Abstract
Objective
Individuals with amnestic Mild Cognitive Impairment (MCI) have few empirically-based treatment options for combating their memory loss. This study sought to examine the efficacy of a calendar/notebook rehabilitation intervention, the Memory Support System (MSS), for individuals with amnestic MCI.
Methods
Forty individuals with single domain amnestic MCI and their program partners were randomized to receive the MSS, either with training or without (controls). Measures of adherence, activities of daily living, and emotional impact were completed at the first and last intervention session and again at 8-weeks and 6 months post intervention.
Results
Training in use of a notebook/calendar system significantly improved adherence over those who received the calendars but no training. Functional ability and memory self efficacy significantly improved for those who received MSS training. Change in functional ability remained significantly better in the intervention group than in the control group out to 8 week follow up. Care partners in the intervention group demonstrated improved mood by 8 week and 6 month follow-up, while control care partners reported worse caregiver burden by 6 month follow up.
Conclusions
MSS training resulted in improvement in ADLs and sense of memory self efficacy for individuals with MCI. While ADL benefits were maintained out to 8 weeks post intervention, future inclusion of booster sessions may help extend the therapeutic effect out even further. Improved mood of care partners of trained individuals and worsening sense of caregiver burden over time for partners of untrained individuals further supports the efficacy of the MSS for MCI.
Keywords: Mild Cognitive Impairment, Rehabilitation, Behavioral Intervention, Activities of Daily Living, Quality of Life, Caregivers
Introduction
Amnestic Mild Cognitive Impairment (MCI) is often the precursor for emerging Alzheimer’s disease (AD). Evidence suggests that while initially thought of as a disorder in which an individual has cognitive decline but no functional impairments, (Petersen et al., 1999) individuals with MCI do have changes in their daily functioning, just not as severe as individuals with AD. (Jefferson et al., 2008) Behavioral rehabilitation interventions may help sustain or even improve functioning in MCI, and in so doing, may additionally delay progression to a clinical AD diagnosis. While many products and services are appearing on the market for older individuals to improve memory, there is little research to support them.
Compensatory rehabilitation strategies attempt to help the individual adapt to memory loss. This approach can both facilitate acquisition of new information through internal strategies such as mnemonic techniques, or can utilize external aids, such as calendars and note taking systems. Most research to date in cognitive rehabilitation has been in AD rather than MCI, with mixed reports of success. In their review, Clare and Woods concluded there is no indication of significant benefit of cognitive training in persons with dementia, largely due to the limited number of randomized controlled trials that currently exist. (Clare, 2008) However, when not limiting results to randomized control trials, Sitzer et al. reported an overall moderate effect size (d = 0.47) in their meta-analysis of various cognitive rehabilitation approaches in AD. (Sitzer, Twamley, & Jeste, 2006)
Most research studies to date in MCI report positive impact on cognitive and mood variables with various rehabilitation approaches (Belleville et al., 2006; Hampstead, Sathian, Moore, Nalisnick, & Stringer, 2008; Kinsella et al., 2009; Kurz, Pohl, Ramsenthaler, & Sorg, 2009; Londos et al., 2008; Rapp, Brenes, & Marsh, 2002; Rozzini et al., 2007). In a review of 15 cognitive intervention programs in memory loss from MCI, Jean and colleagues reported an overall 44% improvement in objective measures of memory and 49% in subjective measures of memory. (Jean, Bergeron, Thivierge, & Simard) Like in AD, this research is limited by few randomized trials and little to no longitudinal follow up. The current study will utilize a randomized control design and follow participants out 6 months post intervention. Further, no MCI rehabilitation research to date has focused on a specific external memory compensatory strategy or on the intervention’s impact on functional ability relevant to MCI participants.
