Summary
Background
Preclinical studies have shown synergistic antitumour activity by inhibition of insulin-like growth factor-1 receptor (IGF-1R) and mTOR. The expression of IGF-1R seems to be crucial for this effect. We investigated the safety and efficacy of the combination of the IGF-1R antibody cixutumumab and the mTOR inhibitor temsirolimus in patients with chemotherapy-refractory bone and soft-tissue sarcomas according to IGF-1R expression by immunohistochemistry.
Methods
We undertook a multicentre, open-label, phase 2 study in 19 cancer centres in the USA. Patients aged at least 16 years with a histologically confirmed diagnosis of bone or soft-tissue sarcoma were allocated on the basis of IGF-1R expression by immunohistochemistry to one of three treatment groups: IGF-1R-positive soft-tissue sarcoma (group A), IGF-1R-positive bone sarcomas (group B), or IGF-1R-negative bone and soft-tissue sarcoma (group C). Patients received weekly treatment with cixutumumab (6 mg/kg, intravenous) and temsirolimus (25 mg, intravenous flat dose) in 6-week cycles. A Simon optimal two-stage design was used for every arm. The primary endpoint was progression-free survival (PFS) at 12 weeks by intention-to-treat analysis in the first 54 patients assigned to every treatment arm. Although patients still remain on treatment, this trial has completed enrolment and this represents the final analysis. This study is registered with ClinicalTrials.gov, number NCT01016015.
Findings
Between Nov 18, 2009, and April 11, 2012, 388 patients were screened for IGF-1R expression and 54 were assigned to each arm. 17 of 54 patients in the IGF-1R-positive soft-tissue sarcoma group (31%; one-sided 95% CI lower bound 21%; two-sided 90% CI 21–43), 19 of 54 in IGF-1R-positive bone sarcoma group (35%; one-sided 95% CI lower bound 24%; two-sided 90% CI 24–47), and 21 of 54 in the IGF-1R-negative group (39%, one-sided 95% CI lower bound 28%; two-sided 90% CI 28–51) were progression free at 12 weeks. On April 6, 2011, the protocol was amended to include three additional patients in the IGF-1R-positive soft-tissue sarcoma group (total of 57 patients) and nine more in the IGF-1R-negative group (total of 63 patients). There were 2546 adverse events reported during the study, 214 (8%) of which were grade 3–4. The most common grade 3–4 toxicities in the 174 treated patients were anaemia in 16 (9%) patients, hyperglycaemia in 18 (10%), hypophosphataemia in 16 (9%), lymphopenia in 25 (14%), oral mucositis in 19 (11%), and thrombocytopenia in 19 (11%).
Interpretation
The combination of cixutumumab and temsirolimus shows clinical activity in patients with sarcoma and forms a basis for future trials. However, IGF-1R expression by immunohistochemistry is not predictive of clinical outcome after treatment with this combination.
Funding
National Cancer Institute and Cycle for Survival Fund, Memorial Sloan-Kettering Cancer Center.
Introduction
About 13 000 cases of soft-tissue and bone sarcoma are diagnosed annually in the USA.1 The median survival from diagnosis for patients with metastatic disease is about 10–18 months.2 In view of the toxicity and limited efficacy of chemotherapy, patients with advanced and metastatic disease are appropriate candidates for investigational treatments. Insulin-like growth factor-1 (IGF-1), IGF-2, and IGF-binding protein (IGF-BP) are expressed in various sarcoma subtypes, which suggests that IGF-1 receptor (IGF-1R) inhibition might be applicable in sarcomas.3 IGF-1R is activated by the growth factor ligands IGF-1 and IGF-2, resulting in receptor autophosphorylation, which leads to the activation of many signalling cascades, including the PI3K–Akt–mTOR pathway. Several lines of evidence have suggested that IGF-1R signalling is crucial to the biological changes in Ewing's sarcoma and that targeting IGF-1R can inhibit tumour growth.4–8 However, in two large phase 2 trials in Ewing's sarcoma, treatment with IGF-1R-targeting monoclonal antibodies, R1507 and figitumumab, resulted in overall response rates of only 10% and 14% and median progression-free survival (PFS) of 1.3 and 1.9 months, respectively.9,10
Combined inhibition of both IGF-1R and mTOR signalling represents a novel approach for treatment of sarcoma.11,12 Blockade of mTOR alone paradoxically activates Akt.13 This finding might explain the disappointing single-drug activity with mTOR inhibitors, such as temsirolimus, in patients with soft-tissue sarcoma.14 However, IGF-1R inhibition suppresses mTOR-induced Akt activation and sensitises tumour cells to mTOR inhibitors.13 Pretreatment of rhabdomyosarcoma cell lines with the IGF-1R antibody h7C10 resulted in blockade of rapamycin-induced Akt activation and in an enhanced antiproliferative effect compared with either drug alone.12 The IGF-1R antibody R1507 similarly enhanced the effect of rapamycin by downregulating IGF-1R and blocking the reactivation of phosphorylated Akt (p-Akt) in a broad range of sarcoma cell lines.15 For sarcoma cell lines in which there was no IGF-1R expression, the combination of R1507 and rapamycin was ineffective.15 Based on such data, phase 1 studies of the combination of IGF-1R and mTOR inhibitors in sarcoma have been undertaken.16,17 The fully humanised IgG1-monoclonal-antibody-targeting IGF-1R cixutumumab can be combined safely with the mTOR inhibitor temsirolimus at respective doses of 6 mg/kg and 25 mg (flat dose) weekly.16 We therefore initiated a phase 2 trial of cixutumumab and temsirolimus in bone and soft-tissue sarcoma with stratification according to IGF-1R status. We hypothesised that IGF-1R expression by the tumour would be crucial to identify the clinical benefit of this drug combination and would be independent of histological subtype. Because response to IGF-1R antibody treatment on the basis of histological subtype alone has been scarce, we believed that upfront stratification by IGF-1R expression would result in an improved clinical benefit. Therefore, rather than stratify by histological subtype, we assessed clinical benefit according to IGF-1R expression by immunohistochemistry.
Methods
Patients
We undertook a multicentre, open-label, phase 2, non-randomised trial in 19 cancer centres in the USA. Patients aged at least 16 years were eligible if they had an Eastern Cooperative Oncology Group performance status of 0 or 1, histologically or cytologically confirmed sarcoma of soft tissue or bone, and measurable metastatic or locally advanced disease by Response Evaluation Criteria in Solid Tumors (RECIST) 1.1, and if they had received at least one but not more than four previous treatments and had adequate organ function (defined by a normal complete blood count [absolute neutrophil count ≥1.5 × 109/L, platelet count ≥100 × 109/L], liver function tests [total bilirubin ≤1.5 × institutional upper limit of normal [ULN] and aspartate aminotransferase and alanine aminotransferase ≤3 × ULN], and renal function [serum creatinine ≤1.5 × ULN). Patients with hyper glycaemia, defined as fasting serum glucose above 6.66 mmol/L, or those already on oral antidiabetic or insulin treatment were excluded from the study, as were those who received previous IGF-1R or mTOR inhibitors.
The study was undertaken in accordance with the Declaration of Helsinki. All patients provided written informed consent before enrolment after being informed about the purpose and the investigational nature of the study. The institutional review boards of all participating centres reviewed and approved the protocol.
