Abstract
Objective
The objective of this study was to examine the influence of persistence of the MetS (MetS) and its individual components over a 3-year period on carotid intima media thickness (CIMT) in overweight Latino children.
Methods
Ninety-seven healthy male and female overweight Latino children (mean age at baseline: 11.0±1.8 yrs) were assessed for MetS on four annual evaluations and classified according to the persistence of MetS: NEVER (0 annual visits with the MetS, n=53), INTERMITTENT (1 or 2 visits with the MetS, n=28), and PERSISTENT (3 or 4 visits with the MetS, n=16). CIMT was measured with high-resolution B-mode ultrasound (7.9±0.7 months after the most recent MetS assessment; mean age: 14.6±1.8 yr).
Results
PERSISTENT MetS was associated with significantly higher CIMT (0.647mm±0.018 compared to (0.600mm±0.007 in those who NEVER had MetS, p<0.01). This difference remained significant after controlling for gender, baseline age, total fat mass, total lean tissue mass and insulin sensitivity. PERSISTENT high waist circumference and PERSISTENT high blood pressure were also significantly associated with higher mean CIMT, but these differences were no longer significant after controlling for total fat and lean tissue mass. Baseline systolic blood pressure and 2-hour glucose were significantly related to CIMT independent of all other MetS components (p<0.05).
Conclusions
Persistence of the MetS over a 3-year period was uniquely associated with increased CIMT during childhood. Children with hypertension, persistent abdominal adiposity and impaired glucose tolerance may also be at higher risk for elevated CIMT.
Keywords: CIMT, obesity, children, MetS
Introduction
In adults, a clustering of risk factors collectively known as the MetS has been linked to cardiovascular disease (CVD) and atherosclerosis 1, 2. Some longitudinal studies have shown that MetS during childhood predicts the development of CVD by adulthood 3, 4. The escalating obesity rates in the pediatric population 5 and the association between obesity and MetS in childhood 6 substantiates the need to investigate the relationship of MetS and its components with atherosclerosis, especially in obese children. High-risk children such as those who are overweight and have family history of CVD or diabetes are of particular interest due to the concomitant occurrence of metabolic and vascular disease.
Common carotid artery intima media thickness (CIMT) has been used predominately in adults as a non-invasive measure of atherosclerosis. CIMT is a significant correlate to traditional vascular disease risk factors, such as cholesterol and blood pressure 7, 8 and has been shown to be predictive of CVD in adults 9-11. In pediatric populations, recent cross-sectionaly studies have shown that obesity 12-14 and the MetS 15 are associated with increased CIMT. Our cross-sectional study in overweight Latino children showed there was no association between the MetS and CIMT 16. No studies to date have investigated the tracking of cardiometabolic risk factors and its effects on CIMT during childhood and adolescence. Our current objectives are therefore: 1) to investigate the effects of persistent MetS over a 3 year period on a subsequent single measure of CIMT and 2) to examine the individual effects of the persistence of each MetS component on a subsequent single measure of CIMT. We hypothesize that persistent MetS will have a significant adverse effect on CIMT.
Methods
Subjects
Participants were enrolled in the Study of Latino Adolescents at Risk for Diabetes (SOLAR), a longitudinal study exploring metabolic risk factors for type 2 diabetes. Study participants satisfied the following criteria for inclusion at the initial baseline visit: 8-13 years of age, Latino ethnicity (i.e., parents and grandparents of Latino descent), age- and gender-specific BMI ≥ 85th percentile, positive family history for type 2 diabetes, and absence of diabetes as assessed by an oral glucose tolerance test (OGTT). Participants were excluded if they were using a medication or diagnosed with a condition known to influence body composition or insulin / glucose metabolism. Prior to testing procedures, written informed consent from parents and assent from the children were obtained. This investigation was approved by the Institutional Review Board of the University of Southern California. To be included in these analyses participants must have 3 or 4 annual MetS assessments prior to CIMT measurement.
