Abstract
IL-17 is a pleiotropic cytokine produced by Th17 T cells that induces a myriad of proinflammatory mediators. However, different models of inflammation report opposite functional roles of IL-17 signal in terms of its effects on bone destruction. In the present study we determined the role of IL-17 receptor A (RA) signal in bone resorption stimulated by dentoalveolar infections. Infrabony resorptive lesions were induced by surgical pulp exposure and microbial infection of mouse molar teeth. IL-17 was strongly induced in periapical tissues in wild-type (WT) mice by 7 days after the infection but was not expressed in uninfected mice. Dentoalveolar infections of IL-17RA knockout (KO) mice demonstrated significantly increased bone destruction and more abscess formation in the apical area compared to WT mice. Infected IL-17RA KO mice exhibited significantly increased neutrophils and macrophages compared to the WT littermates at day 21, suggesting a failure of transition from acute to chronic inflammation in the IL-17RA KO mice. The expression of IL-1 (both α and β isoforms) and MIP2 were significantly up-regulated in the IL-17RA KO compared to WT mice at day 21 post infection. The development of periapical lesion in IL-17RA KO mice was significantly attenuated by neutralization of IL-1β and MIP2. Taken together, these results demonstrate that IL-17RA signal seems to be protective against infection-induced periapical inflammation and bone destruction via suppression of neutrophil and mononuclear inflammation.
Introduction
IL-17 is a proinflammatory cytokine secreted by various immune cell types including Th17 cells, γδ T cells, neutrophils and macrophages (1). IL-17 primarily binds to IL-17RA and initiates a proinflammatory signal, which is strongly involved in the progression of many autoimmune or inflammatory diseases in humans such as rheumatoid arthritis (RA), systemic lupus erythematousus, asthma, and allograft rejection (2, 3). However, in term of inflammatory bone destruction, opposite functional roles for IL-17 have been reported. In different rodent RA models, IL-17 was elevated in synovial fluid (4–6) and blocking of IL-17 reduced inflammation and bone damage, whereas IL-17 excess led to disease exacerbation (7, 8). In human periapical lesions, which are caused by an infection in the root canal system of teeth and ultimately result in alveolar bone loss, higher IL-17 levels and greater numbers of neutrophils were observed in symptomatic lesions compared to asymptomatic lesions (9). In addition, IL-17 expression was higher in the gingival crevicular fluid and tissues of periodontitis patients compared to healthy control subjects (10–12). These findings strongly suggested that IL-17 plays a proinflammatory role in the dentoalveolar infection and inflammation. However, IL-17RA signal is protective in Porphyromonas gingivalis-induced marginal alveolar bone loss in mice (13, 14). By contrast, IL-17 mediates age-related alveolar bone loss in mice (15). In addition, IL-17 KO mice are resistant to develop experimental periapical lesions (16). These basic findings observed in gene modified mouse models seem to be contradictory and are not always accordant with the clinical findings. Thus, the definitive role of IL-17RA signal in dentoalveolar inflammation and bone loss is still unclear. In the present study, we demonstrate that IL-17RA signal is protective in the development of periapical lesions using a well-established mouse periapical lesion model(17) in IL-17RA KO and IL-17-neutralized wild-type (WT) mice. Our data also suggest that IL-17RA signal potently regulates myeloidcell -mediated periapical inflammation.
Material and Methods
Animals
C57BL/6 (wild-type; WT) mice were obtained from Charles River Laboratories (Wilmington, MA). IL-17RA knockout (KO) mice were generously provided by Dr. Sarah Gaffen, (University of Pittsburgh) under the authorization from Amgen Corporation (Seattle, WA). All animals were maintained in a specific pathogen free environment at the Forsyth Institute Animal Facility, in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC). All experimental protocols were approved by the Forsyth IACUC.
Induction of Periapical lesions
Adult male mice, 8 weeks of age, on day 0 were anesthetized via intraperitoneal injection with ketamine HCl (80 mg/kg) and xylazine (10 mg/kg), and were placed on a jaw retraction board. The dental pulps of the mandibular first molars were exposed using an electric dental hand piece with a no. 1/4 round bur under a surgical microscope (MC-M92; Seiler, St. Louis, MO), as described previously(17). The pulp chambers were opened until the entrance of the canals could be visualized and probed with a size 6 endodontic file. Common human pathogens including Prevotella intermedia (American Type Culture Collection (ATCC) 25611), Streptococcus intermedius (ATCC 27335), Fusobacterium nucleatum (ATCC 25586), and Peptostreptococcus micros (ATCC 33270) were grown as previously described(18). A total of 10 μl (1010 cells/ml) of the pathogen mixture in pre-reduced anaerobically sterilized Ringer’s solution were inoculated into the pulp chamber and introduced into the root canal with the endodontic file. One group of mice from each strain served as non-infected controls. On day 1, 10 and 21 after pulp exposure, all groups of mice were killed, mandibles were isolated and dissected free of soft tissues. The left hemi-mandibles were fixed in fresh 4% paraformaldehyde in PBS and used for histological analysis. For the right hemi-mandibles, the periapical tissues surrounding the mesial and distal root apices were carefully extracted, together with surrounding bone in a block specimen under the surgical microscope. Periapical tissues were rinsed in PBS, freed of clots, weighed, and immediately frozen at −70°C for subsequent protein extraction.
Antibody treatment
In one experiment, infected WT mice were treated with either rat anti-mouse IL-17 monoclonal antibody (MAB421, R&D Systems, MN) to neutralize major ligands of IL-17RA including IL-17A and IL-17A/F heterodimer, or isotype-matched control IgG. Antibodies were intramuscularly injected (14μg/mouse/time) on days –1, 2, 5, 8, 11, 14, and 17 relative to the pulpal infection. In the other experiment, infected IL-17RA KO mice received either mouse anti-mouse IL-1α monoclonal antibody (I-658, Leinco Technologies, MO), rat anti-mouse IL-1β monoclonal antibody (I-659, Leinco Technologies), rat anti-mouse MIP2 monoclonal antibody (MAB452, R&D systems), or isotype-matched control IgG. Antibodies were injected into caudal vein (50 μg/mouse/time) on days -1, 2, 7, 12 and 17 relative to pulpal infection. Antibody treated mice were killed on day 21 post infection and subjected to micro-computed tomography (μCT) and histology.
