Abstract
Context
Abnormalities in associative memory processes, such as Pavlovian fear conditioning and extinction, have been observed in schizophrenia. The retrieval of fear extinction memories (‘safety signals’) may be particularly affected; although schizophrenia patients can extinguish conditioned fear, they show a deficit in retrieving fear extinction memories after a delay. The neurobiological basis of this abnormality is unknown, but clues have emerged from studies in rodents and humans demonstrating that the ventromedial prefrontal cortex (vmPFC) is a key mediator of extinction memory retrieval.
Objective
To measure autonomic and neural responses during the acquisition and extinction of conditioned fear and the delayed recall of fear and extinction memories in patients with schizophrenia and healthy controls.
Design
Cross-sectional case-control, functional magnetic resonance imaging study.
Setting
Academic medical center.
Participants
Twenty patients with schizophrenia and 17 healthy control participants, demographically-matched to the patient group.
Main Outcome Measures
Skin conductance and blood oxygen level dependent (BOLD) responses.
Results
During fear conditioning, patients with schizophrenia showed blunted autonomic responses and abnormal BOLD responses, relative to controls, within the posterior cingulate gyrus, hippocampus and other regions. Several of these abnormalities were linked to negative symptoms. During extinction learning, patients with schizophrenia and controls showed comparable autonomic and neural responses. Twenty-four hours after the learning phases, the control subjects exhibited decreased fear and increased vmPFC responses in the extinction (safe) context as expected, indicating successful retention of the extinction memory. In contrast, the schizophrenia patients showed inappropriately elevated fear and poor vmPFC responses in the safe context.
Conclusion
Failure of extinction memory retrieval in schizophrenia is associated with vmPFC dysfunction. In future studies, abnormalities in fear learning and extinction recall may serve as quantitative phenotypes that can be linked to genetic, symptom or outcome profiles in schizophrenia and those at risk for the disorder.
Although cognitive impairment is a central, debilitating feature of schizophrenia, recent evidence suggests that abnormalities in emotion-related processes play an important role in the core symptoms of the disorder. For example, negative symptoms have been linked to a diminished capacity to learn information about rewards or pleasure 1–3 and to use this information to guide behavior 4. Also, associations found between depression and anxiety and 1) elevated risk for the development of psychosis 5–7 and 2) positive symptom severity in schizophrenia 8–11 suggest that dysregulation of the neural systems mediating emotional function contributes to psychosis. Evidence for a bias to respond to neutral information as negatively-valenced or threatening in delusional patients 12, 13 further suggests that the encoding or retrieval of the affective values of stimuli in the environment may be impaired in psychotic patients. One possible explanation for these abnormalities is that they arise from disruptions of the mechanisms governing emotional learning and memory processes. Supporting this hypothesis is evidence for abnormalities in basic appetitive 14, 15 and aversive 16–22 associative learning and memory in schizophrenia.
One common model of emotional learning and memory is Pavlovian fear conditioning and extinction. In experimental paradigms based on this model, the presentation of an aversive stimulus (the unconditioned stimulus, US), such as a loud noise or an electrical shock, follows the presentation of a neutral stimulus, such as a tone or picture 23. This pairing is repeated several times until the animal learns that the neutral stimulus (the conditioned stimulus, CS) predicts the US; the animal then exhibits autonomic responses reflecting fear (such as increased heart rate, blood pressure and sweating) before the onset of the US. In human fear conditioning studies, a second, control CS is also usually presented which is not followed by a US and does not elicit anticipatory fear (the CS−). Repeated presentations of the CS that was previously paired with the US (the CS+) without the US leads to a gradual decrease in the conditioned physiological fear responses— a process known as fear extinction learning. Importantly, it has been demonstrated that both the fear and the extinction memory trace can be retrieved independently at a later time, in a context-gated manner 24–26. The context can be the physical environment, time, or a mood or physiological state that was present at the time of learning 27.
Studies conducted in rodents have found that both fear and extinction learning are initiated in the amygdala 28–31, whereas the medial prefrontal cortex (mPFC) plays a key role in the retrieval of fear extinction memories 32. This role of the mPFC in fear extinction recall was demonstrated by experiments showing that ablation 33–35 or inhibition 36 of a region within the mPFC in rats, the infralimbic cortex (IL), reduces, abolishes or delays extinction recall, whereas electrical stimulation 37 of IL can simulate it. Recent neuroimaging studies in humans have found evidence for a human homologue of IL in the perigenual and orbitofrontal cortex (the ventromedial prefrontal cortex, vmPFC) 38–40. This region in humans responds selectively during the retrieval of extinction memories 38, 40, and its thickness has been correlated with the success of extinction memory retrieval in healthy subjects 41, 42.
Previously we examined fear and extinction learning and memory in patients with schizophrenia by measuring skin conductance responses (SCRs), using a validated two-day Pavlovian fear conditioning and extinction paradigm 17. We found that both healthy control subjects and schizophrenia patients were able to successfully acquire and extinguish conditioned fear responses. Twenty-four hours following successful fear conditioning and extinction learning to a CS+, healthy controls exhibited lower SCRs to the CS+ presented in the extinction learning context compared to the fear conditioning context, similar to the pattern previously observed in humans 43 and rodents 27. In contrast, the schizophrenia patients showed an excessive fear response (high SCRs) to the CS+ in the extinction (‘safe’) context, thus failing to demonstrate appropriate context gating of extinction memory retrieval.
In the present study we sought to identify changes in brain activity associated with deficient fear extinction recall in schizophrenia by measuring fear and extinction learning and memory while simultaneously collecting functional MRI (fMRI) data. We predicted that the schizophrenia patients would show impaired delayed extinction recall and, based on the known critical role of the vmPFC in extinction memory, and evidence for mPFC impairment in schizophrenia during emotional 44–48 and social 49–52 perception, that this extinction recall deficit would be associated with dysfunction of the vmPFC.
METHODS
PARTICIPANTS
For all subjects, exclusion criteria included severe medical illness, significant head trauma, neurologic illness, substance abuse during the past six months and contraindications for MRI scanning (e.g., implanted metal objects, claustrophobia). We limited our cohort to males to avoid introducing heterogeneity into our measures related to potential sex differences 53. Seventeen healthy male subjects were recruited via advertisement and screened for psychiatric illness using the structured clinical interview for DSM-IV (SCID) 54; subjects with past or present psychiatric diagnoses were excluded. Twenty male patients who met DSM-IV criteria for schizophrenia (12 treated and 8 untreated with antipsychotic medication, see Table 1) according to the SCID were recruited and characterized by the MGH Schizophrenia Program. The schizophrenia and control groups were matched with respect to age, mean parental education and handedness (see Table 1). Written informed consent was obtained from all subjects prior to enrollment in accordance with the guidelines of the Partners Healthcare Institutional Review Board. Levels of positive and negative symptoms of schizophrenia were evaluated in each patient by one trained rater (the first author) using the Positive and Negative Symptom Scale (PANSS) 55 on the first day of the experimental protocol. Also, symptoms of anxiety and depression were measured on Day 1 of the protocol in all subjects using the Spielberger State and Trait Anxiety Inventory 56 and the Beck Depression Inventory 57, respectively.
Table 1. Demographic characteristics of the participants.
A group of patients with schizophrenia (n = 20) and a group of healthy controls (n = 17), matched for age, parental education and IQ, were enrolled in the study. One of the patients and one of the controls were left-handed; the remaining subjects were right-handed. Eleven patients were taking second generation antipsychotics (aripiprazole (3); risperidone (2); clozapine (2); olanzapine (1); quetiapine (1); clozapine and aripiprazole (1); paliperidone (1)). One patient was taking haloperidol and eight were not taking any antipsychotic medication. Within the patient group, scores on all subscales of the PANSS (Negative, Positive and General) were correlated with one another (Rs > .46; ps < .02), and state anxiety levels correlated with trait anxiety levels (R = .67; p = .002), depressive symptoms (R = .60; p < .006) and positive symptoms (R = .54; p = .01). Mean levels of electrical stimulation set by each subject were lower in the schizophrenia patients than in the controls (p = .004). Also, the patients with schizophrenia showed lower levels of explicit learning of the CS identities at the end of Day 1 (p = .01) and lower levels of explicit recall of the context identities at the beginning of Day 2 (p = .03), compared to the controls.
| CON n = 17 | SCZ n = 20 | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
|
| ||||
| Age (years) | 34.2 | 9.9 | 34.7 | 9.8 |
| premorbid IQa | 111.1 | 7.1 | 106.1 | 9.8 |
| mean years of parental education | 14.7 | 2.0 | 13.7 | 3.2 |
| trait anxietyb* | 28.6 | 9.9 | 42.3 | 12.4 |
| state anxietyb* | 25.4 | 4.7 | 37.5 | 12.0 |
| depressionc* | 1.3 | 2.0 | 9.5 | 9.4 |
| intensity of electrical stimulation* | 2.0 | 1.0 | 1.3 | 0.5 |
| % of subjects who learned the CS identities* | 88 | 50 | ||
| % of subjects who recalled the CS identities | 82 | 65 | ||
| % of subjects who learned the context identities | 100 | 90 | ||
| % of subjects who recalled the context identities* | 100 | 75 | ||
| PANSS Total score | 52.7 | 13.8 | ||
| PANSS Positive Symptoms Subscale score | 13.5 | 5.7 | ||
| PANSS Negative Symptoms Subscale score | 13.9 | 6.3 | ||
| PANSS General Symptoms Subscale score | 25.3 | 5.9 | ||
| Mean duration of illness (years) | 12.9 | 9.4 | ||
| Mean chlorpromazine equivalents | 301.0 | 347.5 | ||
Premorbid IQ was measured using the American National Adult Reading Test (ANART).
Trait and state anxiety levels were measured using the Spielberger State and Trait Anxiety Inventory (STAI).
Symptoms of depression were measured using the Beck Depression Inventory (BDI).
Significant difference between schizophrenia and control subjects (p < .05). CON = control group; SCZ = schizophrenia group; PANSS = Positive and Negative Symptom Scale.
