Abstract
Objectives
To confirm genotype diversities of clinical isolates of Bordetella pertussis and to evaluate the risk of pertussis outbreak in Korea.
Methods
Seven housekeeping genes and 10 antigenic determinant genes from clinical B. pertussis isolates were analyzed by Multilocus sequence typing (MLST).
Results
More variant pattern was observed in antigenic determinant genes. Especially, PtxS1 gene was the most variant gene; five genotypes were observed from eight global genotypes. In the bacterial type, the number of observed sequence types in the isolates was seven and the most frequent form was type 1 (79.6%). This major sequence type also showed a time-dependent transition pattern. Older isolates (1968 and 1975) showed type 1 and 6 in housekeeping genes and antigenic determinant genes, respectively. However, these were changed to type 2 and 1 in isolates 1999–2008. This transition was mainly attributed to genotype change of PtxS1 and Fim3 gene; the tendency of genotype change was to avoid vaccine-derived genotype. In addition, there was second transition in 2009. In this period, only the sequence type of antigenic determinant genes was changed to type 2. Based Upon Related Sequence Types (BURST) analysis confirmed that there were two clonal complexes (ACCI and ACCII) in the Korean isolates. Moreover, the recently increased sequence type was revealed as AST2 derived from AST 3 in ACCI.
Conclusions
Genotype changes in Korean distributing strains are still progressing and there was a specific driving force in antigenic determinant genes. Therefore continuous surveillance of genotype change of the distributing strains should be performed to confirm interrelationship of genotype change with vaccine immunity.
Keywords: genotype, Multilocus sequence typing (MLST), pertussis, virulence factors
1. Introduction
Pertussis is an infectious respiratory disease that is especially prevalent in newborn babies and infants who have not completed the basic vaccine schedule [1]. The pathogen of pertussis has been known as Bordetella pertussis since 1906 [2]; it is disseminated to only humans by infectious droplets released from carriers [3]. In the initial stage of infection, symptoms akin to a common cold appear, followed by characteristic symptoms of pertussis, such as paroxysmal cough and whoop sounds in breathing. After this paroxysmal stage, patients usually recover, with a long convalescent stage accompanied by coughing [1,4]. However, some patients may develop more severe symptoms such as pneumonia, convulsion, encephalopathy, rib fracture, and even death [4].
The global incidence rate of pertussis has greatly decreased following the introduction of a vaccine. However, there are several considerations in control of pertussis incidence. One is that local outbreaks occur even in highly vaccinated countries [5]; another is the transition of the infected age group from infants to adults [6]. One of the inferred reasons is that distributing strains evade the protection effect of vaccination by alteration of their surface antigens [7–9]. Currently, genetic polymorphisms were reported in four major antigenic determinant genes, such as pertussis toxin, filamentous hemagglutinin, pertactin, and fimbriae [10–15], which encode protein components of acellular pertussis vaccine. It was also reported that the distributing strains show distinct genotype from vaccine strain [9,16]. A third inferred reason is waning of vaccine-induced immunity with increasing age [1,9,17]. It is known that the vaccine’s efficacy starts to decrease after 6–8 years from the last vaccination. Therefore, vaccine-induced immunity is maintained for approximately 12–15 years, then immunity gradually diminishes [1,18].
In Korea, the average pertussis incidence rate from 2000 has been maintained at 11.5 cases per year [19]. However, in 2009, the number of pertussis patients rapidly increased to 66 cases, a 500% increment. Most patients (85.2%) were infants aged <1 year [20]. Although the number of patients decreased to 27 in 2010, it was still higher than the annual average for 2000–2008. Furthermore, 36.6% of contacted persons from eight cases were confirmed as PCR-positive, and most (80%) were adults.
Considering the above, analysis of the reason for this transient increase of pertussis incidences in 2009 was clearly needed, so as better to control pertussis outbreaks. Therefore we confirmed the genotypes in antigenic determinant genes and housekeeping genes of the clinical B. pertussis strains. Then, the genotype variations were analyzed by multilocus sequence typing (MLST).
MLST is a sequence-based typing method to identify isolates into clusters defined as sequence type (ST) with an identical genotype set [21]. The major advantages of the MLST method in bacterial typing are to allow rapid generation of clear results and easy comparisons between STs. Usually, seven core metabolic housekeeping genes originating from multilocus enzyme electrophoresis (MLEE) analysis are used in MLST analysis. However, other genes apart from housekeeping genes are also applicable if there are low genetic variations in housekeeping genes [22].
In this report, we analyzed genotype variations of seven housekeeping genes and 10 antigenic determinant genes to evaluate the genotype variation level of domestically distributed strains. In addition, the tendency of genotype change was analyzed to confirm the current status of pertussis incidence and to produce reliable data for pertussis control.
2. Materials and Methods
2.1. Bacterial isolates and culture condition
A total of 74 B. pertussis strains, mainly isolated between 2000 and 2009 in Korea, were analyzed (Figure 1). All isolated strains were preserved in deep freezer (–80°C) until analysis. Beside these clinical isolates, additionally five clinical strains isolated before 2000 and 3 reference strains (ATCC 10380, ATCC 9797, Tohama I) were included in all experiments. Reagan Lowe medium containing 10% horse blood was used to culture the isolated strains; inoculated medium was incubated in a humid incubator at 37°C for 5–7 days.
Figure 1.
