Abstract
The directions of differentiation and the molecular features of ductal pancreatic cancer have by now been explored in reasonable detail. Already, diagnoses and therapeutic strategies benefit from observations distinguishing the major variant types of pancreatic cancer and the differing stages of disease at presentation. Additionally, individual patients differ within each variant type. In certain high-risk groups, this permits focused screening efforts. The tumorigenic influences that characterize individual patients are increasingly considered appropriate in defining clinical treatment plans. As a result, multiple variables affect success when individualizing screening or therapy. These competing variables often limit the potential for success: some variables dominate and should receive greater consideration than others. Simplistic expectations, often falsely optimistic, for individualized care may fail to ‘pan out’ in the real world. The development of individualized care will be efficient only when the full complexity of the disease is embraced.
Keywords: pancreas, carcinoma, gene, genetic, therapy, heterogeneity, personalized treatment
Introduction
As the ‘disease’ of pancreatic cancer has been transformed over the past two decades to the ‘field’ of pancreatic cancer, expectations for the practitioner have risen. After a dispersion of experience and interest, the number of experienced pancreatic cancer treatment centres has grown from a handful in 1990 to dozens. Attending this has been a daunting proliferation in the number of relevant scientific publications—a growing literature focused both inside and outside the disease itself, but with which familiarity will be assumed.
Consequently, a ‘standard of care’ is emerging both for clinical practice and research efforts in this disease, reflected in higher expectations for doctors in contact with the patients or their diagnostic samples. Individualized, accurate decisions are increasingly expected from attending clinicians and pathologists regarding even the newest opinions in presymptomatic screening and individualized therapeutic options. Associated with a specialized pancreatic cancer research centre, it is necessary to have cornerstone individuals deeply knowledgable in the all three diagnostic arenas: the histological classification, the anatomical extent and the molecular features of these tumours. As an example, it is the experience of the Johns Hopkins Pancreas Multidisciplinary Cancer Clinic (http://pathology.jhu.edu/pancreas/MDC/index.php; accessed 5 September 2010) that 25% of the outside diagnoses are significantly changed upon review.
Pancreatic cancer is bad. It is the fourth leading cause of cancer death in the USA and, perhaps owing to its high association with age and smoking, the incidence of pancreatic cancer has been rising worldwide. A significant subset (perhaps 10%?) arises due to inherited risks. A minute fraction arises as a delayed consequence of the inherited or acquired conditions of recurrent or chronic pancreatitis. Only about 20% of pancreatic cancers are detected early enough to be surgically resectable; palliative procedures are often employed to manage the localized consequences of the disease. The expected survival after resection is typically judged on the anatomical stage (the size, nodal spread, distant metastases) and status of the surgical margins. Five-year survival is not uncommon in favourable categories, such as margin-negative, node-negative primary tumours of smaller size. The survival of non-resected patients is often less than a year and is not fundamentally altered by any particular general therapy. Thus, in both sets of patients, individualized therapeutic strategies, or even earlier diagnosis through use of an individualized screening protocol, is the hope of the patient and his/her caregivers.
The recent completion of the Pancreatic Cancer Genome Project marked a notable milestone [1]. The protein-determining exons of all coding genes (numbering 20 661) were determined in two dozen pancreatic cancers. We can use the resultant database of mutations (an average of 63 somatic mutations per tumour!) and copy number variations to set some practical boundaries on the frequencies of individual variation occurring in the conventional ductal form of pancreatic cancer. It seems an appropriate time to look back at where we are in this field and to consider the implications for individualized, targeted therapy. We hope to present a practical review aimed at the latter goal, rather than to recite a catalogue of disease fundamentals, as can be found in reviews directed towards a more general audience.
Precursor lesions
The curable stage of pancreatic neoplasms is the precursor lesion, the epithelial neoplasm as it exists in a non-invasive in situ state. If we consider the entire spectrum of these lesions, the overwhelming majority do not progress to an invasive carcinoma within the lifespan of the individual. It is thus presumptuous to call these pre-invasive, for most are not. Most of them are merely ‘pre-non-invasive’. This type of neoplasm serves as the apparently-required precursor of a pancreatic cancer, and so they as a group can reasonably be termed ‘precursors’ without any presumption of future invasion implied. Yet, we do want to know which subset of the precursor lesions, however small that subset is, can be suspected as truly pre-invasive, i.e., destined to become a cancer. In search of such clues, these lesions can be imaged or biopsied or their secretions can be sampled.
Precursor lesions to pancreatic ductal cancer fall into three major types: PanINs, IPMNs and MCNs. There are histological and practical distinctions among them, and the lesions of each can be graded in terms of their apparent severity.
Pancreatic intraepithelial neoplasm
The pancreatic intraepithelial neoplasm (PanIN) is typically a diagnosis made after a pancreatic resection. It is the most common precursor to pancreatic cancer, but it is possible that as few as 1 in 500 PanIN lesions progresses to cancer. The epithelium is columnar, in contrast to the usual cuboidal ductal lining cells. The architectural complexity and cytological atypia have been used to grade the lesions by severity for nearly a century, although for most of these decades the originating 1905 report of Hulst had apparently been forgotten [2,3].
Intraductal papillary mucinous neoplasm
The intraductal papillary mucinous neoplasm (IPMN) is a diagnosis often identified upon imaging or endoscopy. Duct dilatation is accompanied by thick mucinous inspissated secretions, which can be visualized endoscopically as thick mucin extruding from the papilla of Vater. The epithelium is columnar and usually of pancreato-biliary, gastrofoveolar, oncocytic, or a molecularly distinct intestinal type.
Mucinous cyctic neoplasm
The mucinous cyctic neoplasm (MCN) can be suspected from imaging or can be recognized grossly— usually to the great relief of the patient—after a pancreatic tumour has been excised. It occurs predominantly in women and is the least common of the precursor lesions.
Each precursor type has characteristic molecular features. Additionally, the higher grades of a given precursor tend to harbour a greater accumulation of genetic mutations and other molecularly defined abnormalities and to convey a higher risk of progression to the invasive lesion (i.e., cancer). A clinically evident precursor lesion is believed to have arisen from an earlier stage lesion, establishing a progression model of neoplasia. A staged progression series offers hope for a stratified approach to managing patients, one in which a more aggressive surveillance or treatment mode can be selectively offered to a subset of the patients. The difficulty is that grading of the lesion is only reliably performed histologically, meaning that non-invasive management is not yet practical or is accompanied by a considerable anxiety from incomplete information.
It might be possible to individualize the clinical management by a molecular grading of the precursor lesions. Alternately, a cytological approach, performed on captured secretions or a needle biopsy, is possible because many of the cellular changes are seen specifically in the advanced grades, but such an approach is limited in that the number of intact cells is generally too few in the instance of an in situ ductal lesion. RNA expression patterns may be adequately characteristic of isolated lesions, but sample degradation often preferentially reduces RNA quality, and the analysis of RNA in natural settings can be confounded by admixture with the greater numbers of transcripts from non-lesional epithelia.
For these reasons, neoplasm-specific DNA markers have garnered most of the attention as aids to the diagnosis and grading of precursor lesions. Some of the most common mutations, including KRAS mutations in PanINs and IPMNs [4] and telomere shortening in PanINs [5], occur at such an early stage that they have been generally thought not to indicate significant risk. To overcome such concerns, it might be possible to assay not for the presence of such alterations but for their quantity. Presumably, the more severe, riskiest lesions would create altered poorly-cohesive cells at a higher rate than do the trivial, earliest lesions. Other genetic targets, eg TP53 and SMAD4, do not have focal ‘hotspots’ of mutation, impairing the creation of a sensitive assay that could detect most of them. Genetic deletions and other manifestations of aneuploidy could serve as a logical marker of severe neoplastic grades but, as with RNA measurements, are impaired when admixed with normal cells. Hypermethylation occurs in a characteristic subset of genes, is sufficiently narrow (i.e., not too diverse), associated with the more severe grades of dysplasia in the precursors, and detectable with both sensitivity and specificity; yet, it is a complex subject that has not yet yielded a test ready for implementation. Perhaps the greatest limitation to such assays (i.e., a major variable), however, is the difficulty of obtaining the appropriate samples on which to perform the measurements from the general population. Until this problem is solved, the other considerations, such as the choice of assay (i.e., the minor variables) may not greatly matter.
