Abstract
The role of social interactions in entrainment has not been extensively studied in the invertebrates. Leucophaea maderae is a gregarious species of cockroach that exhibits extensive social interactions. Social interactions associated with copulation between the sexes have been shown to be regulated by the circadian system. We show here that social interactions between males are also under circadian control. We examined the question of whether or not these rhythmic social contacts could function as zeitgebers capable of regulating circadian phase and period. Animals initially in phase that were housed as groups or pairs of single sex or mixed sex in constant darkness for 2–7 weeks were found to drift out of phase. Their behavior was not significantly different from individual animals maintained in isolation. Further, animals that were initially out of phase by 12 hours housed as groups or pairs were not significantly different in phase from animals that were isolated. The results show that the circadian clocks of cockroaches are remarkably insensitive to the extensive social interactions that occur between individuals.
Keywords: circadian, social entrainment, cockroach, aggression, locomotor activity
Introduction
Light cycles have been clearly established as the dominant external cue for entrainment; however, there is evidence that in some instances other, non-photic environmental cues can bring about entrainment. Entrainment by temperature cycles is widespread among ectotherms and has also been demonstrated in at least one mammal (Rajaratnam and Redman, 1998). Food availability has also been shown to be effective in entrainment in several species and is particularly well studies in mammals (e.g., Feillet et al., 2007; Davidson et al., 2005). Other than light, food, and temperature cycles, evidence is quite limited for the effectiveness of other environmental variables in entrainment. Several studies have examined the effectiveness of social interactions in entrainment (reviewed by Davidson and Menaker, 2003). Given the potential adaptive utility of social synchronization for reproduction and parental care, protection from predators, and food gathering, there are surprisingly few clear demonstrations that social interactions are able to synchronize circadian rhythms between or among individuals who are otherwise isolated from other periodic environmental cues. Among the vertebrates social cues appears to be effective in the house sparrow (Menaker and Eskin, 1966) and in some mammals (e.g., beavers, Bovet and Oertli, 1974; deer mice, Crowley et al,, 1980; and bats, Viswanathan and Chandrashekaran, 1985). In contrast, efforts to demonstrate social entrainment in adult rats or hamsters have not been successful (Davis et al., 1987). In humans, the results are conflicting, but suggest that social cues may be able to act as weak zeitgebers sufficient for entrainment to a period close to that of the person's free-running period, but are not capable of independently producing phase shifts (Davidson and Menaker, 2003).
There have been surprisingly few attempts to examine the effects of social interactions on the circadian rhythms of invertebrates. In Drosophila melanogaster, there are data that suggest that social interactions can influence the phase of the locomotor activity rhythm (Levine et al., 2002) although the effect does not seem to be adequate for entrainment since individuals housed as groups in constant darkness for two weeks show widely dispersed phases. It has also been demonstrated that there are differential, gender-dependent effects on patterning of locomotor activity in D. melanogaster (Fujii et al., 2007). These results indicate that social interactions between flies can affect both pattern and phase of circadian rhythms of locomotor activity; however, the cues were unable to maintain a stable phase relationship among individuals that would indicate entrainment. Similar results have been obtained in honey bees. Here it has been speculated that daily changes in forager body temperatures might affect the phase of rhythms by modulating the temperature in the hive (reviewed by Moore, 2001).
In the present study we sought to extend the information on the effect of social interactions on the phase of circadian activity rhythms in insects through an analysis of social entrainment in the cockroach Leucophaea maderae. There is reason to believe that the cockroach is a particularly good model for this question. First, circadian rhythms of locomotor activity are well-characterized in cockroaches (reviewed in Page, 1990; Homberg et al., 2003). Second, many species (including Leucophaea maderae) are gregarious and exhibit extensive social interactions (Cornwell, 1968). These interactions involve both pheromone initiated mating behavior (e.g., Roth and Barth, 1967; Sreng, 1993) and aggressive behaviors in males that establish social dominance hierarchies (e.g., Moore et al., 1995). Finally, in order for any social interaction to function as an entraining signal, it must itself be periodic; and there is evidence that social interactions among cockroaches are rhythmically regulated. In the cockroach Leucophaea maderae, the timing of copulatory behavior is regulated by the circadian system and is controlled by circadian clocks of both the male and female (Rymer et al., 2007). In addition, in the cockroach Supella longipalpa release of sex pheromone has been shown to be under circadian regulation (Smith and Schal, 1991) as has the behavioral response to sex pheromone in Periplaneta americana (Zhukovskaya, 1995). Finally, as we report here, we have examined the aggressive interactions between males in Leucophaea maderae and find that they are robustly rhythmic. Thus in L. maderae the requisite element necessary for social entrainment to occur (i.e., periodic social interaction) appears to be in place.
