Abstract
Warburg suggested that the alterations in metabolism that he observed in cancer cells were due to the malfunction of mitochondria. In the past decade, we have revisited this idea and reached a better understanding of the ‘metabolic switch’ in cancer cells, including the intimate and causal relationship between cancer genes and metabolic alterations, and their potential to be targeted for cancer treatment. However, the vast majority of the research into cancer metabolism has been limited to a handful of metabolic pathways, while other pathways have remained in the dark. This Progress article brings to light the important contribution of fatty acid oxidation to cancer cell function.
The process of cellular transformation and cancer progression involves genetic mutations and epigenetic alterations, as well as the rewiring of cellular signalling and the reprogramming of metabolic pathways1. We now perceive these processes as intimately interconnected and interdependent2. Emanating from the initial hypothesis of Warburg3 (now known as Warburg's hypothesis), the latest research has revealed that metabolic reprogramming occurs as a consequence of mutations in cancer genes and alterations in cellular signalling. Much of the hype in cancer metabolism comes from the genuine observation that most cancer cells are programmed to increase glucose uptake, but to reduce the proportion of glucose oxidized in the Krebs cycle. Rather than oxidizing glucose for ATP production, glucose in cancer cells tends to be used for anabolic processes, such as ribose production, protein glycosylation and serine synthesis4–7. Cancer cells use additional nutritional inputs for anabolism besides glucose. From its metabolism to pyruvate, glutamine is key for providing reduced NADPH, which is needed for lipid synthesis, and to refill the Krebs cycle (anaplerosis)8,9. The control of this pathway by key oncogenes, such as MYC and mutant RAS, has further enforced the importance of this route in cancer. This view of cancer metabolism takes the focus away from ATP as the key product of glucose and glutamine catabolism. The fact is that in most biological contexts (but not all, as we discuss below), ATP production is sufficient for cancer cell function.
In addition to glucose and glutamine, fatty acids are an extremely relevant energy source. They can be incorporated from the extracellular media, or can be potentially obtained from hydrolysed triglycerides (in cells accumulating lipid droplets) by neutral (N) hydrolases in the cytoplasm or acid (A) hydrolases through a novel autophagic pathway: lipophagy10. De novo synthesis of fatty acids is required for membrane synthesis and therefore for cell growth and proliferation. Fatty acid synthesis is an anabolic process that starts from the conversion of acetyl CoA to malonyl CoA by acetyl CoA carboxylase. Malonyl CoA is then committed to fatty acid synthesis (FAS) and is involved in the elongation of fatty acids through fatty acid synthase (FASN). Additional modifications of fatty acids can be carried out by elongases and desaturases. Fatty acids are catabolized by the fatty acid oxidation (FAO; also known as β-oxidation) pathway.
With most cancer researchers focusing on glycolysis, glutaminolysis and fatty acid synthesis, the relevance of FAO for cancer cell function has not been carefully examined, and its relevance has remained obscure. However, studies in the past 4 years have started to bring to light a relevant role for this metabolic pathway in cancer, and this is accompanied by new and exciting therapeutic implications. The focus of this Progress article is to enumerate, highlight and integrate these recent findings into our current understanding of metabolic reprogramming in cancer cells.
Extra ATP when needed
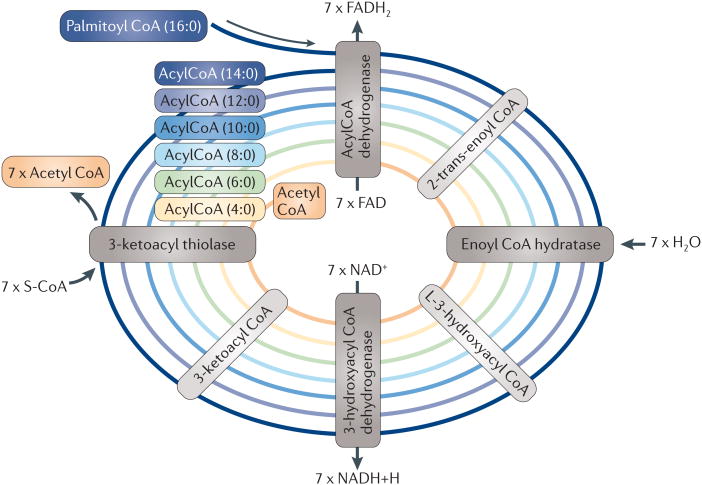
Relative to their dry mass, fatty acids provide twice as much ATP as carbohydrates (six times more when comparing stored fatty acids to stored glycogen), and in turn they are the preferred nutrient for storage (in the form of triglycerides in adipose tissue) under conditions of nutrient abundance. FAO is composed of a cyclical series of reactions that result in the shortening of fatty acids (two carbons per cycle) and that generate in each round NADH, FADH2 and acetyl CoA, until the last cycle when two acetyl CoA molecules are originated from the catabolism of a four-carbon fatty acid (FIG. 1). NADH and FADH2 that are generated by FAO enter the electron transport chain (ETC) to produce ATP (FIG. 2). FAO is carried out in energy-demanding tissues (such as the heart and skeletal muscle) and in the liver as a central organ for nutrient supply and conversion.
