Abstract
Objective
To assess up to 3-year follow-up of a health information technology system that facilitated population-based breast cancer screening.
Study Design
Cohort study with 2-year follow-up after completing a 1-year cluster randomized trial.
Methods
Women 42-69 years old receiving care within a 12-practice primary care network. The trial tested an integrated, non-visit-based population management informatics system that: 1) identified women overdue for mammograms, 2) connected them to primary care providers using a Web-based tool, 3) created automatically-generated outreach letters for patients specified by providers, 4) monitored for subsequent mammography scheduling and completion, and 5) provided practice delegates a list of women remaining unscreened for reminder phone calls. All practices also provided visit-based cancer screening reminders. Eligible women overdue for a mammogram during a one-year study period included those overdue at study start (prevalent cohort) or becoming overdue during follow-up (incident cohort). The main outcome measure was mammography completion rates over three years.
Results
Among 32,688 eligible women, 9,795 (30%) were overdue for screening including 4,487 in intervention and 5,308 in control practices. Intervention patients were somewhat younger, more likely to be non-Hispanic white, and have health insurance compared to control patients. Among patients in the prevalent cohort (n=6,697), adjusted completion rates were significantly higher among intervention compared to control patients after 3 years (51.7% vs. 45.8%, p=0.002). For patients in the incident cohort (n=3,098), adjusted completion rates after 2 years were 53.8% vs. 48.7%, p=0.052, respectively.
Conclusions
Population-based informatics systems can enable sustained increases in mammography screening rates beyond that seen with office-based visit reminders.
Keywords: population management, cancer screening, health information technology, primary care
INTRODUCTION
The U.S. health care system is dramatically expanding the use of health information technology as a way to improve the quality and efficiency of care.1, 2 In primary care networks, population-based surveillance is being used to identify specific individuals for prevention or disease management interventions. To date most interventions have focused on the use of electronic health records to facilitate care during office-based visits or inpatient hospital admissions.3-5
A novel informatics system to facilitate population-based preventive cancer screening was developed and implemented within a large primary care network.6 Breast cancer screening was chosen because of it being the leading cause of cancer in women and the second most common cause of cancer-related death,7 the scientific evidence supporting screening to decrease breast cancer mortality,8, 9 and many women not being regularly screened despite broad consensus, especially for postmenopausal women.10, 11 The study's goal was to increase breast cancer screening rates by identifying eligible women overdue for a mammogram and allowing primary care providers to use an informatics tool to quickly review overdue patients and initiate outreach for those selected for contact. The system then automatically mailed reminder letters to the selected patients, tracked mammogram ordering and completion, and facilitated the scheduling of reminder phone calls by practice delegates for women remaining unscreened.
Previous results demonstrate that among women overdue for screening at the start of the study period (prevalent cohort), this system increased breast cancer screening rates over 1-year of follow-up.12 Outcomes of women who became overdue during the 1-year intervention period (incident cohort), representing those just becoming overdue after prior testing or are newly eligible for screening based upon age criteria, have not been previously reported. Since this incident cohort represents the ongoing population for reminder systems, the current report compares results in incident and prevalent cohorts and assesses the durability of the one-time intervention benefit over a 3-year period.
METHODS
Study Design and Randomization
The informatics system used in this study, the controlled cluster randomized trial methods, and primary outcome results over 1 year among individuals who were overdue for screening at the study start are described elsewhere.6, 12 Twelve primary care practices were allocated to intervention (n=6) or usual care (n=6) control groups after stratifying by practice type, the number of eligible patients, baseline mammography rates, and unaffiliated outside facility screening rates. Providers could not be blinded to group assignment. The study was approved by the institutional review board at Massachusetts General Hospital.
Setting and Participants
The study population consisted of 163,028 individuals seen in the Massachusetts General Primary Care Practice-Based Research Network during the three years ending December 31, 2006. All patients were linked to either a specific primary care physician (PCP), or (for patients who could not be linked to a specific physician) to the primary care practice where they received most of their care using a previously validated algorithm.13, 14 This ensured that the review of women overdue for breast cancer screening was by the PCPs or practices most directly responsible for each patient's care.
Eligible study subjects were women 42 to 69 years of age who had no record of mammography in the prior two years. This included women who were overdue as of the intervention start date (March 20, 2007; prevalent cohort), or became overdue during the first year of follow-up (March 20, 2007 – March 19, 2008; incident cohort). Patients were excluded if their listed PCP was outside of the MGH network, had previously undergone bilateral mastectomy, or had died. All practices used electronic health records that provided visit-based cancer screening reminders.
