Abstract
Objectives
Many natural compounds have been investigated as drug candidates to prevent human immunodeficiency virus (HIV) with low cytotoxicity. We tested whether ingenol from Euphorbia ingens exerts anti-HIV effects in human T cell lines.
Methods and Results
Ingenol effectively maintained high cell viability (CD50, >1 mM) in H9 and MT4 T cells. The efficacy of ingenol to inhibit HIV-1 infection was dose dependent. ED50 for 100 and 200 TCID50 of HIV-1 was 5.06 and 16.87 μM, respectively. Gag p24 antigen production in ingenol-treated MT4 cells was reduced by 24.5% on day 6 post-infection. While p24 antigen was reduced in ingenol-treated cells, levels of cytokines such as TNF-α and IL-6 and chemokines such as RANTES and MCP-1 were increased. dUTP level related to late apoptotic events was increased on day 2 post-infection of HIV by ingenol treatment, whereas expression of annexin V was unchanged. Reduced levels of iNOS and ZAP-70 after HIV infection were recovered by ingenol treatment.
Conclusion
Ingenol helps T cells to survive longer against viremia after HIV-1 infection, without exerting cytotoxic effects. Ingenol can be considered a safe and efficacious candidate for immune-boosting therapy for AIDS patients.
Keywords: anti-HIV activity, Euphorbia ingens, Gag p24, HIV-1, natural products
1. Introduction
Chemotherapy using natural products extracted from various resources, including plants and microorganisms, has long been of interest in the study of anti-human immunodeficiency virus (HIV) drug development [1,2]. Many natural compounds have been widely tested, and some have been reported as possible candidates for inhibition of HIV replication or boosting of host immune response. They are mostly secondary metabolites, including terpenoids and polyphenols. Natural compounds can provide novel anti-AIDS chemotherapeutic leads that are structurally unique or have new mechanisms of action [3].
The chemical constituents of some species of Euphorbia, which are succulent plants growing mostly in semi-desert areas, have been found to include chemotaxonomically important myrsinane diterpenoids acting as enzyme inhibitors against alpha-glycosidase, urease, HIV-1 reverse transcriptase, and prolyl endopeptidase [4]. Ingenol, one of the active ingredients in an extract from Euphorbia peplus, was shown to have activity against human melanoma cells and the ability to induce the apoptosis-regulating tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) in preclinical study [5]. While latex as well as total leaf extracts of Euphorbia plants exhibit Epstein–Barr virus (EBV) inducing activity [6], some ingenol derivatives were reported as potential HIV reverse transcriptase inhibitors [7].
Ingenol derivatives are structurally close to phorbol 12-myristate 13-acetate (PMA) [8], but have been less studied than PMA because of their structural complexity. Prostratin [9], another nontumorigenic phorbol ester, was reported to have HIV-inhibitory activity related to PKC activation [10]. PMA itself also showed inhibitory effects against HIV-1 [11]. Other reports have indicated that treatment of ingenol derivatives such as ingenol triacetate and aurintricarboxylic acid in HIV-infected T cells inhibited HIV replication through downregulation of CD4 receptors [12,13] and CXCR4 receptors [10].
We tested the anti-HIV activity of ingenol purified from Euphorbia ingens and subsequent viral and host event-influencing immune responses, which remain largely unknown despite some previous work on ingenol’s antiviral [12,14] and anti-inflammatory effects [15,16]. In addition to anti-HIV activity screening, it is essential to understand the mechanism of action related to HIV inhibition or immune response during ingenol action against infection, which might contribute to developing novel anti-HIV reagents or new therapeutic strategies for AIDS.
2. Materials and Methods
2.1. Cells culture and reagents
MT4 and H9 human T lymphocyte cell lines were obtained from the NIH AIDS Research and Reference Reagent Program (Bethesda, MD). Cells were maintained in RPMI-1640 medium (GIBCO, Carlsbad, NY) supplemented with 10% fetal bovine serum, penicillin 100 U/mL, streptomycin 100 μg/mL, and glutamine 2 mM.