Methods
Participants
Emory Alzheimer’s Disease Research Center participants and consecutive patients from the Neurology and Neuropsychology clinics with single domain, amnestic MCI were asked to take part in a study of how using a calendar and note taking system [the Memory Support System (MSS)], either with the aid of a therapist or independently, may impact daily life with memory decline. Subjects were diagnosed with amnestic MCI based on the algorithm provided by Petersen (Petersen, 2004) and thought to represent individuals with a high likelihood of eventual progression to clinically probable AD (Petersen & Morris, 2005). Subjects were interviewed with an informant to determine there was 1) a cognitive complaint that represented a decline in function and 2) activities of daily living were essentially intact. Detailed neuropsychological testing demonstrated objective memory impairment in the context of intact functioning in other cognitive domains. Memory testing varied depending upon the provider, but always included the Wechsler Memory Scale Revised or Third Edition Logical Memory, Auditory Verbal Learning Test or Consortium to Establish a Registry for Alzheimer’s Disease Word List, and Benton Visual Memory Test-Revised or WMS R/III Visual Reproduction. Impairment was defined as at least one standard deviation below age matched peers on testing that was judged to represent memory decline by the clinician. Finally, information from interview and neuropsychological testing were utilized by the clinician to judge the individual was not currently demented. Use of human subjects was done in accord with the ethical standards of the Committee on Human Experimentation and the Helsinki Declaration with protocol approval from the Institutional Review Board at Emory University. Participants all gave informed consent and met the following inclusion/exclusion criteria:
Inclusion
Had a study program partner with at least twice weekly contact with the participant
Dementia Rating Scale-2 (DRS-2)(Jurica, 2001) score ≥ 120
Not taking or stable on nootropic medications for at least three months
Exclusion
Dementia diagnosis
Visual/hearing impairment or reading/writing disability sufficient to interfere with training
Severe depression or psychiatric illness
We contacted all potential subjects within 2 hours driving distance of Emory University. Sixty-one potential subjects were contacted via telephone, twenty-one declined enrollment (66% enrollment rate). The most common reason given for declining participation was distance to the treatment facility. There were no significant differences in age, education, gender, or ethnicity of those who did or did not participate.
Intervention
Participants were randomized to the control or intervention group, given the MSS, and briefly instructed to “begin using the calendar to help with your memory.” At the next session (7–10 days later), baseline use of the MSS was assessed, and intervention participants began training. Controls received no intervention. They were provided the MSS calendar and encouraged to use it on their own without further verbal or written instruction.
The MSS includes three sections: 1) appointments, 2) “to do” items, and 3) journaling section. The appointment and “to do” sections allow participants to write things that need to be done either in a calendar time slot, or in list format if not due at a certain time. The journaling section allows participants to log important information that happened to them that day, like a phone call they received, their thoughts on a day’s event, or an update received on a loved one. Sessions follow a manualized training program that provides orientation, modeling, practice use, and homework assignments. A detailed description of the intervention can be found in a previous publication. (Greenaway, Hanna, Lepore, & Smith, 2008) Intervention group dyads received a total of twelve, one-hour MSS training sessions over six weeks. It was hypothesized that training in the MSS may help with the maintenance of memory related activities of daily living (ADLs) in individuals with MCI, thus potentially helping offset the progression to dementia. Such an impact on daily functioning was hypothesized to have a positive impact on mood and quality of life related variables for the individual with MCI and their care partner. As this training was meant to teach adaptation to memory loss rather than primary memory improvement, no change in cognitive testing was expected.
Outcome measures
Participants and their program partners next completed measures of cognition, ADLs, mood, self-efficacy, quality of life and caregiver burden at baseline, 8 week, and 6 month follow up. Measures of ADLs and self-efficacy were additionally given at training end (See Table 1.)
Table 1.
Outcome Measures
| Completed By: | Cognition | IADL | Mood | Quality of Life | Burden | Self Efficacy |
|---|---|---|---|---|---|---|
| MCI Subject | DRS-2 MMSE | CES-D | QOL-AD | Self-Efficacy in MCI* | ||
| Partner | E-Cog | CES-D | QOL-AD | CB |
Note. E-Cog = Everyday Cognition (Farias et al., 2008), DRS-2 = Dementia Rating Scale-2 (Jurica, 2001), MMSE = Mini Mental State Examination (Folstein, Folstein, & McHugh, 1975), CES-D = Centers for Epidemiological Studies-Depression (Radloff, 1977), QOL-AD = Quality of Life-AD (Logsdon, Gibbons, McCurry, & Teri, 2002), CB = Caregiver Burden (Zarit, 1990);
Modified from (Lorig, 1996)
Cognition and ADLs
The DRS-2 and Mini Mental Status Examination (MMSE) are widely used cognitive screening measures utilized to track global cognitive status. The Everyday Cognition (E-Cog) is an informant-based measure created for use in MCI that assesses ability to perform everyday tasks in memory, language, visuospatial abilities, planning, organization, and divided attention. The current study focused on the 8 item Memory subscale (each item ranges 1–4 points, lower scores equal more intact function). The informant was the program partner.
