the results from the immunoblot assay could not be made, because there was not enough data to determine the causative pathogen.
Table 2.
A comparison of the symptoms with the immunoblot IgG and IgM positive categories from Lyme borreliosis positive cases
| Symptoms | No. of positive | Rates of positivity | p100b | VlsEb | p41/bb | p39b | OspAb | OspCa,b |
p41/gb | p41/afb | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g | b/af | g | ||||||||||
| Skin rash, fever, pain | 35 | 66.0 | 5, 14 | 8, 16 | 19, 23 | 3, 5 | 4, 4 | 5, 20 | 6, 13 | 5, 12 | 7, 12 | 5, 4 |
| Neuropathy, paralysis, meningoencephalitis | 15 | 28.3 | 3, 4 | 1, 3 | 5, 11 | 0, 1 | 3, 1 | 4, 5 | 3, 2 | 3, 4 | 4, 4 | 5, 3 |
| Arthritis, knee pain, swelling | 3 | 5.7 | 0, 1 | 0, 1 | 1, 1 | 0, 0 | 0, 0 | 0, 1 | 1, 1 | 1, 1 | 0, 1 | 0, 0 |
| Total | 53 | 100 | 30 | 22 | 43 | 10 | 12 | 34 | 21 | 23 | 23 | 16 |
Genus specific recombinant antigens; g = B. garinii; b = B. burgdorferi; af = B. afzelii;
The numbers in the antigen column indicate the number of cases in which IgG and IgM, separated by a comma, were detected.
4. Discussion
In the 1990s, the level of antibody titer for LB in cases with fever was investigated and antibody-positive findings were found in 8.1–9.2% of total cases. These results suggested the possible presence of LB in Korea [6,7]. Comparative studies have also been conducted to examine the seasonal endemic characteristics seen in May and December and to examine diagnostic regimens. The symptoms of Korean LB cases and their antigenic characteristics have also been reported [6]. The characteristics of positive LB cases in this study were similar to the characteristics reported in previous studies.
Immunoblot assay results for the early infectious stage of LB have shown that the p41 and OspC antigens are the most powerful immunogens [1]. With the progression of the disease, the OspA and OspB antigens have been reported to be powerful immunogens in the late stage of infection. In this study, the OspC and p41 antigen bands were predominantly observed for IgM positive results. Further laboratory evaluations are required for the early stage of LB. In Korea, it is imperative that, to confirm the infection, suspected cases are tested using diagnostic antibody titer and culture tests in the early stage of infection. For the isolation and identification of Borrelia spp., it is essential that, prior to antibiotic treatment, blood samples of suspected cases are referred for diagnosis based on the laboratory tests.
LB occurs worldwide, and it is one of the most common etiologies of vector infections developed by bacteria. The distribution of Borrelia spp. in ticks and rodents captured in Gangwon, Jeollanam and Chungcheongbuk provinces has been examined and bacterial strains such as B. burgdorferi and B. afzeli have been isolated [3,8]. In Korea, the isolation of Borrelia spp. from humans has not yet been reported; however, based on cases of LB diagnosed both serologically and genetically, the current status of the isolation of pathogenic bacteria from the vector and the increased number of cases referred to us for further laboratory diagnosis, we assume that the number of ticks infected with LB is increasing. Our diagnostic results of LB occurrence in Korea imply that cases of LB are no longer restricted to the regional areas previously identified. Indeed, it is highly probable that LB has spread nationwide.
Acknowledgements
This study was supported by an intramural grant (4837-300-210-13) from the National Institute of Health, Korea. The authors thank A-Ram Kee, Ju-Hyum Kim and Kum-Ran Hong for their assistance with IFA and immunoblot diagnosis.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Aguero-Rosenfeld M.E., Wang G., Schwartz I. Diagnosis of lyme borreliosis. Clin Microbiol Rev. 2005 Jul;18(3):484–509. doi: 10.1128/CMR.18.3.484-509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LoGiudice K., Ostfeld R.S., Schmidt K.A. The ecology of infectious disease: effects of host diversity and community composition on Lyme borreliosis risk. Proc Natl Acad Sci USA. 2003 Jan 21;100(2):567–571. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kee S.H., Hwang K.J., Oh H.B. Isolation and Identification of Borrelia burgdorferi in Korea. J Korean Soc Microbiol. 1994 Apr;29(4):301–310. doi: 10.1111/j.1348-0421.1994.tb02157.x. [DOI] [PubMed] [Google Scholar]
- 4.Choi Y.J., Han S.H., Park J.M. First molecular detection of Borrelia afzelii in clinical samples in Korea. Microbiol Immunol. 2007 Dec;51(12):1201–1207. doi: 10.1111/j.1348-0421.2007.tb04015.x. [DOI] [PubMed] [Google Scholar]
- 5.Wilske B., Fingerle V., Schulte-Spechtel U. Microbiological and serological diagnosis of Lyme borreliosis. FEMS Immunol Med Microbiol. 2007 Feb;49(1):13–21. doi: 10.1111/j.1574-695X.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 6.Park K.H., Lee S.H., Jang W.J. Serological and antigenic analysis against Borrelia burgdorferi of febrile cases in Korea. J Korean Soc Microbiol. 1993 Oct;28(5):397–408. [Google Scholar]
- 7.Song J.W., Baek L.J., Lee Y.J. Seroepidemiologic analysis of acute febrile illness from Korea in 1996. J Korean Soc Virol. 1998 Dec;28(4):377–382. [Google Scholar]
- 8.Park K.H., Chang W.H., Schwan T.G. Identification and characterization of Lyme borreliosis spirochetes, Borrelia burgdorferi sensu lato, isolated in Korea. J Clin Microbiol. 1993 Jul;31(7):1831–1837. doi: 10.1128/jcm.31.7.1831-1837.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]