Ingenol (Figure 1) purified from E. ingens was obtained from Sigma-Aldrich (St. Louis MO). Compound was dissolved in dimethylsulfoxide (DMSO) and further diluted with RPMI-1640 medium. The final concentration of DMSO in stock solution was <1.5% (v/v).
Figure 1.
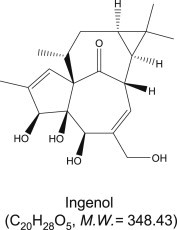
Chemical structure of ingenol.
2.2. Preparation of virus stock and titration
To obtain HIV-1 virus stock we incubated HTLV-IIIB/H9 cells (CRL 8543; American Type Culture Collection, Manassas, VA) for 14 days and harvested cell culture supernatants twice/week. The virus was then harvested from cell culture supernatant by centrifugation at 4500 rpm for 20 minutes followed by filtration through 0.22-μm filter (Millipore, Bedford, MA). To quantify viable HIV-1 virions in culture supernatant we evaluated 50% tissue culture infectious dose (TCID50) using HIV-1 gag p24 antigen ELISA (BioMerieux, Durham, NC). Titration for TCID50 infectivity was measured by the Spearman-Karber statistical method. The titer of virus stock was determined in MT4 cells (6.56 × 105 TCID50/mL) then divided into aliquots of fixed volume. Prepared viruses were stored at –80°C until use.
2.3. Cytotoxicity and anti-HIV activity assay
Cytotoxicity of ingenol was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide method (MTT assay; Roche, Basel, Switzerland) after 24-h incubation using MT4 and H9 cells. Anti-HIV infection activity was measured by cell viability of infected MT4 cells on day 6 postinfection of HIV-1 in the presence of serially diluted ingenol (0.1–250 μM). MT4 cells were infected with HIV-1 at various multiplicities of infection (MOI; 0.002–0.016 [25–200 TCID50]). The 50% effective dose (ED50) was calculated based on inhibition of virus-induced cell death after HIV infection, and 50% cytotoxic dose (CD50) was calculated based on reduction of viability in noninfected cells. Absorbance was measured at 550 nm wavelength with 690 nm reference wavelength using Bio-Tek microplate reader (EL800; Winooski, VT).
2.4. HIV-1 p24 antigen ELISA assay and luminex assay for cytokine measurement
MT4 cells were infected with HIV-1 at 0.004 MOI (50 TCID50) after treatment of 5 μM ingenol. Gag p24 antigen level in culture supernatant was measured by Vironostika HIV-1 Antigen MicroELISA kit (BioMerieux, Durham, NC). Absorbance was read at 450 nm wavelength using EL800 Bio-Tek microplate reader.
Cytokine and chemokine levels in culture supernatants were measured at the same day of p24 antigen measurement. Procarta™ human cytokine 5-plex kit and Procarta™ human chemokine 5-plex kit were purchased from Parnomics (Fremont, CA); assay was performed following the manufacturer’s protocol. Results were read using Bio-Plex 200 Suspension array system (Bio-Rad, Hercules, CA) and calculated with Bio-Plex Manager software 4.1 (Bio-Rad).
2.5. Flow cytometric assay
Cells were incubated with Golgi-Plug™ (BD Biosciences, San Jose, CA) for 4 hours and permeabilized with PermFix/PermWash (BD Biosciences). Then, cells were stained using Annexin V-Mitotracker Vybrant® Apoptosis assay kit (Molecular Probes, Eugene, OR) and APO-BrdUTM TUNEL assay kit (Invitrogen, Carlsbad, CA) following the manufacturers’ protocols. Stained cells were analyzed by FC500 flow cytometer (Beckman Coulter, Fullerton, CA).
