Abstract
Nanometer-sized gold, due to its beautiful and bountiful color and unique optical properties, is a versatile material for many industrial and societal applications. We have studied the effect of gold nanoparticles on Salmonella typhimurium strain TA 102. The gold nanoparticles in solution prepared using the citrate reduction method is found not to be toxic or mutagenic but photomutagenic to the bacteria; however, careful control experiments indicate that the photomutagenicity is due to the co-existing citrate and Au3+ ions, not due to the gold nanoparticle itself. Au3+ is also found to be photomutagenic to the bacteria at concentrations lower than 1 µM, but toxic at higher concentrations. The toxicity of Au3+ is enhanced by light irradiation. The photomutagenicity of both citrate and Au3+ is likely due to the formation of free radicals, as a result of light-induced citrate decarboxylation or Au3+ oxidation of co-existing molecules. Both processes can generate free radicals that may cause DNA damage and mutation. Studies of the interaction of gold nanoparticles with the bacteria indicate that gold nanoparticles can be absorbed onto the bacteria surface but not able to penetrate the bacteria wall to enter the bacteria.
Keywords: gold nanoparticle, gold (III), citrate, phototoxicity, Salmonella TA 102
Introduction
The use of colloidal gold, or gold nanoparticles (GNPs), as a coloring agent dates all the way back to the fourth century, and its use in artwork and other materials dates back to the seventeenth century (Edwards and Thomas, 2007; Jennings and Strouse, 2007). As early as 1920, GNPs were used in clinical trials to treat rheumatoid arthritis (Aaseth et al., 1998). GNPs have great biomedical applications in drug delivery and gene therapy (Jain, 2005; Paciotti et al., 2004), imaging and photothermal therapy (EI-Sayed et al., 2005; Gobin et al., 2007; Higby 1982; Huang et al., 2006; Loo et al., 2005; Murphy et al., 2008; Sokolov et al., 2003), and nanoprobes (Darbha et al., 2008; He et al., 2008; Huang et al., 2006; Ray et al., 2005) because of their ease of preparation, readiness for bio-conjugation, and extraordinary optical properties. While enjoying the great variety of applications of GNPs, researchers are aware that some of the GNP forms may be toxic or harmful to humans (Goodman et al., 2004; Hauck et al., 2008; Mao et al., 2007; Mironava et al., 2010; Murphy et al., 2008; Pan et al., 2007; Pernodet et al., 2006; Ray et al., 2009; Takahashi et al., 2006; Wang et al., 2008; Wiwanitkit et al., 2008). Reports indicate that GNPs’ toxicity is related to their surface chemistry including coating material, size, shape, and biological targets tested (Chithrani et al., 2006; Connor et al., 2005; Goodman et al., 2004; Hauck et al., 2008; Huff et al., 2007; Mao et al., 2007; Mironava et al., 2010; Murphy et al., 2008; Pan et al., 2007; Patra et al., 2007; Pernodet et al., 2006; Ray et al., 2009; Wang et al., 2008; Yang et al., 2008). Pan et al. reported that 15 nm GNPs are nontoxic at even high concentrations, while 1–2 nm particles cause predominantly rapid cell death by necrosis (Pan et al., 2007). Chithrani et al. showed that the kinetics for GNP alteration and saturation inside the mammalian cells depend on their size and shape (Chithrani et al., 2006). Pernodet et al. described the influence of GNPs on the proliferation, spreading and adhesion, morphological structure, migration, and protein synthesis in human dermal fibroblast cells (Pernodet et al., 2006). The surface modifiers included a range of anionic, neutral, and cationic groups, and surface charge also play an important role in toxicity of GNPs (Connor et al., 2005; Goodman et al., 2004; Takahashi et al., 2006; Wang et al., 2008). Goldman et al. suggested that the cationic GNPs were more cytotoxic than the same-size anionic ones due to the affinity of cationic particles to the negatively charged cell membrane. Several research groups examined the cytotoxicity of GNPs in different cell types (Goodman et al., 2004; Patra et al., 2007). Parta et al. found that citrate-capped GNPs were not cytotoxic to baby hamster kidney cells and human hepatocellular liver carcinoma cells, but cytotoxic to human carcinoma cells (A549) at certain concentrations (Patra et al., 2007).
