Summary
Objective
We performed this study to examine the metabolic differences arising from higher liver fat accumulation in obese Hispanic adolescents, with a particular focus on circulating levels of adipocytokines and insulin resistance.
Methods
Forty-one obese Hispanic adolescents (15.3 ± 1.0 years, body mass index percentile: 97.0 ± 3.9) were assessed for: visceral adipose tissue (VAT), subcutaneous adipose tissue (SAT) and hepatic fat fraction (HFF) by magnetic resonance imaging; fasting measures of serum glucose, insulin and adipocytokines; homeostasis model assessment of insulin resistance (HOMA-IR); and insulin sensitivity (SI) and the acute insulin response to glucose (AIR) by intravenous glucose tolerance test. Subjects with normal levels of HFF (below 5%; n = 25) were compared to those with HFF > 5% (n = 16).
Results
The two groups differing in HFF were similar for total body fat, VAT and SAT. The group with HFF > 5% had significantly (P < 0.05) higher interleukin-8 (IL-8) (6.1 ± 1.6 vs. 3.2 ± 0.4 pg mL−1), NGF (30.2 ± 9.9 vs. 13.9 ± 1.6 pg mL−1), HOMA-IR (8.8 ± 1.1 vs. 5.5 ± 0.5), AIR (1869 ± 206 vs. 1092 ± 165) and a tendency for lower SI (1.2 ± 0.4 vs. 2.1 ± 0.3; P = 0.06), with no significant differences in any of other factors measured.
Conclusions
These data suggest that elevated liver fat is most closely associated with elevated serum IL-8 and NGF levels as well as increased AIR and HOMA-IR. These elevated factors may play significant roles in the metabolic abnormalities associated with elevated liver fat in obese Hispanics.
Keywords: Adipocytokine, fatty liver, Hispanics, insulin resistance
Introduction
Non-alcoholic fatty liver disease (NAFLD) is defined by accumulation of liver fat > 5% per liver weight in the presence of < 10 g of daily alcohol consumption, and represents a spectrum of liver disease ranging from simple steatosis to non-alcoholic steatohepatitis (NASH), a progressive form that can lead to extensive fibrosis, cirrhosis and even hepatocellular carcinoma (1). NAFLD is now the most common cause of chronic liver disease in children and adolescents in the United States (2), paralleling the burgeoning prevalence of childhood obesity. A large paediatric autopsy study revealed that 38% of obese children had NAFLD (3).
Previous studies show that NAFLD is found to be more common in Hispanics than Caucasian or African Americans. For example, NAFLD was highest in Hispanics (45%) and lowest in African Americans (24%) in 2287 adults (4). This ethnic disparity has also been reported in the paediatric population (3,5). Hispanics may have higher rates of insulin resistance and visceral adiposity at equivalent body mass index (BMI), predisposing this ethnicity to NAFLD (6).
The adipose tissue has long been considered an energy storage organ but is also now considered a metabolically active endocrine organ and a key regulator of both metabolic and inflammatory pathways (7). Recently, the important role of adipocytokines, peptide factors derived significantly from the adipose tissue, in the pathogenesis of obesity, insulin resistance and also NAFLD has been highlighted (8). However, serum levels of adipocytokines and their association with liver fat accumulation in obese Hispanic adolescents are unknown. Thus, we performed this study to examine the metabolic differences arising from higher liver fat accumulation in obese Hispanic adolescents, with a particular focus on circulating levels of adipocytokines and insulin resistance.
