Abstract
Background
Methamphetamine (METH) use has increased substantially in the last 10 years and poses a serious health concern, especially for young populations. Drug abuse primarily begins during adolescence, when uninhibited and excessive and drug intake is a common occurrence; thus, understanding the developmental patterns of addiction during this critical period is an essential step in its prevention. In the present study, the effect of age on the vulnerability to METH abuse was examined using a rat model of bingeing (i.e., escalation).
Methods
Adolescent and adult rats were compared during short (ShA, 2-h) and long-access (LgA, 6-h) to METH self-administration. On postnatal (PN) days 23 (adolescents) and 90 (adults), rats were implanted with i.v. catheters and trained to lever press for infusions of METH (0.05 mg/kg) during 2-h sessions. Once the rats reached a steady rate of METH self-administration, they were divided into ShA or LgA groups and allowed to self-administer METH for 15 additional days.
Results
Results indicated that adolescent rats earned significantly more infusions than adults under the LgA condition, but the age groups did not differ during ShA. Adolescents, but not adults, also significantly increased (i.e., escalated) METH self-administration across the 15 days of testing under the LgA condition. Further analysis indicated excessive responding during infusions in the LgA METH-exposed adolescents compared to the other groups, suggesting elevated impulsivity or motivation for drug.
Conclusion
These results demonstrate that adolescents are more vulnerable to the escalation of METH than adults during LgA.
Keywords: METH, Adolescence, Escalation, Bingeing, Rat
1. Introduction
Methamphetamine (METH) use is a widespread problem (Rawson et al., 2007). The United Nations Office of Drug and Crime estimates that between 13.7 and 52.9 million people used an amphetamine-like substance at least once in 2010 (nearly half the North American users consumed METH), figures similar to or exceeding cocaine or heroin on a global scale. The growing popularity of METH is attributed to its potency and price. The street cost of METH has continually decreased over the past decade while the drug's purity and availability have increased, thus making first time use more accessible and more addictive (World Drug Report, 2010). Adolescents may be particularly vulnerable to METH abuse given the drug's growing popularity, availability, potency, and low price, and data from U.S. treatment programs indicate that adolescents comprise more than 20% of those admitted for METH abuse or dependence (Rawson et al., 2007; Gonzales et al., 2008).
Drug use generally starts and progresses more rapidly during adolescence. For example, the time from the initial use to the onset of dependence for most drugs of abuse is shorter in adolescents compared to adults (Clark et al., 1998), and once dependence develops, adolescents are more likely to engage in harmful and potentially lethal drug binges (Baumeister and Tossmann, 2005; Estroff et al., 1989; McCambridge and Strang, 2005). Even if abstinence does occur, adolescents are more resistant to treatment interventions and are at an increased risk for relapse compared to adults (Brown and D'Amico, 2001; Catalano et al., 1990; Chung et al., 2006; Dennis et al., 2004; Perepletchikova et al., 2008; Winters and Lee, 2008). These drug abuse patterns are enduring, as epidemiological evidence indicates that adolescence marks a period when individuals are most susceptible to developing lifelong drug addiction (Schramm-Sapyta et al., 2009; Spear, 2004, 2010; Spear and Varlinskaya, 2005, 2010; Doremus-Fitzwater et al., 2010).
Animal models have helped characterize underlying factors that influence drug abuse vulnerability in adolescents. This work has indicated that adolescents (vs. adults) are less sensitive to the aversive aspects of drugs of abuse such as negative withdrawal-related effects that may otherwise limit drug intake (Doremus et al., 2003; Infurna and Spear, 1979; Kota et al., 2007; O'Dell et al., 2007a,b; Schramm-Sapyta et al., 2006; Spear, 2010; Spear and Varlinskaya, 2005; Varlinskaya and Spear, 2004a,b; Doremus-Fitzwater et al., 2010). Furthermore, several studies have indicated that adolescents consume greater amounts of cocaine per kg of body weight (Anker and Carroll, 2010; Anker et al., 2011; Perry et al., 2007), are more resistant to extinction of responding previously reinforced with i.v. cocaine (Anker and Carroll, 2010; Anker et al., 2011), and are more vulnerable to the reinstatement of cocaine-seeking behavior than adult rats (Anker and Carroll, 2010). Thus, animal work supports findings from clinical studies indicating that adolescents have greater drug abuse vulnerability than adults.
The purpose of the present study was to examine METH self-administration using an animal model of drug bingeing (i.e., escalation). This pattern of drug intake was examined because evidence suggests that adolescent humans are particularly prone to engaging in dangerous levels of binge-like drug intake (Baumeister and Tossmann, 2005; Estroff et al., 1989; McCambridge and Strang, 2005), yet it remains unknown if this occurs with respect to METH.