Procedures
All patients had IGF-1R testing on archival tissue by immunohistochemistry at Memorial Sloan-Kettering Cancer Center (MSKCC; New York, NY, USA). Immunohistochemistry was done on 4-μm formalin-fixed paraffin embedded slides using pre-diluted IGF-1R antibodies (Ventana, Ventana Medical Systems, Tucson, AZ, USA) according to published methods.18 The scoring for IGF- 1R expression was quantitated on a 0–3+ scale: 0–1+, no staining or faint or weak staining; 2+, moderate staining; and 3+, strong staining. Only staining of 2+ to 3+ (cytoplasmic or membrane or both) was deemed positive. Scoring for IGF-1R expression was done by central pathology review (Cristina Antonescu, MSKCC) so as to minimise the inter-patient variability with immunohistochemistry.
Patients were assigned to one of three groups: those in group A had IGF-1R-positive sarcomas of soft tissue; those in group B had IGF-1R-positive sarcomas of bone; and those in group C had IGF-1R-negative sarcomas of bone and soft tissue. All patients received weekly treatment with cixutumumab (6 mg/kg intravenous) over 1 h followed 1 h later (30 min if the first two doses were tolerated) by temsirolimus (25 mg intravenous) in 6-week cycles.
If grade 3 or 4 neutropenia or thrombocytopenia occurred, temsirolimus and cixutumumab were respectively reduced by one (20 mg and 5 mg/kg) or two (15 mg and 4 mg/kg) dose levels. For hyperglycaemia of grade 1 toxicity or greater, oral antidiabetic treatment or insulin was started. For symptomatic grade 3 or any grade 4 hyperglycaemia, cixutumumab was reduced by one or two dose levels. For any grade 1 hepatic toxicity (bilirubin, alanine aminotransferase, or aspartate aminotransferase) the temsirolimus dose was reduced immediately by two dose levels (15 mg). Hyperlipidaemia was treated with medical management rather than by dose reduction. Patients were assessed weekly for adverse events.
Clinical and radiological (contrast CT or MRI of chest, abdomen, and pelvis) assessments were obtained every 6 weeks. Plasma samples for measurement of IGF-1 and IGF-BP3 concentrations by ELISA assay (R&D Systems, Minneapolis, MN, USA) were taken before treatment and on weeks 3 and 7. Plasma measurements were a requirement at MSKCC but were optional at other sites.
All patients treated at MSKCC were required to undergo tumour biopsies before and after treatment unless deemed clinically inappropriate. Biopsies were flash frozen in liquid nitrogen before treatment and between weeks 2 and 3 for assessment of total IGF-1R, p-Akt, total Akt, phosphorylated S6 (p-S6), and total S6 by western blots according to standard techniques.15 Equal loading of protein was confirmed by tubulin and GAPDH staining, and relative protein expression was quantified by densitometry assessment with ImageJ. All primary antibodies were obtained from Cell Signaling (Boston, MA, USA).
The primary endpoint was PFS at 12 weeks in the first 54 patients assigned to each treatment group, as assessed by RECIST 1.1. Secondary endpoints were overall survival, overall disease response, and correlation of plasma bio markers with clinical outcome. A post-hoc analysis was done by histological subtypes on progression-free survival, overall survival, and response.
Statistical analysis
Based on historical controls, a PFS of over 40% at 3 months was deemed acceptable for second-line treatment and a PFS of less than 20% was deemed unacceptable.19 We used a Simon optimal two-stage design for every arm such that the outcome of the study was assessed by the decision rule of the study design.20 In particular, in stage 1, 19 patients were to be accrued. If fewer than five patients (26%) were progression free at 12 weeks, further accrual would cease and the combination therapy would be declared ineffective. If at least five patients in stage 1 were progression free then an additional 35 patients would be accrued in stage 2 for a total of 54 patients. If at least 16 patients were progression free at 12 weeks among the first 54 patients, the arm would be judged to have a positive result and the combination therapy would be worthy of further testing. This design had a power of 0.90 for establishing the treatment effect for a population 12-week PFS proportion of 40% using a type I error of 0.05. The probability of stopping each arm of the study early was 67% if the population 12-week PFS proportion was 20%.
Analyses of efficacy data were done for all patients who had received any study drug (intention-to-treat). For every arm, the proportion of patients remaining free of progression at 12 weeks was estimated and a one-sided 95% CI and a two-sided 90% CI were calculated. Median event-free times for all treated patients were estimated by the Kaplan–Meier method with a two-sided 95% CI and were compared between groups by the log-rank test with a two-sided p value. Correlation of plasma biomarkers for IGF-1 and IGF-BP3 to PFS at 12 weeks was examined by the Wilcoxon rank sum test with a two-sided p value for absolute levels at baseline, week 3, or week 7 and for change of levels at week 3 or week 7 from baseline. R software (version 2.14.2) was used for all statistical analyses.
Role of the funding source
The sponsor of the study (National Cancer Institute) was involved in study design, data collection, and the decision to submit for publication in conjunction with the authors. The study sponsor had no role in data analysis, data interpretation, or writing of the report. GKS, L-XQ, VY, CB, AA, CRA, MC, MAD, SDV, ALH, and RGM had access to the raw data. The corresponding author (GKS) had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
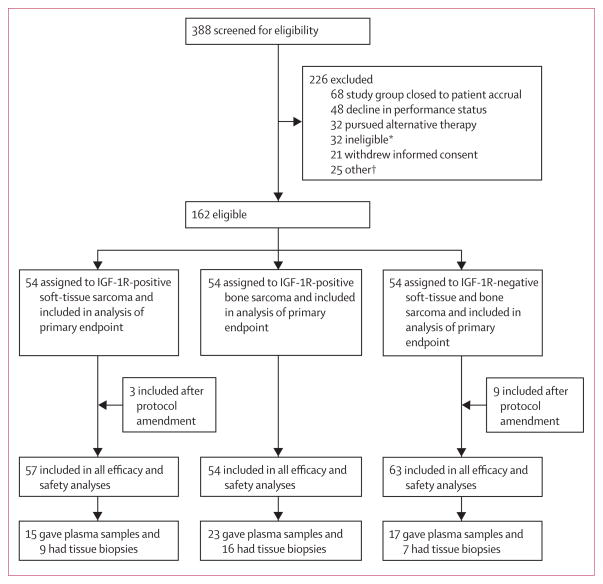
From Nov 18, 2009, to April 11, 2012, 388 patients were screened for IGF-1R expression by immunohistochemistry. Of these, 211 (54%) were IGF-1R positive and 177 (46%) were IGF-1R negative across many sarcoma subtypes (table 1). Patients were then enrolled into a treatment group at the discretion of the treating physicians until 54 patients had been enrolled in each group. All groups met the requirements for stage 1 before enrollment continued. The remainder of the screened patients were excluded because the study arm was closed to patient accrual or the patient did not meet protocol eligibility, had poor performance status, withdrew consent, elected to seek hospice or other treatments, or for other reasons (figure 1). On April 6, 2011, the protocol was amended to include three additional patients in the IGF-1R-positive soft-tissue sarcoma group and nine in the IGF-1R-negative sarcoma group, giving a total of 57 patients with IGF-1R-positive soft-tissue sarcoma (group A), 54 with IGF-1R-positive bone sarcoma (group B), and 63 with IGF-1R-negative sarcoma (group C). This protocol amendment was made because of over enrolment into group A (patients who consented before enrolment was halted) and on the basis of promising results in first 54 patients in group C. Table 2 shows the baseline demographics and patient characteristics, including the most common histological subtypes by IGF-1R status, for all treated patients. The mean number of previous systemic regimens was two (range one to four).
Table 1.