Micro-computed tomography (mCT) analysis
Hemi-mandibles were scanned as previously described(18) using a compact fan-beam-type tomograph (μCT40, Scanco Medical, Bassersdorf, Switzerland) providing a 10μm nominal resolution. The most centrally located section, which included the distal root canal of the mandibular first molar and that exhibited a patent root canal apex, was selected for quantitation. The cross-sectional area of periapical lesions was selected with Adobe Photoshop CS5 (Adobe Systems, San Jose, CA) and measured with ImageJ (Wayne Rasband, National Institutes of Health, Bethesda, MD).
Protein Preparations and ELISA
For protein extraction, frozen periapical tissue samples were disrupted in a cell lysis buffer (Cell Signaling Technology, Danvers, MA) supplemented with 50μg/ml gentamicin (Sigma Aldrich, St. Louis, MO) using Fastprep-24 with matrix A (both MP Biomedicals, Solon, OH). The supernatant was collected after centrifugation and stored at −70°C until assay. Assays for cytokines in extracts employed commercially available ELISA kits obtained from R&D Systems (DuoSets) and were used according to the manufacturer’s instructions to evaluate periapical tissue levels of IL-1α, IL-1β IFN-γ, TNF-α, RANKL, MIP2 and Groα/KC. The concentration of each cytokine was calculated with reference to a standard curve constructed using recombinant cytokines provided with each kit. Results were expressed as picogram cytokine/milligram periapical tissue.
Semi-quantitative RT-PCR
To generate IL-17A mRNA for positive controls, spleen cells were obtained from C57BL/6 mice. Mononuclear leukocytes were isolated by Ficoll-Hypaque centrifugation, washed in RPMI1640 medium supplemented with 5% FBS, and adjusted to a concentration of 2×106 /ml. Cells were stimulated with E. coli LPS (1 μg/ml) and Con A (1μg/ml) for 20 hr. in a humidified atmosphere of 5%CO2/95% air at 37°C. Extraction of whole cellular RNA from stimulated cells or from periapical tissue samples was carried out using Trizol reagent and PureLink Total RNA Purification Kit (both from Invitrogen). The concentration of RNA was determined spectrophotometrically. cDNA was constructed from mRNA using reverse transcription (RT) as described(19). PCR primers specific for IL-17A were chosen using the Primer3 program (Whitehead Institute for Biomedical Research, Cambridge, MA): forward 5′-CAGCACCAGCTGATCAGGAC-3′; reverse 5′-GGGGGTTTCTTAGGGGTCAG-3′ (538 bp). Twenty eight cycles of PCR amplification was carried out using 1μl of cDNA from the RT reaction and other reagents as described(19). The beta-actin gene served as a reference gene (forward 5′-ACTGGGACGACATGGAGAAG-3′; reverse 5′-TCTCAGCTGTGGTGGTGAAG-3′ (384 bp). PCR products were visualized on 2% agarose gels under UV illumination after ethidium bromide staining.
Macrophage culture
Resident peritoneal macrophages were harvested from WT and IL17RA KO mice as we previously described (18, 20). Macrophages (105 cells/well in 96-well plate) were stimulated with a cocktail of fixed endodontic pathogens described above (106 cells/well) for 24 hrs. The level of IL-1α, IL-1β, and MIP2 in cell culture supernatants was determined by ELISA. The results were expressed as nanogram cytokine per milliliter supernatant.
Histologic Sample Preparation
Fixed hemi-mandible samples used for micro-CT analysis were decalcified using 10% formic acid and sodium citrate, dehydrated in ethanol and embedded in paraffin. Serial sections of 6μm thickness were cut; every 5th sample was mounted and stained with hematoxylin and eosin (H&E). Sections containing the region of interest (patent root canal with localized periapical lesion) were selected, mounted and stained with tartrate-resistant acidic phosphatase (TRAP). Methyl green staining was employed as counter staining for the TRAP staining. Adjacent sections were used for immunohistochemical analysis. Identification of neutrophils, B-cells and macrophages were performed by help of the antibodies Ly-6B.2 (dilution 1: 4000; AbD-Serotec, Oxford, UK ), CD20 (1:200, Santa Cruz Biotechnology, Inc, USA), F4/80 (dilution 1:50, Santa Cruz Biotechnology, Inc, Dallas, TX), ab3523 (anti-iNOS, dilution 1:400, Abcam, Cambridge, MA), and N20 (anti-ArgI, dilution 1:50, Santa Cruz Biotechnology, Inc) respectively. Primary antibodies were detected either with biotinylated goat anti-rat antibody or biotinylated rabbit anti-goat using the Vector ELITE ABC kits (Vector Laboratories, Burlingame, CA).
Omissions of the primary antibodies were used as negative controls and the sections showed no positive immune cell staining. Cell enumeration was carried out in grid by light microscopy at 400x magnification.
Statistical Analysis
Descriptive statistics, including the mean and standard errors or standard deviations were calculated for all data. Statistical analysis was performed using one-way ANOVA and Tukey’s multiple comparison tests or Bonferroni post hoc tests, and unpaired t-test for comparison between two groups using GraphPad Prism (GraphPad Software, San Diego, CA, USA).
