Abstract
Osteoporosis is a highly prevalent chronic disease in the US and worldwide. The most serious consequence of this disorder is fractures, which have a serious negative impact on quality of life and are often the trigger for accelerated deterioration, ultimately ending in death. Despite the availability of effective preventive treatments, osteoporosis is frequently underdiagnosed and/or undertreated, particularly among the elderly, who are also at greatest risk. In addition, the presence of co-morbid medical conditions may be both a barrier to osteoporosis care and a risk factor for falls; thus individuals with multiple co-morbid conditions may be a particularly high-risk group.
The management of osteoporosis involves improving bone health via adequate nutrition, calcium and vitamin D supplements, and fall prevention strategies. Although these measures are important in the management of all patients, most elderly patients are likely to need additional pharmacological therapy to adequately reduce their fracture risk. Several pharmacological treatments have been shown to significantly reduce the risk of fracture, including bisphosphonates (e.g. alendronate, risedronate, ibandronate, zoledronic acid), denosumab, raloxifene, calcitonin and teriparatide.
Despite recent advances in osteoporosis care, additional action is urgently needed to improve the quality of life of osteoporotic patients in general and of elderly patients in particular, since fracture outcomes are typically poorer in older than in younger patients. This article reviews the current status of osteoporosis management, emphasizing the need to improve osteoporosis care, with a particular focus on the US, by the use of quality- improvement measures and incentives, which might result in an increased awareness and improved treatment for this debilitating disease.
1. The Burden of Osteoporosis
Osteoporosis is a chronic skeletal disease that causes reduced bone mass and deterioration of the bone microarchitecture, resulting in an increased risk of fracture.[1] It has been estimated that 10 million people in the US alone have osteoporosis, with almost 34 million more having low bone mass.[2] The elderly population accounts for most of the burden, with 70% of all fractures sustained by those aged at least 65 years.[3] In 2005, more than 2 million fractures in the US resulted from osteoporosis,[3] which exceeds the yearly incidence of new-onset diabetes, coronary heart disease events, stroke, heart failure, breast cancer and overall cancer cases (figure 1).[3-6]
Fig. 1.
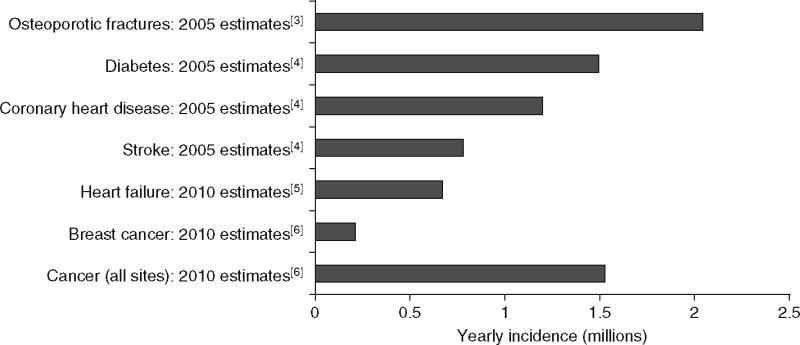
Yearly incidence of common chronic diseases and osteoporotic fractures in the US. [3-6]
2. Mortality and Morbidity Related to Osteoporotic Fractures
Fractures often result in devastating consequences. Approximately 24% of hip fracture patients aged ≥50 years die during the year following their fracture, and 20% of those who were ambulatory before a hip fracture event require subsequent long-term care.[2] A recent study using a random 5% sample of US Medicare beneficiaries reported that the 5-year risk of mortality following a hip fracture was 38% for those aged 65–74 years, 49% for those aged 75–84 years, and 64% for those aged ≥85 years.[7] Among beneficiaries with a clinical (i.e. symptomatic) vertebral fracture, the 5-year risk of mortality for the same age groups was 29%, 36% and 50%, respectively. When co-morbidities such as stroke, diabetes and dementia are taken into account, the risk of death after hip fracture increases much more than for those without co-morbidities at ages >65 years. For example, patients aged >85 years with a hip fracture, without co-morbidities had a 57.4% risk of death, compared with 80.8% of those with dementia.[7]
Fracture outcomes among the elderly are typically poorer than in younger individuals and have been linked with greater mortality, an increased requirement for long-term care, and significant deterioration in health-related quality of life (HR-QOL), as well as an increase in the utilization of healthcare resources.[8] Fractures of the hip, spine and distal forearm can result in 7% of patients losing the ability to carry out the basic activities of daily living, such as dressing or taking care of personal hygiene. A hip fracture can cause a patient to lose the ability to walk and may result in placement in a long-term care facility. It has been estimated that among women who were independent before a hip fracture, half of those who required long-term care after a hip fracture remained in long-term care for a year after the fracture. This also carries the increased risk of some individuals requiring permanent institutionalization.[9]
The Short Form (SF)-36 has been used to measure HR-QOL in women aged 55–75 years who have had hip or other types of fractures. After 2 years, SF-36 scores were significantly lower for women with hip or vertebral fractures compared with age-matched controls, whereas those with other types of fractures had more similar scores.[10]
In light of the high prevalence of osteoporosis among the elderly and the substantial impact of hip and other fractures on quality of life and mortality, the need to identify osteoporosis at a stage prior to fracture is compelling.
3. Osteoporosis: Screening, Diagnosis, and Treatment
Several effective treatments are currently available for osteoporosis. Timely screening, diagnosis and management can reduce the debilitating consequences of the disease.
3.1 Screening and Diagnosis for the Elderly and Those with Co-Morbidities
The general definition of osteopenia established by the World Health Organization (WHO) is a bone mineral density (BMD) level that is 1.0–2.5 standard deviations (SD) below the mean, and osteoporosis is defined as a BMD level ≥2.5 SD below the mean.[1] While these definitions are useful, the sensitivity of this approach is low, and BMD screening and a diagnosis of osteoporosis are unlikely to detect more than 50% of patients experiencing a fracture. Although a low BMD is a strong predictor of the likelihood of fracture, factors other than BMD play a role in fracture risk for those aged >60 years and those with co-morbidities.[2] A 2-year study demonstrated that among women aged 60–79 years who sustained fractures, approximately half did not have a BMD score indicative of osteoporosis;[11] most had only osteopenia. To help identify those patients who would benefit from therapy despite not being classified as having osteoporosis, the WHO has recently developed the FRAX® fracture risk assessment tool. FRAX® weights the relative influence of various clinical risk factors to quantify fracture risk, calculating the likelihood of fracture occurring within a 10-year time frame.[2] It integrates femoral neck BMD with clinical risk factors that are independent of BMD, such as age, sex, race/ethnicity, prior fragility fracture, parental history of hip fracture, current smoking, long-term glucocorticoid use, rheumatoid arthritis or other secondary causes of osteoporosis, and the consumption of >3 alcoholic drinks per day. Although the algorithm can only be applied to treatment-naive patients, it can be used to calculate fracture risk in the absence of BMD testing, using body mass index as a surrogate.[2] Using FRAX® without BMD may be particularly helpful for long-term care patients, or in settings where access to BMD testing is limited. The tool can be used in both sexes and in any race, and gives the 10-year probability of hip fracture or any major osteoporotic fracture. While the FRAX® tool provides an estimation of fracture probability, it does not give an intervention threshold (i.e. the probability at which point intervention is appropriate). Intervention thresholds for a US population are available from the National Osteoporosis Foundation (NOF) and generally recommend prescription treatment if the patient’s predicted 10-year risk of a major fracture (calculated by FRAX®) is ≥20% or their hip fracture risk is ≥3%.[12] An important issue that is not addressed by FRAX® is the increased risks for falls as people age.[13,14] Several factors such as poor eyesight, decreased muscle strength and poor balance can contribute to increased falls in those aged >65 years. Age-associated co-morbidities such as stroke, Parkinson’s disease, dementia and vitamin D deficiency have also been shown to result in increased falls. Furthermore, some medications that treat co-morbidities may also contribute to the risk of falls, such as drugs that can cause postural hypotension. The Garvan model is another tool that can be used to predict the risk of fractures and may be a valuable additional tool for use in assessing the risk of fracture in geriatric patients.[15] The Garvan model is less complicated than FRAX® with respect to patient history, but takes into account the history and number of recent falls and the number of previous fractures the patient has experienced. Compared with the Garvan model, the 10-year fracture risk may be underestimated using FRAX®, especially in geriatric patients at greater risk of falls.