Insulin-like growth factor-1 receptor status according to histological subtype in at least five patients for all screened patients
| Total | IGF-1R positive | IGF-1R negative | |
|---|---|---|---|
| Chondrosarcoma | 38 | 20 (53%) | 18 (47%) |
| Chordoma | 6 | 1 (17%) | 5 (83%) |
| Clear-cell tumour | 6 | 5 (83%) | 1 (17%) |
| Ewing's sarcoma | 61 | 33 (54%) | 28 (46%) |
| Gastrointestinal stromal tumour | 12 | 3 (25%) | 9 (75%) |
| Leiomyosarcoma | 45 | 26 (58%) | 19 (42%) |
| Liposarcoma | 11 | 5 (45%) | 6 (55%) |
| Malignant peripheral nerve sheath tumour | 11 | 9 (82%) | 2 (18%) |
| Myxofibrosarcoma | 6 | 1 (17%) | 5 (83%) |
| Osteosarcoma | 52 | 33 (63%) | 19 (37%) |
| Undifferentiated pleomorphic sarcoma | 19 | 9 (47%) | 10 (53%) |
| Rhabdomyosarcoma | 10 | 7 (70%) | 3 (30%) |
| Sarcoma, unspecified | 14 | 7 (50%) | 7 (50%) |
| Solitary fibrous tumour | 19 | 11 (58%) | 8 (42%) |
| Spindle-cell tumour | 17 | 8 (47%) | 9 (53%) |
| Synovial sarcoma | 18 | 14 (78%) | 4 (22%) |
| Others | 43 | 19 (44%) | 24 (56%) |
Data are number (%). IGF-1R=insulin-like growth factor-1 receptor.
Figure 1. Trial profile.
IGF-1R=insulin-like growth factor-1 receptor. *Reasons for clinical screen failure: more than four treatments, brain metastases, abnormal laboratory values, congestive heart failure, or no measurable disease. †Other reasons included elected to undergo surgery, observation only, or radiation; unspecified reasons; or treatment was not covered by insurance.
Table 2.
Demographics and baseline characteristics
| IGF-1R-positive soft-tissue sarcoma (n=57) | IGF-1R-positive bone sarcoma (n=54) | IGF-1R-negative sarcoma (n=63) | |
|---|---|---|---|
| Age (years) | 50 (19–82) | 38 (18–73) | 55 (19–78) |
|
| |||
| Sex | |||
| Men | 22 (39%) | 32 (59%) | 40 (63%) |
| Women | 35 (61%) | 22 (41%) | 23 (37%) |
|
| |||
| ECOG PS | |||
| 0 | 39 (68%) | 30 (56%) | 31 (49%) |
| 1 | 18 (32%) | 24 (44%) | 32 (51%) |
|
| |||
| Previous treatments | |||
| 1 | 14 (25%) | 15 (28%) | 18 (29%) |
| 2 | 26 (46%) | 17 (31%) | 17 (27%) |
| 3 | 14 (25%) | 16 (30%) | 24 (38%) |
| 4 | 3 (5%) | 6 (11%) | 4 (6%) |
|
| |||
| Mean number of previous treatments | 2.1 | 2.3 | 2.3 |
|
| |||
| Most common histological subtype | |||
| Ewing's sarcoma or primitive neuroectodermal embriogenic tumour | 1 | 19 | 7 |
| Osteosarcoma | 0 | 18 | 6 |
| Chondrosarcoma | 0 | 11 | 6 |
| Leiomyosarcoma | 15 | 0 | 11 |
| Undifferentiated pleomorphic sarcoma | 6 | 3 | 10 |
| Synovial sarcoma | 8 | 0 | 3 |
| Solitary fibrous tumour | 4 | 0 | 4 |
| Malignant peripheral nerve sheath tumour | 5 | 0 | 1 |
| Well-differentiated and dedifferentiated liposarcoma | 3 | 0 | 1 |
| Gastrointestinal stromal tumour | 2 | 0 | 2 |
| Others | 13 | 3 | 12 |
Data are mean (range), number (%), or number. ECOG PS=Eastern Co-operative Oncology Group performance status.
After the first stage of enrolment, more than five patients in each group were progression free at 12 weeks. By intention-to-treat analysis, of the 54 patients in each group in the original cohort (ie, before the protocol amendment), 17 patients (31%; one-sided 95% CI lower bound 21%; two-sided 90% CI 21–43) in the IGF-1Rpositive soft-tissue sarcoma group, 19 patients (35%; one-sided 95% CI lower bound 24%; two-sided 90% CI 24–47) in the IGF-1R-positive bone sarcoma group, and 21 patients (39%; one-sided 95% CI lower bound 28%; two-sided 90% CI 28–51) in the IGF-1R-negative group were progression free at 12 weeks.
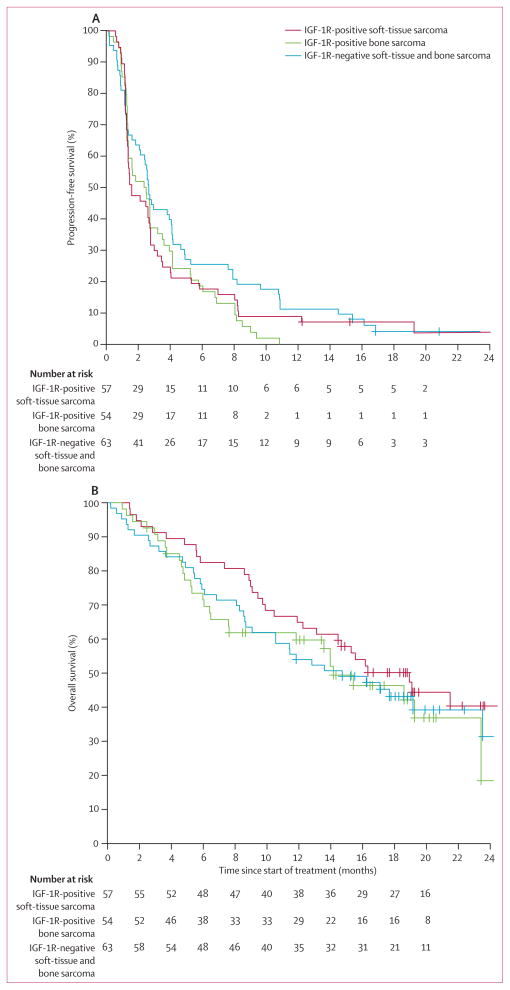
After the protocol amendment and inclusion of additional patients, 17 of 57 patients (30%; one-sided 95% CI lower bound 20%; two-sided 90% CI 20–41) in the IGF-1R-positive soft-tissue sarcoma group and 26 of 63 (41%; one-sided 95% CI lower bound 31%; two-sided 90% CI 31–52) in the IGF-1R-negative group were progression free at 12 weeks. At the time of final analysis, the median follow-up of survivors was 18.6 months (range 2.9–30.9). The median PFS by Kaplan–Meier estimates for all treated patients was 6.9 weeks (95% CI 5.9–12.0) for the IGF-1R-positive soft-tissue sarcoma group, 10.6 weeks (6.0–15.3) for the IGF-1R-positive bone sarcoma group, and 11.6 weeks (9.3–17.9) for the IGF-1R-negative group (figure 2A).
Figure 2. Kaplan–Meier curves for progression-free survival and overall survival.
(A) Progression-free survival and (B) overall survival for all treated patients according to IGF-1R expression groups. Curves were truncated at 24 months for better presentation of the data. IGF-1R=insulin-like growth factor-1 receptor.
Based on 98 deaths and a median duration of followup of 18.6 months (range 2.9–30.9), the median overall survival in all treated patients (n=174) was 18.9 months (95% CI 13.1 to not reached [NR]) for patients with IGF-1R-positive soft-tissue sarcoma, 14.2 months (7.6 to NR) for patients with IGF-1R-positive bone sarcoma, and 14.7 months (10.6 to NR) for patients with IGF-1R-negative sarcoma; figure 2B). All deaths were due to disease progression.
