Abstract
Noninvasive imaging modalities are often used to manage patients with cardiovascular disease. Cardiovascular magnetic resonance (CMR) is increasingly used for diagnosing and evaluating myocardial ischemia and viability; moreover, stress CMR study results can be used to determine cardiac prognosis. In this article, we review recently published material regarding the performance of stress testing with CMR including a brief update regarding techniques, stress agents, diagnostic accuracy, prognosis, economic implications, and ongoing trials and future developments.
Keywords: CMR, Ischemia, Testing, Prognosis
Introduction
Over the past 15 years, cardiovascular magnetic resonance (CMR) has been developed for clinical use to detect myocardial ischemia and viability, and to define cardiac prognosis [1–5].
When compared with other diagnostic stress imaging techniques, such as dobutamine stress echocardiography [6], single photon emission computed tomography (SPECT) [7], or positron emission tomography [8], CMR depicts wall motion and myocardial perfusion with high spatial resolution in virtually any imaging plane without exposure to ionizing radiation (Fig. 1) [9]. These features make CMR an excellent imaging choice for patients in need of cardiac stress imaging [10•]. In this article, we review recently published material regarding the performance of CMR ischemic stress testing. Specifically, these include the agents and techniques used to perform stress, the diagnostic accuracy, prognostic utility, and economic implications of testing procedures, and ongoing trials and future developments in the field of stress CMR.
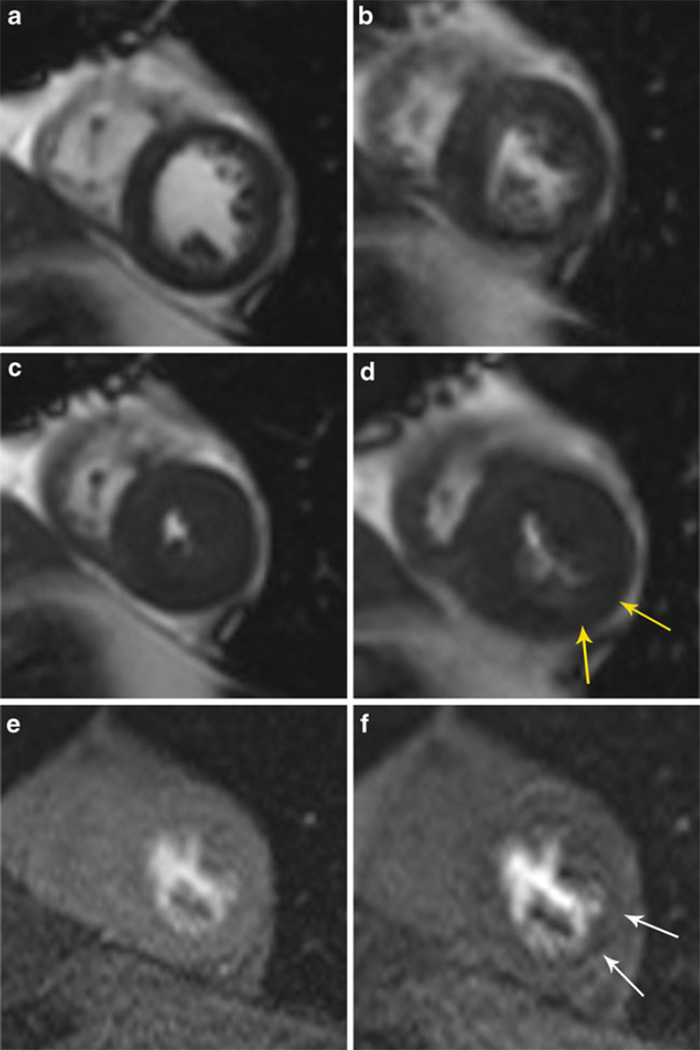
Fig. 1.
A–F Stress cardiovascular magnetic resonance using dobutamine-induced left ventricular (LV) wall motion abnormalities and adenosine-induced perfusion defects. Resting end-diastolic (A) and end-systolic (C) frame from a shortaxis view demonstrated normal LV contraction with no wall motion abnormalities. Peak dobutamine end-diastolic (B) and end-systolic cine view; the yellow arrows (D) highlight hypokinesis of the anterior region at end-systole. Resting (E) and adenosine stress (F) mid left ventricular shortaxis gadolinium enhanced first-pass perfusion slices demonstrating perfusion abnormalities in the postero-lateral (white arrow) regions in the region of the wall motion abnormality (yellow arrows)
Stress Agents
Stress tests incorporating CMR can be performed using inotropic or vasodilator stimuli. Inotropic stimuli, such as with dobutamine [11, 12], promote myocardial ischemia by creating a myocardial supply–demand mismatch in areas perfused by coronary arteries with flow-limiting stenoses. Alternatively, adenosine [13], regadenoson [14], or dipyridamole [15] promote systemic arterial vasodilation. Because the coronary microcirculation is maximally dilated at rest in the setting of a flow-limiting coronary arterial stenosis, the administration of a systemic vasodilator will dilate other territories not subserved by a flow-limiting stenosis and promote a preferential distribution of flow to these areas. Manifestations of ischemia, including abnormalities of perfusion or wall motion, are then identified using various CMR imaging techniques. In general, perfusion abnormalities are recognized after the first-pass of gadolinium contrast on T1-weighted images. Disorders of cardiac muscle function or left ventricular (LV) wall motion are observed using one of several cine white blood imaging techniques.
