Abstract
Objectives
High-density lipoprotein cholesterol (HDL-C) is an independent risk factor for cardiovascular diseases that has shown a remarkable increase, but little is known about the prevalence of low HDL-C in Korea. This study aimed to evaluate changing trends of low HDL-C prevalence, and indicate other risk factors associated with low HDL-C.
Methods
We selected subjects aged ≥20 years from the Korean National Health and Nutrition Examination Survey (KNHANES) 1998, 2001, and 2005 (n = 7962, 6436, and 6412). The mean level of HDL-C and the prevalence of low HDL-C was calculated, and cardiovascular risk factors associated with low HDL-C, as well as demographic, anthropometric, lifestyle, and nutrition factors, were assessed using the KNHANES 2005 data.
Results
Mean HDL-C levels in men and women between KNHANES 1998 and 2005 decreased significantly, from 48.1 to 42.3 and from 51.6 to 47.1 mg/dL, respectively (both p < 0.001). The decrease was slightly less for women compared with men for the same period, and women had higher HDL-C levels at all periods. Covariate-adjusted OR revealed that body mass index, waist circumference, and non-alcohol drinker in both men and women were associated with low HDL-C levels by KNHANES 2005, as were employed and light physical activity in men and low fat intake in women.
Conclusion
The prevalence of low HDL-C increased significantly from KNHANES 1998 to 2001 and 2005 (p < 0.001) in both men and women. body mass index, waist circumference, and non-alcohol drinker were identified as associated with low HDL-C in Korean adults.
Keywords: cardiovascular disease, low high-density lipoprotein cholesterol (HDL-C), risk factors
1. Introduction
Many epidemiological studies have shown that elevated levels of serum total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) are independent risk factors for cardiovascular diseases (CVD) [1–3]. High-density lipoprotein cholesterol (HDL-C) level is also known as an inverse predictor of CVD, and has recently received considerable attention as a therapeutic target for regression of atherosclerosis [3].
Low HDL-C levels play a crucial role in the development of CVD; people with low HDL-C (<35 mg/dL) have a 2.5–4.1-fold risk of dying from CVD than those with high HDL-C (≥60 mg/dL) [4]. A 1-mg/dL increase of HDL-C is associated with 2% reduction in the relative risk of CVD in men and 3% reduction in women [4].
Asian populations tend to have lower levels and prevalence of low HDL-C than European and American populations, and previously reported finding may not be applicable to Asia populations [5–7]. This inter-ethnic diversity in HDL-C levels is considered to be the main factor cause [6,8]. However, the prevalence of and modifiable lifestyle factors that have been associated with low HDL-C have not been investigated fully among Asian populations.
Therefore we investigated the prevalence of low HDL-C levels in the general population using the Korean National Health and Nutrition Examination Surveys (KNHANES) in 1998, 2001, and 2005, and examined cardiovascular risk factors associated with low HDL-C, as well as demographic, anthropometric, lifestyle and nutrition factors.
2. Methods
2.1. Study population
Our research was based on the 1998, 2001, and 2005 KNHANES performed by the Korea Institute for Health and Social Affairs for the Korean Ministry of Health and Welfare. KNHANES is a repeated cross-sectional survey and consists of four components, namely the Health Interview Survey, Health Behavior Survey, Health Examination Survey and Nutrition Survey. The target population for surveys was community-dwelling individuals. The total population included in the Health Interview Survey in each year was n = 39,060, 37,769, and 34,145 primary sampling units randomly sampled throughout South Korea. We selected subjects aged ≥20 years with blood biochemistry data including TC, HDL-C, and LDL-C levels (for KNHANES 1998, 2001, and 2005, n = 7962, 6436, and 5438, respectively).
For the analysis of associated risk factors for low HDL-C, subjects on lipid-altering medications were excluded (Table 3). Self-reported questionnaires were administered to determine residential area, marital status, education level, occupation, economic status, alcohol intake, smoking status, and physical activity. Components of socioeconomic status (residential area, marital status, education level, occupation, and economic status) were obtained by interview. Residential area was categorized into three groups according to the Korean administrative district as large city, medium or small city, and rural area. Educational background was determined in terms of total years of formal education, namely <7, 7–9, 10–12, and >12 years. Occupational status was grouped into three categories: white collar (professional, administrative, office work, or service job); blue collar (agricultural, fishing, industrial, or other manual job); and unemployed (including students and persons who worked in their homes without pay). Economic level was divided into four groups by monthly income.
Table 3.