2.6. Western blot analysis
Cellular lysate was prepared using ice-cold RIPA buffer (50 mM Tris pH 7.5, 0.5% deoxycholate, 0.5% NP-40, 0.5% SDS, and 100 mM NaCl) supplemented with freshly added protease inhibitor cocktail. Protein concentration in cell lysate was determined by BCA protein assay kit (Pierce, Rockford, IL); proteins were separated by SDS-PAGE. After transfer of separated proteins to Immobilon™–P PVDF membrane (Millipore, Danvers, MA), primary antibodies were applied for hybridization: rabbit anti-human inducible nitric oxide synthase (iNOS) antibody (Abcam, Cambridge, MA) and rabbit anti-human zeta chain-associated protein kinase 70 (ZAP-70) antibody (Cell Signaling, Danvers, MA). Hybridized bands were developed with HRP-conjugated anti-rabbit secondary antibody. β-Actin antibody was used as a loading control, TMB chemiluminescence substrate for visualization.
2.7. Statistical analysis
Data are expressed as mean ± SD. Student’s t test was used to analyze significance of differences between test and control. Statistical significance was set at p < 0.05.
3. Results
3.1. Ingenol exerts low cytotoxicity and dose-dependent anti-HIV infection activity
Ingenol revealed no toxic effects in MT4 cells and comparatively low cytotoxicity in H9 cells at the range 0.1–100 μM (Figure 2A). Survival of MT4 cells was more stable up to high concentration than that of H9 cells (Figure 2A).
Figure 2.
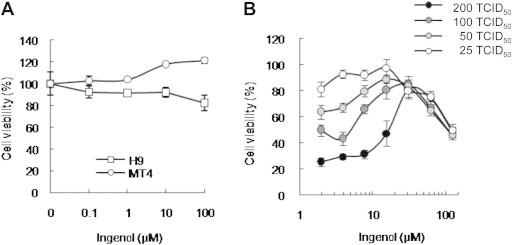
Ingenol revealed low cytotoxicity and dose-dependent anti-HIV infection activity. Viable cells were counted using a tetrazolium-based colorimetric method (MTT). (A) Ingenol revealed good cell viability in MT4 cells and H9 cells. MT4 and H9 cells were allowed to proliferate for 24 hours in the presence of serially diluted ingenol. (B) Anti-HIV infection activity was measured on day 6 postinfection. MT4 cells were infected with HIV-1IIIB at multiplicity of infection (MOI; 0.002–0.016 [25-200 TCID50]) with 1.95–250 μM ingenol. Data represent means ± SD of three independent experiments.
Cell viability after HIV infection in MT4 cells preincubated with ingenol at concentrations 1.95–250 μM was observed as a dose- and MOI-dependent pattern around day 6 postinfection (Figure 2B). ED50 for 100 and 200 TCID50 of HIV-1 was 5.06 and 16.87 μM, respectively. This dose-dependent anti-HIV infection activity of ingenol was similar to that of azidothymidine (AZT), which is widely used for AIDS treatment (data not shown).
3.2. Ingenol reduced HIV-1 gag p24 antigen production by inducing cytokines and chemokines
Gag p24 antigen production in HIV-infected MT-4 cells when 5 μM ingenol was treated into cell culture media was reduced by approximately 25% on day 6 postinfection (Figure 3A).
Figure 3.
