Abstract
Background
Pooled viral load (VL) testing with two different testing strategies was evaluated as a potential cost-saving method to monitor antiretroviral therapy (ART) in HIV-infected children receiving ART in a resource-limited setting.
Methods
Archived samples collected from 250 HIV-1 infected children on first-line ART at various time-points post-ART initiation were evaluated for pooled VL testing using a minipool+algorithm strategy. Additionally, samples collected in real-time from 125 children on ART were assessed for virologic failure using a minipool strategy for pooled viral load testing. Virologic failure was determined as HIV-1 RNA viral loads >1500 copies/ml.
Results
Minipool+algorithm strategy for pooled VL testing of archived samples had estimated viral failure of 13.6%, with a relative efficiency (RE) of 23.6% (95% CI; 18.5, 29.4), and negative predictive value (NPV) of 88%. This testing strategy would have resulted in 24% fewer assays needed, for a cost savings of $1,180 per 100 samples. The minipool strategy for pooled viral load testing of samples obtained in real-time yielded an estimated 23.2% of samples with viral failure and a RE of 8.0 % (95% CI; 3.9, 14.2); however had a minipool+algorithm pooling strategy been used the RE would increase to 20%.
Conclusions
The minipool+algorithm strategy for pooled VL testing to detect virologic failure in HIV-1 infected children on ART was determined to be relatively efficient in detecting virologic failure, had high NPV, with substantial cost savings. Pooling strategies may be important components of cost-effect strategies to reduce rates of viral failure and resistance, thus improving clinical outcomes.
Introduction
Of the estimated 2.2 million HIV-1 infected children globally, over 90% reside in Africa. A global response to provide drug treatment has resulted in an unprecedented scale-up in the access to antiretroviral therapy (ART) in Africa. In Kenya approximately 24% of HIV-1 infected children were receiving ART in 2010, most of whom were on first-line ARV therapy1. HIV-infected children have substantially higher viral loads than adults and consequently take longer to suppress after ART initiation2–6. Two recent separate meta-analyses found that between 20% and 40% of children in Sub-Saharan Africa had incomplete viral suppression after 12 months on ART, which raises concerns regarding durability of first line ART regimens in children7, 8.
Current monitoring of response to ART in resource-limited settings is based on clinical and immunologic criteria and does not include viral load (VL) testing due to its high cost9–13. The WHO-based clinical and immunologic criteria for detecting virologic failure has low sensitivity: adult studies in developing countries have reported sensitivity rates of 12% – 17%, and a recent pediatric study in Tanzania found a sensitivity rate of 3.5%14–18. A recent study in children on ARV therapy, aged between 24–84 months in South Africa found that virologic monitoring improved the sensitivity and predictive values for detecting virologic failure, compared to use of immunologic criteria alone19. A WHO survey in high HIV burden countries found 98% of children on first-line ART regimens with only 3% of children receiving second-line regimens20. The main reason for low usage of second-line regimens is failure to diagnose treatment failure promptly due to lack of regular virologic testing21.
Moreover, studies in resource-limited settings have reported high rates of drug resistance at the time of virologic failure3. Patients in programs that are not routinely monitored for VL had higher levels of resistance which compromise efficacy of currently recommended second-line regimens14, 15, 17. Among patients with faltering adherence, in addition to enhanced counseling, targeted VL testing may conserve first line ART by differentiating those with and without true virologic failure. Previous studies in adults have shown that targeted counseling in patients with detectable viral breakthrough resulted in a 3% to 5% drop in switching of patients to second line ART, thus promoting retention of first-line regimens10, 11. Infrequent monitoring of VL is commonly associated with delayed switch to second line ART regimens leading to accumulation of ARV resistance mutations and potential for poor outcomes of ART. A large multi-country study in adults found that use of VL testing led to decisions to switch subjects to second-line treatment that are made earlier and at higher CD4 counts and may translate to better clinical outcomes16.
These factors highlight an urgent need to find cheaper technologies and strategies for VL testing and more cost-effective strategies for utilizing available technologies. While development of cheaper VL testing is desirable, progress to decrease VL test costs has been slow and unit cost of virologic testing remains about US $50 per test, which rules out the possibility of individual VL testing in many program settings.
Strategies of pooling specimens for testing were developed to decrease the cost of testing by reducing the number of tests required, and were initially used for detecting acute HIV infection among blood donors with a negative antibody test in areas where HIV prevalence is typically low (range 1% –30%)22–24. Several researchers have evaluated pooled sample viral testing strategies for detection of virologic failure in patients on ART. In studies conducted among populations of varying prevalence of ART failure, different cut-offs to define virologic failure have been evaluated25–27. These studies have shown that pooled VL testing can decrease the cost of virologic monitoring especially in populations with low prevalence of virologic failure25, 26. Strategies including minipool, minipool+algorithm and matrix have been described and evaluated to detect virologic failure using pooled samples25. Under these strategies, pools of individual samples are prepared for initial testing and compared against pre-defined viral detection thresholds; following viral detection above threshold of a given pool, varying approaches are then used to test individual samples.