Study Intervention
The informatics tool was implemented in the six intervention practices on March 20, 2007 and remained available to providers through March 19, 2010. During the intervention year (through March 19, 2008), providers received reminders to use the tool. After this 1-year period, providers could still use the tool, but they received no additional reminders and the original patient registry was not updated. For intervention providers, the informatics tool consisted of a Web page listing their eligible patients linked to the network's electronic health record.
Physician and Population Manager Role
Separate list views were visible for PCPs for their own patients and for practice-designated population managers (nurses, medical assistants, or non-clinical staff) for patients in each practice not linked to a specific PCP. Physicians and population managers received three email reminders (start date, 3 months, 8 months) with a direct link to the population screening Web page during the intervention year. A mailed reminder with step-by-step instructions was sent to physicians not yet using the system after 2 months. The Web page could also be accessed directly from the hospital's intranet and included: 1) a list of overdue patients, 2) clinically relevant decision support information to help determine whether or not to initiate patient contact, and 3) an actionable component to initiate or defer the mammography screening process. If a provider initiated patient contact, a centralized process was started with a letter. Providers could also defer screening (for example, if the patient had previously declined screening after a discussion or had screening done elsewhere) and remove a patient from their list for the remainder of the study. Patient letters were sent centrally, electronically signed, and included information about the value of screening and how to schedule a mammogram.
Practice Delegate Role
Physicians and population managers were linked with a practice-specific delegate (non-clinical staff or medical assistant) who used their own version of the informatics tool to facilitate tracking and scheduling patients needing contact. Practice delegates were responsible for contacting patients who did not schedule screening on their own. When speaking with patients, delegates could schedule a mammogram by directly accessing the hospital's radiology ordering system using the informatics tool.
Outcome Assessment
Patient characteristics and mammography reports and dates of completion were obtained from an electronic clinical and billing data repository.15 Physician characteristics were obtained from the hospital registrar.
The primary outcome was mammography completion rates among patients overdue for screening at the start of the study (prevalent cohort) and among women newly overdue for mammography (incident cohort) during the first study year, comparing intervention and control practices.12 The maximum length of follow-up was three years for those in the prevalent cohort, and at least two years among those in the incident cohort.
A mammogram was considered to have been completed if there was an electronic report for an imaging test at a network-affiliated institution or if a mammogram was listed in billing data for the patient. Secondary outcomes included time to mammography completion among all overdue patients (prevalent and incident cohorts), censored by cancer diagnosis, death, or end of follow-up, and new cancer diagnoses using Partners Cancer Registry data compared among intervention and control groups.
Statistical Analyses
Baseline patient and physician/practice characteristics were compared between intervention and control groups and between prevalent and incident cohorts using two-sample t-tests or Chi-square tests, as appropriate. For the primary outcome, adjusted mammography completion rates and 95% confidence intervals were calculated for both the prevalent and incident cohorts at 1, 2, and 3 years of follow-up using Cox proportional hazard models with the robust sandwich covariance matrix estimate to account for clustering while adjusting for potential confounders (PROC PHREG in SAS version 9.2, SAS Institute Inc., Cary, North Carolina). In these models, physicians were considered as the unit of cluster for physician-connected patients and the population manager was considered the unit of cluster for practice-connected patients. To control for differences in patient and practice characteristics among intervention and control practices, patient age, race/ethnicity, insurance status, English language proficiency, practice type (health center vs. non-health center), and number of months since last practice visit were included in the models as covariates. All adjusted rates were calculated by holding these covariates at the population mean levels. Unadjusted time to screening completion survival distributions were depicted with Kaplan-Meier curves and compared using a log-rank test. Adjusted hazard ratios comparing intervention to control practices for the entire follow-up period were also reported from the Cox proportional hazards models. Percent of patients with new cancer diagnoses were compared between intervention and control groups using Chi-square tests.