The individualized care of a known or suspected precursor lesion today rests mainly with the opinion of an experienced clinician and includes the consideration of multiple and all-too-often subjective evidence. A targeted assay has not removed the ‘art’ from decision-making.
Pancreatic adenocarcinoma (the invasive stage)
The ‘targeting’ of a therapy refers to a narrowing of possibilities. It can refer to selecting the more potent or specific treatment for a particular tumour type, to a tumour’s individual anatomical distribution, or to the molecular features that distinguish a patient’s neoplasm from normal tissues. A variety of meanings for the term are in current use during routine patient care, a situation which at times causes confusion.
The histological classification provides the major label of a cancer, enabling at least the diagnosis to be narrowed. A histological classification can be often accurately approximated by cytological examination of a brush or aspirate biopsy, but is definitively established by a combination of gross and histological tissue examination. No specialized assay is conventionally required, although this may change. In unselected patients, the most common pancreatic exocrine cancer is the conventional ductal adenocarcinoma with desmoplasia, which arises from either a PanIN or an IPMN. Other distinguishable types include the medullary cancer [6], the colloid/mucinous non-cystic cancer, the discohesive/signet ring cancer (which can occur in pure form but is most often a local feature within a larger mass of a conventional adenocarcinoma), and undifferentiated carcinoma with osteoclast-like giant cells (the giant cells were molecularly shown to be non-neoplastic phagocytic cells) [7]. Other undifferentiated or neuroendocrine tumours and even lymphomas can occur, but are outside the scope of this review.
It is easy to overlook that it is the cancer’s anatomical features, and not the molecular features or even the full histological classification, that in most patients governs the form of individualized therapy to be given. Surgical resectability, indications for palliative procedures and decisions regarding radiation therapy are largely anatomical decisions. The anatomical mass and boundaries dictate the major presenting signs and symptoms: jaundice, obstruction of the gastrointestinal tract, some types of pain, recent-onset diabetes, weight loss and cachexia, the presence or absence of local and distant metastases and the diverse features specific to particular locations of metastases.
Additionally, and surprisingly, the pattern of anatomical spread may offer a novel binary and perhaps fundamental classification system for conventional pancreatic ductal adenocarcinoma. Using the thorough examinations permitted by a rapid-autopsy research design, Iacobuzio-Donahue and colleagues reported [8] that metastatic spread followed one or two patterns but rarely a mixture of both: local and peritoneal spread, or widespread distant metastases (hundreds, if not thousands, of metastases). The tumours exhibiting widespread distant spread harboured a SMAD4 mutation rate three times that of the locally invasive tumours, suggesting not that SMAD4 has a special role in metastatic patterns, but instead that there had been a fundamental molecular difference in the tumorigenic origin underlying the two categories. The histological similarities of SMAD4-mutant and SMAD4-intact cancers had for years obscured what might represent an essential classification schema. Unfortunately, a study to confirm or disprove this group’s observations has not yet been reported. Confirmation is needed. How could this binary Iacobuzio-Donahue classification aid in individualized therapy? Speculatively, it might be therapeutically useful in the decisions regarding tumours considered as borderline resectable, which present frequent dilemmas in specialized centres for pancreatic cancer care. Perhaps a cancer, if found by immunohistochemistry to have lost Smad4 protein expression (a marker of SMAD4 gene inactivation) would be prioritized for systemic rather than local (radiation, surgical) treatment.
Certain of the perplexing histological and anatomical features of pancreatic neoplasia continue to provide intriguing considerations. Precursor lesions can be histologically mimicked when an invasive cancer erodes and ‘colonizes’ a duct, relining the surface of the duct with ‘invasive’ cancer cells. When this happens, only a resection of the lesion can provide the correct diagnosis. As another example, the primary mass of a pancreatic adenocarcinoma (unlike the findings in colorectal, lung and many other cancers) often has a surprising paucity of mitotic figures [9]; a low mitotic rate would be expected to impair the action of most anticancer drugs and radiation, which depend upon the process of mitogenesis and active cycling through the cell division cycle. These distinctions seem to be potentially important. In contrast, the conventional practice of attempting to distinguish distal bile duct cancers from distal pancreatic ductal cancers on the basis of anatomical and histological clues may be of little practical importance, due to the high fundamental similarity of these cancers in most other respects.
One of the most recent histological realizations concerns the observed hypovascularity of primary pancreatic cancers and the possible clinical implications. Tuveson and colleagues were perplexed by the apparent resistance of pancreatic cancers to a conventional chemotherapy in a transgenic mouse model [10]. Upon histological examination, the tumour cells were noticed to often lie at a considerable distance from the blood vessels, due to an expansion of the non-neoplastic stromal compartment of the tumours. Treating the mice with a hedgehog inhibitor compound caused the stroma to collapse, permitting the more conventional chemotherapeutic agents to become effective. Presumably, the tumour cells were not resistant, and the therapeutic difficulty lay only in the impaired distribution of the anticancer drug. An informed re-examination of human pancreatic cancers then revealed a similar, unusually large, distance between blood vessels, which fits well with their known desmoplastic (stromal, fibrotic) reaction. Thus, attempts to collapse the stroma of human pancreatic cancers were proposed as a basis for new clinical trials targeting a presumed poor distribution of drug (presumably a major variable governing responses in primary tumours and an occasional consideration in those metastatic deposits sharing the desmoplastic reaction). By this perspective, isolated attempts to overcome any presumed cell-based therapeutic resistance (a minor variable, in contrast) might be poised to fail.
Molecular classifications based on gene expression
Unlike the histological/anatomical classifications, molecular typing implies specialized assays. Fortunately, convenient, sensitive and diverse molecular assays are already in clinical practice. These include immunohistochemical markers for specific proteins, which can serve as a surrogate and often reliable assay for a gene’s genetic mutations. In the routine analysis of pancreatic biopsies for cancer evaluation, for example, immunohistochemical studies for the mutational target protein Smad4 and for the cancer-specific over-expression of mesothelin proteins are used routinely in clinical diagnosis [11,12]. Assays for nucleoside transporters have been proposed to predict tumour sensitivity to nucleoside therapeutic agents such as gemcitabine, owing to the dependence on transporter proteins for nucleoside drugs to enter cancer cells [13,14]. Assays based on nucleic acid sequences are less common (they are not used to guide the treatment of most cancer patients) but may become commercially available as their utility becomes better established.
Arguably, an unmet clinical need, greater than the need for analysing the cancers, arises from the need to define the malignant potential of pancreatic cysts. Pancreatic cysts are surprisingly common; they are detected in 2% or more of adults subjected to abdominal imaging [15,16]. The cysts often can be needle-aspirated, but a reliable technique is not yet available to exclude the possibility of a clinically significant neoplasm. Because the cumulative lifetime risk of pancreatic cancer is <1%, any test would need to be highly specific in order not to generate a flood of false-positive results. Molecular markers have been nominated as the solution but, as with precursor lesions, the difficulties (in accessing samples and in replicating results, given the small sample sizes) may prove to the major variables dictating the speed of progress.
DNA methylation patterns, often cursorily termed ‘epigenetics’, do not yet provide a broad classification having clinical utility in pancreatic cancer. The exceptions suggest a potential future utility, however. The E-cadherin cell-surface protein is a hallmark of differentiated epithelial linings, and its expression is lost (occasionally in association with gene promoter methylation) in some discohesive pancreatic cancers [17]. About 15% of pancreatic cancers have methylation-associated transcriptional silencing of the tumour-suppressor gene p16 [18]. Additional hypermethylated genes may be combined to form an aberrant methylation score indicative of cancer [19] or to distinguish subclasses of cancers in diagnostic situations. A potential role in determining a particular therapy is more speculative.