Study Protocol
As part of the full study protocol previously described 17, participants attended their annual visit to at the USC General Clinical Research Center (GCRC) for a comprehensive medical history and physical examination by a licensed health care provider, an oral glucose tolerance test (OGTT) and body composition measure by dual-energy x-ray absorptiometry (DEXA). Approximately 7-14 days following the outpatient visit, participants were admitted to the USC GCRC for their inpatient hospital visit and examined once more by a licensed health care provider and an MRI was completed. A single-slice axial TR 400/16 view of the abdomen at the level of the umbilicus was analyzed for cross-sectional area of visceral and subcutaneous adipose tissue. Following an overnight fast, a frequently-sampled intravenous glucose tolerance test (FSIVGTT) was performed the following morning.
Fasting lipids were assessed using Vitros Chemistry DT Slides (Johnson and Johnson Clinical Diagnostics, Inc, Rochester, New York). Glucose was assayed using a Yellow Springs Instruments analyzer (YSI INC., Yellow Springs, OH) that uses a membrane bound glucose oxidase technique. Insulin was assayed using a specific human insulin enzyme-linked immunosorbent assay kit from Linco (St. Charles, MO; intra-assay coefficient of variation 4.7-7.0%, interassay coefficient of variation 9.1-11.4%; cross-reaction with human proinsulin 0%).
CIMT was determined at the USC Atherosclerosis Research Unit Core Imaging and Reading Center as previously described 11, 18-22. High resolution B-mode ultrasound images were obtained using a Seimens Acuson CV70 (13 MHz linear array) imager. C-IMT was measured from computer processed images of the right distal common carotid artery approximately 1-2 cm from the bifurcation into external and internal carotids.
We defined the MetS using ATP-like criteria adapted for children, as previously described by our laboratory 17. The MetS was determined at each annual visit, and the subjects were then classified into 1 of 3 categories according to the persistence of the MetS over the repeated annual visits: NEVER group (0 annual visits with the MetS), INTERMITTENT group (1 or 2 annual visits with the MetS), or PERSISTENT groups (3 or 4 annual visits with the MetS). The persistence of each MetS component was examined in the same fashion.
Statistical Analyses
The Shapiro-Wilk's W test was used to test the Gaussian distribution of the dependent variable, CIMT, which did not deviate from normality (p>0.05). Chi-square tests and analysis of variance (ANOVA) with Bonferroni corrections were used to compare baseline characteristics of the 3 MetS groups. The analysis of covariance (ANCOVA) test was used to test for differences in CIMT by MetS group while adjusting for gender and the following covariates at baseline: age, total fat mass, total lean tissue mass, LDL-cholesterol, and insulin sensitivity. To test for differences in CIMT by persistence of the individual MetS components, ANOVA and ANCOVA analyses were performed to determine which component contributed the most to our initial result. To determine which of the MetS components contributed most to the results, linear regression models were employed with dependent variable, CIMT. Both models were adjusted for gender, age, total fat mass and total lean tissue mass (either at baseline or at time of CIMT measurement). Data were analyzed using SPSS for Windows version 13.0 (SPSS Inc., Chicago, IL), with an apiori significance level set at p < 0.05. Data are reported as mean ± SD.
Results
The total sample was composed of 57.7% male participants with a mean overall age of 11.0 ± 1.8 yrs at baseline). In Table 1, baseline physical and metabolic characteristics are shown by the three MetS categories (NEVER, INTERMITTENT and PERSISTENT). There were significantly more males in the INTERMITTENT and PERSISTENT groups. The adiposity measures of BMI, total body fat mass and abdominal adipose tissue were not statistically different in any group. Insulin sensitivity was lowest in the PERSISTENT MetS group, but this difference did not reach statistical significance. Differences at baseline of the MetS components are shown in Table 2. The PERSISTENT MetS group had a significantly higher percentage of participants with the MetS at baseline and the highest mean number of MetS components. Mean waist circumference, systolic blood pressure and triglycerides were significantly higher in the PERSISTENT MetS group whereas mean HDL-cholesterol was significantly lower than in the other MetS groups (p<0.05).