RESULTS
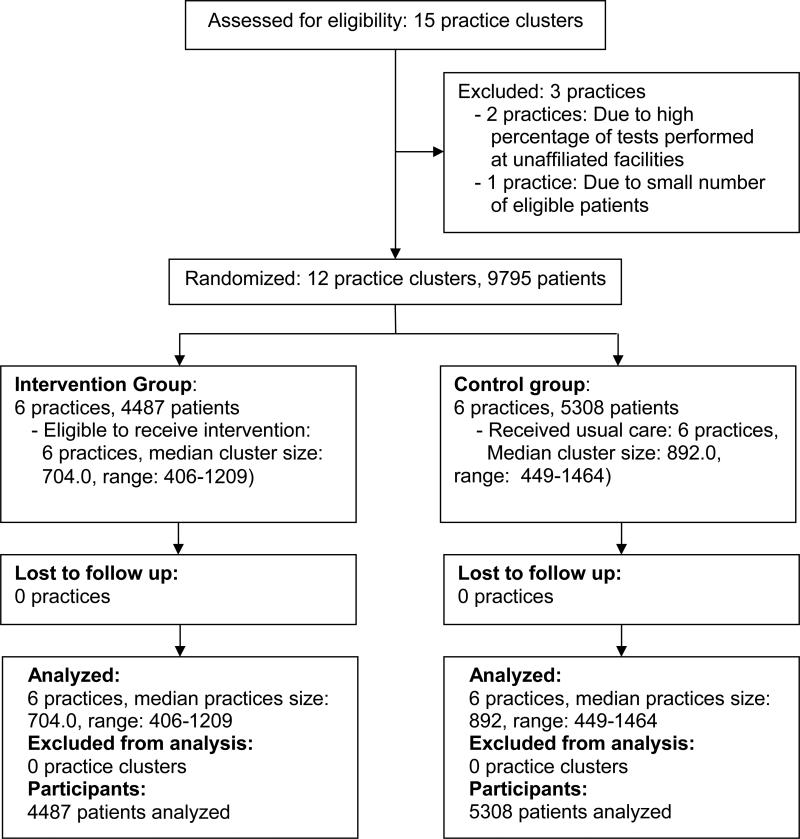
There were 64 eligible physicians and 6 practice population managers in the 6 intervention arm practices and 74 eligible physicians in the 6 control arm practices. Among intervention providers, 65 of 70 (92.9%) used the system. There were no significant differences between intervention and control practice physicians with regard to age (47.4 vs. 46.9, p=0.78), years since medical school graduation (19.9 vs. 19.2, p=0.67), and gender (48.4% vs. 51.4% were women, p=0.86). Two practices in each arm were community health centers. Screening rates at baseline were similar in intervention and control practice groups (79.5% vs. 79.3%, p=0.73). Figure 1 depicts practice randomization and follow-up.
Figure 1.
Diagram depicting the flow of study practice clusters and patients through randomization, intervention, and outcome analysis.
Among 32,688 eligible women, 9,795 (30%) were overdue for screening during the 1-year study period including 4,487 patients in intervention practices and 5,308 patients in control practices (Table 1). Intervention and control patients were equally likely to be connected to a specific physician (58.9% vs. 58.8%) and overdue for screening at the start of the study (prevalent cohort, 67.9% vs. 68.8%). Intervention patients were slightly younger, more likely to speak English, to be non-Hispanic white, have health insurance, and to have their last clinic visit further in the past than control patients. Compared to patients who were overdue at the start of the study (prevalent cohort), patients who became overdue during 1-year follow-up (incident cohort) were more likely to be connected to a physician (67.6% vs. 54.8%, p<0.001), have commercial health insurance (70.9% vs. 61.9%, p<0.001), and to have been seen more recently for a practice visit (mean 8.6 [8.6 SD] vs. 13.8 months [8.1 SD], p<0.001).
Table 1.
Patient Characteristics by Intervention Group Status at Baseline
| Total | Intervention Group | Control Group | P-Value | |
|---|---|---|---|---|
| Patient characteristics, N (%) | N=9,795* | N=4487 | N=5308 | |
| Patient-physician connectedness | 0.87 | |||
| Physician-Connected | 5,763 | 2,644 (58.9) | 3,119 (58.8) | |
| Practice-Connected | 4,032 | 1,843 (41.1) | 2,189 (41.2) | |
| Timing of overdue status | 0.32 | |||
| At study start (prevalent) | 6,697 | 3,045 (67.9) | 3,652 (68.8) | |
| During first year (incident) | 3,098 | 1,442 (32.1) | 1,656 (31.2) | |
| Age, mean (SD) | 54.1 (7.8) | 53.8 (7.7) | 54.3 (7.9) | 0.003 |
| Ethnicity | <0.001 | |||
| Non-Hispanic white | 7,359 | 3,546 (79.0) | 3,813 (71.8) | |
| Hispanic | 840 | 190 (4.2) | 650 (12.3) | |
| African American | 701 | 301 (6.7) | 400 (7.5) | |
| Asian | 466 | 249 (5.6) | 217 (4.1) | |
| Other/unknown | 429 | 201 (4.5) | 228 (4.3) | |
| Primary language, English | 8,803 | 4,173 (93.0) | 4,630 (87.2) | <0.001 |
| Insurance status | <0.001 | |||
| Commercial health insurance | 6,344 | 3,163 (70.5) | 3181 (59.9) | |
| Medicare | 1,261 | 531 (11.8) | 730 (13.8) | |
| Medicaid | 1,178 | 454 (10.1) | 724 (13.6) | |
| No insurance, self-pay | 1,012 | 339 (7.6) | 673 (12.7) | |
| Months since last practice visit, median (IQR) | 9.8 (5.6-18.4) | 10.0 (5.7-18.7) | 9.5 (5.5-18.2) | 0.05 |
33 patients who died prior to the study start date were excluded.