Adjusted OR for low HDL-C: associated factors men and women: KNHANES 2005
| Variable | KNHANES 2005 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Men (2280) |
Women (3077) |
|||||||||
| Low HDL–C (n = 1 023) | Age-adjusted OR (95% CI) | p for trend | Covariate-adjusted OR (95% CI) | p for trend | Low HDL–C (n = 1 966) | Age-adjusted OR (95% CI) | p for trend | Covariate-adjusted OR (95% CI) | p for trend | |
| Age (yr) | ||||||||||
| 20–29 | 87 (8.5) | 1.0 | 0.195 | 1.0 | 0.852 | 222 (11.3) | 1.0 | <0.001 | 1.0 | 0.448 |
| 30–39 | 236 (23.1) | 2.0 (1.45–2.69) | 1.4 (0.86–2.14) | 417 (21.2) | 1.1 (0.87–1.42) | 0.8 (0.55–1.14) | ||||
| 40–49 | 279 (27.3) | 1.9 (1.44–2.63) | 1.2 (0.75–2.01) | 467 (23.8) | 1.4 (1.10–1.80) | 0.7 (0.49–1.07) | ||||
| 50–59 | 197 (19.3) | 2.1 (1.54–2.94) | 1.4 (0.81–2.35) | 320 (16.3) | 1.5 (1.18–2.02) | 0.7 (0.43–1.06) | ||||
| 60–69 | 153 (15.0) | 1.6 (1.14–2.20) | 1.1 (0.63–1.96) | 291 (14.8) | 2.1 (1.58–2.84) | 0.9 (0.54–1.47) | ||||
| ≥70 | 71 (6.9) | 1.6 (1.05–2.32) | 1.1 (0.57–2.12) | 249 (12.7) | 2.8 (2.03–3.91) | 1.4 (0.79–2.562 | ||||
| BMI (kg/m2) | ||||||||||
| <18.5 | 15 (1.5) | 1.0 | <0.001 | 1.0 | <0.001 | 63 (3.2) | 1.0 | <0.001 | 1.0 | 0.021 |
| 18.5–22.9 | 234 (23.2) | 2.0 (1.10–3.53) | 1.6 (0.81–3.00) | 734 (37.7) | 1.6 (1.14–2.28) | 1.4 (0.97–2.13) | ||||
| 23–24.9 | 285 (28.3) | 3.9 (2.15–6.95) | 2.0 (1.02–4.10) | 475 (24.4) | 2.6 (1.79–3.75) | 1.9 (1.20–3.00) | ||||
| ≥25 | 474 (47.0) | 6.1 (3.43–10.98) | 2.7 (1.31–5.55) | 676 (34.7) | 3.3 (2.30–4.77) | 2.0 (1.21–3.25) | ||||
| WC (cm) | ||||||||||
| Lower tertile | 154 (15.2) | 1.0 | <0.001 | 1.0 | <0.001 | 473 (24.2) | 1.0 | <0.001 | 1.0 | <0.001 |
| Middle tertile | 289 (28.4) | 2.2 (1.75–2.82) | 1.7 (1.24–2.32) | 616 (31.5) | 1.8 (1.48–2.15) | 1.7 (1.34–2.12) | ||||
| Higher tertile | 573 (56.4) | 4.1 (3.23–5.08) | 2.5 (1.72–3.65) | 864 (44.2) | 2.7 (2.24–3.33) | 2.1 (1.55–2.98) | ||||
| Residential area | ||||||||||
| Large city | 441 (43.1) | 1.0 | 0.555 | 856 (43.5) | 1.0 | 0.645 | ||||
| Medium city | 343 (33.5) | 1.0 (0.83–1.21) | 649 (33.0) | 1.1 (0.92–1.30) | ||||||
| Rural | 239 (23.4) | 0.9 (0.75–1.15) | 461 (23.4) | 1.0 (0.84–1.25) | ||||||
| Marital status | ||||||||||
| Married | 838 (82.1) | 1.0 | 0.008 | 1.0 | 0.793 | 1365 (69.5) | 1.0 | 0.370 | 1.0 | 0.683 |
| Unmarried | 124 (12.1) | 0.6 (0.44–0.76) | 1.0 (0.68–1.55) | 183 (9.3) | 0.8 (0.62–1.03) | 0.8 (0.53–1.12) | ||||
| Others | 59 (5.8) | 0.9 (0.62–1.26) | 1.1 (0.75–1.72) | 415 (21.1) | 1.0 (0.79–1.23) | 0.9 (0.70–1.19) | ||||
| Education (yr) | ||||||||||
| <7 | 155 (15.2) | 1.0 | 0.098 | 1.0 | 0.884 | 667 (33.9) | 1.0 | 0.002 | 1.0 | 0.963 |
| 7–9 | 131 (12.8) | 1.3 (0.96–1.83) | 1.2 (0.81–1.74) | 254 (12.9) | 1.1 (0.84–1.48) | 1.4 (0.97–1.90) | ||||
| 10–12 | 353 (34.5) | 1.3 (0.95–1.66) | 1.0 (0.73–1.45) | 636 (32.3) | 0.9 (0.67–1.13) | 1.3 (0.91–1.72) | ||||
| ≥13 | 384 (37.5) | 1.3 (1.00–1.83) | 1.1 (0.72–1.57) | 409 (20.8) | 0.6 (0.48–0.87) | 1.0 (0.70–1.47) | ||||
| Occupation | ||||||||||
| White collar | 437(42.8) | 1.0 | <0.001 | 1.0 | 0.006 | 525 (26.7) | 1.0 | 0.683 | 1.0 | 0.347 |
| Blue collar | 382 (37.4) | 0.7 (0.59–0.86) | 0.9 (0.72–1.23) | 381 (19.4) | 1.0 (0.80–1.27) | 0.9 (0.67–1.18) | ||||
| Unemployed | 202 (19.8) | 0.6 (0.48–0.76) | 0.7 (0.47–0.94) | 1 060 (53.9) | 1.0 (0.87–1.24) | 0.8 (0.67–1.04) | ||||
| Income (million won) | ||||||||||
| <1.00 | 168 (16.6) | 1.0 | 0.001 | 1.0 | 0.269 | 431 (22.1) | 1.0 | 0.453 | 1.0 | 0.973 |
| 1.00–2.49 | 394 (39.0) | 1.1 (0.84–1.40) | 1.0 (0.71–1.31) | 762 (39.0) | 1.1 (0.91–1.43) | 1.1 (0.83–1.40) | ||||
| 2.50–3.99 | 253 (25.0) | 1.2 (0.90–1.55) | 0.9 (0.62–1.25) | 466 (23.9) | 1.0 (0.80–1.32) | 1.1 (0.82–1.46) | ||||
| ≥4.00 | 196 (19.4) | 1.6 (1.20–2.20) | 1.2 (0.84–1.84) | 294 (15.1) | 1.0 (0.74–1.27) | 0.8 (0.77–1.47) | ||||
| Alcohol | ||||||||||
| Nondrinker | 84 (8.4) | 1.0 | <0.001 | 1.0 | <0.001 | 451 (23.5) | 1.0 | 0.066 | 1.0 | 0.029 |
| Current drinker | 911 (91.6) | 0.4 (0.30–0.64) | 0.4 (0.29–0.67) | 1472 (76.5) | 0.8 (0.68–1.01) | 0.8 (0.62–0.97) | ||||
| Smoking | ||||||||||
| Nonsmoker | 172 (17.3) | 1.0 | 0.172 | 1748 (90.9) | 1.0 | 0.812 | ||||
| Current smoker | 823 (82.7) | 0.9 (0.68–1.07) | 175 (9.1) | 1.0 (0.79–1.35) | ||||||
| Physical activity | ||||||||||
| Light | 442 (44.4) | 1.0 | 0.003 | 1.0 | 0.040 | 979 (50.9) | 1.0 | 0.387 | 1.0 | 0.618 |
| Moderate | 389 (39.1) | 0.9 (0.74–1.08) | 0.8 (0.62–1.03) | 805 (41.9) | 1.0 (0.87–1.19) | 1.0 (0.83–1.23) | ||||
| Heavy | 164 (16.5) | 0.7 (0.54–0.87) | 0.7 (0.48–0.95) | 139 (7.2) | 1.2 (0.87–1.68) | 1.2 (0.79–1.77) | ||||
| Fat (% of energy) | ||||||||||
| <6 | 246 (27.8) | 1.0 | 0.515 | 1.0 | 0.111 | 617 (35.3) | 1.0 | <0.001 | 1.0 | <0.001 |
| 6–11 | 411 (46.4) | 1.1 (0.80–1.24) | 0.9 (0.73–1.18) | 798 (45.7) | 0.8 (0.63–0.93) | 0.8 (0.62–0.94) | ||||
| ≥12 | 228 (25.8) | 0.9 (0.71–1.19) | 0.8 (0.59–1.05) | 331 (19.0) | 0.6 (0.48–0.78) | 0.6 (0.49–0.80) | ||||
Data are expressed as n(%).