Mood and life quality
The Center for Epidemiological Studies-Depression (CES-D) consists of 20-items about the frequency of depressive symptoms in the last week. The Quality of Life-AD (QOL-AD) is 13-item measure developed for individuals with dementia that has been utilized in MCI for rating relationships, concerns about finances, physical condition, mood, energy level, memory, aspects of daily functioning, and overall life quality on a four-point scale. The 22-item Caregiver Burden questionnaire (CB) was used to gauge the degree of stress in care partners.
Self efficacy
The Self-Efficacy in MCI scale is a 9-item measure of self-efficacy created by modifying selected items from the Chronic Disease Self-Efficacy Scales, (Lorig, 1996) a template self-efficacy scale available for adaptation and use in diseases of interest. Items focused on confidence in managing activities, tasks, and emotional distress caused by MCI; confidence in medication management, chores, and errand ability; and confidence in maintaining hobbies and relationships.
Adherence
Adherence was defined a priori as a score of seven or greater on the Adherence Assessment. The Adherence Assessment was given on four occasions: on the first day of the intervention (+ 7 days from possession of the MSS for controls), the last day of the intervention (6 weeks after initial Adherence Assessment for controls), and 8 weeks and 6 months post intervention. The MSS Instructor examined MSS adherence for two days randomly selected from the prior week. Adherence was based upon four criteria (maximum of 10 points):
Patient brought the MSS to the appointment (1 point)
Patient has at least one entry for today’s date (1 point).
Patient has entries for events happening at a certain time (2 points) and happening any time on that day (2 points)
At least two entries for each of the two days in the journaling section (4 points).
Data was analyzed using the Statistical Package for the Social Sciences (SPSS) program. Intragroup change was analyzed using Wilcoxon or paired sample t-test as appropriate, and differences on raw or change scores between intervention and controls were analyzed using Mann-Whitney or independent t-tests.
Results
Randomization led to 20 participants each in the intervention and control groups (Table 2).
Table 2.
Patient Characteristics.
| Age | Educ | Male | Caucasian | Program Partner as Spouse | AChE Use | |
|---|---|---|---|---|---|---|
| Treatment | 72.7 ( 6.9) | 16.4 (2.8) | 40% | 90% | 60% | 85% |
| Control | 72.3 (7.9) | 16.4 (2.8) | 38% | 85% | 75.0% | 70% |
Note. Differences between groups were all nonsignificant. AChE = Acetylcholinesterase Inhibitor
Two intervention subjects withdrew before beginning the intervention (house fire; increased social commitment). One control discontinued before 8 week follow up (failed to return calls), and 2 additional controls discontinued before 6 month follow up (discomfort with cognitive testing; medical issues). There were no significant demographic or cognitive differences between those who completed the study versus those who withdrew.
Cognition
There were no significant differences in DRS-2 or MMSE either within groups or between groups at any time point (Table 3). There were no significant differences based on cholinesterase inhibitor use.
Table 3.