Figure 1. Representation of the β-oxidation of palmitic acid in the mitochondria.
AcylCoAs enter the fatty acid oxidation (FAO) pathway in which they are dehydrogenated, hydrated and decarboxylated cyclically, which results in the progressive shortening of the fatty acid. The production of NADH and FADH2 will be used for ATP production in the electron transport chain, and acetyl CoA can enter the Krebs cycle. S-CoA, free coenzme A.
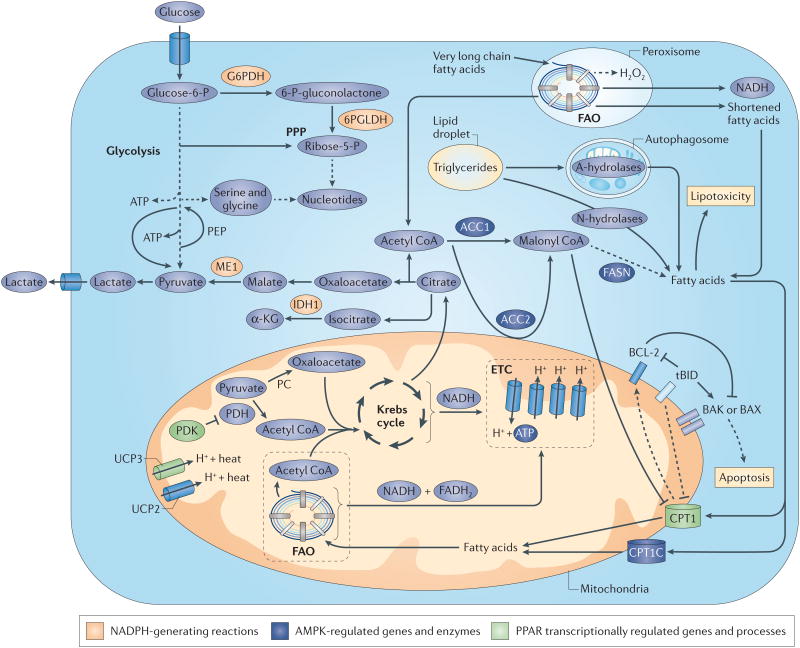
Figure 2. Effect of FAO on cancer cell metabolism, growth and survival.
A schematic representation of the metabolic routes interconnected with fatty acid oxidation (FAO) is shown. Central metabolic processes are depicted such as glycolysis, the pentose phosphate pathway (PPP), the Krebs cycle, the electron transport chain (ETC) and FAO. Fatty acids for FAO can either be of extracellular origin (through a first shortening in the peroxisomal FAO for very long fatty acids) or be obtained through the metabolism of triglycerides from lipid droplets. The fate of FAO products is summarized. NADH and FADH2 are oxidized in the ETC for ATP production and acetyl CoA enters the Krebs cycle to produce citrate, which can be exported to the cytoplasm to engage NADPH-producing reactions. Importantly, the accumulation of fatty acids that do not undergo β-oxidation can induce lipotoxicity. Of note, FAO-independent activities of components in this route are depicted. Dashed arrows represent indirect effects or serial reactions. 6PGLDH, 6-phosphogluconate dehydrogenase; α-KG, α-ketoglutarate; ACC, acetyl CoA carboxylase; AMPK, AMP kinase; CPT1, carnitine palmitoyltransferase; FASN, fatty acid synthase; G6PDH, glucose-6-phosphate dehydrogenase; IDH1, isocitrate dehydrogenase 1; ME1, malic enzyme; PC, pyruvate carboxylase; PDK, pyruvate dehydrogenase (PDH) kinase; PEP, phosphoenol pyruvate; PPAR, peroxisome proliferator-activated receptor; UCP, uncoupling protein.