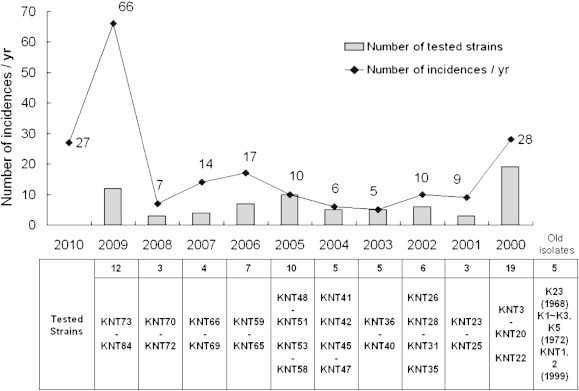
Annual incidence of pertussis in Korea and list of tested strains in this study. Data of annual incidences in Korea were retrieved from the WHO data and disease web statistics system of KCDC. The upper line and number indicate annual pertussis incidence of Korea from 2000. No isolate tested in this study is shown in the lower table. Old isolates are defined as strains isolated before 1999, as indicated. The strain number of the isolates before 1980 was assigned as K00 and the strains named as KNT00 were the isolates after 1990.
2.2. Isolation of genomic DNA
Genomic DNA of the isolates was prepared by QIAamp DNA mini kit (QIAGEN) according to the manufacturer’s instructions.
2.3. PCR of target genes and sequencing
PCR primers and PCR condition of most test genes were cited from previous reports [12,23–25] and the Bordetella Multi Locus Sequence Typing website (http://pubmlst.org/bordetella/) (Table 1). In addition, new primers for FHA, OmpQ, BapC, CyaA, Vag8, and TcfA genes were applied. After confirming amplification of target genes by specific primer sets, the PCR products were purified by QIAquick PCR purification kit (QIAGEN) and their nucleotide sequences confirmed by direct sequencing method.
Table 1.
PCR primers used in this study
| Gene | Size of gene | Primer sequence | Size of analyzed region | Reference |
|---|---|---|---|---|
| Housekeeping genes | ||||
| Adenylate kinase (Adk) | 657 bp |
|
530 bp (30 bp–560 bp) | Bordetella MLST sequence type database (www.Pubmlst.org) |
| Fumarate hydratase class II (FumC) | 1392 bp |
|
498 bp (396 bp–894 bp) | |
| Serine hydroxymethyl-transferase (GlyA) | 1248 bp |
|
499 bp (402 bp–901 bp) | |
| Aromatic amino-acid aminotransferase (TyrB) | 1203 bp |
|
487 bp (462 bp–949 bp) | |
| Isocitrate dehydrogenase (Icd) | 1257 bp |
|
512 bp (704 bp–1216 bp) | |
| Cytosol aminopeptidase (PepA) | 1500 bp |
|
508 bp (454 bp–962 bp) | |
| Phosphoglucomutase (Pgm) | 1383 bp |
|
548 bp (672 bp–1216 bp) | |
| Antigenic determinant genes | ||||
| Pertussis toxin subunit 1 (PtxS1) | 810 bp |
|
935 bp (−29 bp to +98 bp) | [25] |
| Pertactin (Prn) | 2739 bp |
|
1,410 bp (649 bp–2059 bp) | [23] |
| Filamentous hemagglutinin (FHA) | 10,772 bp |
|
372 bp (2348 bp–2720 bp) | This study |
| Fimbriae 2 (Fim2) | 624 bp |
|
388 bp (334 bp to +98 bp) | [12] |
| Fimbriae 3 (Fim3) | 615 bp |
|
810 bp (−68 bp to +119 bp) | [24] |
| Outer-membrane protein Q (OmpQ) | 1095 bp |
|
1,095 bp (1 bp–1,095 bp) | This study |
| Bordetella antitransporter-protein C (BapC) | 2268 bp |
|
935 bp (1,327 bp–2,262 bp) | This study |
| Adenylate cyclase toxin (CyaA) | 5121 bp |
|
1,043 bp (2,935 bp–3,987 bp) | This study |
| Virulence-activated gene (Vag8) | 2748 bp |
|
741 bp (1 bp–741 bp) | This study |
| Tracheal colonizing factor (TcfA) | 2019 bp |
|
1,105 bp (1 bp–1,105 bp) | This study |
2.4. Determination of genotype profiles and completion of sequence types
To determine the genotypes of housekeeping genes, sequence information of tested strains was queried to Bordetella MLST sequence-type database. For antigenic determinant genes the reported DNA sequences of each genotype were compared with those of the tested strains. Table 2 summarizes variation sites of 10 genes. Except Prn and TcfA the variation types of the other eight genes were point mutations at a single indicated site. However, Prn and TcfA genes showed systemic deletion of repeat units in the indicated region. To determine the genotype of each gene, reported DNA sequences were retrieved from the NCBI GenBank and aligned with tested sequences by the multiple alignment method using the MEGA program [26]. From this aligned DNA matrix the genotypes of tested strains were confirmed by comparison with the variable sites. If new variation sites were observed, the sequence was defined as new genotype and submitted to NCBI GenBank.
Table 2.