Nine patients achieved a partial response: one (2%) of 57 patients in the IGF-1R-positive soft-tissue sarcoma group, six (11%) of 54 patients in the IGF-1R-positive bone sarcoma group, and two (3%) of 63 patients in the IGF-1Rnegative group. There were no complete responses.
Analysis of plasma biomarkers in 55 patients (15 in the IGF-1R-positive soft-tissue sarcoma group, 23 in the IGF-1R-positive bone sarcoma group, and 17 in the IGF-1R-negative group) for IGF-1 and IGF-BP3 showed no correlation to PFS or overall survival (data not shown). All patients with disease progression at week 12 had significant increases in plasma biomarkers from baseline to weeks 3 or 7 (p<0.01 appendix).
32 patients (nine in the IGF-1R-positive soft-tissue sarcoma group, 16 in the IGF-1R-positive bone sarcoma group, and seven in the IGF-1R-negative group) at MSKCC had serial tumour biopsies undertaken before treatment and between weeks 2 and 3 of the first cycle of therapy and analysed by western blot. Although suppression of IGF-1R and inhibition of both p-S6 and p-Akt was noted in individual patients in all treatment arms, semi-quantitative analysis did not suggest any consistent association between the percent decrease in IGF-1R, p-S6, or p-Akt expression and clinical outcome (table 3). All patients in the immunohistochemistry defined IGF-1R-negative group—in which IGF-1R was assessable by western blot (five of the seven)—were IGF-1R positive by western blot (table 3).
Table 3.
Percent change from baseline in protein expression for p-Akt, p-S6, and IGF-1R, relative to best response
| p-Akt (% change from baseline) | p-S6 (% change from baseline) | IGF-1R (% change from baseline) | Best RECIST response | |
|---|---|---|---|---|
| IGF-1R-positive soft-tissue sarcoma | ||||
|
| ||||
| 1 | −75% | −66% | −92% | Stable disease |
| 2 | −20% | NA | −18% | Stable disease |
| 3 | −33% | NA | −34% | Stable disease |
| 4 | −50% | −90% | −60% | Stable disease |
| 5 | −88% | −60% | −88% | Progressive disease |
| 6 | −90% | −99% | −98% | Progressive disease |
| 7 | +200% | NA | +171% | Stable disease |
| 8 | 0% | −20% | −40% | Progressive disease |
| 9 | NA | NA | NA | Progressive disease |
|
| ||||
| IGF-1R-positive bone sarcoma | ||||
|
| ||||
| 1 | −80% | −100% | −92% | Stable disease |
| 2 | +239% | +207% | −27% | Partial response |
| 3 | −33% | −97% | −94% | Stable disease |
| 4 | −50% | −89% | −65% | Stable disease |
| 5 | −57% | −97% | −57% | Stable disease |
| 6 | −64% | +200% | +111% | Progressive disease |
| 7 | −88% | −98% | −98% | Progressive disease |
| 8 | −91% | −100% | −97% | Progressive disease |
| 9 | −91% | −98% | −99% | Stable disease |
| 10 | −94% | −97% | −97% | Stable disease |
| 11 | +200% | NA | +127% | Progressive disease |
| 12 | NA | NA | NA | Progressive disease |
| 13 | +236% | −70% | −40% | Stable disease |
| 14 | NA | NA | NA | Progressive disease |
| 15 | NA | −97% | −99% | Stable disease |
| 16 | NA | NA | NA | Progressive disease |
|
| ||||
| IGF-1R-negative soft-tissue and bone sarcoma | ||||
|
| ||||
| 1 | +121% | NA | +117% | Stable disease |
| 2 | −48% | −77% | −37% | Stable disease |
| 3 | −86% | NA | −82% | NA |
| 4 | −89% | −73% | −45% | Progressive disease |
| 5 | NA | NA | NA | Stable disease |
| 6 | +137% | −73% | −64% | Stable disease |
| 7 | NA | −97% | NA | Stable disease |
Data are shown for each patient (numbered) according to their assigned group.
p-Akt=phosphorylated Akt. p-S6=phosphorylated S6. IGF-1R=insulin-like growth factor-1 receptor. RECIST=Response Evaluation Criteria in Solid Tumors. NA=not assessable.
Of 2546 adverse events reported, 214 (8%) were grade 3–4. Grade 1–2 and 3–4 events that occurred in at least 5% of all patients treated are listed by stratification groups (table 4) and by histological subtype (appendix). The incidence of grade 3 and 4 events was similar between the three study arms. The most common grade 3–4 toxicities in the 174 patients were anaemia in 16 (9%) patients, hyperglycaemia in 18 (10%) patients, hypo phosphataemia in 16 (9%) patients, lymphopenia in 25 (14%) patients, oral mucositis in 19 (11%) patients, and thrombocytopenia in 19 (11%) patients. Although hyperglycaemia was noted in 119 (68%) of 174 patients, this was generally grade 1–2 and was well controlled. Of 174 patients treated on the study, 83 (48%) experienced a serious adverse event; 43 (52%) of these patients had a serious adverse event reported as possibly, probably, or definitely related to drug therapy. Among the serious adverse events for which a causal relation to drug therapy was suggested, only oral mucositis (12 [7%]) and thrombocytopenia (nine [5%]) occurred in at least 5% of 174 patients treated. There were four (2%) deaths on study and all of these were attributable to disease progression. Except for one episode of hypoxia, there were no reports of pneumonitis.
Table 4.