2. Materials and methods
2.1. Subjects
Fourteen adult (postnatal (PN) day 90) and thirty adolescent (PN day 23) male Wistar rats served as subjects in the study. Adolescent rats were bred at the University of Minnesota from parents obtained from Harlan Sprague-Dawley, Inc., Indianapolis, IN, USA and housed in temperature- (24 °C) and humidity-controlled holding rooms under a constant light/dark cycle (12:12 h with room lights on at 6:00 a.m.)where they had ad libitum access to food and water. Adults were obtained from the same supplier and were housed under the same conditions. Adult male rats weighed 450–500g at the beginning of the study, and adolescent rats weighed 70–90g. Male rats are considered adults around PNday 60 (Spear, 2000); thus, adolescence was defined as PN days 21–60. On PN days 23–25 for adolescents and 90–100 for adults, rats were implanted with an intravenous catheter following the procedure outlined by Carroll and Boe (1982). During experimental sessions, adolescent and adult rats had free access to water and were given 20 g ground food (Purina Laboratory Chow) at the end of each session (3:00 p.m.). The experimental protocol (1008A87754) was approved by the University of Minnesota Institutional Animal Care and Use Committee (IACUC), and the experiment was conducted in accordance with the Principles of Laboratory Animal Care (National Research Council, 2003) in laboratory facilities accredited by the American Association for the Accreditation of Laboratory Animal Care.
2.2. METH self-administration
Operant conditioning chambers previously described in detail were used in the present study (Carroll et al., 2002). Three days following surgery, rats were trained to self-administer 0.05mg/kg i.v. METH under a fixed-ratio 1 (FR-1) schedule of reinforcement following methods reported by Anker et al. (2010). Briefly, training sessions began at 9:00 a.m. with the illumination of the house light and ended at 11:00 a.m. with its termination. During each self-administration session, a response on the left lever (active lever) delivered a single infusion of 0.05 mg/kg METH and activated the set of stimulus lights located above the lever for the duration of the infusion. Responses on the active lever that occurred during infusions were recorded but did not result in subsequent infusions. There was no timeout period following infusions. A response on the right lever (inactive lever) also illuminated the stimulus lights above that lever (to control for responding maintained by cues) but did not produce i.v. infusions. Acquisition of METH self-administration occurred once rats earned at least 35 unassisted infusions with a 2:1 active/inactive lever response ratio.
Due to the brief duration of adolescence in rats, and the goal of comparing adolescents and adults on escalation of intake, an abbreviated escalation procedure was used (15 days) as opposed to the standard 21-day procedure (e.g., Anker et al., 2010; Perry et al., 2007). Following acquisition, rats were allowed to maintain stable METH intake under experimental conditions identical to those described for training with the exception that self-administration took place during a short access 2-h session (9:00 a.m.–11:00 a.m.) or an extended access 6-h session (9:00 a.m.–3:00 p.m.) for 15 additional days.
2.3. Drugs
METH was supplied by National Institute of Drug Abuse (Research Triangle Institute, Research Triangle Park, NC, USA), dissolved in 0.9% NaCl at a concentration of 0.2 mg METH/1 ml saline, and refrigerated. Heparin (1/200 ml saline) was added to the METH solution to prevent catheter occlusion from thrombin build-up. The flow rate of each METH infusion was 0.025 ml/s, and the duration of pump activation (1 s/100 g of body weight) was adjusted to maintain rats at a 0.05 mg/kg METH dose throughout self-administration testing. Due to the rapid weight gain in the adolescent group, the infusion duration was adjusted approximately every three days for all rats to maintain the 0.05 mg/kg METH dose.
2.4. Data analysis
Infusions and active and inactive lever responses during the differential access condition served as the primary dependent measures. Responses and infusions were averaged across the first and last 3 days of the differential access condition for the ShA and LgA conditions and were compared with 2-factor repeated-measures ANOVAs (age × days). Responses per second during an infusion were also analyzed for the ShA and LgA groups during the differential access condition to examine age differences in cocaine seeking during brief periods of nonavailabilityto additional i.v. cocaine. After a significant main effect, post hoc tests were conducted using Fisher's LSD protected t-tests. All data analyses were conducted using GB Stat (Dynamic Microsystems, Inc., Silver Spring, MD, USA).
3. Results
Table 1 shows the mean food intake and weight at the start and the end of the differential access phase (days 1–3 vs. days 13–15) for adolescent and adult rats under the ShA and LgA conditions. There were no significant differences in food intake due to age or access condition. Examination of weights indicated that adolescent rats under the ShA and LgA condition significantly increased weight during the end of the procedure compared to the beginning (F1, 59 = 53.49, p < 0.01), while adult rats did not show a significant change. Average infusion duration during the study ranged from 1.12 to 2.29 s for adolescents and 3.45 to 4.06 s for adults.