We have summarized the metabolic switch as a programme in which the utilization of metabolic intermediates for anabolism prevails beyond ATP production. But there are situations in which cancer cells seem to require increased ATP production. This is exemplified by loss of attachment (LOA) to the extracellular matrix. Cells derived from solid tumours that undergo LOA display inhibition of glucose uptake and catabolism, which results in the loss of ATP, NADPH (as a result of decreased flux through the pentose phosphate pathway (PPP)) and increased production of reactive oxygen species (ROS) (FIG. 3). Schafer and co-workers11 showed that in these settings ROS inhibit FAO, and that antioxidants counteract ROS accumulation and can reactivate FAO, increase ATP levels and prevent LOA-induced anoikis, although the exact mechanism by which an increase in ATP rescues anoikis remains unclear.
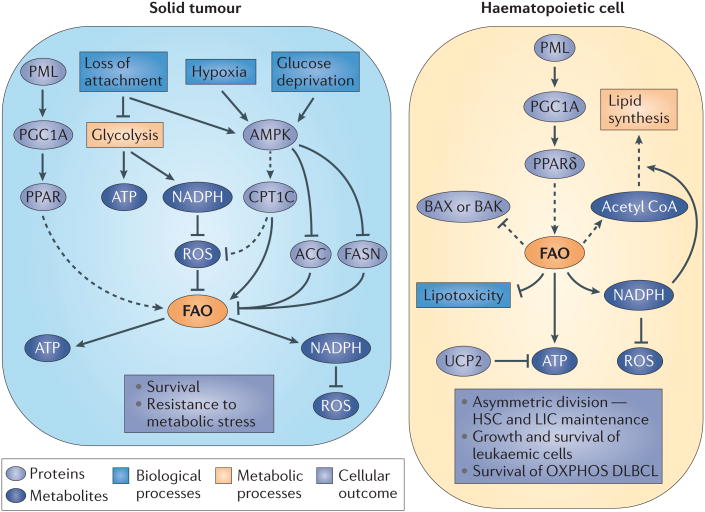
Figure 3. Summary of the regulation of FAO and its effect on cell fate in cancer cells from different origins.
Activation of fatty acid oxidation (FAO) by upstream (AMP kinase (AMPK) and promyelocytic leukaemia (PML)–peroxisome proliferator-activated receptor (PPAR) pathways) regulators is shown, and the FAO products relevant for cancer cell function are indicated, together with the biological outcomes derived from FAO activation. Dashed arrows represent indirect effects or serial reactions. ACC, acetyl CoA carboxylase; CPT1, carnitine palmitoyltrans-ferase; DLBCL, diffuse large B cell lymphoma; FASN, fatty acid synthase; G6PDH, glucose-6-phosphate dehydrogenase; HSC, haematopoietic stem cell; LIC, leukaemia-initiating cell; OXPHOS, oxidative phosphorylation; PGC1A, PPARγ coactivator 1α; ROS, reactive oxygen species; UCP, uncoupling protein.
The relevance of FAO for cell survival on LOA was further supported by findings from the Pandolfi laboratory12. They described a novel mechanism in which FAO is regulated by the promyelocytic leukaemia (PML) protein, and this regulation is dependent on peroxisome proliferator-activated receptors (PPARs). In agreement with the study from Joan Brugge's group11, the ability of PML to increase FAO activity promoted cell survival on LOA. This mechanism of action provides an explanation for the upregulation of PML that is observed in a subset of aggressive breast cancers with increased PPAR activity and a poor prognosis12.
The requirement of FAO for ATP production in conditions of metabolic stress is recapitulated in other cancer types and conditions. The group of Tak W. Mak13 identified an atypical carnitine palmitoyltransferase 1 (CPT1) isoform — CPT1C — as a potential oncogene13. CPT1 conjugates fatty acids with carnitine to translocate them to the mitochondria, where the acylcarinitines undergo FAO. CPT1C is normally primarily expressed in the brain, but these authors found that CPT1C expression in cancer cells promoted FAO and ATP production, tumour growth, rescue from metabolic stress and resistance to mTOR complex 1 (mTORC1) inhibitors. Despite this newly identified oncogenic activity of CPT1C, information regarding its subcellular localization and its substrate specificity is limited and warrants further study14–16.
