Abstract
Objectives
Our goal was to determine the diversity and abundance of Staphylococcus bacteria on different components of a public transportation system in a mid-sized US city (Portland, Oregon) and to examine the level of drug resistance in these bacteria.
Methods
We collected 70 samples from 2 cm × 4 cm sections from seven different areas on buses and trains in Portland, USA, taking 10 samples from each area. We isolated a subset of 14 suspected Staphylococcus spp. colonies based on phenotype, and constructed a phylogeny from16S rRNA sequences to assist in identification. We used the Kirbye–Bauer disk diffusion method to determine resistance levels to six common antibiotics.
Results
We found a range of pathogenic Staphylococcus species. The mean bacterial colony counts were 97.1 on bus and train floors, 80.1 in cloth seats, 9.5 on handrails, 8.6 on seats and armrests at bus stops, 3.8 on the underside of seats, 2.2 on windows, and 1.8 on vinyl seats per 8 cm2 sample area. These differences were significant (p < 0.001). Of the 14 isolates sequenced, 11 were staphylococci, and of these, five were resistant to penicillin and ampicillin, while only two displayed intermediate resistance to bacitracin. All 11 isolates were sensitive to trimethoprim-sulfamethoxazole, vancomycin, and tetracycline.
Conclusions
We found six different strains of Staphylococcus, and while there were varying levels of drug resistance, we did not find extensive levels of multidrug-resistant bacteria, and no S. aureus was found. We found floors and cloth seats to be areas on buses and trains that showed particularly high levels of bacteria.
Keywords: antibiotic resistance, community-acquired infections, environmental microbiology, methicillin-resistant Staphylococcus aureus, pathogen transmission, Staphylococcus, transportation
1. Introduction
Microbes in public areas can be a critical issue in public health, because of the ease of transfer of pathogens from individual to individual [1-5]. This is a particular cause for concern when microbes are drug resistant and pathogenic. Public areas such as restaurants, public transportation systems, parks, schools, daycare centers, and other community areas can bring a large number of people together and facilitate the transmission of microbes [5,6]. Although many drugresistant pathogens have been primarily nosocomial, some, including methicillin-resistant Staphylococcus aureus (MRSA), are also increasingly acquired in the community [7-10]. Therefore, increased attention has been paid to environmental microbes and to the numbers and strains of bacteria found in public places [6,11-13].
Here, we focus on microbes in public transportation, because mass transit systems have become increasingly important in urban areas. With the revitalization of many downtown areas in large urban centers [14,15], and the increased awareness surrounding energy-saving methods of transportation, there has been a push both nationally in the United States [16,17], as well as globally [18,19], towards an increased use of mass transit systems.
Previous studies have indicated that varying levels of bacteria can be found in transport systems [6,13,20]. Within Portland, Oregon, a mid-sized city in the United States (city population approximately 500,000 and metropolitan population around 2.2 million) [21], 17.3% of residents commute to work using bikes and public transportation [22]. The Portland public transit system (TriMet) consists primarily of buses and trains. An estimated 321,100 people per day ride public transportation on weekdays [23], and the system covers a broad geographic area, including suburbs in the greater Portland metropolitan area. As a result, there is potential for widespread interpersonal transfer of bacteria.
The Staphylococcus genus comprises many pathogenic species often found on the skin. These include S. aureus, which has been a pathogen of concern due to the existence of MRSA, as well as strains resistant to vancomycin (vancomycin-resistant S. aureus), a drug often referred to as the “drug of last resort” [24,25]. Furthermore, within the last 5 years, MRSA has moved from being primarily a nosocomial pathogen to one that is also found in community areas and public places [7,26]. Community-acquired strains have been documented in areas such as day-care centers [27-29], fire stations [30], and universities [31]. Furthermore, there are many other species of Staphylococcus that are pathogenic, and our understanding of the distribution of these species in public areas is poor [6]. Therefore, it is becoming increasingly important to understand the risks of Staphylococcus transmission in public areas.
We examined the abundance and distribution of Staphylococcus spp. over several microlocations on Portland’s public transportation system. We were interested in which areas on a bus or train harbored the most bacteria, whether these bacteria were drugresistant, and what the diversity of Staphylococcus was - would we primarily find S. aureus or would we find a range of other species in this genus? Our findings would be able to inform both personal hygiene and public policy regarding cleaning frequency or the areas on which to focus cleaning efforts, as well as the choice of seats type (cloth vs. vinyl), for minimizing exposure to potentially harmful bacteria.
2. Materials and Methods
2.1. Collecting samples
We collected samples on two separate days, April 19 and July 2, 2011. We sampled seven different location types: cloth seats on buses, vinyl seats on trains, handholds and handrails, windows, floors, under seats, and on metal armrests at bus stops. For each of these location types, we obtained 10 different samples. In total, we sampled six different buses, six different bus stops, and four different trains. Using a sterile swab dipped in sterile water, we swiped 2 cm × 4 cm sections. Using aseptic techniques, each sample was streaked onto a Luria–Bertani (LB) agar plate in angled arcs, eight streaks per 100 mm diameter Petri dish, and incubated at 37℃ for 48 hours.