Table 1.
Mean food intake and weight (g) in adolescent and adult rats under ShA(2h) and LgA(6h) to i.v. METH.
| ShA | LgA | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Adolescent | Adult | Adolescent | Adult | |||||
|
|
|
|
|
|||||
| Days | 1–3 | 13–15 | 1–3 | 13–15 | 1–3 | 13–15 | 1–3 | 13–15 |
| Food intake ± SEM (g) | 15.6 ± 0.9 | 17.1 ± 0.6 | 20.2 ± 0.1 | 20.0 ± 0.0 | 14.8 ± 0.6 | 16.7 ± 1.1 | 19.9 ±0.1 | 20.0 ±0.1 |
| Weight ± SEM (g) | 128.9 ± 5.4 | 194.7 ± 11.3a | 374.2 ± 4.2 | 378.8 ± 6.5 | 146.1 ± 8.4 | 185.8 ± 5.9a | 373.6 ± 8.3 | 373.4 ± 8.7 |
Adolescents (ShA and LgA) weighed significantly more at day 15 of the study compared today 1 (p < 0.01).
3.1. Differential access
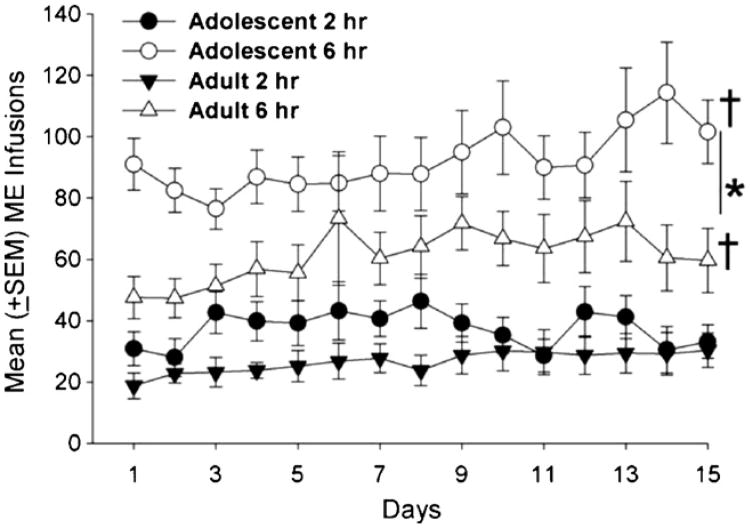
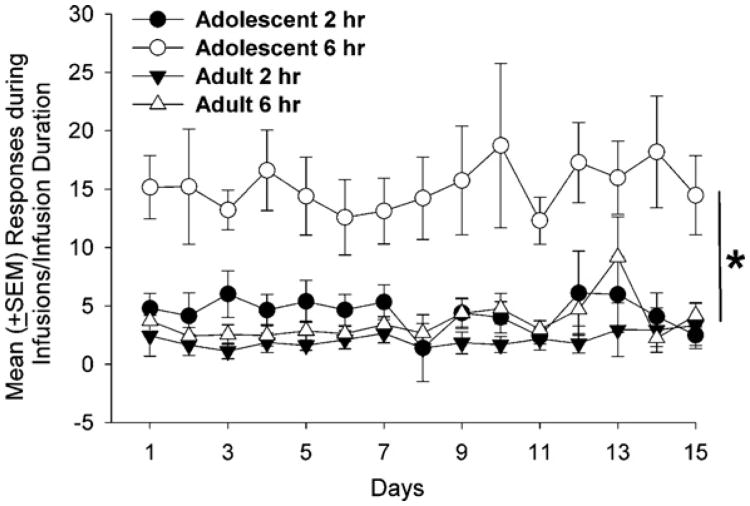
METH infusions (Fig. 1) and active lever presses (Table 2) did not significantly differ between adolescent and adult rats, and there were no differences between the first and last 3 days of the ShA condition. However, during LgA, adolescents earned significantly more METH infusions (F1, 61 =19.00, p < 0.01) and pressed the active lever (F1, 61 = 19.66, p < 0.01) significantly more than adults. In addition, both adolescents and adults earned more infusions (F1, 61 =4.50, p < 0.05) and made more active lever responses (F1, 61 =6.56, p < 0.05) during the last 3 days compared to the first 3 days of the LgA condition. Adolescents also made more inactive lever responses under the ShA (F1, 25 = 8.34, p < 0.05) and LgA (F1, 61 = 6.98, p < 0.05) conditions during this time (Table 2). In addition, under the LgA (but not the ShA) condition, adolescent rats (vs. adult rats) made significantly more responses during infusions (t = 5.49, df = 25, p < 0.01; Fig. 2).