Results
Kinetic Expression of IL-17 in Periapical Granulomas
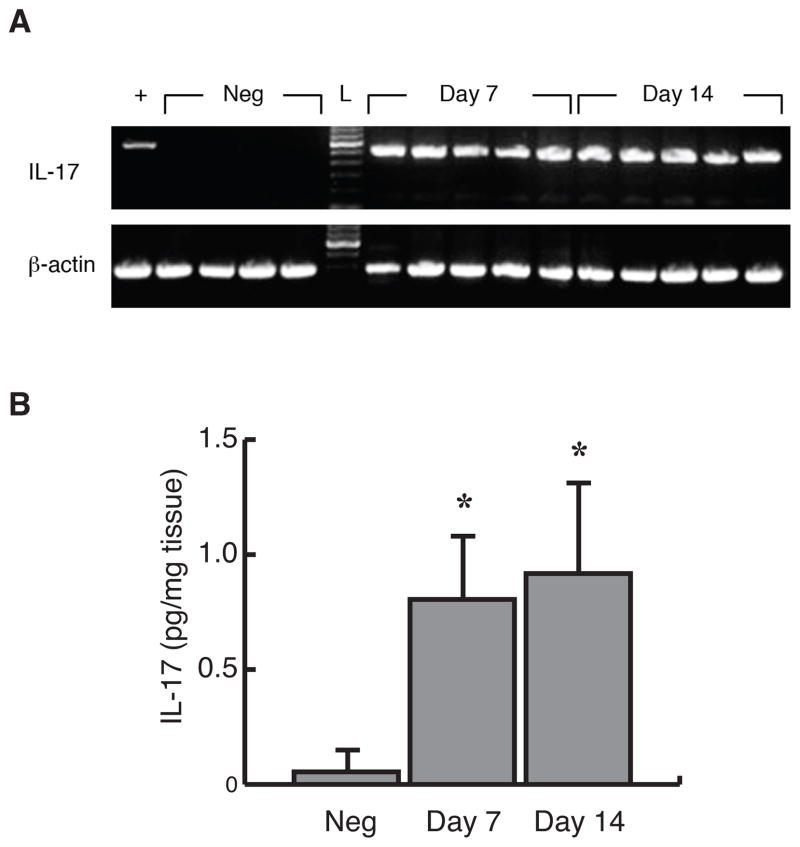
To determine the kinetics of expression of IL-17 in periapical granuloma, pulpal infection was induced in WT mice. The expression of IL-17 gene and protein was kinetically determined on days 7 and 14 after the infection. As shown in Figure 1A, IL-17 gene was strongly induced in all samples in both observation periods, whereas it was not detectable in periapical tissues isolated from non-infected mice. Infection-induced IL-17 expression was also confirmed at a protein level by ELISA. As shown in Figure 1B, in agreement with the gene expression results, IL-17 protein was significantly up-regulated on days 7 and 14 (p < 0.01) compared to the uninfected controls. No significant difference was observed between days 7 and 14.
Figure 1. IL-17 was expressed in infrabony periapical granulomas following pulpal infection.
A mRNA expression. Total RNA was isolated from radicular granulomas in C57BL/6 mice on days 7 and 14 after dental pulp infection (n = 5/group), and was analyzed for IL-17 mRNA by RT-PCR. Periapical tissues from uninfected mice served as controls (n=4). B IL-17 protein expression. Protein samples were extracted from isolated periapical granulomas developed in C57BL/6 mice on days 0 (uninfected controls), 7 and 14 relative to pulpal infection (n = 5/group). The level of IL-17 was determined by ELISA. Vertical bars: SD, *: p<0.01 vs. uninfected controls.
Effect of Genetic Deletion of IL-17RA and IL-17 neutralization on Infection-Induced Bone Destruction
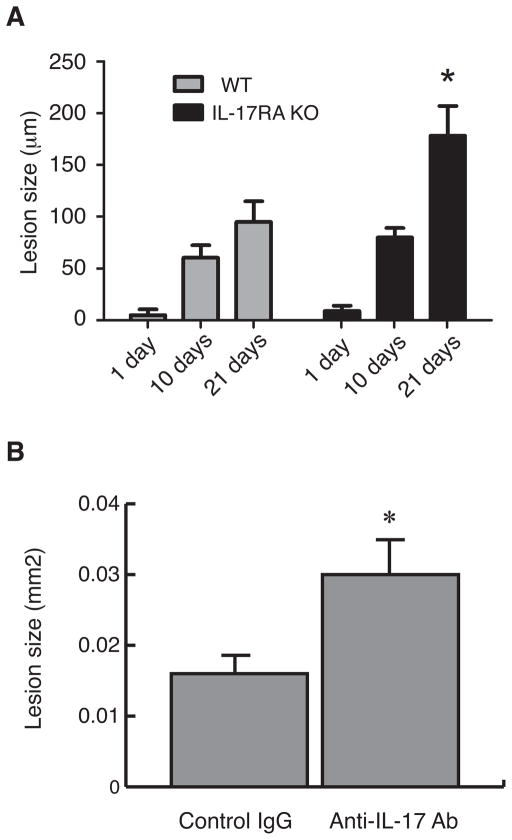
IL-17 has been reported to exacerbate bone and cartilage destruction in models of experimental autoimmune arthritis(7, 8), but was protective against alveolar bone loss stimulated by infection(14). Accordingly, we examined the role of IL-17RA signal in dentoalveolar infection using IL-17RA KO and corresponding WT mice. As shown in Figure 2A, the size of periapical lesion was significantly up-regulated in a time-dependent manner in both IL-17RA KO and WT mice. Although the extent of periapical lesion was similar by day 10 post infection, the KO mice exhibited further extended periapical lesion (approximately 2-folds) compared to WT mice on day 21. This finding was confirmed in other two separated experiments (data not shown). We further confirmed this finding by functional neutralization of IL-17RA ligands in WT mice. As shown in Figure 2B, animals treated with anti-IL-17 antibody had a significant increase of periapical inflammatory bone destruction compared to the control group treated with with isotype-matched IgG (185% in average, p=0.0006). Taken together, these results demonstrate that the role of IL-17RA signal is probably protective in the development of periapical granuloma.
Figure 2. IL-17RA KO mice exhibited up-regulated periapical bone destruction compared to wild-type controls.
A Kinetics of periapical bone destruction in IL-17RA KO and C57BL/6J mice. IL-17 RA KO and C57BL/6 mice were subjected to pulpal infection, and alveolar bone loss was determined by micro-computed tomography on days 1, 10, and 21 post infection. Vertical bars: SD, *: p<0.001 vs. C57BL/6J Day 21 group.
B Periapical bone loss in anti-IL-17 antibody treated mice. Infected C57BL/6 mice received either anti-mouse IL-17 antibody (experimental) or rat IgG (control) intramuscularly on days –1, 2, 5, 8, 11, 14, and 17 post infection (n =10/group). The extent of periapical lesion was quantified on Day 21 using histological sections. Vertical bars: SD, *: p<0.001 vs. control IgG group.