Methods
Study subjects
This cross-sectional analysis used the baseline values of data from the cohort studies conducted in our laboratory (9). These studies are randomized controlled trials of nutrition education and various types of exercise on obesity and metabolic risk factors for type 2 diabetes; however, we have not assessed the relationship between adipocytokines and liver fat accumulation. This analysis includes 41 obese (age- and gender-specific BMI ≥85th percentile) adolescents (aged 14 to 17 years, 27 boys and 14 girls) with Hispanic ethnicity (i.e. parents and grandparents of Hispanic descent by parental self-report). Participants were excluded from the study based on the following criteria: (i) were using medication or were diagnosed with any disease that could influence dietary intake, exercise ability, body composition, fat distribution or insulin resistance; (ii) were previously diagnosed with any major illness; (iii) met any diagnostic criteria for diabetes or (iv) participated in a structured exercise, nutrition or weight loss programme in the past 6 months. Written informed consent and assent was obtained from all participants and their parents. This study was approved by the Institutional Review Board of the University of Southern California (USC), Health Science Campus.
Anthropometry and body composition
Weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively, using beam medical scale and wall-mounted stadiometer, and BMI and BMI percentile were determined (CDC, 2000). Total body fat and soft lean tissue were measured by dual energy X-ray absorptiometry using a Hologic QDR 4500W (Hologic, Bedford, MA, USA). Central fat distribution was measured by magnetic resonance imaging (MRI), on a Siemens Magnetom 1.5-Tesla Symphony Maestro Class Syngo 2004A (Siemens AG, Erlangen, Germany) with a Numaris/4 software at the USC imaging centre. Slices were acquired using a 420-mm field of view (FOV) and FOV phase factor of 75%. Three abdominal scans were performed consecutively and total acquisition time was 24 s per total abdominal scan. Each scan obtained 19 axial images of the abdomen with a slice thickness of 10 mm. After image acquisition, visceral and subcutaneous adipose tissue (VAT and SAT) volumes were segmented using the SliceOmatic software (SliceOmatic Tomovision, Montreal, Canada). SAT and VAT volumes were calculated from all image slices in each subject.
Quantification of hepatic fat fraction
Hepatic fat fraction (HFF) was assessed by MRI using a modification of the Dixon three-point technique. Abdominal slices were acquired contiguously using a breath-holding dual-echo spoiled gradient-recalled echo sequence with repetition time of 156 ms and echo time of 2.3 ms for out-of-phase (OP) images and 4.78 ms for in-phase (IP) images. Images were acquired with flip angles of 70° and then 20° to provide T1 -weighted and intermediate-weighted images. A third dual-echo gradient-echo breath-hold gradient-recalled echo sequence with two IP echoes (4.8 and 9.6 ms) was also performed to calculate T2. The HFF was estimated from the signal intensity index (SII) obtained from IP and OP images. HFF = (SIIP - SIOP)/2SIIP, where SIIP and SIOP are the signal intensities of IP and OP images. Quantitative corrections for the influence of T2 decay on the fat fraction estimates are taken into account by the third dual-echo sequence, where T2 for the liver is estimated on a pixel-by-pixel basis. As the HFF is estimated from flip-angle images (20°), the effect of T1 relaxation on the quantification is minimized. Once the HFF images are calculated, three consecutive slices with maximum axial coverage of the liver are selected. Region of interests (ROIs) are drawn on each slice, ranging from 1.8 cm2 to 14.1 cm2, while avoiding any major blood vessels, to report the average HFF value. We set the cut-off point of hepatic steatosis as 5% of HFF.
Frequently sampled intravenous glucose tolerance test
An insulin-modified frequently sampled intravenous glucose tolerance test (FSIVGTT) was performed after an overnight fast. Upon arrival, a topical anaesthetic (EMLA cream; Astrazeneca, Wilmington, DE, USA) was applied to the antecubital area of both arms, and 1 h later a flexible intravenous catheter was inserted into both of the arms. At time 0 min, glucose (25% dextrose, 0.3 g/kg body wt) was administered intravenously. Insulin (0.02 units/kg body wt, Humulin R [regular insulin for human injection]; Eli Lilly, Indianapolis, IN, USA) was injected intravenously at 20 min. Blood samples of glucose and insulin were collected at time points −15, −5, 2, 4, 8, 19, 22, 30, 40, 50, 70, 100 and 180 min, and of free fatty acids (FFA) at time −15. The MINMOD Millenium 2003 computer program (version 5.16, Bergman, USC) was used to determine insulin sensitivity (SI), acute insulin response (AIR), glucose effectiveness and disposition index.