BMI, body mass index; WC, waist circumference.
Covariates adjusted for age, BMI, WC, marital status, education, occupation, income, alcohol, physical activity, fat (% of energy).
Height was measured to the nearest 0.1 cm by portable stadiometer (850–2069 mm; Seriter®) and weight to nearest 0.1 kg by balance beam scale (Giant-150N; HANA®). Body mass index (BMI; kg/m2) was calculated by dividing weight (kg) by the square of height (m2). WC was measured at the midpoint between the bottom of the rib cage and the top of the lateral border of the iliac crest during minimal respiration. BMI was divided into four categories, namely <18.5, 18.5–22.9, 23.0–24.9, and ≥25 kg/m2. WC was divided into tertiles arbitrarily: <79, 79–85, and ≥86 cm in men and <73, 73–80, and ≥81 cm in women. Blood pressure was measured by mercury sphygmomanometer (Baumanmeter®) in a seated position after a 10-min rest period. Two measurements were made in all subjects at 5-min intervals, and the average of two measurements was used in data analysis. Blood samples were collected from subjects in the morning after overnight fasting and analyzed for TC, LDL-C, HDL-C, and fasting plasma glucose (FPG) at a central laboratory.
Alcohol consumption was estimated from the subjects’ usual daily intake of alcoholic beverages. Drinking was divided into 2 groups by lifetime drinking experience in adults: drinker or non-drinker. Smoking habit was likewise classified as smoker or nonsmoker. Physical activity was categorized into 3 groups, namely light, moderate, and high.
Daily energy and nutrient intakes were assessed by 24-h recall. As one relevant measure of risk factors, fat intake was selected and expressed as percent total food energy. Fat intake percentage are define by fat intake over total energy intake. Percent intake of fat was divided into categories of 25%, 50%, and 75%.
2.2. Definition of cardiovascular risk factors
According to NCEP ATP III [9] and IDF [10] definitions, low HDL-C in men and women was defined as <40 and <50 mg/dL, respectively. Definitions of CVD risk factors were as follow: high LDL-C (≥160 mg/dL); high TC (≥240 mg/dL); high triacylglycerides (TG) (≥200 mg/dL); diabetes mellitus (DM); high FPG (≥126 mg/dL) and/or use of oral hypoglycemic agents; hypertension (HTN; systolic blood pressure [SBP] ≥140 mmHg and/or diastolic blood pressure [DBP] ≥90 mmHg and/or use of antihypertensive medications or previously diagnosed hypertension). We also applied the Korea Society for the Study of Obesity (KSSO) criteria for abdominal obesity in Korean men and women such as WC ≥90 and ≥85 cm, respectively [11].
2.3. Statistical analysis
Statistical analyses were performed using the SPSS statistical package version 12.0 (SPSS Inc., Chicago, IL, USA). Baseline variables were described as group number (percent). HDL-C values were expressed as sex- and age-specific mean. Trends were analyzed by χ2 test. Regression analysis was used to test the significance of increases or decreases of low HDL-C values in each sex. Analysis of variance was used to examine differences between sexes between 3 surveys (KNHANES 1998, 2001, 2005). Demographic factors (age, residential area, marital status, education level, occupation and economic status), anthropometric factors {body mass index (BMI) and waist circumference (WC), lifestyle factors (alcohol consumption, cigarette smoking, and physical activity)}, and nutrition factors (fat intake, protein intake, and sugar intake) were created as dummy variables. Multiple logistic regression analysis was performed to examine the risk of low HDL-C (as a dependent variable) according to the demographic, anthropometric, lifestyle, and nutrition factors (as independent variables) in men and women. Adjusted odds ratio (OR) was presented together with 95% confidence interval (95%CI). All reported p-values were based on two-sided tests; those <0.05 were considered significant.
3. Results
3.1. Baseline characteristics
In this analysis, data from 19,836 individuals (3,597, 2,810, and 2,314 men and 4,365, 3,626, and 3,124 women by KNHANES 1998, 2001, and 2005) were included. Table 1 shows the basic characteristics of the study population. Between KNHANES 1998 and 2005, the prevalence of obesity (BMI, ≥25 kg/m2), resident population in city, high education, family monthly income ≥2.5 million won, and moderate physical activity increased in men and women. Moreover, the prevalence of higher WC (highest tertile, ≥86 cm) in men and current drinker in women increased from KNHANES 1998 to 2001 to 2005 (Table 1).
Table 1.