Effect of IL-17RA Gene Knockout on Osteoclasts and infiltrating Immune Cells
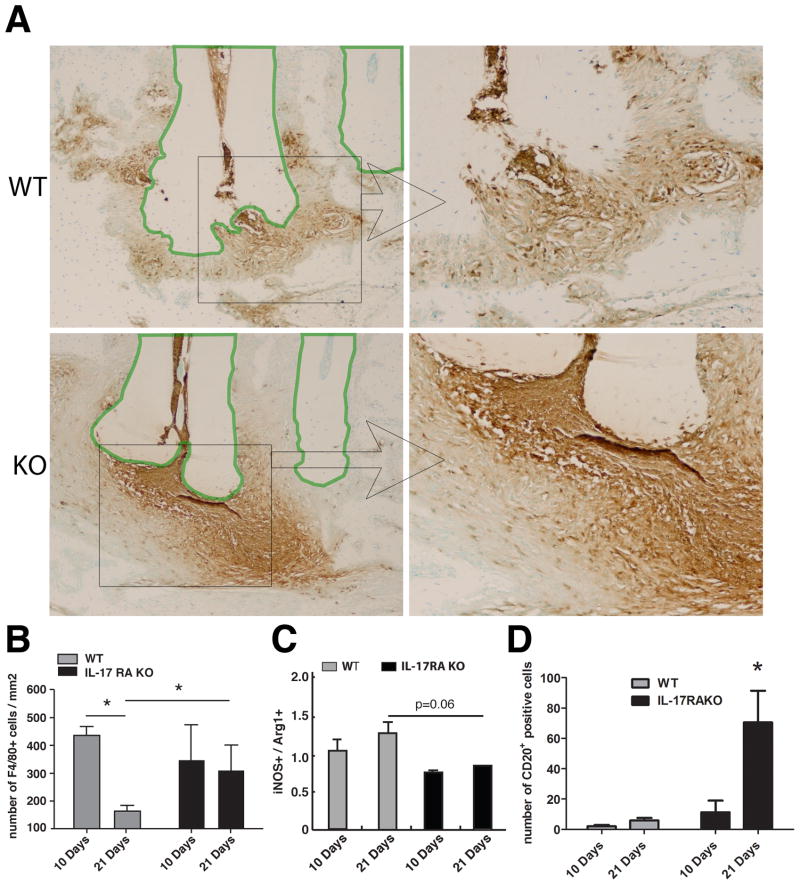
The effect of IL-17RA deficiency on osteoclasts and inflammatory cell infiltration in periapical granuloma was histologically examined. At day 10, no obvious histological difference was observed between the genotypes (data not shown). As shown in Figure 3, all mice with pulp exposures had developed fibrous granulomatous tissue in the apical area, but the KO mice showed more severe inflammatory cell infiltration than the WT littermate on day 21 after the pulpal infection.
Figure 3. Endodontic infection induced elevated TRAP(+) cells in IL-17RA KO mice compared to WT controls.
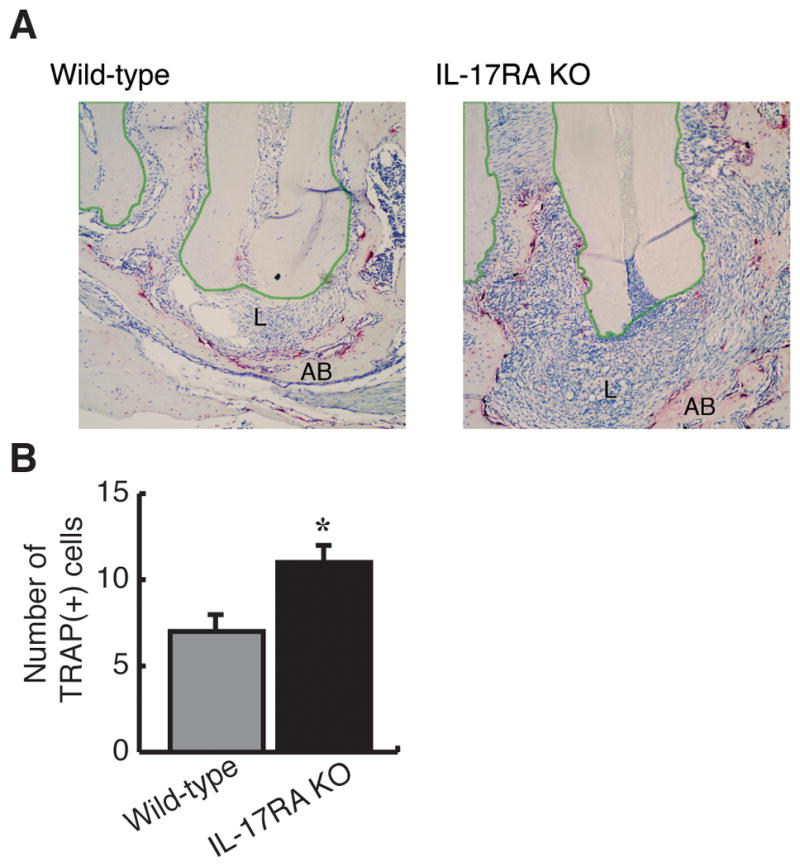
Representative images of TRAP staining (100x) were shown (n=5 per group). Cells exhibiting magenta or red are TRAP(+) cells, which are considered as osteoclasts. IL-17RA KO exhibited large periapical lesion with severe inflammatory cell infiltration and elevated osteoclasts compared to WT. The polygonal regions enclosed with green lines indicate dental roots. AB: alveolar bone, L: periapical lesion, Vertical bar: SD, *: p<0.05 vs. WT mice.
The number of TRAP(+) osteoclasts appeared to be increased in IL-17RA KO animals at day 10 (Figure 3B), whereas, at day 21, the bone surrounding the lesions were in part totally resorbed in the KO mice, making controlled comparisons between the two strains impossible. This observation indicates an elevated osteoclastic activity in the KO model. In general, most osteoclasts were associated with bone at the periphery of the periapical granuloma, although some TRAP(+) cells were also present within the periapical granuloma, possibly associated with residual bone spicules.
As presented in Figure 4A, immunohistochemistry revealed such an extensive apical infiltration of Ly-6B.2(+) neutrophils in KO mice 21 days post infection, that the enumeration of neutrophilswas impossible. In WT mice, such severe infiltration of Ly-6B.2(+) neutrophils was not observed in the lesioned area. Macrophages play a prominent role in periapical lesion development as an important source of chemotactic factors(21–23) and proinflammatory cytokines(24), and are important in phagocytosis of apoptotic cells(25). Enumeration of F4/80(+) cells (Figure 4B) revealed that macrophages in WT mice were significantly reduced on day 21 vs. day 10 after infection (p<0.05). Such reduction of macrophages was not observed in the KO mice. We also examined the ratio of iNOS(+) cells to ArgI(+) cells within lesion areas where F4/80(+) cells were numerous. The former represents classically activated M1 macrophages and the latter alternatively activated M2 cells. As presented in Figure 4C, we observed a trend that infected IL-17RA KO mice consistently exhibited a M2-dominant macrophage profile (ratio < 1). The ratio difference between the two genotypes was chronologically increased by Day 21 post infection. These findings indicate that IL-17RA signal seems to play an important role in accumulation and transition of myeloid cells in periapical lesion.