Outcome Variables for Individuals with MCI and their Program Partners
| Baseline | 8-Week Follow Up | 6-Month Follow Up | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Exp Mean (SD) | Control Mean (SD) | Exp Mean (SD) | Control Mean (SD) | Exp Mean (SD) | Control Mean (SD) | |
| MCI Subject | ||||||
| DRS-2 | 131.1 (6.3) | 133.8 (4.2) | 131.6 (6.8) | 134.8 (5.1) | 130.7 (7.3) | 135.2 (5.7) |
| Total Change | .22 (6.0) | 1.0 (4.8) | –0.9 (5.8) | 0.8 (4.6) | ||
| Cohen’s d | .15 | .34 | ||||
| MMSE | 26.4 (2.2) | 27.2 (2.4) | 26.0 (2.9) | 27.3 (2.2) | 26.1 (2.2) | 27.3 (1.8) |
| Total Change | –0.4 (2.1) | –0.3 (1.8) | –0.2 (1.4) | –0.4 (2.2) | ||
| Cohen’s d | .03 | 0.1 | ||||
| Self Efficacy | 74.9 (12.9) | 79.3 (11.2) | 80.2 (9.0) | 77.6 (12.3) | 79.3 (12.2) | 80.5 (8.3) |
| Total Change | 3.9 (11.1) | –0.9 (8.9) | 2.9 (13.5) | –0.5 (7.0) | ||
| Cohen’s d | .47 | .32 | ||||
| QOL | 43.4 (6.0) | 43.0 (5.1) | 43.4 (5.5) | 41.5 (5.6) | 43.8 (6.2) | 43.5 (4.0) |
| Total Change | 0.2 (3.1) | –1.6 (4.4) | 0.6 (2.4) | –0.6 (3.1) | ||
| Cohen’s d | .48 | .41 | ||||
| Depression | 9.2 (9.6) | 8.9 (6.4) | 9.0 (9.8) | 8.7 (8.0) | 8.7 (10.0) | 8.1 (6.1) |
| Total Change | 0.0 (5.0) | –0.4 (4.4) | –0.3 (5.8) | –0.5 (3.7) | ||
| Cohen’s d | .09 | .03 | ||||
| Anxiety | 16.1 (5.6) | 14.8 (4.6) | 15.4 (5.2) | 14.9 (5.0) | 14.5 (5.2) | 14.4 (3.6) |
| Total Change | –0.4 (3.9) | –0.2 (3.8) | –1.3 (4.6) | –0.4 (2.6) | ||
| Cohen’s d | .06 | .26 | ||||
|
| ||||||
| Program Partner | ||||||
| Burden | 19.8 (13.7) | 16.9 (10.3) | 21.7 (14.5) | 18.8 (10.8) | 21.3 (15.6) | 20.2 (11.1)* |
| Total Change | 0.4 (6.6) | 1.1 (9.3) | –0.1 (6.2) | 4.5 (7.6) | ||
| Cohen’s d | .09 | .63 | ||||
| QOL | 42.2 (6.3) | 41.2 (6.2) | 42.2 (6.6) | 40.7 (4.7) | 42.0 (7.4) | 40.8 (5.1) |
| Total Change | –0.7 (4.2) | –0.1 (2.9) | –0.8 (5.7) | –1.6 (3.1) | ||
| Cohen’s d | .15 | .15 | ||||
| Depression | 11.3 (7.7) | 7.0 (6.0) | 8.2 (8.4) | 8.6 (8.2) | 8.6 (6.3) | 9.3 (6.9) |
| Total Change | –3.3 (7.2) | 1.5 (5.9)* | –2.8 (5.6) | 2.8 (5.6)** | ||
| Cohen’s d | .69 | .90 | ||||
| Anxiety | 19.7 (8.0) | 16.9 (5.6) | 17.3 (6.4) | 16.8 (4.3) | 18.4 (5.2) | 17.9 (6.5) |
| Total Change | –1.9 (7.4) | 0.1 (4.7) | –0.8 (7.1) | 1.9 (6.0) | ||
| Cohen’s d | .31 | .40 | ||||
p < .05,
p <.01
DRS-2 = Dementia Rating Scale-2; MMSE = Mini Mental Status Exam
Adherence
Differences between the intervention and control group in adherence scores were not significant at baseline (intervention = M = 3.3, SD = 2.8; control = M = 5.3, SD = 3.2), z = −1.9, p = .06. In the intervention group, adherence improved significantly by training end (M = 8.8, SD = 1.5), z = −3.7, p < .001. Those in the control group demonstrated a decline in adherence over the same period (training end M = 3.1, SD = 3.3), z = −2.7, p < .01. Improvements in adherence scores for the intervention group remained significant at 8 weeks (M = 6.3, SD = 3.2), z = −3.1, p < .01, while adherence was low in the control group (M = 2.7, SD = 3.8). Improvements in adherence for the intervention group were no longer significant by 6 months compared to their own baseline (M = 3.8, SD = 3.4), and the control group remained lower than baseline (M = 1.8, SD = 3.5), z = −3.0, p < .01. Examining between group differences, the intervention group demonstrated significantly better adherence to the MSS than the control group at training end, z = −4.6, p < .001, 8 week, z = −2.6, p = .01, and 6 month follow up, z = −2.1, p < .05.