Table 1. Baseline physical and metabolic characteristics by MetS Group.
| NEVER MetS (n=53) | INTERMITTENT MetS (n=28) | PERSISTENT MetS (n=16) | Overall p- value (χ square) or significant comparisons (ANOVA) | |
|---|---|---|---|---|
| Male Gender (%) | 47.2% | 64.3% | 81.3% | 0.03 |
| Age (yrs) | 10.9 ± 1.9 | 11.3 ± 1.6 | 10.6 ± 1.8 | NS |
| Maturation stage by Tanner | 2.2 ± 1.3 | 2.0 ± 1.1 | 1.7 ± 1.4 | NS |
| Height (cm) | 146.3 ± 11.6 | 150.3 ± 11.4 | 149.5 ± 14.8 | NS |
| Weight (kg) | 59.3 ± 20.2 | 66.8 ± 20.4 | 67.6 ± 21.2 | NS |
| BMI (kg/m2) | 26.9 ± 5.4 | 28.9 ± 5.9 | 29.4 ± 4.5 | NS |
| Total Lean Tissue Mass (kg) | 34.4 ± 9.9 | 37.7 ± 9.9 | 38.7 ± 12.0 | NS |
| Total Fat Mass (kg) | 22.7 ± 10.3 | 26.6 ± 11.1 | 26.4 ± 9.6 | NS |
| Subcutaneous Adipose Tissue (cm2) | 306.7 ± 158.8 | 344.6 ± 134.9 | 348.1 ± 120.7 | NS |
| Visceral Adipose Tissue (cm2) | 43.6 ± 20.7 | 50.5 ± 16.1 | 45.7 ± 17.9 | NS |
| LDL-cholesterol (mg/dL) | 91.9 ± 21.6 | 96.4 ± 25.8 | 96.2 ± 24.7 | NS |
| Total cholesterol(mg/dL) | 150.4 ± 26.0 | 151.5 ± 28.6 | 155.6 ± 25.9 | NS |
| Adiponectin (μg/mL) | 10.9 ± 3.1 | 10.2 ± 3.3 | 9.1 ± 2.3 | NS |
| Fasting Glucose (mg/dl) | 90.5 ± 6.3 | 90.3 ± 5.5 | 91.1 ± 5.8 | NS |
| Fasting Insulin (μU/ml) | 13.5 ± 8.2 | 18.0 ± 10.7 | 18.3 ± 12.6 | NS |
Chi-square test used to test for gender differences. ANOVA was used to compare means with Bonferroni corrections for multiple comparisons and data are means ± standard deviations.
Table 2. Baseline descriptive statistics of the individual MetS components by MetS group.
| NEVER MetS (n=53) | INTERMITTENT MetS (n=28) | PERSISTENT MetS (n=16) | Overall p-value (χ square) or significant comparisons (ANOVA) | |
|---|---|---|---|---|
| Subjects with MetS (%) | 0% | 32.1% | 87.5% | P=0.000 |
|
| ||||
| Mean Number of MetS components | 1.4 ± 0.7 | 2.2 ± 0.9 | 2.9 ± 0.4 | N vs. I**, N vs. P* |
|
| ||||
| Waist Circumference (WC) (cm) | 85.5 ± 14.4 | 90.2 ± 10.9 | 93.1 ± 13.4 | N vs. P† |
| Subjects meeting criteria (%) | 58.5% | 82.1% | 100% | P=0.002 |
|
| ||||
| HDL-Cholesterol (HDL-C) (mg/dL) | 41.0 ± 10.1 | 34.4 ± 6.4 | 33.7 ± 6.9 | N vs. I**, N vs. P* |
| Subjects meeting criteria (%) | 43.4% | 75.0% | 81.3% | P=0.003 |
|
| ||||
| Systolic blood pressure (SBP)(mmHg) | 106.0 ± 8.4 | 110.3 ± 10.8 | 114.2 ± 8.8 | N vs. P** |
| Subjects meeting criteria (%) | 11.3% | 25.0% | 31.3% | NS |
|
| ||||
| Diastolic blood pressure (DBP) (mmHg) | 62.5 ± 6.3 | 63.8 ± 6.7 | 65.8 ± 4.6 | NS |
| Subjects meeting criteria (%) | 0% | 0% | 0% | NS |
|
| ||||
| Triglycerides (TG) (mg/dL) | 87.6 ± 43.2 | 103.5 ± 35.5 | 128.6 ± 44.8 | N vs. P** |
| Subjects meeting criteria (%) | 9.4% | 21.4% | 50% | P=0.002 |
|
| ||||
| 2-hr Glucose (mg/dL) | 124.8 ± 16.6 | 123.4 ± 17.0 | 128.6 ± 13.4 | NS |
| Subjects meeting criteria (%) | 20.8% | 14.3% | 31.3% | NS |
p=0.06
p<0.05
p<0.01
p<0.001
Chi-square test used for data that were reported as percentages. ANOVA test was used to compare means with Bonferroni corrections for multiple comparisons and data are means ± standard deviations.