The current research attempts to examine whether social cues are sufficient for entrainment in Leucophaea maderae. Three questions concerning entrainment were addressed. First, are social interactions between males periodic? Second, when animals that are initially in phase are housed as a group or in pairs, is phase coherence maintained in constant environmental conditions as would be predicted if social cues are capable of bringing about entrainment? Third, can social cues bring about phase shifts in rhythms of animals that are initially out of phase? Our results indicate that the circadian clocks of cockroaches are remarkably insensitive to their extensive social interactions.
Materials and Methods
Animals
Virgin male and female Leucophaea maderae were obtained from laboratory colonies maintained on twenty-four hour light cycles, LD 12:12, controlled by electronic timers. Colonies were kept on either of two light cycles: one with lights on at 8:00 CST (LON 08), or one with lights on at 20:00 CST (LON 20). Colonies were maintained at 25°C in environmental chambers or incubators. The general strategy for the experiments was to house animals either individually (controls), in pairs, or in groups of 10–12 animals (either single sex or equal numbers of the two sexes) in constant darkness. Individuals and pairs of animals were housed in 135 mm plastic Petri dishes while groups were housed in larger plastic containers. Following the period of social interactions (2–7weeks), animals were placed individually in running wheels (each housed in a separate light-tight box) for measurement of the phase and period of the activity rhythm of each individual. Throughout all experiments food and water were available ad lib. The investigative protocol conformed to the ethical principles stipulated by the journal for the conduct of animal biological rhythm research (Portaluppi et al., 2008).
Time-lapse video recording
Animals were housed in square plastic containers (20 cm × 20 cm) with food and water available ad lib. In addition, each container contained a small “hide” – a black plastic cylinder, enclosed at one end (3 cm dia. × 5 cm long). Animals were continuously monitored over the course of the experiment by videotaping in time lapse (one frame being recorded every 0.2 seconds), utilizing a Panasonic Time-Lapse Video Recorder (Audio-Video Supply, San Diego, CA, USA) coupled to a CCD (Sony XC-77, New York, NY, USA) camera. Each frame of the tape contained a time and date stamp. Constant, very dim red illumination for the videotape was provided by a darkroom safelight (15 W light bulb) equipped with a filter (Kodak 1A, Rochester, New York, USA) that limited wavelengths to > 600 nm. This minimized any effect of the light on the animals' behavior since this wavelength/intensity has no detectable effect on the cockroaches circadian clock and the animals behave as though they were in darkness (Mote and Black, 1981 and unpublished observation). Light intensity was adjusted with a rheostat giving a final light intensity at the floor of the plastic tub of less than 1 μE-m−2-sec−1 (measured with a LiCor Photometer, Lincoln, NE, USA). A grid of 3.5 cm squares was drawn on the bottom of the container. The measure of activity reported are the number of crossings of gridlines/30 minutes. When pairs of males were housed together, one animal was identified by a dot of white paint on the pronotum. Physical contacts between males were also scored in half-hour bins. The vast majority of these contacts involved aggressive behavior by one of the two males.
Locomotor activity recording
A central goal in this study was to determine, following periods of social interaction, whether or not these interactions had altered the phase of circadian clock compared to animals that had been isolated from social contacts. The labor intensive scoring of video-tapes is not practical for measuring phase or period in large numbers of individuals. Thus for most of the data reported here these measures were made using automated recording of activity in running wheels. Following the individual, paired, or group housing, the animals were each isolated and placed in separate light-tight boxes in a running wheel for a two week period to evaluate the phase of each individual's circadian clock at the end of the period of social interaction. Rotation of the wheel activated a switch, and the number of switch closures was counted in six minute bins using a computer based data acquisition system (Actimetrics, Coulbourn Instruments, Whitehall, PA, USA).