Despite these efforts, there are still questions about GNPs and their toxicity that need to be understood. In this report, we present data on the interaction of 16 nm gold nanospheres stabilized by citrate ions with Salmonella typhimurium bacteria strain TA 102. The study takes into account the effect by co-existing chemicals, Au3+ and citrate, and light irradiation.
Materials and method
Salmonella typhimurium strain TA 102 was provided by Dr Bruce Ames from the University of California (Berkeley, California, USA). Hydrogen tetrachloroaurate (HAuCl4), trisodium citrate, and 8-methoxypsoralen were purchased from Sigma-Aldrich (St Louis, Missouri, USA). Other solvents and chemicals were in their highest purity grade.
Synthesis of gold nanoparticles
GNPs were synthesized using the citrate reduction method as reported (Kimling et al., 2006; Ray 2006; Ray et al., 2005; Turkevich et al., 1951). In short, 10 mM of HAuCl4 and 1% of sodium citrate was prepared in water. Then, 2 mL of 10 mM of HAuCl4 were added to 98 mL of water and was brought to boiling while stirred. Then 3 mL of the 1% sodium citrate was added and the boiling continued for further 30 min. The final concentrations of HAuCl4 and sodium citrate were 0.2 mM and 1.2 mM, respectively. A series of color changes occurred from light purple to wine red. The solution was cooled to room temperature. Transmission electron microscope (TEM) images and UV-Vis absorption spectra were used to characterize the nanoparticles. The absorption of the gold nanoparticle has a peak at 522 nm, indicative of 16 nm diameter nanospheres (Kim et al., 2006; Kimling et al., 2006; Kumar et al., 2007).
TEM imaging of bacteria exposed to GNPs
A solution of 100 µL GNPs (16 nm, 1.25 mg/mL) was mixed with 100 µL of 10 mM sodium phosphate buffer and 5 µL of the bacteria in solution. The mixture was allowed to stay for 15 min in the dark or with 15 min irradiation by a 300 W Xenon lamp before TEM images were taken with a JEOL-1011 Transmission electron microscope.
Mutagenicity tests of GNPs, sodium citrate, and HAuCl4
The mutagenicity test was carried out with Salmonella TA102 as previously described (Utesch and Splittgerber, 1996; Wang et al., 2003; Yan et al., 2004). Test tubes containing the mixture of 20 mM sodium phosphate (pH 7), TA102, and GNPs with a volume ratio of 5:1:1 were pre-incubated for 20 min in the gyrorotatory incubator at 210 rpm to homogenize. Then 0.7 mL each of this mixture was pipetted into test tubes containing 2.0 mL of top agar in a Dri-bath at 45°C. The resulting 2.7 mL mixtures were vortexed and poured onto minimal agar petri dishes. Six plates were prepared for each sample, and the experiment was repeated a second time a week later to ensure repeatability of results. The final concentration of the 16 nm GNPs in the agar plates was 5 µg/plate, sodium citrate was 0, 0.48, 2.4, 12, and 60 µM, and HAuCl4 was 0, 0.15, 0.75, 3.7, and 18.5 µM. 8-Methoxypsoralen at 10 µg/plate was irradiated for 2 min as positive control. There were two treatments for each concentration. One was without light irradiation while the other was irradiated for 15 min by a 300 W Xe lamp (Wang et al., 2003, 2009) After irradiation, both Petri dishes were incubated for 48 h at 37°C and the number of revertant colonies was counted with a colony counter (Bantex, Model 920A). If more than twice the number of revertant colonies was observed than the negative control, a positive mutagenic response is scored.