To date, several studies involving thousands of patients have documented the protocols and recommendations for safely performing CMR stress tests [12]. To date, adverse and serious adverse event rates are similar to or lower than those reported with other imaging modalities [16]. The Society for Cardiovascular Magnetic Resonance (http://www.scmr.org), an international society composed of radiologists, cardiologists, physicists, and biomedical engineers, has established criteria for performing and reporting results of stress CMR studies [9].
New Developments in Dobutamine Stress
New Dobutamine Cardiovascular Magnetic Resonance Techniques
Qualitative dobutamine stress wall motion analyses have been used to diagnose coronary artery disease (CAD), identify inducible ischemia, and forecast cardiac prognosis [1–5]. Using myocardial tissue tagging [17], strain-encoding (SENC) [18] or displacement encoding with stimulated echoes [19], several investigators have recently documented the utility of quantitative wall motion analyses for identifying inducible ischemia. In the first, Korosoglou et al. [20] demonstrated that SENC analysis can be used during dobutamine stress to detect patients with moderate (50% to 75%) coronary arterial stenosis. In another study by Korosoglou et al. [21], quantitative measures of myocardial strain were proven useful for detecting early evidence of ischemia during low-dose dobutamine. These quantitative measures were sufficient to detect ischemia at 7.5 to 10 µg/kg per minute of dobutamine and forecasted future LV wall motion abnormalities (WMAs) visualized at high-dose stress. This relatively promising innovation may prove useful in designing future dobutamine cardiovascular magnetic resonance (DCMR) protocols that use online quantitation to facilitate visual identification of ischemia at lower rather than high doses of intravenous dobutamine infusion.
Another technical development in the area of dobutamine stress has been the addition of gadolinium to wall motion assessments. Recently, Kelle et al. [22] performed high-dose DCMR in combination with contrast agent administration at 3T for the identification of myocardial ischemia. The high field strength allowed for identification of small subendocardial perfusion defects. Future studies with large numbers of patients will need to be performed to determine if imaging at 3T exhibits clinical benefit when performing stress CMR perfusion [23–25] or wall motion [26] analyses [27].
DCMR in Women
Many of the original studies of DCMR involved men. For this reason, several investigators recently performed studies to determine the utility of DCMR stress testing in women.
Gebker et al. [28] performed a comparative study to assess the diagnostic value of DCMR for the detection of CAD in women versus men. They found that the diagnostic values (sensitivity/specificity/accuracy) for identifying myocardial ischemia indicative of coronary arterial luminal narrowings of greater than 70% were similar respectively for men (86%, 83%, 85%) and women (85%, 86%, 85%).
In another study, Wallace et al. [29] determined the utility of DCMR results for predicting cardiac prognosis in women. In 266 consecutively referred women for DCMR followed for an average of 6.2 years, DCMR results were found efficacious for identifying women at risk for myocardial infarction (MI) and cardiac death after accounting for known risk factors for cardiac events or CAD. Importantly the prognostic utility of CMR stress results in women was similar to those historically reported in men.
Limitations of DCMR Wall Motion
Studies published in the past 2 years indicate that the results of dobutamine stress wall motion testing may not confer additional prognostic information above and beyond that acquired from resting study results. Several studies indicate that increased LV wall thickness or left ventricular hypertrophy (LVH) confer an increased risk of cardiac events even when there is an absence of inducible LV WMAs indicative of ischemia. Recently, Walsh et al. [30] identified that increased LV end-diastolic wall thickness in the base of the septum or lateral wall was associated with MI and cardiac death in individuals with a resting left ventricular ejection fraction (LVEF) greater than 55% and no inducible LV WMA indicative of ischemia. In a second study, Charoenpanichkit et al. [31•] found that LVH was an independent prognostic marker above and beyond assessments of LV WMA. In fact, in those with LVH but without dobutamine-induced WMA, the future risk of MI and cardiac death was found similar to those with an inducible LV WMA indicative of ischemia. These results suggest that LVH, and perhaps LV mass, should be measured and reported in those referred for dobutamine wall motion testing.
Dall’Armellina et al. [1] addressed the association between dobutamine-induced LV WMA and resting LVEF in patients undergoing stress CMR. In 200 participants followed for 5 years, inducible LV WMA did not offer incremental prognostic information in participants with a resting LVEF less than 40%. In 2010, Korosoglou et al. [32•] provided the outcomes of 1493 patients with suspected or known CAD undergoing CMR dobutamine wall motion and perfusion stress. After a 2±1 year’s follow-up period using multivariable regression analysis, inducible LV WMA or perfusion defects observed during stress exhibited the strongest independent predictors of major adverse cardiac events (MACE, defined as cardiac death, nonfatal MI, and late revascularization). The presence of inducible LV WMA or myocardial perfusion defects were associated with hazard ratios of 5.9 and 5.4, respectively, for MACE. This relatively large study also allowed one to draw important conclusions regarding the potential benefits of incorporating perfusion into dobutamine wall motion studies. Adding perfusion to dobutamine wall motion studies is not trivial because perfusion assessments require the addition of gadolinium contrast and contrast is associated with incremental expense and minor risks to participants. As shown in their study, the implementation of gadolinium contrast was helpful for identifying adverse prognosis when a patient did not exhibit a new inducible LV WMA at peak dobutamine, but exhibited a resting LV WMA, known CAD, or LVH. The data from this study and others [31•] raise an interesting question as to whether DCMR stress perfusion studies should be considered as a first-line dobutamine stress modality (rather than echocardiography alone with wall motion) in appropriately equipped and credentialed centers when patients exhibit resting LV WMAs, CAD, or LVH, and there is no contraindication to contrast.