Data Analysis
Analysis of the actograms was conducted using Clocklab software (Coulbourn Instruments, Whitehall, PA, USA) Chi-squared periodogram analysis was used to determine if a significant rhythm in locomotor activity existed. A linear regression of activity onset was used to determine the phase of each individual's circadian clock at the end of the social interaction.
Statistical analyses of phase were conducted using non-parametric (Sigma-Stat, Systat Software, Inc.Chicago, IL, USA) and circular statistics (Oriana version 2.02a, Kovach Computing Services, Anglesey, Wales). Circular statistics provide an unbiased measure of phase coherence among a population. Using the time of activity onset projected for the first day in the activity monitor as a phase comparison point between animals, a vector (r) was generated for each experimental trial. The length of this mean vector indicates the degree of phase coherence among individual animals, with 0.0 indicating no correlation and 1.0 indicating perfect correlation. The angle of the mean vector depicts the mean time of activity onset for a given set of animals. Comparisons between groups were made using the Mardia-Watson-Wheeler test which tests for differences in either mean angle or in angular variance (Batschelet, 1981).
Results
In Leucophaea mating behavior between males and females are clearly periodic (Rymer et al., 2007). We also wanted to determine the impact on the circadian pattern of activity of social interactions between males (largely aggressive behaviors) and to discover if the interactions were also periodic. We housed males either individually (N=4) or in pairs (N=3 pairs) in constant dim red light, and monitored their activity via time-lapse videography for consecutive days. Videotapes were visually scored for both the times of activity and (when animals were housed in pairs) the times of social contact (Fig. 1). Isolated males in constant dim red light exhibited clear circadian rhythms of locomotor activity with activity onset typically occurring near the beginning of the subjective night. As is evident from the data in Fig. 1, the phases of peak activity did vary slightly from one animal to the next even when animals from the same colony were monitored simultaneously. This variation in phase is examined in more detail below.
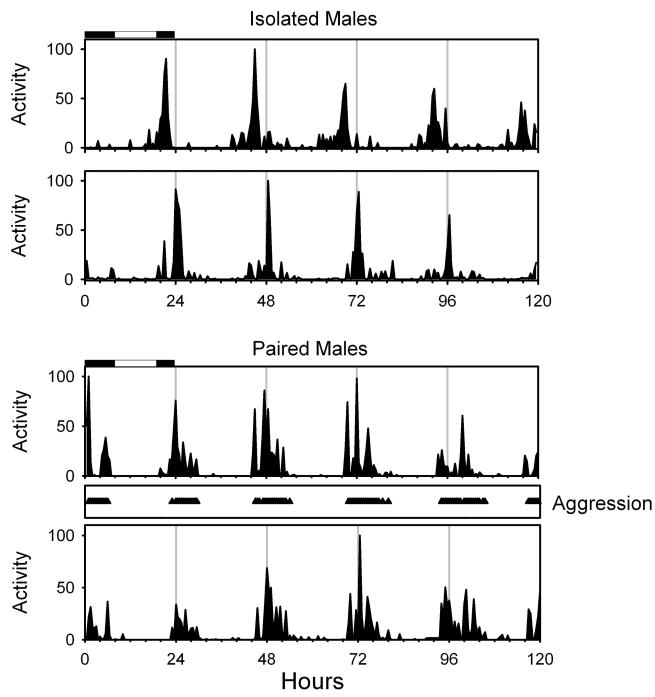
Figure 1. Males exhibit a circadian rhythm in both activity and aggressive behavior that tends to follow the rhythm in locomotor activity.
The locomotor activity of individual male Leucophaea maderae in DD plotted over a five day period. The bars at the top of the first day illustrate the prior LD cycle. The activity levels for each animal were normalized to allow for comparison of rhythms. The top two records show activity of isolated individuals. The lower records show the activity of the two animals housed as a pair and tracks the times of aggressive interactions between the two (triangles). All animals exhibited a rhythm in locomotor activity, with a period of greatest activity during the subjective night. Aggressive interactions between the paired animals coincide with the nocturnal period of activity. Activity onset of the dominant male of the pair (bottom panel) coincided each day with the onset of aggressive interactions. Activity onset of the submissive male phase leads the dominant male by about 30 minutes each day.