Results
Interaction of GNPs with Salmonella TA 102 bacteria
To see how GNPs interact with Salmonella TA 102 bacteria, the bacteria were exposed to the 16 nm GNPs (0.60 mg/mL) for 20 min in 20 mM phosphate buffer with or without 15 min light irradiation. After exposure, the bacteria were loaded onto the copper grid to take TEM images of the bacteria as shown in Figure 1. As can be seen, GNPs are attached to the bacteria surface and light irradiation has no effect (Figure 1B and C). The GNPs attached to the bacteria surface can be washed off by phosphate buffer during filtration on a 0.2 µM filter paper (Figure 1D).
Figure 1.
Transmission electron microscope (TEM) images of Salmonella TA 102 bacteria exposed to gold nanoparticles (GNPs): A: bacteria only, B: bacteria with GNPs, C: bacteria with GNPs with 15 min light irradiation, D: bacteria with GNPs after washing with 20 mM sodium phosphate buffer.
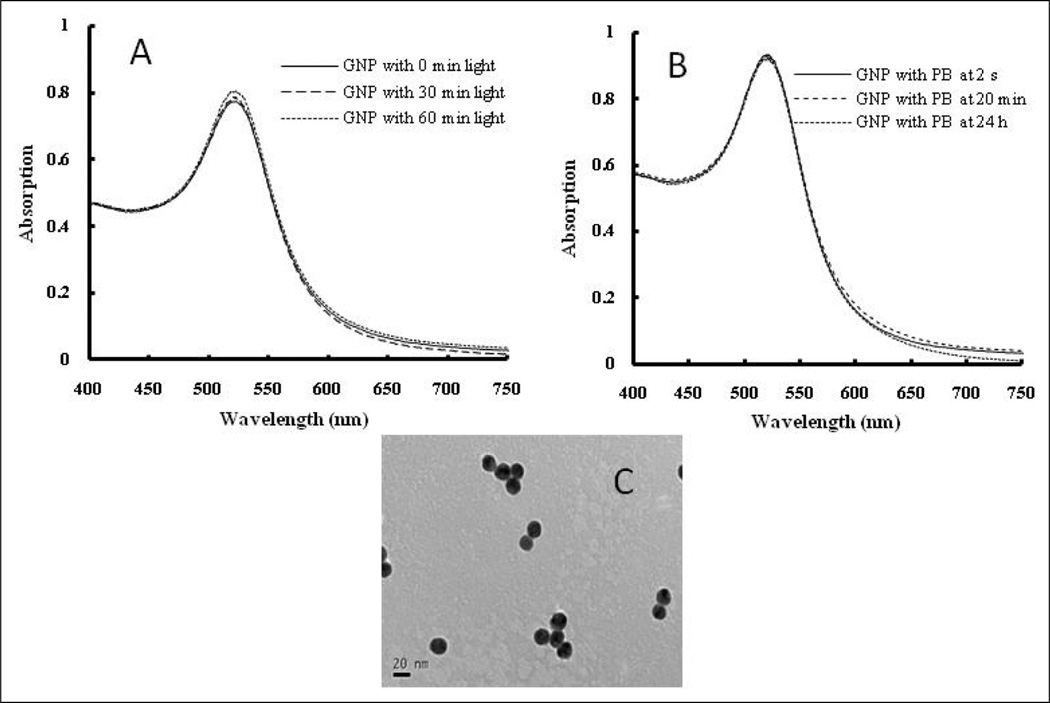
Stability of GNPs under light irradiation and in phosphate buffer
GNPs synthesized with the citrate reduction method are stable in solutions containing adequate amount of citrate ions as the capping agent. They absorb visible light and the absorption wavelength depends strongly on the size of the nanoparticle. The larger the nanoparticle, the longer wavelength light they absorb. Since GNPs applied for cancer imaging or as color coating agents are exposed to a variety of chemicals and light irradiation, their stability is studied under these conditions. To find out whether GNPs transform into other shapes or sizes under light irradiation, they are irradiated with a 300 W Xe lamp or treated with the 20 mM phosphate buffer. The plasmon resonance band of GNPs in solution is recorded using the Ocean Optics USB4000 spectrometer (Dunedin, Florida, USA) after 0, 30, and 60 min irradiation or after 2 sec (shortest time possible), 20 min, and 24 h treatment with the phosphate buffer. The peak at 522 nm does not change after the 30 or 60 min of irradiation (Figure 2A) neither does the 24-h phosphate buffer treatment (Figure 2B). This indicates that GNPs are stable under light irradiation or phosphate buffer treatment. The result of the phosphate buffer treatment also has been confirmed by TEM(Figure 2C) in that GNPs are still the same size and shape as before light irradiation. In our previous study, GNPs formed aggregates as soon as it is introduced into cell culture medium such as DMEM or 1 × PBS during cytotoxic test in skin cells (Wang et al., 2008).