The use of pooled VL testing of blood samples collected in real-time, to monitor ART failure, particularly from HIV-1 infected children on ART in low-resource settings have not been fully evaluated. This study describes the evaluation of minipool and minipool+algorithm strategies for pooled VL testing to detect virologic failure in children receiving ART in Kenya.
Materials & Methods
Study Population
Two source populations of HIV-1 infected children on ART were evaluated: (i) Archived samples collected previously from a research cohort of HIV-1 infected children on ART, and (ii) Real-time samples collected from HIV-1 infected children on ART in routine follow-up at the HIV clinic in the same hospital.
Archived Samples Cohort
The study used archived blood plasma specimens that were previously collected from HIV-1 infected children enrolled in an ongoing study on Long-term Efficacy of Pediatric ART in Nairobi, which has been described previously6, 28, 29. Briefly, in this prospective, observational study HIV-1 infected children at Kenyatta National Hospital (KNH) were recruited between the years 2004 – 2006. Children aged 18 months to 15 years were enrolled in the study if they presented with advance disease (WHO clinical stage 3–4) or CD4% <15%, and were ART naïve. Children were initiated on first-line ARV regimens in tandem with National Guidelines: two nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) plus one non-nucleoside reverse transcriptase inhibitor (NNRTI), except for those with pulmonary tuberculosis co-infection who were initiated on a regimen consisting of three drugs of the NRTI class. Following ART initiation, children were followed prospectively at monthly intervals during the first year and quarterly thereafter. At each clinic visit, information about medical illness and self-reported ART adherence was collected and complete physical examination including anthropometry was performed. Blood samples were collected at baseline and every 6 months thereafter to monitor immunologic response and drug toxicity. Response to ART was monitored using clinical (growth, opportunistic infection) and immunologic parameters.
Blood plasma samples were collected in duplicate: one sample was stored at −80°C while the other sample was previously tested for HIV-1 RNA VL using Gen-Probe HIV-1 Viral Load Assay (San Diego, CA), which has been validated for detection of HIV-1 subtypes prevalent in Kenya30. Stored frozen plasma were available for the present study for pooled VL testing, and 50 samples were selected at random from each of five time-intervals post-ART initiation. The study received approval from the Kenyatta National Hospital and University of Washington IRB ethical review boards.
Real-time Samples Cohort
Plasma samples were obtained from HIV-infected children on ART enrolled in a PEPFAR-supported KNH treatment program in the same comprehensive HIV care clinic but not from participants in the previous study described above. Children were initiated on treatment between 2005 and 2011 according to the then existing Kenyan National Guidelines. Before 2007 the criteria was similar to that used for the archived samples (advanced disease (WHO clinical stage 3–4) or CD4% <15%) while after 2007 the national guidelines were adjusted to reflect age-specific CD4 criteria. Thus, for children < 5 years of age, CD4 percentage < 20% qualified for HAART initiation while those above 5 years of age the corresponding CD4 percentage threshold was 15%. Following ART initiation, children were monitored monthly for the first 6–12 months and quarterly thereafter. ART monitoring in the children was based on WHO-based clinical criteria (intercurrent illness, growth and development) and immunologic criteria (CD4 count and percentage). Prescriptions for ART were refilled every 1–3 months and information on adherence, toxicity and drug switches was recorded. HIV-1 RNA VL testing was not routinely done except for suspected treatment failure.
A total of 125 eligible children attending the clinic during the period between June and August 2011 were selected for pooled VL testing. Children initiated HAART between August 2005 and January 2011. A baseline venous blood sample was obtained from the child and he/she was given an appointment to return to the clinic after 2 weeks for VL results. If VL above 1500 copies/ml was detected (suggesting virologic failure), clinical and immunologic (e.g. CD4+ T cell count and percentage) assessments of failure were performed. For children with possible clinical, immunologic and virologic failure, a confirmation re-bleed sample for VL testing was requested. If the re-bleed sample had VL results above 1500 copies/ml, HIV viral resistance tests were performed to confirm treatment failure before switching to a second-line treatment.
Approval of the present study was obtained from the KNH ethical review board. Informed consent for study participation was obtained from a parent or caregiver, and verbal assent was acquired for children above 8 years as per Kenya national guidelines.