Second, the presence of dobutamine-induced LV WMA forecasts cardiac prognosis in individuals regardless of the pretest probability of CAD (low, intermediate, or high). Third, the results (positive or negative for ischemia) of wall motion or perfusion stress tests do not add incremental information regarding cardiovascular prognosis in individuals with a severely reduced LVEF at rest. Thus, for individuals with a resting LVEF of less than 35%, dobutamine stress testing will only be useful in identifying myocardial ischemia or viability when selecting individuals who may be candidates for coronary artery revascularization procedures to relieve symptoms.
New Development Using Vasodilator Stress
Identification of Ischemia
Although several studies have reported on the utility of CMR using dobutamine stress, worldwide, the majority of CMR stress studies are performed using vasodilating agents such as adenosine, dipyridamole, or regadenoson. During these studies, the signal intensity of the LV myocardium on T1-weighted images after the first pass of gadolinium contrast is observed visually or in a quantitative fashion. Ischemia is identified as regions of low signal intensity after vasodilator administration that do not exhibit a corresponding region of low intensity on rest images, or an area of high intensity on delayed enhancement images. In a meta-analysis by Hamon et al. [33•], vasodilator perfusion assessed with gadolinium contrast was found to have a high sensitivity (89%) and a moderate specificity (80%) for the identification of ≥70% coronary arterial luminal narrowings as assessed with contrast coronary angiography (Table 1).
Table 1.
Sensitivity and specificity of recent cardiovascular magnetic resonance perfusion studies on a per-patient basis for detecting coronary arterial luminal narrowings greater than 50%
| Investigators | n | Stress agent | Sensitivity,% | Specificity,% |
|---|---|---|---|---|
| Burgstahler et al. | 23 | Adenosine | 100 | 83 |
| Cheng et al. | 61 | Adenosine | 90 | 67 |
| Cury et al. | 46 | Dipyridamole | 97 | 75 |
| Doyle et al. | 184 | Dipyridamole | 57 | 78 |
| Gebker et al. | 101 | Adenosine | 90 | 71 |
| Greenwood et al. | 35 | Adenosine | 72 | 100 |
| Giang et al. | 44 | Adenosine | 93 | 75 |
| Ishida et al. | 104 | Dipyridamole/exercise | 90 | 85 |
| Kawase et al. | 50 | Nicorandil | 94 | 94 |
| Klein et al. | 54 | Adenosine | 87 | 88 |
| Klem et al. | 92 | Adenosine | 89 | 87 |
| Klem et al. | 147 | Adenosine | 84 | 88 |
| Merkle et al. | 228 | Adenosine | 96 | 72 |
| Meyer et al. | 60 | Adenosine | 89 | 79 |
| Nagel et al. | 84 | Adenosine | 88 | 90 |
| Paetsch et al. | 79 | Adenosine/dobutamine | 91 | 62 |
| Pilz et al. | 171 | Adenosine | 96 | 83 |
| Pilz et al. | 218 | Adenosine | 92 | 100 |
| Plein et al. | 82 | Adenosine | 88 | 74 |
| Seeger et al. | 51 | Adenosine | 92 | 85 |
| Sakuma et al. | 40 | Dipyridamole | 81 | 68 |
| Schwitter et al. | 47 | Dipyridamole | 86 | 70 |
| Takase et al. | 102 | Dipyridamole | 93 | 85 |
| Thiele et al. | 32 | Adenosine | 75 | 97 |
| Thomas et al. | 60 | Adenosine | 93 | 84 |
Data from Hamon et al. [33•]
Perfusion abnormalities with CMR have recently been shown to be associated with abnormalities of fractional flow reserve (FFR) obtained during cardiac catheterization. In 101 patients with suspected angina, Watkins et al. [34] found that CMR perfusion abnormalities were 91% sensitive and 94% specific for determining abnormalities of FFR measured with intracoronary guidewire methods in coronary arteries with stenoses of intermediate severity.
CMR Perfusion Stress in the Emergency Department
Conventional coronary angiography is a well-established technique for diagnosing coronary arterial luminal abnormalities. Importantly however, it is associated with an interventional procedure and exposes patients to risk. For patients at intermediate risk of a cardiovascular event, noninvasive imaging strategies are often preferred due to their more favorable benefit to risk of adverse event profile. To this end, several recent studies have focused on the use of vasodilator stress CMR in patients presenting to the emergency department (ED) with low- or intermediate-risk chest pain.
In 103 patients presenting to the ED with low-risk chest pain as indicated by negative serial electrocardiograms and cardiac biomarkers for myocardial injury, Lerakis et al. [35] demonstrated that the results of adenosine CMR performed within 24 h of arrival to the ED can be used to identify myocardial ischemia. In this study, the negative predictive value of adenosine CMR for identifying myocardial ischemia was 100%. In 277 days of longitudinal follow-up, there were no cardiac deaths, nonfatal acute MIs, rehospitalizations for chest pain, nor coronary revascularization procedures; patients with negative adenosine CMR exhibited an excellent short- and mid-term prognosis.