FAO is also relevant to cells that do not undergo LOA-induced metabolic stress, such as lymphoma and leukaemia cells (FIG. 3). The group of Michael Andreeff17 suggested that the FAO pathway is not relevant for the production of ATP in certain leukaemias17. Instead, FAO is required for cell survival and may influence BAX- and BAK-dependent mitochondrial permeability transition pore formation17. Proteins involved in FAO, such as CPT1, have been shown to have an anti-apoptotic function that is attributed to crosstalk with the BH3 family of pro-apoptotic proteins18,19. Although the BH3-regulatory activity of proteins involved in FAO (presumably CPT1 (REFS 18,19)) is thought to be instrumental in promoting the survival of certain leukaemia cells17, this might not be the whole story. FAO inhibition leads to a cytotoxic increase of lipids, and preventing this cytotoxicity might also be a contributing factor17,20. Additional papers have also shown that the excessive production of FAO-derived ATP is detrimental to the survival of leukaemia cells, and that this might be avoided through an increase in the expression of mitochondrial uncoupling proteins (UCP2 and UCP3)21. UCPs are mitochondrial anion carriers at the inner mitochondrial membrane. UCPs dissipate the proton gradient, therefore reducing the yield of ATP from oxidative phosphorylation (OXPHOS). UCP2 and UCP3 seem to regulate the production of free radicals, and they are also involved in insulin secretion and fatty acid metabolism, respectively. The catalytic activity of UCP1 is unclear. It might be a fatty acid-activated proton carrier or a flippase of protonated fatty acids to the inner mitochondrial side (which results in the loss of the proton gradient).
Recent studies in diffuse large B cell lymphoma (DLBCL) show a more complex picture. The group of Nika Danial22 recently found that a previously defined subset of DLBCLs that do not require the activation of the B cell receptor for survival have an OXPHOS gene expression signature22, indicating that they are highly dependent on mitochondrial metabolism for survival23. This DLBCL subtype catabolizes fatty acids at a higher rate than other subtypes of DLBCL and is highly dependent on FAO for survival and growth. In this cellular system, fatty acids are responsible for the majority of cellular respiration, a large proportion of ATP production and potentially the recycling of reduced glutathione (GSH) to cope with oxidative stress (as discussed below).
Recent evidence suggests that normal and cancer stem cells benefit from active FAO for their maintenance and function. Samudio et al.17 suggested that inhibition of FAO could affect leukaemia progenitor cells or leukaemia-initiating cells (LICs). In a more recent study, Ito, Carracedo et al.24 characterized the contribution of FAO downstream of PML and PPARδ to the maintenance and function of haematopoietic stem cells (HSCs) and potentially to LICs. They showed that PPARδ is required for the maintenance of HSCs, a function that is associated with FAO-derived ATP production. This PML–PPAR–FAO axis that Pandolfi and colleagues initially described is involved in HSC maintenance through the regulation of asymmetric cell division. Pharmacological or genetic interference at any level in this pathway unequivocally resulted in the exhaustion of the stem cell pool. It is worth noting that the genetic ablation of another FAO-regulatory protein, liver kinase B1 (LKB1; also known as STK11) similarly results in the exhaustion of the HSC pool25–27. In addition, a recent report has demonstrated that neuronal stem and progenitor cells (NSPCs) require FAS to ensure their proliferation and therefore to sustain neurogenesis28, which suggests that a potential transition exists from FAO in the quiescent stem cells to FAS in the proliferating progenitors. To what extent the requirement for FAO by undifferentiated haematopoietic cells can be extrapolated to other LICs or cancer-initiating cells remains to be evaluated, but the concept of targeting FAO for early intervention is novel and of high therapeutic relevance29.
FAO is an important source of NADPH
As stated above, cancer cells are dependent on ATP production for survival only in certain conditions. In the majority of situations, however, the potential of cancer cells to grow and survive will be limited by the levels of cytosolic NADPH. Therefore, metabolic reprogramming must meet the requirement of producing reduced NADP+. This is achieved in four main enzymatic reactions: the oxidation of glucose through glucose-6-phosphodehydrogenase (G6PDH) and 6-phosphogluconolactone dehydrogenase (6PGLDH) in the PPP; the metabolism of malate to pyruvate that is catalysed by malic enzyme (ME1); and the oxidation of isocitrate to α-ketoglutarate by isocitrate dehydrogenase (IDH1) (FIG. 2). NADPH exerts two main functions in the cell30. On the one hand, it provides redox power to counteract oxidative stress, which has proved to be crucial for cancer cell survival in conditions of metabolic stress. On the other hand, it is a co-enzyme for anabolic enzymes, and is thus key for the generation of new building blocks to sustain cell growth and proliferation.