Providers took action on 3415 of 4487 (76.1%) intervention patients (2865 [63.9%] were contacted by letter and 550 (12.3%) were deferred). The most common reasons for deferral included test completion at an outside facility (312 [56.7%]) and patient refusal (89 [16.2%]). Among 3045 intervention patients overdue at baseline (prevalent cohort), action was taken on 2629 (86.3%) patients (2212 [72.6%] were contacted by letter and 417 [13.7%] were deferred). Among 1442 intervention patients who became overdue during the 1-year follow-up period (incident cohort), action was taken on 786 (54.5%) patients (653 [45.3%] were contacted by letter and 133 [9.2%] were deferred).
Mammography Screening Rates over Time
Percent of Overdue Population Screened over 3-Year Follow-up
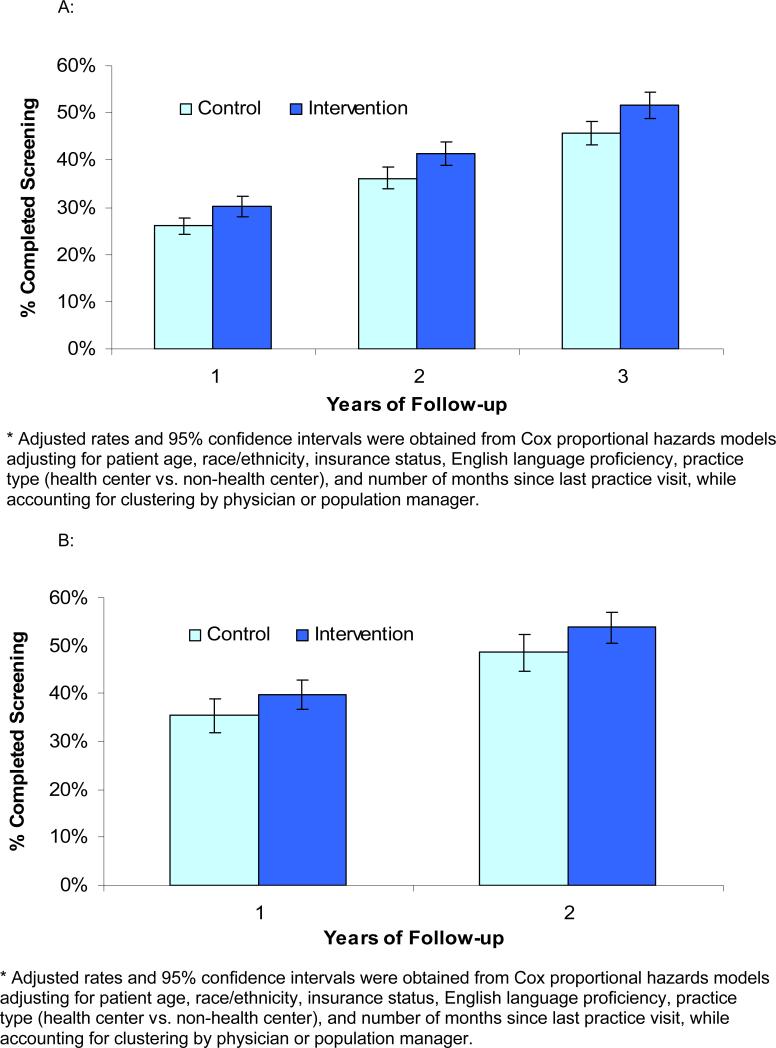
The percent of patients in intervention and control groups who completed screening over 3-year follow-up was analyzed separately for prevalent and incident cohorts. Among patients overdue at baseline (prevalent cohort), adjusted completion rates were significantly higher among patients in the intervention group compared to the control group at 1 year (30.1% vs. 26.0%, p=0.004), 2 years (41.5% vs. 36.2%, p=0.002) and 3 years (51.7% vs. 45.8%, p=0.002) of follow-up (Figure 2A). Among patients becoming overdue during the first year (incident cohort), adjusted completion rates were higher but of borderline statistical significance in the intervention compared to the control group at 1 year (39.8% vs. 35.5%, p=0.07) and 2 years (53.8% vs. 48.7%, p=0.052) of follow-up (Figure 2B).