Fig. 1.
Mean (±SEM) METH infusions earned by adolescent and adult male rats during fifteen ShA (2 h) or LgA (6 h) sessions. Adolescents earned significantly more METH infusions than adults under LgA sessions (*p < 0.01). However, there were no significant age differences in the ShA groups. Adolescents and adults under the LgA condition escalated METH intake during the last 3 days compared to the first 3 days of testing (†p < 0.01).
Table 2.
Mean responses on the active and inactive levers by adolescent and adult male rats during fifteen ShA (2 h) or LgA (6 h) sessions.
| ShA | LgA | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Adolescent | Adult | Adolescent | Adult | |||||
|
|
|
|
|
|||||
| Days | 1–3 | 13–15 | 1–3 | 13–15 | 1–3 | 13–15 | 1–3 | 13–15 |
| Active lever± SEM | 40.8 ± 7.2 | 41.9 ± 7.6 | 28.2 ± 6.7 | 42.3 ±11.0 | 104.1 ± 7.2a | 135.4 ± 16.3a,b | 59.4 ± 7.4 | 85.6 ± 12.9b |
| Inactive lever ± SEM | 20.4 ± 7.2a | 25.1±7.1a | 4.2 ±1.4 | 4.9 ±1.6 | 54.6 ± 15.3a | 67.1 ± 26.7a | 8.6 ± 4.9 | 17.6 ± 13.5 |
Adolescents > adults (p < 0.05).
Days 13–15 >days 1–3 (p < 0.05).
Fig. 2.
Mean (±SEM) nonreinforced active lever presses per 1 s of infusion time by adolescent and adult rats with ShA or LgA to i.v. METH. Adolescents (vs. adults) made significantly more nonreinforced responses during LgA sessions (*p < 0.05), but both groups responded equally under ShA conditions.
An additional analysis was conducted to determine if the elevated METH intake in adolescents (vs. adults) during LgA sessions was a result of METH-seeking behavior and not due to general activity. Results confirmed that under the LgA condition adolescents responded more on the active lever (paired with METH infusion) compared to the inactive lever (t = 4.40, df = 39, p < 0.01). Thus, age differences were not due to indiscriminate responding in adolescents under the LgA condition.
4. Discussion
In the present study, adolescent rats earned significantly more METH infusions than adult rats under conditions of prolonged access (LgA) to i.v. METH. These results suggest that adolescence marks a period of increased vulnerability to the self-administration of METH. Similarly, in a recent study, Zakharova et al. (2009) demonstrated that the conditioned rewarding effects of METH were significantly greater in adolescent vs. adult rats under a conditioned place preference (CPP) procedure. Results during the LgA condition also support other work indicating enhanced sensitivity of adolescents vs. adults to cocaine-induced locomotor activity (Caster et al., 2005; Parylak et al., 2008; Snyder et al., 1998) and cocaine induced CPP (Badanich et al., 2006; Brenhouse and Andersen, 2008; Brenhouse et al., 2008; Zakharova et al., 2009). Adolescents have also been reported to exceed adults in self-administration of other drugs of abuse such as amphetamine (Shahbazi et al., 2008), cocaine (Anker and Carroll, 2010; Anker et al., 2011), nicotine (Chen et al., 2007; Levin et al., 2003, 2007), and alcohol (Fullgrabe et al., 2007; Vetter et al., 2007).
Escalation of METH self-administration occurred in adolescent and adult rats under the LgA condition which resembled the escalation of cocaine (Anker et al., 2010; Gipson et al., 2011; Perry et al., 2007) and heroin (McNamara et al., 2010; Vendruscolo et al., 2011) intake reported in previous studies.The pattern of escalating METH intake in the LgA groups resembled the binge-like patterns of drug use prevalent in adolescent humans (Baumeister and Tossmann, 2005; Estroff et al., 1989; McCambridge and Strang, 2005). The higher intake of METH by adolescent compared to adults under the LgA condition supports results from several animal studies demonstrating increased vulnerability in adolescent (vs. adult) rats during other critical phases of the drug abuse process such as acquisition (Anker and Carroll, 2010; Perry et al., 2007), extinction (Anker and Carroll, 2010; Anker et al., 2011), and reinstatement (Anker and Carroll, 2010). In contrast to the LgA condition, adolescent and adult rats earned a similar number of METH infusions during ShA. This is in line with several other findings demonstrating no age differences in cocaine self-administration under similar ShA conditions (2 h) (Frantz et al., 2007; Kantak et al., 2007; Kerstetter and Kantak, 2007; Li and Frantz, 2009). Furthermore, the lack of escalation in both the adolescent and adult ShA groups supports previous reports demonstrating that escalation of METH self-administration in rats is restricted to LgA conditions and does not occur in ShA conditions (Kitamura et al., 2006; Marusich et al., 2010; Schwendt et al., 2009).