2.2. Bacterial counts and identification of Staphylococcus
After a 48-hour incubation period, all bacterial colony-forming units (cfu, or colonies) were counted from each sample plate. From the 70 plates with a varying number of colonies, we chose a subset of 64 colonies identified as potentially pathogenic Staphylococcus species based on phenotypic traits (size, color, and borders of the colony) [32]. Single colonies from this subset were inoculated into LB broth medium, grown overnight at 37℃ in a shaker and stored at –80℃ in 17% glycerol.
In order to further differentiate these isolates, they were streaked onto LB and mannitol salt agar plates. For each isolate, liquid cultures were streaked onto one quarter of a petri dish and incubated for 48 hours. Growth on LB plates confirmed that the bacteria were viable, and growth on mannitol salt agar (7.5% NaCl by weight in solution) plates indicated likely Staphylococcus spp. due to the relative halotolerance of this genus. Phenol red indicator used in mannitol salt agar changes from red to bright yellow if acidic byproducts from mannitol fermentation are present. This fermentation has traditionally been used to identify coagulase-positive, often pathogenic species of Staphylococci [33-38]. We used one-way analysis of variance (ANOVA) to determine whether the number of cfu per plate results was significantly different among the samples, and Tukey’s post-hoc tests were used to assess which pairwise differences showed significance.
2.3. Determining antibiotic resistance levels
We used the Kirby–Bauer disk diffusion method to test bacterial resistance to six antibiotics: ampicillin, penicillin, tetracycline, bacitracin, trimethoprim-sulfamethoxazole, and vancomycin. We tested 51 strains for resistance. We plated enough bacterial cells to grow a lawn of bacteria and then placed small disks infused with antibiotics onto the petri dishes [BD ampicillin 10 μg, penicillin 10 IU (approximately 6 μg), tetracycline 30 μg, bacitracin 10 IU (approximately 135 μg), trimethoprim-sulfamethoxazole (1.25 μg/23.75 μg), and vancomycin (30 μg)]. These samples were then grown for 24–48 hours before measuring the diameter of the zone of inhibition around each disk. These numbers were then compared to the measurements for disk diffusion standards for Staphylococcus spp. [39].
2.4. Identification using 16S rRNA
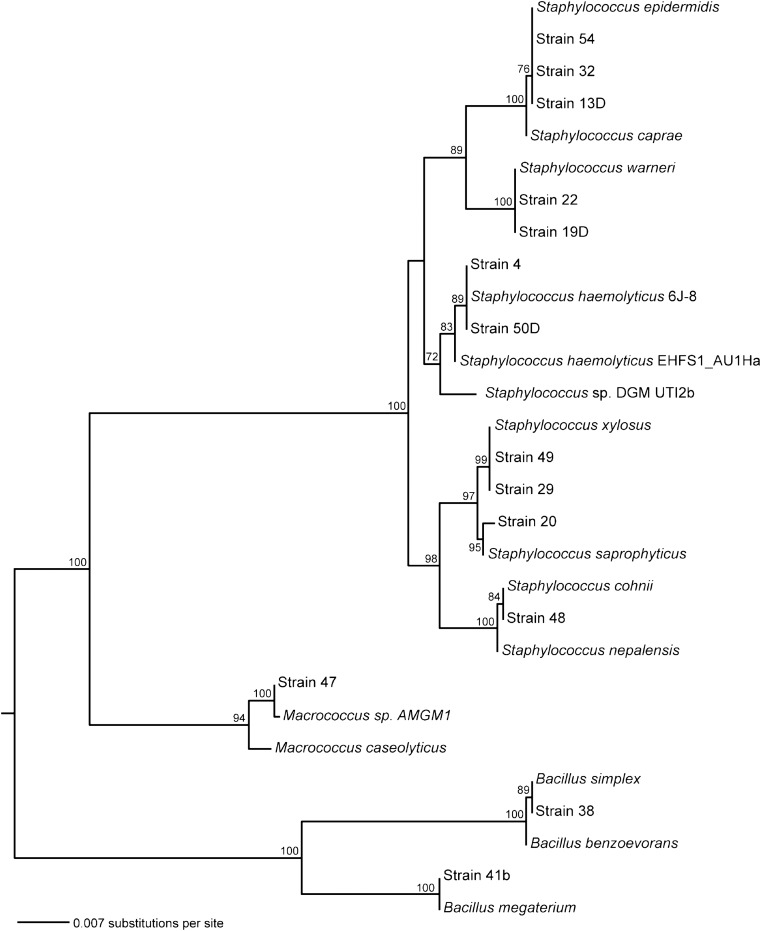
For isolates that grew in mannitol salt agar and fermented mannitol, 1.4 kb of the 16S rRNA was sequenced (Genewiz, South Plainfield, NJ). We used the National Center for Biotechnology BLAST (Information’s Basic Local Alignment Search Tool) to acquire close matches for each of the sequences: Bacillus benzoevorans (AY043085.1), B. megaterium (JF496506.1), B. simplex (JF496314.1), Macrococcus sp. AMGM1(GU322006.1), M. caseolyticus (FJ263452.1), Micrococcus luteus (AB079788.1), S. caprae (NR_024665.1), S. cohnii (HQ169121.1), S. epidermidis (D83362.1), S.haemolyticus 6 J-8 (EU379301.1), S. haemolyticus EHFS1_AU1Ha (EU071616.1), S. nepalensis (NR_028996.1), S. saprophyticus subsp. saprophyticus (JF798360.1), S. saprophyticus V (GQ384358.1), Staphylococcus sp. DGM-UTI2b (JF923460.1), S. warneri (HQ831388.1), and S. xylosus (NR_036907.1). All sequences were aligned using MUSCLE (Multiple Sequence Comparison by Log-Expectation) [40]. Unambiguously aligned positions were identified using Gblocks software [41], with a minimum block size of five characters. The remaining 1,350 positions were submitted to RAxML analysis [42] to generate a maximum likelihood tree.