A considerable number of ongoing immunotherapeutic clinical trials are attempting to activate the patient’s immune system (most commonly T cells), overcome tolerance to tumour-specific antigens and mount anticancer cytotoxic responses. The ‘cancer vaccine’ strategies can employ whole cancer cells or individually chosen protein antigens. The antigens identified as sustaining an augmented immune response in pancreatic cancer patients include mutant genes, such as KRAS, and over-expressed ‘tumour marker’ proteins, such as mesothelin and Muc1 [20,21]. In the strategies used to date, the goal is not so much to take advantage of the individual differences distinguishing patients, but instead to overcome the antigen tolerance maintained in the immune networks of individual patients. One method is to use the patients’ (the hosts’) antigen-presenting cells to overcome the individuals’ MHC restrictions usually encountered when working with T cell antigens [22]. When this is done, the antigen can be presented with any variety of system; inactivated cancer cells, peptide-pulsed dendritic cells, and even Listeria bacteria have been harnessed to do this [23]. T cells have been activated in vitro by exposure to cancer [21]. Also, methods exist to counteract CTLA-4 and other immune-suppressive mechanisms of the host; the therapeutic anti-CTLA4 antibody (Ipilimumab) is an example [24]. It may be possible to assemble a manageably small panel of common antigens that together would correspond to some of the tumour-specific features found in a majority of patients. Ideally, the chosen antigens should be the immunodominant ones; along these lines, mesothelin was identified as an immunodominant antigen in patients who received a vaccine of pancreatic cancer cells engineered to express tolerance-abrogating GM-CSF [20].
The major variable affecting clinical course is the cancer’s stage. In order to detect specifically the impact of a therapeutic vaccine (a lesser variable), it is advisable to minimize the major variable. Thus, stage 2 and stage 3 vaccine trials are usually performed in the setting of minimal disease, generally using post-resection patients having no clinically detectable metastases.
A different approach has also been pursued, in which antibodies against tumour-specific over-expressed proteins are administered; antibodies against mesothelin [25] were tested in a cursory clinical trial, and antibodies against PSCA now are being tested in a clinical trial. A related concept is to perform diagnostic imaging of tumours using tumour-localizing antibodies conjugated to detectable isotopes. A major variable in antibody trials is the degree to which the antibodies are ‘titrated out’ by the comparatively large reserve of antigen molecules present on the more numerous normal cells of bodily organs. This diversion to the antigenic background causes noise in imaging and a decrease in the agent available for localization to the tumours. These background effects are selectively missing from many murine models used to develop imaging techniques, for this variable is selectively minimized owing to species differences. Results from animal models may thus systematically produce a false expectation of clinical success. In pancreatic cancer there is not yet an accepted imaging antibody.
A conceptually distinct concept is to administer receptor-targeting antibodies to inhibit oncogenic receptor proteins, such as Egfr or Erbb2 (Her2/neu). Both of these are over-expressed in many pancreatic cancers and may have an oncogenic role [26,27]; rare examples of EGFR oncogenic point mutations are even reported [28]. Inhibitor-antibody strategies have been superficially explored in pancreatic cancer. In the case of an anti-Egfr antibody [29] and the anti-Erbb2 antibody [30], trial results were not promising.
There are at least four variables that would be expected to strongly affect the success of therapeutic antibody trials: whether the antibodies are delivered to the tumour surface at an adequate concentration; whether the receptors are adequately inactivated by the antibodies; whether a substantial subset of the enrolled patients had tumours driven by these particular oncogenes; and whether this oncogenic drive was essential for cancer cell survival. It is unsatisfying that when antibody trials are performed, the variable(s) responsible for failure are not identified and, by design, are generally not sought.
We note in passing that some cancer markers are secreted and detectable in serum, such as CA19-9 and CEA. These markers are not now adequately sensitive or specific for reliable use in screening for asymptomatic cancers, but they are often used after diagnosis for monitoring disease, in order to judge therapeutic success or to alert caregivers to tumour recurrence.
Molecular classifications based on common genetic mutations
Histologically, most pancreatic adenocarcinomas are moderately differentiated, desmoplastic and have infiltrative to indistinct borders with adjacent non-neoplastic tissues. They are aneuploid, with many chromosomal translocations; their chromosome instability is accompanied by the finding of anaphase bridges in haematoxylin and eosin (H&E)-stained sections due to a defect in a cellular mitotic checkpoint. A KRAS mutation is present in over 90–95% of these typical cancers [31,32]. The adenocarcinomas must be histologically distinguished from other sizable lesions noted earlier, including the IPMNs, the MCNs and the carcinomas that can arise in them.
A few percent, however, have a medullary histology, with syncytium-like ‘sheets’ of closely apposed neoplastic cells having a ‘pushing’ tumour edge. These medullary cancers are genetically distinct, often containing a wild-type KRAS gene, a diploid chromosome content with the genetic pattern of microsatellite instability (MSI), a defect in the DNA mismatch-repair system, and an absence of anaphase bridges [6,32]. Clinically, patients with medullary carcinomas and/or microsatellite instability may survive longer (clinical reports are yet inconclusive) and are more likely to have a family history of cancer than patients with conventional ductal adenocarcinomas of the pancreas [32,33]. Whether all tumours having defects in DNA mismatch-repair will have the medullary phenotype is unsettled, due to the low reported case numbers, but a convincing exception has not yet been published. It is advised to segregate out the medullary cancers in any study of pancreatic carcinomas, for they are too distinct to be lumped in with the conventional adenocarcinomas. In therapeutic trials, for example, the MSI phenotype in the cancers of other organs is a major variable; it can predict resistance to alkylating agents. Indeed, in brain cancers, treatment with the alkylating agent temozolomide can cause selection for the MSI defect, and a tumour recurrence can be found to have acquired both an MSH6 mutation (conveying the MSI defect) and temozolomide resistance, although the original tumour had neither [34]. The MSI pancreatic cancers have mutations typical of the MSI tumours of other organs, including mutations of intragenic mononucleotide tracts within the TGFβ receptor type II (TGFBR2) gene and the activin receptor type II gene [6,35]. The H&E histological finding of conventional ductal differentiation in a pancreatic cancer may suffice to exclude the possibility of MSI [6].
Among the conventional type adenocarcinomas, the KRAS, p16, TP53 and SMAD4/DPC4 genes have highly prevalent mutations [36]. Despite a high degree of attention paid to these genes by researchers and pharmaceutical companies, it remains unsettled whether they provide practical therapeutic targets. The disappointments attending the development of anti-RAS drugs have been well discussed, although indeed a mutation-specific RAS inhibitor would be eagerly received. There has been a general failure to develop optimal drugs for these common mutational targets; because of this, clinical trials have not yet had to deal with the equally difficult task of identifying the major and minor variables that would affect trials designed upon pharmacogenetic principles.
The p16/CDKN2A gene is clinically important. Germline mutations cause an inherited syndrome of multiple dysplastic (atypical) cutaneous naevi, a high risk of melanoma and an increased risk of conventional pancreatic ductal adenocarcinoma [37–39]. Because the p16 protein inhibits the Cdk4 and Cdk6 cyclindependent kinases [40], it was plausible that a selective anti-kinase drug might be particularly appropriate for treating p16-mutant tumours. About 15% of these cancers inactivate p16 through promoter methylation and transcriptional silencing [18]; this suggested that DNA-demethylating compounds might be promising. About 20% of tumours have p16 homozygous deletions large enough to also inactivate the neighbouring MTAP purine salvage gene; this suggested that one might be able on occasion to therapeutically target the gene defects adjacent to a tumour-suppressor gene [41]. Unfortunately, none of these approaches have yet been implemented in the pancreatic cancer clinic.
Interestingly, and perhaps perplexingly, the almost universal activation of KRAS and the virtually certain inactivation of p16 in pancreatic cancer might confound certain clinical trials designed upon pharmacogenetic principles. First, it might be impossible to assemble an adequately sized mutation-negative control group. Second, it is possible that these mutations might interfere with other therapies. For example, in colorectal and small cell lung cancers, KRAS mutation predicts unresponsiveness to anti-Egfr therapy (PMID: [42,43]). As might have been predicted, in pancreatic cancer, anti-Egfr therapies have not shown promise [29].