Figure 4. IL-17RA deficiency led to severe inflammatory cell infiltration into periapical lesions after pulpal infection compared to wild-type controls.
A Representative images of neutrophil staining. Histological sections of day 21 samples (n=6 in WT and n=7 in IL-17RA KO) were stained with Ly-6B.2. The polygonal regions enclosed with green lines indicate dental roots. Left panels: 100X, Right panels: rectangular area specified in the left panels (400X). B Number of macrophages per mm2 in the lesions. Histological sections were stained with anti-F4/80 antibody for the enumeration of macrophages (n=3 per group). Vertical bar: SEM, * p<0.05. C Ratio of M1/M2 macrophages. Histological sections were stained with either anti-iNOS antibody or anti-Arg1 antibody. The number of iNOS(+) and Arg1(+) cells within areas of numerous F4/80(+) cells, was enumerated as M1 and M2 macrophages, respectively (n=3 per group). The ratio represents the number of M1 cells divided by the number of M2 cells. D Total number of CD20(+) cells in the lesioned area. Histological sections were stained with anti-CD20 antibody for the enumeration of B cells. Vertical bar: SEM, *: p<0.001.
IL-17RA signaling also plays an important role in recruitment of B cell to the site of inflammation(26), and we therefore investigated if number of infiltrating CD20(+) B-cells were different in the KO versus WT mice. As shown in Figure 4D, we observed that in the absence of IL-17RA activation in the former, a significant increase in the number of CD20(+) cells was found 21 days after dental pulp infection.
Mediator Responses in Inflammatory Granulomas
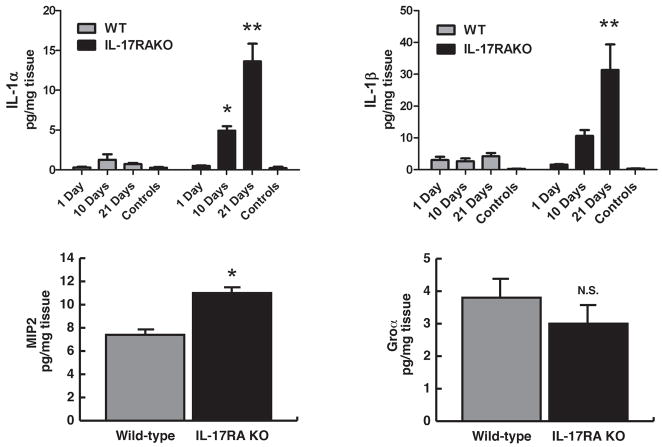
We confirmed the level of pro-inflammatory cytokines in infected mice by cytokine ELISA. As shown in Figure 5, the production of IL-1α and β was up-regulated in a time-dependent manner in the infected KO mice. In contrast, the peak of IL-1α production in WT mice was at day 10 post infection, and the level declined thereafter. The progress of periapical inflammation did not affect IL-1β production during 21 days in the WT mice.
Figure 5. IL-17RA KO mice exhibited elevated inflammatory cytokine expression profiles compared to wild-type controls.
Protein extracts of periapical lesions were subjected to ELISA to determine IL-1 levels on days 1, 10, and 21 post infection (n=6–8/group). In addition, the expression of neutrophil chemotactic factors, MIP2 and Groα, was quantified on Day 21 (n=10/group). Bars: mean ± SEM. * p<0.05 vs. corresponding WT control group, **: p<0.001 vs. corresponding WT control group, N.S: not statistically significant.
MIP2 is a strong chemotactic factor of neutrophils (27). The IL-17RA KO mice expressed significantly higher level of MIP2 (p<0.001 vs. WT), which may contribute to the elevated accumulation of neutrophils. This finding suggests that IL-17 signal seems to be involved in the recruitment of neutrophils via up-regulation of MIP2 in this periapical disease model. The endodontic infection did not modulate the level of another neutrophil chemoattractant, KC/Groα, in any mouse strain.
On the other hand, the effect of IL-17RA deficiency on the level of the pro-inflammatory cytokine TNF-α and the central Th1 cytokine IFNγ was redundant in this model (data not shown), suggesting that these cytokines are unlikely to contribute to the active inflammation and up-regulated bone resorption in the KO mice. The level of the osteoclast-inducing cytokine RANKL was not different between the strains 21 days post infection (data not shown).
IL-17RA deficient macrophages produce higher level of pro-inflammatory cytokines in response to endodontic pathogens
Macrophages are the most important source of pro-inflammatory cytokines in periapical inflammation (28). We therefore determined whether IL-17RA deficiency alters the production of pro-inflammatory cytokines, which were elevated in periapical lesions, in vitro. As shown in Figure 6, IL-17RA KO macrophages exhibited elevated production of MIP2 (≃115% vs. WT cells), IL-1α (≃240%), and IL-1β (≃125%) in response to fixed endodontic pathogens. This result suggests that deficiency of IL-17RA impacts activity of macrophages in response to bacteria.
Figure 6. IL-17RA deficient macrophages exhibited elevated pro-inflammatory cytokine productions in vitro.
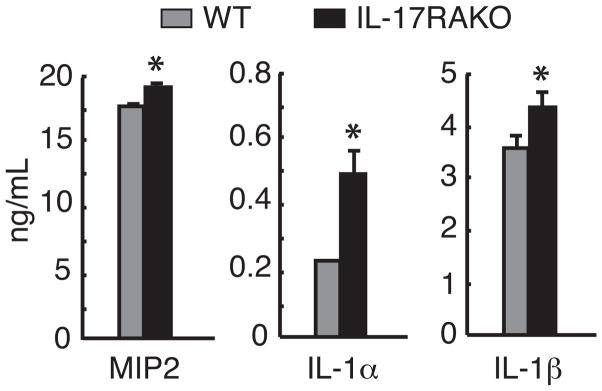
Resident peritoneal macrophages harvested from IL-17RA KO and WT mice were stimulated with a cocktail of fixed endodontic pathogens in triplicates. After 24-hr stimulation, the level of IL-1α, IL-1β, and MIP2 was determined by ELISA. Cells cultured without bacteria served as background controls, and all cytokines were not detectable in the controls (data not shown). Vertical bars: SD, *: p<0.01 vs. WT cells.