FEAR CONDITIONING AND EXTINCTION PROCEDURE
A two-day fear conditioning and extinction protocol used by our group in previous studies 40, 58, 59 was administered during fMRI data collection. The protocol consisted of three phases on Day 1 (Habituation, Fear Conditioning, and Extinction Learning) and two phases on Day 2 (Extinction Recall and Fear Renewal). During both days, recording electrodes were placed on the palm of the participant’s non-dominant hand. Electrodes were also attached to the second and third fingers of the participant’s dominant hand for the purpose of delivering the unconditioned stimulus, US (a 500 ms mild electrical stimulus). The intensity of the US was set by each participant before the beginning of the procedure to a level that was “annoying but not painful.” Electrical stimulations were only delivered during the Fear Conditioning phase, but participants were told that they “may or may not receive electrical stimulations” before every phase other than Habituation. The visual stimuli consisted of digital photographs of two rooms that contain lamps (Figure 1) that were presented via a projector in the magnet bore. The two rooms (a library and an office) comprised the two virtual contexts. Three colors of the lit lampshade of the lamp (blue, red or yellow) comprised the three conditioned stimuli, CSs. During the Fear Conditioning phase, two of the CSs were paired at a 60% reinforcement rate (CS+), and one was not paired (CS−), with the US. The US occurred during 500 ms following the offset of the CS+s. During the Extinction Learning phase, only one of the two CS+s was presented again, without being followed by the US (the extinguished CS+: CS+E). The other CS+ never underwent extinction (the unextinguished CS+: CS+U). All phases of the experiment included 16 CS+ (all phases except Extinction Learning: 8 CS+E, 8 CS+U; Extinction Learning: 16 CS+E) and 16 CS− trials (see Supplementary Figure 1). For the three phases that included both the CS+E and CS+U, these two trial types were presented sequentially, in an order that was counterbalanced across participants. The CS− trials were intermixed among the CS+ trials. For each trial, the context was presented for 9 seconds: 3 seconds alone, followed by 6 seconds in combination with a CS+ or CS−. The trials of the Fear Conditioning and Fear Renewal runs included the conditioning context. The trials of the Extinction Learning and Extinction Recall runs included the extinction context. The selection of the CS colors and contexts was counterbalanced across participants. The design of the paradigm was event-related; the mean inter-trial interval was 15 seconds (range: 12–18 seconds).
Figure 1. The two-day experimental paradigm.
In this example, the blue light is the CS+ that is presented during the Extinction Learning phase, the CS+E. Also, the fear learning context here is the ‘office’, whereas the extinction learning context is the ‘library’. s = seconds.
Throughout the procedure, participants passively viewed the stimuli and each participant’s attention to the stimuli was monitored by study staff via the ISCAN® fMRI Remote Eye Tracking Laboratory. Functional runs during which subjects closed their eyes were excluded from the analyses. At the end of Day 1 and at the start of Day 2, each participant was asked if he could recall the color of the light and describe the room that was or was not associated with the electrical stimulation.
SKIN CONDUCTANCE DATA COLLECTION AND ANALYSES
During the above procedure, skin conductance was recorded for 5 seconds before the presentation of the context, during the 3-second presentation of the context alone, and during the 6-second presentation of the context plus the CS. Skin conductance response (SCR) magnitude for each CS was calculated by subtracting the mean skin conductance during the 2 seconds immediately before CS onset (i.e., the response to the context alone) from the highest skin conductance recorded during the 6-second CS duration. SCRs were square-root transformed prior to analysis. Differential fear conditioning was calculated as the mean SCR for the CS+ trials minus the mean SCR for the CS− trials during Fear Conditioning (Early Conditioning: first 4 trials; Late Conditioning: last 4 trials). Extinction learning was calculated as the mean SCR for the last 4 CS+ trials minus the mean SCR for the last 4 CS− trials during Extinction Learning. Success of extinction recall was measured using an Extinction Retention Index: 100-((the average SCR for the first four trials of Extinction Recall, divided by the largest SCR of Fear Conditioning) x 100). The direction of the effect for within-group differential fear conditioning (CS+ > CS−), extinction recall context-dependence (CS+U > CS+E) 17, 43 and the reduction in the Extinction Retention Index in the schizophrenia patients 17 were each predicted a priori; thus, one-tailed t-tests were planned for those comparisons. Two-tailed t-tests were used for all other comparisons.
FUNCTIONAL MRI DATA ACQUISITION AND ANALYSES
Scanning occurred in a 3 Tesla MR scanner (Siemens TIM Trio) with echoplanar imaging capability and a 12-channel gradient head coil. For each functional run, T2*-weighted EP images were acquired (45 x 3 mm thick slices, 3.1 x 3.1 x 3 mm in-plane resolution) using a gradient echo sequence (TR = 3000 ms; TE = 30 ms; flip angle = 90 degrees). The fMRI data were processed using the FreeSurfer functional analysis stream (FS-FAST) (https://surfer.nmr.mgh.harvard.edu/fswiki). Each functional run was motion corrected, spatially smoothed (full width at half maximal [FWHM] = 5 mm) with a three-dimensional Gaussian filter and intensity normalized. Functional runs were excluded from the fMRI analyses if greater than 15 instances of more than 1 mm of head movement between TRs occurred during the run. The following conditions were included in the general linear model for the Day 1 experimental phases: a blank screen/fixation period (which included the electrical stimulations), the context presented alone, early CS+, late CS+, early CS− and late CS− (see Supplementary Figure 1). Conditions for the Day 2 phases included: a blank screen/fixation period, the context presented alone, early CS+E, late CS+E, early CS+U, late CS+U and CS−. Data collected during the fixation periods and the context presented alone were not included in the subsequent analyses. Statistical maps of group averaged data and between-group differences were created in Talairach space by calculating a t statistic at each voxel for the contrasts of interest, including a weighted-least-squares adjustment, using random effects analyses. Responses during Fear Conditioning and Extinction Learning were measured by comparing responses during the first (Early) or last (Late) 4 CS+ trials to the accompanying 4 CS− trials. Since we did not have a strong a priori basis for making predictions about neural responses during Fear Conditioning and Extinction Learning in schizophrenia (since our previous study did not demonstrate between-group differences for these phases 17), for these two phases we used a conservative whole brain cluster-correction, calculated using a Monte Carlo simulation (10,000 iterations, height threshold of p < .005), to identify voxels showing significant within-group responses or between-group differences in activation. Extinction Recall and Fear Renewal - associated activation was measured by comparing responses during the first 4 trials of the CS+E to responses during the first 4 trials of the CS+U 40. Activation for this contrast during Extinction Recall was consider significant if clusters of voxels within the vmPFC (BA 25, 11, 10) met a threshold of ≥10 contiguous voxels at p < .001 (a small volume correction). Locations of activation peaks were identified using the Talairach atlas 60.
ASSOCIATIONS WITH CLINICAL VARIABLES
Correlations (Spearman’s Rho) between skin conductance measures and symptom levels were deemed significant if they met a statistical threshold of p < .05, Bonferroni-corrected. A whole brain regression analysis, with a cluster correction calculated using a Monte Carlo simulation (10,000 iterations, height threshold of p < .005), was used to identify significant correlations (Pearson’s R) between activation magnitudes and symptom levels. Secondary, exploratory analyses comparing the antipsychotic treated versus untreated patients, and the patients with active delusions versus those without (score on the PANSS delusion item ≥ 3 or ≤ 2, respectively), were also conducted because of (1) concern about the potential confounding effects of antipsychotic treatment on our outcomes 61, 62 and (2) prior evidence for abnormal affective function in delusional patients 12, 13, 44, 47, 63.
RESULTS
FEAR CONDITIONING AND EXTINCTION LEARNING
Skin conductance responses
Both the controls and patients with schizophrenia showed differential fear conditioning (CS+ > CS−) during Early (Controls: t = 4.6, df = 16, p = 2 x 10−4; Schizophrenia patients: t = 2.05, df = 19, p = .027) and to a lesser extent, Late (Controls: t = 1.9, df = 16, p = .035; Schizophrenia patients: t = 1.6, df = 19, p = .059) Fear Conditioning (Figure 2A). At a trend level, controls showed greater magnitudes of differential fear conditioning than patients during Early (t = 1.8, df = 35, p = .07), but not Late, Fear Conditioning.
Figure 2. Skin conductance responses, Day 1 of the experiment.

Mean skin conductance responses (SCR) for the Fear Conditioning (A) and Extinction Learning (B) phases are plotted. Means for each phase (Early and Late Conditioning, end of Extinction Learning) are calculated using 4 CS+ and 4 CS− trials. Because it is only possible to measure levels of fear extinction and delayed fear extinction memory in participants who show some fear conditioning, participants who did not have two or more trials with a response magnitude of ≥ 0.3 microseimens during the Fear Conditioning phase were excluded from the SCR analyses for all of the phases that followed (two controls and three patients) (see Holt et al, 2009 for further details). One additional control was excluded from the Extinction Learning analysis because of poor electrode contact during data collection. Thus, the Fear Conditioning SCR analyses include data of 17 controls and 20 schizophrenia patients, and the Extinction Learning SCR analysis includes data of 14 controls and 17 schizophrenia patients. Both the control and schizophrenia groups acquired differential conditioned fear responses (CS+ > CS) during Early Conditioning. Although there was a trend towards a difference between the two groups in differential Early Fear Conditioning (p = .07, see text), there were no significant differences between the two groups in skin conductance responses to the CS+ and CS− alone. Red bars = mean skin conductance response to the CS+; black bars = mean skin conductance response to the CS−. CON = control group; SCZ = schizophrenia group; SCR = skin conductance response; sqrt = square root transformed. * p < .05; ** p < .0005, for the results of the within-group paired t-tests.
Both the controls and the schizophrenia patients were able to successfully extinguish conditioned fear responses (CS+ minus CS− in Late Extinction Learning, Controls vs. Schizophrenia patients: t = .41, df = 29, p = .96; Figure 2B).