Figure 2.
A: Absorption spectral change of gold nanoparticles (GNPs) after 0, 30, and 60 min irradiation; B: After mixing with 20 mM sodium phosphate buffer at 2 sec, 20 min, and 24 h. C: Transmission electron microscope (TEM) image of GNPs in 20 mM sodium phosphate buffer.
Mutagenicity of GNPs and co-existing chemicals in Salmonella TA 102
Possible toxicity of freshly prepared 16 nm GNPs in solution was examined using Salmonella TA 102. TA 102 bacteria were exposed to GNPs in solution with or without light irradiation. Exposure to light alone without GNPs slightly increases the number of revertant colonies from 320 to 500, while exposure to light irradiation in the presence of GNPs at a dose of 5 µg/plate increases the number of revertant colonies to 1150, more than twice the number of revertant colonies of the three controls (Figure 3), indicating that GNPs solution is photomutagenic in Salmonella TA102.
Figure 3.
Number of revertant colonies of Salmonella TA 102 due to exposure to gold nanoparticles (GNPs) with or without light irradiation by a 300 W Xe lamp.
Since GNPs used was not purified and the solution contained both citrate as a capping agent, and possibly unreacted HAuCl4 (Au3+), photomutagenicity of Au3+, and sodium citrate were tested under the same conditions. The initial concentrations of HAuCl4 and sodium citrate used for the synthesis were 200 µM and 1020 µM, respectively. It is expected that greater than 97% of the Au3+ is converted to GNPs as will be discussed later. Therefore, the experiments were performed at concentrations of 0, 10.15, 0.75, 3.7, and 18.5 µM for HAuCl4 and 0.48, 2.4, 12, and 60 µM for sodium citrate. As can be seen in Figure 4, neither Au3+ nor citrate is mutagenic to Salmonella TA 102 without light irradiation. Concomitant exposure to each of them at increasing concentrations and light irradiation produces increased number of revertant colonies. The number of revertant colonies caused by citrate at 0.48, 2.4, 12, and 60 µM are 1.5, 1.5, 1.9, and 1.7 times of the control (Figure 4A), close to the required ratio of 2.0 to score a positive mutagenicity. This indicates that citrate is borderline photomutagenic to TA 102. Under the same conditions, the number of revertant colonies due to concomitant exposure to 0.15 µM Au3+ and light irradiation are 2 times the control, but rapidly decreases at the three higher Au3+ concentrations. The decrease must be due to bacteria death caused by Au3+ phototoxicity as it was demonstrated by many other phototoxic compounds (Wang et al., 2009; Yan et al., 2004). When Au3+ concentration reaches 18.5 µM, all the Salmonella bacteria are killed with or without light irradiation. This indicates that Au3+ is photomutagenic at low concentrations (<1 µM) and is toxic as well as phototoxic at >1 µM (Figure 4B). The fact that the GNPs solution is not toxic in Salmonella TA 102 (Figure 3) indicates that the concentration of Au3+ must be less than 5 µM. This suggests that at least 97% of the starting Au3+ at 200 µM is converted to GNPs during the synthesis.
Figure 4.