Genotype polymorphism of antigenic determinant genes analyzed in this study
| Gene | Polymorphic sites | Allele | References |
|---|---|---|---|
| Pertussis toxin subunit 1 (PtxS1) | PtxS1A(1)PtxS1B(2)PtxS1C(3)PtxS1D(4)PtxS1E(5)PtxS1F(6)PtxS1G(7)PtxS1H(8) | AJ245366AJ245367[25]AJ245368AJ006151AJ506994AJ506995HM212424 | |
| Pertactin (Prn) | Prn(1)Prn(2)Prn(3)Prn(4)Prn(5)Prn(6)Prn(7)Prn(8)Prn(9)Prn(10)Prn(11) | AF456359AF348484AF48485AJ011015AJ011016AF456357AJ133748AJ133245AF456356AJ784875AJ507642 | |
| Filamentous hemagglutinin (FHA) | FHA(1)FHA(2) | X52156AJ420989 | |
| Fimbriae2 (Fim2) | Fim2(1)Fim2(2) | AY845256AJ420988 | |
| Fimbriae3 (Fim3) | Fim3(1)Fim3(2)Fim3(3)Fim3(4)Fim3(5) | X51543AY464179AY464180AY464181AY845257 | |
| Outer membrane protein Q (OmpQ) | OmpQ(1)OmpQ(2) | U16266AJ420989 | |
| Bordetella autotransporter protein C (BapC) | BapC(1)BapC(2) | AF081499[12] | |
| Adenylate cyclase toxin (CyaA) | CyaA(1)CyaA(2) | Y00545BX470248 | |
| Virulence activated gene (Vag8) | Vag8(1)Vag8(2) | U90124AJ420992 | |
| Tracheal colonizing factor (TcfA) | TcfA(1)TcfA(2)TcfA(3)TcfA(4)TcfA(5)TcfA(6)TcfA(7) | U16754AJ009785AJ420991AJ507643AJ420992AY375533AM238667 |
For sequence types of housekeeping genes, genotype profiles of each isolate were queried to Bordetella MLST sequence-type database. For antigenic determinant genes we independently assigned STs according to the frequencies of genotype profiles confirmed in this study.
The relatedness among each ST was analyzed by the START program package [27]. From this analysis, a clustering result was represented as a dendrogram by the Unweighted Pair Group Method with Arithmetic Mean (UPGMA) method. In addition, we compared the two dendrograms produced by analysis of housekeeping genes and antigenic determinant genes. The clonality of each ST was also analyzed by BURST algorithm and represented as a minimum spanning tree.
3. Results
3.1. Genotype polymorphisms in housekeeping genes and antigenic determinant genes
Briefly, the polymorphic level of antigenic determinant genes was higher than housekeeping genes. As indicated in Table 3, the Korean genotypes of 7 housekeeping genes showed similar patterns of reported global genotypes [28]. Except Adk and Tyr B genes, only single a genotype was observed in the other five genes. There were two different genotypes in Adk and Tyr B genes; the major type was type 1 and type 3, respectively. However, in the case of antigenic determinant genes a more complicated genotype pattern was observed. Currently, the reported global genotypes were 45 in 10 tested genes. From these reported types 23 genotypes (52.3%) were confirmed in the Korean isolates (Table 3). Under the consideration of average genotype per gene, antigenic determinant gene and housekeeping gene showed 2.2 and 1.3, respectively, suggesting that while the genetic variations were rare events in housekeeping genes, more genetic variations occurred in antigenic determinant genes.
Table 3.
Summary of genotype and frequency observed in Korean isolates of B. pertussis
| Gene | No. reported genotypes | No. confirmed genotypes | Type and frequency (%) |
|---|---|---|---|
| Housekeeping genes | |||
| Adk | 2 | 2 | Type 1 (96.7), type 2 (3.3) |
| Fum C | 1 | 1 | Type 1 (100) |
| Gly A | 1 | 1 | Type 1 (100) |
| Tyr B | 2 | 2 | Type 1 (9.8), type 3 (90.2) |
| Icd | 1 | 1 | Type 1 (100) |
| Pep A | 1 | 1 | Type 1 (100) |
| Pgm | 1 | 1 | Type 1 (100) |
| Antigenic determinant genes | |||
| PtxS1 | 8 | 5 | Type 1 (93.2), type 2 (1.4), type 4 (1.4), type 5 (2.7%), type 8 (1.4) |
| Prn | 11 | 2 | Type 1 (96.7), type 6 (3.3) |
| Fha | 2 | 2 | Type 1 (96.7), type 2 (3.3) |
| Fim2 | 2 | 1 | Type 1 (100) |
| Fim3 | 5 | 2 | Type 1 (21.3), type 2 (78.7) |
| Omp Q | 2 | 2 | Type 1 (3.3), type 2 (96.7) |
| Bap C | 2 | 2 | Type 1 (96.7), type 2 (3.3) |
| Cya A | 2 | 2 | Type 1 (3.3), type 2 (96.7) |
| Vag 8 | 2 | 2 | Type 1 (3.3), type 2 (96.7) |
| Tcf A | 9 | 2 | Type 1 (3.3), type 2 (96.7) |
Adk, adenylate kinase; fumC, fumarate hydratase class II, glyA, serine hydroxymethyltransferase; tyrB, aromatic amino-acid aminotransferase; icd, isocitrate dehydrogenase; pepA, cytosol aminopeptidase; pgm, phosphoglucomutase; ptxS1, pertussis toxin; prn, pertactin; fha, filamentous hemagglutinin; fim2, fimbriae2; fim3, fimbriae3; ompQ, outer-membrane protein Q; bapC, Bordetella autotransporter protein C; cyaA, adenylate cyclase toxin; vag8, virulence-activated gene; tcfA, tracheal colonizing factor.
From the 10 antigenic determinant genes global genotype variations were mainly reported in PtxS1, Prn, Fim3, and TcfA genes (Table 3). However, in the Korean isolates, polymorphisms of Fim3 and TcfA were relatively low as compared with global genotypes. The most polymorphic gene was PtxS1; 5 types (1, 2, 4, 5, and 8) were observed and the major form was type 1 (93.2%). Especially, the PtxS1 genotype of KNT 72 was confirmed as a new genotype. This variation was a silent point mutation occurring at site 600 bp where base C was changed to T. We listed up this new sequence to GenBank (HM212424) and assigned this genotype as type 8.