3. Results
3.1. Abundance of bacteria, by microlocation
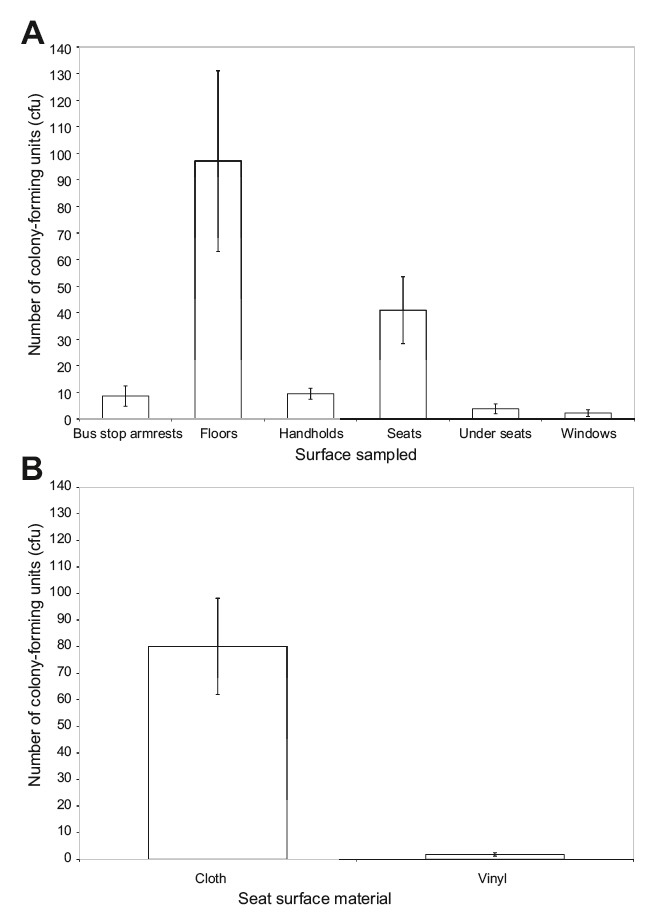
We found the following bacterial colonies per 2 cm × 4 cm swab sample: 97.1 on bus and train floors, 80.1 in cloth seats, 9.5 on handrails, 8.6 on seats and armrests at bus stops, 3.8 on the underside of seats, 2.2 on windows, and 1.8 on vinyl seats (Figure 1). These differences were significant (p < 0.001, F = 7.83, N = 70). Tukey’s post-hoc tests indicated that the following pairs were significantly different in terms of bacterial counts: floor-vinyl seats (p < 0.001), floor- window (p < 0.001), floor-underneath seats (p < 0.001), floor-bus stop armrest (p = 0.001), floor-handrails (p = 0.002), cloth seats-vinyl seats (p = 0.006), cloth seats-window (p = 0.007), cloth seats-underneath seats (p = 0.009), cloth seats-bus stop armrest (p = 0.02), and cloth seats-handrails (p = 0.02).
Figure 1. Comparison of the number of colony-forming units (cfu) found in 8 cm2 locations within the Portland public transit system. There were significant mean differences in the number of cfu between these types of surface. (A) Number of cfu shown for various surface types within TriMet buses and trains, and for bus stops (mean ± standard error). (B) Number of cfu for all seats broken down into surface type:cloth seats found on buses, and vinyl seats found on trains (mean ± standard error).
3.2. Staphylococcal diversity
All of the 64 isolates grew on LB; of these, 51 grew on mannitol salt agar, and of these, 15 turned the red plates yellow, indicating a fermentation of mannitol. We
sequenced the 16S rRNA from 14 of the 15 isolates that grew on and fermented mannitol, which indicates possibly pathogenic Staphylococcus species. Phylogenetic analyses indicate that our sequences fall into three distinct clusters (Figure 2). Specifically, our sequences include two members of the genus Bacillus, one of Macrococcus, and 11 of Staphylococcus. From the subset of staphylococci sequenced, 16S similarities were found with the following taxa: S. xylosus, S. saprophyticus, S. cohnii, S. haemolyticus, S. epidermidis, and S.warneri. Our phylogenetic analysis of these species as
Figure 2. Maximum likelihood tree for 16S sequences of all mannitol-fermenting samples taken from the transit system. The tree is rooted at the midpoint, and bootstrap values (100 replicates) over 70 are shown. It includes a selection of near relatives identified using BLAST for each new sequence. Our samples include 11 strains clustered tightly with other Staphylococcus strains, as well as two strains that match Bacilli and one Macrococcus.
well as S. aureus indicates that none of the samples in this study was S. aureus.