No cancer gene has supported greater research efforts than the TP53 (p53) gene. TP53 analysis in tumours may convey prognostic information, but prognosis is seldom a major factor in clinical decision-making in pancreatic cancer: one cannot conceive of using the information to exclude patients from any particular therapeutic options. TP53 mutations usually are missense, retaining the expression of an intact but modified protein; unfortunately, the capability to reactivate mutant proteins is not yet convincing. The compound nutlin was found to restore p53 activity in MDM2-amplified tumours [44], but these are rare in pancreatic cancer and, to date, a clinically useful form of nutlin has not convincingly been formulated. A subset of p53 mutations cause nonsense-mediated decay (NMD) of the p53 mRNA (C Iacobuzio-Donahue et al, manuscript in preparation); it was thus suggested that known pharmacological methods to suppress NMD might have antitumour effects, but this has not yet been much explored.
The SMAD4 gene mutations convey no obvious therapeutic target. Their recognition, however, does provide a diagnostic tool and a potential patient classifier (see above).
Molecular classifications based on infrequent genetic mutations
Another irony of the genetic pattern is that the less-frequent targets of mutation (ie <10% of unselected patients) do often provide important clinical tools and even therapeutic targets. Perhaps this is a statistical expectation, for there are so many of them.
Inherited risks for developing pancreatic cancer are elevated in families carrying inactivating mutations of the LKB1/STK11 (Peutz–Jeghers) gene [45], the p16 gene (see above), the PRSS1 gene (familial pancreatitis) [46] and the genes of the DNA mismatch-repair system (see above). They are also elevated due to inherited mutations of the distal members of the Fanconi anaemia DNA-repair pathway, the genes BRCA2 [47,48] and PALB2 [49]. Although reported examples are very few, germline mutations of the proximal Fanconi anaemia genes FANCC and FANCG may also convey an inherited risk of pancreatic cancer [50,51]. For most of these (excepting PRSS1 and PALB2), somatic mutations that inactivate the gene are also reported in the absence of germline mutations [52].
LKB1 mutations seem to be more characteristic of IPMNs than of non-IPMN-associated cancers. The pancreatic cancer lesions found in Peutz–Jeghers families tend to be IPMNs. Among sporadic IPMNs, there is a moderate prevalence of these mutations [53,54] and, as part of a screening protocol for high-risk individuals employing CT and endoscopic ultrasound, an asymptomatic IPMN having high-grade dysplasia was identified and resected in a patient with Peutz–Jeghers syndrome [55]. It is increasingly evident that LKB1 mutations may mark a clinically and histopathologically distinct subset of pancreatic neoplasms. Although pharmacological targeting of the LKB1-mutant signalling pathway is plausible, a clinical implementation still appears distant.
Hereditary acute relapsing pancreatitis and chronic pancreatitis in children and adolescents is caused by autosomal dominant mutations of the cationic trypsinogen gene PRSS1 or autosomal recessive mutations of the SPINK1 gene [56]. In PRSS1-mutant individuals, a cumulative pancreatic cancer rate of up to 40% is reported by age 70 [46]. Clearly, these patients require special attention clinically, and in some instances patients chose prophylactic pancreatectomy.
Therapeutic targets are provided by the mutations of the Fanconi anaemia genes, which govern DNA repair, especially repair employing homologous recombination and repair of damage from collisions of the replication form with crosslinks and single-stranded breaks. The enzyme poly ADP-ribose polymerase (PARP) is needed to stabilize the intermediate repair structures, among other possible roles. A roughly 1000-fold increased sensitivity to PARP-inhibitor compounds is characteristic of cells defective in the distal pathway [57,58], and a trial in breast cancer reported clinical responses [59]. Defects of the proximal pathway, however, may not convey as strong a hypersensitivity. The PARP gene family has more than a half-dozen members, and not all PARP inhibitors produce such dramatic responses. The numerical ‘pharmacogenetic windows’ are measured by comparing the dose–response curves of matched cells having and lacking the pathway function. Reversion to a resistant phenotype can occur when pathway-mutant cells are cultured in the presence of a PARP inhibitor, due to the emergence of clones harbouring second mutations of the mutant pathway gene [60]. A clinical trial of a PARP inhibitor has opened in pancreatic cancer.
DNA-crosslinking agents include the drugs mitomycin C, cisplatin, carboplatin and melphalan. Crosslinker sensitivity has long served as a reliable diagnostic blood test for Fanconi anaemia. Likewise, defects in genes of both the proximal and distal Fanconi anaemia pathway convey as 9- to 25-fold hypersensitivity to these crosslinkers when tested in culture. Pharmacogenetic windows of this size are clinically promising. When tested in human pancreatic cancer xenografted into mice, a single dose of crosslinker therapy produces confluent necrosis in cancers harbouring one of these gene defects. Anecdotal cases of beneficial therapeutic responses in BRCA2-mutant pancreatic cancer patients have been reported [61,62,63]. As with the PARP inhibitors, reversion to a resistant phenotype have occurred upon exposure of BRCA2-mutant cancer cells, including a tumour of a treated patient, to a crosslinker drug [64].
Inhibitors of topoisomerases I and II constitute some of the most commonly used of the conventional anticancer drugs in oncology. Etoposide, an inhibitor of topoisomerase II, is conventionally taken orally by patients at home. A 10-fold hypersensitivity appears to attend BRCA2 mutations but not the defects of the proximal pathway [65,66]. This hypersensitivity was first reported by Abbott et al in a naturally occurring BRCA2-mutant human cancer cell line [67]. It may represent a reliable property, having been observed in a well-controlled mutagen-induced hamster cell model [68], in an incompletely controlled murine cellular model [69] and in human cancer cells genetically engineered to lack the BRCA2 gene [65]. An anecdotal report in pancreatic cancer used a drug combination including camptothecin (a topoisomerase I inhibitor) to induce response, followed by maintenance therapy using a crosslinker [62]. The attraction of topoisomerase therapy is that they are generally better tolerated than are crosslinkers.
BRAF mutations are found in the medullary cancers having MSI. An anti-Braf drug is in clinical trials for cancers of other organs and has produced anti-tumour effects [70]. The first generation of drugs inhibiting the constitutively active, mutant Braf protein has an undesirable effect of stimulating wild-type Braf protein [71], causing cutaneous side-effects. EGFR oncogenic missense mutations are similarly rare in pancreatic cancer [28], but the histological subtype in which the mutations were identified was not reported.
PIK3CA mutations are among the most common mutations in colorectal and breast carcinoma. In the pancreas, they appear to specifically affect and thus distinguish the mucinous (colloid) carcinomas and the intestinal-type IPMNs from which the mucinous carcinomas may generally arise [72]. PIK3CA mutations create an oncogenic and activated kinase; an early clinical trial of a Pik3ca inhibitor is under way.
Broadly applicable therapeutic targets
There are a number of gene-specific therapeutic targets that are yet largely independent of tumour classification, gene mutation or abnormal gene expression.
The process of angiogenesis is targeted by drug inhibitors of vascular endothelial growth factor (VEGF). Interesting considerations appear when one ponders the major variables affecting patient survival. These drugs characteristically debulk the interior, hypoxia-prone regions of cancers. Patient survival, however, often depends on the aggressive nature of the neoplastic cells at the interface of tumour and normal tissues, or the ability of metastatic cancer cells to lodge and survive in distant organs. Neither of these latter features (major variables) is dependent upon neovascular processes, and neither is hindered by the collapse of hypovascular interior regions of tumours (a minor variable). In theory, one would not expect patient survival to be greatly influenced by these agents.
Recently, Abraxane, a nanoparticle albumin-bound form of paclitaxel, was reported as promising in pancreatic cancer [73]. It is not known what are the major variables conferring the effectiveness of Abraxane, but the tumour-specific over-expression of Sparc protein has been suggested. The SPARC gene is known to be over-expressed in the stromal cells of pancreatic cancers [74]. A possible mechanism of action was indicated by the finding that Abraxane binds to the albumin-binding protein Sparc in other cancer types [75].