In addition to BMD testing where appropriate, NOF guidelines recommend that patients should be screened for secondary causes of osteoporosis,[2] including lifestyle factors (e.g. alcohol overuse, smoking, and calcium and vitamin D deficiency),[14,16] conditions and diseases that cause or contribute to osteoporosis (e.g. hypogonadism, gastrointestinal conditions and renal disease),[17-19] and concomitant use of medications with adverse skeletal effects (e.g. cancer treatments, proton pump inhibitors, peroxisome proliferator-activated receptor [PPAR]-γ activators and corticosteroids).[18-20] Where possible, these factors should be addressed, for example, reversal of vitamin D deficiency, using diet or supplementation with ergocalciferol 50 000 IU once or twice weekly for several months.
3.2 Management of Osteoporosis
The NOF released updated recommendations to clinicians on the monitoring and management of osteoporosis, including pharmacological treatment guidelines that are partially based on the US-adapted FRAX® tool (table I).[2] These guidelines are valuable for establishing the best management strategies for osteoporosis, but they do not necessarily take into account the complications associated with co-morbidities in those aged >65 years.[21] In an analysis of Medicare beneficiaries in 1999, 48% of those aged ≥65 years had ≥3 co-morbid conditions, and 21% had ≥5 co-morbid conditions.[22] Some of the common conditions found in those aged >65 years include diseases such as chronic obstructive pulmonary disease, which requires medication such as glucocorticoids, which exacerbate osteoporosis. Other conditions such as Parkinson’s disease may increase the risk of falls. Unfortunately, guidelines that provide information on how to optimize overall health in the presence of multiple medical conditions are not yet available, but the recent investment in comparative effectiveness research may soon fill this gap.
Table I.
National Osteoporosis Foundation major recommendations for the clinician (adapted with permission from the National Osteoporosis Foundation,[2] Washington, DC 20036. All rights reserved)
| Recommendations apply to postmenopausal women, and men aged ≥50 years |
| – Counsel on the risk of osteoporosis and related fractures |
| – Check for secondary causes of osteoporosis |
| – Advise on adequate intakes of calcium (≥1200 mg per day) and vitamin D (800 –1000 IU per day), including supplements if necessary |
| – Recommend regular weight-bearing and muscle-strengthening exercise to reduce the risk of falls and fractures |
| – Advise avoidance of tobacco smoking and excessive alcohol intake |
| – In women aged ≥65 years and men aged ≥70 years, recommend BMD testing |
| – In men aged 50–69 years and postmenopausal women, recommend BMD testing when there is concern based on the patient’s risk factor profile |
| – Recommend BMD testing to patients who have suffered a fracture, to determine the degree of disease severity |
| – Initiate treatment in patients with hip or vertebral (clinical or morphometric) fractures |
| – Initiate treatment in patients with BMD T-scores of –2.5 or less at the femoral neck, total hip or spine by DXA, after appropriate evaluation |
| – Initiate treatment in postmenopausal women and men aged ≥50 years with low bone mass (T-score –1 to –2.5, osteopenia) at the femoral neck, total hip or spine and a 10-year hip fracture probability of ≥3% or a 10-year major osteoporotic fracture probability of ≥20%, based on the US-adapted FRAX® fracture risk assessment tool |
| – Current US FDA-approved pharmacological options for osteoporosis prevention and/or treatment are bisphosphonates (alendronate, risedronate, ibandronate and zoledronic acid), calcitonin, oestrogens and/or hormone therapy, oestrogen agonist/antagonist (raloxifene) and parathyroid hormone (teriparatide) |
| – BMD testing performed in DXA centres using accepted quality-assurance measures is appropriate for monitoring bone loss. For patients on pharmacotherapy, BMD assessment is typically performed 2 years after initiating therapy and at 2-year intervals thereafter; however, more frequent testing may be warranted in certain clinical situations |
BMD = bone mineral density; DXA = dual-energy x-ray absorptiometry; FDA = Food and Drug Administration.
While it should be remembered that only a minority of falls result in a fracture, most osteoporotic fractures occur after a fall; thus, fall prevention measures can reduce fracture risk.[2] A recent Cochrane review concluded that home safety interventions have questionable effectiveness, but exercise interventions reduced the risk of falls in the elderly.[23] Insufficiencies in dietary calcium and vitamin D are more pronounced in the elderly and may lead to an increase in falls and fracture risk; treatment of such insufficiencies is generally well tolerated in older patients although caution must be exercised with those who may be prone to kidney stones or who have kidney disease. A number of studies and meta-analyses have indicated that vitamin D supplementation can result in a reduction in falls and fall-related fractures. In a meta-analysis of randomized controlled trials, oral vitamin D with additional calcium demonstrated a significant 18% reduction in hip fracture risk compared with no treatment.[24] Another meta-analysis of 45 trials examined vitamin D and calcium for the prevention of fractures in men and postmenopausal women aged >65 years.[25] Results from this Cochrane analysis indicated that although vitamin D alone is unlikely to prevent fractures, treatment with vitamin D with calcium may reduce fractures in frail elderly residents in institutions. Other studies, however, have failed to find that vitamin D supplementation results in a reduction in falls.[26-31] In one randomized, open-label, secondary prevention trial conducted in 3314 women aged ≥70 years with one or more risk factors for hip fracture, for example, no significant difference in clinical fracture rates at 2 years was found between patients receiving daily oral supplementation with vitamin D (800 IU cholecalciferol) and calcium (1000 mg calcium) compared with controls. [27] The odds ratio of a fall was 0.99 (95% CI 0.81, 1.20) at 6 months and 0.98 (95% CI 0.79, 1.20) at 12 months. In a randomized controlled trial conducted in 3717 elderly care home residents (mainly women aged around 85 years), patients were randomized to a group receiving vitamin D supplementation (equivalent to a daily dose of 1100 IU) or to a control group.[30] No significant difference was found between the vitamin D and control groups; after 10 months, 64 (3.6%) of 1762 vitamin D-treated residents and 51 (2.6%) of 1955 residents in the control group had one or more non-vertebral fractures, and 24 (1.3%) of the vitamin D-treated residents and 20 (1.0%) of the control-group residents had a hip fracture. A total of 44% of the vitamin D-treated and 43% of control group patients had experienced a fall. A recently published review of the literature from the past 15 years proposed that vitamin D had a beneficial effect on postural adaptations (i.e. muscles and central nervous system) that might explain the observation in some studies of a reduction in falls and bone fracture rates.[32] In general, however, interpreting the findings of studies investigating calcium and vitamin D for falls and fracture prevention is likely to be complicated by the heterogeneity of study populations, treatment doses and formulations, baseline intake and compliance with treatment.[33]
Given the importance of fall prevention in the elderly, studies have been conducted on multifactorial risk assessment for falls.[34] By assessing risk, interventions can be implemented to reduce the incidence of falls. In a meta-analysis of 19 studies that assessed multifactorial fall prevention measures, it was shown that evidence of the effectiveness of multifactorial fall prevention programmes in primary-care, community-care or emergency-care settings is limited, and further studies are required to show their effectiveness.
Aside from hormone therapy, which is probably less appropriate for geriatric patients, the available pharmacological treatments for osteoporosis include antiresorptive agents that reduce bone loss, anabolic agents that increase bone formation, and denosumab. Antiresorptive agents that have been approved by the US Food and Drug Administration (FDA) include bisphosphonates (alendronate, risedronate, ibandronate and zoledronic acid), the oestrogen agonist/antagonist raloxifene, and calcitonin. Teriparatide [recombinant human parathyroid hormone analogue (1–34)] is the only anabolic agent approved for the treatment of osteoporosis.[2] Absolute risk reduction (ARR) at key skeletal sites for each of the medications approved for osteoporosis treatment is summarized in table II.[35-48] Efficacy and safety data for these agents are presented below. In addition, two trials with strontium ranelate, which has not been approved by the US FDA, are included in the table. Although the mechanism of action of strontium ranelate is not completely understood, it may act by reducing bone resorption while maintaining bone formation.[14] In women aged >80 years, strontium ranelate treatment has resulted in a significant reduction in vertebral and non-vertebral fracture risk over 5 years and also showed cost benefits.[49] It should be remembered, however, that ARR (like number needed to treat [NNT]) is dependent on the severity of disease in recruited patients and is not necessarily a true reflection of drug efficacy.
Table II.