Preparation of Pools for HIV-1 RNA VL Testing
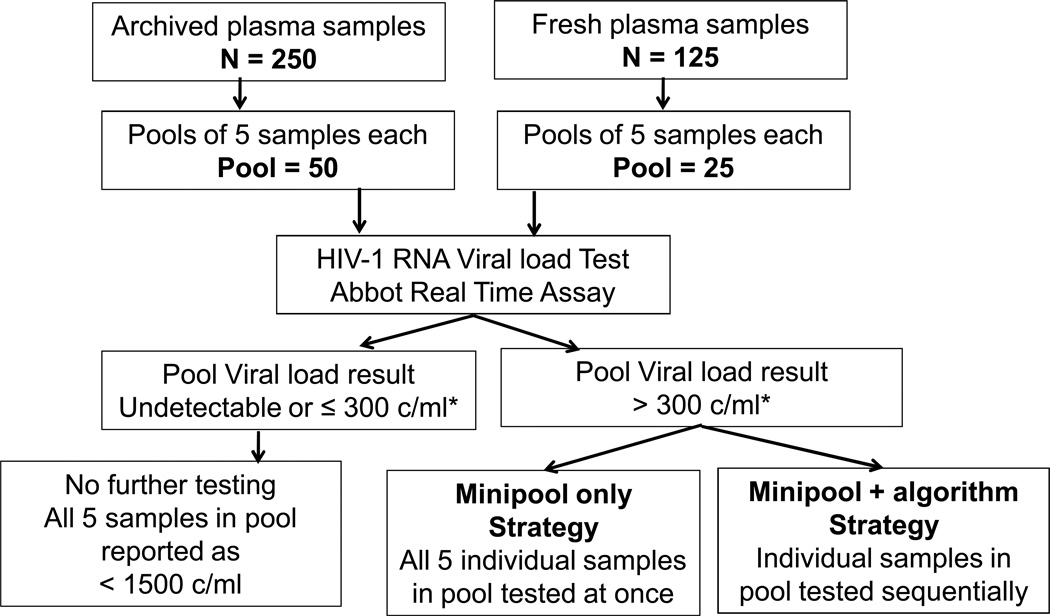
Pools of either archived samples or samples obtained in real-time were prepared in the laboratory using 5 individual samples per pool (Figure 1). For the pools an approximate volume of 600 ul of pooled plasma was prepared, using 125 ul from each of the 5 individual samples. All pools and individual samples were coded and all staff involved in sample preparation or VL testing using pooled methods were blinded to the child’s previous VL results. Samples were tested for HIV-1 RNA VL using a commercial Abbot™ Real-Time HIV-1 Assay, which has a lower limit of detection of 50 copies/ml (and a standard deviation of 0.25). Virologic failure was defined as HIV-1 RNA greater than 1500 copies/ml.
Figure 1.
Strategy for pooled HIV-1 VL testing for all samples. The minipool and minipool+algorithm testing strategies have been previously described by May et al25.
*300 c/ml is the pool threshold of interest for pool size of 5 and definition of virologic failure as viral load > 1500 c/ml
Minipool+algorithm Strategy for Pooled VL Testing of Archived Samples
Pools of archived samples were prepared based on time of sample since ART initiation. Under a minipool+algorithm strategy if the VL result of the pool was less than 300 copies/ml, then it was assumed that all five individual samples in the pool had VL ≤1500 copies/ml (Figure 1). If the VL result of the pool was greater than 300 copies/ml, individual samples in the pool were tested sequentially; individual results (divided by the pool size) were subtracted from the pool VL estimate, until the threshold value was reached (≤300 copies/ml). Hence, the remaining samples in the pool that had not been tested individually were assumed to be below the cut point defining virologic failure (<1500 copies/ml). One to two pools were prepared and tested per week. Implementation of this testing strategy required waiting for results from individual samples and depended on the number of individual samples tested; time for testing varied from 5 to 20 days.
Minipool strategy for Pooled VL Testing of Samples Obtained in Real-time
For testing of plasma samples obtained in real-time, the minipool strategy was evaluated because results were needed in real-time for monitoring of virologic failure and this strategy has a shorter turn-around time than the minipool+algorithm strategy.
Under this minipool strategy, if the VL result for the pool was <300 copies/ml then it was assumed that all individual samples had VL below 1500 copies/ml; while if the VL results of the pool were >300 copies/ml then all individual samples in the pool were tested (Figure 1). About one to two pools were prepared and tested per week, based on the volume of clinic attendance. Time for testing varied from 5 to 10 days.
Statistical Analysis
Relative Efficiency of Pooled VL Testing Strategies
Relative efficiency (RE) of each strategy was defined as one minus the number of assays performed divided by the number of samples25, and thus determines the percentage of assays saved by the pooling strategies compared with individual testing of all samples. A relative efficiency of zero has no advantage over individual testing while a relative efficiency of 50% uses half as many samples as individual testing. As reported by May et al25, an increased RE is function of decreased prevalence of virologic failure in the population and the number of individual samples comprising a pool. Exact binomial 95% confidence intervals of relative efficiency were also calculated.
Negative Predictive Value of Pooled VL Testing Strategy
Negative predictive value (NPV) was calculated for the minipool+algorithm strategy for pooled testing of archived samples, as individual results were available from a previous analysis6. NPV represents the percentage of samples that truly do not have virologic failure under individual testing, among those samples determined to not have virologic failure by the pooling strategy. A high NPV indicates that most subjects determined to not have virologic failure by a testing strategy will be confirmed by individual testing.