Basic characteristics of the study population by in 3 KNHANES phases
| Variable | KNHANES |
|||||
|---|---|---|---|---|---|---|
| Men |
Women |
|||||
| 1998 | 2001 | 2005 | 1998 | 2001 | 2005 | |
| No. subjects (%) | 3597 (45.2) | 2810 (43.7) | 2314 (42.6) | 4365 (54.8) | 3626 (56.3) | 3124 (57.4) |
| Age (yr) | ||||||
| 20–29 | 659 (18.3) | 458 (16.3) | 274 (11.8) | 834 (19.1) | 619 (17.1) | 403 (12.9) |
| 30–39 | 923 (25.7) | 711 (25.3) | 497 (21.5) | 1 045 (23.9) | 920 (25.4) | 723 (23.1) |
| 40–49 | 775 (21.5) | 675 (24.0) | 595 (25.7) | 855 (19.6) | 820 (22.6) | 743 (23.8) |
| 50–59 | 575 (16.0) | 425 (15.1) | 407 (17.6) | 685 (15.7) | 502 (13.8) | 502 (16.1) |
| 60–69 | 445 (12.4) | 351 (12.5) | 370 (16.0) | 562 (12.9) | 434 (12.0) | 426 (13.6) |
| ≥70 | 220 (6.1) | 190 (6.8) | 171 (7.4) | 384 (8.8) | 331 (9.1) | 327 (10.5) |
| BMI (kg/m2) | ||||||
| <18.5 | 173 (4.8) | 89 (3.2) | 79 (3.4) | 235 (5.4) | 205 (5.7) | 147 (4.7) |
| 18.5–22.9 | 1 630 (45.4) | 1 092 (39.1) | 762 (33.2) | 1 969 (45.2) | 1 553 (43.1) | 1 318 (42.6) |
| 23–24.9 | 883 (24.6) | 700 (25.1) | 621 (27.1) | 924 (21.2) | 819 (22.7) | 700 (22.6) |
| ≥25 | 902 (25.1) | 913 (32.7) | 830 (36.2) | 1 227 (28.2) | 1 028 (28.5) | 932 (30.1) |
| WC (cm) | ||||||
| Lower tertile | 1 197 (33.3) | 714 (25.5) | 604 (26.2) | 1 377 (31.5) | 1 152 (31.9) | 962 (31.0) |
| Middle tertile | 1 139 (31.7) | 912 (32.5) | 683 (29.7) | 1 315 (30.1) | 1 068 (29.6) | 962 (31.0) |
| Higher tertile | 1 261 (35.1) | 1 177 (42.0) | 1 015 (44.1) | 1 673 (38.3) | 1 389 (38.5) | 1 181 (38.0) |
| Residential area | ||||||
| Large city | 1 412 (39.3) | 1 281 (45.6) | 994 (43.0) | 1 699 (38.9) | 1 680 (46.3) | 1 391 (44.5) |
| Medium city | 905 (25.2) | 870 (31.0) | 771 (33.3) | 1 073 (24.6) | 1 115 (30.8) | 1 029 (32.9) |
| Rural | 1 278 (35.5) | 659 (23.5) | 549 (23.7) | 1 593 (36.5) | 831 (22.9) | 704 (22.5) |
| Marital status | ||||||
| Married | 2 835 (78.8) | 2 200 (78.3) | 1 815 (78.6) | 3 095 (70.9) | 2 587 (71.3) | 2 155 (69.0) |
| Unmarried | 636 (17.7) | 488 (17.4) | 353 (15.3) | 488 (11.2) | 410 (11.3) | 359 (11.5) |
| Others | 126 (3.5) | 122 (4.3) | 140 (6.1) | 782 (17.9) | 629 (17.3) | 607 (19.4) |
| Education (yr) | ||||||
| <7 | 770 (21.4) | 431 (15.3) | 374 (16.2) | 1 659 (38.0) | 1 047 (28.9) | 959 (30.7) |
| 7–9 | 508 (14.1) | 359 (12.8) | 282 (12.2) | 621 (14.2) | 477 (13.2) | 367 (11.7) |
| 10–12 | 1 346 (37.4) | 1 031 (36.7) | 800 (34.6) | 1 370 (31.4) | 1 285 (35.4) | 1 026 (32.8) |
| ≥13 | 973 (27.1) | 989 (35.2) | 857 (37.1) | 715 (16.4) | 817 (22.5) | 772 (24.7) |
| Occupation | ||||||
| White collar | 1 259 (35.0) | 1 146 (40.8) | 886 (38.4) | 1 051 (24.1) | 991 (27.3) | 879 (28.1) |
| Blue collar | 1 561 (43.4) | 1 005 (35.8) | 906 (39.2) | 1 028 (23.6) | 551 (15.2) | 585 (18.7) |
| Unemployed | 777 (21.6) | 659 (23.5) | 518 (22.4) | 2 286 (52.4) | 2 083 (57.5) | 1 660 (53.1) |
| Income (million won) | ||||||
| <1.00 | 1 214 (33.8) | 484 (17.2) | 400 (17.5) | 1 588 (36.4) | 743 (20.5) | 641 (20.7) |
| 1.00–2.49 | 1 923 (53.5) | 1 490 (53.0) | 936 (40.9) | 2 207 (50.6) | 1 768 (48.8) | 1 185 (38.2) |
| 2.50–3.99 | 353 (9.8) | 510 (18.1) | 574 (25.1) | 450 (10.3) | 667 (18.4) | 776 (25.0) |
| ≥4.00 | 107 (3.0) | 326 (11.6) | 381 (16.6) | 120 (2.7) | 448 (12.4) | 497 (16.0) |
| Alcohol | ||||||
| Nondrinker | 377 (10.9) | 331 (12.9) | 130 (5.8) | 1 868 (44.0) | 1 308 (38.6) | 647 (21.2) |
| Current drinker | 3 075 (89.1) | 2 235 (87.1) | 2 093 (94.2) | 2 382 (56.0) | 2 080 (61.4) | 2 408 (78.8) |
| Smoking | ||||||
| Nonsmoker | 600 (17.4) | 525 (20.5) | 359 (16.1) | 3 856 (90.7) | 3 173 (93.7) | 2 786 (91.2) |
| Current smoker | 2 852 (82.6) | 2 041 (79.5) | 1 864 (83.9) | 394 (9.3) | 215 (6.3) | 269 (8.8) |
| Physical activity | ||||||
| Light | 1 414 (41.0) | 1 174 (45.8) | 932 (41.9) | 1 943 (45.7) | 1 825 (53.9) | 1 556 (50.9) |
| Moderate | 1 148 (33.3) | 945 (36.8) | 867 (39.0) | 1 761 (41.4) | 1 413 (41.7) | 1 301 (42.6) |
| Heavy | 890 (25.8) | 447 (17.4) | 424 (19.1) | 546 (12.8) | 150 (4.4) | 198 (6.5) |
Data are expressed as n(%).