Compared of the prevalence of PtxS1 genotype from previous reports of other countries [10,12,16,29–33] confirmed that the most frequent global genotype was type 1, except in Japan (Table 4) and the next most frequent form was type 2. Other genotypes (types 3–8) were rarely reported. Especially, in Australia and Italy only type 1 was reported. However, Japan and the USA showed different patterns; in the former, type 2 showed higher frequency than type 1, whereas in the latter the ratio of type 2 was higher than in other reported countries. In the case of the Korean isolates the major genotype was type 1 similar to other countries, but rare types (type 4 and 5) were also observed, and even a new type (type 8). Therefore Korean isolates showed the most diverse pattern in the PtxS1 gene among countries.
Table 4.
Comparison of PtxS1 genotypes with the reports from other countries
| Allele type | GenBank accession no. or ref. | Korea (n = 81b)1) |
Japan (n = 107)2) |
Taiwan (n = 80)3) |
USA (n = 152)4) |
Finland (n = 122)5) |
UK (n = 335)6) |
Sweden (n = 1006)7) |
Australia (n = 46)8) |
Italy (n = 30)9) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1968–2010 | 1988–2001 | 1998–2004 | 1935–1996 | 1953–2003 | 1920–2002 | 1970–2003 | 1970–2003 | 1993–1995 | ||
| S1A (1) | AJ245366 | 71a | 23 | 79 | 98 | 115 | 288 | 998 | 46 | 30 |
| S1B (2) | AJ245367 | 6 | 84 | 1 | 51 | 7 | 36 | 8 | – | – |
| S1C (3) | [25] | – | – | – | – | – | – | – | – | – |
| S1D (4) | AJ245368 | 1 | – | – | 3 | – | – | – | – | – |
| S1E (5) | AJ006151 | 2 | – | – | – | – | – | – | – | – |
| S1F (6) | AJ506994 | – | – | – | – | – | – | – | – | – |
| S1G (7) | AJ506995 | – | – | – | – | – | – | – | – | – |
| S1G (8) | HM212424 | 1 | – | – | – | – | – | – | – | – |
Strain number reconstructed from data of the indicated reference.
Number of tested strain was reconstructed from cited references (superior number). 1) In this study; 2) Kodama et al., 2004 [29]; 3) Yao et al., 2005 [30]; 4) Cassisay et al., 2000 [10]; 5) Elomaa et al., 2005 [31]; 6) Packard et al., 2004 [12]; 7) Hallander et al., 2005 [16]; 8) Byrne and Slack, 2006 [32]; 9) Mastrantonio et al., 1999 [33].
While the PtxS1 genotype was most diverse in the Korean isolates, the Prn genotype showed a different pattern from those in the reports by other countries [10,12,16,29–33]. Currently, 11 different prn genotypes were reported and the major difference between genotypes was attributed to deletion of repeated amino acid sequence units (GGxxP and PQP) [13]. From these genotypes the frequently observed genotypes were type 1, 2, and 3. Other genotypes (types 4–11) were rarely observed. As indicated in Table 5, more than types were reported from other countries, and the major genotype was also different in each country. Japan, the UK, and Australia showed type 1 as the major genotype, whereas type 2 was major genotype in Taiwan, Finland, and Sweden. In the Korean isolates, 3 genotypes were observed; the major genotype was type 1. Notably, the rare type 6 was also observed. Therefore, the Prn gene of Korean isolates was not as diverse a gene as those reported from other countries.
Table 5.
Comparison of Prn genotypes with reports from other countries
| Allele type | GenBank accession no. or ref. | Korea (n = 81b)1) |
Japan (n = 107)2) |
Taiwan (n = 80)3) |
USA (n = 152)4) |
Finland (n = 122)5) |
UK (n = 335)6) |
Sweden (n = 919)7) |
Australia (n = 46)8) |
Italy (n = 129)9) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1968–2010 | 1988–2001 | 1998–2004 | 1935–1996 | 1953–2003 | 1920–2002 | 1992–2000 | 1970–2003 | 1993–1995 | ||
| Prn (1) | AF456359 | 72a | 88 | 6 | 86 | 26 | 213 | 51 | 38 | 8 |
| Prn (2) | AF348484 | 7 | 18 | 72 | 66 | 92 | 111 | 748 | 3 | 53 |
| Prn (3) | AF.48485 | – | 1 | 2 | – | 2 | 6 | 116 | 5 | 65 |
| Prn (4) | AJ011015 | – | – | – | – | 2 | – | – | – | – |
| Prn (5) | AJ011016 | – | – | – | – | – | – | – | – | 3 |
| Prn (6) | AF456357 | 2 | – | – | – | – | – | – | – | – |
| Prn (7) | AJ133784 | – | – | – | – | – | – | – | – | – |
| Prn (8) | AJ133245 | – | – | – | – | – | – | – | – | – |
| Prn (9) | AF456356 | – | – | – | – | – | – | – | – | – |
| Prn (10) | AJ784875 | – | – | – | – | – | – | – | – | – |
| Prn (11) | AJ507642 | – | – | – | – | – | – | – | – | – |
Strain number reconstructed from data of the indicated reference.
Number of tested strain reconstructed from cited references (superior number). 1) In this study; 2) Kodama et al., 2004 [29]; 3) Yao et al., 2005 [30]; 4) Cassisay et al., 2000 [10]; 5) Elomaa et al., 2005 [31]; 6) Packard et al., 2004 [12]; 7) Hallander et al., 2005 [16]; 8) Byrne and Slack, 2006 [32]; 9) Mastrantonio et al., 1999 [33].