How is FAO relevant to the production of NADPH? As described above, FAO generates one molecule of acetyl CoA in each oxidation cycle and two in the last cycle (FIG. 1). Acetyl CoA enters the Krebs cycle, and together with oxaloacetate gives rise to citrate, which on export to the cytoplasm, can enter two metabolic chain reactions that produce cytosolic NADPH (FIG. 2). The production of FAO-derived cytosolic NADPH by cancer cells is key to counteract oxidative stress. For example, glioma cells in which FAO is inhibited exhibit a profound decrease in NADPH levels, accumulate increased levels of ROS and undergo cell death31. The relevance of FAO for NADPH homeostasis has been recently investigated by the group of Nissim Hay32. On metabolic stress, FAO serves to sustain ATP levels and NADPH production11,13,32. They observed that availability of NADPH is controlled by the LKB1–AMP kinase (AMPK) axis. AMPK is involved in maintaining the equilibrium of NADPH-consuming (FAS) and NADPH-producing (FAO-derived) reactions. AMPK promotes FAO through the inhibitory phosphorylation of acetyl CoA carboxylase (ACC)33, and potentially through the regulation of PPAR signalling34 and CPT1C expression13. Therefore, although the LKB1–AMPK axis is thought to be tumour suppressive, AMPK activity is essential for a cancer cell to ‘sense’ stressful environments and to respond by activating a catabolic switch that increases ATP and NADPH reserves.
FAO-dependent NADPH production could also be relevant to the survival of leukaemia cells17,23,25–27, although this possibility has not been formally tested. Indeed, in the haematopoietic lineage, it is well established that HSCs are extremely sensitive to ROS levels35–37. Increased levels of ROS result in a loss of maintenance and exhaustion of HSCs. As FAO-regulatory pathways are also relevant for the maintenance of this cell subpopulation, it is tempting to speculate that FAO could provide stem cells with the NADPH required to prevent ROS from driving cell differentiation.
Perspectives
Cancer metabolism can be perceived as a network of pathways with plasticity, feedback loops and crosstalk that ensure the fitness of tumour cells. Plasticity is key, and FAO might provide some of this plasticity by enabling the production of ATP and NADPH when required, eliminating potentially toxic lipids, inhibiting pro-apoptotic pathways and providing metabolic intermediates for cell growth. However, FAO cannot be perceived as a metabolic pathway that is active independently of the microenvironment of the cancer cell. Indeed, in ovarian cancers, which have a predilection to metastasize to the omentum (an adipocyte-rich tissue), the interaction with adipocytes is necessary for the transfer of lipids to the cancer cell, the activation of FAO and the establishment of metastasis38.
A big challenge is to unify the idea of FAO as an essential pathway in cancer cells with the fact that cancer cells also require active FAS in order to grow and divide. Dogma states that FAO and FAS are incompatible. In principle, ACC determines which pathway is active, on the basis of acetyl CoA and malonyl CoA levels. Therefore, as ACC is a ‘one-way street’, both metabolic activities cannot coexist. However, we might need to rethink such a rigid regulatory framework. The group of Nissim Hay32 showed that genetic manipulation of ACC1 or ACC2 in cancer cells yielded different outcomes in terms of FAS and FAO. In addition, FAO metabolism can contribute to the accumulation of acetyl CoA in the cytoplasm that is needed to initiate FAS, so that FAS and FAO can support each other23. On the basis of this idea, we can speculate that, rather than a total pool of acetyl CoA and malonyl CoA, there might be ACC1 and ACC2 localization-dependent compartmentalization39 of these metabolites that allows both metabolic pathways to be active simultaneously and independently from each other.
The data suggesting a greater requirement of FAO in undifferentiated cells also raise an interesting possibility. It is plausible that in quiescent and undifferentiated cells the competition between FAS and FAO may be less prominent (as these cells display a lower membrane synthesis rate), thus indicating that these cells might derive a full survival benefit from FAO activation and its biological output. In turn, their dependence on FAO could make them vulnerable, providing a unique therapeutic opportunity from the pharmacological manipulation of this metabolic pathway.
For all the reasons stated above, there is an exciting therapeutic potential for the pharmacological blockade of FAO in cancer. Two key enzymes in the FAO pathway are particularly interesting as potential targets for pharmacological intervention. CPT1 is considered the rate-limiting enzyme in FAO and can be pharmacologically targeted. Drugs that target 3-ketoacylthiolase (3-KAT), which catalyses the final step in FAO, are also available (TABLE 1).