Osteoporosis therapies: absolute risk reduction at key skeletal sites
| Therapy | Study population
|
Study follow-up (y) | ARR (fracture incidence in treatment group vs placebo) [%]
|
|||
|---|---|---|---|---|---|---|
| Mean age (range) [y] | Prior fractures | Vertebral | Non-vertebral | Hip | ||
| Alendronate | ||||||
| Black et al.[35] | 71 (55–81) | Included | 3 | 7 (8 vs 15) | NS | 1.1 (1.1 vs 2.2) |
| Cummings et al.[36] | 68 (54–81) | Excluded | 4 | 1.7 (2.1 vs 3.8) | NS | NS |
| Pols et al.[37] | 63 (<85) | NE | 1 | NE | 2 (2.4 vs 4.4) | Not specified |
| Risedronate | ||||||
| Harris et al.[38] | 69 (<85) | Included | 3 | 5 (11.3 vs 16.3) | 3.2 (5.2 vs 8.4) | Not specified |
| Reginster et al.[39] | 71 (≤85) | Included | 3 | 10.9 (18.1 vs 29.0) | NS | Not specified |
| McClung et al.[40] | 74 (70–79); 83 (≥80) | Included | 3 | NE | 1.8 (9.4 vs 11.2) | 1.1 (2.8 vs 3.9) |
| Ibandronate | ||||||
| Chesnut et al.[41] | 69 (55–80) | Included | 3 | 4.9 (4.7 vs 9.6) | NS | NE |
| Zoledronic acid | ||||||
| Black et al.[42] | 73 (65–89) | Included | 3 | 7.6 (3.3 vs 10.9) | 2.7 (8.0 vs 10.7) | 1.1 (1.4 vs 2.5) |
| Lyles et al.[43] | 75 (≥50) | Included | 2 | 2.1 (1.7 vs 3.8)a | 3.1 (7.6 vs 10.7) | NS |
| Calcitonin | ||||||
| Chesnut et al.[44] | (68–69) | Included | 5 | 8 (18 vs 26) | NS | NS |
| Raloxifene | ||||||
| Ettinger et al.[45] | (65–69) | Included | 3 | 3.5 (6.6 vs 10.1) | NS | NS |
| Teriparatide | ||||||
| Neer et al.[46] | (69–71) | Included | 1.8 | 9 (5 vs 14) | 3 (3 vs 6) | Not specified |
| Strontium ranelateb | ||||||
| Meunier et al.[47] | 69 (≥50) | Included | 3 | 11.9 (20.9 vs 32.8) | NS | Not specified |
| Reginster et al.[48] | 76 (≥74, or ≥70 with1 extra fracture risk factor) | Included | 3 | 6.3 (7.7 vs 14) | 1.7 (11.2 vs 12.9) | NS |
Clinical vertebral fracture.
Not US FDA approved.
ARR = absolute risk reduction; FDA = Food and Drug Administration; NE = not evaluated; NS = not significant.
A newly approved treatment for osteoporosis is denosumab, which is a human monoclonal antibody targeting the receptor activator of nuclear factor kappa-B ligand (RANKL), that is a highly effective antiresorptive agent.[50] In phase II and III clinical trials, denosumab was shown to be effective, with a safety profile that is similar to bisphosphonates, although long-term safety data are not yet available. Denosumab treatment resulted in increases in BMD in the total hip (0.6%), femoral neck (1.0%), greater trochanter (1.1%) and lumbar spine (1.1%) compared with alendronate (p ≤ 0.0002).[51] In women aged 60–90 years with a BMD T-score of −2.5 or less, denosumab reduced the risk of new vertebral fracture and hip fracture compared with placebo by 68% (p < 0.001) and 40% (p = 0.04), respectively.[52] Denosumab also reduced the risk of non-vertebral fracture by 20% compared with placebo (p = 0.01). The long-term significance of this new treatment in those aged >75 years may require additional analysis of efficacy and safety in this population.
Most trials evaluating the antifracture efficacy of anti-osteoporotic drugs are in postmenopausal women aged <80 years.[8] Results for fracture incidence following 3 years of osteoporosis treatment from several post hoc analyses of trials in older women are shown in table III.[40,49,53-55] These findings indicate that osteoporotic agents can achieve significant reductions in vertebral and non-vertebral fracture risk in women aged 70–100 years.[40,49,53-55]
Table III.
Three-year, post hoc analyses of treatment benefits for women aged >70 years
| Treatment | Age (y) | Fracture incidence (%) [treatment vs placebo] (ARR %) | p-Value |
|---|---|---|---|
| Zoledronic acid[53] | ≥75 | Vertebral: 1.1 vs 3.7 (2.6) | <0.001 |
| Non-vertebral: 9.9 vs 13.7 (3.8) | 0.002 | ||
| Any: 10.8 vs 16.6 (5.8) | <0.001 | ||
| Risedronate[40,54] | 70–79 | Hip: 1.9 vs 3.2 (1.3) | 0.009 |
| ≥80 | Vertebral: 18.2 vs 24.6 (6.4) | 0.003 | |
| Teriparatide[55] | ≥75 | Vertebral: 5.2 vs 15.1 (9.9) | <0.05 |
| Strontium ranelate[49] | 80–100 | Vertebral: 19.1 vs 26.5 (7.4) | 0.013 |
| Non-vertebral: 14.2 vs 19.7 (5.5) | 0.011 |
ARR = absolute risk reduction.
Long-term adherence with osteoporosis drugs, similar to the situation with treatment for many chronic conditions without obvious symptoms or benefits from treatment, is known to be problematic. While the bisphosphonates, for example, have proven fracture reduction efficacy, optimal treatment effects in individual patients (and cost effectiveness from a public health perspective) are dependent on long-term patient adherence.[56] Approaches such as extending oral dosing intervals and the development of intravenous rather than oral administration have been explored in an effort to improve long-term adherence. Several osteoporosis treatments can now be taken on a monthly basis, which may be helpful in elderly patients who are likely to be receiving multiple daily medications for other chronic conditions. Intravenous bisphosphonate formulations also provide alternative administration options (3-monthly and yearly administration). While treatment decisions tend to be based primarily on antifracture efficacy, individual patient circumstances and preferences should be taken into account when tailoring therapy to maximize adherence in individual patients, particularly among the elderly, who may have additional limitations (e.g. memory disorders) and be already taking multiple medications.
Cochrane reviews have assessed the efficacy of alendronate and risedronate for the prevention of osteoporotic fractures in postmenopausal women and included women aged 50–90+ years.[57,58] In a review of 11 trials, alendronate treatment was associated with a significant and clinically important reduction in the risks of vertebral (ARR 6%), non-vertebral (ARR 2%), hip (ARR 1%) and wrist (ARR 2%) fractures among women with BMD T-scores of −2 or less and/or at least one vertebral compression fracture.[57] In a review of seven trials, risedronate treatment was found to result in a significant and clinically important reduction in the risks of vertebral (ARR 5%), non-vertebral (ARR 2%) and hip (ARR 1%) fractures in women with BMD T-scores of −2 or less and/or at least one vertebral compression fracture.[58]
In addition to efficacy, a well established tolerability and long-term safety profile are important in treatments for chronic diseases. Bisphosphonates are generally well tolerated, although oral formulations are associated with gastrointestinal tract intolerance. Unfortunately, the use of proton pump inhibitors to treat such symptoms may diminish the effectiveness of the bisphosphonate.[59] Acute-phase reactions have been reported with weekly and monthly oral and intravenous bisphosphonates in approximately 5% of patients, although these symptoms usually resolve within a few days.[42,60] The long-term safety of alendronate and risedronate has been established in studies with follow-up for up to 10 and 7 years of treatment, respectively.[61,62] As the mean age of the patients in the alendronate study was 73 years and the mean age of the patients in the risedronate study was 69 years, these safety results might not apply to the very elderly.[61,62] Safety data for ibandronate and zoledronic acid have not been reported beyond 3 years in the peer-reviewed literature.[41,42]
Osteonecrosis of the jaw is a rare condition that has been associated with high-dose or long-term bisphosphonate therapy, but has a low incidence when bisphosphonates are used in the treatment of osteoporosis.[2] Another potential risk associated with long-term bisphosphonate therapy is that of atypical fractures of long bones with no or very-low-impact trauma.[63] Awareness of the possibility of atypical femoral fractures associated with bisphosphonate use was initially raised by the publication of a number of case reports.[64-66] These, in turn, generated concern that these agents might result in oversuppression of bone turnover leading to impaired bone remodelling, accumulation of microdamage in bone and increased skeletal fragility.[67-69] Findings from retrospective studies supported this possibility,[70,71] although a register-based national cohort study found that the ratio of classical to atypical fractures was identical in bisphosphonate-treated individuals and matched, untreated controls.[72] The American Society for Bone and Mineral Research (ASBMR) appointed a task force to address key questions related to this problem; this multidisciplinary group reviewed the data and developed a case definition to help with further identification and diagnosis.[73] It was concluded that, based on currently available data and the widespread use of bisphosphonates, the incidence of bisphosphonate-associated atypical femoral fractures appears to be very low, particularly compared with the number of fractures that are prevented by this therapy. Furthermore, a causal association between these agents and atypical fractures has not been established. It would appear, however, that risk rises with increasing duration of exposure, and awareness of the problem should be maintained and further research conducted. For patients who have experienced an atypical fracture while receiving long-term bisphosphonate treatment, switching to teriparatide treatment may be an alternative.[74]
There have been a number of case reports of oesophageal cancer among patients receiving oral bisphosphonate therapy, with a median time from use to diagnosis of 1–2 years.[75] In contrast to these case reports, two analyses, one using data from European national registries and the second from a US Medicare database, have not shown an increased risk of oesophageal cancer among individuals who were receiving oral bisphosphonates compared with those who were not.[76,77] In view of the conflicting findings from studies evaluating this risk, the FDA issued a Drug Safety Communication in July 2011, announcing that it was undertaking an ongoing safety review of bisphosphonates and the potential increased risk of oesophageal cancer.[78] At this time, however, the FDA believes that the benefits of oral bisphosphonate drugs in reducing the risk of serious fractures in people with osteoporosis continue to outweigh their potential risks. The FDA’s review is ongoing and the Agency has not concluded that patients taking oral bisphosphonate drugs have an increased risk of oesophageal cancer.