Mutagenicity test of citrate (A) and Au3+ (B) in Salmonella typhimurium TA 102 with or without irradiation by a 300 W Xe lamp.
Discussions
The results presented here show that GNPs in solution, freshly prepared using citrate reduction of Au3+, is not mutagenic or toxic in Salmonella TA 102 but is photomutagenic to the bacteria. Exposure to the 16 nm GNPs solution at a concentration of 5 µg/plate causes the formation of revertant colonies in TA102 bacteria more than twice the control, an indication of a positive mutagenic response. Further careful tests point to the remnant citrate and Au3+ being the photomutagenic chemicals. The citrate ions are both the reducing agent to convert Au3+ to GNPs and the capping agent to stabilize the newly formed GNPs. Exposure to pure sodium citrate solutions in the concentration range of 0–60 µM, the estimated concentration range for the remnant sodium citrate in GNP solutions, and light irradiation causes increased revertant colonies of 1.5–1.9 times of the control, borderline photomutagenic. There is one related report on hydroxycitric acid which induces micronuclei formation in cells (Lee and Lee, 2007). Citrate is also known to undergo light-induced or heat-induced decarboxylations, resulting in acetone-1,3-dicaboxylate, acetoacetic acid, and finally acetone (Borer et al., 2007; Hay and Leong, 1971; Munro et al., 1995; Quici et al., 2008; Quici et al., 2007; Zeldes and Livingston, 1971). During the decarboxylation process, redox reactions occur, leading to the generation of reactive species and free radicals that may cause damage to the bacteria.
We also demonstrated that Au3+ ions are toxic and phototoxic at concentrations >1 µM and photomutagenic at <1 µM. The photomutagenicity of Au3+ has not been reported before. A recent report indicates that Au3+ is cytotoxic to human K562 cells at concentrations >50 µM(Connor et al., 2005), but our results clearly indicate that phototoxicity of Au3+ in Salmonella starts at a much lower concentration (1–5 µM range) and light irradiation enhances the toxicity. It has been reported that Au3+ can oxidize HEPES or components of the Good’s buffer to nitrogen-centered free radicals and cause DNA strand cleavage with light irradiation (Habib and Tabata, 2004; Tabata, et al., 2005). Therefore, we believe that light irradiation of Au3+ in solution may promote formation of free radicals that cause mutation in bacteria or even kill the bacteria. It is conceivable that the strong phototoxicity of Au3+ may be taken advantage of for killing of bacteria or control of bacteria outbreak. Further experiment is needed to understand the mechanism of Au3+ photomutagenicity.
Finally, the toxic effects of GNPs are complex due to co-existing chemicals citrate (or its oxidation products) and Au3+ ions. Citrate is tested to be borderline photomutagenic in Salmonella TA102 bacteria at the concentration range of 15–60 µM. Although there is no indication that a toxic level of Au3+ is present in freshly prepared GNPs solutions, Au3+ is both cytotoxic/photocytotoxic and photomutagenic to Salmonella TA 102 bacteria. As we reported previously, cetyl triammonium bromide was the main ingredient for cytotoxicity of gold nanorods to human skin cells (Wang et al., 2008). It seems that GNPs, whether nanorods or nanospheres, are not toxic in skin cells or bacteria, but the co-existing chemicals are the actual culprits. Therefore, co-existing chemicals, no matter how nontoxic they seem to be, must be tested as controls during toxicity assessment of any nanomaterials including GNPs.
Acknowledgments
Funding
We thank the National Science Foundation for grants: Partnership for Research and Education in Materials (NSF PREM DMR-0611539) and Research Experience for Undergraduates (NSF REU DMR-0755433), and the National Institutes of Health (NIH/NCRR RCMI Grant G12RR013459) grant for equipments usage in the Core Research Facilities.