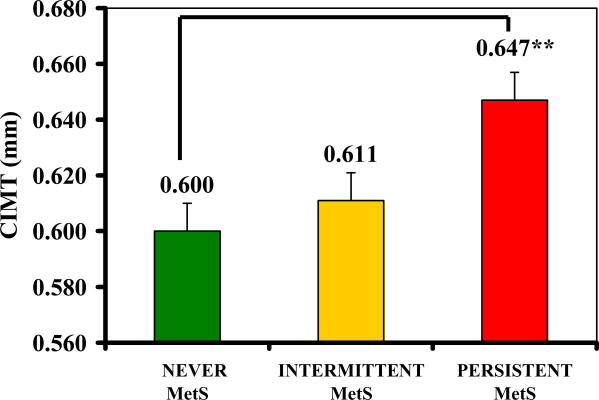
Figure 1 shows the significantly higher CIMT with increasing persistence of the MetS (p=0.01), and this significance remained after adjusting for covariates (gender, baseline age, total fat mass, total lean tissue mass and insulin sensitivity, p<0.05). Post-hoc analyses further revealed a significantly higher mean CIMT in the PERSISTENT than in the NEVER group (ANOVA means: 0.647 ± 0.018 mm vs. 0.600 ± 0.007 mm, p <0.01) and a marginally significantly higher mean CIMT in PERSISTENT than in the INTERMITTENT group (0.647 ± 0.018 mm vs. 0.611 ± 0.008 mm, p=0.09). Statistical significance remained in ANCOVA analyses.
Figure 1. CIMT by MetS persistence group.
Persistence of MetS defined as: NEVER (0 annual visits with the MetS, n=53), INTERMITTENT (1 or 2 visits with the MetS, n=28), and PERSISTENT (3 or 4 visits with the MetS, n=16).
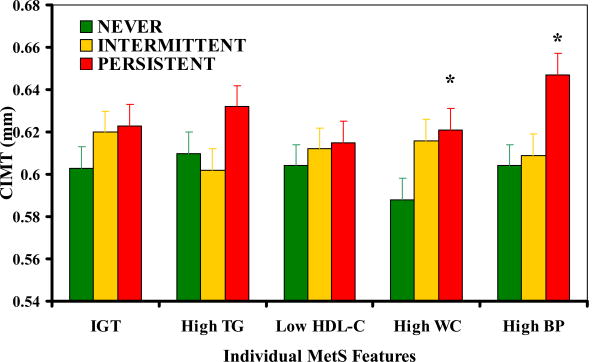
Examination of the persistence of each MetS component and their individual influences on CIMT are reported in Figure 2. Participants with either PERSISTENT high waist circumference (HWC) or PERSISTENT high blood pressure (HBP) had significantly higher mean CIMT than those in the NEVER HWC or HBP groups (p<0.05). Both models were no longer significant after adjustment for total body fat and lean tissue mass. All other PERSISTENT component groups had higher CIMT than the INTERMITTENT or NEVER group, but these differences did not reach statistical significance (p>0.05).
Figure 2. CIMT by persistence of individual MetS component.
In Table 3, it is shown that CIMT was significantly related with baseline SBP (p=0.018) and impaired glucose tolerance (p=0.02), independent of gender, baseline age, body composition and all other MetS components. At time of CIMT measurement, CIMT was significantly related with age (p=0.008) and total lean tissue mass (p<0.001), but it was not associated with any of the MetS components.