Hypocalcaemia is at least a theoretical risk when patients are treated with bisphosphonates[79] and could be related to vitamin D insufficiency; it has been suggested that patients be pretreated with supplementary calcium and vitamin D before treatment with bisphosphonates is initiated, especially for those at increased risk of vitamin D deficiency, patients with chronic kidney disease, or those with Paget’s disease.[80] As with other pharmacological therapies, treatment with bisphosphonates should be offered to patients in whom the potential benefits of treatment outweigh the risks. However, in light of the substantial risk of fracture for older patients with osteoporosis, the risk-to-benefit balance will likely favour treatment.
Raloxifene has shown efficacy in the prevention and treatment of vertebral fractures in postmenopausal women (ARR 3.5% following 3 years of follow-up), but has not demonstrated efficacy in the reduction of non-vertebral fractures;[45] it is indicated for the treatment and prevention of osteoporosis in postmenopausal women.[81] A total of 15.5% of the patients in placebo-controlled clinical studies of raloxifene were aged ≥75 years; no overall differences in safety or effectiveness were observed between these and younger participants.[81] Tolerability and safety data for raloxifene have been reported for up to 8 years of treatment. A common adverse effect of raloxifene is an increased incidence of hot flashes.[82] An increased risk of deep-vein thrombosis, pulmonary embolism and fatal stroke has also been documented.[45,82] However, raloxifene has the extraskeletal benefit of reducing the risk of invasive breast cancer, so the risk-to-benefit ratio of treatment should be carefully considered for each patient.[83] Approximately 39% of the patients in the study by Barrett-Connor et al.,[82] investigating the effects of raloxifene on cardiovascular events and breast cancer, were aged ≥70 years, suggesting that these raloxifene safety data are applicable to the older patient.
Calcitonin nasal spray has shown efficacy in the treatment of vertebral fractures (ARR 8% following 5 years of follow-up), but has not demonstrated efficacy in reducing non-vertebral fractures;[44] it is indicated for the treatment of postmenopausal osteoporosis in women greater than 5 years post menopause.[84] Calcitonin nasal spray is well tolerated, with a safety profile that has been established for up to 5 years of treatment; common adverse effects include nasal irritation.[44] Although approximately a fifth of the patients in the long-term calcitonin study were aged ≥75 years, there were no differences in safety reported for those who were aged <75 years compared with those aged ≥75 years.
Teriparatide has shown efficacy in the treatment of both vertebral and non-vertebral fractures (ARR 9% in vertebral and 3% in non-vertebral fractures following 1.8 years of follow-up); its efficacy in the treatment of hip fractures remains unclear.[46] It is indicated for the treatment of postmenopausal women at high risk for fracture.[85] A total of 75% of the women participating in clinical trials were aged ≥65 years and 23% were aged ≥75 years; no overall differences in safety or effectiveness were observed between the young and older participants.[85] Teriparatide is well tolerated, although some patients experience leg cramps and dizziness. Because of safety concerns regarding the development of osteosarcoma in animal studies, teriparatide is only indicated for those at high risk of fracture and for a maximum of 2 years. It is contraindicated in patients with a history of radiation therapy of the skeleton, skeletal malignancy or hypercalcaemia.[2]
Osteoporosis treatments are sometimes costly, and this often represents a common barrier to therapy. Alendronate is now available as a generic medication, which may assist in attenuating the impact of cost. Additional generic bisphosphonates will likely become available within the next few years. Reimbursement options associated with different medication formulations (e.g. intravenous vs oral bisphosphonates) combined with secondary medical insurance can also reduce out-of-pocket costs for many patients.
4. Osteoporosis and the Need to Improve Quality of Care: Benefits and Risks of Pharmacological Therapy
Osteoporosis is asymptomatic until a fracture occurs and, therefore, may not receive the clinical attention that other chronic diseases are likely to command. Although some improvements have occurred in the quality of osteoporosis care, the disease remains largely underdiagnosed and undertreated. A recent US study reported that the proportion of individuals aged ≥65 years who had been assessed for BMD at any time over a 7-year period was just 31% for Caucasian women, 15% for African-American women and <5% for men.[83] Even among those who suffer a fracture, diagnosis is underutilized. Only 22% of elderly patients who suffer a fracture receive BMD testing or pharmacological treatment in the 6 months following the fracture, yet most of these fractures are attributable to osteoporosis, and an osteoporotic fracture is a dominant risk factor for subsequent fracture.[86]
The National Committee for Quality Assurance has developed a Health Plan Employer Data and Information Set measure for osteoporosis. This can be used to compare health system data on the number of women aged ≥67 years who suffered a fracture and received either BMD testing or a prescription for osteoporosis treatment in the 6 months following fracture.[86] In 2006, only 22% of all fractures led to BMD testing or pharmacological treatment, and the proportion of patients tested or treated has not meaningfully increased in more recent years. The NOF recommends that BMD testing should be done in women aged ≥65 years and in men aged ≥70 years. Testing should be done earlier if the patient has other risk factors for osteoporosis or if they have had a fracture.[2]
Osteoporosis treatment rates have also been shown to be low in elderly patients referred to receive home therapy for recent fracture, with only 35% of patients prescribed medications or recommended over-the-counter supplements.[87] In addition, higher degrees of medical co-morbidity appeared to be associated with a decrease in the likelihood of receiving prescription medications.[87] Although osteoporosis prevalence and fracture risk are significantly higher among elderly patients living in residential or nursing homes, diagnosis and treatment in this population are inadequate. A study of residents in 34 nursing homes in the US state of Maryland indicated that BMD values in these individuals were 15% lower than those in others in the community; in female residents, the prevalence of osteoporosis was 79%, rising to 86% in women aged ≥85 years.[88] Despite this prevalence, BMD testing does not occur routinely in long-term care settings, and even when a diagnosis of osteoporosis is established, a large proportion of patients are not treated.[86] Treatment of osteoporosis in this population has been shown to be effective.[89] In a study of ambulatory female residents aged >65 years (mean age 78.5 years) with a T-score of −2.0 or less at the posterior–anterior lumbar spine or total hip, significant increases in BMD at all sites measured over a 24-month period were seen after treatment with alendronate compared with placebo.[90] In this study, alendronate was well tolerated, with incidences of gastrointestinal adverse effects that were similar in the treated and placebo groups. This study and others suggest that treatment of patients with osteoporosis in long-term care facilities is efficacious.[90] The barriers to providing osteoporosis care include multiple patient co-morbidities, reimbursement issues, length of stay, regulatory oversight, wheelchair use, cognitive impairment, depression and swallowing difficulties.[91] Cognitive impairment and polypharmacy can create challenges for adherence to medications, especially in those patients with multiple co-morbidities.[21]
Low treatment rates are not related to a lack of efficacy of available treatments. Compared with therapies for other chronic diseases, osteoporosis medications have been shown to have at least equivalent, if not better, effectiveness. Reductions in fracture risk with osteoporosis therapies are in the range of 30–70% for vertebral fractures,[42,45] 20–53% for non-vertebral fractures[46,57] and 26–53% for hip fractures.[57,58] In comparison, tamoxifen has been reported to reduce breast cancer recurrence by 21–47%[92] and statins are associated with a 22–27% reduction in the incidence of cardiac events.[93] Two meta-analyses examined the efficacy of alendronate and risedronate, respectively, in the prevention of osteoporotic fractures in postmenopausal women aged 50–90+ years.[57,58] These analyses indicated that there were no treatment differences associated with increasing age. Furthermore, in one study of 7705 postmenopausal women aged 31–80 years (mean age 67 years), the reduction in vertebral fracture risk associated with raloxifene treatment was similar in tertiles of age.[45] As the event rates for fractures are higher in older people, the ARR with osteoporosis medication often exceeds the ARR for other therapies. Coupled with the high 1-year mortality rate following osteoporosis fractures, which is typically much higher than the 1-year mortality following a diagnosis of breast cancer or a heart attack, the case for treating osteoporosis in elderly patients is compelling.