The drug 5-fluorouracil (5-FU) is still the most commonly employed chemotherapeutic agent in gastrointestinal malignancies and was often used in pancreatic cancer. One of the major drug targets is the thymidylate synthase protein, which becomes bound covalently to the deoxynucleotide metabolite of the drug. In colorectal cancer metastases persisting after 5-FU therapy, the thymidylate synthase gene is genetically amplified [76]; a similar amplification reliably occurs and causes 5-FU resistance in cultured cells exposed to the drug. Despite a considerable literature, the major variables affecting 5-FU success are unclear. 5-FU resistance is found in colorectal cancers having MSI [77]. Except possibly for avoiding the MSI tumours, it is not known how best to select the most appropriate patients for 5-FU therapy, or how best to optimize the dosing.
Inhibitors of the hedgehog pathway have been suggested for use in pancreatic cancer. No intragenic mutations (e.g., affecting the PTC or SMO proximal pathway genes) are reported in pancreatic cancer, and GLI gene amplification (a distal pathway effector) is rare, yet the pathway may be overactive due to other mechanisms [78]. A role for hedgehog signalling in the stroma was suggested by a study in which a hedgehog inhibitor was used to collapse the stroma in a transgenic mouse model of pancreatic cancer [10]. Much is yet unexplored, however. For example, it is not self-evident that in mutation-negative cells the pathway’s overactivity would be as promising a target as in tumours having mutant hedgehog pathways.
In short, for many of the broad therapeutic targets, we do not have a strong grasp of the numerical predictors for tumour-specific therapy or of the major and minor variables that would determine success. In the absence of a strong theoretical and (ideally) numerical foundation, the empirical results of clinical trials must guide us. Even in the instances of success, the targeted cell type and the precise molecular ‘reason why’ will remain murky. Thus, we expect that efforts to further optimize these therapies will be hindered for some time.
Individualized care of the cancer patient: variables affecting success
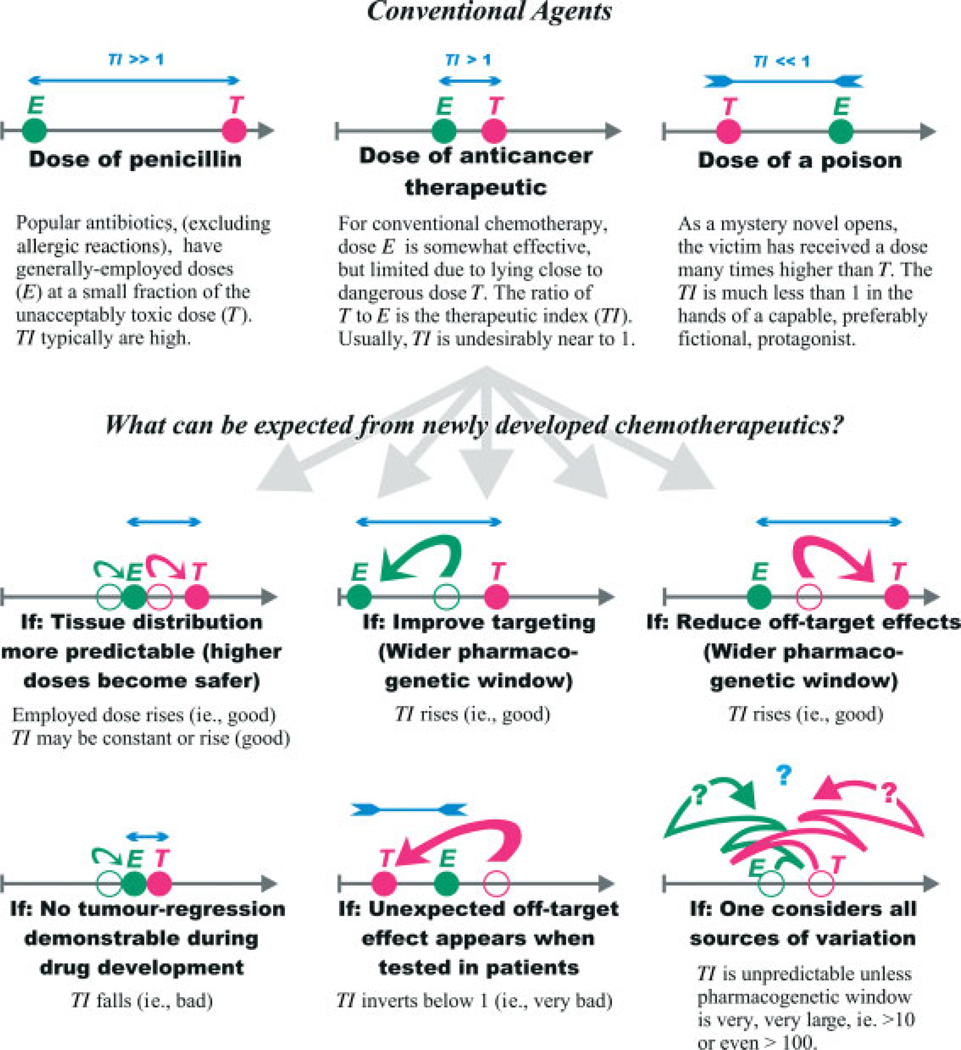
When a new therapeutic strategy is suggested, it is usually based on the attractiveness of a single variable. For example, the affinity of a drug to bind a cellular molecule is simplistically and often termed ‘targeting’ (Figure 1). This can be criticized. Seldom is a list presented of competing variables (see also Figure 1). The consequence is that the chosen variable is often over-emphasized, leading to a falsely elevated expectation that successful strategies can be devised.
Figure 1.
A schematic diagram of variables affecting therapeutic success and failure. The grey arrow represents a spectrum of administered doses, from no treatment at the left endpoint to a very high dose on the right. Filled circles indicate the dose (green for the generally-employed dose E, red for the minimal dose T giving unacceptable toxicity) chosen after considering the illustrated variable. Open circles, baseline doses that might have been assumed in ignorance of the same variable.
In clinical trials, other important variables include the existence of differing patient subpopulations. Overlooking these distinctions impairs an effective stratification of the trial. Many of the relevant variables may be frankly unsuspected; they then contribute ‘noise’ to the data, swamping attempts to identify a hint of treatment success. If we presume that on occasion new ‘targeted’ therapies actually will work, the lack of attention to these additional variables would risk a false result, that of trial failure.
If individualized care is a valued goal, and if attention to multiple variables affecting success will be needed to evaluate new strategies competently, it is important to discuss the range of variables affecting seemingly ‘targeted’ trials.
A distinctive mechanism of drug resistance can affect the drug target. In chronic myelogenous leukaemia and gastrointestinal stromal tumours, secondary mutations of the BCR-ABL or KIT oncogenes, respectively, modify the protein pocket bound by imatinib; these mutations emerge during therapy and weaken the association of the drug and the protein [79,80]. Another example, less well-established in the clinic but repeatedly occurring in the laboratory, is the amplification of the thymidylate synthase gene after 5-FU therapy. A theoretical possibility, shown to date in the laboratory and in a single patient treated with mitomycin C, is the emergence of secondary mutations in the BRCA2 or BRCA1 genes following clinical treatment with PARP inhibitors or crosslinkers [60,64]. A theoretical possibility, convincingly shown to date only in the laboratory [81], is the emergence of topoisomerase gene mutations that cause resistance to topo-inhibitor drugs.
Somewhat less obvious are the variables identified from empirical clinical findings. In examples introduced above, the resistance of RAS-mutant cancers to Egfr inhibitors, or the emergence of MSH6 mutations and drug-resistance of brain tumours treated with an alkylating agent, were not quite predictable, since the drug target and the resistance mechanism involved different genes.
Yet other forms of resistance emerge from mechanical considerations. Tuveson and colleagues found evidence that the delivery of a drug was impaired by hypovascularity in a genetically engineered mouse model of pancreatic cancer [10]. The novelty of the finding belies its obviousness, for the hypovascularity of human pancreatic cancer as a possible major variable was previously overlooked in hundreds of prior attempts to improve the drug-responsiveness of this cancer type. It will be important to systematically evaluate this variable, for we do not yet know how to measure it, or its variability among a group of patients, or whether it greatly differs in primary tumours as compared to metastatic lesions or between the metastases of differing organs. Tuveson and colleagues may or not be proved right when translating their observations on drug distribution to human cancers (Figure 1), but the fact that the variable was systematically overlooked by most investigators planning novel clinical trials in pancreatic cancer is documented.