Adolescents (vs. adults) under the LgA condition made significantly more active lever responses during brief periods of nonavailability to i.v. METH that occurred during infusions. Since the duration of METH infusions was based on the rat's weight, the adolescents' infusions were far shorter in duration than those of adults. Despite this, LgA adolescents responded more than LgA adults during these brief periods suggesting they were more persistent in seeking METH despite not being rewarded. Other studies support this preservative nonreinforced responding in adolescent rats. For example, adolescents were more likely than adults to respond for amphetamine (Shahbazi et al., 2008) and cocaine (Anker et al., 2011) infusions during periods of signaled nonavailability to drug, a possible indicator of an underlying deficit in response inhibition. Alternately, the nonreinforced responding in adolescents may be due to a general increase in behavior or to a difference in cue reactivity compared to adults.
Similar to a previous study (Anker and Carroll, 2010), adolescent rats responded significantly more on the inactive lever compared to adults during the LgA condition suggesting that increased METH seeking was a result of indiscriminate lever pressing rather than motivation to receive METH infusions. However, adolescents responded significantly more on the lever reinforced with i.v. infusions of METH compared to the inactive lever. Furthermore, indiscriminate active lever responding due to stereotypy was controlled by placing levers at a height the required rats to make a concerted effort to achieve a lever press (i.e., partially stand on haunches and exert pressure downward). Thus, despite increased responding over the 15 days in adolescent vs. adults during LgA, behavior was primarily directed toward drug reinforcement. Results from the differential access phase in the present study indicated that adolescent rats, compared to adults, were more sensitive to the reinforcing effects of METH, and they had a decreased ability to control responding as measured by responding that occurred during an infusion that did not yield additional reinforcement.
Findings from animal and human studies suggest that these age differences may be due to differential activation of brain regions that control reward-related behaviors. Several neurodevelopmental changes occur in the mesolimbic pathway and prefrontal cortex during adolescence (Crews et al., 2007). Dopamine release in the mesolimbic pathway in response to stimulant self-administration is involved in attributing salience to drug and nondrug rewards (Carelli and Deadwyler, 1994; Schultz et al., 1993). In both animals and humans, adolescence is characterized by elevated neuronal dopamine activity in the mesolimbic (reward) pathway of the brain (Chambers et al., 2003) suggesting that adolescents are hypersensitive to reward (Casey et al., 2008). In contrast, during adolescence, the frontal cortex involved in the inhibition of potentially harmful and dangerous behaviors are underdeveloped (Montague et al., 1999). Further, deficits in prefrontal cortex function are associated with decreased executive control and increased vulnerability to relapse in adults with METH dependence (Hester et al., 2010). Overactive mesolimbic and underactive prefrontal cortical function in adolescents may result in behaviors that are driven by rewards rather than executive decision making. This is supported by both human and animal studies that show amplified mesolimbic responses and diminished prefrontal cortical responses in anticipation of rewards (Casey et al., 2008). Thus, with respect to the current study, increased METH self-administration in adolescent rats may have been a result of underdeveloped frontal cortical and mesolimbic areas that guide reward-related behaviors.
The growing occurrence of METH abuse worldwide imposes a pressing need to understand its consequences and patterns of use. Our findings suggest that adolescents are more likely to engage in binge-like METH intake that can be exceedingly harmful and potentially lethal. While METH use is dangerous regardless of the user's age, the consequences adolescent users face are far more serious and detrimental than those faced by adults. Our findings reinforce the importance of METH abuse prevention at young ages before serious drug abuse can occur.
Acknowledgments
We would like to thank Alex Claxton, Nathan Holtz, Seth Johnson, Sean Navin, Paul Regier, Tyler Rehbein, Amy Saykao, and Troy Velie for assistance on this project.
Role of funding source: This research was supported by the National Institute on Drug Abuse (NIDA) grants R01 DA003240, R01 DA019942, and K05 DA015267 (MEC). NIDA had no further role in the study design; in the collection, analysis, and interpretation of data; in writing; nor in the decision to submit the manuscript for publication.
Footnotes
Contributors: Justin Anker and Marilyn Carroll were involved in the design of the experiment, data analysis, graphic presentation, and manuscript preparation. Thomas Baron and Natalie Zlebnik were involved in data analysis, graphic presentation, and manuscript preparation. All authors were involved in the final preparation of the manuscript, and have approved the final version.
Conflict of interest: No conflict declared.