4.1 Treating Osteoporosis among Elderly Patients with Other Chronic Conditions: Evaluating the Expected Benefits of Treatment
Although the consequences of a fracture in the elderly are often devastating, there may still be reluctance on the part of the physician to prescribe osteoporosis medications, possibly stemming from an expectation of a lack of treatment benefit for the older patient. The elderly have competing medical co-morbidities with associated polypharmacy, and may be assumed to have a short life expectancy that would obviate the need for osteoporosis treatment late in life. It has been estimated that the average additional life span for a person aged 75 years or 85 years in the US is 11.8 or 6.8 years, respectively.[94] Most drug therapies for osteoporosis show significant decreases in vertebral fracture rates as early as 6 months to 1 year after initiation of treatment,[42,95,96] suggesting that for many patients, the time to reach effective treatment is much shorter than the expected life span. Furthermore, the magnitude of risk of a second fracture may be underestimated. A recent study of Medicare beneficiaries evaluated the risk of a second fracture versus death in the 5 years following a hip, clinical vertebral or wrist/forearm fracture.[7] In patients aged 74–84 years, the risk of a second fracture following a hip fracture was 26%, compared with a mortality risk of 49%; for clinical vertebral fractures, the risks were 39% and 36%, respectively. In patients aged >85 years, the risk of mortality increased dramatically compared with the risk of a second fracture; the risk of a second fracture after a hip fracture was 23% and the mortality risk was 64%; for clinical vertebral fractures, the risks were 36% and 50%, respectively.[7] Overall, although the 5-year mortality risk usually exceeded the risk of a second fracture, the 5-year risk of a second fracture was considerable (13–43%) and generally fell into a range where patients are recommended to receive prescription osteoporosis medications.[12] As a consequence of the effectiveness of treatments available to prevent a second fracture, treatment is almost always cost effective, especially in those aged >60 years.[11] Furthermore, the cost effectiveness of treating osteoporosis increases with age.[14]
The benefit of preventive screening and treatment of chronic diseases in the elderly is less clear than for other age groups, since the frail elderly and the very old have often been excluded from clinical trials. For example, there is substantial disagreement about whether chemotherapy is used appropriately in terminally ill, elderly patients, because of cost, test-related morbidity, and effects on survival and quality of life.[97] Similarly, physicians in the osteoporosis field have to make individualized patient management decisions on preventive screening and treatment of the very old or the frail. Fortunately, data on the NNT to prevent fracture are available, and compare favourably to many well accepted preventive treatments. In a study by Curtis et al.,[7] the NNT to prevent a fracture for almost all demographic and fracture groups are comparable to the NNT for accepted secondary prevention strategies, such as aspirin after myocardial infarction (MI), statins after MI or β-blockers after MI.[98] Specifically, for Caucasian women aged 75–84 years with a clinical vertebral fracture, taking an oral bisphosphonate for 5 years, the NNT to prevent one additional subsequent fracture is 7.7. The largest NNT was 45.6 for African-American men aged 75–84 years with a hip fracture. In comparison, aspirin treatment after MI has been shown to have an NNT of 150, statins after MI have an NNT of 94, and β-blockers after MI have an NNT of 54.[98] Indeed, these data support the proposition that individuals of any age or with any co-morbidities should receive adequate screening and care to prevent fractures, unless there is a very high expectation of mortality within 6–12 months. It should be remembered, however, that NNT (like ARR) is dependent on the severity of disease in recruited patients and is not necessarily a true reflection of drug efficacy.
Another dimension that can aid in the treatment decision is whether treatment improves quality of life, especially for those who have suffered any kind of fracture. In a study of quality-of-life improvement in patients treated with zoledronic acid, Adachi et al.[99] showed that zoledronic acid treatment significantly improved HR-QOL, as assessed using the EuroQol (EQ)-5D visual analogue scale (VAS), in patients with low-trauma hip fracture. The mean change (± SE) from baseline in EQ-5D VAS score was greater for zoledronic acid versus placebo in all patients (7.67 ± 0.56 vs 5.42 ± 0.56), but was greatest in patients with clinical vertebral fractures (8.86 ± 4.91 vs −1.69 ± 3.42) compared with non-vertebral fractures (5.03 ± 2.48 vs −1.07± 2.16).
4.2 Osteoporosis Quality-Improvement Measures and Incentives to Improve Quality of Care
To address the suboptimal nature of osteoporosis care in the US, several quality-improvement measures have been implemented at local and national levels, with variable degrees of success. In 2005, the American Orthopaedic Association instigated a pilot initiative for the prevention of secondary fractures.[100] This was a hospital-based, quality-improvement approach aimed at preventing future fractures and improving physician and patient education. Improvements occurred in the percentage of patients who received advice about calcium and vitamin D supplementation, exercise, and fall prevention, but the intervention did not increase the proportion of patients receiving BMD testing or prescribed pharmacological therapy.[100] Another approach using electronic medical record reminders, which give patient-specific advice to providers caring for post-fracture patients, resulted in over half of patients receiving either BMD testing or osteoporosis treatment, compared with 6% in the control group.[101]
The Physician Quality Reporting Initiative, authorized by the US 2006 Tax Relief and Health Care Act, was established and implemented by the Centers for Medicare and Medicaid Services. The scheme included a bonus payment for eligible professionals who satisfactorily reported data on three or more of the chosen applicable quality measures, which included 153 quality measures, of which six are relevant to osteoporosis (table IV).[102]
Table IV.
The 2010 Physician Quality Reporting Initiative: osteoporosis-related measures[102]
| Quality measure | Key quality indicator |
|---|---|
| Communication with the physician managing on-going care post-fracture | Percentage of patients aged ≥50 years treated for a hip, spine or distal radial fracture with documentation of communication with the physician managing the patient’s ongoing care that a fracture occurred and that the patient was or should be tested or treated for osteoporosis |
| Screening or therapy for women aged ≥65 years | Percentage of female patients aged ≥65 years who have a central DXA measurement ordered or performed at least once since age 60 years, or pharmacological therapy prescribed within 12 months |
| Management following fracture | Percentage of patients aged ≥50 years with fracture of the hip, spine or distal radius who had a central DXA measurement ordered or performed, or pharmacological therapy prescribed |
| Pharmacological therapy | Percentage of patients aged ≥50 years with a diagnosis of osteoporosis who were prescribed pharmacological therapy within 12 months |
| Falls: risk assessment | Percentage of patients aged ≥65 years with a history of falls who had a risk assessment for falls completed within 12 months |
| Falls: plan of care | Percentage of patients aged ≥65 years with a history of falls who had a plan of care for falls documented within 12 months |
DXA = dual-energy x-ray absorptiometry.