In the situation of intermediate- to high-risk chest pain with potential acute coronary syndrome (ACS), the results of stress CMR may be used to cost effectively manage patients presenting to the ED. Miller et al. [36•] performed a single-center trial of 110 non–low-risk ACS patients randomized to stress CMR in an observation unit versus standard inpatient care. The observation unit CMR strategy was equivalent to a hospital admission for identifying those with ACS yet was accomplished at a cost savings of $588 per patient (95% CI, $336 to $811). These results suggest CMR stress may be beneficial in managing these presenting to the ED and this form of management may reduce health care costs relative to hospital admission.
Evaluating patients presenting to the ED with suspected ACS, Vogel-Claussen et al. [37] studied the prognostic importance of the size and duration of perfusion defects identified during perfusion stress. In ED patients with chest pain and an intermediate probability for CAD, Vogel-Claussen et al. demonstrated that diffuse subendocardial hypoperfusion defects (<1/2 of the myocardial wall thickness in at least two different coronary artery territories of six beats in duration) were associated with return to the ED with chest pain compared with patients who had no CMR perfusion defect (P=0.02). Thus, whereas large perfusion defects may represent ischemia due to epicardial coronary arterial luminal narrowings, smaller defects may represent a microvascular process associated with recurrent chest pain.
Efficacy of CMR Stress Perfusion in Prior Revascularization
Over the past 5 years, several investigators have focused on the utility of CMR perfusion stress testing for identifying restenosis of coronary arterial segments sustaining prior percutaneous revascularization, or narrowings of implanted coronary arterial conduits. Klein et al. [38] showed in patients after surgical revascularization that the combination of stress perfusion and late gadolinium enhancement (LGE) yields a reasonable diagnostic accuracy for the detection and localization of significant bypass stenoses. However, the sensitivity (77%) of this form of testing was reduced in comparison with published data (84% to 93%) in patients without coronary artery bypass graft surgery.
Bernhardt et al. [39•] performed CMR perfusion stress in those receiving prior percutaneous or bypass revascularization. In patients who previously were treated by percutaneous revascularization, the sensitivity and specificity for the results of adenosine-induced, gadolinium-enhanced firstpass perfusion to detect to identify flow-limiting stenoses were 0.91 and 0.90, respectively. In those sustaining prior coronary artery bypass grafting, they were 0.79 and 0.77, respectively.
Several explanations are possible for these results. First, the timing of myocardial perfusion may differ after bypass surgery as the first-pass kinetics of a contrast bolus may be altered due to the difference in distance that the bolus must travel through native vessels versus bypass grafts to reach the LV myocardium. In addition, after bypass, myocardial perfusion may occur to a greater degree in systole versus diastole (as in native coronary conduits). Second, after bypass surgery, patients may sustain small MIs. Residual effects of small infarcts may confound image interpretation as physicians struggle to differentiate small regions of ischemia versus prior infarcted tissue. Although the etiologies remain uncertain, to date, the recently published literature suggests that the sensitivity of CMR stress perfusion for identifying flow-limiting stenosis after coronary artery bypass grafting is reduced compared with identifying stenosis in native coronary arteries.
Prognosis and Risk Stratification
As with dobutamine stress LV wall motion studies, the results of vasodilator perfusion stress testing have been used to determine adverse cardiac prognosis (Table 2). Bodi et al. [40] evaluated 601 patients with severe ischemia who exhibited dipyridamole stress-induced perfusion deficits and WMAs. After a median follow-up of 553 days, the incidence of MACE (defined as cardiac deaths, nonfatal MIs, and admissions for unstable angina with documented abnormal angiography) was 20% in those with versus 4% in those without a perfusion or WMA. Interestingly in those with a perfusion and wall motion defect, the MACE rate was 39%.
Table 2.
Prognostic evidence base of stress cardiovascular magnetic resonance
| Investigators | n | Stress agent | Study performance | Follow-up | End point |
|---|---|---|---|---|---|
| Dall’Armellina et al. [1] | 200 | Dobutamine | WMA | 5 y | MI/mortality/CHF |
| Wallace et al. [29] | 266 | Dobutamine | WMA | 6.2 y | MI/mortality |
| Walsh et al. [30] | 157 | Dobutamine | WMA | 5.5 y | MI/mortality/CHF |
| Charoenpanichkit et al. [31] | 62 | Dobutamine | WMA | 6 y | MI/mortality |
| Korosoglou et al. [32] | 1,493 | Dobutamine | Perfusion and WMA | 2 y | MI/mortality/revascularization |
| Bodi et al. [40] | 601 | Dipyridamole | Perfusion and WMA | 553 d | MACE/mortality |
| Doesch et al. [41] | 81 | Adenosine | Perfusion | 30 mo | MI/mortality/revascularization |
| Lerakis et al. [35] | 103 | Adenosine | Perfusion | 277 d | MACE/mortality |
| Steel et al. [42] | 254 | Adenosine | Perfusion and LGE | 30 mo | MACE/mortality |
| Husser et al. [43] | 192 | Adenosine | Perfusion and LGE | 655 d | MACE/mortality |
CHF congestive heart failure; LGE late gadolinium enhancement; MACE major adverse cardiac events; MI myocardial infarction; WMA wall motion abnormality
Doesch et al. [41] demonstrated that patients with coronary artery stenoses of angiographic intermediate severity causing a perfusion defect on CMR are at higher risk for MACE within the following 18 months after the procedure. Steel et al. [42] demonstrated complementary prognostic associations with CMR stress myocardial perfusion and LGE imaging in 254 patients with symptoms of myocardial ischemia. Patients with neither a CMR perfusion defect nor LGE exhibited a 98.1% negative annual event rate for cardiac death and MI in 17 months of postprocedure surveillance. In a multivariable analysis including cardiac deaths, acute infarctions, and cardiac hospitalizations, reversible CMR perfusion defects were the most highly associated (hazard ratio, 10.92; P<0.0001) variables for predicting future adverse cardiac events.