BOLD responses
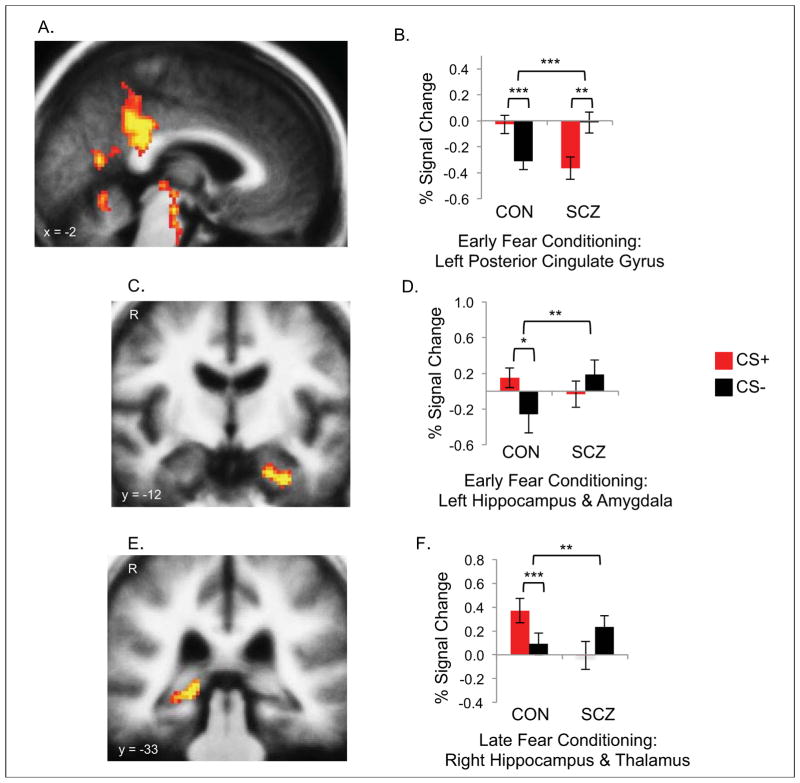
During Early Fear Conditioning, the controls showed larger responses to the CS+ compared to the CS− in limbic (hippocampus, entorhinal cortex, amygdala, insula, thalamus, brainstem and superior temporal sulcus) and visual (fusiform and lateral occipital cortices) areas (Table 2). During Late Fear Conditioning, the controls showed larger responses to the CS+ compared to the CS− in the right hippocampus. In contrast, the schizophrenia patients showed a reversal of the expected pattern of response during Early Fear Conditioning, with larger response to the CS− compared to the CS+ in the inferior parietal cortex, precuneus and posterior cingulate gyrus. Direct comparisons between the responses of the two groups revealed that the controls showed significantly greater activation (CS+ > CS−) of the thalamus (Early and Late Fear Conditioning), brainstem and left posterior cingulate gyrus (Early Fear Conditioning) and the right hippocampus (Late Fear Conditioning) than the schizophrenia patients (Figure 3). Also, below the whole brain corrected level of significance, there was greater activation in the control compared to the schizophrenia group during Early Fear Conditioning in the left medial temporal lobe (amygdala, hippocampus and entorhinal cortex) (Talairach coordinates (x, y, z) of peak difference: −30, −12, −27, z = 4.1, p = 5 x 10−5).
Table 2. Neural responses during fear conditioning and extinction learning.
Peak activations during fear conditioning and extinction learning in the control and schizophrenia groups, and clusters of activation which showed significantly greater activation in the control compared to the schizophrenia group are listed. There were no regions that showed significantly greater activation in the schizophrenia group compared to the controls. Within-group or between-group differences for the CS+ > CS− contrast are listed, except in the case of Early Fear Conditioning for the schizophrenia group, for which only clusters of CS− > CS+ activation were found (indicated with gray shading). 17 controls and 18 patients are included in these analyses (two patients were excluded due to excessive head movement, see Methods). BA = Brodmann Area; Tal = Talairach; R = right; L = left.
| Controls: | |||||
|---|---|---|---|---|---|
| Early Fear Conditioning (CS+ > CS−) | |||||
| Region | BA | Area (mm3) | Tal (x,y,z) | Peak P-value | Z |
| L thalamus, brainstem | 84392 | −10, −21, −7 | 4 x 10−8 | 5.51 | |
| R lateral occipital cortex | 18 | 3784 | 42, −85, −8 | 2 x 10−7 | 5.21 |
| L entorhinal cortex, hippocampus, amygdala | 28 | 6304 | −20, −14, −24 | 6 x 10−6 | 4.53 |
| L superior temporal sulcus | 22 | 4808 | −50, −55, −17 | 9 x 10−6 | 4.44 |
| R fusiform gyrus | 37 | 3592 | 32, −56, −12 | 5 x 10−5 | 4.06 |
| L insula | 3760 | −38, 13, −1 | 3 x 10−4 | 3.62 | |
| Late Fear Conditioning (CS+ > CS−) | |||||
| Region | BA | Area (mm3) | Tal (x,y,z) | Peak P-value | Z |
|
| |||||
| R hippocampus | 2792 | 20, −34, −1 | 1 x 10−4 | 3.89 | |
| Early Extinction Learning (CS+ > CS−) | |||||
| Region | BA | Area (mm3) | Tal (x,y,z) | Peak P-value | Z |
|
| |||||
| R brainstem, thalamus | 6432 | 4, −18, −20 | 1 x10−5 | 4.42 | |
| Schizophrenia Patients: | |||||
| Early Fear Conditioning (CS− > CS+)* | |||||
| Region | BA | Area (mm3) | Tal (x,y,z) | Peak P-value | Z |
|
| |||||
| L posterior cingulate gyrus, inferior parietal cortex | 31, 39 | 5552 | −32, −58, 22 | 3 x 10−5 | 4.17 |
| R posterior cingulate gyrus, precuneus, inferior parietal cortex | 23, 40 | 4528 | 30, −52, 27 | 8 x 10−5 | 3.95 |
| Control > Schizophrenia: | |||||
| Early Fear Conditioning (CS+ > CS−) | |||||
| Region | BA | Area (mm3) | Tal (x,y,z) | Peak P-value | Z |
|
| |||||
| L posterior cingulate gyrus thalamus, brainstem | 23 | 53632 | −8, −42, 27 | 4 x 10−7 | 5.08 |
| 4880 | −8, −19, −3 | 3 x 10−5 | 4.17 | ||
| Late Fear Conditioning (CS+ > CS−) | |||||
| Region | BA | Area (mm3) | Tal (x,y,z) | Peak P-value | Z |
|
| |||||
| R hippocampus, thalamus | 2968 | 18, −34, −1 | 5 x 10−5 | 4.06 | |
Figure 3. Neural responses during fear conditioning.
Voxel-wise maps (A, C, E) and bar plots (B, D, F) showing responses of regions with significantly greater activation for the CS+ minus CS− contrast in the controls (n = 17), compared to the schizophrenia patients (n = 18), during Fear Conditioning: the left posterior cingulate gyrus (A, B), left hippocampus and amygdala (C, D) during Early Fear Conditioning and the right hippocampus and thalamus (E, F) during Late Fear Conditioning. Red bars = blood oxygen level dependent (BOLD) response (percent signal change) to the CS+; black bars = BOLD response (percent signal change) to the CS−. Percent signal change values, relative to a low-level baseline condition, were extracted using 3 millimeter radius spheres centered on the coordinate of the voxel showing the peak between-group difference (see Table 2 for coordinates and p values). The low level baseline condition consisted of the average signal intensity over the fMRI run. * p < .05; ** p < .005; *** p < .0005. Astericks which are closest to the bar plots represent p values for the within-group paired t-tests; those that are further from the plots represent p values for between-group comparisons. The threshold used for the maps displayed in A, C and E is p = .005.
During Early Extinction Learning, the controls showed activation of the right brainstem and thalamus, however there were no significant differences between the two groups in the magnitude of responses during Early or Late Extinction Learning.
CONTEXT-DEPENDENT EXTINCTION RECALL
Skin conductance responses
Twenty-four hours after the fear conditioning and extinction learning phases, the controls showed a mean Extinction Retention Index of 76.6%, whereas the schizophrenia patients showed significant impairment in extinction memory, with a mean Extinction Retention Index of 42.9% (t = 2.06, df = 24, p = .025; Figure 4A). In addition, the healthy control subjects demonstrated context gating of extinction memory retrieval, showing significantly lower SCRs (i.e., lower fear) to the CS+E presented with the extinction context compared to the fear context (Extinction Recall vs. Fear Renewal: t = 2.16, df = 12, p = .026; Figure 4B and Supplementary Figure 2). However, the patients with schizophrenia failed to show the expected pattern of context gating, in fact showing larger SCRs to the CS+E with the extinction compared to the fear context (t = 3.38, df = 12, p = .003).
Figure 4. Skin conductance and neural responses during retrieval of extinction and fear memories.
A: Bar plots showing mean Extinction Retention Index values for the control (n = 13, orange bar) and schizophrenia (n = 13, purple bar) groups (an additional two controls and four patients were excluded from the Day 2 skin conductance response (SCR) analyses because of poor electrode contact during data collection). B: Bar plots showing the expected pattern of context-dependence of SCRs during Day 2 to the CS+ presented during Extinction Learning (the CS+E) in the control group (n = 13): lower SCRs (i.e., lower fear) to the CS+E in the extinction, compared to the fear, context. In contrast, the schizophrenia patients (n = 13) show an aberrant pattern of responses on Day 2, showing lower SCRs to the CS+E in the fear, compared to the extinction, context. Responses to the CS+ that was not presented during Extinction Learning (the CS+U) are not shown here. C: A voxel-wise map of the results of the comparison between the mean activation levels (for the CS+E minus CS+U contrast) during Extinction Recall in the control (n = 17) and schizophrenia (n = 15) groups, shows that the ventromedial prefrontal cortex (vmPFC) exhibited significantly greater activation in the controls compared to the schizophrenia patients. D: Bar plots showing the expected context gating of vmPFC responses in the control group: higher responses to the CS+E (vs. CS+U) in the extinction, compared to the fear, context. In contrast, the schizophrenia patients fail to recruit the vmPFC in either context. Percent signal change data were extracted using a 3 mm radius sphere centered on the voxel showing the peak between-group difference in the vmPFC during Extinction Recall. Five schizophrenia patients were excluded from the Day 2 fMRI analyses because of excessive head motion. Blue bars = skin conductance (B) or BOLD (D) responses during Extinction Recall; red bars = skin conductance (B) or BOLD (D) responses during Fear Renewal. CON = control group; SCZ = schizophrenia group. * p = .025.
BOLD responses
As expected, the controls successfully recruited the vmPFC (BA25/BA11 (2, 16, −17), z = 4.16, p = 3 x 10−5) during Extinction Recall. The schizophrenia patients failed to show this response. Moreover, the vmPFC response during Extinction Recall was significantly larger in the control group compared to the schizophrenia group (peak difference: BA25 (0, 12, −18), z = 3.98, p = 8 x 10−5, Figure 4C). In addition, the responses of the vmPFC were modulated by context in the control but not the schizophrenia group (Figure 4D and Supplementary Figure 2); in the controls, the portion of the vmPFC showing significant activation during Extinction Recall showed no responses during Fear Renewal (t = 2.57, df = 13, p = .012).