Table 1. Pharmacological tools to manipulate FAO.
| Compound | Effect on FAO | Target | Approved for human use |
|---|---|---|---|
| Trimetazidine | Inhibition | 3-KAT inhibitor | Europe and Asia |
| Ranolazine | Inhibition | 3-KAT inhibitor | Europe and United States |
| Etomoxir | Inhibition | CPT1 inhibitor | Tested in clinical trials; retired owing to hepatotoxicity |
| Perhexiline | Inhibition | CPT1 inhibitor | Australia and Asia |
| Oxfenicine | Inhibition | CPT1 inhibitor | No |
| 4-bromocrotonic acid | Inhibition | Mitochondrial thiolase inhibitor | No |
| Fenofibrate | Activation | PPARα agonist | Yes |
| GW5011516 | Activation | PPARδ agonist | In clinical trials |
| TOFA | Activation | ACC inhibitor | No |
| Metformin | Activation | ETC complex | inhibitor | Yes |
| Phenformin | Activation | ETC complex | inhibitor | Withdrawn owing to high incidence of lactic acidosis |
| AICAR | Activation | AMP mimetic | No |
| Palmitate and carnitine | Activation | PPAR activator and substrate for FAO | No |
3-KAT, trimetazidine hydrochloride; ACC, acetyl CoA carboxylase; AICAR, 5-amino-1-β-D-ribofuranosyl-imidazole-4-carboxamide; CPT1, carnitine palmitoyltransferase 1; ETC, electron transport chain; FAO, fatty acid oxidation; PPAR, peroxisome proliferator-activated receptor; TOFA, 5-tetradecyloxy-2-furonic acid.
The pharmacological blockade of FAO has been pursued for the treatment of heart diseases. As a consequence, FAO inhibitors have been approved for human use. Perhexiline, a CPT1 inhibitor, has been approved in Australia and some Asian countries for the treatment of heart disease. Other CPT1 inhibitors either exhibited toxic side effects in clinical trials (etomoxir treatment in patients results in hepatotoxicity40 but has promising antitumoural effects in mice17) or are still at the preclinical stage (oxfenicine). Trimetazidine41 and ranolazine42, which are 3-KAT inhibitors, are both licensed in Europe for the treatment of angina, as is trimetazidine in Asia and ranolazine in the United States. Notably, other non-clinical compounds that activate FAO have been tested in cell culture models and mice12,23,24,32 (TABLE 1), and could be useful to elucidate the contribution of FAO to tumour cell survival. FAO can be indirectly activated by PPAR activators, AMPK activators or ACC inhibitors, or by exposing cells to high concentrations of fatty acids and carnitine. Although these indirect mechanisms for FAO activation are useful, the variety of pathways that become activated by these approaches complicates the interpretation of the results.
In summary, the study of FAO in the context of cancer metabolism has unveiled new and exciting therapeutic opportunities, together with a more profound comprehension of the metabolic wiring of cancer cells. We have learned over the past few decades that targeting single enzymes or pathways rarely results in cures for cancer, so it is likely that agents that target FAO will need to be combined with chemotherapies or with other targeting agents to be successful. Clearly, further knowledge of the dependence of cancer cells on FAO will be necessary to refine rational approaches to combination therapies that target fatty acid catabolism.
Acknowledgments
The authors apologize to those whose publications related to the discussed issues could not be cited owing to space limitations. The authors would like to thank the members of the Carracedo laboratory (N. Martín, V. Torrano, A. Zabala, A. Arruabarrena, P. Zuñiga and S. Fernández) for the insightful discussion and critical comments. The work of A.C. is supported by the Ramón y Cajal award (Spanish Ministry of Education), the Basque Department of Industry, Tourism and Trade (Etortek), Marie Curie Reintegration grant (277043), Movember Global Action Plan, ISCIII (PI10/01484) and the Basque Government of health (2012111086) and education (PI2012-03). The work of P.P.P. is supported by grants from the US National Cancer Institute (NCI). The work of L.C.C. is supported by grants from both the US National Institutes of Health and the NCI.