3.2. Multilocus sequence type determination and comparative analysis of sequence types
Currently, a total of 43 STs (housekeeping gene sequence types; HSTs) for seven housekeeping genes were reported in Bordetella MLST database (http://pubmlst.org). These HSTs were established from analysis of collected genotype data from three Bordetella species (B. pertussis, Bordetella bronchiseptica, and Bordetella parapertussis) by Diavatopoulos et al. [28]. From these HSTs, only three HST types (1, 2 and 24) are originated from B. pertussis species. According to the analysis of clonality by Bordetella MLST database, 43 STs are reorganized into four clonal complexes. From these clonal complexes, clonal complex II is the group of B. pertussis comprising HST1, HST2 and HST24.
In the Korean isolates (Table 6), 3 ST types (HST1, HST2, and HST24) were also observed and the frequencies of each HST were 92.6% for HST2, 4.9% for HST1, and 2.5% for HST24 as similar to the pattern of MLST database. Therefore the distributing strains in Korea were in a single clonal complex II, as indicated in the MLST database.
Table 6.
Summary of genotype profiles from Korean isolates of B. pertussis
| ST of Housekeeping genes (HST) | Genotype |
||||||
|---|---|---|---|---|---|---|---|
| GlyA | Adk | Icd | Tyr B | Pep A | Pgm | Fum C | |
| 1 (4.9%) | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2 (92.6%) | 1 | 1 | 1 | 3 | 1 | 1 | 1 |
| 24 (2.5%) | 1 | 2 | 1 | 1 | 1 | 1 | 1 |
| ST of Antigenic determinant genes (AST) | Genotype |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PtxS1 | Prn | FHA | Fim2 | Fim3 | OmpQ | BapC | CyaA | Vag8 | TcfA | |
| 1 (72.8%) | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 2 | 2 | 2 |
| 2 (8.6%) | 1 | 2 | 1 | 1 | 1 | 2 | 1 | 2 | 2 | 2 |
| 3 (6.2%) | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 2 | 2 |
| 4 (2.5%) | 5 | 6 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| 5 (1.2%) | 4 | 1 | 1 | 1 | 2 | 2 | 1 | 2 | 2 | 2 |
| 6 (7.4%) | 2 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 2 | 2 |
| 7 (1.2%) | 8 | 1 | 1 | 1 | 2 | 2 | 1 | 2 | 2 | 2 |
Adk, adenylate kinase; fumC, fumarate hydratase class II, glyA, serine hydroxymethyltransferase; tyrB, aromatic amino-acid aminotransferase; icd, isocitrate dehydrogenase; pepA, cytosol aminopeptidase; pgm, phosphoglucomutase; ptxS1, pertussis toxin; prn, pertactin; fha, filamentous hemagglutinin; fim2, fimbriae2; fim3, fimbriae3; ompQ, outer-membrane protein Q; bapC, Bordetella autotransporter protein C; cyaA, adenylate cyclase toxin; vag8, virulence-activated gene; tcfA, tracheal colonizing factor.
In the case of STs of antigenic determinant genes (antigenic determinant gene sequence types; ASTs), a more divergent pattern was observed. The Korean isolates were divided into seven ASTs based on the genotype profiles of 10 antigenic determinant genes. Because there was no database for ASTs of 10 antigenic determinant genes, we independently assigned ASTs according to the observed frequencies. As shown in Table 6, type 1 (most frequent form in the Korean isolates) showed 72.8% of frequency and other types (2–7) showed frequencies <10%.
When we analyzed relatedness among these HSTs by BURST algorithm using the START program package [27], the STs confirmed in the Korean isolates were grouped in single clonal complex as expected and the central ST type was determined as HST1 (Table 7). The strains included in HST1 were K1, K2, K5, and K23. These isolates were old strains isolated in 1968 and 1972 (Figure 1). In the old strains, the frequently observed ST profile was HST1, but the major sequence type was changed to HST2 in the strains isolated from 2000. Although HST24 was also observed, the frequency was too low and only two strains were transiently observed in 2000 and 2001. Therefore a major sequence-type transition from HST1 to HST2 was confirmed according to time period in the Korean isolates.
Table 7.
Lineage assignment analysis by genotype profiles of housekeeping genes and antigenic determinant genes
| Gene type | Groupa | ST | Frequency | SLV | DLV | SAT |
|---|---|---|---|---|---|---|
| Housekeeping genes |  |
1b224 | 4752 | 211 | 11 | ––– |
| Antigenic determinant genes |
 Singleton Singleton |
61b35724 | 65951172 | 133221– | 422222– | 000112– |
SLV, single locus variation; DLV, double locus variation; SAT, satellite strain.
Group definition (N−1).
Central types.
In the AST analysis, as shown in Table 7, it was revealed that the Korean isolates were categorized into two clonal complexes, except AST4. AST4 showed a unique and quite different genotype as compared with other genotype profiles. This sequence type was not a frequently observed form in the Korean isolates, and only two strains (KNT22 and KNT23) were observed in 2000–01. Therefore it was inferred that this sequence type was transiently introduced type from outside Korea.
These two clonal complexes were assigned as antigenic clonal complex (ACC) I and II. ACC I was composed of AST2, AST3, and AST6. The property of this clonal complex was old or new sequence type. AST3 was the central type of ACC I and emerged in 2000 and reemerged in 2009. AST6 was only confirmed in old strains isolated in 1968 and 1972. However, AST2 was the new sequence type that appeared in 2009, and the major difference was a change of genotype of Prn from 1 to 2.
The other clonal complex, ACC II, was composed of AST1, AST5, and AST7. The major sequence type of ACC II was AST1 and AST1 was also determined to be the central type. The strains of AST1 were frequently observed from 1999 to 2008. However, the frequency was greatly decreased in 2009.