ASSOCIATIONS WITH SYMPTOMS AND POTENTIAL CONFOUNDS
In the schizophrenia group, negative symptom severity was inversely correlated with: 1) skin conductance (R = −.59, p = .006; Figure 5A) and 2) posterior cingulate gyrus (p = 7 x 10−5; Figure 5B) responses during Early Fear Conditioning. There were no significant correlations between the abnormalities found in the schizophrenia group described above and levels of positive symptoms, anxiety, depression, electrical stimulation level, antipsychotic dose or duration of illness. Secondary analyses revealed no significant differences between the antipsychotic treated and untreated patients (Supplementary Figure 3), or between the delusional and non-delusional patients (Supplementary Figure 4), in skin conductance or neural responses during any phase. However, the delusional patients showed significantly lower vmPFC responses than the healthy control subjects during Extinction Recall (BA25 (−4, 1, −11), z = 3.14, p = .002), whereas the non-delusional patients and control subjects did not differ in vmPFC response magnitude during this phase.
Figure 5. Correlations between skin conductance and neural responses during fear conditioning and negative symptom levels.

A: Scatter plot illustrating the relationship between negative symptom severity, as measure by the Positive and Negative Symptom Scale (PANSS) Negative Symptom Subscale Score, and early differential fear conditioning (CS+ minus CS− skin conductance responses, SCR). Values for antipsychotic-treated (n = 12) and antipsychotic-free (n = 8) schizophrenia patients are presented as blue and orange diamonds, respectively. B: A map of the clusters of voxels which showed less differential activation during Early Fear Conditioning in patients with greater levels of negative symptoms (inverse correlations between CS+ minus CS− activation and negative symptom severity, n = 18) is shown (cluster-corrected for the whole brain, p < .005). The Talairach coordinates and location of the voxel with the lowest p value for this correlation are: 2, −41, −20 (BA23), z = 3.98, p = 7 x 10−5 (see white arrow). Also, the location and lowest p value for the more dorsal and anterior peak found in the posterior cingulate gyrus for this correlation are: 0, −11, 33 (BA23/24), z = 3.54, p = 4 x 10−4. Because during Early Fear Conditioning the controls and schizophrenia patients showed opposite patterns of responses within the posterior cingulate gyrus (see Figure 3B), this correlation suggests that the between-group difference in activation during this phase was driven largely by abnormal (reversed) responses of the schizophrenia patients with high levels of negative symptoms. When the contributions of the individual items of the PANSS Negative Symptom subscale to these two correlations were examined, it was found that all of the items, except Social Withdrawal (r = −.16, p = .5) and Emotional Withdrawal which showed only a trend (r = −.40, p = .08), showed significant inverse correlations (ps < .05) with SCR during Early Fear Conditioning. Also, significant inverse correlations were found between Blunted Affect (r = −.60, p = .008), Poor Rapport (r = −.47, p = .049) and Stereotyped Thinking (r=−.58, p = .01) and posterior cingulate gyrus responses during Early Fear Conditioning.
COMMENT
SUMMARY OF MAIN FINDINGS
During fear conditioning, patients with schizophrenia showed blunted autonomic responses and either absent or reversed (larger responses to the CS− than to the CS+) neural responses compared to controls. Several of these abnormalities were linked to negative symptoms. In contrast, autonomic and neural responses during extinction learning in the schizophrenia and control groups did not differ. Twenty-four hours following extinction learning, the controls exhibited the expected pattern of decreased fear and increased vmPFC responses in the extinction, compared to the fear, context. However, the schizophrenia patients showed inappropriately elevated fear and no vmPFC activity in the extinction context, failing to retain the extinction memory encoded one day earlier.
FEAR CONDITIONING AND EXTINCTION LEARNING IN SCHIZOPHRENIA
The results of older studies of Pavlovian or other types of aversive conditioning in schizophrenia have been mixed 19–22, 64, possibly reflecting methodologic variation 17. However, several recent studies have demonstrated that schizophrenia patients can successfully acquire differential conditioned fear 17, 18 (also see 16), but they often show lower responses to the CS+ 16, 18 and/or higher responses to the CS− 16, 17, compared to control subjects. In the present study, this reversed pattern of responses was observed in the schizophrenia group during fear conditioning in the posterior cingulate gyrus, precuneus and inferior parietal cortex, and to a lesser extent in the hippocampus and thalamus. This overall pattern of larger responses to non-salient relative to salient stimuli has been observed previously in the posterior cingulate gyrus 44 and parahippocampal gyrus 65 in schizophrenia patients, as well as in the medial frontal and parietal cortices, thalamus and hippocampus in young people at elevated risk for developing schizophrenia 66. Given that many previous studies have reported abnormally reduced activation of limbic brain regions, particularly the amygdala 67, in schizophrenia, the present findings support the proposal 67, 68 that many of these previously reported ‘hypoactivations’ may in fact reflect a combined effect of abnormally elevated responses to neutral stimuli and reduced responses to aversive stimuli. The present results suggest that this pattern of responses may arise from abnormalities in emotional learning.
The inverse correlations seen here between (1) skin conductance and (2) posterior cingulate responses during fear conditioning, and the severity of negative symptoms, although unexpected, are generally reminiscent of findings of impaired positive reinforcement learning in schizophrenia patients with prominent negative symptoms 1–3. Together the findings of the present study and these prior studies suggest that negative symptoms may be related to a general impairment in learning conditioned associations (whether linked to aversive or rewarding unconditioned stimuli).
The posterior cingulate gyrus has not been studied extensively in schizophrenia, possibly because its function is not well understood 69, 70. However a number of recent studies have reported abnormalities in its function or connectivity in schizophrenia 44, 45, 49, 71. In light of evidence for its involvement in episodic memory processes 72, 73, we speculate that dysfunction of the posterior cingulate gyrus and hippocampus during fear conditioning in schizophrenia may interfere with the encoding of episodic memory traces of the CS+/US and CS−/noUS associations. The reduced accuracy in encoding the CS+ and CS− identities shown here by the schizophrenia patients compared to the controls (see Table 1) is consistent with this possibility. Given that humans may rely on episodic memory processes during fear conditioning to a greater extent than other species, one possible interpretation of our findings is that nonconscious, automatic associative learning during fear conditioning is preserved (as reflected by the patients’ ability to acquire some differential skin conductance responses) in patients with schizophrenia to a greater extent than conscious, episodic learning.
The finding of relatively preserved skin conductance responses accompanied by abnormal neural responses during fear conditioning in schizophrenia is consistent with previous reports of inconsistencies between peripheral and central nervous system measures of fear responses in schizophrenia patients 47, 48. This pattern of findings may be related to a disruption in communication between central and peripheral autonomic system centers in schizophrenia, or, in the current study, may simply reflect a greater sensitivity of functional MRI (due to its anatomic resolution) compared to skin conductance measurements.
Although the schizophrenia patients showed aberrant neural responses during fear conditioning, their extinction learning responses were comparable to those of controls. This dissociation between our findings for fear and extinction learning may be partly explained by evidence for independence of the fear and extinction systems. Fear and extinction learning are mediated by distinct cell populations in the amygdala 30, and fear and extinction memories are retrieved independently, in a context-gated manner 27.
ABNORMALITIES IN FEAR EXTINCTION MEMORY IN SCHIZOPHRENIA
Following successful extinction learning, both peripheral (skin conductance) and central (vmPFC responses) nervous system components of extinction recall were deficient in schizophrenia. Interestingly, impairment in vmPFC activity during the extinction recall phase was particularly prominent in the patients with active delusions, suggesting that deficient retrieval of safety–related information may confer a vulnerability to delusional thinking. It is not yet clear whether extinction recall impairment in schizophrenia reflects a selective derangement of the medial prefrontal-emotional memory system or one manifestation of a more global abnormality in limbic function or memory consolidation 74–76. In a recent study that used a preference conditioning paradigm, patients with schizophrenia showed intact learning but, twenty-four hours later, failed to recall the more frequently rewarded stimulus, whereas the controls retained this association 76. In light of the established role of the vmPFC in reward processing 77, 78, this previous finding and the present result suggest that inaccurate assessments of both reward and safety-related information in schizophrenia may result from disruptions of affective discrimination, memory consolidation and retrieval processes mediated by the vmPFC.
LIMITATIONS
The majority of the patients enrolled in this study were taking antipsychotic medications, which have known effects on associative learning. Treatment with D2 dopamine receptor antagonists interferes with the expression of conditioned avoidance motor responses 79, 80 and the acquisition of conditioned fear responses 61 in rodents. Results of studies of the effect of antipsychotics on extinction learning and extinction memory recall in rodents have been mixed, with evidence for facilitation of extinction learning 80, 81 and extinction recall 82, as well as evidence for inhibition of extinction recall by D2 62, as well as D1 83, receptor antagonists. It is not clear whether similar effects occur in humans. Functional MRI studies have shown that treatment with first-generation antipsychotics is associated with reduced activation of the striatum during aversive 84 and reward 85,86, 87 learning. However, to our knowledge, the effect of antipsychotic medication on emotional memory in humans has not been investigated. Here we did not observe any significant differences between the control and schizophrenia groups in striatal activation during fear conditioning or other phases of the experiment, nor did we find any differences between the antipsychotic treated and untreated schizophrenia patients in the extent of the abnormalities reported here. However, to fully resolve this issue, follow-up studies conducted in a larger number of unmedicated schizophrenia patients or individuals in the prodromal phase of the illness must be conducted.
POTENTIAL MOLECULAR MEDIATORS AND FUTURE DIRECTIONS
Studies in rodents have demonstrated that fear extinction recall can be induced or augmented by stimulation of NMDA 36 and metabotropic 88 glutamate receptors within the medial prefrontal cortex. Other studies suggest that a partial NMDA receptor agonist, d-cycloserine, can facilitate consolidation of extinction memories 89–91. Also, it has been shown that neurotrophins such as brain-derived neurotrophic factor (BDNF) play a central role in extinction and fear memory formation in the medial prefrontal cortex 92, 93. Because schizophrenia has been associated with NMDA receptor hypofunction 94, 95, reductions in serum BDNF 96, 97, and neural changes linked to a specific BDNF genotype 98, it will be important to determine whether abnormalities in any of these molecular mediators play a role in deficits in fear extinction memory and vmPFC function in schizophrenia. Preliminary work by our group suggests that once-weekly treatment with d-cycloserine facilitates memory consolidation and reduces negative symptom burden in patients with schizophrenia 99, and that d-cycloserine may also potentiate responses to cognitive treatments of delusions 100. Follow-up studies will determine whether d-cycloserine or other therapeutic agents can selectively reverse deficits in vmPFC-mediated extinction recall and affective dysfunction in schizophrenia patients. These data also support the use of psychosocial approaches for treating schizophrenia that influence the fear and extinction memory system by reducing negative affect and arousal, or by promoting consolidation of extinction memories.