Functional role of proinflammatory cytokines in the development of periapical lesion in IL-17RA KO mice
Our findings above led to a hypothesis that the elevated pro-inflammatory cytokines mediates enhanced periapical bone loss in IL-17RA KO mice. To test this hypothesis, we blocked these mediators using neutralizing antibodies in infected IL-17RA KO mice. As shown in Figures 7A and B, neutralization of MIP2 and IL-1β, but not IL-1α, suppressed the development of periapical lesions (by 55% and 80% on average, respectively) compared to mice that received the control IgG treatment. By histology, we found that the neutralization of MIP2 and IL-1β markedly inhibited accumulation of neutrophils, which was a characteristic observation in infected IL-17RA KO mice. These results support a protective role of IL-17RA signaling via suppression of MIP2 and IL-1βin neutrophil induced inflammation.
Figure 7. Neutralization of MIP2 and IL-1β, but not IL-1α suppressed neutrophil accumulation andperiapical bone loss in IL -17RA KO mice.
A Representative μCT images of periapical lesions (anterior-posterior section). The effect of neutralization of proinflammatory cytokines was determined on Day 21 post infection (n=4/group). R: dental root, I: incisor; Arrow: periapical lesion. B Periapical lesion size. The development of periapical lesion was suppressed by neutralization of MIP2 and IL-1β. Vertical bar: SD, *: p<0.05, **: p<0.01. C Representative images of H&E staining (100X). Characteristic neutrophilic inflammation in IL-17RA KO mice (see Fig. 4A) was dramatically suppressed by neutralization of MIP2 and IL-1β. R: dental root, Arrow: accumulation of neutrophils.
Discussion
The IL-17 cytokine family contains six members (IL-17 A, B, C, D, E and F) and five receptors (IL-17RA, RB, RC, RD and RE) (29). The major immunoregulatory ligand is IL-17A that primarily binds IL-17RA. Increasing evidence supports an essential role for the IL-17 family of ligands and receptors in tissue homeostasis in health and disease (1). Differing models of inflammation have reported opposite functional roles for IL-17 in term of bone resorption. In rodent models of rheumatoid arthritis, IL-17 level was elevated in synovial fluid (4–6) and blocking of IL-17 reduced the inflammation and bone damage, whereas IL-17 excess led to disease exacerbation (7, 8). In old mice, gingival IL-17 is up-regulated by age-related reduction of Del-1, which is an antagonist of a neutrophil chemoattractant LFA-1, and triggers marginal periodontitis with elevated neutrophil accumulation (15). In the same study, IL-17 was also shown to be harmful in induction of ligature-induced bone loss in both young and old mice. In contrast, IL-17 is protective in reducing alveolar bone resorption in periodontal disease induced by Porphyromonas gingivalis infection, possibly due to defective neutrophil recruitment (13, 14). According to these findings, we utilized IL-17RA KO mice and IL-17 neutralization to determine the role of IL-17RA signal in the development of periapical lesion. Our data suggest that the susceptibility of IL-17RA KO mice to lesion development appeared to be dependent on persistent active inflammation during 21-day observation period compared to WT mice.
Periapical lesion is a bone destructive dental disease caused by infection in the dental root canal system. In contrast to periodontal disease, in which the bacteria have to invade the periodontal tissue through the gingival epithelium barrier, endodontic infections involve direct tissue invasion of bacteria and may subsequently lead to different host immune responses. In the present study, infected IL-17RA KO and IL-17-neutralized WT mice exhibited increased periapical lesions. The results show that IL-17RA signalling was protective in the development of periapical lesion in both situations and similar to that in P. gingivalis-induced periodontitis (14). However, the mechanism of IL-17RA signal-mediated protection in the periapical lesions seems to be different from that in the periodontitis model. In the periodontitis model (14), the expression of neutrophil chemotactic factors MIP2 and GROα/KC where shown to be significantly lower in IL-17RA KO compared to infected WT controls. The reduction of these chemotactic factors leads to a neutropenic condition resulting in deteriorated periodontitis. In contrast, mobilization of neutrophils was more abundant in infected IL-17RA KO compared to WT controls in the present study. Although the absence of IL-17RA decreased the expression of MIP-2 in the periodontitis model, MIP2 expression was contrarily up-regulated along with IL-1α and β in IL-17RA KO in our study. These findings suggest the existence of an IL-17RA signal-independent pathway for proinflammatory mediator production. It is important to emphasize that neutrophil function per se in IL-17RA KO mice seems to be normal, since the cells produce normal levels of myeloperoxidase and migrate normally ex vivo to chemokines and other stimuli (14). In addition, the phagocytic function of IL-17RA null neutrophils is not impaired compared to WT neutrophils (30). Therefore, our results suggest that periapical lesion is caused by persistent neutrophilic inflammation in IL-17RA KO mice.
In WT mice, the number of macrophages was significantly reduced on day 21 compared to day 10 post infection, whereas the level remained elevated in IL-17RA KO mice in the same time period. Furthermore, the level of IL-1α and β was not significantly changed in any observation period in the WT mice compared to IL-17RA KO mice, which exhibited increased expression of IL-1α and β from day 10. In the 21 days observation period, the up-regulation of both IL-1α and β in KO mice was more than 10-folds compared to its own controls. We previously reported that macrophages are prominent in inflammatory periapical tissues, and produce large quantities of IL-1 (24, 31). Macrophages are also an important source of MIP-2(32) as a neutrophil chemoattractant in the present study. The production of these pro-inflammatory cytokines by IL-17RA KO macrophages in response to fixed endodontic pathogens in vitro was higher compared to that by WT cells. Thus, increased pro-inflammatory cytokines in periapical lesion in IL-17RA KO mice in this study appeared to be dependent on the elevated cytokine productivity and the number of macrophages.