BMI, body mass index; WC, waist circumference.
Table 2 shows the cardiovascular risk factors in the study population in men and women. Between KNHANES 1998 and 2005, the overall mean age, BMI, and TC/HDL-C increased significantly in men and women (p < 0.05). In men mean WC and TG were increased, whereas mean HDL-C, FPG, and SBP decreased (p < 0.001). In women mean TC, HDL-C, FPG, and SBP decreased (p < 0.001). The prevalence of low HDL-C increased significantly from KNHANES 1998 to 2001 to 2005 (p < 0.001) in men and women. In addition, the prevalence of abdominal obesity increased gradually in men, whereas DM, high TC, and high LDL-C decreased from KNHANES 1998 to 2001 to 2005 (p < 0.05) (Table 2).
Table 2.
Cardiovascular disease risk factors in men and women: KNHANES 1998, 2001, and 2005
| Variable | KNHANES |
|||||||
|---|---|---|---|---|---|---|---|---|
| Men |
Women |
|||||||
| 1998 (n = 3597) | 2001 (n = 2810) | 2005 (n = 2314) | p-value | 1998 (n = 4365) | 2001 (n = 3626) | 2005 (n = 3124) | p-value | |
| Age (yr) | 44.2 ± 14.8b | 44.8 ± 15.0b | 47.3 ± 14.7a | <0.001 | 45.1 ± 16.1b | 45.1 ± 15.8b | 47.1 ± 15.7a | <0.001 |
| BMI (kg/m2) | 23.1 ± 3.0c | 23.7 ± 3.1b | 24.0 ± 3.1a | <0.001 | 23.3 ± 3.4b | 23.3 ± 3.4b | 23.5 ± 3.4a | 0.003 |
| WC (cm) | 82.8 ± 8.4b | 84.3 ± 8.4a | 84.3 ± 8.8a | <0.001 | 78.5 ± 9.7 | 78.4 ± 9.6 | 78.5 ± 9.6 | 0.693 |
| TC (mg/dL) | 187.5 ± 37.0a | 189.2 ± 34.6a | 185.0 ± 34.6b | <0.001 | 189.0 ± 37.8a | 187.8 ± 35.2a | 184.8 ± 35.5b | <0.001 |
| HDL–C (mg/dL) | 48.1 ± 12.3a | 43.6 ± 10.1b | 42.3 ± 10.2c | <0.001 | 51.6 ± 12.7a | 47.9 ± 10.4b | 47.1 ± 10.9c | <0.001 |
| LDL–C (mg/dL) | 112.0 ± 33.8 | 112.9 ± 31.4 | 112.9 ± 30.4 | 0.450 | 114.9 ± 33.7 | 114.3 ± 30.2 | 115.1 ± 30.4 | 0.543 |
| TC/HDL–C (mg/dL) | 4.1 ± 1.3b | 4.5 ± 1.2a | 4.6 ± 1.3a | <0.001 | 3.9 ± 1.2b | 4.1 ± 1.1a | 4.1 ± 1.1a | <0.001 |
| TG (mg/dL) | 136.4 ± 65.9b | 157.8 ± 84.9a | 160.3 ± 131.0a | <0.001 | 112.5 ± 55.9b | 123.3 ± 71.2a | 115.2 ± 79.7b | <0.001 |
| FPG (mg/dL) | 101.9 ± 31.0a | 98.3 ± 17.8b | 98.2 ± 26.2b | <0.001 | 100.8 ± 32.8a | 96.8 ± 17.1b | 93.2 ± 19.9c | <0.001 |
| SBP (mmHg) | 128.1 ± 18.2a | 126.1 ± 17.4b | 122.9 ± 16.1c | <0.001 | 124.2 ± 21.2a | 119.6 ± 19.6b | 116.6 ± 18.6c | <0.001 |
| DBP (mmHg) | 81.0 ± 11.7 | 80.3 ± 11.1 | 80.8 ± 10.4 | 0.072 | 76.5 ± 11.7a | 74.6 ± 11.3b | 74.8 ± 10.4b | <0.001 |
| DM (%) | 429 (12.0) | 268 (9.7) | 255 (11.1) | 0.015 | 415(9.5) | 295 (8.2) | 225 (7.3) | 0.002 |
| HTN (%) | 1 099 (30.6) | 805 (31.2) | 717 (31.1) | 0.868 | 1 084 (24.9) | 795 (23.6) | 745 (24.0) | 0.363 |
| High TC (%) | 289 (8.0) | 230 (8.2) | 135 (5.8) | 0.002 | 429 (9.8) | 312 (8.6) | 209 (6.7) | <0.001 |
| High LDL–C (%) | 275 (7.6) | 198 (7.5) | 135 (6.1) | 0.068 | 420 (9.6) | 276 (7.8) | 216 (7.0) | <0.001 |
| High TG (%) | 561 (15.6) | 68.5 (25.4) | 512 (22.1) | <0.001 | 319 (7.3) | 470 (13.2) | 305 (9.8) | <0.001 |
| Low HDL–C (%) | 893 (24.8) | 1 049 (37.3) | 1 045 (45.2) | <0.001 | 2 084 (47.7) | 2 160 (59.6) | 1 997 (63.9) | <0.001 |
| Abdominal obesity (%) | 731 (20.3) | 681 (24.3) | 612 (26.6) | <0.001 | 1 077 (24.7) | 881 (24.4) | 771 (24.8) | 0.921 |
Data are expressed as mean ± SD or n(%).
BMI, body mass index; WC, waist circumference; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triacylglycerides; SBP, systolic blood pressure; DBP, diastolic blood pressure; DM, diabetes mellitus; HTN, hypertension.
a b cMeans in the same row not sharing a common superscript are significantly different between groups (p < 0.05).
3.2. HDL-C levels and prevalence of low HDL-C
Age-specific mean HDL-C levels and prevalence of low HDL-C from KNHANES 1998 to 2001 to 2005 are presented in Figures 1 and 2. Overall mean HDL-C was higher in women than in men. During the observation period, mean HDL-C decreased in all age groups <60 years except in men aged >50 years and women aged 50–69 years during 2001 and 2005 (Figure 1). The overall prevalence of low HDL-C was higher in women than in men in all three surveys. From 1998 to 2005, the prevalence of low HDL-C increased in all age groups exceps in men aged >70 years and in women 60–69 years of age between 2001 and 2005 (Figure 2).