These different results between HSTs and ASTs indicate that there were different driving forces for genetic events. This was clearly confirmed when these two gene categories were compared by UPGMA tree. As shown in Figure 2, all the strains belonging to HST2 showed more diverse genotype variations in antigenic determinant genes, and finally diverged into six ASTs, indicating that there were different evolutional changes, such as vaccination in antigenic determinant genes [9,11,25,30,33–36].
Figure 2.
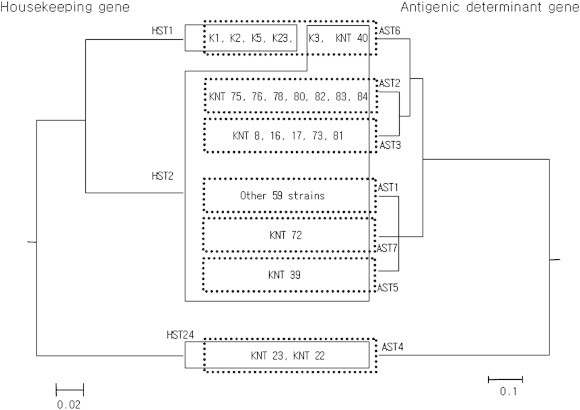
Comparison of UPGMA trees originating from analysis of housekeeping genes and antigenic determinant genes. Two UPGMA trees of genotype profiles deriving from housekeeping genes (HSTs) and antigenic determinant genes (ASTs) represented as merged form. The left-side tree was derived from housekeeping genes and the right side tree from antigenic determinant genes. The line box indicates sequence type clustered by housekeeping genes analysis and dot box strain sequence type clustered by antigenic determinant genes analysis.
4. Discussion
The emergence mechanisms of variants are not exactly known. However, it is considered that there are two major ways for variants to emerge. One is that existing strains acquire different genotype and phenotype by mutation; the other is that distributing strains are selected from the population by factors such as vaccination. From these two considerations, it is not clear which is predominant. In the case of B. pertussis, this species is known to be a homologous population by MLEE pattern analysis [37]. The variant sequence was also rarely confirmed in housekeeping genes [11]. However, there were many reports about genotype changes in antigenic determinant genes. Especially it was also suggested by Smith et al. [4] that because B. pertussis is an exquisitely adapted pathogen to the human respiratory tract by evolutionary process, its rapid evolution rate should be considered in developing a vaccine and protecting efficacy. Therefore it is inferred that emergence of the genotype variants in antigenic determinant genes is the active process of B. pertussis to avoid the protective effect of vaccination.
These genotype variations also showed a transition pattern according to the vaccine introduction. In the Netherlands, the UK, Sweden, Taiwan, and Japan [9,11–14,25,32] it was reported that the major sequence types before vaccine introduction gradually changed thereafter. That is, the distributed strains containing the same genotype with the vaccine strain were gradually reduced after vaccination, while other strains containing different genotypes appeared. In Korea, similar genotype changes were confirmed by MLST analysis. One of the advantages of MLST is that it is easy to see transitional trends of bacterial type over the long term. In this analysis, the Korean isolates also showed a transitional pattern in antigenic determinant genes. The first transition was observed in the isolates of 1999. In this stage, the genotype profiles of the major isolates were changed from AST6 (2,1,1,1,1,2,1,2,2,2) to AST1 (1,1,1,1,2,2,1,2,2,2). As indicated in Table 8, this was attributed to genotype change of PtxS1 and Fim3 genes. In addition, major ST of housekeeping gene also changed from HST1 (1,1,1,1,1,1,1) to HST2 (1,1,1,3,1,1,1). When we considered that the acellular vaccine derived from Tohama I strain was introduced from 1982 [19], these genotype change patterns also showed a tendency to avoid vaccine-derived genotypes as described above.
Table 8.
Transition pattern according to time period of major STs in housekeeping genes and antigenic determinant genes
| Time periods | Housekeeping genes |
Antigenic determinant genes |
||||
|---|---|---|---|---|---|---|
| Major ST | Genotype profilea | Frequency (%) | Major ST | Genotype profileb | Frequency (%) | |
| Tohama I | 1 | 1,1,1,1,1,1,1 | – | – | 2,1,2,1,1,2,1,2,2,2 | – |
| Old (1968/1975) | 1 | 1,1,1,1,1,1,1 | 80 | 6 | 2,1,1,1,1,2,1,2,2,2 | 100 |
| 1999 ∼ 2008 | 2 | 1,1,1,3,1,1,1 | 96.9 | 1 | 1,1,1,1,2,2,1,2,2,2 | 87.5 |
| Outbreak in 2009 | 2 | 1,1,1,3,1,1,1 | 100 | 2 | 1,2,1,1,1,2,1,2,2,2 | 58.3 |
Gene order of housekeeping genes: GlyA, Adk, Icd, TyrB, PepA, Pgm, FumC.
Gene order of antigenic determinant genes: PtxS1, Prn, FHA, Fim2, Fim3, OmpQ, BapC, CyaA, Vag8, TcfA.
A second transition was also observed, in 2009. At that time, pertussis incidence was greatly increased by 500% as compared with the average for the previous decade. AST of the isolates in 2009 changed from AST1 to AST2 (1,2,1,1,1,2,1,2,2,2). This ST change was attributed to genotype changes of Prn and Fim3 genes. Especially, the genotype change of Prn gene was first confirmed in the Korean isolates and new ST was diverged. It was also clearly confirmed by minimum spanning tree (Figure 3). As shown in Figure 3, the ASTs of the Korean isolates were divided into two clonal complexes, ACC I and ACC II. From these clonal complexes it was revealed that AST1 of ACC II was distributed until 2008 and AST2 of ACC I newly appeared from 2009.