Blood analysis
Blood samples from all time points taken during the FSIVGTT were centrifuged immediately for 10 min at 2500 RPM and 8–10°C to obtain plasma, and aliquots were frozen at −70°C until assayed. Glucose was assayed in duplicate on a Yellow Springs Instrument 2700 Analyzer (Yellow Springs Instrument, Yellow Springs, OH, USA) using the glucose oxidase method. Insulin was assayed in duplicate using a specific human insulin enzyme-linked immunosor-bent assay kit from Linco (St. Charles, MO, USA), and FFA using a colorimetric kit (NEFA-HR (2), Wako Diagnostics, VA, USA). Circulating inflammatory mediators, including adiponectin, leptin, resistin, plasminogen activator inhibitor-1, monocyte chemoattractant protein-1, interleukin-8 (IL-8), tumour necrosis factor-alpha (TNF-alpha), nerve growth factor (NGF) and hepatocyte growth factor (HGF), were measured in batch using multiplex Luminex assays (Linco Research, St. Charles, MO, USA). High sensitivity C-reactive protein (hsCRP) was measured chemically using ADVIA® 1800 Chemistry System (Siemens Healthcare Diagnostics, Deerfield, IL, USA). The homeostasis model assessment of insulin resistance (HOMA-IR) was determined as the product of the fasting plasma insulin level (μU/mL) and the fasting plasma glucose level (mg dL−1), divided by 405.
Statistical analysis
All data are presented as means ± SE. Mean variable differences between two groups were analysed by independent t-test and Chi-square test. Adjustments of comparisons for potential confounders including age, gender, BMI, total fat mass, visceral fat and SI markers were performed using analysis of covariance (ancova) when appropriate. Correlation analyses were done using Pearson's correlation test. Variables not normally distributed were log-transformed before the analysis. All statistical analyses were performed using SPSS version 13.0 (SPSS, Chicago, IL, USA), and a significance level was set at P < 0.05.
Results
Clinical characteristics, body composition and serum adipocytokines
Sixteen adolescents (39%) had HFF of 5% or greater and were assigned to the group with high HFF, while the remaining 25 adolescents were assigned to the control group having HFF less than 5%. The clinical characteristics, anthropometric and metabolic parameters of the subjects are shown in Table 1. There were no significant differences between the groups in regard to age, gender, BMI, BMI percentile, adiposity measures and circulating FFA. However, the group with high HFF had significantly higher HOMA-IR and AIR compared with the control group (P < 0.05), a tendency for lower SI (P = 0.06). The circulating adipocytokines and hsCRP levels of the subjects are shown in Table 2, and were similar between groups with the exception of IL-8 and NGF which were significantly elevated in the group with high HFF (P < 0.05).
Table 1. Clinical characteristics and metabolic parameters of the subjects.