All analyses were performed using Stata Intercooled v9.2 (College Station, StataCorp).
Results
Cohort Characteristics
Two hundred and fifty archived plasma samples were obtained from 100 children who participated in the Long-term Pediatric HAART efficacy cohort. At ART initiation children had a median age of 4.7 years (inter-quartile range (IQR), 2.7–6.6 years) and were initiated on first-line ART regimens (64 on nevirapine-containing regimens, 32 on efavirenz-containing regimens, and 4 on triple NRTI regimens) (Table 1). Children had a median follow-up of 3 years (range 4–7 years). Archived blood samples collected within the following time intervals post ART initiation were available: 94 samples at 6 months, 86 samples at 15 months, 85 samples at 27 months, 77 samples at 45 months and 55 samples at 57 months. For the present study, 50 samples from each of the intervals were included, contributing a total of 250 samples collected from 100 children (Table 2). One sample was later determined to fall out of the desired range, and was excluded from examinations by time interval. Each child contributed a median of 3 samples (IQR, 2–3).
Table 1.
Enrollment characteristics and ART regimens of children providing samples for pooled VL testing.
| Enrollment characteristics* | Archived samples (N=100) N (%) or Median (IQR) |
Real-time samples (N=125) N (%) or Median (IQR) |
|---|---|---|
| Age (years) | 4.7 (2.7, 6.6) | 5.9 (4.0, 7.9) |
| Male | 56 (56) | 75 (60) |
| Height (cm) | 96 (83, 108) | 111 (100, 121) |
| Weight (kg) | 14 (10, 17) | 19 (15, 22) |
| CD4%a | 7 (4, 13) | N/A |
| CD4 countb | 352 (102, 659) | 843 (470, 1167) |
| HIV-1 viral load (copies//ml)c | 872,400 | N/A |
| (287,900 – 2,822,300) | ||
| HAART regimen | ||
| NVP/3TC/AZT | 52 (52) | 29 (23) |
| NVP/3TC/ABC | 0 | 27 (22) |
| NVP/3TC/D4T | 12 (12) | 8 (6) |
| EFV/3TC/AZT | 23 (23) | 26 (21) |
| EFV/3TC/ABC | 0 | 30 (24) |
| EFV/3TC/D4T | 9 (9) | 2 (2) |
| LPVr//3TC/AZT | 0 | 1 (1) |
| LPVr//3TC/ABC | 0 | 1 (1) |
| 3TC/AZT/ABC | 2 (2) | 1 (1) |
| 3TC/D4T/ABC | 1 (1) | 0 |
| 3TC/AZT/NFV | 1 (1) | 0 |
Children in the archived samples cohort were initiated on ART soon after enrollment, while children in the real-time samples cohort had been on ART for 4 to 72 months at the time of enrollment.
CD4% results were available from N=88 children in the archived samples cohort, and were not collected from children in the real-time samples cohort.
CD4 count results were available from N=86 children in the archived samples cohort, and for N=20 children in the real-time samples cohort.
HIV-1 VL results were available from N=88 children in the archived samples cohort, and were not collected from children in the real-time samples cohort.
Table 2.
Relative efficiency and cost-savings of Minipool+algorithm strategy for pooled viral load testing of Archived samples.
| Time Post- HAART |
No. samples |
Viral Failure (95% CI) |
No. pools |
No. individual assays performed |
Total no. assays performed |
No. assays saved |
Relative Efficiency (%) (95% CI) |
Cost savings (per 100 assays saved) |
|---|---|---|---|---|---|---|---|---|
| Overall | 250 | 13.6 (9.6–18.5) | 50 | 141 | 191 | 59 | 23.6 (18.5–29.4) | $1,180 |
| 6 month | 49* | 12.2 | 10 | 22 | 32 | 17 | 35 (22–50) | $1,735 |
| 1–2 years | 50 | 12.0 | 10 | 25 | 35 | 15 | 30 (18–45) | $1,500 |
| 2–3 years | 50 | 16.0 | 10 | 40 | 50 | 0 | 0 (0–7) | $0 |
| 3–4 years | 50 | 14.0 | 10 | 29 | 39 | 11 | 22 (12–36) | $1,100 |
| 4–5 years | 50 | 14.0 | 10 | 24 | 34 | 15 | 30 (18–45) | $1,500 |
A total of 250 archived plasma samples were obtained for ART monitoring and pooled in groups of 5 samples according to time post-ART for detection of virologic failure.
After compilation and testing of pools, one sample was found to be out of the desired range and is excluded from the 6 month time interval.
Samples obtained in real-time were collected from 125 children. Children had a median age of 5.9 years (IQR, 4.0 – 7.9 years) and were on the following ART regimens at the time of sample collection: 64 on nevirapine-containing regimens, 58 on efavirenz-containing regimens, 2 on ritonavir-boosted lopinavir containing regimens, and one on a triple NRTI regimen (Table 1). At the time of sample collection, children had been on ART between 4 and 72 months: one child between 4–6 months, 24 between 6 months to one year, 33 between 1–2 years, 23 between 2–3 years, 31 between 3–4 years, 9 between 4–5 years and 4 between 5–6 years.