When housed in pairs, the timing of activity of males was quite similar to the activity of isolated individuals (cf. Fig. 1 A, B with Fig. 1 C,D). Activity remained nocturnal, peaking in the early subjective night, and activity during the subjective day was very limited. Thus social interactions did not appear to disrupt the circadian regulation of activity in any substantial way. In addition, the interactions between males were clearly periodic and exhibited a rhythm in constant darkness that was essentially identical in phase with the activity rhythms. Though the numbers of animals do not allow a rigorous comparison, we did not find any evidence for significant changes in either the level or duration of activity between individually housed or paired animals. Not surprisingly these interactions resulted in clear “masking” effects on the activity as aggressive attacks by one male led the other to flee. Interestingly, these aggressive behaviors, which occurred throughout the night, essentially ceased during the subjective day. These results and those previously published on the circadian regulation of mating behavior (Rymer et al., 2007) indicate that in both male groups and male/female groups social interactions are periodic and could conceivably function as a rhythmic entrainment cue. They also suggest that circadian patterns of activity exhibited by isolated individuals are generally similar to the circadian patterns exhibited in a setting that allows for social interaction.
Do animals housed together in constant conditions maintain phase synchrony? As a first step in evaluating the potential of these social cues to function in entrainment, we examined the extent to which phase and freerunning period differed among animals. Leucophaea are nocturnal and they typically begin activity at subjective dusk (Fig. 2A). When individuals from the colony entrained to LD 12:12 (lights on at 08:00 and lights off at 20:00) were isolated and placed in DD, they showed a strong degree of phase coherence (r=0.964, p<0.001) around the predicted time of activity onset, with a mean onset time at 19.9 CST just prior to subjective dusk (Fig. 2B). Phases exhibited a spread of 4.1 hours and ranged from 17.4 CST to 21.5 CST. The average freerunning period (τ) was 23.60 ± 0.22 (mean ± SD) and for females it was 23.78 ± 0.15. These values are similar to those previously reported (e.g., Page and Block, 1980, Barrett and Page, 1989).
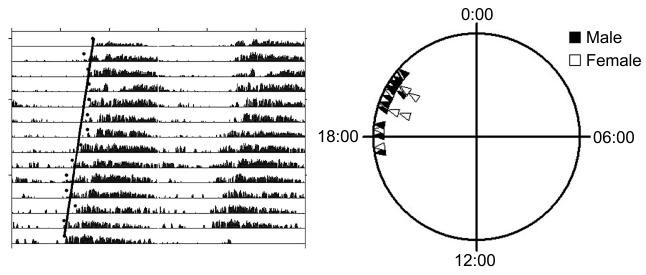
Figure 2. Leucophaea maderae entrained to a light cycle start out their locomotor activity in phase with one another.
Left, sample actogram from one cockroach. The days are double-plotted, with each horizontal line corresponding to a 48 hr period. Left edge is 0:00 (CST). The gray bars correspond to active periods, with higher bars indicating higher levels of activity. A linear regression (black line) was used to determine the time of onset on the day the animal was placed in the wheel. The intersection of this line with the x-axis for day one (22:58 CST) was taken as the point for the time of activity onset. Right, Triangles mark the phase of activity onset (male, filled, N=14; female, unfilled, N=10) on the first day in DD following entrainment to LD 12:12 cycle (light off at 20:00). In cases where more than one animal shared the same time of activity onset, triangles are depicted in layers. Mean time of activity onset was 19:55 CST (r-0.964).
The central question to be addressed was whether individuals housed in a group environment would maintain phase coherence. If social interaction is a cue for entrainment, then one would expect animals that start out in phase with one another to maintain synchrony in their rhythms compared to isolated individuals who would drift out of synchrony due to inter-individual variation in free-running period. The free-running periods of 90% roaches used in these experiments ranged from 23.4 hours to 24.0 hours, with a mean τ of 23.7 hours. The mean phase, determined from running wheel activity of individuals in constant darkness, was 19.5 CST and the distribution of phases was about 4 hours (r=0.964). Given this range of τ, in the absence of other entrainment forces, animals could be expected to diverge in phase substantially during two weeks in DD assuming the period expressed by individuals in running wheels accurately reflected the period expressed during isolation or group housing. By the same token, social interactions would only have to be capable of generating daily phase shifts of, at most, about 30 minutes for successful mutual entrainment. Based on the mean τ, one would expect the mean time of activity onset to have moved back to about 15.8 CST after two weeks in DD, and given the range of periods phase should be substantially more scattered (i.e., there should be a substantial decrease in r) for isolated animals. Individuals who were isolated for two weeks in DD and then placed on running wheels exhibited a mean phase of 14.75 CST (slightly earlier than predicted) and the range of phases increased from four hours to 11.7 hours with a standard deviation of 3.09 hours (r = 0.721). Group housed animals behaved in a very similar manner. The mean phase exhibited by the individuals when isolated in a running wheel at the end of the period of group housing was 14.9 CST, and the range of phases increased to about 8.95 hours with a standard deviation of 2.14 hours (r=0.854). There was no significant difference in either phase or, critically, the coherence of phase between the isolated and group housed animals (p=0.33), suggesting that over this short time period, social entrainment had little or no effect (Fig. 3).