References
- Aaseth J, Haugen M, Forre O. Rheumatoid arthritis and metal compounds–perspectives on the role of oxygen radical detoxification. Analyst. 1998;123:3–6. doi: 10.1039/a704840h. [DOI] [PubMed] [Google Scholar]
- Borer P, Hug SJ, Sulzberger B, Kraemer SM, Kretzschmar R. Photolysis of citrate on the surface of lepidocrocite: An in situ attenuated total reflection infrared spectroscopy study. The Journal of Physical Chemistry C. 2007;111:10560–10569. [Google Scholar]
- Chithrani BD, Ghazani AA, Chean WCW. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Letters. 2006;6:662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1:325–327. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- Darbha GK, Rai US, Singh AK, Ray PC. Gold-nanorod-based sensing of sequence specific HIV-1 virus DNA by using Hyper-Rayleigh scattering spectroscopy. Chemical European Journal. 2008;14:3896–3903. doi: 10.1002/chem.200701850. [DOI] [PubMed] [Google Scholar]
- Edwards PP, Thomas JM. Gold in a metallic divided state-from Faraday to present-day nanoscience. Angewandte Chemie (International Education) 2007;46:5480–5486. doi: 10.1002/anie.200700428. [DOI] [PubMed] [Google Scholar]
- EI-Sayed IH, Huang X, EI-Sayed MA. Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancer. Nano Letters. 2005;5:829–834. doi: 10.1021/nl050074e. [DOI] [PubMed] [Google Scholar]
- Gobin AM, Lee MH, Halas NJ, Janes WD, Drezek RA, West JL. Near-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy. Nano Letters. 2007;7:1929–1934. doi: 10.1021/nl070610y. [DOI] [PubMed] [Google Scholar]
- Goodman C, McCusker C, Yilmaz T, Rotello V. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjugate Chemistry. 2004;15:897–900. doi: 10.1021/bc049951i. [DOI] [PubMed] [Google Scholar]
- Habib A, Tabata M. Oxidative DNA damage induced by HEPES (2-[4-(2-hydroxyethyl)-1-piperazinyl] ethanesulfonic acid) buffer in the presence of Au(III) Journal of Inorganic Biochemistry. 2004;98:1696–1702. doi: 10.1016/j.jinorgbio.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Hauck TS, Ghazani AA, Chan WCW. Assessing the effect of surface chemistry on gold nanorod uptake, toxicity, and gene expression in mammalian cells. Small. 2008;4:153–159. doi: 10.1002/smll.200700217. [DOI] [PubMed] [Google Scholar]
- Hay RW, Leong KN. The uncatalysed and metal-ion catalysed decarboxylation of 3-oxoglutaric acid: a model for an enzyme system. Journal of the Chemical Society A. 1971;1:3639–3647. [Google Scholar]
- He W, Huang CZ, Li YF, Xie JP, Yang RG, Zhou PZ, Wang J. One-step label-free optical genosensing system for sequence-specific DNA related to the human immunodeficiency virus based on the measurements of 6 Toxicology and Industrial Health 000(00) light scattering signals of gold nanorods. Analytical Chemistry. 2008;80:8424–8430. doi: 10.1021/ac801005d. [DOI] [PubMed] [Google Scholar]
- Higby GJ. Gold in medicine. Gold Bulletin. 1982;15:130–140. doi: 10.1007/BF03214618. [DOI] [PubMed] [Google Scholar]
- Huang X, EI-Sayed IH, Qian W, EI-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. Journal of American Chemical Society. 2006;128:2115–2120. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- Huff TB, Hansen MH, Zhao Y, Cheng J-X, and Wei A. Controlling the cellular uptake of gold nanorods. Langmuir. 2007;23:1596–1599. doi: 10.1021/la062642r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain KK. Role of nanobiotechnology in developing personalized medicine for cancer. Technology in Cancer Research & Treatment. 2005;4:407–416. doi: 10.1177/153303460500400608. [DOI] [PubMed] [Google Scholar]