Relative Efficiency and Virologic Failure of Minipool+algorithm Strategy for Pooled VL Testing of Archived Samples
Overall, the minipool+algorithm strategy for pooled VL testing of archived samples had relative efficiency of 23.6% (95% CI, 18.5–29.4). A total of 34 (13.6%) of 250 samples were determined to have virologic failure (VL >1500 copies/ml). Relative efficiency was highest among samples collected either at 6 months, 1–2 years or 4–5 years post-ART, with relative efficiencies ≥30% (Table 2). Overall, 59 individual samples did not require individual testing. Based on the current local cost of $50 per test, the program thus would have saved $2950 for the testing of 250 samples, or $1,180 per 100 tests.
Negative Predictive Value of Minipool+algorithm Strategy for Pooled VL Testing of Archived samples
Of the 216 archived samples determined to have VL <1500 copies/ml under a minipool+algorithm strategy, 179 samples had individual results from a prior analysis. A total of 158 samples were confirmed below threshold yielding overall NPV of 88% (95% CI, 83–93) (Table 3). Of the 21 samples that the strategy misclassified as VL < 1500 copies/ml, one sample was misclassified at the initial pool stage and 20 samples were misclassified at the individual, sequential testing stage.
Table 3.
NPV of Minipool+algorithm strategy for pooled viral load testing of Archived samples.
| Time since ART initiation |
No. samples without VF |
No. samples with individual results* |
No. confirmed without VF |
NPV (95% CI) |
|---|---|---|---|---|
| Overall | 216 | 179 | 158 | 88 (83–93) |
| 6 month | 43 | 41 | 39 | 95 (83–99) |
| 1–2 years | 44 | 41 | 37 | 90 (77–97) |
| 2–3 years | 42 | 33 | 28 | 85 (68–95) |
| 3–4 years | 43 | 30 | 25 | 83 (65–94) |
| 4–5 years | 43 | 34 | 29 | 85 (69–95) |
Individual sample VL results were available from a prior study.
Relative Efficiency and Virologic failure of Minipool Strategy for Pooled VL Testing of Samples Obtained in Real-time
For the minipool strategy for pooled VL testing of samples obtained in real time, overall relative efficiency was 8.0% (95% CI, 3.9–14.2) (Table 4). Ten samples did not have to be tested and thus the program would have saved only $500, or $400 per 100 tests. A total of 29 (23.2%) of 125 children were found to have virologic failure (VL >1500 copies/ml). Of the 29 children, only 14 children met the additional clinical and/or immunologic criteria for classification of failure, and of those 9 were available for re-bleed and resistance testing; of these, 6 children were identified with ART resistant mutations indicating treatment failure.
Table 4.
Overall Relative efficiency and cost-savings of Minipool and Minipool+algorithm strategies for pooled viral load testing of samples obtained in Real-time.
| Testing Strategy | No. of samples* | No. of assays performed |
No. of assays saved |
Relative Efficiency (95% CI) |
Cost savings (per 100 assays saved) |
|---|---|---|---|---|---|
| Minipool | 125 | 115 | 10 | 8.0 (3.9–14.2) | $400 |
| Minipool+algorithm | 125 | 100 | 25 | 20.0 (13.4–28.1) | $1,000 |
A total of 125 plasma samples were obtained from children attending clinic for ART monitoring in real-time: at various times after ART initiation: 1 within 4–6 months, and 24 within 6–12 months, 33 within 1–2 years, 23 within 2–3 years, 3–4 within 31 years, 9 within 4–5 years, and 4 within 5–6 years post ART initiation.
Had the minipool+algorithm strategy been used for pooled VL testing of this samples obtained in real-time, estimated virologic failure would have decreased to 18.4% and RE would have increased to 20.0%.
Discussion
We analyzed pooled HIV-1 viral load testing using two strategies, the minipool and minipool+algorithm strategies, as has been described earlier25. We observed that pooled VL testing using a minipool+algorithm strategy was a more efficient and cost-saving strategy compared to use of a minipool strategy.
For archived samples using a minipool+algorithm strategy, we observed virologic failure of 13.6% and relative efficiency of 23.6%. According to simulations done by May & others25, for a population with prevalence of virologic failure of about 13–14%, standard deviation of the assay of 0.12, and a minipool+algorithm strategy with pool size of 5, relative efficiency would be estimated at about 40–45%. However, use of an assay with standard deviation of 0.25 like the Abbot™ Real-Time HIV-1 Assay used here may result in a somewhat lower RE, perhaps in the range of what we have observed. NPV was high at 88%, and cost savings would have been substantial at $1,180 per 100 tests.