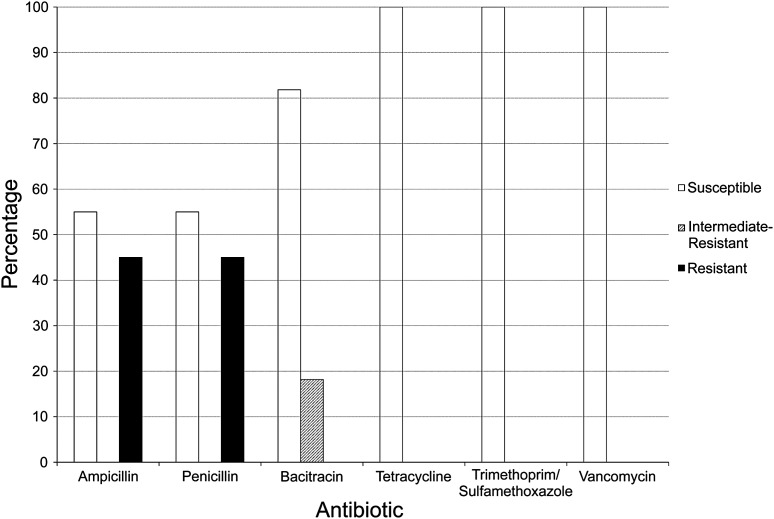
3.3. Staphylococcal antibiotic resistance
Of the 11 confirmed isolates of mannitol-fermenting Staphylococcus we examined, five were resistant to penicillin and ampicillin, two showed intermediate resistance to bacitracin, and none was resistant to tetracycline, trimethoprim-sulfamethoxazole, or vancomycin (Figure 3). Among the 36 strains that grew on but did not ferment mannitol, 11 were resistant to ampicillin
Figure 3. Resistance to antibiotics among confirmed Staphylococcus isolates. Among the 14 isolates sequenced, 16S sequences indicated that 11 were staphylococci. The resistance of these strains to several antibiotics, as determined by a disk diffusion method, is shown on the graph in percentages. The percentages of isolates that were susceptible are shown as white bars, isolates with intermediate resistance are shown as hatched bars, and resistant isolates are shown as black bars. There is a diversity of resistance levels among strains and antibiotics.
and 12 were resistant to penicillin. Three strains were resistant to bacitracin, and one strain was resistant to vancomycin. This strain may have simply been Gramnegative, as Gram-negative bacteria in general are not affected by vancomycin; however, further analysis would be required. None of these bacteria was resistant to trimethoprim-sulfamethoxazole or tetracycline.
4. Discussion
It is not surprising that many bacteria were found on the different surfaces on Portland’s buses and trains. This is consistent with results found in other studies of microbes in public transit systems [6,13,20] (D. Franklin, unpublished data).
Previous studies have shown that nonporous items that hands frequently touch, for example remote controls, door knobs, phones, and computer keyboards [2-4,12,43] harbor smaller numbers of bacteria that change over time due to higher rates of transfer and low retention, despite frequent skin contact. We had therefore expected a lower number of bacteria to be found on the nonporous handrails and handholds in the buses and trains, and our study confirmed this to some degree. The number of bacteria on the handrails was approximately an order of magnitude lower than that found on the floor and on cloth seats. This may also be partially due to the ease of cleaning metal handrails, or to the fact that Portland’s transit system, despite being very popular, is not particularly crowded.
The seat material had a significant effect on the number of bacteria present. Seats with cloth padding had almost 40 times the number of bacteria as those made of vinyl. This is likely due to the low retention and efficient transfer of bacteria on nonporous surfaces, as well as the ease and low cost of cleaning these surfaces in comparison to cloth. Cloth padding may also harbor bacteria beneath the surface areas that cannot be directly washed. These findings were similar to a previous study on the public transport systems in San Francisco, California (D. Franklin, unpublished data), where cloth seats harbored many more bacteria.
Bacteria were most numerous on floors, which was an unexpected result. Floors are generally easy to clean because they can be washed and swept, so large areas can be covered with less human power. The floor bacteria levels from this study were approximately 12 cfu/cm2, which was higher than levels of bacteria on the floor from other studies, examples of which show approximately 3–5 cfu/cm2 [44], < 5 cfu/cm2 [45], 4 cfu/cm2 [46], and approximately 4 cfu/cm2 and 7 cfu/cm2 for semi-clean and dirty floors, respectively [47].
Other findings were less surprising: metal seats and armrests at bus stops had very few bacteria. This may be due to the material, possibly the limited use of these seats, and the fact that many are exposed to the elements, including rain. Windows and areas under seats on buses showed a low number of bacteria, likely because of limited human contact.
A diversity of Staphylococcus species were identified in this study, although none was S. aureus. Although this may be heartening, it should be emphasized that only bacteria that grew on and fermented mannitol were sequenced. Fermentation of mannitol has been a standard method to obtain pathogenic Staphylococcus, but is not always accurate as a diagnostic for pathogenesis or the production of coagulase [48,49]. In this study, two Bacillus species, known to be a false positive for Staphylococcus in the mannitol fermentation test [48], and one member of genus Macrococcus were present alongside many Staphylococci pathogens such as S. cohnii, S. epidermidis, S. haemolyticus, S. saprophyticus, S. warneri, and S. xylosus. Furthermore, Staphylococcus species that could not grow in 7.5% NaCl [50,51] would not have been represented.