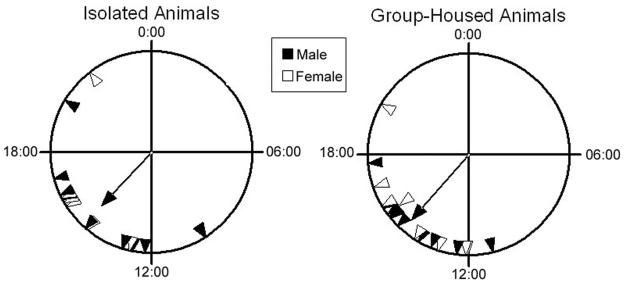
Figure 3. Group-housing does not result in significantly greater phase coherence in the time of activity onset.
Triangles indicate the time of activity onset for individual males (filled) or females (unfilled). The mean vector is indicated by a line ending in an unfilled arrowhead marked with a dot. A, shows animals that had been isolated for 14days; B shows animals that had been housed as a group. Grouped animals did not show a difference in phase coherence compared to isolated animals (r=0.721 for isolated animals, N=16; and r=0.854 for group-housed animals; p=0.891, N=18). Results from two trials of each condition are depicted together since both trials gave similar results and no significant differences were found between individuals trials. n=16 for isolated, n=18 for group-housed.
However, both the isolated and group housed animals still exhibited a significant cluster of phases indicating that two weeks was not a long enough period to produce a complete loss of phase coherence. This did occur when male and female cockroaches from LON 08 were housed together in DD for a forty-five day period (individual adult life span is over 12 months and during this period the freerunning period is quite stable – Page and Block, 1980). When the time of activity onset for individual animals was compared (Fig. 4A), no significant phase coherence was observed (r=0.291; p=0.439, Rayleigh test) and the range of phases extended virtually around the clock.
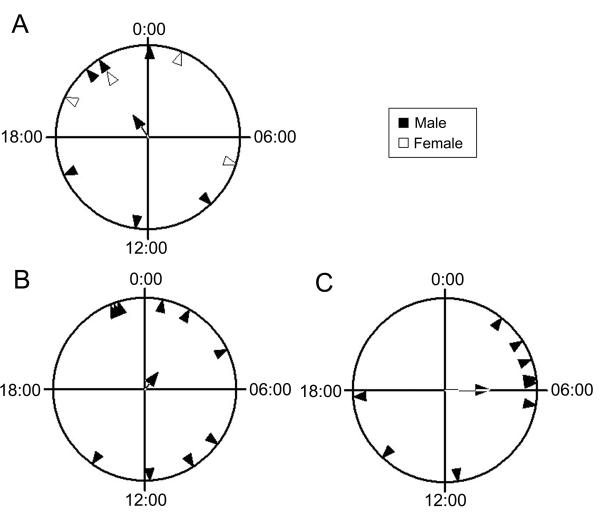
Figure 4. Extended group-housing does not result in significant phase coherence in the time of locomotor activity onset, regardless of gender composition.
Triangles indicate the phase of activity onset for individual male (filled) or female (unfilled) cockroaches.. Mean vector is depicted as a line terminated in an arrowhead that is unfilled and marked with a dot (A) or filled (B, C) A. After a period of 45 days of group-housing in DD, animals from LON 08 showed a large phase scatter with no significant coherence (r=0.291, p=0.439, n=10). The vector angle depicts a mean onset time of 21:42 CST. B. When males were housed together for fifty-one days in DD, they showed no significant degree of phase coherence (r=0.23, p=0.601, n=10). C. A second trial housing males together for forty-six days in DD also showed no significant degree of phase coherence (r=0.481, p=0.097, n=10).