We selected the minipool VL testing strategy to monitor virologic failure of samples in real-time from children attending an ART clinic. We found the minipool testing strategy to have RE of 8% and not particularly cost-saving. However, had we subjected the same samples to a minipool+algorithm testing strategy relative efficiency would have increased to 20%. Thus we observed that pooled VL testing using the minipool+algorithm was a more efficient and cost-saving strategy compared to minipool. It should be noted that due to the differing strategies of testing individual samples within pools that initially test above threshold, the minipool+algorithm may miss additional true positives that the minipool would not miss. In the archived samples cohort, all but one sample was missed at the individual, sequential testing stage. Further, in the samples obtained in real-time, the minipool strategy captured 6 additional samples with virologic failure than did minipool+algorithm strategy; this would have contributed to the higher VF and lower RE seen. It is thus important that immunologic, growth, and other factors be examined to identify any samples with virologic failure that a testing strategy could miss.
To our knowledge this study is one of the first to perform pooled VL testing in a real-time, clinical management setting to confirm virologic failure in children in a resource-limited setting. In addition to evaluating a minipool+algorithm strategy, we also assessed a minipool testing strategy. While the minipool+algorithm testing strategy had higher relative efficiency, use of this strategy may lead to delay in VL results for individual samples in the pool. In a laboratory that performs VL testing on a routine basis, for example at least 3 days per week typical of a large busy lab, the minipool+algorithm strategy could be suitable as the turn-around time for testing is shorter and confirmatory VL results for an individual may be available within 2 to 3 weeks. However, in many smaller settings samples accrue more slowly, and a minipool testing strategy with shorter turnaround time may be more feasible.
Our study however did include a number of limitations. First, samples obtained in real-time were from children attending the clinic for ART monitoring, therefore samples were taken at varying lengths of time after ART initiation. Pooling and testing for VL according to time since ART initiation can be more efficient: time points with lower prevalences of virologic failure will yield more pools falling below the limit of detection, and thus require fewer tests of individual samples. However, this pooling of samples with varying times since ART initiation is likely to arise in smaller lab settings.
Second, for archived samples under the minipool+algorithm pooling strategy, samples from pool results above the threshold limit were tested individually using the Abbot™ Real-Time HIV-1 assay. However, individual results previously obtained in our collaborator’s laboratory and used to assess negative predictive value were tested using a Gen-Probe HIV-1 VL assay. Thus, the NPVs reported in our study represent both the shortcomings of a pooled VL testing strategy and use of different assays for viral load testing. Further, we could not calculate NPV of the minipool testing strategy used for samples obtained in real-time. However, under a minipool testing strategy that tests all individual samples from positive pools, the source of undetected true positives arises only from pools that test below threshold.
Another limitation of our study is that we only used the minipool and minipool+algorithm strategies for pooled VL testing and there is a possibility that under certain conditions there may be other testing strategies that might be more efficient. A recent study conducted in adults on ART in a low-resource setting employed the matrix strategy, and found that with a prevalence 22% of virologic failure in the population the strategy was cost-effective and saved more than 33% of the VL assays27. However, implementation of a matrix strategy is more complex and can increase the necessary technician time. Further exploration is needed in order to compare the performance of a matrix strategy to the strategies examined here.
In this study in children from a resource-limited setting, we demonstrated that pooled HIV-1 RNA VL testing using the minipool+algorithm strategy to resolve positive pools, can reduce the cost of monitoring for virologic failure by up to a quarter compared with individual VL testing. This efficiency was demonstrated in our archived samples from pediatric HIV-1 infected population with ~14% virologic failure, and was accompanied by a high negative predictive value. Moreover, while the minipool strategy for pooled VL testing is an appealing approach in real-time for clinical management, our study showed that use of minipool strategy was not efficient. Hence, with the use of pooled VL testing with a minipool+algorithm strategy and employment of low-cost technologies for VL assays, HIV-1 VL monitoring may become more accessible for low resource-settings. More research is needed to refine these methods toward achieving greater efficiency and simplicity.
Acknowledgements
The parent study was funded by NIH-Fogarty grants R01 D43-TW000007 and R01 TW007632. Antiretrovirals for the KNH HIV clinic was funded through CDC PEPFAR grants to the University of Nairobi (CCU 024513 CDC 2004–2010, and 5U2GPS002182 CDC 2010–2011). The present study was supported by the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) and the NIH Office of AIDS Research, through supplemental funding of NIH grant 3R01 TW007632-05S1.