In addition to the prognostic utility of visually identified perfusion defects, quantitative analyses of perfusion images have also been performed. In a single-center study of 192 participants, Husser et al. [43] evaluated CMR perfusion images at 1 week and 6 months after ST-segment elevation MI. This group evaluated the imaging using three quantitative (initial slope, maximal signal intensity, and contrast delay in first-pass imaging) and two visual perfusion indexes (hypoenhancement in first-pass and microvascular obstruction in late enhancement imaging). Quantification of infarct mass and visual assessment of the extent of transmural necrosis were also performed. Perfusion quantification was time consuming (P<0.001) and was not superior to visual assessment to predict a future decline in LVEF or MACE (defined as death, reinfarction, or readmission for heart failure [P = not significant]). In addition, from multivariate analyses, only visual assessment of extent transmural necrosis predicted either LVEF or MACE (hazard ratios of 1.3 to 1.4).
Whereas large perfusion defects suggestive of epicardial coronary arterial luminal narrowings forecast a poor cardiac prognosis, smaller, more subendocardial defects may also confer a future adverse cardiovascular outcome. In a retrospective study, Yilmaz et al. [44] evaluated 42 patients who presented with unstable angina who underwent an adenosine-stress perfusion CMR without coronary arterial luminal narrowings ≥50% by contrast coronary angiography. They found that reversible perfusion defects depicted by perfusion CMR imaging occurred in 22 of 42 patients without significant CAD. These were related to coronary epicardial vasospasm in 10 of 42 patients (24%), and microvascular dysfunction (identified with intracoronary acetylcholine infusion) in 20 of 42 patients (48%). The results of this study indicate that there are causes of perfusion defects that are due to processes other than epicardial CAD.
Given the potential clinical utility of CMR stress perfusion, investigators working in the United Kingdom have proposed the CE-MARC (Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease) [45•] study in a population of 750 outpatients presenting with stable angina. In this study, stress CMR perfusion, cine imaging at rest and stress, and CMR coronary angiography will be compared with x-ray coronary angiography, nuclear scintigraphy (SPECT), and exercise tolerance testing. The prognostic value of CMR and its cost effectiveness will also be compared with these modalities. CE-MARC will be the largest prospective trial to date to compare CMR against standard noninvasive investigations for the diagnosis of CAD, and may have important implications for determining the optimal role of stress CMR for managing patients with or suspected of ischemic heart disease.
CMR Methods to Assess Myocardial Oxygenation and Perfusion Without Contrast
Currently, clinical CMR perfusion stress tests involve the administration of gadolinium. Blood oxygen leveldependent (BOLD) CMR has the potential to noninvasively measure myocardial oxygenation without exogenous contrast administration. Vöhringer et al. [46] demonstrated oxygenation-sensitive CMR using a T2*-sensitive steadystate free-precession BOLD sequence in vivo. In seven mongrel dogs, changes in BOLD signal were found proportional to changes in coronary sinus oxygen saturation.
Using a two-compartment model, McCommis et al. [47] demonstrated that CMR-derived calculations of oxygen extraction fraction could be measured at rest and/or during hyperemia induced with intracoronary acetylcholine. These results raise the possibility that a CMR-derived measure of myocardial oxygenation may be useful in identifying regions of myocardial ischemia involving the left ventricle.
Importantly however, Karamitsos et al. [48] recently demonstrated that regional myocardial perfusion and oxygenation may be dissociated, indicating that in patients with CAD, reduced perfusion does not always lead to deoxygenation. Therefore, although BOLD imaging may provide important information relative to myocardial tissue oxygenation, noninvasive, noncontrast methods such as arterial spin labeling (ASL) [49] that directly measure organ perfusion may also be necessary to determine myocardial ischemia. In addition, it is important to recognize that these research initiatives (BOLD and ASL) require high signal to noise and are susceptible to motion artifacts. To enhance signal to noise on these images, investigators have implemented BOLD imaging at 3.0T. In 50 participants, Jahnke et al. [50•] demonstrated reduced oxygenation identified on BOLD imaging was as efficacious as first-pass gadolinium-enhanced contrast results for identifying flow-limiting stenosis.
Conclusions
Stress wall motion or perfusion CMR assessments, are safe, reliable, effective diagnostic methods for identifying ischemia. The prognostic value of stress CMR results has high utility for forecasting future cardiac events. Ongoing research is underway to determine the cost effectiveness of CMR stress testing and to define new, noncontrast methods for measuring myocardial ischemia and injury.
Acknowledgment
Research was supported in part by the following grants from the National Institutes of Health (NIH): RO1HL076438, R33CA1219601, T32HL091824, P30AG21332, and MO1-RR07122.
Footnotes
Disclosure Conflicts of interest: R. Chotenimitkhun: none; W.G. Hundley: serves as a board member, is a consultant for, and has stock in MRI Cardiac Services, Inc.; is employed at Wake Forest University School of Medicine; has received research funding from the NIH, the Susan G. Komen Foundation, and from Astellas Pharmaceuticals; and has served on the speakers’ bureau for Bracco Diagnostics.