| Control group (n = 25) |
High HFF group (n = 16) |
|
|---|---|---|
| Gender (males/females) | 17/8 | 10/6 |
| Age (years) | 15.4 ± 0.2 | 15.3 ± 0.3 |
| Weight (kg) | 94.9 ± 4.4 | 95.8 ± 6.3 |
| Height (cm) | 168.1 ± 1.5 | 164.7 ± 1.9 |
| Waist circumference (cm) | 97.1 ± 2.7 | 99.7 ± 3.6 |
| BMI (kg/m2) | 33.4 ± 1.3 | 35.7 ± 2.0 |
| BMI percentile | 96.7 ± 0.9 | 97.7 ± 0.8 |
| Total fat mass (kg) | 32.4 ± 2.6 | 34.5 ± 3.2 |
| Total fat-free mass (kg) | 57.3 ± 1.7 | 53.2 ± 2.5 |
| Serum FFA (mmol L−1) | 0.65 ± 0.03 | 0.62 ± 0.04 |
| HFF (%) | 1.9 ± 0.3 | 17.2 ± 2.3* |
| SAT (L) | 8.6 ± 0.8 | 10.0 ± 1.0 |
| VAT (L) | 1.6 ± 0.2 | 1.8 ± 0.2 |
| HOMA-IR | 5.5 ± 0.5 | 8.8 ± 1.1* |
| AIR (μU/mL × 10 min) | 1092 ± 120 | 1869 ± 272* |
| SI (×10−4 min−1/μU/mL) | 2.1 ± 0.4 | 1.2 ± 0.2 |
| Glucose effectiveness (%/min) | 0.03 ± 0.005 | 0.04 ± 0.008 |
| Disposition index (×10−4/min) | 1535.8 ± 187.4 | 1718.5 ± 206.1 |
Data expressed as mean ± SE. Independent t-tests were used to test for differences between groups, and Chi-square test was used for gender differences.
P < 0.05.
AIR, acute insulin response to glucose; BMI, body mass index; FFA, free fatty acid; HFF, hepatic fat fraction; HOMA-IR, homeostasis model assessment of insulin resistance; SAT, subcutaneous adipose tissue; SI, insulin sensitivity; VAT, visceral adipose tissue.
Table 2. Circulating adipocytokines and hsCRP levels of the subjects.
| Control group (n = 25) |
High HFF group (n = 16) |
|
|---|---|---|
| Adiponectin (μg mL−1) | 20.8 ± 2.3 | 18.1 ± 3.0 |
| PAI-1 (ng mL−1) | 133.5 ± 12.3 | 130.2 ± 23.4 |
| Resistin (ng mL−1) | 52.4 ± 6.0 | 38.3 ± 7.0 |
| IL-8 (pg mL−1) | 3.2 ± 0.4 | 6.1 ± 1.6* |
| Leptin (ng mL−1) | 41.6 ± 6.5 | 41.4 ± 4.9 |
| TNF-alpha (pg mL−1) | 11.4 ± 1.4 | 15.4 ± 2.4 |
| MCP-1 (pg mL−1) | 259.7 ± 21.2 | 279.0 ± 39.1 |
| HGF (ng mL−1) | 1.2 ± 0.1 | 1.4 ± 0.3 |
| NGF (pg mL−1) | 13.9 ± 1.6 | 30.2 ± 9.9* |
| hsCRP (mg L−1) | 4.0 ± 1.9 | 3.6 ± 1.1 |
Data expressed as mean ± SE. Independent t-tests were used to test for differences between groups.
P < 0.05.
HGF, hepatocyte growth factor; hsCRP, high sensivity C-reactive protein; IL-8, interleukin-8; MCP-1, monocyte chemoat-tractant protein-1; NGF, nerve growth factor; PAI-1, plasminogen activator inhibitor-1; TNF-alpha, tumour necrosis factor-alpha.
Correlations between hepatic fat fraction and body composition/markers of inflammation and insulin resistance
In univariate analysis, there were no significant relationships between HFF and body composition variables, including BMI, SAT, VAT, total fat mass, total fat-free mass as well as circulating level of FFA. HFF was significantly correlated with the value of HOMA-IR (r = 0.56, P < 0.001) and AIR (r = 0.40, P = 0.009). There was a significant negative correlation between serum levels of adiponectin and HOMA-IR (r = −0.44, P = 0.004).