Adverse events of grade 1 or higher experienced by at least 5% of patients
| IGF-1R-positive soft-tissue sarcoma (n=57) | IGF-1R-positive bone sarcoma (n=54) | IGF-1R-negative sarcoma (n=63) | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | |
| Abdominal pain | 6 (11%) | 2 (4%) | 0 | 1 (2%) | 9 (14%) | 0 |
|
| ||||||
| Activated partial thromboplastin time prolonged | 4 (7%) | 1 (2%) | 5 (9%) | 1 (2%) | 8 (13%) | 2 (3%) |
|
| ||||||
| Alanine aminotransferase increased | 18 (32%) | 0 | 19 (35%) | 0 | 18 (29%) | 1 (2%) |
|
| ||||||
| Alkaline phosphatase increased | 16 (28%) | 0 | 18 (33%) | 1 (2%) | 19 (30%) | 1 (2%) |
|
| ||||||
| Allergic rhinitis | 3 (5%) | 0 | 2 (4%) | 0 | 7 (11%) | 0 |
|
| ||||||
| Alopecia | 6 (11%) | 0 | 4 (7%) | 0 | 5 (8%) | 0 |
|
| ||||||
| Anaemia | 22 (39%) | 7 (12%) | 29 (54%) | 4 (7%) | 42 (67%) | 5 (8%) |
|
| ||||||
| Anorexia | 24 (42%) | 0 | 17 (31%) | 0 | 26 (41%) | 1 (2%) |
|
| ||||||
| Anxiety | 4 (7%) | 0 | 3 (6%) | 0 | 8 (13%) | 0 |
|
| ||||||
| Arthralgia | 1 (2%) | 0 | 5 (9%) | 0 | 4 (6%) | 0 |
|
| ||||||
| Aspartate aminotransferase increased | 17 (30%) | 1 (2%) | 14 (26%) | 0 | 17 (27%) | 1 (2%) |
|
| ||||||
| Back pain | 10 (18%) | 1 (2%) | 6 (11%) | 1 (2%) | 14 (22%) | 0 |
|
| ||||||
| Bruising | 3 (5%) | 0 | 2 (4%) | 0 | 4 (6%) | 0 |
|
| ||||||
| Chest wall pain | 4 (7%) | 0 | 4 (7%) | 0 | 6 (10%) | 0 |
|
| ||||||
| Cholesterol high | 4 (7%) | 1 (2%) | 3 (6%) | 0 | 3 (5%) | 0 |
|
| ||||||
| Constipation | 20 (35%) | 0 | 18 (33%) | 0 | 25 (40%) | 0 |
|
| ||||||
| Cough | 12 (21%) | 1 (2%) | 11 (20%) | 1 (2%) | 20 (32%) | 0 |
|
| ||||||
| Creatine increased | 13 (23%) | 1 (2%) | 16 (30%) | 0 | 15 (24%) | 0 |
|
| ||||||
| Dehydration | 6 (11%) | 0 | 2 (4%) | 1 (2%) | 8 (13%) | 1 (2%) |
|
| ||||||
| Depression | 5 (9%) | 0 | 8 (15%) | 0 | 5 (8%) | 0 |
|
| ||||||
| Diarrhoea | 18 (32%) | 2 (4%) | 14 (26%) | 1 (2%) | 26 (41%) | 6 (10%) |
|
| ||||||
| Dizziness | 4 (7%) | 0 | 4 (7%) | 0 | 5 (8%) | 0 |
|
| ||||||
| Dry mouth | 3 (5%) | 0 | 5 (9%) | 0 | 8 (13%) | 0 |
|
| ||||||
| Dry skin | 14 (25%) | 0 | 11 (20%) | 0 | 9 (14%) | 0 |
|
| ||||||
| Dysgeusia | 9 (16%) | 0 | 9 (17%) | 0 | 18 (29%) | 0 |
|
| ||||||
| Dyspnoea | 15 (26%) | 0 | 11 (20%) | 1 (2%) | 19 (30%) | 2 (3%) |
|
| ||||||
| Epistaxis | 11 (19%) | 0 | 9 (17%) | 0 | 14 (22%) | 0 |
|
| ||||||
| Fatigue | 30 (53%) | 3 (5%) | 35 (65%) | 2 (4%) | 43 (68%) | 1 (2%) |
|
| ||||||
| Fever | 11 (19%) | 1 (2%) | 6 (11%) | 1 (2%) | 10 (16%) | 0 |
|
| ||||||
| Flu-like symptoms | 4 (7%) | 0 | 1 (2%) | 0 | 3 (5%) | 0 |
|
| ||||||
| Gastro-oesophageal reflux disease | 2 (4%) | 0 | 0 | 0 | 7 (11%) | 0 |
|
| ||||||
| Generalised muscle weakness | 3 (5%) | 0 | 1 (2%) | 0 | 3 (5%) | 0 |
|
| ||||||
| Headache | 11 (19%) | 0 | 13 (24%) | 0 | 15 (24%) | 0 |
|
| ||||||
| Hypercalcaemia | 7 (12%) | 0 | 4 (7%) | 0 | 4 (6%) | 0 |
|
| ||||||
| Hyperglycaemia | 28 (49%) | 7 (12%) | 35 (65%) | 4 (7%) | 38 (60%) | 7 (11%) |
|
| ||||||
| Hyperkalaemia | 2 (4%) | 1 (2%) | 1 (2%) | 0 | 9 (14%) | 0 |
|
| ||||||
| Hypernatraemia | 2 (4%) | 1 (2%) | 1 (2%) | 0 | 8 (13%) | 0 |
|
| ||||||
| Hypertriglyceridaemia | 19 (33%) | 4 (7%) | 24 (44%) | 2 (4%) | 26 (41%) | 2 (3%) |
|
| ||||||
| Hypoalbuminaemia | 15 (26%) | 1 (2%) | 13 (24%) | 0 | 16 (25%) | 0 |
|
| ||||||
| Hypocalcaemia | 10 (18%) | 0 | 7 (13%) | 1 (2%) | 12 (19%) | 2 (3%) |
|
| ||||||
| Hypoglycaemia | 3 (5%) | 0 | 4 (7%) | 0 | 2 (3%) | 0 |
|
| ||||||
| Hypokalaemia | 12 (21%) | 2 (4%) | 7 (13%) | 1 (2%) | 11 (17%) | 1 (2%) |
|
| ||||||
| Hyponatraemia | 10 (18%) | 2 (4%) | 14 (26%) | 0 | 19 (30%) | 2 (3%) |
|
| ||||||
| Hypophosphataemia | 5 (9%) | 5 (9%) | 7 (13%) | 4 (7%) | 5 (8%) | 7 (11%) |
|
| ||||||
| Hypotension | 3 (5%) | 0 | 1 (2%) | 1 (2%) | 3 (5%) | 0 |
|
| ||||||
| International normalised ratio increased | 3 (5%) | 1 (2%) | 4 (7%) | 0 | 3 (5%) | 1 (2%) |
|
| ||||||
| Insomnia | 3 (5%) | 0 | 6 (11%) | 0 | 11 (17%) | 0 |
|
| ||||||
| Leucopenia | 23 (40%) | 3 (5%) | 24 (44%) | 2 (4%) | 28 (44%) | 4 (6%) |
|
| ||||||
| Limb oedema | 7 (12%) | 0 | 3 (6%) | 0 | 9 (14%) | 1 (2%) |
|
| ||||||
| Lymphocytopenia | 8 (14%) | 9 (16%) | 6 (11%) | 7 (13%) | 13 (21%) | 9 (14%) |
|
| ||||||
| Musculoskeletal and connective tissue disorder—other | 2 (4%) | 0 | 1 (2%) | 0 | 6 (10%) | 0 |
|
| ||||||
| Myalgia | 8 (14%) | 0 | 8 (15%) | 0 | 7 (11%) | 0 |
|
| ||||||
| Nail infection | 3 (5%) | 0 | 0 | 0 | 5 (8%) | 0 |
|
| ||||||
| Nail ridging | 2 (4%) | 0 | 2 (4%) | 0 | 4 (6%) | 0 |
|
| ||||||
| Nasal congestion | 2 (4%) | 0 | 6 (11%) | 0 | 5 (8%) | 0 |
|
| ||||||
| Nausea | 20 (35%) | 0 | 20 (37%) | 0 | 24 (38%) | 0 |
|
| ||||||
| Neutropenia | 8 (14%) | 2 (4%) | 13 (24%) | 5 (9%) | 13 (21%) | 4 (6%) |
|
| ||||||
| Non-cardiac chest pain | 3 (5%) | 0 | 3 (6%) | 2 (4%) | 2 (3%) | 0 |
|
| ||||||
| Oral mucositis | 36 (63%) | 5 (9%) | 27 (50%) | 9 (17%) | 42 (67%) | 5 (8%) |
|
| ||||||
| Pain | 20 (35%) | 2 (4%) | 22 (41%) | 1 (2%) | 26 (41%) | 0 |
|
| ||||||
| Papulopustular rash | 5 (9%) | 1 (2%) | 2 (4%) | 0 | 2 (3%) | 0 |
|
| ||||||
| Paraesthesia | 2 (4%) | 0 | 2 (4%) | 0 | 5 (8%) | 0 |
|
| ||||||
| Paronychia | 4 (7%) | 0 | 3 (6%) | 0 | 3 (5%) | 0 |
|
| ||||||
| Peripheral sensory neuropathy | 5 (9%) | 0 | 7 (13%) | 0 | 9 (14%) | 0 |
|
| ||||||
| Pruritus | 11 (19%) | 0 | 5 (9%) | 0 | 12 (19%) | 0 |
|
| ||||||
| Rash acneiform | 2 (4%) | 0 | 13 (24%) | 0 | 12 (19%) | 0 |
|
| ||||||
| Rash maculopapular | 12 (21%) | 2 (4%) | 12 (22%) | 0 | 16 (25%) | 0 |
|
| ||||||
| Sinusitis | 2 (4%) | 0 | 1 (2%) | 0 | 5 (8%) | 0 |
|
| ||||||
| Skin infection | 4 (7%) | 0 | 3 (6%) | 0 | 6 (10%) | 0 |
|
| ||||||
| Sore throat | 6 (11%) | 0 | 4 (7%) | 0 | 6 (10%) | 0 |
|
| ||||||
| Upper respiratory infection | 2 (4%) | 0 | 2 (4%) | 0 | 5 (8%) | 0 |
|
| ||||||
| Urinary tract infection | 5 (9%) | 2 (4%) | 1 (2%) | 1 (2%) | 3 (5%) | 1 (2%) |
|
| ||||||
| Thrombocytopenia | 30 (53%) | 3 (5%) | 30 (56%) | 7 (13%) | 32 (51%) | 9 (14%) |
|
| ||||||
| Vomiting | 15 (26%) | 1 (2%) | 13 (24%) | 0 | 14 (22%) | 0 |
|
| ||||||
| Weight loss | 10 (18%) | 0 | 9 (17%) | 0 | 21 (33%) | 1 (2%) |
|
| ||||||
| Total | 712 | 75 | 675 | 62 | 945 | 77 |
Data are number (%).