References
- 1.Anker JJ, Carroll ME. Reinstatement of cocaine seeking induced by drugs, cues, and stress in adolescent and adult rats. Psychopharmacology. 2010;208:211–222. doi: 10.1007/s00213-009-1721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anker JJ, Zlebnik NE, Carroll ME. Differential effects of allopregnanolone on the escalation of cocaine self-administration and sucrose intake in female rats. Psychopharmacology. 2010;212:419–429. doi: 10.1007/s00213-010-1968-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anker JJ, Zlebnik NE, Navin SF, Carroll ME. Responding during signaled availability and nonavailability of iv cocaine and food in rats: age and sex differences. Psychopharmacology. 2011;215:785–799. doi: 10.1007/s00213-011-2181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badanich KA, Adler KJ, Kirstein CL. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur J Pharmacol. 2006;550:95–106. doi: 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 5.Baumeister SE, Tossmann P. Association between early onset of cigarette, alcohol and cannabis use and later drug use patterns: an analysis of a survey in European metropolises. Eur Addict Res. 2005;11:92–98. doi: 10.1159/000083038. [DOI] [PubMed] [Google Scholar]
- 6.Brenhouse HC, Andersen SL. Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behav Neurosci. 2008;122:460–465. doi: 10.1037/0735-7044.122.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J Neurosci. 2008;28:2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown SA, D'Amico EJ. Outcomes of alcohol treatment for adolescents. Recent Dev Alcohol. 2001;15:307–327. doi: 10.1007/978-0-306-47193-3_18. [DOI] [PubMed] [Google Scholar]
- 9.Carelli RM, Deadwyler SA. A comparison of nucleus accumbens neuronal firing patterns during cocaine self-administration and water reinforcement in rats. J Neurosci. 1994;14:7735–7746. doi: 10.1523/JNEUROSCI.14-12-07735.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carroll ME, Boe IN. Increased intravenous drug self-administration during deprivation of other reinforcers. Pharmacol Biochem Behav. 1982;17:563–567. doi: 10.1016/0091-3057(82)90319-7. [DOI] [PubMed] [Google Scholar]
- 11.Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology (Berl) 2002;161:304–313. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- 12.Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caster JM, Walker QD, Kuhn CM. Enhanced behavioral response to repeated-dose cocaine in adolescent rats. Psychopharmacology (Berl) 2005;183:218–225. doi: 10.1007/s00213-005-0159-4. [DOI] [PubMed] [Google Scholar]
- 14.Catalano RF, Hawkins JD, Wells EA, Miller J, Brewer D. Evaluation of the effectiveness of adolescent drug abuse treatment, assessment of risks for relapse, and promising approaches for relapse prevention. Int J Addict. 1990;25:1085–1140. doi: 10.3109/10826089109081039. [DOI] [PubMed] [Google Scholar]
- 15.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen H, Matta SG, Sharp BM. Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacology. 2007;32:700–709. doi: 10.1038/sj.npp.1301135. [DOI] [PubMed] [Google Scholar]
- 17.Chung T, Maisto SA. Relapse to alcohol and other drug use in treated adolescents: review and reconsideration of relapse as a change point in clinical course. Clin Psychol Rev. 2006;26:149–161. doi: 10.1016/j.cpr.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Clark D, Kirisci BL, Tarter RE. Adolescent versus adult onset and the development of substance use disorders in males. Drug Alcohol Depend. 1998;49:115–121. doi: 10.1016/s0376-8716(97)00154-3. [DOI] [PubMed] [Google Scholar]
- 19.Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dennis M, Godley SH, Diamond G, Tims FM, Babor T, Donaldson J, Liddle H, Titus JC, Kaminer Y, Webb C, Hamilton N, Funk R. The Cannabis Youth Treatment (CYT) study: main findings from two randomized trials. J Subst Abuse Treat. 2004;27:197–213. doi: 10.1016/j.jsat.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2003;75:411–418. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- 22.Doremus-Fitzwater TL, Varlinskaya EL, Spear LP. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 2010;72:114–123. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Estroff TW, Schwartz RH, Hoffmann NG. Adolescent cocaine abuse. Addictive potential, behavioral and psychiatric effects. Clin Pediatr (Phila) 1989;28:550–555. doi: 10.1177/000992288902801201. [DOI] [PubMed] [Google Scholar]
- 24.Frantz KJ, O'Dell LE, Parsons LH. Behavioral and neurochemical responses to cocaine in periadolescent and adult rats. Neuropsychopharmacology. 2007;32:625–637. doi: 10.1038/sj.npp.1301130. [DOI] [PubMed] [Google Scholar]