The kinetic of macrophages and neutrophils in IL-17RA KO indicates a failure of resolution of inflammation. A serial transition of inflammation involving predominantly pro-inflammatory M1 and anti-inflammatory/pro-fibrotic M2 macrophages is important for resolution of inflammation and tissue repair (33). IL-17RA KO mice exhibited prolonged infiltration of macrophages with a M2-dominant profile in response to endodontic infection. It is also likely that M2 macrophages can derive IL-1 as a pro-fibrotic cytokine (34, 35). However, IL-1β may play a harmful role in the development of periapical lesion in IL-17RA KO mice. A potential pathway for the susceptibility of IL-17RA KO to endodontic infection involving elevated pro-inflammatory cytokines and persistent neutrophil infiltration, was further supported by our data showing dramatic reduction of neutrophilic inflammation and periapical bone loss by neutralization of MIP2 and IL-1β in this mouse strain. Our data demonstrate that IL-17RA signal contributes to the resolution of periapical inflammation.
We previously demonstrated that B cells are important in prevention of dissemination of endodontic infection(36). In this study, we observed an augmented mobilization of B cells in periapical lesions of IL-17RA KO mice. This finding is in accordance to a previous study, which found elevated gastric B cell number in a model of Helicobacter pylori infection in the same strain of mice(26). However, the role of accumulated B cells, whether protective or harmful, is still unknown in the IL-17RA KO model.
On the other hand, the functional role of IL-17 in periapical lesions was recently assessed using IL-17 KO mice(16), and showed significantly less bone loss in infected IL-17 KO mice than infected WT littermates. In opposite, the extent of bone loss in IL-17RA KO was significantly increased compared to WT controls in this study. We also observed enlarged periapical lesions when we neutralized IL-17 ligands using IL-17 neutralizing antibody in WT mice. One possible reason for this discordance is pathogen-dependent alteration of macrophage activation. Oseko et al. (16) employed P. gingivalis 33277 that can induce myeloid suppressor cell-dependent immunosuppression (37), while we employed four different pathogens as described above. Another possible reason is that IL-17F compensates for the role of IL-17A via IL-17RA in IL-17 KO mice. IL-17F utilizes IL-17RA and has similar biological effect compared to IL-17A (38). These factors might affect the discordant outcome.
Taken together, we concluded that IL-17 signal is protective in the development of periapical lesion depending on its regulation of myeloid cell-mediated inflammation. The detailed mechanism behind the IL-17 signal-mediated protection in periapical lesion is however still unclear. In particular, the mechanism of up-regulated proinflammatory cytokine production by IL-17RA null macrophages, inclusion of other immune cells, and effect of pathogen-dependent immunomodulation needs to be determined in future studies.
Acknowledgments
This work was supported by grants from the Krakow Endodontic Research Fund (E.A., J.R.), Norwegian Research Council (Leiv Erikson Grant), The JSPS Strategic Young Researcher Overseas Visits Program for Accelerating Brain Circulation (H.F), DE-09018 (P.S.) from the NIDCR/NIH, and RR027553 (H.S.) from NCRR/NIH.
Authors acknowledge Amgen Inc. and Dr. Sarah Gaffen (University of Pittsburgh, School of Medicine, Division of Rheumatology & Clinical Immunology) for providing IL-17RA KO mice. We also acknowledge Drs. Thomas Kohler (b-cube AG, Switzerland) and Ralph Müller (Institute for Biomechanics, ETH Zurich, Switzerland) for μCT scanning. We thank Drs. Antonio Campos-Neto and Thomas Van Dyke (both The Forsyth Institute) for helpful discussions. We are grateful to Susan Orlando for her assistance in preparation of this manuscript.
References
- 1.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 2.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155–174. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal S, Gurney AL. IL-17: prototype member of an emerging cytokine family. J Leukoc Biol. 2002;71:1–8. [PubMed] [Google Scholar]
- 4.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, Cua DJ, Takayanagi H. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, Martin TJ, Suda T. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van bezooijen RL, Farih-Sips HC, Papapoulos SE, Lowik CW. Interleukin-17: A new bone acting cytokine in vitro. J Bone Miner Res. 1999;14:1513–1521. doi: 10.1359/jbmr.1999.14.9.1513. [DOI] [PubMed] [Google Scholar]
- 7.Chabaud M, Lubberts E, Joosten L, van Den Berg W, Miossec P. IL-17 derived from juxta-articular bone and synovium contributes to joint degradation in rheumatoid arthritis. Arthritis research. 2001;3:168–177. doi: 10.1186/ar294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lubberts E, Joosten LA, van de Loo FA, Schwarzenberger P, Kolls J, van den Berg WB. Overexpression of IL-17 in the knee joint of collagen type II immunized mice promotes collagen arthritis and aggravates jointdestruction. Inflamm Res. 2002;51:102–104. doi: 10.1007/BF02684010. [DOI] [PubMed] [Google Scholar]
- 9.Colic M, Gazivoda D, Vucevic D, Vasilijic S, Rudolf R, Lukic A. Proinflammatory and immunoregulatory mechanisms in periapical lesions. Mol Immunol. 2009;47:101–113. doi: 10.1016/j.molimm.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Vernal R, Dutzan N, Chaparro A, Puente J, Antonieta Valenzuela M, Gamonal J. Levels of interleukin-17 in gingival crevicular fluid and in supernatants of cellular cultures of gingival tissue from patients with chronic periodontitis. J Clin Periodontol. 2005;32:383–389. doi: 10.1111/j.1600-051X.2005.00684.x. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi K, Azuma T, Motohira H, Kinane DF, Kitetsu S. The potential role of interleukin-17 in the immunopathology of periodontal disease. J Clin Periodontol. 2005;32:369–374. doi: 10.1111/j.1600-051X.2005.00676.x. [DOI] [PubMed] [Google Scholar]
- 12.Cardoso CR, Garlet GP, Crippa GE, Rosa AL, Junior WM, Rossi MA, Silva JS. Evidence of the presence of T helper type 17 cells in chronic lesions of human periodontal disease. Oral Microbiol Immunol. 2009;24:1–6. doi: 10.1111/j.1399-302X.2008.00463.x. [DOI] [PubMed] [Google Scholar]