Figure 2.
Percent completing mammography during 3-years of follow-up in intervention and control practices among, A. women overdue at the start of follow-up (prevalent cohort) and B. women becoming overdue during the first year of follow-up (incident cohort).
Figure 2A: Percent of women overdue for mammography at the start of follow-up (prevalent cohort) in intervention and control groups who completed screening in each year of follow-up.
Figure 2B: Percent of women becoming overdue for mammography during the first year of follow-up (incident cohort) in intervention and control groups who completed screening in each year of follow-up.
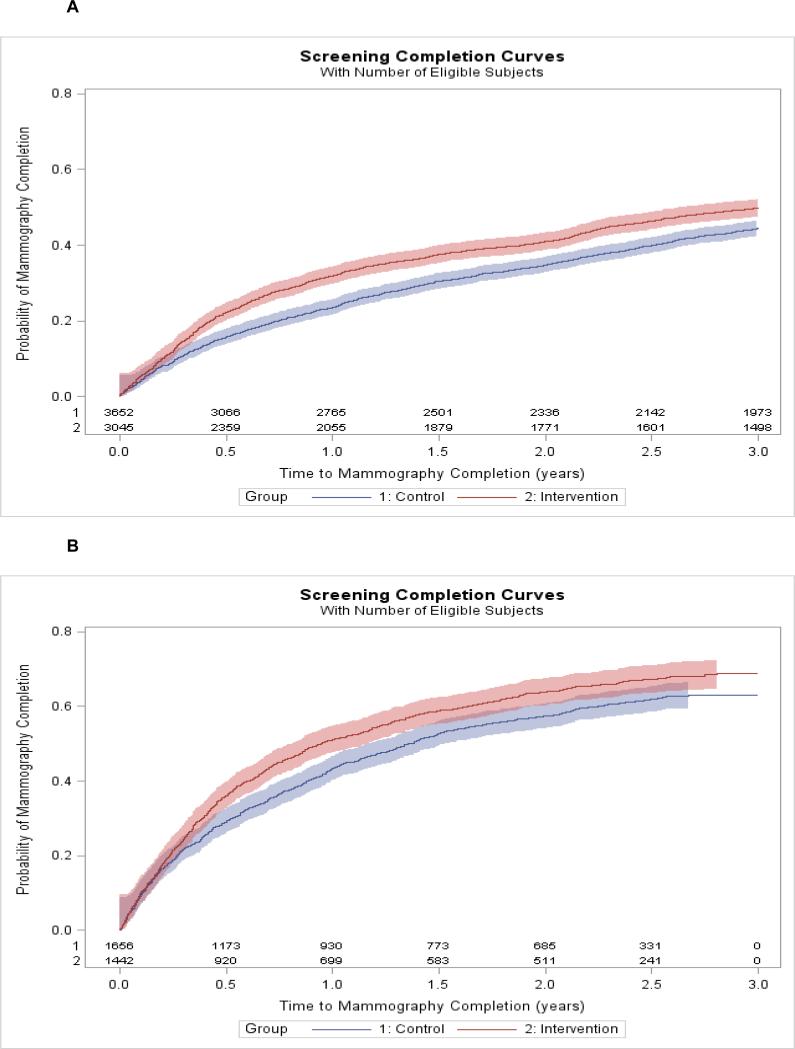
Time to First Completed Mammogram
Time to first completed mammogram over three years of follow-up was stratified by whether patients were overdue for screening at the start of the study (prevalent cohort, Figure 3A) or became overdue during the first year of follow-up (incident cohort, Figure 3B). Screening rates were higher in the incident compared to prevalent cohorts, and in both cohorts intervention patients completed screening sooner than control patients (log-rank p<0.001 for both cohorts). Multivariable Cox regression models controlling for potential confounding factors including age, insurance information, race/ethnicity, language spoken, practice type (community health center or not), and months since last practice visit, resulted in adjusted hazard ratios demonstrating a benefit in both prevalent and incident cohorts over the follow-up period (prevalent hazard ratio: 1.19, 95% CI: 1.07-1.32, p=0.001; incident hazard ratio: 1.16, 95% CI: 1.05-1.28, p=0.004). In addition to intervention status, other significant predictors of time to first completed mammogram in multivariable Cox regression modeling included patient age, insurance status, practice type and months since last practice visit (Table 2).