91 (52%) of 174 patients needed either one (40 patients [23%]) or two (51 patients [29%]) dose reductions of temsirolimus. Of the 91 patients who needed one or more dose reductions, the most common causes were for thrombocytopenia (24 patients [26%]), oral mucositis (18 [20%]), or grade 1 or greater increases in aminotransferases (17 [19%]). 47 (27%) of 174 patients needed one (32 patients [18%]) or two (15 [9%]) dose reductions of cixutumumab. 16 (34%) of the 47 dose reductions were related to thrombocytopenia.
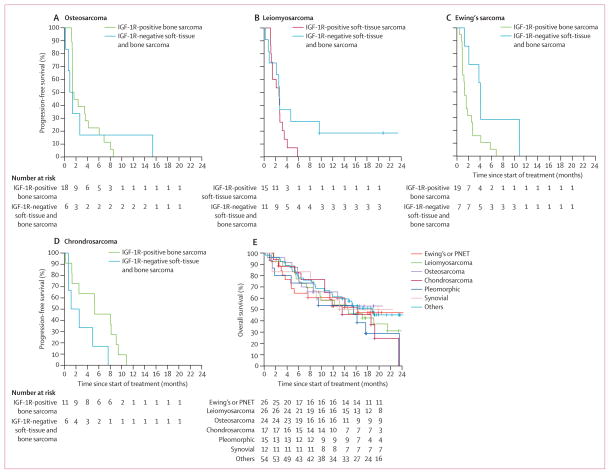
In a post-hoc analysis, we analysed median PFS, overall survival, and response according to histological subtype and IGF-1R status. The median PFS for the most common histological subtypes were 7.5 weeks (95% CI 5.6–17.7) for Ewing's sarcoma, 6.0 weeks (5.9–15.7) for osteosarcoma, 21.4 weeks (5.7–35.4) for chondrosarcoma, 11.4 weeks (6.3–15.3) for leiomyosarcoma, and 12.9 weeks (5.6–46.9) for undifferentiated pleomorphic sarcoma. For patients with osteosarcoma and leiomyosarcoma, IGF-1R status did not have an effect on median PFS (figure 3A and B). However, for Ewing's sarcoma and chondrosarcoma, IGF-1R immunohistochemistry expression had a statistically significant effect on median PFS (figure 3C and D). Nine (82%) of 11 IGF-1R-positive chondrosarcomas were histologically grade 2 and 3 and five (56%) of these nine were dedifferentiated. For patients with Ewing's sarcoma, the median PFS was 5.7 weeks (95% CI 4.3–11.9) for those with IGF-1Rpositive disease and and 17.7 weeks (9.0 to NR) for those with IGF-1R-negative disease (p=0.027, figure 3C). For patients with chondrosarcoma, the median PFS was 23.0 weeks (11.0 to NR) for those with IGF-1R-positive disease and 8.1 weeks (3.0 to NR) for those with IGF-1R-negative disease (p=0.048, figure 3D). For the eight patients with solitary fibrous tumours, the median PFS for the four patients with IGF-1R-positive disease was 89.6 weeks (4.0 to NR) and 16.1 weeks (5.0 to NR) for those with IGF-1Rnegative disease, but this was not statistically significantly different (p=0.22). This analysis included two patients with IGF-1R-positive solitary fibrous tumours who remained on study for 126 weeks and another who remains on study for 96 weeks.
Figure 3. Kaplan–Meier curves for post-hoc analyses.
Progression-free survival curves for (A) osteosarcoma, (B) leiomyosarcoma, (C) Ewing's sarcoma, and (D) chondrosarcoma. (E) Overall survival curves by histological subtype. Survival curves were truncated at 24 months for better presentation of the data. IGF-1R=insulin-like growth factor-1 receptor. PNET=primitive neuroectodermal embryogenic tumour.
Median overall survival was 16.2 months (95% CI 5.2 to NR) for Ewing's sarcoma, 14.6 months (8.6 to NR) for leiomyosarcoma, not reached (7.6 to NR) for osteosarcoma, 13.6 months (10.6 to NR) for chondrosarcoma, 15.5 months (8.5 to NR) for undifferentiated pleomorphic sarcoma, and 18.9 months (11.9 to NR) for others (figure 3E). In a post-hoc analysis, patients with IGF-1R-negative Ewing's sarcoma also had a numerical but not statistically significant (p=0.16) improvement in median overall survival (NR, 95% CI 16.2 to NR) when compared with those who were IGF-1R positive (14.0 months, 4.6 to NR).
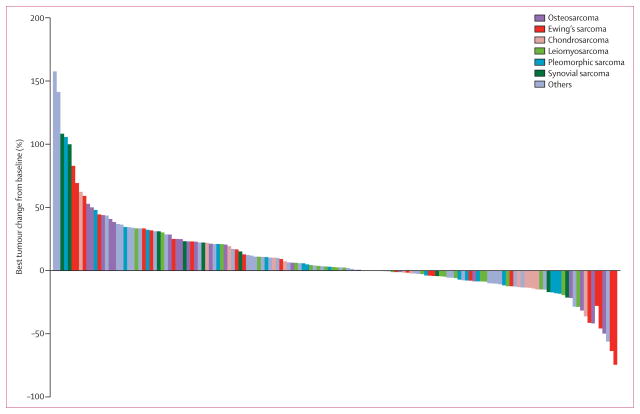
Figure 4 shows the waterfall plot for best RECIST response according to histology. Four of 27 (15%) patients with Ewing's sarcoma, three of 24 (13%) with osteosarcoma, one of 17 (6%) with chondrosarcoma, and one of eight (13%) with solitary fibrous tumour (hemangiopericytoma) had partial responses. There were no complete responses. Of the four patients with a diagnosis of gastrointestinal stromal tumour, one (IGF-1R positive) had a 13% decrease in tumour size from baseline as measured by RECIST.
Figure 4.