- Jennings T, Strouse G. Past, present, and future of gold nanoparticles. Advances in Experimental Medicine and Biology. 2007;620:34–47. doi: 10.1007/978-0-387-76713-0_3. [DOI] [PubMed] [Google Scholar]
- Kim CK, Kalluru RR, Singh JP, Fortner A, Griffin J, Darbha GK, et al. Gold-nanoparticle-based miniaturized laser-induced fluorescence probe for specific DNA hybridization detection: Studies on size-dependent optical properties. Nanotechnology. 2006;17:3085–3093. [Google Scholar]
- Kimling J, Maier M, Okenve B, Kotaidis V, Ballot H, Plech A. Turkevich method for gold nanoparticle synthesis revisited. Journal of Physical Chemistry B. 2006;110:15700–15707. doi: 10.1021/jp061667w. [DOI] [PubMed] [Google Scholar]
- Kumar S, Gandhi KS, Kumar R. Modeling of formation of gold nanoparticles by citratemethod. Industrial & Engineering Chemistry Research. 2007;46:3128–3136. [Google Scholar]
- Lee KH, Lee BM. Evaluation of the genotoxicity of (-)-hydroxycitric acid (HCA-SX) isolated from Garcinia cambogia. Journal of Toxicology and Environmental Health–Part A: Current Issues. 2007;70:388–392. doi: 10.1080/15287390600882192. [DOI] [PubMed] [Google Scholar]
- Loo C, Lowery A, Halas N, West J, Drezek R. Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Letters. 2005;5:709–711. doi: 10.1021/nl050127s. [DOI] [PubMed] [Google Scholar]
- Mao Z, Wang B, Ma L, Gao C, Shen J. The influence of polycaprolactone coating on the internalization and cytotoxicity of gold nanoparticles. Nanomedicine. 2007;3:215–223. doi: 10.1016/j.nano.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Mironava T, Hadjiargyrou M, Simon M, Jurukovski V, Rafailovich MH. Gold nanoparticles cellular toxicity and recovery: Effect of size, concentration and exposure time. Nanotoxicology. 2010;4:120–137. doi: 10.3109/17435390903471463. [DOI] [PubMed] [Google Scholar]
- Munro CH, Smith WE, Garner M, Clarkson J, White PC. Characterization of the surface of a citratereduced colloid optimized for use as a substrate for surface-enhanced resonance Raman scattering. Langmuir. 1995;11:3712–3720. [Google Scholar]
- Murphy CJ, Gole AM, Stone JW, Sisco PN, Alkilany AM, Goldsmith EC, et al. Gold nanoparticles in biology: Beyond toxicity to cellular imaging. Accounts of Chemical Research. 2008;41:1721–1730. doi: 10.1021/ar800035u. [DOI] [PubMed] [Google Scholar]
- Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, et al. Colloidal gold: A novel nanoparticle vector for tumor directed drug delivery. Drug Delivery: Journal of Delivery Targeting Therapeutic Agents. 2004;11:169–183. doi: 10.1080/10717540490433895. [DOI] [PubMed] [Google Scholar]
- Pan Y, Neuss S, Leifert A, Fischler M, Wen F, Simon U, et al. Size-dependent cytotoxicity of gold nanoparticles. Small. 2007;3:1941–1949. doi: 10.1002/smll.200700378. [DOI] [PubMed] [Google Scholar]
- Patra HK, Banerjee S, Chaudhuri U, Lahiri P, Dasgupta AK. Cell-selective response to gold nanoparticles. Nanomedicine. 2007;3:111–119. doi: 10.1016/j.nano.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Pernodet N, Fang X, Sun Y, Bakhtina A, Ramakrishna A, Sokolov J, et al. Adverse effects of citrate/gold nanoparticles on human dermal fibroblasts. Small. 2006;6:766–773. doi: 10.1002/smll.200500492. [DOI] [PubMed] [Google Scholar]
- Quici N, Litter MI, Braun AM, Oliveros E. Vacuum-UV-photolysis of aqueous solutions of citric and gallic acids. Journal of Photochemistry and Photobiology A: Chemistry. 2008;197:306–312. [Google Scholar]