Figure 3.
Kaplan-Meier curves of time to mammography completion during 3 years of follow-up among, A. women overdue at the start of follow-up (prevalent overdue) and B. women becoming overdue during the first year of follow-up (incident overdue) in intervention and control practices. Shaded area around each line represents the 95% confidence interval for that Kaplan-Meier curve.
Table 2.
Multivariable Cox Model Results for the Association between Time to Mammography Completion and Intervention Status among All Patients
| Parameter | Hazard Ratio | 95% Confidence Interval | P-Value |
|---|---|---|---|
| Intervention | 1.19 | 1.10 – 1.29 | <0.001 |
| Age (10-year increment) | 0.93 | 0.89 – 0.98 | 0.003 |
| Insurance status | |||
| Medicare | 0.67 | 0.59 – 0.76 | <0.001 |
| Medicaid | 0.65 | 0.58 – 0.74 | <0.001 |
| No insurance, self-pay | 0.70 | 0.59 – 0.83 | <0.001 |
| Commercial health insurance | - | - | - |
| Ethnicity | |||
| Asian | 1.14 | 0.997 – 1.31 | 0.06 |
| Black | 1.05 | 0.94 – 1.18 | 0.38 |
| Hispanic | 1.09 | 0.96 – 1.24 | 0.18 |
| Other/unknown | 1.01 | 0.87 – 1.16 | 0.99 |
| Non-Hispanic white | - | - | - |
| Primary language | |||
| Non-English | 1.05 | 0.94 – 1.18 | 0.40 |
| English | - | - | - |
| Practice Type | |||
| Community health center | 0.90 | 0.81 – 0.99 | 0.03 |
| Non-community health center | - | - | - |
| Months since last practice visit (3-month increment) | 0.83 | 0.82 – 0.84 | <0.001 |
Identification of New Breast Cancers
Though the intervention group had higher rates of mammography screening, the number of breast cancers diagnosed (n=82) were similar among intervention and control groups (8.7 vs. 8.1 per 1000 eligible women, respectively, p=0.75) as well as among patients in the prevalent and incident populations (data not shown).
DISCUSSION
This study evaluated a novel informatics system delivering integrated population-based preventive care that was designed to supplement and be independent of visit-based reminders. By following patients treated in a cluster randomized trial in one primary care network, we demonstrated that such a system could increase mammography rates over a three-year follow-up period. The intervention was more effective in patients who were overdue at the start of the study than in patients who became overdue over the first year of study follow-up. The Web-based informatics tool was used by over 90% of intervention providers supporting that it was an easy and feasible way to screen patients without needing a face-to-face encounter.
The benefits of reminder systems for improving preventive cancer screening rates have been demonstrated in both visit-based and population-based settings, but most studies have only examined outcomes over short follow-up periods.16-22 We previously reported increased mammography rates over a 1-year period,12 but it is possible that such interventions speed up time to testing completion without raising overall completion rates. By following patients up to 3 years, we demonstrated the durability of the intervention among patients who were overdue at the start of the study. Though differences between intervention and control group rates narrowed over time, patients from intervention practices remained significantly more likely to be screened after 2 or 3 years.
Another limitation of existing studies of reminder systems is that most focus on one-time screening among patients who have failed to be screened over long periods of time (our prevalent cohort).21 However, once reminder systems are started, most patients becoming overdue will be new to screening based upon age or just becoming overdue after prior testing. As expected, screening rates differ among patients in incident and prevalent cohorts (Figure 2). For reminder systems such as this one that are designed to continue to identify patients as they become overdue over time, their true benefit needs to be evaluated in this incident cohort. We demonstrate that patients in intervention practices who became overdue after the start of the study (incident cohort) were more likely to complete screening than patients in control practices, but the benefit was smaller than for the prevalent cohort. Factors that may explain these results include higher screening rates among patients in the incident than prevalent cohort regardless of intervention status (Figure 2). In addition, fewer patients in the incident cohort were screened than in the prevalent cohort (45.3% vs. 72.6%, respectively). Additional reminders to providers over time to screen newly overdue patients are likely necessary to sustain long-term outcomes in this population.