Contributor Information
Runyawan Chotenimitkhun, Department of Internal Medicine (Section on Cardiology), Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157-1045, USA.
W. Gregory Hundley, Email: ghundley@wfubmc.edu, Department of Internal Medicine (Section on Cardiology), Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157-1045, USA; Department of Radiology, Wake Forest University School of Medicine, Winston-Salem, NC, USA.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Dall’Armellina E, Morgan TM, Mandapaka S, et al. Prediction of cardiac events in patients with reduced left ventricular ejection fraction with dobutamine cardiovascular magnetic resonance assessment of wall motion score index. J Am Coll Cardiol. 2008;52:279–286. doi: 10.1016/j.jacc.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hundley WG, Morgan TM, Neagle CM, et al. Magnetic resonance imaging determination of cardiac prognosis. Circulation. 2002;106:2328–2333. doi: 10.1161/01.cir.0000036017.46437.02. [DOI] [PubMed] [Google Scholar]
- 3.Hundley WG, Rerkpattanapipat P, Little WC, et al. Relation of cardiac prognosis to segment location with apical left ventricular ischemia. Am J Cardiol. 2003;92:1206–1208. doi: 10.1016/j.amjcard.2003.07.033. [DOI] [PubMed] [Google Scholar]
- 4.Jahnke C, Nagel E, Gebker R, et al. Prognostic value of cardiac magnetic resonance Stress tests: adenosine stress perfusion and dobutamine stress wall motion imaging. Circulation. 2007;115:1769–1776. doi: 10.1161/CIRCULATIONAHA.106.652016. [DOI] [PubMed] [Google Scholar]
- 5.Kuijpers D, van Dijkman PR, Janssen CH, et al. Dobutamine stress MRI. Part II. Risk stratification with dobutamine cardiovascular magnetic resonance in patients suspected of myocardial ischemia. Eur Radiol. 2004;14:2046–2052. doi: 10.1007/s00330-004-2426-x. [DOI] [PubMed] [Google Scholar]
- 6.Hundley WG, Hamilton CA, Thomas MS, et al. Utility of fast cine magnetic resonance imaging and display for the detection of myocardial ischemia in patients not well suited for second harmonic stress echocardiography. Circulation. 1999;100:1697–1702. doi: 10.1161/01.cir.100.16.1697. [DOI] [PubMed] [Google Scholar]
- 7.Schwitter J, Wacker CM, van Rossum AC, et al. MR-IMPACT: Comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J. 2008;29:480–489. doi: 10.1093/eurheartj/ehm617. [DOI] [PubMed] [Google Scholar]
- 8.Schwitter J, Nanz D, Kneifel S, et al. Assessment of myocardial perfusion in coronary artery disease by magnetic resonance: A comparison with positron emission tomography and coronary angiography. Circulation. 2001;103:2230–2235. doi: 10.1161/01.cir.103.18.2230. [DOI] [PubMed] [Google Scholar]
- 9.SCMR: Society for CardiovascularMagnetic Resonance. [Accessed November 2010];Stress and safety equipment. Available at http://www.scmr.org/navigation/CMR-in-specific-circumstances/1462.html.
- 10. Hundley WG, Bluemke DA, Finn JP, et al. ACCF/ACR/AHA/NASCI/SCMR 2010 Expert consensus document on cardiovascular magnetic resonance: A report of the American college of cardiology foundation task force on expert consensus documents. Circulation. 2010;121:2462–2508. doi: 10.1161/CIR.0b013e3181d44a8f. This is a recently expert consensus document on CMR.
- 11.Wahl A, Paetsch I, Gollesch A, et al. Safety and feasibility of high-dose dobutamine-atropine stress cardiovascular magnetic resonance for diagnosis of myocardial ischaemia: experience in 1000 consecutive cases. Eur Heart J. 2004;25:1183–1184. doi: 10.1016/j.ehj.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 12.Kuijpers D, Janssen CH, van Dijkman PR, et al. Dobutamine stress MRI. Part I. Safety and feasibility of dobutamine cardiovascular magnetic resonance in patients suspected of myocardial ischemia. Eur Radiol. 2004;14:1823–1828. doi: 10.1007/s00330-004-2425-y. [DOI] [PubMed] [Google Scholar]
- 13.Pennell DJ. Cardiovascular magnetic resonance and the role of adenosine pharmacologic stress. Am J Cardiol. 2004;94:26D–31D. doi: 10.1016/j.amjcard.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 14.Botvinick EH. Current methods of pharmacologic stress testing and the potential advantages of new agents. J Nucl Med Technol. 2009;37:14–25. doi: 10.2967/jnmt.108.057802. [DOI] [PubMed] [Google Scholar]
- 15.Javadi H, Shariati M, Mogharrabi M, et al. The Association of Dipyridamole Side Effects with Hemodynamic Parameters, ECG Findings, and Scintigraphy Outcomes. J Nucl Med Technol. 2010 Sep;38:149–152. doi: 10.2967/jnmt.109.072629. [DOI] [PubMed] [Google Scholar]
- 16.Karamitsos TD, Arnold JR, Pegg TJ, et al. Tolerance and safety of adenosine stress perfusion cardiovascular magnetic resonance imaging in patients with severe coronary artery disease. Int J Cardiovasc Imaging. 2009;25:277–283. doi: 10.1007/s10554-008-9392-3. [DOI] [PubMed] [Google Scholar]