The authors thank and appreciate the assistance provided by Susanne May in the initial discussion and design of the study. The authors thank the clinic team, laboratory staff and data management team in Nairobi, Kenya for their participation and cooperation; the Pediatric Molecular Biology Laboratory at Department of Pediatrics, University of Nairobi for performing the VLs; and Barbara Lohman-Payne for her helpful discussion and scientific output throughout the study. Support for statistical analysis was provided by the University of Washington Center For AIDS Research (P30 AI027757), an NIH-funded program. Most of all we thank all the children and their caregivers who participated in the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors declare no conflicts of interest
Data presented at Meeting: Presented as poster P-05 at 6th International Workshop on HIV Treatment, Pathogenesis and Prevention Research in Resource-Limited Settings, 8–11 May, 2012; Mombasa, Kenya
Author Contributions
BHC, DCW AND EMO contributed to the proposal development and study design. BHC, KT and DW performed the data analysis and interpretation of study findings, and wrote the manuscript. BHC and MM participated in study management and data collection. MM, ACK and BK prepared the pools, interpreted the results of pooled VL with BHC and made decisions for individual VL testing. DCW and EMO provided oversight in clinical management of patients. The clinicians AO and RG were involved in recruiting and monitoring children from the KNH clinic. All authors participated in reviewing and commenting on the manuscript.
References
- 1.National AIDS Control Council. United Nations General Assembly Special Session on HIV and AIDS. 2010 Country Report Kenya. Available at: http://data.unaids.org/pub/Report/2010/kenya_2010_country_progress_report_en.pdf.
- 2.Sabin CA, Smith CJ, d'Arminio Monforte A, et al. Response to combination antiretroviral therapy: variation by age. AIDS. 2008 Jul 31;22(12):1463–1473. doi: 10.1097/QAD.0b013e3282f88d02. [DOI] [PubMed] [Google Scholar]
- 3.Sigaloff KC, Calis JC, Geelen SP, van Vugt M, de Wit TF. HIV-1-resistance-associated mutations after failure of first-line antiretroviral treatment among children in resource-poor regions: a systematic review. Lancet Infect Dis. 2011 Oct;11(10):769–779. doi: 10.1016/S1473-3099(11)70141-4. [DOI] [PubMed] [Google Scholar]
- 4.van Rossum AM, Fraaij PL, de Groot R. Efficacy of highly active antiretroviral therapy in HIV-1 infected children. Lancet Infect Dis. 2002 Feb;2(2):93–102. doi: 10.1016/s1473-3099(02)00183-4. [DOI] [PubMed] [Google Scholar]
- 5.Obimbo EM, Wamalwa D, Richardson B, et al. Pediatric HIV-1 in Kenya: pattern and correlates of viral load and association with mortality. J Acquir Immune Defic Syndr. 2009 Jun 1;51(2):209–215. doi: 10.1097/qai.0b013e31819c16d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wamalwa DC, Farquhar C, Obimbo EM, et al. Early response to highly active antiretroviral therapy in HIV-1-infected Kenyan children. J Acquir Immune Defic Syndr. 2007 Jul 1;45(3):311–317. doi: 10.1097/QAI.0b013e318042d613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciaranello AL, Chang Y, Margulis AV, et al. Effectiveness of pediatric antiretroviral therapy in resource-limited settings: a systematic review and meta-analysis. Clin Infect Dis. 2009 Dec 15;49(12):1915–1927. doi: 10.1086/648079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutcliffe CG, Scott S, Mugala N, et al. Survival from 9 months of age among HIV-infected and uninfected Zambian children prior to the availability of antiretroviral therapy. Clin Infect Dis. 2008 Sep 15;47(6):837–844. doi: 10.1086/591203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kantor R, Diero L, Delong A, et al. Misclassification of first-line antiretroviral treatment failure based on immunological monitoring of HIV infection in resource-limited settings. Clin Infect Dis. 2009 Aug 1;49(3):454–462. doi: 10.1086/600396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orrell C, Harling G, Lawn SD, et al. Conservation of first-line antiretroviral treatment regimen where therapeutic options are limited. Antivir Ther. 2007;12(1):83–88. [PubMed] [Google Scholar]
- 11.Wilson D, Keiluhu AK, Kogrum S, et al. HIV-1 viral load monitoring: an opportunity to reinforce treatment adherence in a resource-limited setting in Thailand. Trans R Soc Trop Med Hyg. 2009 Jun;103(6):601–606. doi: 10.1016/j.trstmh.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Calmy A, Ford N, Hirschel B, et al. HIV viral load monitoring in resource-limited regions: optional or necessary? Clin Infect Dis. 2007 Jan 1;44(1):128–134. doi: 10.1086/510073. [DOI] [PubMed] [Google Scholar]
- 13.Phillips AN, Pillay D, Miners AH, Bennett DE, Gilks CF, Lundgren JD. Outcomes from monitoring of patients on antiretroviral therapy in resource-limited settings with viral load, CD4 cell count, or clinical observation alone: a computer simulation model. Lancet. 2008 Apr 26;371(9622):1443–1451. doi: 10.1016/S0140-6736(08)60624-8. [DOI] [PubMed] [Google Scholar]