Table 3. Associations between CIMT and the individual components of the Mets at baseline and at time of CIMT measurement.
| At Baseline | At time of CIMT measurement | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Unstandardized Beta | SE | p-value | Unstandardized Beta | SE | p-value | |
| Gender | -0.010056 | 0.012073 | 0.407 | 0.023653 | 0.014120 | 0.098 |
| Age | -0.007905 | 0.004942 | 0.113 | -0.010823 | 0.003952 | 0.008 |
| Total Lean Tissue Mass | 0.000000 | 0.000001 | 0.930 | 0.000003 | 0.000001 | 0.000 |
| Total Fat Mass | 0.000002 | 0.000001 | 0.075 | 0.000000 | 0.000001 | 0.509 |
| Systolic Blood Pressure | 0.001625 | 0.000676 | 0.018 | 0.000941 | 0.000827 | 0.259 |
| 2-hour Glucose | 0.000820 | 0.000347 | 0.020 | 0.000146 | 0.000275 | 0.598 |
| Waist | 0.001642 | 0.001078 | 0.113 | 0.000428 | 0.001641 | 0.795 |
| Triglycerides | -0.000034 | 0.000134 | 0.803 | -0.000052 | 0.000136 | 0.700 |
| HDL-cholesterol | -0.000008 | 0.000645 | 0.990 | 0.000144 | 0.000858 | 0.867 |
Discussion
The overall objective of this study was to investigate in overweight Latino youth, the effects of the persistence of the MetS over several years on CIMT (measured 7.9 ± 0.7 months following the most recent MetS evaluation). The analysis showed that children with persistent MetS over a 3-year period was associated with a 7% higher CIMT compared to those who never had MetS. This finding remained significant after adjusting for a variety of potential covariates including gender, baseline age, total body fat mass, total lean tissue mass and insulin sensitivity. Of the individual MetS components, persistent high waist circumference and high blood pressure were related to increased CIMT, although these effects may be explained by baseline total body fat and lean tissue mass. However, baseline measures of systolic blood pressure and 2-hour glucose were independently related to increased CIMT.
To date, there are only two longitudinal studies focusing on the relationship between CVD risk factors in childhood and their long-term effects on CIMT in adulthood. The Bogalusa Heart Study found that clustering of increasing number of MetS components at the lower quartiles of risk predicted about a 0.1mm decrease in CIMT at 26 yr follow-up 3. Secondly, the Young Finns Study found that adverse CVD clustering of risk factors (LDL cholesterol, systolic blood pressure, BMI, smoking) in childhood predicted up to 0.1 mm higher CIMT at 21 yr follow-up 4. The present study is novel in that it shows that a significant 0.047mm greater CIMT can be observed in childhood following only 3 years of persistent MetS.
There are few studies examining the relationships between clustered cardiometabolic risk factors and subclinical atherosclerosis during childhood. A cross-sectional study of various pediatric MetS definitions and CIMT found that the definitions with higher BMI cut-offs and that utilized impaired glucose tolerance (Viner et al. & Weiss et al.)6, 27 were the ones with significant relationships with CIMT 28. The present study showed that persistent MetS was related to elevated CIMT, using our pediatric definition of the MetS. This suggests that our pediatric definition, along with Viner et al. 27 and Weiss et al. 6, may be suitable matches for the concept of MetS in children. Our study also showed that all participants in the PERSISTENT MetS group were also part of the PERSISTENT HWC group, which implies that abdominal adiposity may be driving the relationship between persistent MetS and CIMT. In contrast, only 37.5% of those in the PERSISTENT MetS group were also in the PERSISTENT high blood pressure group, suggesting this feature may be more of a stand-alone risk. Similar to Reinehr et al. 28, we found that baseline systolic blood pressure and 2-hour glucose were the best predictors of CIMT, independent of the other MetS components. These data suggest that hypertension in conjunction with early insulin resistance and high waist circumference may be a plausible explanation for elevated CIMT in overweight Latino youth.