The introduction of this novel informatics system required a fundamental restructuring of the way providers deliver preventive services. Current fee-for-service payment models generally require face-to-face visits and do not provide reimbursement for population-based, visit-independent care.23 Physicians in intervention practices were not compensated for the time they spent reviewing their lists. Though most providers still used the tool, it is possible that decreased use for patients who became overdue after the start of the study reflected the uncompensated nature of panel management outside of an office visit.
The intervention was designed to supplement the primary care network's current visit-based, electronic health record reminder systems. Future work should assess whether preventive services are best delivered outside of the limited time available during office-based visits. Removing routine but time-consuming tasks from office visits may change the nature of patient-physician face-to-face contact, potentially improving care continuity, and providing physicians with the knowledge they need to optimally use such non-visit based systems.24, 25
Though this study involved a large population followed for an extended time period, several limitations warrant comment. By randomizing at the practice rather than patient, there were small differences in patient characteristics between intervention and control groups that were adjusted for in multivariable models. However, residual unmeasured confounding may exist even after adjustment. These results cannot be generalized beyond academic primary care networks with well developed informatics systems, but may represent what can be achieved within a medical home model of care delivery.26 Only one screening cycle was assessed for each patient and only extended follow-up will determine whether ongoing use sustains higher screening rates. Though rates of screening were higher in the intervention group, we did not demonstrate that this led to more breast cancers diagnosed. Future studies examining whether informatics interventions decrease morbidity and mortality will require larger patient populations and considerably longer follow-up periods. Finally, future work should assess the additional cost of the intervention relative to the increase in screening observed.
For health information technology to facilitate transformational change in health care, current models for delivering care will need to undergo a fundamental restructuring. We have demonstrated that a system for preventive breast cancer screening that allows 1) providers to screen their overdue list without a face-to-face visit and identify who should be contacted, 2) tracks those patients for test scheduling and completion, and 3) has practice delegates follow-up those patients remaining overdue, can improve screening rates beyond visit-based reminders in primary care practice settings. Over three years of follow-up, a novel system for breast cancer screening increased mammography rates among intervention patients compared to usual care. These results support the potential value of using integrated informatics tools to help providers deliver care outside of the usual office-based clinical encounter.
Acknowledgements
Registered with ClinicalTrials.gov: NCT00462891, http://clinicaltrials.gov/. Drs. Atlas and Grant are supported by a grant from the Agency for Healthcare Research and Quality (AHRQ R18 HS018161).
Grant funding from the National Cancer Institute (NCI 1 R21 CA121908) and by institutional support through the Massachusetts General Hospital Primary Care Operations Improvement program
REFERENCES
- 1.Jain SH, Seidman J, Blumenthal D. How health plans, health systems, and others in the private sector can stimulate ‘meaningful use’. Health Aff (Millwood) 2010;29:1667–70. doi: 10.1377/hlthaff.2010.0766. [DOI] [PubMed] [Google Scholar]
- 2.Stark P. Congressional intent for the HITECH Act. Am J Manag Care. 2010;16:SP24–8. [PubMed] [Google Scholar]
- 3.Jones SS, Adams JL, Schneider EC, et al. Electronic health record adoption and quality improvement in US hospitals. Am J Manag Care. 2010;16:SP64–71. [PubMed] [Google Scholar]
- 4.Ornstein SM, Garr DR, Jenkins RG, et al. Computer-generated physician and patient reminders. Tools to improve population adherence to selected preventive services. J Fam Pract. 1991;32:82–90. [PubMed] [Google Scholar]
- 5.Poon EG, Wright A, Simon SR, et al. Relationship between use of electronic health record features and health care quality: results of a statewide survey. Med Care. 2010;48:203–9. doi: 10.1097/MLR.0b013e3181c16203. [DOI] [PubMed] [Google Scholar]