In order to assess whether the mixed-gender setting of the group-housing might have some effect on these results, an identical experiment was carried out housing groups of only males or only females. Males showed a marked phase scatter with no significant clustering of phase (Fig. 4B, C). Interestingly, when virgin females were housed together for extended time periods, most of the animals produced very weak rhythms characterized by small amounts of activity, which rendered analysis of the activity records impossible. It is plausible that this is a consequence of the reproductive isolation that prevented mating. For example, reproductive state has been shown to have significant effects on the patterns of activity of the German cockroach (Lin and Lee, 1996).
The results of these experiments indicate that social interactions are not sufficient to maintain a stable phase relationship of circadian rhythms among individuals housed as a group indicating that social interactions are not sufficient for entrainment. In fact, we were unable to detect any difference between group housed and isolated animals. However, as we report below we did obtain some weak evidence that male-female interactions might have an impact on phase distribution when animals start 12 hour out of phase. This prompted one final experiment in which a single male was paired in constant darkness for four weeks with a single female that had been entrained to the same light cycle. Phase differences between individuals of each pair evaluated at the end of the four weeks averaged 7.0 ± 3.57 h and ranged from 1.0 h to 11.8 h (N=8 pairs). The results clearly support the conclusion that social interactions are insufficient for entrainment.
Do same-sex social interactions between animals from different LD cycles have any effect on phase? The data presented above raise the question of whether or not social interactions can have any impact on circadian phase in L. maderae. Previous studies in Drosophila had suggested that animals entrained to one light cycle were able to influence the phase of other animals entrained to a different light cycle (Levine et al., 2002). In order to assess whether such an effect might also be present in Leucophaea maderae, animals from two different light cycles were housed together to evaluate the potential affects of social interaction on phase. The strategy here was to determine if the phase and/or coherence of phase of animals that were allowed social contact differed from animals from the same light cycle that were isolated from social contact. The results are presented in Table 1.
Table 1.
Phase of activity following 14 days of housing in DD with animals from other LD cycles.
| N | LD* / Gender | LD*/ Gender of Cohorts | Phase(deg.) 0 = 0:00 | Phase (CST) | SD** |
|---|---|---|---|---|---|
| 18 | 20 / Males | 08 / Females | 42.88 | 2.86 | 3.97 |
| 20 | 20 / Males | 08 / Males | 9.04 | 0.60 | 4.53 |
| 31 | 20 / Males | None | 34.44 | 2.30 | 3.65 |
| 20 | 08 / Males | 20 / Males | 213.73 | 14.25 | 2.02 |
| 19 | 08 / Males | None | 202.63 | 13.51 | 3.13 |
| 5 | 08 / Females | 20 / Females | 235.38 | 15.69 | 2.08 |
| 21 | 08 / Females | 20 / Males | 251.82 | 16.79 | 2.60 |
| 18 | 08 / Females | None | 219.47 | 14.63 | 3.24 |
Time of lights-on in prior LD 12:12 cycle (20 = 20:00, 08 = 08:00)
Circular standard deviation (h)
LON 20 males allowed social interaction with LON 08 males had a mean phase that was on average, slightly earlier (by 1.7 hours) but was also not significantly different from isolated LON 20 males (Mardia-Watson-Wheeler test, p= 0.168). The LON 8 males showed no significant difference in phase (0.74 hours, p = 0.258) whether isolated or group housed with LON 20 males. Similarly when females were allowed to interact in groups with other females who were 12 hours out of phase there was only a slight difference in phase (1.3 hours) from females who had remained isolated for the same period of time (Table 1). In addition, in all cases the group housed animals maintained significant phase coherence (similar to the isolated animals). The results indicate coherence was not disrupted by interaction with animals from another LD cycle as might be expected if some animals were being delayed and other advanced by the interaction.
One might argue that in a group housed situation animals could be receiving conflicting signals from their cohorts from the two light cycles that might affect the results. To examine this, we housed males raised on the two light cycles as pairs for two weeks (N=5 pairs). An average difference in phase of 11.0 ± 3.23 hours between the pairs was maintained and mean time of activity onset for both the LON 08 and the LON 20 males was within 1.25 hours of the average phase of isolated animals from the corresponding LD cycle. The results of these experiments along with those reported above (Fig. 3,4) indicate that same sex social interactions simply have no significant impact on the phase of the circadian clock of males or females.