The PERSISTENT group's average CIMT was 0.047 mm higher than the NEVER group. The normal rate of progression reported in our control groups ranges from 0.003 mm to 0.005 mm increase/year 20, 22. Therefore, this 0.047mm difference would represent about 10 years of increased atherosclerosis progression. Although we can only speculate, such an elevated CIMT may indicate an early state of diseased vessels in these adolescents, signifying a more rapid progression of atherosclerosis as compared to youth with no history of the MetS. Supportive evidence includes an adult longitudinal study that showed that every 0.030 mm increase in CIMT per year translates into a relative risk for any coronary event of 3.111. Although a direct comparison cannot be made to our current study, a 0.047 mm CIMT difference may signify a potential coronary risk in this high-risk youth group.
Our findings have other important clinical implications. The relatively short-term effects of MetS on CIMT during childhood and adolescence suggest that more regular measurement of MetS and more aggressive treatment for children with the MetS may be appropriate, particularly in high-risk groups such as obese and family history. Waist circumference and blood pressure are easily accessible clinic measures that would allow for cost-effective and repeatable clinical evaluation. This could be an important predictive measure of atherosclerosis and premature CVD events, perhaps as early as young adulthood.
The strengths of this study stem from the methodological aspects, which included the longitudinal measures the MetS and a subclinical atherosclerosis measure completed by the same sonographer and reader for the ultrasound images. In addition, we used accurate measures of adiposity such as total and regional body composition (DEXA and MRI scans) and direct measures of insulin sensitivity (FSIVGTT with minimal modeling). The homogeneous sample of overweight, understudied minority youth also contributed to the strength of this study. Despite these strengths, there were several design limitations. The CIMT measure was only taken at a single time point and consequently, we could only speculate that persistent MetS caused increased thickening of the carotid artery in overweight Latino youth. Repeated measures of CIMT are currently being conducted within this cohort, but have yet to be reported. Another limitation of the study is the unequal gender distribution, resulting in more male participants within the PERSISTENT MetS group. Increased CIMT has been shown to be more prevalent in male adults 29 and male children 30, hence we cannot disregard male gender as a potential predictor as any gender differences may be masked. Finally, the data results could only be generalized to overweight Latino adolescents with a family history of type 2 diabetes.
In summary, our main conclusion is that persistence of the MetS over a 3-year period was associated with increased CIMT during childhood. Persistent abdominal adiposity and/or hypertension may also have an effect on arterial structure even before adulthood. A comprehensive evaluation for presence of the MetS is warranted to intervene and prevent vascular disease in this especially high-risk group of overweight adolescents.
Acknowledgments
We would like to thank the staff of the University of Southern California/Los Angeles County GCRC and the dedicated SOLAR coordinators, Quintilla Avila and Christina Ayala and the rest of the SOLAR staff over the past six years. Our gratitude is especially extended to the loyal participants and their families for their continued participation. This work is supported by NIDDK grants R01-DK59211 (M.I. Goran) and General Clinical Research Center for Health Resources grant (M01 RR 00043). Claudia Toledo-Corral is supported by a NIDDK fellowship (F31-DK081276) under the sponsorship of M.I.Goran.