- Quici N, Morgada ME, Gettar RT, Bolte M, Litter MI. Photocatalytic degradation of citric acid under different conditions: TiO2 heterogeneous photocatalysis against homogeneous photolytic processes promoted by Fe(III) and H2O2. Applied Catalysis B: Environment. 2007;71:117–124. [Google Scholar]
- Ray PC. Diagnostics of single base-mismatch DNA hybridization on gold nanoparticles by using the hyper-Rayleigh scattering technique. Angewandte Chemie–International Education. 2006;45:1151–1154. doi: 10.1002/anie.200503114. [DOI] [PubMed] [Google Scholar]
- Ray PC, Fortner A, Griffin J, Kim CK, Singh JP. Laser-induced fluorecence quenching of tagged oligonucleotide probes by gold nanoparticles. Chemical Physics Letter. 2005;414:259–264. [Google Scholar]
- Ray PC, Yu H, Fu PP. Toxicity and environmental risks of nanomaterials: challenges and future needs. Journal of Environmental Science and Health. Part C, Environmental Carcinogenesis & Ecotoxicology Reviews. 2009;27:1–25. doi: 10.1080/10590500802708267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov K, Follen M, Aaron J, Pavel N, McLaughlin R, Lotan R. Real-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles. Caner Research. 2003;63:1999–2004. [PubMed] [Google Scholar]
- Tabata M, Habib A, Watanabe K. DNA cleavage by Good’s buffers in the presence of Au(III) Bulletin Chemical Society of Japan. 2005;78:1263–1269. [Google Scholar]
- Takahashi H, Niidome Y, Niidome T, Kancko K, Kawasaki H, Yamada S. Modification of gold nanorods using phosphatidylcholine to reduce cytotoxicity. Langmuir. 2006;22:2–5. doi: 10.1021/la0520029. [DOI] [PubMed] [Google Scholar]
- Turkevich J, Stevenson PC, Hillier J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discussions of the Faraday Society. 1951;11:55–75. [Google Scholar]
- Utesch D, Splittgerber J. Bacterial photomutagenicity testing: distinction between direct, enzymemediated and light-induced events. Mutation Research. 1996;361:41–48. doi: 10.1016/s0165-1161(96)90228-9. [DOI] [PubMed] [Google Scholar]
- Wang L, Yan J, Fu PP, Parekh KA, Yu H. Photomutagenicity of cosmetic ingredient chemicals azulene and guaiazulene. Mutation Research. 2003;530:19–26. doi: 10.1016/s0027-5107(03)00131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Lu W, Tovmachenko O, Rai US, Yu H, Ray PC. Challenge in understanding size and shape dependent toxicity of gold nanomaterials in human skin keratinocytes. Chemical Physics Letters. 2008;463:145–149. doi: 10.1016/j.cplett.2008.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Wang L, Yin JJ, Wang Z, Fu P, Yu H. Light-induced toxic effects of tamoxifen: A chemotherapeutic and chemopreventive agent. Journal of Photochemistry and Photobiology A. 2009;201:50–56. doi: 10.1016/j.jphotochem.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiwanitkit V, Sereemaspunm A, Rojanathanes R. Visualization of gold nanoparticle on the microscopic picture of red blood cell: implication for possible risk of nanoparticle exposure. Stochastic Environmental Research and Risk Assessment: Research Journal. 2008;22:583–585. [Google Scholar]
- Yan J, Wang L, Fu P, Yu H. Photomutagenicity of 16 polycyclic aromatic hydrocarbons from the US EPA priority pollutant list. Mutation Research. 2004;557:99–108. doi: 10.1016/j.mrgentox.2003.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zeng J, Kong T, Wang X, Roa W, EI-Bialy T, et al. The effect of surface properties of gold nanoparticles on cellular uptake. IEEE. 2008;1:92–95. [Google Scholar]
- Zeldes H, Livingston R. Paramagnetic resonance study of liquids during photolysis. XI. Citric acid and sodium citrate in aqueous solution. Journal of American Chemical Society. 1971;93:1082–1085. [Google Scholar]