Waterfall plot of best change from baseline according to the most common histological subtypes
Discussion
We undertook a phase 2 study to assess the safety and efficacy of an IGF-1R antibody and a mTOR inhibitor in combination therapy in bone and soft-tissue sarcomas based on IGF-1R immunohistochemistry status. Toxicities were consistent with those reported in the phase 1 study of this combination.16 The primary endpoint of PFS at 12 weeks ranged from 31% in patients with IGF-1Rpositive soft-tissue sarcoma to 39% in patients with IGF-1R-negative sarcoma. Based on historical controls, a PFS in this range suggests that the combination of cixutumumab and temsirolimus is active in sarcoma.19 12-week PFS is a widely accepted benchmark for assessment of new drugs in sarcoma. It has been the primary endpoint of several clinical trials including the single-arm phase 2 study of pazopanib in sarcoma,21 a drug that is now approved by the US Food and Drug Administration for this disease. Additionally, the evidence of stable disease for at least 1 year in two patients with solitary fibrous tumours and partial responses in patients with Ewing's sarcoma, osteosarcoma, solitary fibrous tumour, and chondrosarcoma would support an argument that these drugs could offer disease control to subsets of patients with sarcoma (panel).
We hoped that IGF-1R expression by immunohistochemistry would be predictive of an improved outcome with therapy. The disease control noted in the IGF-1R-negative immunohistochemistry group was at least as good as that in the IGF-1R-positive groups. This inability to show an improved outcome across all tumour histological subtypes on the basis of IGF-1R expression does point out potential limitations to using IGF-1R immunohistochemistry as a screening tool for an IGF-1R-directed treatment. This could be because of limited antibody sensitivity. Therefore, the inability to detect IGF-1R by immunohistochemistry in patients using the Ventana antibody, who were otherwise IGF-1R positive by western blot using the Cell Signaling antibody, suggests that the Ventana antibody might not be sensitive enough to detect the low levels of IGF-1R expression. By standardising our immunohistochemistry assay and by having one sarcoma pathologist for central review of all the immunohistochemistry stained slides, we tried to avoid many of the pitfalls associated with immunohistochemistry staining. These pitfalls include broad inter-laboratory variability in performance of the assay, discrepancies between laboratories with high-volume versus low-volume throughput, use of different antibodies across sites, and deviations from recommended methods in the package insert leading to altered performance characteristics of the assays. Different cutoff points for classifying a positive result can also affect interpretation of the data. In our study we limited enrolment to tumour tissue that was 2–3+ by immunohistochemistry. This stringent cutoff might have excluded patients from the study who would have been otherwise IGF-1R positive. There are several pitfalls associated with western blotting, especially for the detection of phosphoproteins on tumour tissues, including the handling of the tissues, the time from tumour procurement to flash freezing, the amount of tumour available for analysis, and the specificity of the antibodies used. In our study, to minimise several of these issues, we obtained tumour biopsies at only one site (MSKCC) and all samples were flash frozen almost immediately upon biopsy within the interventional radiology suite. For the plasma correlates (IGF-1 and IGF-BP3 by ELISA), even though they were run at one site (MSKCC), they were obtained at multiple cancer centres, leaving us susceptible to some of the same issues mentioned previously for tumour procurement.
Although there was no overall effect of IGF-1R expression by immunohistochemistry on outcome, a post-hoc analysis of IGF-1R immunohistochemistry according to specific sarcoma subtypes did reveal that patients with Ewing's sarcoma who were IGF-1R negative by immunohistochemistry had better median PFS than those who were IGF-1R positive. This finding suggests that in Ewing's sarcoma, with the present drug dosing (6 mg/kg), low concentrations of IGF-1 receptor might be more easily saturated than higher concentrations. Alter natively, Ewing's sarcoma with low IGF-1R immunohistochemistry expression might have less aggressive biological effects. However, this suggestion is con founded by the fact that IGF-1Rpositive chondrosarcomas still had a better median PFS over IGF-1R-negative chondrosarcomas. The higher incidence of high-grade and dedifferentiated chondrosarcomas in the IGF-1R-positive group suggests that this better outcome was not because of less aggressive histological changes. These results, relative to IGF-1R status for specific sarcoma histological changes, are based on small numbers and should still be considered exploratory.
Pappo and colleagues10 suggested that the moderate clinical benefit noted with the IGF-1R antibody R1507 as a single drug in the treatment of Ewing's sarcoma could have been because of under dosing of the drug.10 Because the minimum dose of cixutumumab needed for inhibition of IGF-1R is unknown, the dose reductions needed for cixutumumab-related toxicity could have influenced the outcome of the present study. The same issue applies to temsirolimus, for which we used a starting dose of 25 mg intravenous weekly. Although this dose will modulate p-S6, lower doses have not been assessed and whether lower doses will inhibit mTOR signalling is unknown. This point is particularly relevant to our study because over 50% of the patients needed a dose reduction of temsirolimus and this also could have had a negative effect on the primary study outcome. Therefore, without additional historical data linking the pharmacology of these drugs with pharmacodynamic changes, especially at low doses, identifying how these dose reductions within the trial affected median PFS is difficult. However, the western blots from our study confirmed pathway inhibition in some patients with downregulation of IGF-1R and inhibition of p-Akt and p-S6 in all arms of this study, particularly within the first cycle of therapy, suggesting a sufficient biological effect to inhibit IGF-1R and mTOR signalling at the recommended phase 2 dose of cixutumumab and temsirolimus in the combination therapy. Despite evidence for target inhibition within the tumours, disease control was not achieved in many of these patients. One possibility is that there might be other receptor tyrosine kinases that are activated in sarcoma that need to be targeted in concert with mTOR to maximise p-Akt inhibition.15 However, we do not know whether pathway inhibition was sustained beyond weeks 2–3 when the matched pair biopsies were obtained, especially at later times in the trial when dose reductions for toxicity were started. We were unsuccessful in obtaining tumour tissue at the time of disease progression, and so assessment of the exact mechanism for drug resistance and tumour progression is difficult.
Median overall survival was over 14 months for all patients in the study. Comparison of data regarding single-drug IGF-1R targeting with other IGF-1R-mTOR combinations or even with best supportive care is difficult. The only comparison that can be made is with patients with Ewing's sarcoma. With R1507 alone in Ewing's sarcoma, median PFS was 5.7 weeks, the RECIST response rate was 10%, and median overall survival was 7.6 months.10 In the phase 1, dose-expansion phase of the trial of patients with Ewing's sarcoma treated with cixutumumab and temsirolimus, there was a 29% response rate and a median overall survival of 12.6 months (median PFS not reported).16 With cixutumumab and temsirolimus in the present trial, even though the median PFS for the whole group was 6.0 weeks, the response rate was 15% and the median overall survival was 16.2 months. Furthermore, an examination of patients with Ewing's sarcoma by IGF-1R immunohistochemistry expression suggests that patients who were IGF-1R negative had a better median PFS than those who were IGF-1R positive. Additionally, although not statistically significant, in a post-hoc analysis, patients with IGF-1R-negative Ewing's sarcoma also had a numerical improvement in median overall survival when compared with those who were IGF-1R positive. Collectively, these results suggest that the combination therapy should be explored in future clinical studies, especially for specific sarcoma histological subtypes where IGF-1R immunohistochemistry status might have an effect on clinical benefit and where signs of clinical response were noted (ie, Ewing's sarcoma, chondrosarcoma, and solitary fibrous tumour).
Panel: Research in context.
Systematic review
We searched PubMed for original research articles published in English before Dec 1, 2012 with the terms “sarcoma”, “IGF-1R”, “mTOR”, and “combinations”. We found two phase 1 combination trials and only one of these included a dose expansion with the combination therapy at the maximally tolerated dose in patients with Ewing's sarcoma.16,17 Therefore, we undertook a multicentre phase 2 clinical trial to assess the combination of an inhibitor of insulin-like growth factor-1 receptor (IGF-1R; cixutumumab) and an inhibitor of mTOR (temsirolimus) in patients with sarcoma. We also examined whether IGF-1R expression by immunohistochemistry could be used to identify patient populations who would most benefit from the combination treatment.