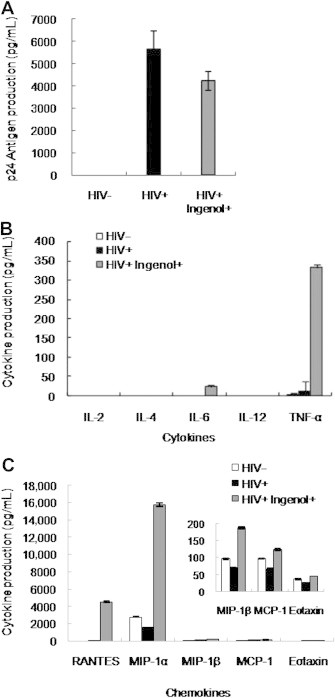
Ingenol treatment reduced Gag p24 production, whereas cytokines and chemokines were induced during HIV infection after ingenol treatment. MT4 cells were infected with HIV-1IIIB at multiplicity of infection (MOI; 0.004 [50 TCID50]) after treatment with 5 μM ingenol and supernatants analyzed every 3 days thereafter . (A) p24 antigen production measured by ELISA showed reduced level by ingenol treatment. (B) Cytokine production including TNF-α and IL-6 was induced by ingenol during HIV infection at day 6 postinfection. (C) Chemokine production was induced by ingenol during HIV infection at day 6 postinfection. RANTES and MIP-1α were highly increased whereas MIP-1β, MCP-1, and eotaxin were moderately or slightly increased by ingenol treatment. Data represent means ± SD of three independent experiments.
Th1 cytokines such as TNF-α and IL-6 were highly induced by ingenol treatment in HIV-infected MT4 cells, whereas Th2 cytokines were not detected (Figure 3B). TNF-α was considerably increased by 14 times and IL-6 was also induced by ingenol treatment.
Chemokines such as RANTES and MIP-1α were remarkably induced by ingenol treatment in HIV-infected cells on day 6 postinfection of HIV (Figure 3C). MIP-1β, MCP-1, and eotaxin were moderately increased or recovered by ingenol treatment compared with HIV-infected cells without ingenol (Figure 3C).
3.3. Apoptosis more advanced to late phase by ingenol in HIV-infected cells
Percent apoptotic events MT4 cells was measured by flow cytometric assay using annexin V as an early phase marker and dUTP as a late phase marker at 24 h postinfection. Annexin-V expression was slightly reduced by 4% in ingenol-treated MT4 cells compared with nontreated cells; however, the change was not statistically significant (Figure 4A). The dUTP level in HIV-infected MT4 cells was further increased by 25% with ingenol treatment (Figure 4B).
Figure 4.
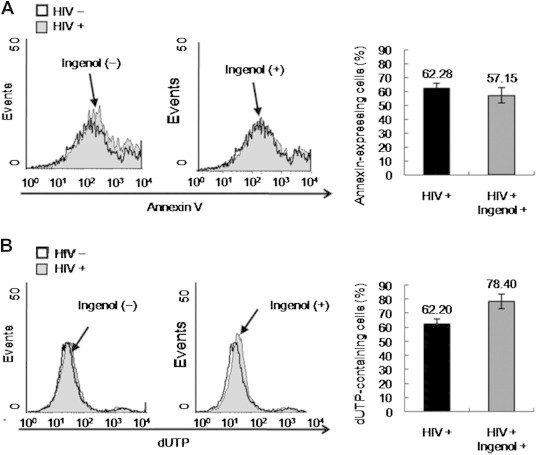
Late apoptotic events induced by ingenol during HIV infection. MT4 cells were infected with HIV-1IIIB at multiplicity of infection (MOI; 0.004 [50 TCID50]) after addition of 5 μM ingenol into culture media, and cells were analyzed using flow cytometry at 24 hours postinfection. (A) Annexin V expression as early apoptotic marker analyzed by flow cytometry. (B) dUTP level as late apoptotic marker investigated by flow cytometry using BrdU staining. Bar graphs on the right side represent calculated ratio of annexin V– or dUTP-expressing cells. Data represent means ± SD of three independent experiments.
3.4. iNOS and ZAP-70 recovered by ingenol treatment in HIV-infected cells
Western blot results of iNOS and ZAP-70 expression are shown in Figure 5. Total cell lysate was analyzed at 24 h postinfection; both iNOs and ZAP-70 were recovered by ingenol treatment compared with the remarkably reduced levels of HIV infection. Especially, iNOS was increased to a higher level than that in uninfected cells, whereas ZAP-70 recovered to almost the same level of uninfected cells.