The different staphylococci found have all been documented in other studies to be pathogenic. Staphylococcus cohnii, for example, has been show to be an opportunistic pathogen with high morbidity in people with a compromised immune system [52-54]. Moreover, antibiotic-resistant clinical isolates [55] have been reported, as have those with antibiotic resistance plasmids [56]. Staphylococcus cohnii has also been reported as a false positive for MRSA on specialized chromogenic media [57]. The danger of transit microbes may lie not in well-known pathogens such as S. aureus, but in potential pathogens like S. cohnii that may also serve as a reservoir for antibiotic resistance, presenting the threat of horizontal transfer to more virulent Staphylococcus
We also found strains such as S. saprophyticus and S.xylosus that are each implicated in a variety of infections [58-61]. Staphylococcus xylosus, like S. cohnii, has been found as antibiotic-resistant clinical isolates [55]. Two strains we tested were closely related to S. epidermidis, a common nosocomial pathogen [62]. Although S. epidermidis is exclusively opportunistic, lacking many of the toxins produced by S. aureus, it, like S. cohnii and other staphylococci, can present a serious threat in immunocompromised individuals. Furthermore, S. epidermidis has a tendency to form biofilms such as on catheters and other foreign bodies, which cause infections within the patient that are difficult to treat [62].
We also found two strains closely related to S. haemolyticus, which is often both pathogenic and resistant to antibiotics [63-66]. Staphylococcus haemolyticus can cause septicemia and peritonitis as well as infect the ears and urinary tract. Staphylococcus haemolyticus, like the other staphylococci in this study, largely infects immunocompromised individuals and can cause serious infections in such situations [65]. Staphylococcus haemolyticus has also been found to have resistance genes to antibiotics such as gentamicin and erythromycin in its plasmids [63], which can be a source of concern for the horizontal transfer of resistance to other, possibly more virulent bacteria.
Finally, we found two Staphylococci with 16S sequences that were very close to those of S. warneri, another occasionally pathogenic [66-68] microbe that has been found with decreased vancomycin susceptibility [69] and is implicated in bacteremia in immunosuppressed patients [67]. Altogether, all the Staphylococcus species we found have been documented as the causative pathogen in infections in immunosuppressed patients [54,59,62,65,67], with the exception of S. saprophyticus, a common cause of urinary tract infections [58].
In conclusion, this study highlights that, in the Portland public transit system, floors and seats are the areas with the highest bacterial densities, and efforts to clean buses and trains should focus on these areas. Furthermore, there is a clear difference in bacterial levels between cloth and vinyl seats, and this may be something to consider as new buses and trains are manufactured and equipped. Finally, although we found a diversity of Staphylococcus species, we did not find S.aureus, nor did we find evidence of extensive and high levels of multidrug resistance.
This study is limited in scope, but indicates that while we might not have extremely multidrug-resistant bacteria in high abundance on public transport, we should continue to keep monitoring carefully, especially in light of the fact that resistance genes can be transferred horizontally to more pathogenic strains of Staphylococcus. This study can serve as a data point for future comparisons of possible changes in antibiotic resistance levels in public transport systems.
Acknowledgments
We thank D. Wilson for help with collecting data, and A. Krykun and K. Huynh for comments on the manuscripts. We thank the Oregon Medical Research Foundation and Portland State University’s Faculty Enhancement Grant for funding. Salary support for D.S. was provided by Grant Number P20 RR16469 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). The contents of this publication are the sole responsibility of the authors and do not necessarily represent the official views of the NCRR or NIH.
References
- 1.Ehrenkranz NJ. Person-to-person transmission of Staphylococcusaureus. Quantitative characterization of nasal carriers spreadinginfection. N Engl J Med. 1964 Jul 30;271:225–30. doi: 10.1056/NEJM196407302710503. [DOI] [PubMed] [Google Scholar]
- 2.Scott E, Bloomfield SF. The survival and transfer of microbialcontamination via cloths, hands and utensils. J Appl Bacteriol. 1990 Mar;68(3):271–8. doi: 10.1111/j.1365-2672.1990.tb02574.x. [DOI] [PubMed] [Google Scholar]
- 3.Brook J, Brook I. Recovery of organisms from the handrails of escalators in the public metro rail system in Washington, D.C. J Environ Health. 1994 Nov;57(4):13–4. [Google Scholar]