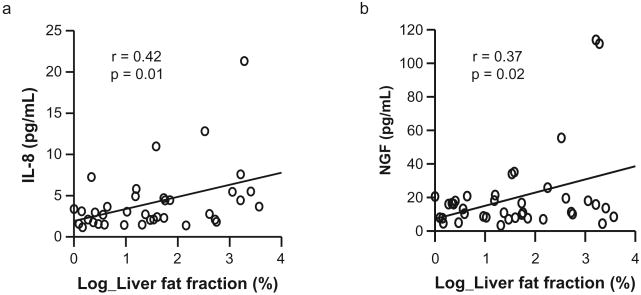
HFF was significantly correlated with serum levels of IL-8 (r = 0.42, P = 0.01) and NGF (r = 0.37, P = 0.02) (Fig. 1). The correlation between HFF and IL-8 remained significant after adjusting for age, gender, BMI, total fat mass, HOMA-IR, SI and AIR (r = 0.45, P = 0.02). But the correlation between HFF and NGF was no longer significant after adjusting the same variables (r = 0.38, P = 0.052). In addition, serum levels of IL-8 were significantly correlated with TNF-alpha (r = 0.72, P < 0.001), NGF (r = 0.78, P < 0.001), FFA (r = 0.43, P = 0.008) as well as the degree of HFF.
Figure 1.
Relationships between hepatic fat fraction and serum levels of (a) interleukin-8 (IL-8) and (b) nerve growth factor (NGF). Hepatic fat fraction was significantly correlated with serum levels of IL-8 (r = 0.42, P = 0.01) and NGF (r = 0.37, P = 0.02).
Discussion
The overall objective of this study was to examine the metabolic differences arising from higher liver fat accumulation in obese Hispanic adolescents, with a particular focus on circulating levels of adipocytokines and insulin resistance. Our main findings were that higher liver fat accumulation was associated with elevated serum IL-8 and NGF levels as well as increased AIR and HOMA-IR. These results suggest that together with insulin resistance, high circulating levels of IL-8 may play an important role in the pathogenesis of obesity-related hepatic steatosis, although further studies are required to elucidate the role of other adipocytokines and the role of these elevated adipocytokines in the progression from elevated liver fat to NAFLD. These mechanisms could include direct lipid toxicity, mitochondrial dysfunction, oxidative stress and inflammation caused by proinflammatory cytokines (10). In addition, obesity has been considered a state of chronic low-grade inflammation in the adipose tissue, which may play a role in the development of obesity-related diseases, including insulin resistance and NAFLD (11). This close relationship may be mediated by inflammatory mediators including adipocytokines (8,10,11).
Adipocytokines are biologically active, polypeptide humoral mediators secreted mainly by the adipose tissue in a regulated manner. Most of adipocytokines except adiponectin are overproduced as the adipose tissue enlarges in obesity (8,11,12). Proinflammatory cytokines, including TNF-alpha, IL-1 and IL-6, mediate macrophage infiltration which causes adipose tissue inflammation through the activation of nuclear factor kappaB (NF-kB) and c-Jun N-terminal kinase (JNK) pathways in obesity, resulting in insulin resistance and dysregulated secretion of other adipokines (13). This dysregulation of adipokine production may promote obesity-related metabolic disorders and cardiovascular disease.
There is increasing evidence that adipose tissue-derived adipocytokines contributed to the pathogenesis of NAFLD and NASH in children (14–17). Among those adipocytokines, circulating levels of adiponectin are inversely correlated with hepatic fat content and insulin resistance. Hypoadiponectinemia may play a predominant role in the pathogenesis of NASH and insulin resistance (11,18). Adiponectin could have a protective effect on progression of hepatic steatosis to NASH through its anti-inflammatory properties because it inhibits the expression of TNF-alpha and other pro-inflammatory cytokines in hepatic stellate cells (19). On the other hand, analysis of the circulating levels of other adipocytokines including leptin, resistin and visfatin in NASH yielded conflicting results (11). Therefore, their role in the pathogenesis of NASH and NAFLD seems to be less clear. Another complex problem in the pathogenesis of NAFLD involves an interplay between adipokines and pro-inflammatory cytokines secreted by both peripheral blood monocytes, lymphocytes and infiltrating macrophages embedded in adipose tissue (20).