Figure 5.
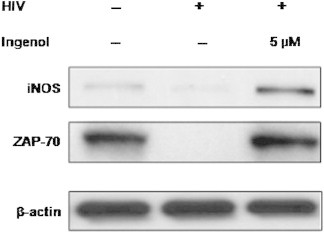
Western blot analysis of iNOs and ZAP-70 in MT4 cells. Ingenol treatment during HIV infection resulted in recovery of iNOS and ZAP-70. MT-4 cells were infected with HIV-1IIIB at multiplicity of infection (MOI; 0.004 [50 TCID50]) after 5 μM ingenol treatment then protein expression in cell lysate was analyzed by western blot 24 hours after HIV infection. iNOS, inducible nitric oxide synthase; ZAP-70, zeta chain-associated protein kinase 70. β-actin was used as loading control.
4. Discussion
Ingenol and its derivatives are known to have several biological activities. In particular, they have a degree of potential to activate protein kinase C (PKC) [17]. In previous studies, some ingenol derivatives were demonstrated to be potent inhibitors of acutely HIV-infected cells [12], whereas others were potent activators of HIV-1 transcription in chronically infected cells [7]. We focused on the anti–HIV-1 activity of ingenol, so as further to elucidate the mechanism of inhibition, and to identify cellular factors related to inhibition of HIV-1 replication in infected cells.
Ingenol-treated MT4 cells showed reduced p24 level compared with PBS-treated HIV-infected cells on day 6 postinfection. Gag p24 antigen of ingenol-treated cells was reduced by approximately 25% compared with mock-infected cells. Cell survival of ingenol-treated MT4 cells on day 6 postinfection was reduced to approximately 20% lower than AZT control (data not shown). Although our results showed lower inhibition of HIV infectivity compared with AZT, we nonetheless observed significant anti-HIV activity of ingenol.
HIV-1 induces apoptotic and anti-apoptotic signaling in infected and uninfected T lymphocytes, and thereby counteracts the antiviral response and immune effector cell functions [18]. Excessive induction of apoptosis may induce downregulation of the cellular immune response and favor pathogen immune evasion [19].
Annexin V binding of ingenol-treated cells was reduced by approximately 4% compared with nontreated cells. On the other hand, dUTP of ingenol-treated MT4 cells was increased by approximately 9% compared with nontreated cells. Inhibition of apoptosis in HIV-1-infected T cells enhances virus production and facilitates persistent infection [20]. HIV-1–infected and ingenol-treated MT4 cells suppressed virus production and early apoptosis arising compared with uninfected and AZT-treated cells. A reduced level of annexin V may suggest apoptosis of uninfected and ingenol-susceptible cells. Otherwise, DNA strand breakages of HIV-1-infected and ingenol-treated MT4 cells were not protected from later apoptosis. However, increased level of dUTP may have been induced by apoptosis of HIV-1 completely infected cells.
Although little is known of the ability of TNF to induce apoptosis in HIV-infected cells, it may contribute to apoptosis induced by gp120-mediated cross-linking of CD4 [21].
TNF-α and p24 antigen production were positively correlated at 6 days postinfection. In addition, IL-6 was very slightly increased in HIV-1-infected and ingenol-treated cells compared with days 1 and 3, suggesting that elevated TNF-α and IL-6 expression may induce high levels of HIV-1 infectivity. Indeed, this result is supported by elevated serum TNF levels seen in symptomatic HIV-infected patients [22,23], but not in asymptomatic patients [24,25]. Although the mechanisms to control the apoptotic pathway by ingenol has not been elucidated in detail, there is evidence that TNF-α may be involved in regulation of apoptosis when HIV-infected cells were treated with ingenol derivatives [26].