- 4.Rusin P, Maxwell S, Gerba C. Comparative surface-to-hand and fingertip-to-mouth transfer efficiency of gram-positive bacteria, Diversity of Staphylococcus spp. in public transit 207 gram-negative bacteria, and phage. J Appl Microbiol. 2002;93(4):585–92. doi: 10.1046/j.1365-2672.2002.01734.x. [DOI] [PubMed] [Google Scholar]
- 5.Kassem II, Sigler V, Esseili MA. Public computer surfaces are reservoirs for methicillin-resistant staphylococci. ISME J. 2007 Jul;1(3):265–8. doi: 10.1038/ismej.2007.36. [DOI] [PubMed] [Google Scholar]
- 6.Stepanovic S, Cirkovic I, Djukic S, et al. Public transport as a reservoir of methicillin-resistant staphylococci. Lett Appl Microbiol. 2008 Oct;47(4):339–41. doi: 10.1111/j.1472-765x.2008.02436.x. [DOI] [PubMed] [Google Scholar]
- 7.Carleton HA, Diep BA, Charlebois ED, et al. Community-adapted methicillin-resistant Staphylococcus aureus (MRSA): population dynamics of an expanding community reservoir of MRSA. J InfectDis. 2004 Nov 15;190(10):1730–8. doi: 10.1086/425019. [DOI] [PubMed] [Google Scholar]
- 8.Zetola N, Francis JS, Nuermberger EL, Bishai WR. Communityacquired meticillin-resistant Staphylococcus aureus: an emerging threat. Lancet Infect Dis. 2005 May;5(5):275–86. doi: 10.1016/S1473-3099(05)70112-2. [DOI] [PubMed] [Google Scholar]
- 9.Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006 Aug 17;355(7):666–74. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 10.Deurenberg RH, Stobberingh EE. The evolution of Staphylococcus aureus. Infect Genet Evol. 2008 Dec;8(6):747–63. doi: 10.1016/j.meegid.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds KA, Watt PM, Boone SA, Gerba CP. Occurrence of bacteria and biochemical markers on public surfaces. Int J EnvironHealth Res. 2005 Jun;15(3):225–34. doi: 10.1080/09603120500115298. [DOI] [PubMed] [Google Scholar]
- 12.Tunc K, Olgun U. Microbiology of public telephones. J Infect. 2006 Aug;53(2):140–3. doi: 10.1016/j.jinf.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 13.Otter JA, French GL. Bacterial contamination on touch surfaces in the public transport system and in public areas of a hospital in London. Lett Appl Microbiol. 2009 Dec;49(6):803–5.. doi: 10.1111/j.1472-765X.2009.02728.x. [DOI] [PubMed] [Google Scholar]
- 14.Levine M. Downtown redevelopment as an urban growth strategy:a critical appraisal of the Baltimore renaissance. J Urban Aff. 1987 Jun;9(2):103–23. [Google Scholar]
- 15.Gotham K. A city without slums: urban renewal, public housing,and downtown revitalization in Kansas City, Missouri. Am J Econ Sociol. 2001 Jan;60(1):285–316. [Google Scholar]
- 16.Environmental Protection Agency. Transportation control strategies for the state implementation plan: city of Philadelphia.Environmental Protection Agency. Research Triangle Park, NC:EPA; 1973.. [Google Scholar]
- 17.Thompson L. High-speed rail (HSR) in the United States—why isn’t there more? Jpn Railw Transp Rev. 1994 Oct;3:32–9. [Google Scholar]
- 18.Bose R, Srinivasachary V. Policies to reduce energy use and environmental emissions in the transport sector: a case of Delhi city. Energ Policy. 1997 Dec;25(14-15):1137–50. [Google Scholar]
- 19.Barrero R. Energy savings in public transport. IEEE Veh Tech. 2008 Sep;3(3):26–36. [Google Scholar]
- 20.Simoes RR, Aires-de-Sousa M, Conceicao T, et al. High prevalence of EMRSA-15 in Portuguese public buses: a worrisome finding. PLoS One. 2011;6(3):e17630.. doi: 10.1371/journal.pone.0017630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.United States Census Bureau. [[updated Mar 19; cited 2011 Aug 8].];Annual estimates of the population of metropolitan and micropolitan statistical areas: April 1, 2000 to July 1, 2008 [Internet]. 2009 U.S. Census Bureau. Available from:www.census.gov/compendia/smadb/TableC-02.xls.
- 22.United States Census Bureau. 2009 American Community Survey:Portland city, Oregon S0801. Commuting characteristics by sex[Internet]. U.S. Census Bureau.; 2009. [[cited 2011 Aug 5].]. Available from: http://factfinder.census.gov/servlet/STTable?_bm=y&-context=st&-qr_name=ACS_ 2009_5YR_G00_S0801&-ds_name=ACS_2009_5YR_G00_&-tree_ id=5309&-redoLog=true&-_caller=geoselect&-geo_id=16000US 4159000&-format=&-_lang=en. [Google Scholar]
- 23.American Public Transport Association. Transit ridership report:first quarter. American Public Transport Association;; Washington, DC:: 2011. [Google Scholar]
- 24.Lewis R. The rise of antibiotic-resistant infections. FDA Consum. 1995 Sep;29(7):11–5. [PubMed] [Google Scholar]
- 25.Normark BH, Novak R, Ortqvist A, et al. Clinical isolates of Streptococcus pneumoniae that exhibit tolerance of vancomycin. Clin Infect Dis. 2001 Feb 15;32(4):552–8. doi: 10.1086/318697. [DOI] [PubMed] [Google Scholar]
- 26.Popovich KJ, Weinstein RA, Hota B. Are community-associated methicillin-resistant Staphylococcus aureus (MRSA) strains replacing traditional nosocomial MRSA strains? Clin Infect Dis. 2008 Mar 15;46(6):787–94. doi: 10.1086/528716. [DOI] [PubMed] [Google Scholar]