In the present study, we found that circulating levels of IL-8 and NGF were significantly higher in the obese Hispanic adolescents with hepatic steatosis than in obese controls with low liver fat. We also demonstrated that the degree of liver fat accumulation is strongly associated with serum IL-8 levels, and this significant association still remained after adjusting for age, gender, BMI, total body fat mass, HOMA-IR, SI and AIR. Our findings support previous studies that showed the association of elevated serum levels of IL-8 with obesity-related diseases, including diabetes, atherosclerosis and NASH, suggesting that IL-8 may play an important role in the pathogenesis of these diseases by causing inflammation and tissue injury (20,21). Unlike previous studies, this study showed that circulating adiponectin levels were not different between the groups. Moreover, our data did not show any relationship between liver fat accumulation hsCRP, the most commonly used marker of inflammation.
IL-8 is a monomeric polypeptide and a member of cysteine X cysteine (CXC) chemokine family, which functions as a critical chemoattractant and activator of neutrophils, lymphocytes and monocytes into the adipose tissues for initiation and maintenance of inflammatory reaction. IL-8 is produced by a variety of cells including human adipocytes, frequently in response to inflammatory stimuli such as TNF-alpha, IL-1β or CRP (23–25). A recent study showed that FFA stimulate hepatocytes to produce IL-8 via the activation of NF-kB and JNK pathways like other pro-inflammatory cytokines (26). We found in this study that serum levels of IL-8 were correlated significantly with TNF-alpha, NGF and FFA as well as the degree of liver fat accumulation. These results suggest that there is complex interplay between IL-8 and other pro-inflammatory cytokines and increased lipolysis with ensuing lipid toxicity in the pathogenesis of NAFLD; eventually, IL-8 may play a crucial role in the pathogenesis of NAFLD.
In this study, serum levels of NGF were significantly elevated in the group with high liver fat. However, the correlation of NGF and HFF did not prove significant in the multivariate analysis. NGF is a member of the neurotrophin family of proteins, including brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (27). In addition to their neurotrophic function, NGF and BDNF act on enhancing survival and activity of a large number of non-neuronal cells. The plasma concentration of NGF is known to be elevated in a number of inflammatory and autoimmune states in response to the strong inducers, such as IL-1β and TNF-alpha (28). In the present study, we postulate that pro-inflammatory cytokines including IL-8 stimulate adipocytes to synthesize NGF, and NGF also could play a role in the inflammatory pathways of obesity-related hepatic steatosis.
At present, liver histopathology is the gold standard for the definitive diagnosis of NAFLD and exclusion of other causes. However, owing to the invasiveness of liver biopsy, the quantitative assessment of hepatic steatosis using non-invasive imaging technique is highly required for the diagnosis, evaluation and follow-up of NAFLD. MRI is the most promising modality to satisfy such need. It is non-invasive, utilizes no ionizing radiation, is reproducible and is applicable to subjects of all ages. The best MR option would be MR spectroscopy, which is considered to be the most accurate method to quantify precisely HFF. We used hepatic MRI with a modification of the Dixon technique to estimate HFF quantitatively in the entire liver, an approach that we have previously validated against MR spectroscopy (29). One limitation of MR spectroscopy is that it can only image isolated voxels of the liver, whereas the modified Dixon method can be used to assess the fat content of the liver.
Finally, we also found that the insulin resistance, determined as HOMA-IR, was significantly higher in Hispanic adolescents with high liver fat than in obese controls with low liver fat. Moreover, there was a significant correlation between the degree of liver fat content and HOMA-IR. These results suggest that insulin resistance may play an important role in the development of obesity-related hepatic steatosis. We confirmed a negative correlation between serum adiponectin and HOMA-IR like previous studies that suggest hypoadiponectinemia plays a crucial role in the development of insulin resistance. We found a significant association of liver fat accumulation with circulating adipocytokines only in IL-8 and NGF. These elevated factors may play significant roles in the metabolic abnormalities associated with elevated liver fat in obese Hispanic adolescents. The limitation of this study was small sample size and conducted only in Hispanics; therefore, our findings need to be confirmed in a larger study.