Supplementary Material
Supplementary Figure 1. The design of the paradigm. One example of the eight possible counterbalanced versions of the paradigm is displayed here. One can see that for the three phases of the experiment that included the CS+E and CS+U (Fear Conditioning, Extinction Recall and Fear Renewal), the CS+E and CS+U trials were presented sequentially, in an order that was counterbalanced across subjects. This design was chosen based on early pilot work showing that sequential, rather than randomized, presentations of these conditions produced the most effective conditioning (see Milad et al, Biological Psychiatry 2007 for additional details).
During Fear Conditioning, two of the conditioned stimuli (CS) are followed by the unconditioned stimulus, US (a mild electrical stimulation, 500 ms in duration, represented by a drawing of a red bolt here) for 60% of the trials. In this example, one of the two CS+s is the blue light of the lamp (CS+1, labeled blue above) and the other CS+ is the red light of the lamp (CS+2, labeled red above). Another CS, the yellow light of the lamp, is never paired with the US, the CS− (labeled yellow above). During Extinction Learning, the CS− and only one of the two CS+ are presented (in this example, the CS+1, the blue light); during this phase the CS+ is not followed by the US, becoming the extinguished CS+ (the CS+E). The other CS+ (in this example, the CS+2, the red light) is not presented during Extinction Learning and becomes the unextinguished CS+ (CS+U). During Extinction Recall and Fear Renewal, both CS+s (the CS+E and the CS+U) are presented without being followed by the US. In every phase of the experiment, 16 CS+ trials are presented (all phases except Extinction Learning: 8 CS+E, 8 CS+U; Extinction Learning: 16 CS+E), as well as 16 CS− trials.
For the analysis of the Fear Conditioning phase, trials were separated into Early and Late Conditioning (Early: first 4 trials of the CS+1 and the CS+2, 8 trials total; Late: last 4 trials of the CS+1 and the CS+2, 8 trials total), and the accompanying CS− trials were separated into the Early and Late Conditioning phases in a similar manner. For the analysis of the Extinction Learning phase, trials were separated into Early and Late Extinction Learning (Early: first 4 trials of the CS+E; Late: last 4 trials of the CS+E), and the accompanying CS− trials were separated into the Early and Late Extinction Learning phases in a similar manner. The eight CS+ and eight CS− trials presented between the Early and Late Extinction Learning phases were not included in the analyses. For the analyses of the Extinction Recall and Fear Renewal phases, trials were separated into Early and Late (Early: first 4 trials of the CS+E and CS+U; Late: last 4 trials of the CS+E and the CS+U). Only the trials of the Early phase were used in the analyses (Early CS+E minus Early CS+U).
Supplementary Figure 2. Context modulation of skin conductance and neural responses during delayed retrieval of extinction and fear memories. A: Bar plots of the difference between the mean SCR to the CS+E (the CS+ presented during Extinction Learning) in the extinction context (the Extinction Recall phase) and the mean SCR to the CS+E in the fear context (the Fear Renewal phase) for the controls (orange bar) and schizophrenia patients (black bar). In the control group, SCR responses to the CS+E in the fear context were greater than in the extinction context (t = 2.16, df = 12, p = .026), whereas schizophrenia patients showed a reversed pattern, with greater responses to the CS+E in the extinction context than the fear context (t = 3.38, df = 12, p = .003). Moreover, the controls showed significantly greater context gating of SCRs than the schizophrenia patients (t = 2.72, df = 24, p = .003). B: Bar plot showing the expected context gating of vmPFC responses in the control group (orange bar): vmPFC responses to the CS+E (vs. CS+U) in the extinction context were higher than in the fear context (t = 2.57, df = 13, p = .012). In contrast, the schizophrenia patients (black bar) showed little vmPFC response in both contexts. Percent signal change data were extracted using a 3 mm radius sphere centered on the voxel showing the peak between-group difference in the vmPFC during Extinction Recall. CON = control group; SCZ = schizophrenia group. * p < .05; ** p < .005. Asterisks which are directly above the bar plot represent p values for the within-group paired t-tests; the asterisks in Panel A further away from the bar plot represent the p value for the between-group comparison (independent sample t-test).
Supplementary Figure 3. Comparisons between the antipsychotic-treated (AT) and the antipsychotic-free (AF) schizophrenia patients. Bar plots (A, B, C) showing the responses of the healthy controls (n = 17), antipsychotic-treated patients (Fear Conditioning: n = 11; Extinction Recall: n = 9), and antipsychotic-free patients (Fear Conditioning: n = 7; Extinction Recall: n = 6) within the posterior cingulate gyrus during Early Fear Conditioning (A), the right hippocampus and thalamus during Late Fear Conditioning (B) and the ventromedial prefrontal cortex (vmPFC) during Extinction Recall (C). Both antipsychotic-treated and antipsychotic-free schizophrenia patients show abnormal responses during these three phases, compared to the control group. Moreover, there were no differences found between the two patient groups. Thus, the responses (CS+ minus CS−) of the posterior cingulate gyrus during Early Fear Conditioning were greater in the control group compared to both the antipsychotic-treated (p = 2 x 10−5) and the antipsychotic-free (p = 4 x 10−5) schizophrenia patients (A). Similarly, the responses (CS+ minus CS−) of the right hippocampus and thalamus during Late Fear Conditioning were greater in the control group compared to both the antipsychotic-treated (p = .002) and the antipsychotic-free (p = .03) schizophrenia patients (B). Lastly, the responses (CS+E minus CS+U) of the right vmPFC during Extinction Recall were greater in the control group compared to the antipsychotic-free patients (p < .05) and, at a trend level, compared to the antipsychotic-treated patients (p = .06) (C). A voxel-wise map of the comparison of the responses of the antipsychotic-free schizophrenia patients and the healthy controls during Extinction Recall shows that the antipsychotic-free patients show significantly impaired vmPFC function (BA25 (0, 14, −18), z = 3.54, p = .0004) relative to the controls (D).
Red bars = blood oxygen level dependent (BOLD) response (percent signal change) to the CS+; black bars = BOLD response (percent signal change) to the CS−. Percent signal change values, relative to the low-level baseline condition (the average signal intensity over the fMRI run), were extracted using 3-millimeter radius spheres centered on the coordinate of the voxel showing the peak between-group difference for the comparison between the full cohort of schizophrenia patients and the controls. * p < .05; ** p < .005; *** p < .0005. Astericks which are closest to the bar plot represent p values for the within-group paired t-tests; those that are further from the plots represent p values for between-group comparisons (independent sample t-tests). CON = control group; AT = antipsychotic-treated schizophrenia group; AF = antipsychotic-free schizophrenia group; SCZ = schizophrenia patients; vmPFC = ventromedial prefrontal cortex.
Supplementary Figure 4. Comparisons between the non-delusional (ND) and delusional (D) schizophrenia patients. The schizophrenia patients were divided into two groups based on whether they exhibited active delusional thinking (PANSS delusion item score > 2) or not (PANSS delusion item score of 1 or 2). Bar plots (A, B, C) showing responses of the healthy controls (n = 17), non-delusional patients (Fear Conditioning: n = 9; Extinction Recall: n = 7), and delusional patients (Fear Conditioning: n = 9; Extinction Recall: n = 8) within the posterior cingulate gyrus during Early Fear Conditioning (A), the right hippocampus and thalamus during Late Fear Conditioning (B) and the ventromedial prefrontal cortex (vmPFC) during Extinction Recall (C). Each plot shows that both non-delusional and delusional patients exhibit abnormal responses during these three phases. Thus, the responses (CS+ minus CS−) of the posterior cingulate gyrus during Early Fear Conditioning were greater in the control group compared to both the non-delusional (p = 7 x 10−7) and delusional (p = .0004) schizophrenia patients (A). Similarly, the responses (CS+ minus CS−) of the right hippocampus and thalamus during Late Fear Conditioning were greater in the control group compared to both the non-delusional (p = .002) and the delusional (p = .01) schizophrenia patients (B). However, the responses of the right vmPFC during Extinction Recall (CS+E minus CS+U) were greater in the control group compared to the delusional (p = .004), but not compared to the non-delusional (p = .30), schizophrenia patients (C). A voxel-wise map of the comparison of the responses of the delusional schizophrenia patients and the healthy controls shows that the delusional patients showed significantly impaired vmPFC function (two peaks in BA25: (−4, 1, −11), z = 3.14, p = .002; (6, 10, −16), z = 2.97, p = .003) relative to the controls (D).
Red bars = blood oxygen level dependent (BOLD) response (percent signal change) to the CS+; black bars = BOLD response (percent signal change) to the CS−. Percent signal change values, relative to the low-level baseline condition (the average signal intensity over the fMRI run), were extracted using 3-millimeter radius spheres centered on the coordinate of the voxel showing the peak between-group difference for the comparison between the full cohort of schizophrenia patients and the controls. * p < .05; ** p < .005; *** p < .0005. Astericks which are closest to the bar plot represent p values for the within-group paired t-tests; those that are further from the plots represent p values for between-group comparisons (independent sample t-tests). CON = control group; ND = non-delusional schizophrenia group (PANSS delusion item = 1 or 2); D = delusional schizophrenia group (PANSS delusion item > 2); SCZ = schizophrenia patients; vmPFC = ventromedial prefrontal cortex.
Acknowledgments
Over the last five years, Dr. Goff has served as a consultant or advisor to: GlaxoSmithKline, Merck, Bristol- Myers Squibb, Wyeth, Organon, Xytis, XenoPort, Proteus, Vanda, Astra-Zeneca, Forest Labs, Pfizer, Indevus Pharmaceuticals, H. Lundbeck, Ortho-McNeil-Janssen, Schering-Plough, Eli Lilly, Takeda, Biovail, Solvay, Hoffman- La Roche, Cypress, Dainippon Sumitomo, Abbott Laboratories, Genentech, and Endo Pharmaceuticals. He served on a DSMB for Otsuka and Wyeth and received research funding from Cephalon, Pfizer, Janssen, Novartis, and GlaxoSmithKline. Dr. Holt takes responsibility for the integrity of the data and the accuracy of the data analysis, and all authors had full access to all the data in the study. We are grateful for the advice of Drs. Doug Greve and Eric Macklin on the imaging and statistical analyses, respectively.