Despite some federal initiatives to improve osteoporosis care, other federal actions have created barriers. For example, the Centers for Medicare and Medicaid Services substantially reduced reimbursement for non-facility (e.g. physician office setting) dual-energy x-ray absorptiometry (DXA) scans to levels that are far below the cost of providing these services.[103] Two-thirds of all DXA scans are currently performed in these settings, but these recent reimbursement cuts will likely result in a discontinuation of services at some centres, leading to increases in the travel distance required for DXA scans, limiting access to BMD tests. This may result in even fewer patients being diagnosed and subsequently treated.[103] This is unfortunate because DXA scans are the gold standard for diagnosing osteoporosis and have been shown to be cost effective for identifying patients with osteoporosis.[14] However, risk assessment using FRAX® with clinical risk factors (and without BMD) may be useful if patients are unable to obtain tests in the current reimbursement climate.
5. Conclusion
Osteoporosis imposes a high burden on the US and global population and because over two-thirds of those affected are aged ≥65 years, the associated cost of osteoporosis for both patients and society is expected to rise sharply over the coming years as the population continues to age. Osteoporosis can be managed effectively by timely screening, patient counselling and, in many cases, pharmacological treatment. Osteoporosis medications are generally well tolerated and highly effective, offering real hope of dramatic risk reduction for fracture and maintenance of functional independence. However, osteoporosis screening and treatment fall far short of recommended care, and even geriatricians find it difficult to adhere to the guidelines for treating osteoporosis, especially when co-morbid conditions are present.[88] Improved knowledge of the comparative effectiveness of various treatments, using well established metrics such as the NNT to prevent a future event, can help clinicians and patients evaluate the relative benefits of treatment of numerous co-occurring conditions; in many cases, osteoporosis treatment compares favourably with other well established and more widely implemented treatments. Additional action is urgently required to improve and maintain quality of life and functional independence for the burgeoning older population.
Acknowledgments
Funding: Editorial and writing support in the preparation of this manuscript was funded by Warner Chilcott (US) LLC, and Sanofi. Dr Curtis and Dr Safford were supported by AHRQ 1U18HS016956-01. Dr Curtis received support from the National Institutes of Health (NIH) [AR053351].
Tam Vo, PhD, and a team from Excerpta Medica provided editorial and writing assistance. The authors were fully responsible for all content and editorial decisions, and received no other financial support or other form of compensation related to the development of the paper.
Footnotes
Conflicts of interest: Dr Curtis has received research grants from Merck, Procter & Gamble, Eli Lilly and Novartis and has acted as a consultant for and received honoraria from Merck, Novartis, Roche, Amgen and Eli Lilly. Dr Safford has received research grants from the NIH, American College of Rheumatology and Peers for Progress (a collaboration between the American Academy of Family Physicians and the Eli Lilly Foundation).
Authorship: Both Dr Curtis and Dr Safford contributed to the conceptualization, drafting, interpretation, critical revisions and final approval of the manuscript, and the decision to submit the manuscript.
References
- 1.Prevention and management of osteoporosis. World Health Organ Tech Rep Ser. 2003;921:1–164. [PubMed] [Google Scholar]
- 2.National Osteoporosis Foundation (NOF) Clinician’s guide to prevention and treatment of osteoporosis. Washington, DC: NOF; 2010. [online]. Available from URL: http://www.nof.org/sites/default/files/pdfs/NOF_ClinicianGuide2009_v7.pdf [Accessed 2011 Aug 18] [Google Scholar]
- 3.Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465–75. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 4.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics – 2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 6.American Cancer Society. Cancer Facts & Figures 2010. Atlanta (GA): American Cancer Society; 2010. [online]. Available from URL: http://www.cancer.org/acs/groups/content/@nho/documents/document/acspc-024113.pdf [Accessed 2011 Aug 10] [Google Scholar]
- 7.Curtis JR, Arora T, Matthews RS, et al. Is withholding osteoporosis medication after fracture sometimes rational? A comparison of the risk for second fracture versus death. J Am Med Dir Assoc. 2010;11:584–91. doi: 10.1016/j.jamda.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boonen S, Dejaeger E, Vanderschueren D, et al. Osteoporosis and osteoporotic fracture occurrence and prevention in the elderly: a geriatric perspective. Best Pract Res Clin Endocrinol Metab. 2008;22:765–85. doi: 10.1016/j.beem.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761–7. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 10.Hallberg I, Rosenqvist AM, Kartous L, et al. Health-related quality of life after osteoporotic fractures. Osteoporos Int. 2004;15:834–41. doi: 10.1007/s00198-004-1622-5. [DOI] [PubMed] [Google Scholar]
- 11.Sanders KM, Nicholson GC, Watts JJ, et al. Half the burden of fragility fractures in the community occur in women without osteoporosis. When is fracture prevention cost-effective? Bone. 2006;38:694–700. doi: 10.1016/j.bone.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Dawson-Hughes B, Tosteson AN, Melton LJ, 3rd, et al. National Osteoporosis Foundation Guide Committee. Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the USA. Osteoporos Int. 2008;19:449–58. doi: 10.1007/s00198-008-0559-5. [DOI] [PubMed] [Google Scholar]
- 13.Van den Bergh JP, van Geel TA, Lems WF, et al. Assessment of individual fracture risk: FRAX and beyond. Curr Osteoporos Rep. 2010;8:131–7. doi: 10.1007/s11914-010-0022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vondracek SF, Linnebur SA. Diagnosis and management of osteoporosis in the older senior. Clin Interv Aging. 2009;4:121–36. doi: 10.2147/cia.s4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen ND, Frost SA, Center JR, et al. Development of prognostic nomograms for individualizing 5-year and 10-year fracture risks. Osteoporos Int. 2008;19:1431–44. doi: 10.1007/s00198-008-0588-0. [DOI] [PubMed] [Google Scholar]
- 16.Berg KM, Kunins HV, Jackson JL, et al. Association between alcohol consumption and both osteoporotic fracture and bone density. Am J Med. 2008;121:406–18. doi: 10.1016/j.amjmed.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peppone LJ, Hebl S, Purnell JQ, et al. The efficacy of calcitriol therapy in the management of bone loss and fractures: a qualitative review. Osteoporos Int. 2010;21:1133–49. doi: 10.1007/s00198-009-1136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katz S, Weinerman S. Osteoporosis and gastrointestinal disease. Gastroenterol Hepatol (NY) 2010;6:506–17. [PMC free article] [PubMed] [Google Scholar]
- 19.VanderWalde A, Hurria A. Aging and osteoporosis in breast and prostate cancer. CA Cancer J Clin. 2011;61:139–56. doi: 10.3322/caac.20103. [DOI] [PubMed] [Google Scholar]
- 20.Pitts CJ, Kearns AE. Update on medications with adverse skeletal effects. Mayo Clin Proc. 2011;86:338–43. doi: 10.4065/mcp.2010.0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294:716–24. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 22.Anderson G, Horvath J. Chronic conditions: making the case for ongoing care. Baltimore (MD): Johns Hopkins University; 2002. Dec, [online]. Available from URL: http://www.partnershipforsolutions.org/DMS/files/chronicbook2002.pdf [Accessed 2011 Aug 10] [Google Scholar]
- 23.Gillespie L, Handoll H. Prevention of falls and fall-related injuries in older people. Inj Prev. 2009;15:354–5. doi: 10.1136/ip.2009.023101. [DOI] [PubMed] [Google Scholar]
- 24.Boonen S, Lips P, Bouillon R, et al. Need for additional calcium to reduce the risk of hip fracture with vitamin D supplementation: evidence from a comparative metaanalysis of randomized controlled trials. J Clin Endocrinol Metab. 2007;92:1415–23. doi: 10.1210/jc.2006-1404. [DOI] [PubMed] [Google Scholar]