- 25.Fullgrabe MW, Vengeliene V, Spanagel R. Influence of age at drinking onset on the alcohol deprivation effect and stress-induced drinking in female rats. Pharmacol Biochem Behav. 2007;86:320–326. doi: 10.1016/j.pbb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Gipson CD, Beckmann JS, El-Maraghi S, Marusich JA, Bardo MT. Effect of environmental enrichment on escalation of cocaine self-administration in rats. Psychopharmacology. 2011;214:557–566. doi: 10.1007/s00213-010-2060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzales R, Ang A, McCann MJ, Rawson RA. An emerging problem: methamphetamine abuse among treatment-seeking youth. Subst Abuse. 2008;29:71–80. doi: 10.1080/08897070802093312. [DOI] [PubMed] [Google Scholar]
- 28.Hester R, Lubman DI, Yücel M. The role of executive control in human drug addiction. Curr Top Behav Neurosci. 2010;3:301–318. doi: 10.1007/7854_2009_28. [DOI] [PubMed] [Google Scholar]
- 29.Infurna RN, Spear LP. Developmental changes in amphetamine-induced taste aversion. Pharmacol Biochem Behav. 1979;11:31–35. doi: 10.1016/0091-3057(79)90293-4. [DOI] [PubMed] [Google Scholar]
- 30.Kantak KM, Goodrich CM, Uribe V. Influence of sex, estrous cycle, and drug-onset age on cocaine self-administration in rats (Rattus norvegicus) Exp Clin Psychopharmacol. 2007;15:37–47. doi: 10.1037/1064-1297.15.1.37. [DOI] [PubMed] [Google Scholar]
- 31.Kerstetter KA, Kantak KM. Differential effects of self-administered cocaine in adolescent and adult rats on stimulus-reward learning. Psychopharmacology (Berl) 2007;194:403–411. doi: 10.1007/s00213-007-0852-6. [DOI] [PubMed] [Google Scholar]
- 32.Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology. 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- 33.Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. J Pharmacol Exp Ther. 2007;322:399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- 34.Levin ED, Lawrence SS, Petro A, Horton K, Rezvani AH, Seidler FJ, Slotkin TA. Adolescent vs. adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicol Teratol. 2007;29:458–465. doi: 10.1016/j.ntt.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology (Berl) 2003;169:141–149. doi: 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- 36.Li C, Frantz KJ. Attenuated incubation of cocaine seeking in male rats trained to self-administer cocaine during periadolescence. Psychopharmacology (Berl) 2009;204:725–733. doi: 10.1007/s00213-009-1502-y. [DOI] [PubMed] [Google Scholar]
- 37.Marusich JA, Beckmann JS, Gipson CD, Bardo MT. Methylphenidate as a reinforcer for rats: contingent delivery and intake escalation. Exp Clin Psychopharmacol. 2010;18:257–266. doi: 10.1037/a0019814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCambridge J, Strang J. Age of first use and ongoing patterns of legal and illegal drug use in a sample of young Londoners. Subst Use Misuse. 2005;40:313–319. doi: 10.1081/ja-200049333. [DOI] [PubMed] [Google Scholar]
- 39.McNamara R, Dalley JW, Robbins TW, Everitt BJ, Belin D. Trait-like impulsivity does not predict escalation of heroin self-administration in the rat. Psychopharmacology. 2010;212:453–464. doi: 10.1007/s00213-010-1974-9. [DOI] [PubMed] [Google Scholar]
- 40.Montague DM, Lawler CP, Mailman RB, Gilmore JH. Developmental regulation of the dopamine D1 receptor in human caudate and putamen. Neuropsychopharmacology. 1999;21:641–649. doi: 10.1016/S0893-133X(99)00062-7. [DOI] [PubMed] [Google Scholar]
- 41.National Research Council. Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. The National Academies Press; Washington D.C: 2003. p. 209. [PubMed] [Google Scholar]
- 42.O'Dell LE, Chen SA, Smith RT, Specio SE, Balster RL, Paterson NE, Markou A, Zorrilla EP, Koob GF. Extended access to nicotine self-administration leads to dependence: circadian measures, withdrawal measures, and extinction behavior in rats. J Pharmacol Exp Ther. 2007a;320:180–193. doi: 10.1124/jpet.106.105270. [DOI] [PubMed] [Google Scholar]