ADLs
Intervention versus Controls
The intervention group showed significant improvement in ADLs using the memory scale of the ECog by training end, t (15) = 3.1, p < .01 (baseline M = 21.2, SD = 5.9; training end M = 17.8, SD = 5.4), and at 8 week follow up (M = 18.5, SD = 4.7), t (17) = 2.4, p < .05 (lower scores represent better ADLs). Change was no longer significant by 6 months (M = 20.5, SD = 4.9). Baseline initial ECog memory scores were not significantly different between the intervention and control group. There was no significant change in the control group from baseline (M = 18.0, SD = 4.2) to training end (M = 18.8, SD = 4.5), 8 week follow up (M = 18.8, SD = 4.6) and 6 month follow up (M = 18.1, SD = 4.2).
Looking at between group differences for change scores to allow for time by group interactions, change in the E-Cog was better in the intervention group compared to the control group by training end, t (33) = −3.4, p < .01, Cohen’s d = 1.0, and at 8 week follow up, t (28.4) = −2.9, p < .01, Cohen’s d = .88. Change was no longer significantly different between the groups by 6 months. There were no differences in ADLs based on cholinesterase inhibitor use.
Adherent versus Non-adherent
Correlation between adherence score and memory ADLs at training end and follow ups failed to reach significance, p > .05. Subjects in the control and intervention groups were further divided into “adherent” (adherence score of 7 or greater) and “non-adherent.” Individuals who were adherent or not at baseline did not differ significantly on baseline ECog memory subscales in the intervention (adherent M = 24.5, SD = .7 and not-adherent M = 20.9, SD = 5.3) or control group (adherent M = 16.5, SD = 5.8 and not adherent M = 19.0, SD = 2.8). At training end (equivalent time frame for control group), controls labeled adherent were significantly more likely to have worse memory ADLs (M = 22.8, SD = 4.3) compared to those who were not adherent (M = 17.5, SD = 4.3), t (17) = 2.1, p < .05. All trained individuals who completed the ECog memory scale were adherent, with significantly improved ADLs as noted above. By 8 week and 6 month follow up, no significant differences were found between those that were adherent or not in the intervention or control group. Effect sizes for those deemed “compliant” were moderate by training end, d = .43, and 8 week follow up, d = .39, and mild to moderate by 6 month follow up, d = .30 (Figure 2).
Figure 2.
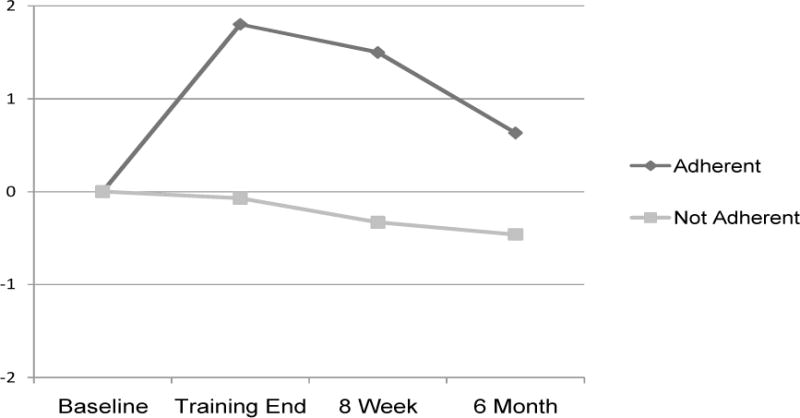
Everyday Cognition (Ecog) Memory Subscale Scores by Adherence. Note: Positive change scores represent improvement.
Mood
Trained individuals with MCI demonstrated significant improvements in sense of memory self-efficacy by training end, t (15) = −3.1, p <.01), which was better than controls, t (33) = 2.4, p=.02. No other mood related variables were significant for individuals with MCI.
Change in mood scores was significantly different between care partners in the two groups at 8 weeks, t (30) = −2.1, p < .05, and 6 months, t (33) = −3.0, p <.01. Care partners in the intervention group had improvement in the CES-D that reached significance by 6 months post intervention, t (17) = 1.1, p < .05. In contrast, those in the control group showed a trend towards worsening mood, p = .06. Care partners of those in the control group also showed a significant increase in caregiver burden by 6 months, t (16) = −2.4, p < .05. No other mood variables were significant for care partners. Table 3 displays mood related variables for individuals with MCI and their care partners.