- 14.Gupta RK, Hill A, Sawyer AW, et al. Virological monitoring and resistance to first-line highly active antiretroviral therapy in adults infected with HIV-1 treated under WHO guidelines: a systematic review and meta-analysis. Lancet Infect Dis. 2009 Jul;9(7):409–417. doi: 10.1016/S1473-3099(09)70136-7. [DOI] [PubMed] [Google Scholar]
- 15.Hosseinipour MC, van Oosterhout JJ, Weigel R, et al. The public health approach to identify antiretroviral therapy failure: high-level nucleoside reverse transcriptase inhibitor resistance among Malawians failing first-line antiretroviral therapy. AIDS. 2009 Jun 1;23(9):1127–1134. doi: 10.1097/QAD.0b013e32832ac34e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keiser O, Tweya H, Boulle A, et al. Switching to second-line antiretroviral therapy in resource-limited settings: comparison of programmes with and without viral load monitoring. AIDS. 2009 Sep 10;23(14):1867–1874. doi: 10.1097/QAD.0b013e32832e05b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumarasamy N, Madhavan V, Venkatesh KK, et al. High frequency of clinically significant mutations after first-line generic highly active antiretroviral therapy failure: implications for second-line options in resource-limited settings. Clin Infect Dis. 2009 Jul 15;49(2):306–309. doi: 10.1086/600044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petersen ML, van der Laan MJ, Napravnik S, Eron JJ, Moore RD, Deeks SG. Long-term consequences of the delay between virologic failure of highly active antiretroviral therapy and regimen modification. AIDS. 2008 Oct 18;22(16):2097–2106. doi: 10.1097/QAD.0b013e32830f97e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies MA, Moultrie H, Eley B, et al. Virologic failure and second-line antiretroviral therapy in children in South Africa--the IeDEA Southern Africa collaboration. J Acquir Immune Defic Syndr. 2011 Mar 1;56(3):270–278. doi: 10.1097/QAI.0b013e3182060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. Recommendations for a public health approach. Geneva: WHO; 2010. [Accessed 2010]. Antiretroviral therapy for HIV infection in infants and children: towards universal access. revision. Available at: http://apps.who.int/medicinedocs/documents/s18809en/s18809en.pdf. [PubMed] [Google Scholar]
- 21.Davies MA, Boulle A, Eley B, et al. Accuracy of immunological criteria for identifying virological failure in children on antiretroviral therapy - The IeDEA Southern Africa Collaboration. Trop Med Int Health. 2011 Nov;16(11):1367–1371. doi: 10.1111/j.1365-3156.2011.02854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Busch MP, Glynn SA, Stramer SL, et al. A new strategy for estimating risks of transfusion-transmitted viral infections based on rates of detection of recently infected donors. Transfusion. 2005 Feb;45(2):254–264. doi: 10.1111/j.1537-2995.2004.04215.x. [DOI] [PubMed] [Google Scholar]
- 23.Patterson KB, Leone PA, Fiscus SA, et al. Frequent detection of acute HIV infection in pregnant women. AIDS. 2007 Nov 12;21(17):2303–2308. doi: 10.1097/QAD.0b013e3282f155da. [DOI] [PubMed] [Google Scholar]
- 24.Pilcher CD, McPherson JT, Leone PA, et al. Real-time, universal screening for acute HIV infection in a routine HIV counseling and testing population. JAMA. 2002 Jul 10;288(2):216–221. doi: 10.1001/jama.288.2.216. [DOI] [PubMed] [Google Scholar]
- 25.May S, Gamst A, Haubrich R, Benson C, Smith DM. Pooled nucleic acid testing to identify antiretroviral treatment failure during HIV infection. J Acquir Immune Defic Syndr. 2010 Feb 1;53(2):194–201. doi: 10.1097/QAI.0b013e3181ba37a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith DM, May SJ, Perez-Santiago J, et al. The use of pooled viral load testing to identify antiretroviral treatment failure. AIDS. 2009 Oct 23;23(16):2151–2158. doi: 10.1097/QAD.0b013e3283313ca9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tilghman MW, Guerena DD, Licea A, et al. Pooled nucleic acid testing to detect antiretroviral treatment failure in Mexico. J Acquir Immune Defic Syndr. 2011 Mar 1;56(3):e70–e74. doi: 10.1097/QAI.0b013e3181ff63d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wamalwa DC, Farquhar C, Obimbo EM, et al. Medication diaries do not improve outcomes with highly active antiretroviral therapy in Kenyan children: a randomized clinical trial. J Int AIDS Soc. 2009;12(1):8. doi: 10.1186/1758-2652-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wamalwa DC, Obimbo EM, Farquhar C, et al. Predictors of mortality in HIV-1 infected children on antiretroviral therapy in Kenya: a prospective cohort. BMC Pediatr. 2010;10:33. doi: 10.1186/1471-2431-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emery S, Bodrug S, Richardson BA, et al. Evaluation of performanceof the Gen-Probe human immunodeficiency virus type 1 viral load assay using primary subtype A, C, and D isolates from Kenya. J Clin Microbiol. 2000 Jul;38(7):2688–2695. doi: 10.1128/jcm.38.7.2688-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]