Figure 3.
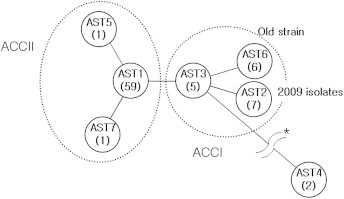
Minimum spanning tree of Korean isolates by MLST profiles of antigenic determinant genes. A minimum spanning tree was constructed by sequence type profiles deriving from antigenic determinant genes. Lines-circles indicate sequence type; numbers in parentheses are the number of strains included in each sequence type. Dots-circles indicate the clonal complex confirmed by BURST analysis. Because the distance between AST4 with AST3 was too long, we omitted (asterisk). AST2 was newly appearing sequence type when the pertussis incidence was greatly increased.
Genotype changes of the distributing strains are still actively progressing events. Although the polymorphic status of Korean isolates was relatively lower than other countries, there is enough evidence to warn of the risk of pertussis outbreak in Korea. For this reason, construction of an efficient and continuous surveillance system to detect emergence of genotype variants is required.
Acknowledgement
This study was supported by an intramural grant (No. 4800-4845-300) of Korea National Institute of Health.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Centers for Disease Control and Prevention . Centers for Disease Control and Prevention; Atlanta, GA: 2000. Guidelines for the control of pertussis outbreaks. [Google Scholar]
- 2.Bordet J., Gengou O. Le microbe de la coqueluche. Ann Inst Pasteur Lille. 1906;20:731–741. [Google Scholar]
- 3.Mattoo S., Cherry J.D. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microb Rev. 2005 Apr;18(2):326–382. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith A.M., Guzman C.A., Walker M.J. The virulence factors of Bordetella pertussis: as matter of control. FEMS Microbiol Rev. 2001 May;25(3):309–333. doi: 10.1111/j.1574-6976.2001.tb00580.x. [DOI] [PubMed] [Google Scholar]
- 5.Broutin H., Guegan J.F., Elguero E. Large-scale comparative analysis of pertussis population dynamics: periodicity, synchrony, and impact of vaccination. Am J Epidemiol. 2005 Jun;161(12):1159–1167. doi: 10.1093/aje/kwi141. [DOI] [PubMed] [Google Scholar]
- 6.Rothstein E., Edwards K. Health burden of pertussis in adolescents and adults. Pediatr Infect Dis J. 2005 May;24(5 Suppl):S44–S47. doi: 10.1097/01.inf.0000160912.58660.87. [DOI] [PubMed] [Google Scholar]
- 7.de Melker H.E., Conyn-van Spaendonck M.A.E., Rumke H.C. Pertussis in the Netherlands: an outbreak despite high levels of immunization with whole-cell vaccine. Emerg Infect Dis. 1997 Apr–Jun;3(2):175–178. doi: 10.3201/eid0302.970211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Melker H.E., Schellekens J.F.P., Neppelenbroek S.E. Reemergence of pertussis in the highly vaccinated population of the Netherlands: observations on surveillance data. Emer Inf Dis. 2000 Jul–Aug;6(4):348–357. doi: 10.3201/eid0604.000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mooi F.R., van Loo I.H.M., King A.J. Adaptation of Bordetella pertussis to vaccination: a cause for its reemergence? Emer Inf Dis. 2001;7(3 Suppl):526–528. doi: 10.3201/eid0707.017708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cassiday P., Sanden G., Heuvelman K. Polymorphism in Bordetella pertussis pertactin and pertussis toxin virulence factors in the United States, 1953–1999. J Inf Dis. 2000 Nov;182(5):1402–1408. doi: 10.1086/315881. [DOI] [PubMed] [Google Scholar]
- 11.van Loo I.H.M., Heuvelman K.J., King A.J., Mooi F.R. Multilocus sequence typing of Bordetella pertussis based on surface protein gene. J Clin Microb. 2002 Jun;40(6):1994–2001. doi: 10.1128/JCM.40.6.1994-2001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Packard E.R., Parton R., Coote J.G., Fry N.K. Sequence variation and conservation in virulence-related genes of Bordetella pertussis isolates from the UK. J Med Microb. 2004 May;53(5):355–365. doi: 10.1099/jmm.0.05515-0. [DOI] [PubMed] [Google Scholar]
- 13.Fry N.K., Neal S., Harrison T.G. Genotypic variation in the Bordetella pertussis virulence factors pertactin and pertussis toxin in historical and recent clinical isolates in the United Kingdom. Infect Immun. 2001 Sep;69(9):5520–5528. doi: 10.1128/IAI.69.9.5520-5528.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mooi F.R., He Q., van Oirschot H., Mertsola J. Variation in the Bordetella pertussis virulence factors pertussis toxin and pertactin in vaccine strains and clinical isolated in Finland. Infect Immun. 1999 Jun;67(6):3133–3134. doi: 10.1128/iai.67.6.3133-3134.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byrne S., Slack A.T. Analysis of Bordetella pertussis pertactin and pertussis toxin types from Queensland, Australia, 1999–2003. BMC Infect Dis. 2006 Mar;6:53–60. doi: 10.1186/1471-2334-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hallander H.O., Advani A., Donnelly D. Shifts of Bordetella pertussis variants in Sweden from 1970 to 2003, during three periods marked by different vaccination programs. J Clin Microb. 2005 Jun;43(6):2856–2865. doi: 10.1128/JCM.43.6.2856-2865.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hochwald O., Bamberger E., Srugo I. The return of pertussis: who is responsible? What can be done? Isr Med Assoc J. 2006 May;8(5):301–307. [PubMed] [Google Scholar]