Funding: This study was supported by the National Institute of Mental Health K23MH076054 (DH) and the National Alliance for Research on Depression and Schizophrenia with the Sidney R. Baer, Jr Foundation (DH). Portions of this work were presented at the 2010 Society for Neuroscience Meeting in San Diego, CA and the 2010 Annual Meeting of the American College of Neuropsychopharmacology in Miami, FL.
Footnotes
The remaining authors do not have any potential financial interests to disclose.
References
- 1.Polgar P, Farkas M, Nagy O, Kelemen O, Rethelyi J, Bitter I, Myers CE, Gluck MA, Keri S. How to find the way out from four rooms? The learning of “chaining” associations may shed light on the neuropsychology of the deficit syndrome of schizophrenia. Schizophr Res. 2008 Feb;99(1–3):200–207. doi: 10.1016/j.schres.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 2.Strauss GP, Frank MJ, Waltz JA, Kasanova Z, Herbener ES, Gold JM. Deficits in positive reinforcement learning and uncertainty-driven exploration are associated with distinct aspects of negative symptoms in schizophrenia. Biol Psychiatry. 2011 Mar 1;69(5):424–431. doi: 10.1016/j.biopsych.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waltz JA, Frank MJ, Wiecki TV, Gold JM. Altered probabilistic learning and response biases in schizophrenia: behavioral evidence and neurocomputational modeling. Neuropsychology. 2011 Jan;25(1):86–97. doi: 10.1037/a0020882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophr Bull. 2010 Sep;36(5):919–934. doi: 10.1093/schbul/sbq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hafner H, Maurer K, Trendler G, an der Heiden W, Schmidt M. The early course of schizophrenia and depression*. Eur Arch Psychiatry Clin Neurosci. 2005 Jun;255(3):167–173. doi: 10.1007/s00406-005-0584-8. [DOI] [PubMed] [Google Scholar]
- 6.Krabbendam L, Janssen I, Bak M, Bijl RV, de Graaf R, van Os J. Neuroticism and low self-esteem as risk factors for psychosis. Soc Psychiatry Psychiatr Epidemiol. 2002 Jan;37(1):1–6. doi: 10.1007/s127-002-8207-y. [DOI] [PubMed] [Google Scholar]
- 7.Krabbendam L, van Os J. Affective processes in the onset and persistence of psychosis. Eur Arch Psychiatry Clin Neurosci. 2005;255:185–189. doi: 10.1007/s00406-005-0586-6. [DOI] [PubMed] [Google Scholar]
- 8.Koreen AR, Siris SG, Chakos M, Alvir J, Mayerhoff D, Lieberman J. Depression in first-episode schizophrenia. Am J Psychiatry. 1993 Nov;150(11):1643–1648. doi: 10.1176/ajp.150.11.1643. [DOI] [PubMed] [Google Scholar]
- 9.Hafner H, Maurer K, Trendler G, an der Heiden W, Schmidt M, Konnecke R. Schizophrenia and depression: challenging the paradigm of two separate diseases--a controlled study of schizophrenia, depression and healthy controls. Schizophr Res. 2005 Sep 1;77(1):11–24. doi: 10.1016/j.schres.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Freeman D, Garety PA. Connecting neurosis and psychosis: the direct influence of emotion on delusions and hallucinations. Behav Res Ther. 2003;41:923–947. doi: 10.1016/s0005-7967(02)00104-3. [DOI] [PubMed] [Google Scholar]
- 11.Norman RM, Malla AK. Correlations over time between dysphoric mood and symptomatology in schizophrenia. Compr Psychiatry. 1994 Jan-Feb;35(1):34–38. doi: 10.1016/0010-440x(94)90167-8. [DOI] [PubMed] [Google Scholar]
- 12.Holt DJ, Titone D, Long LS, Goff DC, Cather C, Rauch SL, Judge A, Kuperberg GR. The misattribution of salience in delusional patients with schizophrenia. Schizophr Res. 2006;83:247–256. doi: 10.1016/j.schres.2005.12.858. [DOI] [PubMed] [Google Scholar]
- 13.Phillips ML, David AS. Abnormal visual scan paths: a psychophysiological marker of delusions in schizophrenia. Schizophr Res. 1998;29(3):235–245. doi: 10.1016/s0920-9964(97)00097-2. [DOI] [PubMed] [Google Scholar]
- 14.Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull. 2008 Sep;34(5):835–847. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiler JA, Bellebaum C, Brune M, Juckel G, Daum I. Impairment of probabilistic reward-based learning in schizophrenia. Neuropsychology. 2009 Sep;23(5):571–580. doi: 10.1037/a0016166. [DOI] [PubMed] [Google Scholar]
- 16.Romaniuk L, Honey GD, King JR, Whalley HC, McIntosh AM, Levita L, Hughes M, Johnstone EC, Day M, Lawrie SM, Hall J. Midbrain activation during pavlovian conditioning and delusional symptoms in schizophrenia. Arch Gen Psychiatry. 2010 Dec;67(12):1246–1254. doi: 10.1001/archgenpsychiatry.2010.169. [DOI] [PubMed] [Google Scholar]
- 17.Holt DJ, Lebron-Milad K, Milad MR, Rauch SL, Pitman RK, Orr SP, Cassidy BS, Walsh JP, Goff DC. Extinction memory is impaired in schizophrenia. Biological Psychiatry. 2009;65:455–463. doi: 10.1016/j.biopsych.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen J, Willeit M, Zipursky RB, Savina I, Smith AJ, Menon M, Crawley AP, Kapur S. The formation of abnormal associations in schizophrenia: neural and behavioral evidence. Neuropsychopharmacology. 2008 Feb;33(3):473–479. doi: 10.1038/sj.npp.1301437. [DOI] [PubMed] [Google Scholar]
- 19.Spain B. Eyelid conditioning and arousal in schizophrenic and normal subjects. J Abnorm Psychol. 1966;71:260–266. doi: 10.1037/h0023596. [DOI] [PubMed] [Google Scholar]
- 20.Sears LL, Andreasen NC, O’Leary DS. Cerebellar functional abnormalities in schizophrenia are suggested by classical eyeblink conditioning. Biol Psychiatry. 2000 Aug 1;48(3):204–209. doi: 10.1016/s0006-3223(00)00247-x. [DOI] [PubMed] [Google Scholar]
- 21.Hofer E, Doby D, Anderer P, Dantendorfer K. Impaired conditional discrimination learning in schizophrenia. Schizophr Res. 2001;51:127–136. doi: 10.1016/s0920-9964(00)00118-3. [DOI] [PubMed] [Google Scholar]
- 22.Kosmidis MH, Breier A, Fantie BD. Avoidance learning in schizophrenia: a dissociation between the effects of aversive and non-aversive stimuli. Schizophr Res. 1999;38:51–59. doi: 10.1016/s0920-9964(98)00181-9. [DOI] [PubMed] [Google Scholar]
- 23.Ledoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 24.Quirk GJ. Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learn Mem. 2002 Nov-Dec;9(6):402–407. doi: 10.1101/lm.49602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouton ME, Moody EW. Memory processes in classical conditioning. Neurosci Biobehav Rev. 2004;28:663–674. doi: 10.1016/j.neubiorev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006 Jul;73(1):61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006 Aug 15;60(4):352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 28.LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008 Jul 31;454(7204):642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008 Jul 31;454(7204):600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- 31.Amano T, Unal CT, Pare D. Synaptic correlates of fear extinction in the amygdala. Nat Neurosci. 2010 Apr;13(4):489–494. doi: 10.1038/nn.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006 Aug 15;60(4):337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000 Aug 15;20(16):6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lebron K, Milad MR, Quirk GJ. Delayed recall of fear extinction in rats with lesions of ventral medial prefrontal cortex. Learn Mem. 2004 Sep-Oct;11(5):544–548. doi: 10.1101/lm.78604. [DOI] [PubMed] [Google Scholar]
- 35.Morgan MA, Schulkin J, LeDoux JE. Ventral medial prefrontal cortex and emotional perseveration: the memory for prior extinction training. Behav Brain Res. 2003 Nov 30;146(1–2):121–130. doi: 10.1016/j.bbr.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 36.Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007 Mar 15;53(6):871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 37.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002 Nov 7;420(6911):70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 38.Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J Neurosci. 2006 Sep 13;26(37):9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phelps EA, Delgado MR, Nearing KI, Ledoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 40.Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007 Sep 1;62(5):446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 41.Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci USA. 2005;102:10706–10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hartley CA, Fischl B, Phelps EA. Brain Structure Correlates of Individual Differences in the Acquisition and Inhibition of Conditioned Fear. Cereb Cortex. Jan 24; doi: 10.1093/cercor/bhq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milad MR, Orr SP, Pitman RK, Rauch SL. Context modulation of memory for fear extinction in humans. Psychophysiology. 2005;42:456–464. doi: 10.1111/j.1469-8986.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 44.Holt DJ, Lakshmanan B, Freudenreich O, Goff DC, Rauch SL, Kuperberg GR. Dysfunction of a cortical midline network during emotional appraisals in schizophrenia. Schizophr Bull. 2011 Jan;37(1):164–176. doi: 10.1093/schbul/sbp067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reske M, Habel U, Kellermann T, Backes V, Jon Shah N, von Wilmsdorff M, Gaebel W, Zilles K, Schneider F. Differential brain activation during facial emotion discrimination in first-episode schizophrenia. J Psychiatr Res. 2009 Mar;43(6):592–599. doi: 10.1016/j.jpsychires.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 46.Taylor SF, Welsh RC, Chen AC, Velander AJ, Liberzon I. Medial frontal hyperactivity in reality distortion. Biol Psychiatry. 2007 May 15;61(10):1171–1178. doi: 10.1016/j.biopsych.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 47.Williams LM, Das P, Harris AW, Liddell BB, Brammer MJ, Olivieri G, Skerrett D, Phillips ML, David AS, Peduto A, Gordon E. Dysregulation of arousal and amygdala-prefrontal systems in paranoid schizophrenia. Am J Psychiatry. 2004;161:480–489. doi: 10.1176/appi.ajp.161.3.480. [DOI] [PubMed] [Google Scholar]
- 48.Williams LM, Das P, Liddell BJ, Olivieri G, Peduto AS, David AS, Gordon E, Harris AW. Fronto-limbic and autonomic disjunctions to negative emotion distinguish schizophrenia subtypes. Psychiatry Res. 2007 May 15;155(1):29–44. doi: 10.1016/j.pscychresns.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 49.Holt DJ, Cassidy BS, Andrews-Hanna JR, Lee SM, Coombs G, Goff DC, Gabrieli JD, Moran JM. An anterior-to-posterior shift in midline cortical activity in schizophrenia during self-reflection. Biol Psychiatry. 2011 Mar 1;69(5):415–423. doi: 10.1016/j.biopsych.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vinogradov S, Luks TL, Schulman BJ, Simpson GV. Deficit in a neural correlate of reality monitoring in schizophrenia patients. Cereb Cortex. 2008 Nov;18(11):2532–2539. doi: 10.1093/cercor/bhn028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brune M, Lissek S, Fuchs N, Witthaus H, Peters S, Nicolas V, Juckel G, Tegenthroff M. An fMRI study of theory of mind in schizophrenic patients with “passivity” symptoms. Neuropsychologia. 2008;46(7):1992–2001. doi: 10.1016/j.neuropsychologia.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 52.Walter H, Ciaramidaro A, Adenzato M, Vasic N, Ardito RB, Erk S, Bara BG. Dysfunction of the social brain in schizophrenia is modulated by intention type: an fMRI study. Soc Cogn Affect Neurosci. 2009 Jun;4(2):166–176. doi: 10.1093/scan/nsn047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Milad MR, Goldstein JM, Orr SP, Wedig MM, Klibanski A, Pitman RK, Rauch SL. Fear conditioning and extinction: influence of sex and menstrual cycle in healthy humans. Behav Neurosci. 2006 Dec;120(6):1196–1203. doi: 10.1037/0735-7044.120.5.1196. [DOI] [PubMed] [Google Scholar]