Therapeutic failure can arise from a drug’s off-target effects. The relative propensity to create on-target and off-target effects is measured in a number of ways. The therapeutic index is the ratio of the maximally tolerated clinical dose to the desired therapeutic dose given to a patient or a test animal (Figure 1). The pharmacogenetic window is the ratio of the effective drug concentrations of two matched test systems: for example, a cell line competent in a pathway and a derived (syngeneic) cell line having a genetic knockout of an essential gene of the pathway. This knockout creates a defect that is the ‘target’ for the therapy. Of the two assessments, the laboratory-based pharmacogenetic window is the more conveniently obtained and creates the more robust numerical comparisons.
For mutation-targeted therapies, the magnitude of the pharmacogenetic window is important [82]. Initial studies comparing imatinib in leukaemic cells lacking and harbouring the BCR-ABL oncogene indicated a wide window, with a ratio of between 10 and 20; imatinib proved clinically useful, but with significant side-effects. The recently derived PARP inhibitors have a window of nearly 1000 when tested on BRCA2-proficient and -deficient cells. They are proving to be clinical promising. In contrast, many ‘targeted’ therapies proposed in the literature appear to have pharmacogenetic windows that are quite low. The low numerical windows are parallelled in animal models, where the new chemical compounds often cause only a slowing of tumour growth (a fairly promiscuous observation when toxins are tested) but unfortunately fail to cause regression of established tumours (Figure 1). Even if the ‘targeting’ were to work in the clinic at the (low) magnitude expected, the other variables affecting patient outcome would dwarf the treatment effect.
There exist other sources of therapeutic unpredictability. For example, some patients sustain unacceptable, idiosyncratic toxicity (Figure 1) from drugs that are generally tolerated well. Unless a cause is found that permits pretreatment testing (eg testing for DPD deficiency in persons about to receive 5-FU), this risk of sporadic toxicity will limit the typical dosing range designed into a clinical trial. This could deny all patients in the trial the potentially effective dose, even for an otherwise ideal and efficacious drug. To extend the example using 5-FU and its analogues, the high level of first-pass liver metabolism and the uncertainties of drug tissue levels needed, combined with the tendency for up to 15% of the patients to sustain significant skin, gastrointestinal or marrow toxicity, produce a situation in which the dosing rules for this half-century-old drug are still hazy. Success in targeted therapy will entail targeting both the optimal dose and the sporadic toxicities.
Evaluating targeted therapy implies capabilities that we may not have. For example, it implies that we should monitor the cancer during the trial. In pancreatic cancer, this can be difficult. At presentation, patients can be severely ill due to cholestasis rather than directly due to the bulk of the cancer. Cachexia and other constitutional signs and symptoms do not clearly parallel the mass of neoplasms, for they are a distant effect, presumably owing to circulating factors released by the stromal or neoplastic cells of the tumour. The metastatic deposits in distant organs, the peritoneal spread and the primary mass of cancer at the pancreas may not respond similarly. Desmoplasia (dense scar) may remain at the site of the tumour even after the death of cancer cells, impairing recognition of a response.
Conventionally, the major variable affecting clinical trials, assuming that the diagnosis is accurate, is the precise stage of the cancer. The word ‘precise’ is important, for any additional variable that carries information associated with the stage will appear to be an additional, relevant variable. This can be misleading unless the new variable is known to also contain additional information not conveyed by a precise staging. Quite possibly, the main reason to carefully stratify a patient population in a clinical trial is that many diverse features of a tumour or characteristics of a patient may, upon close evaluation, unexpectedly be found to associate with stage. Even a pattern of gene mutation may be associated with stage and thereby produce a misleading result in an otherwise logical trial design. When this happens, one may mistakenly think that the mutation represents a prognostic determinant or that it is a ‘druggable’ target, when in reality it is not.
For pancreatic cancer, precise staging is an art in evolution, and it is seldom that a clinical trial can be designed with confidence, even using conventional staging. Staging is best done currently with anatomical exploration, such as that permitted by a surgical resection. When patients have either a minimal lesion (such as a small unchanging cyst identified accidentally) or an advanced disease (such as with multiple-organ metastases), surgical exploration is not indicated and staging can remain imprecise. Perhaps newly proposed technologies will help: (a) invasion by the initial cancer cells may be detectable by blood-based screening to detect the more common mutations and methylated DNA sequences [83,84]; (b) the total burden of disease may be measured by blood monitoring of circulating DNA fragments containing signature translocations specific to each patient [85]; (c) detection of circulating cancer cells might become so reliable that recognition of a new ‘stage V’, a late stage wherein substantial blood-borne cancer dissemination occurs, would be included routinely in designing the optimal stratification of clinical trials; (d) the Iacobuzio–Donahue classes may become validated for routine evaluation, to discriminate tumours that metastasize by local versus distant routes; (e) we may become adept at measuring/monitoring the systemic tumour-effects-at-a-distance (the ‘hormonal’ effects of putative cytokines and cachexia-inducing factors on organs in the absence of neighbouring tumour cells), but even the appropriate analytes for such a method are yet unknown.
The influence of these variables will greatly affect the interpretation of targeted therapy. The same variables lead to misinterpretation if adequate care is not taken. The variables operate simultaneously (Figure 1). There is no single solution for managing the multiple relevant variables affecting trials of targeted therapy. We must be aware of our limitations and work to develop our capabilities.
Footnotes
Conflict of interest: Johns Hopkins University and the co-authors (SEK and RHH) may have intellectual property interests relating to discoveries of the co-authors. All potential conflicts of interest are managed according to the policies of the University.
Author contributions
SEK and RHH collaborated on many of the discoveries presented. SEK created the outline, wrote the final text and prepared the figure. CS wrote the intial text. RHH extensively revised the outline and performed the final edit of the text.
Teaching Materials
PowerPoint slides of the figures from this review are supplied as supporting information in the online version of this article.