Footnotes
Disclosures: The authors have no disclosures.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dekker JM, Girman C, Rhodes T, Nijpels G, Stehouwer CD, Bouter LM, Heine RJ. MetS and 10-year cardiovascular disease risk in the Hoorn Study. Circulation. 2005;112:666–673. doi: 10.1161/CIRCULATIONAHA.104.516948. [DOI] [PubMed] [Google Scholar]
- 2.Hunt KJ, Resendez RG, Williams K, Haffner SM, Stern MP. National Cholesterol Education Program versus World Health Organization MetS in relation to all-cause and cardiovascular mortality in the San Antonio Heart Study. Circulation. 2004;110:1251–1257. doi: 10.1161/01.CIR.0000140762.04598.F9. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Srinivasan SR, Li S, Xu J, Berenson GS. MetS variables at low levels in childhood are beneficially associated with adulthood cardiovascular risk: the Bogalusa Heart Study. Diabetes Care. 2005;28:126–131. doi: 10.2337/diacare.28.1.126. [DOI] [PubMed] [Google Scholar]
- 4.Raitakari OT, Juonala M, Kahonen M, Taittonen L, Laitinen T, Maki-Torkko N, Jarvisalo MJ, Uhari M, Jokinen E, Ronnemaa T, Akerblom HK, Viikari JS. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. Jama. 2003;290:2277–2283. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- 5.Flegal KM, Ogden CL, Carroll MD. Prevalence and trends in overweight in Mexican-american adults and children. Nutr Rev. 2004;62:S144–148. doi: 10.1111/j.1753-4887.2004.tb00085.x. [DOI] [PubMed] [Google Scholar]
- 6.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, Sherwin RS, Caprio S. Obesity and the MetS in children and adolescents. N Engl J Med. 2004;350:2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 7.Poli A, Tremoli E, Colombo A, Sirtori M, Pignoli P, Paoletti R. Ultrasonographic measurement of the common carotid artery wall thickness in hypercholesterolemic patients. A new model for the quantitation and follow-up of preclinical atherosclerosis in living human subjects. Atherosclerosis. 1988;70:253–261. doi: 10.1016/0021-9150(88)90176-1. [DOI] [PubMed] [Google Scholar]
- 8.Heiss G, Sharrett AR, Barnes R, Chambless LE, Szklo M, Alzola C. Carotid atherosclerosis measured by B-mode ultrasound in populations: associations with cardiovascular risk factors in the ARIC study. Am J Epidemiol. 1991;134:250–256. doi: 10.1093/oxfordjournals.aje.a116078. [DOI] [PubMed] [Google Scholar]
- 9.Chambless LE, Folsom AR, Clegg LX, Sharrett AR, Shahar E, Nieto FJ, Rosamond WD, Evans G. Carotid wall thickness is predictive of incident clinical stroke: the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol. 2000;151:478–487. doi: 10.1093/oxfordjournals.aje.a010233. [DOI] [PubMed] [Google Scholar]
- 10.O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 11.Hodis HN, Mack WJ, LaBree L, Selzer RH, Liu CR, Liu CH, Azen SP. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128:262–269. doi: 10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 12.Reinehr T, Kiess W, de Sousa G, Stoffel-Wagner B, Wunsch R. Intima media thickness in childhood obesity: relations to inflammatory marker, glucose metabolism, and blood pressure. Metabolism. 2006;55:113–118. doi: 10.1016/j.metabol.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Zhu W, Huang X, He J, Li M, Neubauer H. Arterial intima-media thickening and endothelial dysfunction in obese Chinese children. Eur J Pediatr. 2005;164:337–344. doi: 10.1007/s00431-005-1642-y. [DOI] [PubMed] [Google Scholar]
- 14.Woo KS, Chook P, Yu CW, Sung RY, Qiao M, Leung SS, Lam CW, Metreweli C, Celermajer DS. Overweight in children is associated with arterial endothelial dysfunction and intima-media thickening. Int J Obes Relat Metab Disord. 2004;28:852–857. doi: 10.1038/sj.ijo.0802539. [DOI] [PubMed] [Google Scholar]
- 15.Iannuzzi A, Licenziati MR, Acampora C, Salvatore V, Auriemma L, Romano ML, Panico S, Rubba P, Trevisan M. Increased carotid intima-media thickness and stiffness in obese children. Diabetes Care. 2004;27:2506–2508. doi: 10.2337/diacare.27.10.2506. [DOI] [PubMed] [Google Scholar]