- 27.Adcock PM, Pastor P, Medley F, et al. Methicillin-resistant Staphylococcus aureus in two child care centers. J Infect Dis. 1998 Aug;178(2):577–80. doi: 10.1086/517478. [DOI] [PubMed] [Google Scholar]
- 28.Shahin R, Johnson IL, Jamieson F, et al. Methicillin-resistant Staphylococcus aureus carriage in a child care center following a case of disease. Toronto Child Care Center Study Group. Arch Pediatr Adolesc Med. 1999 Aug;153(8):864–8. doi: 10.1001/archpedi.153.8.864. [DOI] [PubMed] [Google Scholar]
- 29.Aires de Sousa M, Conceicao T, Simas C, de Lencastre H. Comparison of genetic backgrounds of methicillin-resistant and-susceptible Staphylococcus aureus isolates from Portuguese hospitals and the community. J Clin Microbiol. 2005 Oct;43(10):5150–7. doi: 10.1128/JCM.43.10.5150-5157.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts MC, Soge OO, No D, et al. Isolation and characterization of methicillin-resistant Staphylococcus aureus from fire stations in two northwest fire districts. Am J Infect Control. 2011 Jun;39(5):382–9. doi: 10.1016/j.ajic.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Roberts MC, Soge OO, No D, et al. Characterization of methicillin-resistant Staphylococcus aureus isolated from public surfaces on a university campus, student homes and local community. J Appl Microbiol. 2011 Jun;110(6):1531–7. doi: 10.1111/j.1365-2672.2011.05017.x. [DOI] [PubMed] [Google Scholar]
- 32.Hill LR. The Adansonian classification of the staphylococci. J Gen Microbiol. 1959 Apr;20(2):277–83. doi: 10.1099/00221287-20-2-277. [DOI] [PubMed] [Google Scholar]
- 33.Chapman GH. The significance of sodium chloride in studies of staphylococci. J Bacteriol. 1945 Aug;50:201–3. doi: 10.1128/JB.50.2.201-203.1945. [DOI] [PubMed] [Google Scholar]
- 34.Evans JB. Anaerobic fermentation of mannitol by staphylococci. J Bacteriol. 1947 Aug;54(2):266.. doi: 10.1128/jb.54.2.266-266.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edmiston Jr CE, Schmitt DD, Seabrook GR. Coagulase-negative staphylococcal infections in vascular surgery: epidemiology and pathogenesis. Infect Control Hosp Epidemiol. 1989 Mar;10(3):111–7. doi: 10.1086/645977. [DOI] [PubMed] [Google Scholar]
- 36.Moreillon P, Entenza JM, Francioli P, et al. Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infect Immun. 1995 Dec;63(12):4738–43. doi: 10.1128/iai.63.12.4738-4743.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Eiff C, Peters G, Heilmann C. Pathogenesis of infections due to coagulase-negative staphylococci. Lancet Infect Dis. 2002 Nov;2(11):677–85. doi: 10.1016/s1473-3099(02)00438-3. [DOI] [PubMed] [Google Scholar]
- 38.Longauerova A. Coagulase negative staphylococci and their participation in pathogenesis of human infections. Bratisl Lek Listy. 2006;107(11-12):448–52. [PubMed] [Google Scholar]
- 39.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. CLSI approved standard; Wayne, PA: CLSI;: 2007. pp. M100–S17. [Google Scholar]
- 40.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–7. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Talavera G, Castresana J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol. 2007 Aug;56(4):564–77. doi: 10.1080/10635150701472164. [DOI] [PubMed] [Google Scholar]
- 42.Stamatakis A, Hoover P, Rougemont J. Rougemont J. A rapid bootstrap algorithm for the RAxML Web servers. Syst Biol. 2008 Oct;57(5):758–71. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- 43.Wilkoff LJ, Westbrook L, Dixon GJ. Factors affecting the persistence of Staphylococcus aureus on fabrics. Appl Microbiol. 1969 Feb;17(2):268–74. doi: 10.1128/am.17.2.268-274.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andersen BM, Rasch M, Kvist J, et al. Floor cleaning: effect on bacteria and organic materials in hospital rooms. J Hosp Infect. 2009 Jan;71(1):57–65. doi: 10.1016/j.jhin.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 45.Berry MA. Healthy school environment and enhanced educational performance. Carpet and Rug Institute;; Washington, D.C.:: 2002. [Google Scholar]
- 46.Dharan S, Mourouga P, Copin P, et al. Routine disinfection of patients’ environmental surfaces. Myth or reality? J Hosp Infect. 1999 Jun;42(2):113–7. doi: 10.1053/jhin.1999.0567. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki A, Namba Y, Matsuura M, Horisawa A. Bacterial contamination of floors and other surfaces in operating rooms:a five-year survey. J Hyg (Lond) 1984 Dec;93(3):559–66. doi: 10.1017/s002217240006513x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Evans JB. Studies of staphylococci with special reference to the coagulase-positive types. J Bacteriol. 1948 Jun;55(6):793–800. doi: 10.1128/jb.55.6.793-800.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Finegold SM, Sweeney EE. New selective and differential medium for coagulase-positive staphlococci allowing rapid growth and strain differentiation. J Bacteriol. 1961 Apr;81:636–41. doi: 10.1128/jb.81.4.636-641.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bruins MJ, Juffer P, Wolfhagen MJ, Ruijs GJ. Salt tolerance of methicillin-resistant and methicillin-susceptible Staphylococcus aureus. J Clin Microbiol. 2007 Feb;45(2):682–3. doi: 10.1128/JCM.02417-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kassem II. Concerning public transport as a reservoir of methicillin-resistant staphylococci. Lett Appl Microbiol. 2009 Feb;48(2):268. doi: 10.1111/j.1472-765X.2008.02518.x. [DOI] [PubMed] [Google Scholar]