Figure 1.
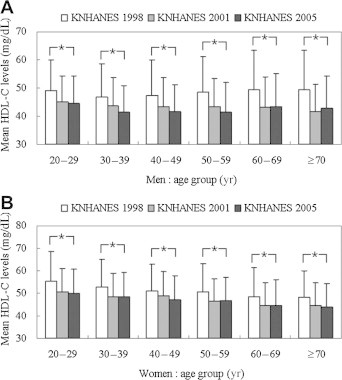
Mean high-density lipoprotein cholesterol (HDL-C) levels by age group in men (A) and women (B): KNHANES 1998, 2001, 2005. Data are mean ± SD. ∗p < 0.05 for differences within the same age group.
Figure 2.
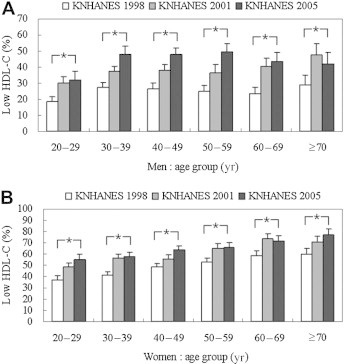
Frequency of low high-density lipoprotein cholesterol (HDL-C) level by age groups in men (A) and women (B): KNHANES 1998, 2001, 2005. Error bars indicate 95%CIs. For men and women low HDL-C was defined as <40 and <50 mg/dL, respectively. ∗p < 0.05 for trends within the same age group.
3.3. Risk factors associated with low HDL-C levels according to sex in KNHANES 2005
Multiple logistic analyses for low HDL-C levels for men and women are shown in Table 3. Age-adjusted OR for low HDL-C increased with increments of BMI and WC in men and women. In men, age-adjusted OR for low HDL-C was higher in those who were married as opposed to unmarried, employed as opposed to unemployed, higher monthly family income (≥4.00 million won), nondrinker as opposed to current smoker drinkers, and light physical activity as opposed to heavy physical activity. In women, age-adjusted OR for low HDL-C was lower for university school educational level and for higher fat intake expressed as a percentage of total energy consumption. Fat intake was statistically significant only for women (Table 3). Covariate-adjusted OR for low HDL-C increased with increments of BMI, WC, and non-alcohol drinker in men and women. In men, covariate-adjusted OR for low HDL-C showed an increasing trend for employed as opposed to unemployed and light physical activity as opposed to moderate physical activity. In women, fat intake was significantly correlated to low HDL-C (Table 3).
4. Discussion
The KNHANES database is a valuable resource for the study of trends in the health status of the Korean population because of its large sample size, complex sampling design, good quality control, and comprehensive content.
In our study, mean HDL-C levels in men and women aged ≥20 years between KNHANES 1998 and 2005 decreased significantly from 48.1 to 42.3 and from 51.6 to 47.1 mg/dL, respectively (both p < 0.001). During a similar time period, in the US National Health and Nutrition Examination Survey (NHANES) mean HDL-C levels for men and women aged 20–74 years increased from 45.4 to 47.2 and from 55.1 to 57.7 mg/dL, respectively (both p < 0.001) [12]. In a large cohort of Japanese subjects aged 20–79 years HDL-C decreased from 55.4 to 54.5 mg/dL in men, but increased from 66.4 to 68.9 mg/dL in women between 1989 and 1998 [13]. In Chinese men and women, HDL-C decreased from 52 to 49 and from 60 to 58 mg/dL, respectively, were noted between 1984–88 and 2001–02 [14].
We demonstrated that the prevalence of low HDL-C increased from 24.8% to 45.2% in men and from 47.7% to 63.9% in women between KNHANES 1998 and KNHANES 2005 (both p < 0.001). In the USA the prevalence of low HDL-C was 35.2% for men and 39.3% for women in 1994 and in Japan 18.5% for men and 30% for women [15,16]. In a pan-European survey, the prevalence of low HDL-C was 33% in men and 40% in women [7].
Epidemiologic studies found lower levels of TC and HDL-C in African and Asian men compared with their European and American counterparts [6,7]. HDL-C values are influenced by various factors, including TG level, obesity, and lifestyle. For example, smoking decreases HDL-C, whereas alcohol consumption and regular exercise increase this parameter [17–19]. Asian populations tend to be less obese than European and American populations [5,6,8]. The decrease of HDL-C in Koreans during the last decade may be associated with an increased prevalence of obesity. Changes in body weight are important independent and consistent predictors of changes in TC, LDL-C, and HDL-C [20]. Obesity is also a significant risk factor of CVD; excess body weight is closely associated with lowered HDL-C levels. Several studies have reported that lowering BMI, WC, and abdominal fat are important factors in reducing HDL-C levels and elevated TC, glycemia, TG, and blood pressure [21–23]. Among US adults, abdominal obesity was higher at 29.8% in men and 46.3% in women than in our data (20.3–26.6% and 24.7–24.8%, respectively, in KNHANES 1998 and 2005) [15]. However, the prevalence of abdominal obesity increased significantly in men from KNHANES 1998 to 2005 (p < 0.001); the OR of low HDL-C levels increased significantly in relation to higher BMI and WC for both sexes in KNHANES 2005. Components of socioeconomic status are important risk factors, and may affect low HDL-C levels. Our study revealed that the covariate-adjusted OR for low HDL-C levels increased significantly for employed as opposed to unemployed and light physical activity as opposed to moderate physical activity in men and low fat intake in women in KNHANES 2005. Recent large-scale epidemiological studies reported a higher prevalence of CVD and higher rates of smoking and DM prevalence among individuals in lower socioeconomic groupings [24].
We showed inverse association between low HDL-C levels and alcohol consumption in men and women. Other researchers have shown that alcohol consumption has a significant inverse relation with the OR for low HDL-C [18], and have directly associated HDL-C with TG [25]. TC and HDL-C increased with alcohol intake; frequent drinkers were associated with increased levels of HDL-C and LDL-C [26]. On the other hand, cigarette smoking is associated with substantially lower levels of HDL-C; furthermore, this association appears dose-dependent, indicating a possible causal relationship between cigarette smoking and lower HDL-C [17,27]. This association is independent of age, obesity, alcohol consumption, and regular exercise [27]. However, we found that smoking status was not a significant independent risk factor for low HDL-C levels for both sexes.
