Objectives
Infections relating to orthopaedic procedures are difficult to treat with systemic antibiotics due to encapsulation of the implant by fibrous tissue and formation of biofilm.1,2
The aim of the study was to develop electrochemically deposited hydroxyapatite (EHA) coating and incorporate silver (Ag) to provide a controlled and sustained release of Ag ions at a bactericidal concentration.
Design
We investigated six groups: electrochemical co-precipitation of HA and Ag (EHA/Ag); EHA pre-coated discs treated in AgNO3 (EHA/AgNO3); plasma-sprayed HA (PHA) pre-coated discs treated in AgNO3 (PHA/AgNO3); EHA with 2 ‘layers’ of Ag (EHA/Ag/2-layers); EHA coating only and PHA coating only.
Setting
The tests were carried out in a laboratory.
Main outcome measures
Bacterial inhibition tests were carried out by placing coated discs on agar plates of Staphylococcus aureus and zones of inhibition were quantified in each group. Scanning electron microscopy and energy dispersive X-ray (EDX) and X-ray diffraction (XRD) analyses quantified coating thickness, calcium/phosphorous ratio and % atomic silver content, respectively.
Results
XRD and EDX analyses confirmed the presence of Ag in all coatings deposited using electrochemical method and silver.
The mean thickness coating was noted to be highest in EHA/AG group (102.20 ± 4.20 um) followed by PHA (76.40 ± 2.20 um). Mean coating thicknesses in other groups were: PHA/AgNO3 (76.20 ± 1.29 um); EHA (32.98 ± 2.50 um); EHA/Ag/2-layers (30.70 ± 2.40 um); EHA/AgNO3 (8.60 ± 0.60 um).
The average silver content measured was highest with 6.55% in EHA/Ag/2-layer followed by 3.92% in EHA/AgNO3, 0.38% in EHA/Ag and 0.10% in PHA/AgNO3.
The electrochemical technique deposited a uniform coating onto the disc surface with clearly defined microcrystals as compared to the PHA-coated discs where the surface appeared smoother. The EHA coating, however, appeared less dense when compared with the PHA coating.
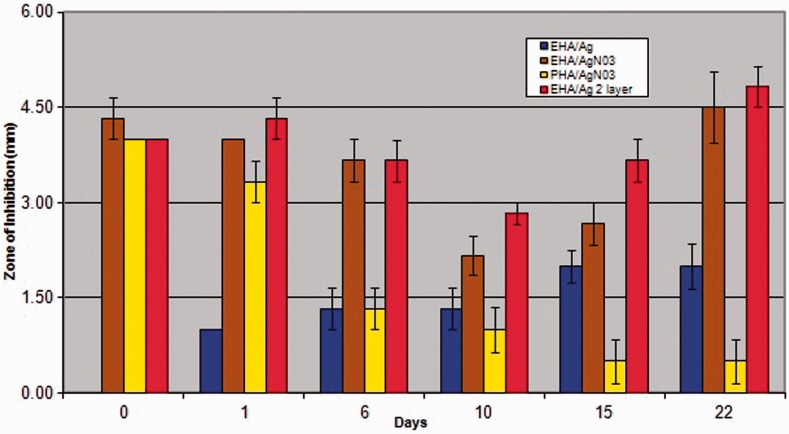
In the bacterial inhibition test no zone of inhibition was seen in the EHA and PHA groups.
No-inhibition zones were seen in the EHA/Ag group on day 0, but appeared on day 1 and gradually increased by day 22. In the PHA/AgNO3 large zones of inhibition were present initially on days 0 and 1 but decreased significantly from day 6 to 22.
Zones of inhibition in EHA/AgNO3 and EHA/Ag/2-layers showed significantly larger zones of inhibition than PHA/AgNO3 at days 6,10,15 and 22 and also significantly larger zones than EHA/Ag at most days (Figure 1).
Figure 1.
A graph comparing bacterial inhibition zones in all groups at all time points.
Conclusions
We have developed a method of electrochemically depositing HA coating and incorporating silver ions within it resulting in a crystal appearance as compared with the plasma-coated HA which has molten appearance.
The bacterial inhibition test signifies that when the smooth PHA-coated discs were dipped in silver nitrate only the surface is coated showing burst release initially and rapidly depleting over days.
Our different methods of incorporating silver within the crystalline EHA entrapped the silver ions within the coating, resulting in sustained release of silver ions at bactericidal concentrations when the coating dissolves.
This study demonstrated that Ag ions can be incorporated into an HA coating using an electrochemical technique to provide an antibacterial implant coating.
DECLARATIONS
Competing interests
None declared
Funding
This study was funded by the Centre for Biomedical Engineering, UCL
Ethical approval
In line with institutional policy, ethical approval was not required
Guarantor
YG
Contributorship
YG conducted literature review, carried out experiments, collected and analysed data and drafted and revised article. MC carried out experiments, analysed data and helped with writing up the article. GB conceived and designed the study and revised the article. Each of the authors has been an active contributor to the article and has seen, approved and is fully conversant with its contents
Acknowledgements
None
Provenance
Submitted; peer reviewed by RSM Orthopaedic section
References
- 1. Parvizi J, Wickstrom E, Zieger AR, et al. Titanium surface with biologic activity against infection. Clin Orthop Relat Res 2004; 429: 33–8 [DOI] [PubMed] [Google Scholar]
- 2. Zimmerli W, Trampuz A, Ochsner PE. Prosthetic joint infections. N Engl J Med 2004; 351: 1645–54 [DOI] [PubMed] [Google Scholar]