- 17.Kuijpers D, Ho KY, van Dijkman PR, et al. Dobutamine cardiovascular magnetic resonance for the detection of myocardial ischemia with the use of myocardial tagging. Circulation. 2003;107:1592–1597. doi: 10.1161/01.CIR.0000060544.41744.7C. [DOI] [PubMed] [Google Scholar]
- 18.Garot J, Lima JA, Gerber BL, et al. Spatially resolved imaging of myocardial function with strain-encoded MR: Comparison with delayed contrast-enhanced MR imaging after myocardial infarction. Radiology. 2004;233:596–602. doi: 10.1148/radiol.2332031676. [DOI] [PubMed] [Google Scholar]
- 19.Kim D, Gilson W, Kramer C, et al. Myocardial tissue tracking with two-dimensional cine displacement-encoded MR imaging: Development and initial evaluation. Radiology. 2004;230:862–871. doi: 10.1148/radiol.2303021213. [DOI] [PubMed] [Google Scholar]
- 20.Korosoglou G, Futterer S, Humpert PM, et al. Strain-encoded cardiac MR during high-dose dobutamine stress testing: comparison to cine imaging and to myocardial tagging. J of MRI. 2009;29:1053–1061. doi: 10.1002/jmri.21759. [DOI] [PubMed] [Google Scholar]
- 21.Korosoglou G, Lehrke S, Wochele A, et al. Strain-encoded CMR for the detection of inducible ischemia during intermediate stress. JACC Cardiovasc Imaging. 2010;3:361–371. doi: 10.1016/j.jcmg.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Kelle S, Hamdan A, Schnackenburg B, et al. Dobutamine stress cardiovascular magnetic resonance at 3 Tesla. J Cardiovasc Magn Reson. 2008;10:44–51. doi: 10.1186/1532-429X-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gebker R, Jahnke C, Paetsch I, et al. Diagnostic performance of myocardial perfusion MR at 3T in patients with coronary artery disease. Radiology. 2008;247:57–63. doi: 10.1148/radiol.2471070596. [DOI] [PubMed] [Google Scholar]
- 24.Meyer C, Strach K, Thomas D, et al. High-resolution myocardial stress perfusion at 3T in patients with suspected coronary artery disease. Eur Radiol. 2008;18:226–233. doi: 10.1007/s00330-007-0746-3. [DOI] [PubMed] [Google Scholar]
- 25.Cheng AS, Pegg TJ, Karamitsos TD, et al. Cardiovascular magnetic resonance perfusion imaging at 3-tesla for the detection of coronary artery disease: a comparison with 1.5-tesla. J Am Coll Cardiol. 2007;49:2440–2249. doi: 10.1016/j.jacc.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 26.Thomas D, Strach K, Meyer C, et al. Combined myocardial stress perfusion imaging and myocardial stress tagging for detection of coronary artery disease at 3 Tesla. J Cardiovasc Magn Reson. 2008;10:59. doi: 10.1186/1532-429X-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nandalur KR, Dwamena BA, Choudhri AF, et al. Diagnostic performance of stress cardiac magnetic resonance imaging in the detection of coronary artery disease: a meta-analysis. J Am Coll Cardiol. 2007;50:1343–1353. doi: 10.1016/j.jacc.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 28.Gebker R, Jahnke C, Hucko T, et al. Dobutamine stress magnetic resonance imaging for the detection of coronary artery disease in women. Heart. 2010;96:616–620. doi: 10.1136/hrt.2009.175521. [DOI] [PubMed] [Google Scholar]
- 29.Wallace EL, Morgan TM, Walsh TF, et al. Dobutamine cardiac magnetic resonance results predict cardiac prognosis in women with known or suspected ischemic heart disease. J Am Coll Cardiol Img. 2009;2:299–307. doi: 10.1016/j.jcmg.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walsh TF, Dall’Armellina E, Chughtai H, et al. Adverse effect of increased left ventricular wall thickness on five year outcomes of patients with negative dobutamine stress. J Cardiovasc Magn Reson. 2009;11:25. doi: 10.1186/1532-429X-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Charoenpanichkit C, Morgan TM, Hamilton CA, et al. Left ventricular hypertrophy influences cardiac prognosis in patients undergoing dobutamine cardiac stress testing. Circ Cardiovasc Imaging. 2010;3:126–133. doi: 10.1161/CIRCIMAGING.109.912071. LVH was an independent prognostic marker above and beyond assessments of LV WMA.
- 32. Korosoglou G, Elhmidi Y, Steen H, et al. Prognostic value of high-dose dobutamine stress magnetic resonance imaging in 1493 consecutive patients. Assessment of myocardial wall motion and perfusion. J Am Cardiol Coll. doi: 10.1016/j.jacc.2010.06.020. (In Press) This is the largest study of CMR dobutamine wall motion and perfusion stress.
- 33. Hamon M, Fau G, Née G, et al. Meta-analysis of the diagnostic performance of stress perfusion cardiovascular magnetic resonance for detection of coronary artery disease. J of Cardiovasc Magn Reson. 2010;12:29–38. doi: 10.1186/1532-429X-12-29. This is a meta-analysis comparing the results of stress CMR.