Physical activity has been significantly associated with blood pressure, BMI and HDL-C level [19]; compared to a sedentary or low physical activity, moderate and high physical activity were associated with higher HDL-C in women [28]. In contrast to these findings, the present study demonstrated statistically significant associations between heavy physical activity and low HDL-C in men.
Several dietary components also affect HDL-C levels. Higher intake of fat is associated with higher levels of TC and HDL-C [29]. Our study showed that fat intake was significantly associated with HDL-C levels in women, in whom low fat intake increased OR of low HDL-C. In a previous study, a high-fat and high-cholesterol diet raised HDL-C and production of apoA-I [30]. Also, reduced apoA-I mRNA related to consumption of a low-fat diet was attributed to low LDL-C and HDL-C [31]. Compared with populations in the Minnesota Heart survey, whose percent fat intake in total diet was 30.7–39.8% in men and 30.4–39.4% in women [32], Koreans consume relatively little fat, because their meals are carbohydrate dominant. This finding may in part explain why Koreans have relatively low TC and HDL-C levels as compared with Westerners. A more balanced diet may improve low HDL-C in Korean women.
Analyses of large US cohorts indicated that a 1-mg/dL increase in HDL-C may decrease the risk of CHD by 2% in men and 3% in women [4]. NCEP ATP III has recognized HDL-C as an independent CVD risk factor and recommends screening measurements of HDL-C for all adults [9].
Our analyses have several limitations. First, our study was based on repeated cross-sectional design, which may be used for development of public health programs to improve detection, prevention, and treatment of CVD in Korea. Therefore, the results reflect only associations between demographic, anthropometric, lifestyle, and nutrition factors and low HDL-C. Second, we were unable to observe genetic factors that are related to the development of low HDL-C. In this regard, further studies aimed at identifying genetic factors associated with low HDL-C are necessary. Third, there was a possible misclassification of risk status, recall bias, and confounding factors because a self-administered questionnaire was used. Estimates from a single 24-h recall method cannot be corrected for intra-individual daily variation in consumption. Although single recalls are useful to estimate population means, the variation of intake might be underestimated or overestimated [33]. Finally, we could not analyze other dietary factors including particular fat intake that might influence low HDL-C levels.
5. Conclusions
In conclusion, our study revealed that the prevalence of low HDL-C has rapidly increased in men and women in Korea. This suggests that attention should be focused to increase HDL-C levels for prevention of CVD. Mean BMI, WC, TC/HDL-C, and TG in men and BMI and TG in women increased significantly between KNHANES 1998 and 2005. KNHANES 2005 study demonstrated that the prevalence of low HDL-C was 45.2% in men and 63.9% in women. Increased BMI, WC, and non-alcohol drinker in men and women were associated with covariate-adjusted low HDL-C levels. Employed status and light physical activity in men and low fat intake in women were also identified as risk factors associated with low HDL-C.
Acknowledgements
This work is supported by a grant of the Korea National Institute of Health intramural research grant (2007-N63001-00).
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Castelli W.P., Garrison R.J., Wilson P.W. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA. 1986 Nov 28;256(20):2835–2838. [PubMed] [Google Scholar]
- 2.Kannel W.B., Neaton J.D., Wentworth D. Overall and coronary heart disease mortality rates in relation to major risk factors in 325,348 men screened for the MRFIT. Multiple Risk Factor Intervention Trial. Am Heart J. 1986 Oct;112(4):825–836. doi: 10.1016/0002-8703(86)90481-3. [DOI] [PubMed] [Google Scholar]
- 3.Despres J.P., Lemieux I., Dagenais G.R. HDL-cholesterol as a marker of coronary heart disease risk: the Quebec cardiovascular study. Atherosclerosis. 2000 Dec;153(2):263–272. doi: 10.1016/s0021-9150(00)00603-1. [DOI] [PubMed] [Google Scholar]
- 4.Gordon D.J., Probstfield J.L., Garrison R.J. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989 Jan;79(1):8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 5.Kesteloot H., Lee C.S., Park H.M. A comparative study of serum lipids between Belgium and Korea. Circulation. 1982 Apr;65(4):795–799. doi: 10.1161/01.cir.65.4.795. [DOI] [PubMed] [Google Scholar]
- 6.Knuiman J.T., West C.E., Burema J. Serum total and high density lipoprotein cholesterol concentrations and body mass index in adult men from 13 countries. Am J Epidemiol. 1982 Oct;116(4):631–642. doi: 10.1093/oxfordjournals.aje.a113446. [DOI] [PubMed] [Google Scholar]
- 7.Bruckert E., Baccara-Dinet M., McCoy F. High prevalence of low HDL-cholesterol in a pan-European survey of 8545 dyslipidaemic patients. Curr Med Res Opin. 2005 Dec;21(12):1927–1934. doi: 10.1185/030079905X74871. [DOI] [PubMed] [Google Scholar]
- 8.Simons L.A. Interrelations of lipids and lipoproteins with coronary artery disease mortality in 19 countries. Am J Cardiol. 1986 May 30;57(14):5G–10G. doi: 10.1016/0002-9149(86)90659-4. [DOI] [PubMed] [Google Scholar]
- 9.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001 May 16;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 10.International Diabetes Federation . 2005. The IDF consensus worldwide definition of the metabolic syndrome.www.idf.org/webdata/docs/IDF_metasyndrome_definition.pdf Available from: [accessed 07.11.05] [Report] [Google Scholar]