- 52.Mastroianni A, Coronado O, Nanetti A, et al. Communityacquired pneumonia due to Staphylococcus cohnii in an HIVinfected patient: case report and review. Eur J Clin MicrobiolInfect Dis. 1995 Oct;14(10):904–8. doi: 10.1007/BF01691498. [DOI] [PubMed] [Google Scholar]
- 53.Fernandes AP, Perl TM, Herwaldt LA. Staphylococcus cohnii:a case report on an unusual pathogen. Clin Perform Qual Health Care. 1996 Apr-Jun;4(2):107–9. [PubMed] [Google Scholar]
- 54.Basaglia G, Moras L, Bearz A, et al. Staphylococcus cohnii septicaemia in a patient with colon cancer. J Med Microbiol. 2003 Jan;52(Pt 1):101–2. doi: 10.1099/jmm.0.05002-0. [DOI] [PubMed] [Google Scholar]
- 55.Pinna A, Zanetti S, Sotgiu M, et al. Identification and antibiotic susceptibility of coagulase negative staphylococci isolated in corneal/external infections. Br J Ophthalmol. 1999 Jul;83(7):771–3. doi: 10.1136/bjo.83.7.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Szewczyk Karlowsky, Rozalska M, Cieslikowski T, Nowak T. Plasmids of Staphylococcus cohnii isolated from the intensive-care unit. Folia Microbiol (Praha) 2004;Rev Infect Dis(2):123–31. doi: 10.1007/BF02931385. [DOI] [PubMed] [Google Scholar]
- 57.Vinh D, Nichol K, Rand F, Karlowsky J. Not so pretty in pink:Staphylococcus cohnii masquerading as methicillin-resistant Staphylococcus aureus on chromogenic media. J Clin Microbiol. 2006 Dec;44(12):4623–4. doi: 10.1128/JCM.01764-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hovelius B, Mardh PA. Staphylococcus saprophyticus as a common cause of urinary tract infections. Rev Infect Dis. 1984 May-Jun;6(3):328–37. doi: 10.1093/clinids/6.3.328. [DOI] [PubMed] [Google Scholar]
- 59.Siqueira Jr JF, Lima KC. Staphylococcus epidermidis and Staphylococcus xylosus in a secondary root canal infection with persistent symptoms: a case report. Aust Endod J. 2002 Aug;28(2):61–3. doi: 10.1111/j.1747-4477.2002.tb00382.x. [DOI] [PubMed] [Google Scholar]
- 60.Kuroda M, Yamashita A, Hirakawa H, et al. Whole genome sequence of Staphylococcus saprophyticus reveals the pathogenesis of uncomplicated urinary tract infection. Proc Natl Acad Sci U S A. 2005 Sep 13;102(37):13272–7. doi: 10.1073/pnas.0502950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dordet-Frisoni E, Dorchies G, De Araujo C, et al. Genomic diversity in Staphylococcus xylosus. Appl Environ Microbiol. 2007 Nov;73(22):7199–209. doi: 10.1128/AEM.01629-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vuong C, Otto M. Staphylococcus epidermidis infections. Microbes Infect. 2002 Apr;4(4):481–9. doi: 10.1016/s1286-4579(02)01563-0. [DOI] [PubMed] [Google Scholar]
- 63.Froggatt JW, Johnston JL, Galetto DW, Archer GL. Antimicrobial resistance in nosocomial isolates of Staphylococcus haemolyticus. Antimicrob Agents Chemother. 1989 Apr;33(4):460–6. doi: 10.1128/aac.33.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bartoszewicz M, Nowicka J, Przondo-Mordarska A. Przondo-Mordarska A. Selected features determine pathogenicity of Staphylococcus haemolyticus. Med Dosw Mikrobiol. 2003;55(3):225–9. [PubMed] [Google Scholar]
- 65.Takeuchi F, Watanabe S, Baba T, et al. Whole-genome sequencing of Staphylococcus haemolyticus uncovers the extreme plasticity of its genome and the evolution of human-colonizing staphylococcal species. J Bacteriol. 2005 Nov;187(21):7292–308. doi: 10.1128/JB.187.21.7292-7308.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bera A, Gotz F, Biswas R, Herbert S. The presence of peptidoglycan O-acetyltransferase in various staphylococcal species correlates with lysozyme resistance and pathogenicity. Infect Immun. 2006 Aug;74(8):4598–604. doi: 10.1128/IAI.00301-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kamath U, Singer C, Isenberg HD. Clinical significance of Staphylococcus warneri bacteremia. J Clin Microbiol. 1992 Feb;30(2):261–4. doi: 10.1128/jcm.30.2.261-264.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arslan F, Saltoglu N, Mete B, Mert A. Recurrent Staphylococcus warnerii prosthetic valve endocarditis: a case report and review. Ann Clin Microbiol Antimicrob. 2011;10:14. doi: 10.1186/1476-0711-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Center KJ, Reboli AC, Hubler R, et al. Decreased vancomycin susceptibility of coagulase-negative staphylococci in a neonatal intensive care unit: evidence of spread of Staphylococcus warneri. J Clin Microbiol. 2003 Oct;41(10):4660–5. doi: 10.1128/JCM.41.10.4660-4665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]