Glossary
- Anoikis
An apoptotic process activated as a result of insufficient or inadequate cell–matrix interactions. This process can be observed during development and epithelial hyperproliferation or can be induced experimentally
- Fatty acid synthase
(FASN). This protein has eight different enzymatic activities in a single polypeptidic chain and gives rise to palmitic acid (16.0)
- Oxidative phosphorylation
(OXPHOS). The oxidation of reduced NAD and FAD for the production of ATP, creating an exchange of electrons between donors and acceptors (oxygen) and a proton gradient between the intermembrane space and the lumen of the mitochondria. This process is carried out by the electron transport chain at the inner mitochondrial membrane and favours the generation of ATP by ATP synthase while dissipating the proton gradient
- Pentose phosphate pathway
(PPP). This metabolic pathway generates pentoses and NADPH, which are both required for cell growth and proliferation. Through its oxidative branch, glucose-6-phosphate is oxidized to generate ribulose-5-phosphate (for nucleotide synthesis) and NADPH for anabolism
- Warburg's hypothesis
Otto Warburg observed that cancer cells, in the presence of oxygen, metabolize glucose anaerobically leading to the production of lactate, instead of oxidizing it through the Krebs cycle. Thus, he hypothesized that cancer cells had dysfunctional mitochondria, and this metabolic rewiring was termed the Warburg effect
Footnotes
Competing interests statement: The authors declare no competing financial interests.
Further Information Arkaitz Carracedo's homepage: http://personal.cicbiogune.es/acarracedo/
Contributor Information
Arkaitz Carracedo, CIC bioGUNE, Bizkaia Technology Park, Derio, Spain, IKERBASQUE, Basque foundation for science, Bilbao 48160, Spain and Biochemistry and Molecular Biology Department, University of the Basque Country (UPV/EHU), P. O. BOX 644, E-48080 Bilbao, Spain.
Lewis C. Cantley, Department of Medicine, Division of Signal Transduction, Beth Israel Deaconess Medical Center, Boston, Massachusetts 02215, USA, and the Department of Systems Biology, Harvard Medical School, Boston, Massachusetts 02115, USA. Present address: Weill Cornell Medical College, New York, New York 10065, USA
Pier Paolo Pandolfi, Cancer Genetics Program, Beth Israel Deaconess Cancer Center; Department of Medicine and Pathology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts 02215, USA.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koppenol WH, Bounds PL, Dang CV. Otto Warburg's contributions to current concepts of cancer metabolism. Nature Rev Cancer. 2011;11:325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 4.Christofk HR, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 5.Locasale JW, et al. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nature Genet. 2011;43:869–874. doi: 10.1038/ng.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vander Heiden MG, et al. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science. 2010;329:1492–1499. doi: 10.1126/science.1188015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hitosugi T, et al. Phosphoglycerate mutase 1 coordinates glycolysis and biosynthesis to promote tumor growth. Cancer Cell. 2012;22:585–600. doi: 10.1016/j.ccr.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeBerardinis RJ, et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci. 2010;35:427–433. doi: 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh R, Cuervo AM. Lipophagy: connecting autophagy and lipid metabolism. Int J Cell Biol. 2012;2012:282041. doi: 10.1155/2012/282041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schafer ZT, et al. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature. 2009;461:109–113. doi: 10.1038/nature08268. This study demonstrated for the first time that FAO has a crucial role in the survival of cancer cells under metabolic stress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carracedo A, et al. A metabolic prosurvival role for PML in breast cancer. J Clin Invest. 2012;122:3088–3100. doi: 10.1172/JCI62129. This study uncovered a pro-survival activity of the PML tumour suppressor in breast cancer through the regulation of FAO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaugg K, et al. Carnitine palmitoyltransferase 1C promotes cell survival and tumor growth under conditions of metabolic stress. Genes Dev. 2011;25:1041–1051. doi: 10.1101/gad.1987211. This study shows that CPT1C overexpression in cancer is important for cancer cell survival and resistance to therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carrasco P, et al. Ceramide levels regulated by carnitine palmitoyltransferase 1C control dendritic spine maturation and cognition. J Biol Chem. 2012;287:21224–21232. doi: 10.1074/jbc.M111.337493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J, Wolfgang MJ. Metabolomic profiling reveals a role for CPT1c in neuronal oxidative metabolism. BMC Biochem. 2012;13:23. doi: 10.1186/1471-2091-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sierra AY, et al. CPT1c is localized in endoplasmic reticulum of neurons and has carnitine palmitoyltransferase activity. J Biol Chem. 2008;283:6878–6885. doi: 10.1074/jbc.M707965200. [DOI] [PubMed] [Google Scholar]