The mechanism of anti-HIV-1 activity of ingenol requires further study. In future, we will focus on elucidating the steps of HIV-1-mediated immune-related mechanisms, so as to gain insight into new applications for anti-HIV treatment.
Acknowledgement
This project was supported by an intramural grant of Korea National Institute of Health (Grant No. 2008-N00387-00).
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Matthee G., Wright A.D., Konig G.M. HIV reverse transcriptase inhibitors of natural origin. Planta Med. 1999 Aug;65(6):493–506. doi: 10.1055/s-1999-14004. [DOI] [PubMed] [Google Scholar]
- 2.De Clercq E. Current lead natural products for the chemotherapy of human immunodeficiency virus (HIV) infection. Med Res Rev. 2000 Sep;20(5):323–349. doi: 10.1002/1098-1128(200009)20:5<323::aid-med1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 3.Yu D., Morris-Natschke S.L., Lee K.H. New developments in natural products-based anti-AIDS research. Med Res Rev. 2007 Jan;27(1):108–132. doi: 10.1002/med.20075. [DOI] [PubMed] [Google Scholar]
- 4.Jassbi A.R. Chemistry and biological activity of secondary metabolites in Euphorbia from Iran. Phytochemistry. 2006 Sep;67(18):1977–1984. doi: 10.1016/j.phytochem.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 5.Gillespie S.K., Zhang X.D., Hersey P. Ingenol 3-angelate induces dual modes of cell death and differentially regulates tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in melanoma cells. Mol Cancer Ther. 2004 Dec;3(12):1651–1658. [PubMed] [Google Scholar]
- 6.Vogg G., Mattes E., Rothenburger J. Tumor promoting diterpenes from Euphorbia leuconeura L. Phytochemistry. 1999 May;51(2):289–295. doi: 10.1016/s0031-9422(99)00016-3. [DOI] [PubMed] [Google Scholar]
- 7.Fujiwara M., Okamoto M., Ijichi K. Upregulation of HIV-1 replication in chronically infected cells by ingenol derivatives. Arch Virol. 1998;143(10):2003–2010. doi: 10.1007/s007050050436. [DOI] [PubMed] [Google Scholar]
- 8.Fujiwara M., Ijichi K., Tokuhisa K. Mechanism of selective inhibition of human immunodeficiency virus by ingenol triacetate. Antimicrob Agents Chemother. 1996 Jan;40(1):271–273. doi: 10.1128/aac.40.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanco-Molina M., Tron G.C., Macho A. Ingenol esters induce apoptosis in Jurkat cells through an AP-1 and NF-kappaB independent pathway. Chem Biol. 2001 Aug;8(8):767–778. doi: 10.1016/s1074-5521(01)00048-5. [DOI] [PubMed] [Google Scholar]
- 10.Kedei N., Lundberg D.J., Toth A. Characterization of the interaction of ingenol 3-angelate with protein kinase C. Cancer Res. 2004 May 1;64(9):3243–3255. doi: 10.1158/0008-5472.can-03-3403. [DOI] [PubMed] [Google Scholar]
- 11.Challacombe J.M., Suhrbier A., Parsons P.G. Neutrophils are a key component of the antitumor efficacy of topical chemotherapy with ingenol-3-angelate. J Immunol. 2006 Dec 1;177(11):8123–8132. doi: 10.4049/jimmunol.177.11.8123. [DOI] [PubMed] [Google Scholar]
- 12.Hasler C.M., Acs G., Blumberg P.M. Specific binding to protein kinase C by ingenol and its induction of biological responses. Cancer Res. 1992 Jan 1;52(1):202–208. [PubMed] [Google Scholar]
- 13.Chowdhury I.H., Koyanagi Y., Kobayashi S. The phorbol ester TPA strongly inhibits HIV-1-induced syncytia formation but enhances virus production: possible involvement of protein kinase C pathway. Virology. 1990 May;176(1):126–132. doi: 10.1016/0042-6822(90)90237-l. [DOI] [PubMed] [Google Scholar]