- 54.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I Disorders. New York: The New York State Psychiatric Institute, Biometrics Research; 1995. [Google Scholar]
- 55.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 56.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, California: Consulting Psychologists’ Press; 1983. [Google Scholar]
- 57.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961 Jun;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 58.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009 Dec 15;66(12):1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry. 2007 Nov 15;62(10):1191–1194. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 60.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers, Inc; 1988. [Google Scholar]
- 61.Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol. 2004 Dec;74(5):301–320. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 62.Mueller D, Bravo-Rivera C, Quirk GJ. Infralimbic D2 receptors are necessary for fear extinction and extinction-related tone responses. Biol Psychiatry. 2010 Dec 1;68(11):1055–1060. doi: 10.1016/j.biopsych.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Phillips ML, Williams L, Senior C, Bullmore ET, Brammer MJ, Andrew C, Williams SC, David AS. A differential neural response to threatening and non-threatening negative facial expressions in paranoid and non-paranoid schizophrenics. Psychiatry Res. 1999;92(1):11–31. doi: 10.1016/s0925-4927(99)00031-1. [DOI] [PubMed] [Google Scholar]
- 64.Howe ES. GSR conditioning in anxiety states, normals, and chronic functional schizophrenic subjects. J Abnorm Psychol. 1958;56:183–189. doi: 10.1037/h0047365. [DOI] [PubMed] [Google Scholar]
- 65.Surguladze S, Russell T, Kucharska-Pietura K, Travis MJ, Giampietro V, David AS, Phillips ML. A reversal of the normal pattern of parahippocampal response to neutral and fearful faces is associated with reality distortion in schizophrenia. Biol Psychiatry. 2006 Sep 1;60(5):423–431. doi: 10.1016/j.biopsych.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 66.Seiferth NY, Pauly K, Habel U, Kellermann T, Shah NJ, Ruhrmann S, Klosterkotter J, Schneider F, Kircher T. Increased neural response related to neutral faces in individuals at risk for psychosis. Neuroimage. 2008 Mar 1;40(1):289–297. doi: 10.1016/j.neuroimage.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 67.Anticevic A, Van Snellenberg JX, Cohen RE, Repovs G, Dowd EC, Barch DM. Amygdala Recruitment in Schizophrenia in Response to Aversive Emotional Material: A Meta-analysis of Neuroimaging Studies. Schizophr Bull. doi: 10.1093/schbul/sbq131. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holt DJ, Kunkel L, Weiss AP, Goff DC, Wright CI, Shin LM, Rauch SL, Hootnick J, Heckers S. Increased medial temporal lobe activation during the passive viewing of emotional and neutral facial expressions in schizophrenia. Schizophr Res. 2006;82:153–162. doi: 10.1016/j.schres.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 69.Vogt BA, Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Prog Brain Res. 2005;150:205–217. doi: 10.1016/S0079-6123(05)50015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008 Mar;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 71.Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RW, Theberge J, Schafer B, Williamson P. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007 Jul;33(4):1004–1012. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005 Sep;9(9):445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 73.Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: II. Cortical afferents. J Comp Neurol. 2003 Nov 3;466(1):48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- 74.Manoach DS, Cain MS, Vangel MG, Khurana A, Goff DC, Stickgold R. A failure of sleep-dependent procedural learning in chronic, medicated schizophrenia. Biol Psychiatry. 2004;56:951–956. doi: 10.1016/j.biopsych.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 75.Leeson VC, Robbins TW, Franklin C, Harrison M, Harrison I, Ron MA, Barnes TR, Joyce EM. Dissociation of long-term verbal memory and fronto-executive impairment in first-episode psychosis. Psychol Med. 2009 Nov;39(11):1799–1808. doi: 10.1017/S0033291709005935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Herbener ES. Impairment in long-term retention of preference conditioning in schizophrenia. Biol Psychiatry. 2009 Jun 15;65(12):1086–1090. doi: 10.1016/j.biopsych.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005 Sep;6(9):691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 78.Grabenhorst F, Rolls ET. Value, pleasure and choice in the ventral prefrontal cortex. Trends Cogn Sci. 2011 Feb;15(2):56–67. doi: 10.1016/j.tics.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 79.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998 Dec;28(3):309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 80.Li M, Parkes J, Fletcher PJ, Kapur S. Evaluation of the motor initiation hypothesis of APD-induced conditioned avoidance decreases. Pharmacol Biochem Behav. 2004 Aug;78(4):811–819. doi: 10.1016/j.pbb.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 81.Blackburn JR, Pfaus JG, Phillips AG. Dopamine functions in appetitive and defensive behaviours. Prog Neurobiol. 1992 Sep;39(3):247–279. doi: 10.1016/0301-0082(92)90018-a. [DOI] [PubMed] [Google Scholar]
- 82.Ponnusamy R, Nissim HA, Barad M. Systemic blockade of D2-like dopamine receptors facilitates extinction of conditioned fear in mice. Learn Mem. 2005 Jul-Aug;12(4):399–406. doi: 10.1101/lm.96605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hikind N, Maroun M. Microinfusion of the D1 receptor antagonist, SCH23390 into the IL but not the BLA impairs consolidation of extinction of auditory fear conditioning. Neurobiol Learn Mem. 2008 Jul;90(1):217–222. doi: 10.1016/j.nlm.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 84.Menon M, Jensen J, Vitcu I, Graff-Guerrero A, Crawley A, Smith MA, Kapur S. Temporal difference modeling of the blood-oxygen level dependent response during aversive conditioning in humans: effects of dopaminergic modulation. Biol Psychiatry. 2007 Oct 1;62(7):765–772. doi: 10.1016/j.biopsych.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 85.Juckel G, Schlagenhauf F, Koslowski M, Filonov D, Wustenberg T, Villringer A, Knutson B, Kienast T, Gallinat J, Wrase J, Heinz A. Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology (Berl) 2006 Aug;187(2):222–228. doi: 10.1007/s00213-006-0405-4. [DOI] [PubMed] [Google Scholar]
- 86.Schlagenhauf F, Juckel G, Koslowski M, Kahnt T, Knutson B, Dembler T, Kienast T, Gallinat J, Wrase J, Heinz A. Reward system activation in schizophrenic patients switched from typical neuroleptics to olanzapine. Psychopharmacology (Berl) 2008 Mar;196(4):673–684. doi: 10.1007/s00213-007-1016-4. [DOI] [PubMed] [Google Scholar]
- 87.Kirsch P, Ronshausen S, Mier D, Gallhofer B. The influence of antipsychotic treatment on brain reward system reactivity in schizophrenia patients. Pharmacopsychiatry. 2007 Sep;40(5):196–198. doi: 10.1055/s-2007-984463. [DOI] [PubMed] [Google Scholar]
- 88.Fontanez-Nuin DE, Santini E, Quirk GJ, Porter JT. Memory for fear extinction requires mGluR5-mediated activation of infralimbic neurons. Cereb Cortex. 2011 Mar;21(3):727–735. doi: 10.1093/cercor/bhq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McCallum J, Kim JH, Richardson R. Impaired extinction retention in adolescent rats: effects of D-cycloserine. Neuropsychopharmacology. 2010 Sep;35(10):2134–2142. doi: 10.1038/npp.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Langton JM, Richardson R. The effect of D-cycloserine on immediate vs. delayed extinction of learned fear. Learn Mem. 2010;17(11):547–551. doi: 10.1101/lm.1927310. [DOI] [PubMed] [Google Scholar]
- 91.Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008 Jun 15;63(12):1118–1126. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 92.Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Induction of fear extinction with hippocampal-infralimbic BDNF. Science. 2010 Jun 4;328(5983):1288–1290. doi: 10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Choi DC, Maguschak KA, Ye K, Jang SW, Myers KM, Ressler KJ. Prelimbic cortical BDNF is required for memory of learned fear but not extinction or innate fear. Proc Natl Acad Sci U S A. 2010 Feb 9;107(6):2675–2680. doi: 10.1073/pnas.0909359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Krystal JH, D’Souza DC, Mathalon D, Perry E, Belger A, Hoffman R. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl) 2003 Sep;169(3–4):215–233. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- 95.Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry. 2001;158(9):1367–1377. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- 96.Buckley PF, Pillai A, Howell KR. Brain-derived neurotrophic factor: findings in schizophrenia. Curr Opin Psychiatry. 2011 Mar;24(2):122–127. doi: 10.1097/YCO.0b013e3283436eb7. [DOI] [PubMed] [Google Scholar]
- 97.Green MJ, Matheson SL, Shepherd A, Weickert CS, Carr VJ. Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol Psychiatry. 2011 Sep;16(9):960–972. doi: 10.1038/mp.2010.88. [DOI] [PubMed] [Google Scholar]
- 98.Ho BC, Andreasen NC, Dawson JD, Wassink TH. Association between brain-derived neurotrophic factor Val66Met gene polymorphism and progressive brain volume changes in schizophrenia. Am J Psychiatry. 2007 Dec;164(12):1890–1899. doi: 10.1176/appi.ajp.2007.05111903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Goff DC, Cather C, Gottlieb JD, Evins AE, Walsh J, Raeke L, Otto MW, Schoenfeld D, Green MF. Once-weekly D-cycloserine effects on negative symptoms and cognition in schizophrenia: an exploratory study. Schizophr Res. 2008 Dec;106(2–3):320–327. doi: 10.1016/j.schres.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gottlieb JD, Cather C, Shanahan M, Creedon T, Macklin EA, Goff DC. D-cycloserine facilitation of cognitive behavioral therapy for delusions in schizophrenia. Schizophr Res. 2011 Sep;131(1–3):69–74. doi: 10.1016/j.schres.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. The design of the paradigm. One example of the eight possible counterbalanced versions of the paradigm is displayed here. One can see that for the three phases of the experiment that included the CS+E and CS+U (Fear Conditioning, Extinction Recall and Fear Renewal), the CS+E and CS+U trials were presented sequentially, in an order that was counterbalanced across subjects. This design was chosen based on early pilot work showing that sequential, rather than randomized, presentations of these conditions produced the most effective conditioning (see Milad et al, Biological Psychiatry 2007 for additional details).