Discussion
Training in the use of the MSS leads to improvements in reported functional ability and sense of self efficacy for individuals with amnestic MCI. Compared to controls who received the calendar system but no training, trained individual’s change in ADLs remained significantly better than untrained individuals out to 8 week follow up. It appears that individuals with MCI can be taught to compensate for their memory loss, and this improves function and confidence.
MSS trainees no longer demonstrated significantly improved ADLs by 6 month follow up. However, the effect size for change scores between the groups was moderate, d = .56, suggesting these differences may be significant in a larger sample. Further investigation is needed for empiric verification.
Correlations between adherence (i.e., how well participants in either group utilized the calendar) and change in functional ability were not significant. This contrasted with the fact that participants repeatedly told us about how much using the calendar helped their day to day ability to “remember” information. Interestingly, the relationship between adherence and ADLs appear to be different between controls and MSS trainees. It is important to note that, while not significant (p < .06), there was a tendency for controls to have higher adherence scores at baseline than those in the intervention group. Participants were told that the purpose of the study was to see how well some individuals did when trained in the use of the MSS compared to others using the MSS on their own. Given these instructions, 45% of the controls were adherent at baseline (based on a cut score of 7), compared to only 11% of those waiting to be trained. Six weeks later, controls labeled adherent were significantly more likely to have worse memory ADLs compared to those who were not adherent. The opposite finding was apparent in the trained individuals, who reported significantly improved ADLs. This suggests that different motivations may affect choice to utilize the notebook for trained and untrained individuals. Namely, those in the intervention group use the MSS because they are being trained, and because it helps their ADLs. In contrast, those in the control group try hard to use the MSS initially because they know they are in a group that is trying to use the calendar on their own. However, controls who are motivated to keep using it are those who perhaps realize (or their partners realize) they need it the most.
In terms of mood and QOL variables, MCI care partners in the intervention group had significantly more improvement in mood symptoms at 6 month follow up compared to control care partners, who had a tendency to decline in mood. These mood findings suggest that the MSS intervention is having a positive long term effect on care partners of those with MCI. Additionally, care partners of controls have an increased sense of caregiver burden at our furthest follow up point (6 months), suggesting that additional cares are needed by this group that was not captured by our ADL data.
Limitations/Future Trials
Despite moderate to large effect sizes on many functional and mood variables, some of these comparisons failed to reach significance given the small sample size. A future larger trial will help to determine if these findings truly are of significance and further assess the long-term benefits to ADLs of this training.
Initial data for 6 month follow up shows adherence to the calendar system roughly returned to baseline for the intervention group, although 53% were still using the calendar to some degree (adherence score of 4 or greater). At 6 months, trained individuals had functional ability similar to baseline. In retrospect, the decline in adherence over the 6 months of minimal contact with subjects could have been anticipated given evidence of the need for refresher or “booster” sessions to maintain subject adherence in both the medical (Fappa et al., 2008) and psychological literature (Lester et al., 2005), as well as other large cognitive rehabilitation trials with the elderly (Loewenstein, Acevedo, Czaja, & Duara, 2004; Willis et al., 2006). This finding stresses the importance of adding booster sessions to the training paradigm that may help improve adherence rates over time, as well as maintenance of the functional gains found out to 8 week follow up.
A placebo social contact group was not utilized, making it difficult to determine how much improvement, particularly in mood variables, may be related to receiving 12 hours of personal contact with a professional, regardless of the MSS intervention itself. While research has reported no cognitive/functional differences between social contact and no-contact control groups in healthy elderly cognitive enhancement trials, (Clark et al., 1997; Willis et al., 2006) further research is necessary to determine to what degree social contact hours affect “therapeutic” outcomes in MCI.
Three subjects carrying diagnoses of MCI may have progressed to AD by the time we began training, despite a brief structured interview and administration of the DRS-2 at eligibility. All of these subjects randomized to the intervention. None of the three subjects could make it through the three stages of training curriculum for the MSS by the end of the 12 sessions, and their results tended to lower adherence rates, ADL performance, and mood and caregiver variables in our final results. These findings reflect a challenge to any MCI study, and suggest individuals with more late stage MCI or transitioning into early AD may not benefit as much from the current MSS training paradigm. By including more detailed assessment of functional ability at the time of enrollment, future results should be able to speak more to how individuals with earlier or more advanced MCI or early AD respond to MSS training.