- 6.Lester WT, Ashburner JM, Grant RW, et al. Mammography FastTrack: an intervention to facilitate reminders for breast cancer screening across a heterogeneous multi-clinic primary care network. J Am Med Inform Assoc. 2009;16:187–95. doi: 10.1197/jamia.M2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jemal A, Siegel R, Xu J, et al. Cancer statistics. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 8.Community Preventive Services Task Force Updated recommendations for client- and provider-oriented interventions to increase breast, cervical, and colorectal cancer screening. Am J Prev Med. 2012;43:92–6. doi: 10.1016/j.amepre.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Mandelblatt JS, Cronin KA, Bailey S, et al. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151:738–47. doi: 10.1059/0003-4819-151-10-200911170-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams EK, Breen N, Joski PJ. Impact of the National Breast and Cervical Cancer Early Detection Program on mammography and Pap test utilization among white, Hispanic, and African American women: 1996-2000. Cancer. 2007;109:348–58. doi: 10.1002/cncr.22353. [DOI] [PubMed] [Google Scholar]
- 11.Smith-Bindman R, Miglioretti DL, Lurie N, et al. Does utilization of screening mammography explain racial and ethnic differences in breast cancer? Ann Intern Med. 2006;144:541–53. doi: 10.7326/0003-4819-144-8-200604180-00004. [DOI] [PubMed] [Google Scholar]
- 12.Atlas SJ, Grant RW, Lester WT, et al. A cluster-randomized trial of a primary care informatics-based system for breast cancer screening. J Gen Intern Med. 2011;26:154–61. doi: 10.1007/s11606-010-1500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atlas SJ, Chang Y, Lasko TA, et al. Is this “my” patient? Development and validation of a predictive model to link patients to primary care providers. J Gen Intern Med. 2006;21:973–8. doi: 10.1111/j.1525-1497.2006.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atlas SJ, Grant RW, Ferris TG, et al. Patient-physician connectedness and quality of primary care. Ann Intern Med. 2009;150:325–35. doi: 10.7326/0003-4819-150-5-200903030-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy SN, Chueh HC. A security architecture for query tools used to access large biomedical databases. Proc AMIA Symp. 2002:552–6. [PMC free article] [PubMed] [Google Scholar]
- 16.Baron RC, Melillo S, Rimer BK, et al. Intervention to increase recommendation and delivery of screening for breast, cervical, and colorectal cancers by healthcare providers a systematic review of provider reminders. Am J Prev Med. 2010;38:110–7. doi: 10.1016/j.amepre.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 17.Baron RC, Rimer BK, Breslow RA, et al. Client-directed interventions to increase community demand for breast, cervical, and colorectal cancer screening a systematic review. Am J Prev Med. 2008;35:S34–55. doi: 10.1016/j.amepre.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Brouwers MC, De Vito C, Bahirathan L, et al. What implementation interventions increase cancer screening rates? a systematic review. Implement Sci. 2011;6:111. doi: 10.1186/1748-5908-6-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabatino SA, Habarta N, Baron RC, et al. Interventions to increase recommendation and delivery of screening for breast, cervical, and colorectal cancers by healthcare providers systematic reviews of provider assessment and feedback and provider incentives. Am J Prev Med. 2008;35:S67–74. doi: 10.1016/j.amepre.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Sohl SJ, Moyer A. Tailored interventions to promote mammography screening: a meta-analytic review. Prev Med. 2007;45:252–61. doi: 10.1016/j.ypmed.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vernon SW, McQueen A, Tiro JA, et al. Interventions to promote repeat breast cancer screening with mammography: a systematic review and meta-analysis. J Natl Cancer Inst. 2010;102:1023–39. doi: 10.1093/jnci/djq223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagner TH. The effectiveness of mailed patient reminders on mammography screening: a meta-analysis. Am J Prev Med. 1998;14:64–70. doi: 10.1016/s0749-3797(97)00003-2. [DOI] [PubMed] [Google Scholar]
- 23.Berwick DM. Launching accountable care organizations--the proposed rule for the Medicare Shared Savings Program. N Engl J Med. 2011;364:e32. doi: 10.1056/NEJMp1103602. [DOI] [PubMed] [Google Scholar]
- 24.Mauksch LB, Dugdale DC, Dodson S, et al. Relationship, communication, and efficiency in the medical encounter: creating a clinical model from a literature review. Arch Intern Med. 2008;168:1387–95. doi: 10.1001/archinte.168.13.1387. [DOI] [PubMed] [Google Scholar]
- 25.Weiner SJ, Barnet B, Cheng TL, et al. Processes for effective communication in primary care. Ann Intern Med. 2005;142:709–14. doi: 10.7326/0003-4819-142-8-200504190-00039. [DOI] [PubMed] [Google Scholar]
- 26.American Academy of Family Physicians (AAFP) American Academy of Pediatrics (AAP) American College of Physicians (ACP) American Osteopathic Association (AOA) [December 28, 2011];Joint principles of the patient-centered medical home, March 2007. http://www.acponline.org/running_practice/pcmh/understanding/what.htm.