- 25.Avenell A, Gillespie WJ, Gillespie LD, et al. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. Cochrane Database Syst Rev. 2009;(2) doi: 10.1002/14651858.CD000227.pub3. CD000227. [DOI] [PubMed] [Google Scholar]
- 26.Latham NK, Anderson CS, Lee A, et al. A randomized, controlled trial of quadriceps resistance exercise and vitamin D in frail older people: the frailty interventions trial in elderly subjects (FITNESS) J Am Geriatr Soc. 2003;51:291–9. doi: 10.1046/j.1532-5415.2003.51101.x. [DOI] [PubMed] [Google Scholar]
- 27.Porthouse J, Cockayne S, King C, et al. Randomized controlled trial of calcium and supplementation with chole-calciferol (vitamin D3) for prevention of fractures in primary care. BMJ. 2005;330:1–6. doi: 10.1136/bmj.330.7498.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grant AM, Avenell A, Campbell MK, et al. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (randomized evaluation of calcium or vitamin D, RECORD): a randomized placebo-controlled trial. Lancet. 2005;365:1621–8. doi: 10.1016/S0140-6736(05)63013-9. [DOI] [PubMed] [Google Scholar]
- 29.Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and risk of fractures. N Engl J Med. 2006;354:669–83. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 30.Law M, Withers H, Morris J, et al. Vitamin D supplementation and the prevention of fractures and falls: results of a randomized trial in elderly people in residential accommodation. Age Ageing. 2006;35:482–6. doi: 10.1093/ageing/afj080. [DOI] [PubMed] [Google Scholar]
- 31.Lyons RA, Johansen A, Brophy S, et al. Preventing fractures among older people living in institutional care: a pragmatic randomised double blind placebo controlled trial of vitamin D supplementation. Osteoporos Int. 2007;18:811–8. doi: 10.1007/s00198-006-0309-5. [DOI] [PubMed] [Google Scholar]
- 32.Annweiler C, Montero-Odasso M, Schott AM, et al. Fall prevention and vitamin D in the elderly: an overview of the key role of the non-bone effects. J Neuroeng Rehabil. 2010;7:50. doi: 10.1186/1743-0003-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ip TP, Leung J, Kung AW. Management of osteoporosis in patients hospitalized for hip fractures. Osteoporos Int. 2010;21(Suppl. 4):S605–14. doi: 10.1007/s00198-010-1398-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gates S, Fisher JD, Cooke MW, et al. Multifactorial assessment and targeted intervention for preventing falls and injuries among older people in community and emergency care settings: systematic review and meta-analysis. BMJ. 2008;336:130–3. doi: 10.1136/bmj.39412.525243.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–41. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 36.Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280:2077–82. doi: 10.1001/jama.280.24.2077. [DOI] [PubMed] [Google Scholar]
- 37.Pols HA, Felsenberg D, Hanley DA, et al. Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Fosamax International Trial Study Group. Osteoporos Int. 1999;9:461–8. doi: 10.1007/pl00004171. [DOI] [PubMed] [Google Scholar]
- 38.Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344–52. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 39.Reginster J, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11:83–91. doi: 10.1007/s001980050010. [DOI] [PubMed] [Google Scholar]
- 40.McClung M, Geusens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344:333–40. doi: 10.1056/NEJM200102013440503. [DOI] [PubMed] [Google Scholar]
- 41.Chesnut CH, 3rd, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241–9. doi: 10.1359/JBMR.040325. [DOI] [PubMed] [Google Scholar]
- 42.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–22. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 43.Lyles KW, Colón-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799–809. doi: 10.1056/NEJMoa074941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chesnut CH, 3rd, Silverman S, Andriano K, et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the Prevent Recurrence of Osteoporotic Fractures study. PROOF Study Group. Am J Med. 109;2000:267–76. doi: 10.1016/s0002-9343(00)00490-3. [DOI] [PubMed] [Google Scholar]
- 45.Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282:637–45. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 46.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 344;2001:1434–41. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 47.Meunier PJ, Roux C, Seeman E, et al. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med. 2004;350:459–68. doi: 10.1056/NEJMoa022436. [DOI] [PubMed] [Google Scholar]
- 48.Reginster JY, Seeman E, De Vernejoul MC, et al. Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) study. J Clin Endocrinol Metab. 2005;90:2816–22. doi: 10.1210/jc.2004-1774. [DOI] [PubMed] [Google Scholar]
- 49.Seeman E, Vellas B, Benhamou C, et al. Strontium ranelate reduces the risk of vertebral and nonvertebral fractures in women eighty years of age and older. J Bone Miner Res. 2006;21:1113–20. doi: 10.1359/jbmr.060404. [DOI] [PubMed] [Google Scholar]
- 50.Pageau SC. Denosumab. MAbs. 2009;1:210–5. doi: 10.4161/mabs.1.3.8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown JP, Prince RL, Deal C, et al. Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res. 2009;24:153–61. doi: 10.1359/jbmr.0809010. [DOI] [PubMed] [Google Scholar]
- 52.Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–65. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 53.Boonen S, Black DM, Colón-Emeric CS, et al. Efficacy and safety of a once-yearly intravenous zoledronic acid 5 mg for fracture prevention in elderly postmenopausal women with osteoporosis aged 75 and older. J Am Geriatr Soc. 2010;58:292–9. doi: 10.1111/j.1532-5415.2009.02673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boonen S, McClung MR, Eastell R, et al. Safety and efficacy of risedronate in reducing fracture risk in osteoporotic women aged 80 and older: implications for the use of antiresorptive agents in the old and oldest old. J Am Geriatr Soc. 2004;52:1832–9. doi: 10.1111/j.1532-5415.2004.52506.x. [DOI] [PubMed] [Google Scholar]
- 55.Boonen S, Marin F, Mellstrom D, et al. Safety and efficacy of teriparatide in elderly women with established osteoporosis: bone anabolic therapy from a geriatric perspective. J Am Geriatr Soc. 2006;54:782–9. doi: 10.1111/j.1532-5415.2006.00695.x. [DOI] [PubMed] [Google Scholar]
- 56.Bock O, Felsenberg D. Bisphosphonates in the management of postmenopausal osteoporosis-optimizing efficacy in clinical practice. Clin Interv Aging. 2008;3:279–97. doi: 10.2147/cia.s2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wells GA, Cranney A, Peterson J, et al. Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. 2008;(1) doi: 10.1002/14651858.CD003376.pub3. CD001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wells GA, Cranney A, Peterson J, et al. Risedronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. 2008;(1) doi: 10.1002/14651858.CD003376.pub3. CD004523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abrahamsen M, Eiken P, Eastell R. Proton pump inhibitor use and the antifracture efficacy of alendronate. Arch Intern Med. 2011;171:998–1004. doi: 10.1001/archinternmed.2011.20. [DOI] [PubMed] [Google Scholar]
- 60.Delmas PD, Benhamou CL, Man Z, et al. Monthly dosing of 75 mg risedronate on 2 consecutive days a month: efficacy and safety results. Osteoporos Int. 2008;19:1039–45. doi: 10.1007/s00198-007-0531-9. [DOI] [PubMed] [Google Scholar]
- 61.Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–38. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- 62.Mellström DD, Sörensen OH, Goemaere S, et al. Seven years of treatment with risedronate in women with postmenopausal osteoporosis. Calcif Tissue Int. 2004;75:462–8. doi: 10.1007/s00223-004-0286-7. [DOI] [PubMed] [Google Scholar]
- 63.Lewiecki EM. Safety of long-term bisphosphonate therapy for the management of osteoporosis. Drugs. 2011;71:791–814. doi: 10.2165/11585470-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 64.Armamento-Villareal R, Napoli N, Panwar V, et al. Suppressed bone turnover during alendronate therapy for high-turnover osteoporosis. N Engl J Med. 2006;355:2048–50. doi: 10.1056/NEJMc062268. [DOI] [PubMed] [Google Scholar]
- 65.Lee P, van der Wall H, Seibel MJ. Looking beyond low bone mineral density: multiple insufficiency fractures in a woman with post-menopausal osteoporosis on alendronate therapy. J Endocrinol Invest. 2007;30:590–7. doi: 10.1007/BF03346353. [DOI] [PubMed] [Google Scholar]