- 16.Toledo-Corral CM, W M, Hodis HN, Goran MI. Influence of adiposity, insulin sensitivity, cardiovascular risk factors, and fasting glucose on Carotid Intima Media Thickness (C-IMT) in overweight Latino adolescents (abstract) Obesity. 2007;15:A125. [Google Scholar]
- 17.Cruz ML, Weigensberg MJ, Huang TT, Ball G, Shaibi GQ, Goran MI. The MetS in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrinol Metab. 2004;89:108–113. doi: 10.1210/jc.2003-031188. [DOI] [PubMed] [Google Scholar]
- 18.Selzer RH, Hodis HN, Kwong-Fu H, Mack WJ, Lee PL, Liu CR, Liu CH. Evaluation of computerized edge tracking for quantifying intima-media thickness of the common carotid artery from B-mode ultrasound images. Atherosclerosis. 1994;111:1–11. doi: 10.1016/0021-9150(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 19.Selzer RH, Mack WJ, Lee PL, Kwong-Fu H, Hodis HN. Improved common carotid elasticity and intima-media thickness measurements from computer analysis of sequential ultrasound frames. Atherosclerosis. 2001;154:185–193. doi: 10.1016/s0021-9150(00)00461-5. [DOI] [PubMed] [Google Scholar]
- 20.Hodis HN, Mack WJ, Lobo RA, Shoupe D, Sevanian A, Mahrer PR, Selzer RH, Liu Cr CR, Liu Ch CH, Azen SP. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;135:939–953. doi: 10.7326/0003-4819-135-11-200112040-00005. [DOI] [PubMed] [Google Scholar]
- 21.Xiang AH, Azen SP, Buchanan TA, Raffel LJ, Tan S, Cheng LS, Diaz J, Toscano E, Quinonnes M, Liu CR, Liu CH, Castellani LW, Hsueh WA, Rotter JI, Hodis HN. Heritability of subclinical atherosclerosis in Latino families ascertained through a hypertensive parent. Arterioscler Thromb Vasc Biol. 2002;22:843–848. doi: 10.1161/01.atv.0000015329.15481.e8. [DOI] [PubMed] [Google Scholar]
- 22.Hodis HN, Mack WJ, LaBree L, Mahrer PR, Sevanian A, Liu CR, Liu CH, Hwang J, Selzer RH, Azen SP. Alpha-tocopherol supplementation in healthy individuals reduces low-density lipoprotein oxidation but not atherosclerosis: the Vitamin E Atherosclerosis Prevention Study (VEAPS) Circulation. 2002;106:1453–1459. doi: 10.1161/01.cir.0000029092.99946.08. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr. 2004;145:439–444. doi: 10.1016/j.jpeds.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 24.Hickman TB, Briefel RR, Carroll MD, Rifkind BM, Cleeman JI, Maurer KR, Johnson CL. Distributions and trends of serum lipid levels among United States children and adolescents ages 4-19 years: data from the Third National Health and Nutrition Examination Survey. Prev Med. 1998;27:879–890. doi: 10.1006/pmed.1998.0376. [DOI] [PubMed] [Google Scholar]
- 25.Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents: a working group report from the National High Blood Pressure Education Program. National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents. Pediatrics. 1996;98:649–658. [PubMed] [Google Scholar]
- 26.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. 1997;20:1183. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 27.Viner RM, Segal TY, Lichtarowicz-Krynska E, Hindmarsh P. Prevalence of the insulin resistance syndrome in obesity. Arch Dis Child. 2005;90:10–14. doi: 10.1136/adc.2003.036467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reinehr T, Wunsch R, de Sousa G, Toschke AM. Relationship between MetS definitions for children and adolescents and intima-media thickness. Atherosclerosis. 2008;199:193–200. doi: 10.1016/j.atherosclerosis.2007.09.041. [DOI] [PubMed] [Google Scholar]
- 29.Ebrahim S, Papacosta O, Whincup P, Wannamethee G, Walker M, Nicolaides AN, Dhanjil S, Griffin M, Belcaro G, Rumley A, Lowe GD. Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: the British Regional Heart Study. Stroke. 1999;30:841–850. doi: 10.1161/01.str.30.4.841. [DOI] [PubMed] [Google Scholar]
- 30.Osika W, Dangardt F, Montgomery SM, Volkmann R, Gan LM, Friberg P. Sex differences in peripheral artery intima, media and intima media thickness in children and adolescents. Atherosclerosis. 2009;203:172–177. doi: 10.1016/j.atherosclerosis.2008.05.054. [DOI] [PubMed] [Google Scholar]