Conclusion
Individuals with amnestic MCI can and will use a memory notebook system to help compensate for their memory loss. MSS trainees report significantly improved functional ability compared to controls out to 8 week follow up, as well as improvements in memory self efficacy. Mood subsequently improved for care partners of MSS trainees. In contrast, care giving burden worsened for partners of control participants by 6 month follow up, suggesting more care may be required by this group over time than those trained in MSS use.
Figure 1.
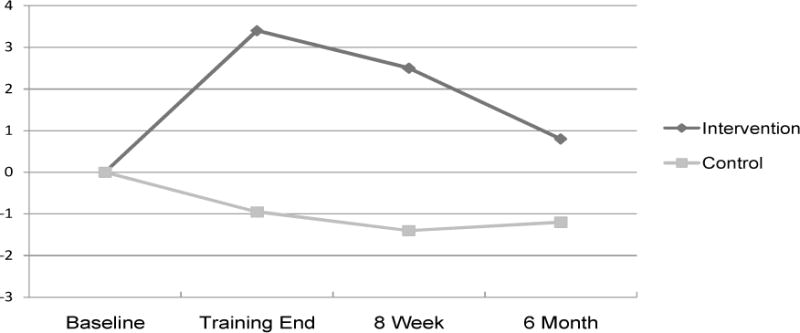
Change in Everyday Cognition (ECog) Memory Subscale Scores. Note: Positive change numbers represent improvement.
Key Points.
1) Training in the use of a notebook/calendar system significantly improved functional ability and memory self efficacy in those with amnestic MCI. 2) Change in functional ability remained significantly better in the intervention group than in the control group out to 8 week follow up. 3) Care partners of those trained to use the notebook/calendar system demonstrated improved mood by 8 week and 6 month follow-up. 4) Care partners of those in the control group reported worse caregiver burden by 6 month follow up.
Acknowledgments
This project was funded in part by the Alzheimer’s Association, NIRG-07-58843 and the Emory Alzheimer’s Disease Research Center, AG025688.
Footnotes
Declaration
The author’s have no conflicts of interest to disclose in relationship to this manuscript. The manuscript is not currently submitted or under consideration for publication elsewhere.
References
- Belleville S, Gilbert B, Fontaine F, Gagnon L, Menard E, Gauthier S. Improvement of episodic memory in persons with mild cognitive impairment and healthy older adults: evidence from a cognitive intervention program. Dement Geriatr Cogn Disord. 2006;22(5–6):486–499. doi: 10.1159/000096316. [DOI] [PubMed] [Google Scholar]
- Clare L, Woods RT. Cognitive rehabilitation and cognitive training for early-stage Alzheimer’s disease and vascular dementia. Cochrane Database Syst Rev. 2008;(4) doi: 10.1002/14651858.CD003260. [DOI] [PubMed] [Google Scholar]
- Clark F, Azen SP, Zemke R, Jackson J, Carlson M, Mandel D, et al. Occupational therapy for independent-living older adults. A randomized controlled trial. Jama. 1997;278(16):1321–1326. [PubMed] [Google Scholar]
- Fappa E, Yannakoulia M, Pitsavos C, Skoumas I, Valourdou S, Stefanadis C. Lifestyle intervention in the management of metabolic syndrome: could we improve adherence issues? Nutrition. 2008;24(3):286–291. doi: 10.1016/j.nut.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Farias ST, Mungas D, Reed BR, Cahn-Weiner D, Jagust W, Baynes K, et al. The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology. 2008;22(4):531–544. doi: 10.1037/0894-4105.22.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Greenaway MC, Hanna SM, Lepore SW, Smith GE. A behavioral rehabilitation intervention for amnestic mild cognitive impairment. Am J Alzheimers Dis Other Demen. 2008;23(5):451–461. doi: 10.1177/1533317508320352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampstead BM, Sathian K, Moore AB, Nalisnick C, Stringer AY. Explicit memory training leads to improved memory for face-name pairs in patients with mild cognitive impairment: results of a pilot investigation. J Int Neuropsychol Soc. 2008;14(5):883–889. doi: 10.1017/S1355617708081009. [DOI] [PubMed] [Google Scholar]