- 66.Lenart BA, Lorich DG, Lane JM. Atypical fractures of the femoral diaphysis in postmenopausal women taking alendronate. N Engl J Med. 2008;358:1304–6. doi: 10.1056/NEJMc0707493. [DOI] [PubMed] [Google Scholar]
- 67.Mashiba T, Hirano T, Turner CH, et al. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res. 2000;15:613–20. doi: 10.1359/jbmr.2000.15.4.613. [DOI] [PubMed] [Google Scholar]
- 68.Visekruna M, Wilson D, McKiernan FE. Severely suppressed bone turnover and atypical skeletal fragility. J Clin Endocrinol Metab. 2008;93:2948–52. doi: 10.1210/jc.2007-2803. [DOI] [PubMed] [Google Scholar]
- 69.Armamento-Villareal R, Napoli N, Diemer K, et al. Bone turnover in bone biopsies of patients with low-energy cortical fractures receiving bisphosphonates: a case series. Calcif Tissue Int. 2009;85:37–44. doi: 10.1007/s00223-009-9263-5. [DOI] [PubMed] [Google Scholar]
- 70.Goh SK, Yang KY, Koh JS, et al. Subtrochanteric in-sufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007;89:349–53. doi: 10.1302/0301-620X.89B3.18146. [DOI] [PubMed] [Google Scholar]
- 71.Lenart BA, Neviaser AS, Lyman S, et al. Association of low-energy femoral fractures with prolonged bisphosphonate use: a case control study. Osteoporos Int. 2009;20:1353–62. doi: 10.1007/s00198-008-0805-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abrahamsen B, Eiken P, Eastell R. Subtrochanteric and diaphyseal femur fractures in patients treated with alendronate: a register-based national cohort study. J Bone Miner Res. 2009;24:1095–102. doi: 10.1359/jbmr.081247. [DOI] [PubMed] [Google Scholar]
- 73.Shane E, Burr D, Ebeling PR, et al. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25:2267–94. doi: 10.1002/jbmr.253. [DOI] [PubMed] [Google Scholar]
- 74.Sellmeyer DE. Atypical fractures as a potential complication of long-term bisphosphonate therapy. JAMA. 2010;304:1480–4. doi: 10.1001/jama.2010.1360. [DOI] [PubMed] [Google Scholar]
- 75.Wysowski DK. Reports of esophageal cancer with oral bisphosphonate use [letter] N Engl J Med. 2009;360:89–90. doi: 10.1056/NEJMc0808738. [DOI] [PubMed] [Google Scholar]
- 76.Abrahamsen B, Eiken P, Eastell R. More on reports of esophageal cancer with oral bisphosphonate use [letter] N Engl J Med. 2009;360:1789. doi: 10.1056/NEJMc096026. [DOI] [PubMed] [Google Scholar]
- 77.Solomon DH, Patrick A, Brookhart MA. More on reports of esophageal cancer with oral bisphosphonate use [letter] N Engl J Med. 2009;360:1789–90. [PubMed] [Google Scholar]
- 78.FDA Drug Safety Communication: Ongoing safety review of oral osteoporosis drugs (bisphosphonates) and potential increased risk of esophageal cancer. Rockville (MD): FDA; 2011. Jul 21, [online]. Available from URL: http://www.fda.gov/Drugs/DrugSafety/ucm263320.htm [Accessed 2011 Nov 14] [Google Scholar]
- 79.Papapetrou PD. Bisphosphonate-associated adverse events. Hormones (Athens) 2009;8:96–110. doi: 10.14310/horm.2002.1226. [DOI] [PubMed] [Google Scholar]
- 80.Actonel® (risedronate sodium) tablets: US prescribing information. Rockaway (NJ): Warner Chilcott (US), LLC; 2011. [Google Scholar]
- 81.Evista: US prescribing information. Indianapolis (IN): Lilly USA, LLC; 2011. Jan 7, [online]. Available from URL: http://pi.lilly.com/us/evista-pi.pdf [Accessed 2011 Nov 15] [Google Scholar]
- 82.Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125–37. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- 83.Curtis JR, Carbone L, Cheng H, et al. Longitudinal trends in use of bone mass measurement among older Americans, 1999-2005. J Bone Miner Res. 2008;23:1061–7. doi: 10.1359/JBMR.080232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miacalcin: US prescribing information. East Hanover (NJ): Novartis; 2011. [online]. Available from URL: http://www.pharma.us.novartis.com/product/pi/pdf/miacalcin-nasal.pdf [Accessed 2011 Nov 15] [Google Scholar]
- 85.Forteo: US prescribing information. Indianapolis (IN): Lilly USA, LLC; 2010. [online]. Available from URL: http://pi.lilly.com/us/forteo-pi.pdf [Accessed 2011 Nov 15] [Google Scholar]
- 86.National Committee for Quality Assurance. The state of health care quality 2007. Washington, DC: National Committee for Quality Assurance; 2007. [online]. Available from URL: http://www.ncqa.org/Portals/0/Publications/Resource%20Library/SOHC/SOHC_07.pdf [Accessed 2011 Aug 10] [Google Scholar]
- 87.Curtis JR, Kim Y, Bryant T, et al. Osteoporosis in the home health care setting: a window of opportunity? Arthritis Rheum. 2006;55:971–5. doi: 10.1002/art.22349. [DOI] [PubMed] [Google Scholar]
- 88.Zimmerman SI, Girman CJ, Buie VC, et al. The prevalence of osteoporosis in nursing home residents. Osteoporos Int. 1999;9:151–7. doi: 10.1007/s001980050129. [DOI] [PubMed] [Google Scholar]
- 89.Greenspan SL, Schneider DL, McClung MR, et al. Alendronate improves bone mineral density in elderly women with osteoporosis residing in long-term care facilities: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2002;136:742–6. doi: 10.7326/0003-4819-136-10-200205210-00009. [DOI] [PubMed] [Google Scholar]
- 90.Parikh S, Avorn J, Solomon DH. Pharmacological management of osteoporosis in nursing home populations: a systematic review. J Am Geriatr Soc. 2009;57:327–34. doi: 10.1111/j.1532-5415.2008.02119.x. [DOI] [PubMed] [Google Scholar]
- 91.Colón-Emeric C, Casebeer L, Saag K, et al. Barriers to providing osteoporosis care in skilled nursing facilities: perceptions of medical directors and directors of nursing. J Am Med Dir Assoc. 2005;6(3 Suppl):S61–6. doi: 10.1016/j.jamda.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 92.Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of randomised trials. Lancet. 1998;351:1451–67. [PubMed] [Google Scholar]
- 93.Messerli F, Pinto L, Tang SS, et al. Impact of systemic hypertension on the cardiovascular benefits of statin therapy: a meta-analysis. Am J Cardiol. 2008;101:319–25. doi: 10.1016/j.amjcard.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 94.Arias E National Vital Statistics Report: United States life tables. Department of Health and Human Services. Centers for Disease Control and Prevention. National Center for Health Statistics. 2003 [online]. Available from URL: http://www.cdc.gov/nchs/data/statab/lewk3_2003.pdf [Accessed 2011 Aug 10]
- 95.Maricic M, Adachi JD, Sarkar S, et al. Early effects of raloxifene on clinical vertebral fractures at 12 months in postmenopausal women with osteoporosis. Arch Intern Med. 2002;162:1140–3. doi: 10.1001/archinte.162.10.1140. [DOI] [PubMed] [Google Scholar]
- 96.Roux C, Seeman E, Eastell R, et al. Efficacy of risedronate on clinical vertebral fractures within six months. Curr Med Res Opin. 2004;20:433–9. doi: 10.1185/030079903125003125. [DOI] [PubMed] [Google Scholar]
- 97.Fojo T, Grady C. How much is life worth: cetuximab, non-small cell lung cancer, and the $440 billion question. J Natl Cancer Inst. 2009;101:1044–8. doi: 10.1093/jnci/djp177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Freemantle N, Cleland J, Young P, et al. Beta blockade after myocardial infarction: systematic review and meta regression analysis. BMJ. 1999;318:1730–7. doi: 10.1136/bmj.318.7200.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Adachi JD, Lyles KW, Colón-Emeric CS, et al. Zoledronic acid results in better health-related quality of life following hip fracture: the HORIZON-Recurrent Fracture Trial. Osteoporos Int. 2011 Jan 20; doi: 10.1007/s00198-010-1514-9. Epub. [DOI] [PubMed] [Google Scholar]
- 100.Tosi LL, Gliklich R, Kannan K, et al. The American Orthopaedic Association’s ‘‘own the bone’’ initiative to prevent secondary fractures. J Bone Joint Surg Am. 2008;90:163–73. doi: 10.2106/JBJS.G.00682. [DOI] [PubMed] [Google Scholar]
- 101.Feldstein A, Elmer PJ, Smith DH, et al. Electronic medical record reminder improves osteoporosis management after a fracture: a randomized, controlled trial. J Am Geriatr Soc. 2006;54:450–7. doi: 10.1111/j.1532-5415.2005.00618.x. [DOI] [PubMed] [Google Scholar]
- 102.Centers for Medicare & Medicaid Services. The 2010 Physician Quality Reporting Initiative measures list. [online]. Available from URL: https://www.cms.gov/PQRS/Downloads/2010_PQRI_MeasuresList_111309.pdf [Accessed 2011 Aug 10]
- 103.Lewiecki EM, Baim S, Siris ES. Osteoporosis care at risk in the United States. Osteoporos Int. 2008;19:1505–9. doi: 10.1007/s00198-008-0716-x. [DOI] [PubMed] [Google Scholar]