During Fear Conditioning, two of the conditioned stimuli (CS) are followed by the unconditioned stimulus, US (a mild electrical stimulation, 500 ms in duration, represented by a drawing of a red bolt here) for 60% of the trials. In this example, one of the two CS+s is the blue light of the lamp (CS+1, labeled blue above) and the other CS+ is the red light of the lamp (CS+2, labeled red above). Another CS, the yellow light of the lamp, is never paired with the US, the CS− (labeled yellow above). During Extinction Learning, the CS− and only one of the two CS+ are presented (in this example, the CS+1, the blue light); during this phase the CS+ is not followed by the US, becoming the extinguished CS+ (the CS+E). The other CS+ (in this example, the CS+2, the red light) is not presented during Extinction Learning and becomes the unextinguished CS+ (CS+U). During Extinction Recall and Fear Renewal, both CS+s (the CS+E and the CS+U) are presented without being followed by the US. In every phase of the experiment, 16 CS+ trials are presented (all phases except Extinction Learning: 8 CS+E, 8 CS+U; Extinction Learning: 16 CS+E), as well as 16 CS− trials.
For the analysis of the Fear Conditioning phase, trials were separated into Early and Late Conditioning (Early: first 4 trials of the CS+1 and the CS+2, 8 trials total; Late: last 4 trials of the CS+1 and the CS+2, 8 trials total), and the accompanying CS− trials were separated into the Early and Late Conditioning phases in a similar manner. For the analysis of the Extinction Learning phase, trials were separated into Early and Late Extinction Learning (Early: first 4 trials of the CS+E; Late: last 4 trials of the CS+E), and the accompanying CS− trials were separated into the Early and Late Extinction Learning phases in a similar manner. The eight CS+ and eight CS− trials presented between the Early and Late Extinction Learning phases were not included in the analyses. For the analyses of the Extinction Recall and Fear Renewal phases, trials were separated into Early and Late (Early: first 4 trials of the CS+E and CS+U; Late: last 4 trials of the CS+E and the CS+U). Only the trials of the Early phase were used in the analyses (Early CS+E minus Early CS+U).
Supplementary Figure 2. Context modulation of skin conductance and neural responses during delayed retrieval of extinction and fear memories. A: Bar plots of the difference between the mean SCR to the CS+E (the CS+ presented during Extinction Learning) in the extinction context (the Extinction Recall phase) and the mean SCR to the CS+E in the fear context (the Fear Renewal phase) for the controls (orange bar) and schizophrenia patients (black bar). In the control group, SCR responses to the CS+E in the fear context were greater than in the extinction context (t = 2.16, df = 12, p = .026), whereas schizophrenia patients showed a reversed pattern, with greater responses to the CS+E in the extinction context than the fear context (t = 3.38, df = 12, p = .003). Moreover, the controls showed significantly greater context gating of SCRs than the schizophrenia patients (t = 2.72, df = 24, p = .003). B: Bar plot showing the expected context gating of vmPFC responses in the control group (orange bar): vmPFC responses to the CS+E (vs. CS+U) in the extinction context were higher than in the fear context (t = 2.57, df = 13, p = .012). In contrast, the schizophrenia patients (black bar) showed little vmPFC response in both contexts. Percent signal change data were extracted using a 3 mm radius sphere centered on the voxel showing the peak between-group difference in the vmPFC during Extinction Recall. CON = control group; SCZ = schizophrenia group. * p < .05; ** p < .005. Asterisks which are directly above the bar plot represent p values for the within-group paired t-tests; the asterisks in Panel A further away from the bar plot represent the p value for the between-group comparison (independent sample t-test).
Supplementary Figure 3. Comparisons between the antipsychotic-treated (AT) and the antipsychotic-free (AF) schizophrenia patients. Bar plots (A, B, C) showing the responses of the healthy controls (n = 17), antipsychotic-treated patients (Fear Conditioning: n = 11; Extinction Recall: n = 9), and antipsychotic-free patients (Fear Conditioning: n = 7; Extinction Recall: n = 6) within the posterior cingulate gyrus during Early Fear Conditioning (A), the right hippocampus and thalamus during Late Fear Conditioning (B) and the ventromedial prefrontal cortex (vmPFC) during Extinction Recall (C). Both antipsychotic-treated and antipsychotic-free schizophrenia patients show abnormal responses during these three phases, compared to the control group. Moreover, there were no differences found between the two patient groups. Thus, the responses (CS+ minus CS−) of the posterior cingulate gyrus during Early Fear Conditioning were greater in the control group compared to both the antipsychotic-treated (p = 2 x 10−5) and the antipsychotic-free (p = 4 x 10−5) schizophrenia patients (A). Similarly, the responses (CS+ minus CS−) of the right hippocampus and thalamus during Late Fear Conditioning were greater in the control group compared to both the antipsychotic-treated (p = .002) and the antipsychotic-free (p = .03) schizophrenia patients (B). Lastly, the responses (CS+E minus CS+U) of the right vmPFC during Extinction Recall were greater in the control group compared to the antipsychotic-free patients (p < .05) and, at a trend level, compared to the antipsychotic-treated patients (p = .06) (C). A voxel-wise map of the comparison of the responses of the antipsychotic-free schizophrenia patients and the healthy controls during Extinction Recall shows that the antipsychotic-free patients show significantly impaired vmPFC function (BA25 (0, 14, −18), z = 3.54, p = .0004) relative to the controls (D).
Red bars = blood oxygen level dependent (BOLD) response (percent signal change) to the CS+; black bars = BOLD response (percent signal change) to the CS−. Percent signal change values, relative to the low-level baseline condition (the average signal intensity over the fMRI run), were extracted using 3-millimeter radius spheres centered on the coordinate of the voxel showing the peak between-group difference for the comparison between the full cohort of schizophrenia patients and the controls. * p < .05; ** p < .005; *** p < .0005. Astericks which are closest to the bar plot represent p values for the within-group paired t-tests; those that are further from the plots represent p values for between-group comparisons (independent sample t-tests). CON = control group; AT = antipsychotic-treated schizophrenia group; AF = antipsychotic-free schizophrenia group; SCZ = schizophrenia patients; vmPFC = ventromedial prefrontal cortex.
Supplementary Figure 4. Comparisons between the non-delusional (ND) and delusional (D) schizophrenia patients. The schizophrenia patients were divided into two groups based on whether they exhibited active delusional thinking (PANSS delusion item score > 2) or not (PANSS delusion item score of 1 or 2). Bar plots (A, B, C) showing responses of the healthy controls (n = 17), non-delusional patients (Fear Conditioning: n = 9; Extinction Recall: n = 7), and delusional patients (Fear Conditioning: n = 9; Extinction Recall: n = 8) within the posterior cingulate gyrus during Early Fear Conditioning (A), the right hippocampus and thalamus during Late Fear Conditioning (B) and the ventromedial prefrontal cortex (vmPFC) during Extinction Recall (C). Each plot shows that both non-delusional and delusional patients exhibit abnormal responses during these three phases. Thus, the responses (CS+ minus CS−) of the posterior cingulate gyrus during Early Fear Conditioning were greater in the control group compared to both the non-delusional (p = 7 x 10−7) and delusional (p = .0004) schizophrenia patients (A). Similarly, the responses (CS+ minus CS−) of the right hippocampus and thalamus during Late Fear Conditioning were greater in the control group compared to both the non-delusional (p = .002) and the delusional (p = .01) schizophrenia patients (B). However, the responses of the right vmPFC during Extinction Recall (CS+E minus CS+U) were greater in the control group compared to the delusional (p = .004), but not compared to the non-delusional (p = .30), schizophrenia patients (C). A voxel-wise map of the comparison of the responses of the delusional schizophrenia patients and the healthy controls shows that the delusional patients showed significantly impaired vmPFC function (two peaks in BA25: (−4, 1, −11), z = 3.14, p = .002; (6, 10, −16), z = 2.97, p = .003) relative to the controls (D).
Red bars = blood oxygen level dependent (BOLD) response (percent signal change) to the CS+; black bars = BOLD response (percent signal change) to the CS−. Percent signal change values, relative to the low-level baseline condition (the average signal intensity over the fMRI run), were extracted using 3-millimeter radius spheres centered on the coordinate of the voxel showing the peak between-group difference for the comparison between the full cohort of schizophrenia patients and the controls. * p < .05; ** p < .005; *** p < .0005. Astericks which are closest to the bar plot represent p values for the within-group paired t-tests; those that are further from the plots represent p values for between-group comparisons (independent sample t-tests). CON = control group; ND = non-delusional schizophrenia group (PANSS delusion item = 1 or 2); D = delusional schizophrenia group (PANSS delusion item > 2); SCZ = schizophrenia patients; vmPFC = ventromedial prefrontal cortex.