In conclusion, we demonstrate that the degree of liver fat accumulation is strongly associated with elevated serum IL-8 and NGF levels as well as increased AIR and HOMA-IR in obese Hispanic adolescents. Our results suggest that together with insulin resistance, high circulating levels of IL-8 may play an important role in the pathogenesis of obesity-related hepatic steatosis. Further studies are required to elucidate the possible role of other adipocytokines and the interaction between adipocytokines and the pathogenesis of NAFLD.
What is already known about this subject
Obese Hispanics tend to accumulate fat in the liver which may contribute to a state of insulin resistance and inflammation.
What this study adds
Obese Hispanic teenagers with fatty liver are more insulin resistant despite similar levels of overall obesity and visceral obesity.
The inflammatory markers that are elevated in obese Hispanic teenagers with fatty liver are interleukin-8 and NGF.
Other inflammatory markers and adipocytokines such as adiponectin, leptin, TNF-alpha and CRP were unaffected by fatty liver in this population.
Footnotes
Conflict of Interest Statement: The authors report no conflicts of interest. The authors are responsible for the content and writing of the paper.
References
- 1.Loomba R, Sirlin CB, Schwimmer JB, Lavine JE. Advances in pediatric nonalcoholic fatty liver disease. Hepatology. 2009;50:1282–1293. doi: 10.1002/hep.23119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shneider BL, Gonzalez-Peralta R, Roberts EA. Controversies in the management of pediatric liver disease: Hepatitis B, C and NAFLD – summary of a single topic conference. Hepatology. 2006;44:1344–1354. doi: 10.1002/hep.21373. [DOI] [PubMed] [Google Scholar]
- 3.Schwimmer JB, McGreal N, Deutsch R, Finegold MJ, Lavine JE. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics. 2005;115:e561–e565. doi: 10.1542/peds.2004-1832. [DOI] [PubMed] [Google Scholar]
- 4.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 5.Taksali SE, Caprio S, Dziura J, et al. High visceral and low abdominal subcutaneous fat stores in the obese adolescent: a determinant of an adverse metabolic phenotype. Diabetes. 2008;57:367–371. doi: 10.2337/db07-0932. [DOI] [PubMed] [Google Scholar]
- 6.Petersen KF, Dufour S, Feng J, et al. Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. Proc Natl Acad Sci USA. 2006;103:18273–18277. doi: 10.1073/pnas.0608537103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 8.Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010;314:1–16. doi: 10.1016/j.mce.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 9.Davis JN, Kelly LA, Lane CJ, et al. Randomized control trial to improve adiposity and insulin resistance in overweight Latino adolescents. Obesity. 2009;17:1542–1548. doi: 10.1038/oby.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCullough AJ. Pathophysiology of nonalcoholic steatohepatitis. J Clin Gastroenterol. 2006;40(Suppl. 1):S17–S29. doi: 10.1097/01.mcg.0000168645.86658.22. [DOI] [PubMed] [Google Scholar]
- 11.Marra F, Bertolani C. Adipokines in liver diseases. Hepatology. 2009;50:957–969. doi: 10.1002/hep.23046. [DOI] [PubMed] [Google Scholar]