Interpretation
The combination of cixutumumab and temsirolimus achieved an acceptable median progression-free survival at 12 weeks in patients with both IGF-1R-positive and IGF-1R-negative bone and soft-tissue sarcoma. This effect seemed to be greatest in specific subsets of patients identified by immunohistochemistry for IGF-1R expression (ie, IGF-1R-negative Ewing's sarcoma and IGF-1R-positive chondrosarcoma). The data also show that in some patients these two drugs together can suppress IGF-1R expression and inhibit both Akt and mTOR signalling. These results provide a basis for future clinical development of IGF-1R and mTOR combinations in patients with both soft-tissue and bone sarcomas.
Supplementary Material
Acknowledgments
We thank all collaborators on this study and all the patients who participated. We also acknowledge the funding provided by the National Cancer Institute under grants RC2 CA148260 (RGM and GKS) and R01 CA140331 (GKS), and CycleforSurvival funding from MSKCC. We thank Noah Goodman-Davis and Jerusa Altema (MSKCC), who contributed substantially to the preparation of the data for this manuscript.
Footnotes
Contributors GKS, WDT, L-XQ, CRA, MAD, ALH, LAD, HXC, and RGM designed and developed the study. GKS, WDT, MBL, SDU, BC, MA, SMS, DRR, SHO, JAL, VK, PR, ASK, DA, BAVT, BB, MC, MAD, and RGM were responsible for patient inclusion. GKS, VY, CB, AA, MC, and SDV collected data. GKS, WDT, L-XQ, and ALH analysed data. GKS, WDT, MBL, SDU, BC, MA, SMS, DRR, SHO, JAL, VK, PR, ASK, DA, BAVT, BB, MC, MAD, and RGM interpreted data. GKS, WDT, L-XQ, MAD, ALH, and RGM wrote the manuscript. All the authors reviewed the article for intellectual content, provided comments, and gave final approval.
Conflicts of interest GKS has received a consultancy fee from Pfizer and a speaker honorarium from Imclone. WDT is the study chair (uncompensated) of a clinical trial funded by Imclone. MAD has received a consultancy fee from Pfizer and is a study chair (uncompensated) of a clinical trial funded by Pfizer. RGM has received a consultancy fee from Imclone. All other authors declare that they have no conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Brennan M, Singer S, Maki R, O'Sullivan B. Soft tissue sarcoma. In: DeVita VT, Lawrence TS, Rosenberg SA, editors. Cancer: principles & practices of oncology. 8. Vol. 2. Philadelphia: Lippincott, Williams & Wilkins; 2008. pp. 1741–94. [Google Scholar]
- 3.Baird K, Davis S, Antonescu CR, et al. Gene expression profiling of human sarcomas: insights into sarcoma biology. Cancer Res. 2005;65:9226–35. doi: 10.1158/0008-5472.CAN-05-1699. [DOI] [PubMed] [Google Scholar]
- 4.Olmos D, Tan DS, Jones RL, Judson IR. Biological rationale and current clinical experience with anti-insulin-like growth factor 1 receptor monoclonal antibodies in treating sarcoma: twenty years from the bench to the bedside. Cancer J. 2010;16:183–94. doi: 10.1097/PPO.0b013e3181dbebf9. [DOI] [PubMed] [Google Scholar]
- 5.Prieur A, Tirode F, Cohen P, Delattre O. EWS/FLI-1 silencing and gene profiling of Ewing cells reveal downstream oncogenic pathways and a crucial role for repression of insulin-like growth factor binding protein 3. Mol Cell Biol. 2004;24:7275–83. doi: 10.1128/MCB.24.16.7275-7283.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scotlandi K, Manara MC, Nicoletti G, et al. Antitumor activity of the insulin-like growth factor-I receptor kinase inhibitor NVP-AEW541 in musculoskeletal tumors. Cancer Res. 2005;65:3868–76. doi: 10.1158/0008-5472.CAN-04-3192. [DOI] [PubMed] [Google Scholar]
- 7.Sekyi-Otu A, Bell RS, Ohashi C, Pollak M, Andrulis IL. Insulin-like growth factor 1 (IGF-1) receptors, IGF-1, and IGF-2 are expressed in primary human sarcomas. Cancer Res. 1995;55:129–34. [PubMed] [Google Scholar]
- 8.Toretsky JA, Kalebic T, Blakesley V, LeRoith D, Helman LJ. The insulin-like growth factor-I receptor is required for EWS/FLI-1 transformation of fibroblasts. J Biol Chem. 1997;272:30822–27. doi: 10.1074/jbc.272.49.30822. [DOI] [PubMed] [Google Scholar]
- 9.Juergens H, Daw NC, Geoerger B, et al. Preliminary efficacy of the anti-insulin-like growth factor type 1 receptor antibody figitumumab in patients with refractory Ewing sarcoma. J Clin Oncol. 2011;29:4534–40. doi: 10.1200/JCO.2010.33.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pappo AS, Patel SR, Crowley J, et al. R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: results of a phase II Sarcoma Alliance for Research through Collaboration study. J Clin Oncol. 2011;29:4541–47. doi: 10.1200/JCO.2010.34.0000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolb EA, Gorlick R, Maris JM, et al. Combination testing (stage 2) of the anti-IGF-1 receptor antibody IMC-A12 with rapamycin by the pediatric preclinical testing program. Pediatr Blood Cancer. 2012;58:729–35. doi: 10.1002/pbc.23157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–40. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 13.O'Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–08. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okuno S, Bailey H, Mahoney MR, et al. A phase 2 study of temsirolimus (CCI-779) in patients with soft tissue sarcomas: a study of the Mayo phase 2 consortium (P2C) Cancer. 2011;117:3468–75. doi: 10.1002/cncr.25928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho AL, Vasudeva SD, Lae M, et al. PDGF receptor alpha is an alternative mediator of rapamycin-induced Akt activation: implications for combination targeted therapy of synovial sarcoma. Cancer Res. 2012;72:4515–25. doi: 10.1158/0008-5472.CAN-12-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naing A, LoRusso P, Fu S, et al. Insulin growth factor-receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with refractory Ewing's sarcoma family tumors. Clin Cancer Res. 2012;18:2625–31. doi: 10.1158/1078-0432.CCR-12-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quek R, Wang Q, Morgan JA, et al. Combination mTOR and IGF-1R inhibition: phase I trial of everolimus and figitumumab in patients with advanced sarcomas and other solid tumors. Clin Cancer Res. 2011;17:871–79. doi: 10.1158/1078-0432.CCR-10-2621. [DOI] [PubMed] [Google Scholar]
- 18.Lasota J, Wang Z, Kim SY, Helman L, Miettinen M. Expression of the receptor for type I insulin-like growth factor (IGF1R) in gastrointestinal stromal tumors: an immunohistochemical study of 1078 cases with diagnostic and therapeutic implications. Am J Surg Pathol. 2013;37:114–19. doi: 10.1097/PAS.0b013e3182613c86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Blabbeke M, Verweij J, Judson I, Nielsen OS. Progression-free rate as the principal end-point for phase II trials in soft-tissue sarcomas. Eur J Cancer. 2002;38:543–49. doi: 10.1016/s0959-8049(01)00398-7. [DOI] [PubMed] [Google Scholar]
- 20.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 21.Sleijfer S, Ray-coquard I, Papi Z, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043) J Clin Oncol. 2009;27:3126–32. doi: 10.1200/JCO.2008.21.3223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.