- 34.Watkins S, McGeoch R, Lyne J, et al. Validation of magnetic resonance myocardial perfusion imaging with fractional flow reserve for the detection of significant coronary heart disease. Circulation. 2009;120:2207–2213. doi: 10.1161/CIRCULATIONAHA.109.872358. [DOI] [PubMed] [Google Scholar]
- 35.Lerakis S, McLean DS, Anadiotis AV, et al. Prognostic value of adenosine stress cardiovascular magnetic resonance in patients with low-risk chest pain. J of Cardiovasc Magn Reson. 2009;11:37. doi: 10.1186/1532-429X-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miller CD, Hwang W, Hoekstra JW, et al. Stress cardiac magnetic resonance imaging with observation unit care reduces cost for patients with emergent chest pain: a randomized trial. Ann Emerg Med. 2010 May 27; doi: 10.1016/j.annemergmed.2010.04.009. [Epub ahead of print] This article discusses stress CMR and its economic implication in emergent chest pain.
- 37.Vogel-Claussen J, Skrok J, Dombroski D, et al. Comprehensive adenosine stress perfusion MRI defines the etiology of chest pain in the emergency room: comparison with nuclear stress test. J. Magn. Reson. Imaging. 2009;30:753–762. doi: 10.1002/jmri.21899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klein C, Nagel E, Gebker R, et al. Magnetic resonance adenosine perfusion imaging in patients after coronary artery bypass graft surgery. J Am Coll Cardiol Img. 2009;2:437–445. doi: 10.1016/j.jcmg.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 39. Bernhardt P, Spiess J, Levenson B, et al. Combined assessment of myocardial perfusion and late gadolinium enhancement in patients after percutaneous coronary intervention or bypass grafts: a multicenter study of an integrated cardiovascular magnetic resonance protocol. J Am Coll Cardiol Img. 2009;2:1292–1300. doi: 10.1016/j.jcmg.2009.05.011. This article discusses a multicenter study of an integrated CMR protocol in patients after revascularization.
- 40.Bodi V, Sanchis J, Lopez-Lereu MP, et al. Prognostic and therapeutic implications of dipyridamole stress cardiovascular magnetic resonance on the basis of the ischemic cascade. Heart. 2009;95:49–55. doi: 10.1136/hrt.2007.139683. [DOI] [PubMed] [Google Scholar]
- 41.Doesch C, Seeger A, Doering J, et al. Risk stratification by adenosine stress cardiac magnetic resonance in patients with coronary artery stenoses of intermediate angiographic severity. J Am Coll Cardiol Img. 2009;2:424–433. doi: 10.1016/j.jcmg.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 42.Steel K, Broderick R, Gandla V, et al. Complementary prognostic values of stress myocardial perfusion and late gadolinium enhancement imaging by cardiac magnetic resonance in patients with known or suspected coronary artery disease. Circulation. 2009;120:1390–1400. doi: 10.1161/CIRCULATIONAHA.108.812503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Husser O, Bodi V, Sanchis J, et al. Head to head comparison of quantitative versus visual analysis of contrast CMR in the setting of myocardial stunning after STEMI: implications on late systolic function and patient outcome. Int J Cardiovasc Imaging. 2010;26:559–569. doi: 10.1007/s10554-010-9601-8. [DOI] [PubMed] [Google Scholar]
- 44.Yilmaz A, Athanasiadis A, Mahrholdt H, et al. Diagnostic value of perfusion cardiovascular magnetic resonance in patients with angina pectoris but normal coronary angiograms assessed by intracoronary acetylcholine testing. Heart. 2010;96:372–379. doi: 10.1136/hrt.2009.174367. [DOI] [PubMed] [Google Scholar]
- 45. Greenwood JP, Maredia N, Radjenovic A, et al. Clinical evaluation of magnetic resonance imaging in coronary heart disease: The CE-MARC study. Trials. 2009;10:62. doi: 10.1186/1745-6215-10-62. This article assesses the diagnostic accuracy and prognostic implication of multiparametric CMR.
- 46.Vöhringer M, Flewitt JA, Green JD, et al. Oxygenation-sensitive CMR for assessing vasodilator-induced changes of myocardial oxygenation. J of Cardiovas Magnetic Resonance. 2010;12:20. doi: 10.1186/1532-429X-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCommis KS, O’Connor R, Lesniak D, et al. Quantification of global myocardial oxygenation in humans: initial experience. J of Cardiovasc Magn Reson. 2010;12:34. doi: 10.1186/1532-429X-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karamitsos TD, Leccisotti L, Arnold JR, et al. Relationship between regional myocardial oxygenation and perfusion in patients with coronary artery disease: insights from cardiovascular magnetic resonance and positron emission tomography. Circ Cardiovasc Imaging. 2010;3:32–40. doi: 10.1161/CIRCIMAGING.109.860148. [DOI] [PubMed] [Google Scholar]
- 49.Kazan SM, Chappell MA, Payne SJ. Modelling the effects of cardiac pulsations in arterial spin labelling. Phys Med Biol. 2010;55:799–816. doi: 10.1088/0031-9155/55/3/017. [DOI] [PubMed] [Google Scholar]
- 50. Jahnke C, Gebker R, Manka R, et al. Navigator-gated 3D blood oxygen level-dependent CMR at 3.0-T for detection of stressinduced myocardial ischemic reactions. J Am Coll Cardiol Img. 2010;3:375–384. doi: 10.1016/j.jcmg.2009.12.008. BOLD is a new technique for detection of stress-induced myocardial ischemia.