References
- 1.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hulst SPL. Zur Kenntnis der Genese des Adenokarzinoms und Karzinoms des Pankreas [trans. van Heek T, Koopman V] Virchows Arch B. 1905;180:288–316. [Google Scholar]
- 3.Hruban RH, Wilentz R, Kern SE. Genetic progression in the pancreatic ducts. Am J Pathol. 2000;156:1821–1825. doi: 10.1016/S0002-9440(10)65054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caldas C, Hahn SA, Hruban RH, et al. Detection of K-ras mutations in the stool of patients with pancreatic adenocarcinoma and pancreatic ductal hyperplasia. Cancer Res. 1994;54:3568–3573. [PubMed] [Google Scholar]
- 5.van Heek NT, Meeker AK, Kern SE, et al. Telomere shortening is nearly universal in pancreatic intraepithelial neoplasia. Am J Pathol. 2002;161:1541–1547. doi: 10.1016/S0002-9440(10)64432-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goggins M, Offerhaus GJA, Hilgers W, et al. Adenocarcinomas of the pancreas with DNA replication errors (RER+) are associated with wild-type K-ras and characteristic histopathology: poor differentiation, a syncytial growth pattern, and pushing borders suggest RER+ Am J Pathol. 1998;152:1501–1507. [PMC free article] [PubMed] [Google Scholar]
- 7.Westra WH, Sturm P, Drillenburg P, et al. K-ras oncogene mutations in osteoclast-like giant cell tumors of the pancreas and liver: genetic evidence to support origin from the duct epithelium. Am J Surg Pathol. 1998;22:1247–1254. doi: 10.1097/00000478-199810000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806–1813. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montgomery E, Wilentz RE, Argani P, et al. Analysis of anaphase figures in routine histologic sections distinguishes chromosomally unstable from chromosomally stable malignancies. Cancer Biol Ther. 2003;2:248–252. doi: 10.4161/cbt.2.3.362. [DOI] [PubMed] [Google Scholar]
- 10.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tascilar M, Offerhaus GJ, Altink R, et al. Immunohistochemical labeling for the Dpc4 gene product is a specific marker for adenocarcinoma in biopsy specimens of the pancreas and bile duct. Am J Clin Pathol. 2001;116:831–837. doi: 10.1309/WF03-NFCE-7BRH-7C26. [DOI] [PubMed] [Google Scholar]
- 12.McCarthy DM, Maitra A, Argani P, et al. Novel markers of pancreatic adenocarcinoma in fine-needle aspiration: mesothelin and prostate stem cell antigen labeling increases accuracy in cytologi-cally borderline cases. Appl Immunohistochem Mol Morphol. 2003;11:238–243. doi: 10.1097/00129039-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Sebastiani V, Ricci F, Rubio-Viqueira B, et al. Immuno-histochemical and genetic evaluation of deoxycytidine kinase in pancreatic cancer: relationship to molecular mechanisms of gemcitabine resistance and survival. Clin Cancer Res. 2006;12:2492–2497. doi: 10.1158/1078-0432.CCR-05-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spratlin J, Sangha R, Glubrecht D, et al. The absence of human equilibrative nucleoside transporter 1 is associated with reduced survival in patients with gemcitabine-treated pancreas adenocarci-noma. Clin Cancer Res. 2004;10:6956–6961. doi: 10.1158/1078-0432.CCR-04-0224. [DOI] [PubMed] [Google Scholar]
- 15.Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. Am J Roentgenol. 2008;191:802–807. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee KS, Sekhar A, Rofsky NM, et al. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 105:2079–2084. doi: 10.1038/ajg.2010.122. [DOI] [PubMed] [Google Scholar]
- 17.Winter JM, Ting AH, Vilardell F, et al. Absence of E-cadherin expression distinguishes noncohesive from cohesive pancreatic cancer. Clin Cancer Res. 2008;14:412–418. doi: 10.1158/1078-0432.CCR-07-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schutte M, Hruban RH, Geradts J, et al. Abrogation of the Rb/p16 tumor-suppressive pathway in virtually all pancreatic carcinomas. Cancer Res. 1997;57:3126–3130. [PubMed] [Google Scholar]
- 19.Parsi MA, Li A, Li CP, et al. DNA methylation alterations in endoscopic retrograde cholangiopancreatography brush samples of patients with suspected pancreaticobiliary disease. Clin Gastroen-terol Hepatol. 2008;6:1270–1278. doi: 10.1016/j.cgh.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas AM, Santarsiero LM, Lutz ER, et al. Mesothelin-specific CD8+ T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondo H, Hazama S, Kawaoka T, et al. Adoptive immunother-apy for pancreatic cancer using MUC1 peptide-pulsed dendritic cells and activated T lymphocytes. Anticancer Res. 2008;28:379–387. [PubMed] [Google Scholar]
- 22.Huang AY, Golumbek P, Ahmadzadeh M, et al. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 23.Le DT, Pardoll DM, Jaffee EM. Cellular vaccine approaches. Cancer J. 16:304–310. doi: 10.1097/PPO.0b013e3181eb33d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peggs KS, Quezada SA, Chambers CA, et al. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassan R, Ebel W, Routhier EL, et al. Preclinical evaluation of MORAb-009, a chimeric antibody targeting tumor-associated mesothelin. Cancer Immun. 2007;7:20. [PMC free article] [PubMed] [Google Scholar]
- 26.Day JD, Digiuseppe JA, Yeo C, et al. Immunohistochemical evaluation of HER-2/neu expression in pancreatic adenocarcinoma and pancreatic intraepithelial neoplasms. Hum Pathol. 1996;27:119–124. doi: 10.1016/s0046-8177(96)90364-0. [DOI] [PubMed] [Google Scholar]
- 27.Yamanaka Y, Friess H, Kobrin MS, et al. Coexpression of epidermal growth factor receptor and ligands in human pancreatic cancer is associated with enhanced tumor aggressiveness. Anticancer Res. [PubMed] [Google Scholar]
- 28.Kwak EL, Jankowski J, Thayer SP, et al. Epidermal growth factor receptor kinase domain mutations in esophageal and pancreatic adenocarcinomas. Clin Cancer Res. 2006;12:4283–4287. doi: 10.1158/1078-0432.CCR-06-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Philip PA, Benedetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28(22):3605–3610. doi: 10.1200/JCO.2009.25.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Safran H, Iannitti D, Ramanathan R, et al. Herceptin and gemc-itabine for metastatic pancreatic cancers that overexpress HER-2/neu. Cancer Invest. 2004;22:706–712. doi: 10.1081/cnv-200032974. [DOI] [PubMed] [Google Scholar]
- 31.Almoguera C, Shibata D, Forrester K, et al. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- 32.Wilentz RE, Goggins M, Redston M, et al. Genetic, immunohis-tochemical, and clinical features of medullary carcinomas of the pancreas: a newly described and characterized entity. Am J Pathol. 2000;156:1641–1651. doi: 10.1016/S0002-9440(10)65035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kastrinos F, Mukherjee B, Tayob N, et al. Risk of pancreatic cancer in families with Lynch syndrome. J Am Med Assoc. 2009;302:1790–1795. doi: 10.1001/jama.2009.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunter C, Smith R, Cahill DP, et al. A hypermutation phenotype and somatic MSH6 mutations in recurrent human malignant gliomas after alkylator chemotherapy. Cancer Res. 2006;66:3987–3991. doi: 10.1158/0008-5472.CAN-06-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hempen PM, Zhang L, Bansal RK, et al. Evidence of selection for clones having genetic inactivation of the activin A type II receptor (ACVR2) gene in gastrointestinal cancers. Cancer Res. 2003;63:994–999. [PubMed] [Google Scholar]
- 36.Rozenblum E, Schutte M, Goggins M, et al. Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res. 1997;57:1731–1734. [PubMed] [Google Scholar]
- 37.Gruis NA, Sandkuiji LA, van der Velden PA, et al. CDKN2 explains part of the clinical phenotype in Dutch familial atypical multiple-mole melanoma (FAMMM) syndrome families. Melanoma Res. 1995;9:169–177. doi: 10.1097/00008390-199506000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Lynch HT, Fusaro RM. Pancreatic cancer and the familial atypical multiple mole melanoma (FAMMM) syndrome. Pancreas. 1991;6:127–131. doi: 10.1097/00006676-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Whelan AJ, Bartsch D, Goodfellow PJ. Brief report: a familial syndrome of pancreatic cancer and melanoma with a mutation in the CDKN2 tumor-suppressor gene. N Engl J Med. 1995;333:975–977. doi: 10.1056/NEJM199510123331505. [DOI] [PubMed] [Google Scholar]
- 40.Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 41.Hahn SA, Hoque ATMS, Moskaluk CA, et al. Homozygous deletion map at 18q21.1 in pancreatic cancer. Cancer Res. 1996;56:490–494. [PubMed] [Google Scholar]
- 42.Lievre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–3995. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 43.Jiang Y, Mackley H, Cheng H, et al. Use of K-Ras as a predictive biomarker for selecting anti-EGF receptor/pathway treatment. Biomark Med. 2010;4:535–541. doi: 10.2217/bmm.10.74. [DOI] [PubMed] [Google Scholar]