- 12.Qureshi K, Abrams GA. Metabolic liver disease of obesity and role of adipose tissue in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol. 2007;13:3540–3553. doi: 10.3748/wjg.v13.i26.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louthan MV, Barve S, McClain CJ, Joshi-Barve S. Decreased serum adiponectin: an early event in pediatric nonalcoholic fatty liver disease. J Pediatr. 2005;147:835–838. doi: 10.1016/j.jpeds.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 15.Mandato C, Lucariello S, Licenziati MR, et al. Metabolic, hormonal, oxidative, and inflammatory factors in pediatric obesity-related liver disease. J Pediatr. 2005;147:62–66. doi: 10.1016/j.jpeds.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 16.Zou CC, Liang L, Hong F, Fu JF, Zhao ZY. Serum adiponectin, resistin levels and non-alcoholic fatty liver disease in obese children. Endocr J. 2005;52:519–524. doi: 10.1507/endocrj.52.519. [DOI] [PubMed] [Google Scholar]
- 17.Lebensztejn DM, Wojtkowska M, Skiba E, Werpachowska I, Tobolczyk J, Kaczmarski M. Serum concentration of adiponectin, leptin and resistin in obese children with non-alcoholic fatty liver disease. Adv Med Sci. 2009;54:177–182. doi: 10.2478/v10039-009-0047-y. [DOI] [PubMed] [Google Scholar]
- 18.Musso G, Gambino R, Durazzo M, et al. Adipokines in NASH: postprandial lipid metabolism as a link between adiponectin and liver disease. Hepatology. 2005;42:1175–1183. doi: 10.1002/hep.20896. [DOI] [PubMed] [Google Scholar]
- 19.Venturi C, Zoppini G, Zamboni C, Muggeo M. Insulin sensitivity and hepatic steatosis in obese subjects with normal glucose tolerance. Nutr Metab Cardiovasc Dis. 2004;14:200–204. doi: 10.1016/s0939-4753(04)80005-x. [DOI] [PubMed] [Google Scholar]
- 20.Tilg H, Hotamisligil GS. Nonalcoholic fatty liver disease: cytokine-adipokine interplay and regulation of insulin resistance. Gastroenterology. 2006;131:934–945. doi: 10.1053/j.gastro.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 21.Straczkowski M, Kowalska I, Nikolajuk A, Dziennis-Strazkowska S, Szelachowska M, Kinalska I. Plasma interleukin-8 concentrations in obese subjects with impaired glucose tolerance. Cardiovasc Diabetol. 2003;16:2–5. doi: 10.1186/1475-2840-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bahcecioglu IH, Yalniz M, Ataseven H, et al. Levels of serum hyaluronic acid, TNF-alpha and IL-8 in patients with nonalcoholic steatohepatitis. Hepatogastroenterology. 2005;52:1549–1553. [PubMed] [Google Scholar]
- 23.Remick DG. Interleukin-8. Crit Care Med. 2005;33(Suppl. 12):S466–S467. doi: 10.1097/01.ccm.0000186783.34908.18. [DOI] [PubMed] [Google Scholar]
- 24.Mackay CP. Chemokines: immunology's high impact factors. Nat Immunol. 2001;2:95–101. doi: 10.1038/84298. [DOI] [PubMed] [Google Scholar]
- 25.Gerhardt CC, Romero IA, Cancello R, Camoin L, Strosberg AD. Chemokines control fat accumulation and leptin secretion by cultured human adipocytes. Mol Cell Endocrinol. 2001;175:81–92. doi: 10.1016/s0303-7207(01)00394-x. [DOI] [PubMed] [Google Scholar]
- 26.Joshi-Barve S, Barve SS, Amancheria K, et al. Palmitic acid induces production of proinflammatory cytokine interleukin-8 from hepatocytes. Hepatology. 2007;46:823–830. doi: 10.1002/hep.21752. [DOI] [PubMed] [Google Scholar]
- 27.Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida K, Gage FH. Cooperative regulation of nerve growth factor synthesis and secretion in fibroblasts and astrocytes by fibroblasts growth factor and other cytokines. Brain Res. 1992;569:14–25. doi: 10.1016/0006-8993(92)90364-f. [DOI] [PubMed] [Google Scholar]
- 29.Hu HH, Nayak KS, Kim HK, Goran MI. Comparison of fat-water MRI and single-voxel MRS in the assessment of hepatic and pancreatic fat fractions in humans. Obesity. 2010;18:841–847. doi: 10.1038/oby.2009.352. [DOI] [PMC free article] [PubMed] [Google Scholar]