- 14.Warrilow D., Gardner J., Darnell G.A. HIV type 1 inhibition by protein kinase C modulatory compounds. AIDS Res Hum Retroviruses. 2006 Sep;22(9):854–864. doi: 10.1089/aid.2006.22.854. [DOI] [PubMed] [Google Scholar]
- 15.Sorg B., Hecker E. Zur chemie des Ingenols, II. On the chemistry of ingenol II. Esters of ingenol and of Δ7,8-isoingenol. Z Naturforsch. 1982;37b:748–756. [Google Scholar]
- 16.Hezareh M., Moukil M.A., Szanto I. Mechanisms of HIV receptor and co-receptor down-regulation by prostratin: role of conventional and novel PKC isoforms. Antivir Chem Chemother. 2004 Jul;15(4):207–222. doi: 10.1177/095632020401500404. [DOI] [PubMed] [Google Scholar]
- 17.El-Mekkawy S., Meselhy M.R., Nakamura N. Anti–HIV-1 phorbol esters from the seeds of Croton tiglium. Phytochemistry. 2000 Feb;53(4):457–464. doi: 10.1016/s0031-9422(99)00556-7. [DOI] [PubMed] [Google Scholar]
- 18.Ameisen J.C. Apoptosis subversion: HIV-Nef provides both armor and sword. Nat Med. 2001 Nov;7(11):1181–1182. doi: 10.1038/nm1101-1181. [DOI] [PubMed] [Google Scholar]
- 19.Savill J., Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000 Oct 12;407(6805):784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 20.Antoni B.A., Sabbatini P., Rabson A.B., White E. Inhibition of apoptosis in human immunodeficiency virus-infected cells enhances virus production and facilitates persistent infection. J Virol. 1995 Apr;69(4):2384–2392. doi: 10.1128/jvi.69.4.2384-2392.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han X., Becker K., Degen H.J. Synergistic stimulatory effects of tumour necrosis factor alpha and interferon gamma on replication of human immunodeficiency virus type 1 and on apoptosis of HIV-1-infected host cells. Eur J Clin Invest. 1996 Apr;26(4):286–292. doi: 10.1046/j.1365-2362.1996.116271.x. [DOI] [PubMed] [Google Scholar]
- 22.Brown C.C., Poli G., Lubaki N. Elevated levels of tumor necrosis factor-alpha in Zairian neonate plasmas: implications for perinatal infection with the human immunodeficiency virus. J Infect Dis. 1994 May;169(5):975–980. doi: 10.1093/infdis/169.5.975. [DOI] [PubMed] [Google Scholar]
- 23.Zangerle R., Gallati H., Sarcletti M. Tumor necrosis factor alpha and soluble tumor necrosis factor receptors in individuals with human immunodeficiency virus infection. Immunol Lett. 1994 Jul;41(2–3):229–234. doi: 10.1016/0165-2478(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 24.Hober D., Haque A., Wattre P. Production of tumour necrosis factor-alpha (TNF-alpha) and interleukin-1 (IL-1) in patients with AIDS. Enhanced level of TNF-alpha is related to a higher cytotoxic activity. Clin Exp Immunol. 1989 Dec;78(3):329–333. [PMC free article] [PubMed] [Google Scholar]
- 25.Maury C.P., Lahdevirta J. Correlation of serum cytokine levels with haematological abnormalities in human immunodeficiency virus infection. J Intern Med. 1990 Apr;227(4):253–257. doi: 10.1111/j.1365-2796.1990.tb00154.x. [DOI] [PubMed] [Google Scholar]
- 26.Klein S.A., Dobmeyer J.M., Dobmeyer T.S. TNF-alpha mediated apoptosis of CD4 positive T-lymphocytes. A model of T-cell depletion in HIV infected individuals. Eur J Med Res. 1996 Feb;1(5):249–258. [PubMed] [Google Scholar]