- 44.Vassilev LT, Vu BT, Graves B, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 45.Giardiello FM, Brensinger JD, Tersmette AC, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119:1447–1453. doi: 10.1053/gast.2000.20228. [DOI] [PubMed] [Google Scholar]
- 46.Lowenfels AB, Maisonneuve P, DiMagno EP, et al. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst. 1997;89(6):442–446. doi: 10.1093/jnci/89.6.442. [DOI] [PubMed] [Google Scholar]
- 47.Goggins M, Schutte M, Lu J, et al. Germline BRCA2 gene mutations in patients with apparently sporadic pancreatic carcinomas. Cancer Res. 1996;56:5360–5364. [PubMed] [Google Scholar]
- 48.Murphy KM, Brune KA, Griffin C, et al. Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: deleterious BRCA2 mutations in 17% Cancer Res. 2002;62:3789–3793. [PubMed] [Google Scholar]
- 49.Jones S, Hruban RH, Kamiyama M, et al. Exomic sequencing identifies P A L B 2 as a pancreatic cancer susceptibility gene. Science. 2009;324:217. doi: 10.1126/science.1171202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van der Heijden MS, Kern SE. Fanconi anemia gene mutations in young-onset pancreatic cancer. Cancer Res. 2003;63:2585–2588. [PubMed] [Google Scholar]
- 51.Couch FJ, Johnson MR, Rabe K, et al. Germ line Fanconi anemia complementation group C mutations and pancreatic cancer. Cancer Res. 2005;65:383–386. [PubMed] [Google Scholar]
- 52.Su GH, Hruban RH, Bova GS, et al. Germline and somatic mutations of the STK11/LKB1 Peutz-Jeghers gene in pancreatic and biliary cancers. Am J Pathol. 1999;154:1835–1840. doi: 10.1016/S0002-9440(10)65440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Su GH, Hruban RH, Bansal RK, et al. Germline and somatic mutations of the STK11/LKB1 Peutz-Jeghers gene in pancreatic and biliary cancers. Am J Pathol. 1999;154:1835–1840. doi: 10.1016/S0002-9440(10)65440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sahin F, Maitra A, Argani P, et al. Loss of Stk11/Lkb1 expression in pancreatic and biliary neoplasms. Mod Pathol. 2003;16:686–691. doi: 10.1097/01.MP.0000075645.97329.86. [DOI] [PubMed] [Google Scholar]
- 55.Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol. 2006;4:766–781. doi: 10.1016/j.cgh.2006.02.005. quiz, 665. [DOI] [PubMed] [Google Scholar]
- 56.Witt H, Luck W, Hennies HC, et al. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat Genet. 2000;25:213–216. doi: 10.1038/76088. [DOI] [PubMed] [Google Scholar]
- 57.Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 58.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 59.Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 60.Edwards SL, Brough R, Lord CJ, et al. Resistance to therapy caused by intragenic deletion in BRCA2 . Nature. 2008;451:1111–1115. doi: 10.1038/nature06548. [DOI] [PubMed] [Google Scholar]
- 61.Showalter SL, Charles S, Belin J, et al. Identifying pancreatic cancer patients for targeted treatment: the challenges and limitations of the current selection process and vision for the future. Expert Opin Drug Deliv. 2010;7:273–284. doi: 10.1517/17425240903544462. [DOI] [PubMed] [Google Scholar]
- 62.James E, Waldron-Lynch MG, Saif MW. Prolonged survival in a patient with BRCA2 associated metastatic pancreatic cancer after exposure to camptothecin: a case report and review of literature. Anticancer Drugs. 2009;20:634–638. doi: 10.1097/CAD.0b013e32832b511e. [DOI] [PubMed] [Google Scholar]
- 63.Chalasani P, Kurtin S, Dragovich T. Response to a third-line mito-mycin C (MMC)-based chemotherapy in a patient with metastatic pancreatic adenocarcinoma carrying germline BRCA2 mutation. Jop. 2008;9:305–308. [PubMed] [Google Scholar]
- 64.Sakai W, Swisher EM, Karlan BY, et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature. 2008;451:1116–1120. doi: 10.1038/nature06633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hucl T, Rago C, Gallmeier E, et al. A syngeneic variance library (SyVaL) for functional annotation of human variation: application to BRCA2 . Cancer Res. 2008;68:5023–5030. doi: 10.1158/0008-5472.CAN-07-6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gallmeier E, Calhoun ES, Rago C, et al. Targeted disruption of FANCC and FANCG in human cancer provides a preclinical model of specific therapeutic options. Gastroenterology. 2006;130:2145–2154. doi: 10.1053/j.gastro.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 67.Abbott DW, Freeman ML, Holt JT. Double-strand break repair deficiency and radiation sensitivity in BRCA2 mutant cancer cells. J Natl Cancer Inst. 1998;90:978–985. doi: 10.1093/jnci/90.13.978. [DOI] [PubMed] [Google Scholar]
- 68.Wiegant WW, Overmeer RM, Godthelp BC, et al. Chinese hamster cell mutant, V-C8, a model for analysis of Brca2 function. Mutat Res. 2006;600:79–88. doi: 10.1016/j.mrfmmm.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 69.Treszezamsky AD, Kachnic LA, Feng Z, et al. BRCA1- and BRCA2-deficient cells are sensitive to etoposide-induced DNA double-strand breaks via topoisomerase II. Cancer Res. 2007;67:7078–7081. doi: 10.1158/0008-5472.CAN-07-0601. [DOI] [PubMed] [Google Scholar]
- 70.Bollag G, Hirth P, Tsai J, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Halaban R, Zhang W, Bacchiocchi A, et al. PLX4032, a selective BRAF(V600E) kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF melanoma cells. Pigment Cell Melanoma Res. 23:190–200. doi: 10.1111/j.1755-148X.2010.00685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schonleben F, Qiu W, Ciau NT, et al. PIK3CA mutations in intra-ductal papillary mucinous neoplasm/carcinoma of the pancreas. Clin Cancer Res. 2006;12:3851–3855. doi: 10.1158/1078-0432.CCR-06-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nyman DW, Campbell KJ, Hersh E, et al. Phase I and pharma-cokinetics trial of ABI-007, a novel nanoparticle formulation of paclitaxel in patients with advanced nonhematologic malignancies. J Clin Oncol. 2005;23:7785–7793. doi: 10.1200/JCO.2004.00.6148. [DOI] [PubMed] [Google Scholar]
- 74.Sato N, Fukushima N, Maehara N, et al. SPARC/osteonectin is a frequent target for aberrant methylation in pancreatic adenocar-cinoma and a mediator of tumor-stromal interactions. Oncogene. 2003;22:5021–5030. doi: 10.1038/sj.onc.1206807. [DOI] [PubMed] [Google Scholar]
- 75.Desai NP, Trieu V, Hwang LY, et al. Improved effectiveness of nanoparticle albumin-bound (nab) paclitaxel versus polysorbate-based docetaxel in multiple xenografts as a function of HER2 and S PARC status. Anticancer Drugs. 2008;19:899–909. doi: 10.1097/CAD.0b013e32830f9046. [DOI] [PubMed] [Google Scholar]
- 76.Wang TL, Diaz LA, Jr., Romans K, et al. Digital karyotyping identifies thymidylate synthase amplification as a mechanism of resistance to 5-fluorouracil in metastatic colorectal cancer patients. Proc Natl Acad Sci USA. 2004;101:3089–3094. doi: 10.1073/pnas.0308716101. Epub17 February 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carethers JM, Smith EJ, Behling CA, et al. Use of 5-fluorouracil and survival in patients with microsatellite-unstable colorectal cancer. Gastroenterology. 2004;126:394–401. doi: 10.1053/j.gastro.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 78.Berman DM, Karhadkar SS, Maitra A, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–851. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 79.Gorre ME, Mohammed M, Ellwood K, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 80.Wakai T, Kanda T, Hirota S, et al. Late resistance to imatinib therapy in a metastatic gastrointestinal stromal tumour is associated with a second KIT mutation. Br J Cancer. 2004;90:2059–2061. doi: 10.1038/sj.bjc.6601819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sinha BK. Topoisomerase inhibitors. A review of their therapeutic potential in cancer. Drugs. 1995;49:11–19. doi: 10.2165/00003495-199549010-00002. [DOI] [PubMed] [Google Scholar]
- 82.Hucl T, Gallmeier E, Kern SE. Distinguishing rational from irrational applications of pharmacogenetic synergies from the bench to clinical trials. Cell Cycle. 2007;6:1336–1341. doi: 10.4161/cc.6.11.4359. [DOI] [PubMed] [Google Scholar]
- 83.Diehl F, Li M, Dressman D, et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc Natl Acad Sci USA. 2005;102:16368–16373. doi: 10.1073/pnas.0507904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li M, Chen WD, Papadopoulos N, et al. Sensitive digital quantification of DNA methylation in clinical samples. Nat Biotechnol. 2009;27:858–863. doi: 10.1038/nbt.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leary RJ, Kinde I, Diehl F, et al. Development of personalized tumor biomarkers using massively parallel sequencing. Sci Transl Med. 2010;2 doi: 10.1126/scitranslmed.3000702. 20ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]