- 11.Lee S.Y., Park H.S., Kim D.J. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract. 2007 Jan;75(1):72–80. doi: 10.1016/j.diabres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Cohen J.D., Cziraky M.J., Cai Q. 30-year trends in serum lipids among United States adults: results from the National Health and Nutrition Examination Surveys II, III, and 1999–2006. Am J Cardiol. 2010 Oct 1;106(7):969–975. doi: 10.1016/j.amjcard.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 13.Kuzuya M., Ando F., Iguchi A. Changes in serum lipid levels during a 10 year period in a large Japanese population. A cross-sectional and longitudinal study. Atherosclerosis. 2002 Aug;163(2):313–320. doi: 10.1016/s0021-9150(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 14.Li J.Z., Wang S., Dong J. Present status of serum lipid levels in Beijing professional populations and its trend of changes over 15 years – a collaborative study of seven research and clinical laboratories in Beijing. Clin Chim Acta. 2005 Feb;352(1-2):199–207. doi: 10.1016/j.cccn.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 15.Ford E.S., Giles W.H., Dietz W.H. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002 Jan 16;287(3):356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 16.Okayama A., Ueshima H., Marmot M.G. Changes in total serum cholesterol and other risk factors for cardiovascular disease in Japan 1980–1989. Int J Epidemiol. 1993 Dec;22(6):1038–1047. doi: 10.1093/ije/22.6.1038. [DOI] [PubMed] [Google Scholar]
- 17.Batic-Mujanovic O., Zildzic M., Beganlic A. [The effect of cigarette smoking on HDL-cholesterol level] Med Arh. 2006;60(6 Suppl. 2):90–92. [PubMed] [Google Scholar]
- 18.Yoon Y.S., Oh S.W., Baik H.W. Alcohol consumption and the metabolic syndrome in Korean adults: the 1998 Korean National Health and Nutrition Examination Survey. Am J Clin Nutr. 2004 Jul;80(1):217–224. doi: 10.1093/ajcn/80.1.217. [DOI] [PubMed] [Google Scholar]
- 19.Eaton C.B., Lapane K.L., Garber C.E. Physical activity, physical fitness, and coronary heart disease risk factors. Med Sci Sports Exerc. 1995 Mar;27(3):340–346. [PubMed] [Google Scholar]
- 20.Ferrara A., Barrett-Connor E., Shan J. Total, LDL, and HDL cholesterol decrease with age in older men and women. The Rancho Bernardo Study 1984–1994. Circulation. 1997 Jul 1;96(1):37–43. doi: 10.1161/01.cir.96.1.37. [DOI] [PubMed] [Google Scholar]
- 21.Berns M.A., de Vries J.H., Katan M.B. Increase in body fatness as a major determinant of changes in serum total cholesterol and high density lipoprotein cholesterol in young men over a 10-year period. Am J Epidemiol. 1989 Dec;130(6):1109–1122. doi: 10.1093/oxfordjournals.aje.a115438. [DOI] [PubMed] [Google Scholar]
- 22.Park H.S., Yun Y.S., Park J.Y. Obesity, abdominal obesity, and clustering of cardiovascular risk factors in South Korea. Asia Pac J Clin Nutr. 2003;12(4):411–418. [PubMed] [Google Scholar]
- 23.Rezende F.A., Rosado L.E., Ribeiro R.C. Body mass index and waist circumference: association with cardiovascular risk factors. Arq Bras Cardiol. 2006 Dec;87(6):728–734. doi: 10.1590/s0066-782x2006001900008. [DOI] [PubMed] [Google Scholar]
- 24.Kanjilal S., Gregg E.W., Cheng Y.J. Socioeconomic status and trends in disparities in 4 major risk factors for cardiovascular disease among US adults, 1971–2002. Arch Intern Med. 2006 Nov 27;166(21):2348–2355. doi: 10.1001/archinte.166.21.2348. [DOI] [PubMed] [Google Scholar]
- 25.Schroder H., Marrugat J., Elosua R. Tobacco and alcohol consumption: impact on other cardiovascular and cancer risk factors in a southern European Mediterranean population. Br J Nutr. 2002 Sep;88(3):273–281. doi: 10.1079/BJN2002655. [DOI] [PubMed] [Google Scholar]
- 26.Peasey A., Bobak M., Malyutina S. Do lipids contribute to the lack of cardio-protective effect of binge drinking: alcohol consumption and lipids in three eastern European countries. Alcohol Alcohol. 2005 Sep-Oct;40(5):431–435. doi: 10.1093/alcalc/agh161. [DOI] [PubMed] [Google Scholar]
- 27.Criqui M.H., Wallace R.B., Heiss G. Cigarette smoking and plasma high-density lipoprotein cholesterol. The lipid research clinics program prevalence study. Circulation. 1980 Nov;62(4 Pt 2):IV70–IV76. [PubMed] [Google Scholar]
- 28.Panagiotakos D.B., Pitsavos C., Chrysohoou C. Effect of leisure time physical activity on blood lipid levels: the ATTICA study. Coron Artery Dis. 2003 Dec;14(8):533–539. doi: 10.1097/00019501-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Yang E.J., Chung H.K., Kim W.Y. Carbohydrate intake is associated with diet quality and risk factors for cardiovascular disease in U.S. adults: NHANES III. J Am Coll Nutr. 2003 Feb;22(1):71–79. doi: 10.1080/07315724.2003.10719278. [DOI] [PubMed] [Google Scholar]
- 30.Wolf G. High-fat, high-cholesterol diet raises plasma HDL cholesterol: studies on the mechanism of this effect. Nutr Rev. 1996 Jan;54(1 Pt 1):34–35. doi: 10.1111/j.1753-4887.1996.tb03772.x. [DOI] [PubMed] [Google Scholar]
- 31.Mooradian A.D., Haas M.J., Wong N.C. The effect of select nutrients on serum high-density lipoprotein cholesterol and apolipoprotein A-I levels. Endocr Rev. 2006 Feb;27(1):2–16. doi: 10.1210/er.2005-0013. [DOI] [PubMed] [Google Scholar]
- 32.Arnett D.K., McGovern P.G., Jacobs D.R., Jr. Fifteen-year trends in cardiovascular risk factors (1980–1982 through 1995–1997): the Minnesota Heart Survey. Am J Epidemiol. 2002 Nov 15;156(10):929–935. doi: 10.1093/aje/kwf133. [DOI] [PubMed] [Google Scholar]
- 33.Lichtman S.W., Pisarska K., Berman E.R. Discrepancy between self-reported and actual caloric intake and exercise in obese subjects. N Engl J Med. 1992 Dec 31;327(27):1893–1898. doi: 10.1056/NEJM199212313272701. [DOI] [PubMed] [Google Scholar]