- Jean L, Bergeron ME, Thivierge S, Simard M. Cognitive intervention programs for individuals with mild cognitive impairment: systematic review of the literature. Am J Geriatr Psychiatry. 18(4):281–296. doi: 10.1097/JGP.0b013e3181c37ce9. [DOI] [PubMed] [Google Scholar]
- Jefferson AL, Byerly LK, Vanderhill S, Lambe S, Wong S, Ozonoff A, et al. Characterization of activities of daily living in individuals with mild cognitive impairment. Am J Geriatr Psychiatry. 2008;16(5):375–383. doi: 10.1097/JGP.0b013e318162f197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurica PJ, Leitten CL. Dementia Rating Scale-2: Professional Manual. Lutz (FL): PAR, Inc; 2001. [Google Scholar]
- Kinsella GJ, Mullaly E, Rand E, Ong B, Burton C, Price S, et al. Early intervention for mild cognitive impairment: a randomised controlled trial. J Neurol Neurosurg Psychiatry. 2009;80(7):730–736. doi: 10.1136/jnnp.2008.148346. [DOI] [PubMed] [Google Scholar]
- Kurz A, Pohl C, Ramsenthaler M, Sorg C. Cognitive rehabilitation in patients with mild cognitive impairment. Int J Geriatr Psychiatry. 2009;24(2):163–168. doi: 10.1002/gps.2086. [DOI] [PubMed] [Google Scholar]
- Lester H, Tait L, Khera A, Birchwood M, Freemantle N, Patterson P. The development and implementation of an educational intervention on first episode psychosis for primary care. Med Educ. 2005;39(10):1006–1014. doi: 10.1111/j.1365-2929.2005.02273.x. [DOI] [PubMed] [Google Scholar]
- Loewenstein DA, Acevedo A, Czaja SJ, Duara R. Cognitive rehabilitation of mildly impaired Alzheimer disease patients on cholinesterase inhibitors. Am J Geriatr Psychiatry. 2004;12(4):395–402. doi: 10.1176/appi.ajgp.12.4.395. [DOI] [PubMed] [Google Scholar]
- Logsdon RG, Gibbons LE, McCurry SM, Teri L. Assessing quality of life in older adults with cognitive impairment. Psychosom Med. 2002;64(3):510–519. doi: 10.1097/00006842-200205000-00016. [DOI] [PubMed] [Google Scholar]
- Londos E, Boschian K, Linden A, Persson C, Minthon L, Lexell J. Effects of a goal-oriented rehabilitation program in mild cognitive impairment: a pilot study. Am J Alzheimers Dis Other Demen. 2008;23(2):177–183. doi: 10.1177/1533317507312622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorig K, Stewart A, Ritter P, Gonzalez V, Laurent D, Lynch J. Outcome Measures for Health Education and other Health Care Interventions. Thousand Oaks (CA): Sage Publications; 1996. [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol. 2005;62(7):1160–1163. doi: 10.1001/archneur.62.7.1160. discussion 1167. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rapp S, Brenes G, Marsh AP. Memory enhancement training for older adults with mild cognitive impairment: a preliminary study. Aging Ment Health. 2002;6(1):5–11. doi: 10.1080/13607860120101077. [DOI] [PubMed] [Google Scholar]
- Rozzini L, Costardi D, Chilovi BV, Franzoni S, Trabucchi M, Padovani A. Efficacy of cognitive rehabilitation in patients with mild cognitive impairment treated with cholinesterase inhibitors. Int J Geriatr Psychiatry. 2007;22(4):356–360. doi: 10.1002/gps.1681. [DOI] [PubMed] [Google Scholar]
- Sitzer DI, Twamley EW, Jeste DV. Cognitive training in Alzheimer’s disease: a meta-analysis of the literature. Acta Psychiatr Scand. 2006;114(2):75–90. doi: 10.1111/j.1600-0447.2006.00789.x. [DOI] [PubMed] [Google Scholar]
- Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. Jama. 2006;296(23):2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarit S, Zarit J. The memory and behavior problems checklist and the burden interview. Penn State: Gerontological Center, College of Health and Human Development; 1990. [Google Scholar]