- 43.O'Dell LE, Torress OV, Natividad LA, Tejeda HA. Adolescent nicotine exposure produces less affective measures of withdrawal relative to adult nicotine exposure in male rats. Neurotoxicol Teratol. 2007b;29:17–22. doi: 10.1016/j.ntt.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parylak SL, Caster JM, Walker QD, Kuhn CM. Gonadal steroids mediate the opposite changes in cocaine-induced locomotion across adolescence in male and female rats. Pharmacol Biochem Behav. 2008;89:314–323. doi: 10.1016/j.pbb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perepletchikova F, Krystal JH, Kaufman J. Practitioner review: adolescent alcohol use disorders: assessment and treatment issues. J Child Psychol Psychiatry. 2008;49:1131–1154. doi: 10.1111/j.1469-7610.2008.01934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perry JL, Anderson MM, Nelson SE, Carroll ME. Acquisition of i.v. cocaine self-administration in adolescent and adult rats bred for high and low saccharin intake. Physiol Behav. 2007;91:126–133. doi: 10.1016/j.physbeh.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rawson RA, Gonzales R, McCann M, Ling W. Use of methamphetamine by young people: is there reason for concern? Addiction. 2007;102:1021–1022. doi: 10.1111/j.1360-0443.2007.01899.x. [DOI] [PubMed] [Google Scholar]
- 48.Schramm-Sapyta NL, Morris RW, Kuhn CM. Adolescent rats are protected from the conditioned aversive properties of cocaine and lithium chloride. Pharmacol Biochem Behav. 2006;84:344–352. doi: 10.1016/j.pbb.2006.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schramm-Sapyta NL, Walker QD, Caster JM, Levin ED, Kuhn CM. Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacology. 2009;206:1–21. doi: 10.1007/s00213-009-1585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schultz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J Neurosci. 1993;13:900–913. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwendt M, Rocha A, See RE, Pacchioni AM, McGinty JF, Kalivas PW. Extended methamphetamine self-administration in rats results in a selective reduction of dopamine transporter levels in the prefrontal cortex and dorsal striatum not accompanied by marked monoaminergic depletion. J Pharmacol Exp Ther. 2009;331:555–562. doi: 10.1124/jpet.109.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shahbazi M, Moffett AM, Williams BF, Frantz KJ. Age- and sex-dependent amphetamine self-administration in rats. Psychopharmacology (Berl) 2008;196:71–81. doi: 10.1007/s00213-007-0933-6. [DOI] [PubMed] [Google Scholar]
- 53.Snyder KJ, Katovic NM, Spear LP. Longevity ofthe expression of behavioral sensitization to cocaine in preweanling rats. Pharmacol Biochem Behav. 1998;60:909–914. doi: 10.1016/s0091-3057(98)00078-1. [DOI] [PubMed] [Google Scholar]
- 54.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 55.Spear LP. Adolescence and the trajectory of alcohol use: introduction to part VI. Ann N Y Acad Sci. 2004;1021:202–205. doi: 10.1196/annals.1308.025. [DOI] [PubMed] [Google Scholar]
- 56.Spear LP. The Behavioral Neuroscience of Adolescence. W.W. Norton; New York: 2010. [Google Scholar]
- 57.Spear LP, Varlinskaya EI. Adolescence: alcohol sensitivity, tolerance, and intake. In: Galanter M, editor. Recent Developments in Alcoholism. Vol. 17. Alcohol Problems in Adolescents and Young Adults. Kluwer Academic Publishers; Hinham, MA: 2005. pp. 143–159. [PubMed] [Google Scholar]
- 58.Spear LP, Varlinskaya EL. Sensitivity to ethanol and other hedonic stimuli in an animal model of adolescence: implications for prevention science? Dev Psychobiol. 2010;52:236–243. doi: 10.1002/dev.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Varlinskaya EI, Spear LP. Acute withdrawal (hangover) and social behavior in adolescent and adult male and female Spraue-Dawley rats. Alcohol Clin Exp Res. 2004a;28:40–50. doi: 10.1097/01.ALC.0000108655.51087.DF. [DOI] [PubMed] [Google Scholar]
- 60.Varlinskaya EI, Spear LP. Changes in sensitivity to ethanol-induced social facilitation and social inhibition from early to late adolescence. Ann N Y Acad Sci. 2004b;1021:459–461. doi: 10.1196/annals.1308.064. [DOI] [PubMed] [Google Scholar]
- 61.Vendruscolo LG, Schlosburg JE, Misra KK, Chen SA, Greenwell TN, Koob GF. Escalation patterns of varying periods of heroin access. Pharmacol Biochem Behav. 2011;98:570–574. doi: 10.1016/j.pbb.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntaryaccess conditions. Alcohol Clin Exp Res. 2007;31:1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Winters KC, Lee CY. Likelihood of developing an alcohol and cannabis use disorder during youth: association with recent use and age. Drug Alcohol Depend. 2008;92:239–247. doi: 10.1016/j.drugalcdep.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.World Drug Report. United Nations Office of Drug and Crime; New York: 2010. p. 153. [Google Scholar]
- 65.Zakharova E, Leoni G, Kichko I, Izenwasser S. Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behav Brain Res. 2009;198:45–50. doi: 10.1016/j.bbr.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]