- 18.World Health Organization Pertussis vaccines. Wkly Epidemiol Rec. 2005 Jan;80(4):31–39. [PubMed] [Google Scholar]
- 19.Korea Centers for Disease Control and Prevention Pertussis. Communicable Diseases Monthly Report (CDMR) 2007;18:2–8. [Google Scholar]
- 20.Korea Centers for Disease Control and Prevention Increasing incidence of pertussis in Korea, 2009. PHWR. 2009;2(42):709. [Google Scholar]
- 21.Spratt B.G. Multilocus sequence typing: molecular typing of bacterial pathogens in an era of rapid DNA sequencing and the Internet. Curr Opin Microbiol. 1999 Jun;2(3):312–316. doi: 10.1016/S1369-5274(99)80054-X. [DOI] [PubMed] [Google Scholar]
- 22.Tankouo-Sandjong B., Sessitsch A., Liebana E. MLST-v, multilocus sequence typing based on virulence genes, for molecular typing of Salmonella enteric subsp. enteric serovars. J Microbiol Method. 2007 Apr;69(1):23–36. doi: 10.1016/j.mimet.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Charles I.G., Dougan G., Pickard D. Molecular cloning and characterization of protective outer membrane protein P.69 from Bordetella pertussis. Proc Natl Acad Sci U S A. 1989 May;86(10):3554–3558. doi: 10.1073/pnas.86.10.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsang R.S., Lau A.K., Sill M.L. Polymorphisms of the fimbria fim3 gene of Bordetella pertussis strains isolated in Canada. J Clin Microbiol. 2004 Nov;42(11):5364–5367. doi: 10.1128/JCM.42.11.5364-5367.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mooi F.R., van Oirschot H., Heuvelman K. Polymorphism in the Bordetella pertussis virulence factors P.69/pertactin and pertussis toxin in the Netherlands: temporal trends and evidence for vaccine-driven evolution. Infect Immun. 1998 Feb;66(2):670–675. doi: 10.1128/iai.66.2.670-675.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar S., Tamura K., Nei M. MEGA3: integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief Bioinform. 2004 Jun;5(2):150–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- 27.Jolley K.A., Feil E.J., Chan M.S., Maiden M.C. Sequence type analysis and recombinational tests (START) Bioinformatics. 2001 Dec;17(12):1230–1231. doi: 10.1093/bioinformatics/17.12.1230. [DOI] [PubMed] [Google Scholar]
- 28.Diavatopoulos D.A., Cummings C.A., Schouls L.M. Bordetella pertussis, the causative agent of whooping cough, evolved from a distinct, human-associated lineage of B. bronchiseptica. PLos Pathog. 2005 Dec;1(4):e45. doi: 10.1371/journal.ppat.0010045. doi:10.1371/journal.ppat.0010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kodama A., Kamachi K., Horiuchi Y. Antigenic divergence suggested by correlation between antigenic variation and pulsed-field gel electrophoresis profiles of Bordetella pertussis isolates in Japan. J Clin Microbiol. 2004 Dec;42(12):5453–5457. doi: 10.1128/JCM.42.12.5453-5457.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao S.M., Lin Y.C., Chou C.Y. Antigenic divergence of Bordetella pertussis isolates in Taiwan. J Clin Microbiol. 2005 Nov;43(11):5457–5461. doi: 10.1128/JCM.43.11.5457-5461.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elomaa A., Advani A., Donnelly D. Strain variation among Bordetella pertussis isolates in Finland, where the whole-cell pertussis vaccine has been used for 50 years. J Clin Microbiol. 2005 Aug;43(8):3681–3687. doi: 10.1128/JCM.43.8.3681-3687.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mastrantonio P., Spigaglia P., van Oirschot H. Antigenic variants in Bordetella pertussis strains isolated from vaccinated and unvaccinated children. Microbiology. 1999 Aug;145(8):2069–2075. doi: 10.1099/13500872-145-8-2069. [DOI] [PubMed] [Google Scholar]
- 33.Bottero D., Gaillard M.E., Fingermann M. Pulsed-field gel electrophoresis, pertactin, pertussis toxin S1 subunit polymorphisms, and surfaceome analysis of vaccine and clinical Bordetella pertussis strains. Clin Vaccine Immunol. 2007 Nov;14(11):1490–1498. doi: 10.1128/CVI.00177-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diavatopoulos D.A., Hijinen M., Mooi F.R. Adaptive evolution of the Bordetella autotransporter pertactin. J Evol Biol. 2006 Nov;19(6):1931–1938. doi: 10.1111/j.1420-9101.2006.01154.x. [DOI] [PubMed] [Google Scholar]
- 35.King A.J., Berbers G., van Oirschot H.F. Role of the polymorphic region 1 of the Bordetella pertussis protein pertactin in immunity. Microbiology. 2001 Nov;147(11):2885–2895. doi: 10.1099/00221287-147-11-2885. [DOI] [PubMed] [Google Scholar]
- 36.Musser J.M., Hewlett E.L., Peppler M.S., Selander R.K. Genetic diversity and relationships in populations of Bordetella spp. J Bacteriol. 1986 Apr;166(1):230–237. doi: 10.1128/jb.166.1.230-237.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Zee A., Mooi F., van Embden Musser J. Molecular evolution and host adaptation of Bordetella spp.: phylogenetic analysis using multilocus enzyme electrophoresis and typing with three insertion sequences. J Bacteriol. 1997 Nov;179(21):6609–6617. doi: 10.1128/jb.179.21.6609-6617.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