- 13.Kramer JM, Gaffen SL. Interleukin-17: a new paradigm in inflammation, autoimmunity, and therapy. J Periodontol. 2007;78:1083–1093. doi: 10.1902/jop.2007.060392. [DOI] [PubMed] [Google Scholar]
- 14.Yu JJ, Ruddy MJ, Wong GC, Sfintescu C, Baker PJ, Smith JB, Evans RT, Gaffen SL. An essential role for IL-17 in preventing pathogen-initiated bone destruction: recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals. Blood. 2007;109:3794–3802. doi: 10.1182/blood-2005-09-010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eskan MA, Jotwani R, Abe T, Chmelar J, Lim JH, Liang S, Ciero PA, Krauss JL, Li F, Rauner M, Hofbauer LC, Choi EY, Chung KJ, Hashim A, Curtis MA, Chavakis T, Hajishengallis G. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nature immunology. 2012;13:465–473. doi: 10.1038/ni.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oseko F, Yamamoto T, Akamatsu Y, Kanamura N, Iwakura Y, Imanishi J, Kita M. IL-17 is involved in bone resorption in mouse periapical lesions. Microbiol Immunol. 2009;53:287–294. doi: 10.1111/j.1348-0421.2009.00123.x. [DOI] [PubMed] [Google Scholar]
- 17.Kawashima N, Stashenko P. Expression of bone-resorptive and regulatory cytokines in murine periapical inflammation. Arch Oral Biol. 1999;44:55–66. doi: 10.1016/s0003-9969(98)00094-6. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki H, Hou L, Belani A, Wang CY, Uchiyama T, Muller R, Stashenko P. IL-10, but not IL-4, suppresses infection-stimulated bone resorption in vivo. J Immunol. 2000;165:3626–3630. doi: 10.4049/jimmunol.165.7.3626. [DOI] [PubMed] [Google Scholar]
- 19.Sasaki H, Okamatsu Y, Kawai T, Kent R, Taubman M, Stashenko P. The interleukin-10 knockout mouse is highly susceptible to Porphyromonas gingivalis-induced alveolar bone loss. J Periodontal Res. 2004;39:432–441. doi: 10.1111/j.1600-0765.2004.00760.x. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki H, Balto K, Kawashima N, Eastcott J, Hoshino K, Akira S, Stashenko P. Gamma interferon (IFN-gamma) and IFN-gamma-inducing cytokines interleukin-12 (IL-12) and IL-18 do not augment infection-stimulated bone resorption in vivo. Clin Diagn Lab Immunol. 2004;11:106–110. doi: 10.1128/CDLI.11.1.106-110.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linden A. Role of interleukin-17 and the neutrophil in asthma. International archives of allergy and immunology. 2001;126:179–184. doi: 10.1159/000049511. [DOI] [PubMed] [Google Scholar]
- 22.Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 23.Huang S, Paulauskis JD, Godleski JJ, Kobzik L. Expression of macrophage inflammatory protein-2 and KC mRNA in pulmonary inflammation. Am J Pathol. 1992;141:981–988. [PMC free article] [PubMed] [Google Scholar]
- 24.Tani-Ishii N, Wang CY, Stashenko P. Immunolocalization of bone-resorptive cytokines in rat pulp and periapical lesions following surgical pulp exposure. Oral MicrobiolImmunol. 1995;10:213–219. doi: 10.1111/j.1399-302x.1995.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 25.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nature immunology. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 26.Algood HM, Allen SS, Washington MK, Peek RM, Jr, Miller GG, Cover TL. Regulation of gastric B cell recruitment is dependent on IL-17 receptor A signaling in a model of chronic bacterial infection. J Immunol. 2009;183:5837–5846. doi: 10.4049/jimmunol.0901206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng L, Xia Y, Yoshimura T, Wilson CB. Modulation of neutrophil influx in glomerulonephritis in the rat with anti-macrophage inflammatory protein-2 (MIP-2) antibody. J Clin Invest. 1995;95:1009–1017. doi: 10.1172/JCI117745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang CY, Stashenko P. The role of interleukin-1 alpha in the pathogenesis of periapical bone destruction in a rat model system. Oral Microbiol Immunol. 1993;8:50–56. doi: 10.1111/j.1399-302x.1993.tb00543.x. [DOI] [PubMed] [Google Scholar]
- 29.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 30.van de Veerdonk FL, Kullberg BJ, Verschueren IC, Hendriks T, van der Meer JW, Joosten LA, Netea MG. Differential effects of IL-17 pathway in disseminated candidiasis and zymosan-induced multiple organ failure. Shock. 2010;34:407–411. doi: 10.1097/SHK.0b013e3181d67041. [DOI] [PubMed] [Google Scholar]
- 31.Wang CY, Tani-Ishii N, Stashenko P. Bone-resorptive cytokine gene expression in periapical lesions in the rat. Oral Microbiol Immunol. 1997;12:65–71. doi: 10.1111/j.1399-302x.1997.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 32.Wolpe SD, Sherry B, Juers D, Davatelis G, Yurt RW, Cerami A. Identification and characterization of macrophage inflammatory protein 2. Proc Natl Acad Sci U S A. 1989;86:612–616. doi: 10.1073/pnas.86.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anders HJ, Ryu M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney international. 2011;80:915–925. doi: 10.1038/ki.2011.217. [DOI] [PubMed] [Google Scholar]
- 34.Fairweather D, Cihakova D. Alternatively activated macrophages in infection and autoimmunity. Journal of autoimmunity. 2009;33:222–230. doi: 10.1016/j.jaut.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanke ML, Angle A, Kielian T. MyD88-dependent signaling influences fibrosis and alternative macrophage activation during Staphylococcus aureus biofilm infection. PloS one. 2012;7:e42476. doi: 10.1371/journal.pone.0042476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hou L, Sasakj H, Stashenko P. B-Cell deficiency predisposes mice to disseminating anaerobic infections: protection by passive antibody transfer. Infect Immun. 2000;68:5645–5651. doi: 10.1128/iai.68.10.5645-5651.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ezernitchi AV, Vaknin I, Cohen-Daniel L, Levy O, Manaster E, Halabi A, Pikarsky E, Shapira L, Baniyash M. TCR zeta down-regulation under chronic inflammation is mediated by myeloid suppressor cells differentially distributed between various lymphatic organs. J Immunol. 2006;177:4763–4772. doi: 10.4049/jimmunol.177.7.4763. [DOI] [PubMed] [Google Scholar]
- 38.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]