- 17.Samudio I, et al. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. J Clin Invest. 2010;120:142–156. doi: 10.1172/JCI38942. This study demonstrated the therapeutic potential of FAO inhibition in leukaemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giordano A, et al. tBid induces alterations of mitochondrial fatty acid oxidation flux by malonyl-CoA-independent inhibition of carnitine palmitoyltransferase-1. Cell Death Differ. 2005;12:603–613. doi: 10.1038/sj.cdd.4401636. [DOI] [PubMed] [Google Scholar]
- 19.Paumen MB, et al. Direct interaction of the mitochondrial membrane protein carnitine palmitoyltransferase I with Bcl-2. Biochem Biophys Res Commun. 1997;231:523–525. doi: 10.1006/bbrc.1997.6089. [DOI] [PubMed] [Google Scholar]
- 20.Vickers AE. Characterization of hepatic mitochondrial injury induced by fatty acid oxidation inhibitors. Toxicol Pathol. 2009;37:78–88. doi: 10.1177/0192623308329285. [DOI] [PubMed] [Google Scholar]
- 21.Samudio I, Fiegl M, McQueen T, Clise-Dwyer K, Andreeff M. The warburg effect in leukemia-stroma cocultures is mediated by mitochondrial uncoupling associated with uncoupling protein 2 activation. Cancer Res. 2008;68:5198–5205. doi: 10.1158/0008-5472.CAN-08-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monti S, et al. Molecular profiling of diffuse large B-cell lymphoma identifies robust subtypes including one characterized by host inflammatory response. Blood. 2005;105:1851–1861. doi: 10.1182/blood-2004-07-2947. [DOI] [PubMed] [Google Scholar]
- 23.Caro P, et al. Metabolic signatures uncover distinct targets in molecular subsets of diffuse large B cell lymphoma. Cancer Cell. 2012;22:547–560. doi: 10.1016/j.ccr.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito K, et al. A PML–PPAR-δ pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nature Med. 2012;18:1350–1358. doi: 10.1038/nm.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gan B, et al. Lkb1 regulates quiescence and metabolic homeostasis of haematopoietic stem cells. Nature. 2011;468:701–704. doi: 10.1038/nature09595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gurumurthy S, et al. The Lkb1 metabolic sensor maintains haematopoietic stem cell survival. Nature. 2011;468:659–663. doi: 10.1038/nature09572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakada D, Saunders TL, Morrison SJ. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature. 2011;468:653–658. doi: 10.1038/nature09571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knobloch M, et al. Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Nature. 2013;493:226–230. doi: 10.1038/nature11689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valent P, et al. Cancer stem cell definitions and terminology: the devil is in the details. Nature Rev Cancer. 2012;12:767–775. doi: 10.1038/nrc3368. [DOI] [PubMed] [Google Scholar]
- 30.Chiarugi A, Dolle C, Felici R, Ziegler M. The NAD metabolome--a key determinant of cancer cell biology. Nature Rev Cancer. 2012;12:741–752. doi: 10.1038/nrc3340. [DOI] [PubMed] [Google Scholar]
- 31.Pike LS, Smift AL, Croteau NJ, Ferrick DA, Wu M. Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochim Biophys Acta. 2011;1807:726–734. doi: 10.1016/j.bbabio.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 32.Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485:661–665. doi: 10.1038/nature11066. This study demonstrated that FAO counteracts the accumulation of ROS in conditions of metabolic stress through the generation of NADPH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nature Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diradourian C, Girard J, Pegorier JP. Phosphorylation of PPARs: from molecular characterization to physiological relevance. Biochimie. 2005;87:33–38. doi: 10.1016/j.biochi.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Hosokawa K, et al. Function of oxidative stress in the regulation of hematopoietic stem cell-niche interaction. Biochem Biophys Res Commun. 2007;363:578–583. doi: 10.1016/j.bbrc.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Ito K, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nature Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 37.Ito K, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 38.Nieman KM, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nature Med. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abu-Elheiga L, et al. The subcellular localization of acetyl-CoA carboxylase 2. Proc Natl Acad Sci USA. 2000;97:1444–1449. doi: 10.1073/pnas.97.4.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holubarsch CJ, et al. A double-blind randomized multicentre clinical trial to evaluate the efficacy and safety of two doses of etomoxir in comparison with placebo in patients with moderate congestive heart failure: the ERGO (etomoxir for the recovery of glucose oxidation) study. Clin Sci (Lond) 2007;113:205–212. doi: 10.1042/CS20060307. [DOI] [PubMed] [Google Scholar]
- 41.Kantor PF, Lucien A, Kozak R, Lopaschuk GD. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res. 2000;86:580–588. doi: 10.1161/01.res.86.5.580. [DOI] [PubMed] [Google Scholar]
- 42.Nash DT, Nash SD. Ranolazine for chronic stable angina. Lancet. 2008;372:1335–1341. doi: 10.1016/S0140-6736(08)61554-8. [DOI] [PubMed] [Google Scholar]