Do social interactions between sexes have any effect on phase? When LON 20 Males were allowed to interact in groups with LON 08 females they showed essentially the same time of activity onset as males who had been isolated (2.86 vs. 2.30 CST, p=0.115). Activity onsets for the LON 08 females in this experiment were on average 2.16 hours later than isolated females (Table 1). This difference was not statistically significant (p = 0.11), but was the largest effect that we had observed.
To further examine the potential for male-female interactions to phase shift, LON 20 males and LON 08 female were housed as pairs for a two-week period. At the end of the two weeks, the average phase for the males was 2.15 (± 2.28). This value is virtually identical to that of isolated males (Table 1). The average phase of the females was 17.14 (± 3.44) which again about 2.5 hours later than isolated females (Table 1), but failed to reach statistical significance (p > 0.05).
These results indicate that male clocks are completely impervious to the effects of females, but could suggest that the presence of males may delay the phase of the female clocks either via a phase shift or slight lengthening of the period. However, the effect was small (about 10 minutes/day) and not statistically significant.
Discussion
In view of our observations that both male-female social interactions (mating behavior – Rymer et al., 2007) and male-male interactions (aggressive behavior - this paper) were strongly circadian in expression it was clearly plausible that social interactions might have a significant impact on the regulation of the phase or period of the cockroaches' circadian clocks. This plausibility was bolstered by the fact that Leucophaea maderae is a gregarious species, lending credence to the proposition that rhythmic social interactions in nature could form the mechanistic basis for the evolution of a pathway for social entrainment, assuming a selective advantage to maintaining synchrony among individuals. However, the data provided little or no evidence that social interactions have any significant impact on the cockroach's clock. These data are consistent with the observation on the German cockroach that although males could affect the pattern of activity in females, entrainment of the female rhythms was not apparent (Lin and Lee, 1996).
Despite a concerted effort to identify effects of social interactions on circadian phase, at best we were only able to detect a small (and not statistically significant) effect of the presence of out-of-phase males on the phase of the female's circadian system when compared to isolated virgin females. Even this result may not have been directly due to the social interactions between males and females, but could have been a physiological consequence of the effects of insemination of the females. It is well-documented that insemination can have effects on the expression and pattern of circadian activity in a variety of insects including ants (McCluskey and Carter, 1969), bees (Harano et al., 2007), mosquitoes (reviewed in Klowden, 1999); and the German cockroach (Lin and Lee, 1996). Since virtually 100% of virgin female L. maderae housed with males mate within 10–14 days (Rymer et al., 2007) it is certain that most of the females in our experiments had been inseminated.
One question that arises concerns the general absence of widespread evidence that social cues are relevant to entrainment of circadian clocks. If synchronization is advantageous, one might expect there would be selection for the evolution of social entrainment pathways. Two possibilities could explain the relative paucity of systems in which social cues appear to function as Zeitgebers. One is that the potential “advantages” of synchronization among individuals of a population is more imagined than real. Rigorous demonstrations of the selective advantage of circadian rhythms are hard to come by (Johnson, 2005), and rigorous demonstrations on the selective advantage of synchronization of the circadian rhythms of individuals of a population are even more limited. Nevertheless, some observations certainly suggest that such synchronization can be important. For example, in the moth Spodoptera littoralis, mating behavior is regulated by the circadian system and mating between males and females whose circadian clocks are out of phase is severely disrupted (Silvergren et al., 2005). The data show that in this species synchronization of the circadian clocks of males and females is crucial to reproductive success (though the same is not true for Leucophaea – Rymer et al., 2007). In those cases where synchronization is clearly important, the salient question then becomes how this synchronization is achieved.
A second potential explanation for the limited examples of social entrainment is that in those cases where commonality of phase is important, it may be that entrainment by other environmental factors such as light or temperature cycles is perfectly adequate to maintain phase coherence within the population. As a consequence, the selective pressure for the evolution of social entrainment is simply very weak or absent. This would certainly seem to be the case for most diurnal organisms who can not avoid the daily entrainment signal of the solar light cycle. A priori then one might expect social entrainment to be more prominent in organisms that, as a consequence of their natural history, might miss the daily entrainment cues of light or temperature cycles. In this context it is interesting to note that one of the earliest compelling demonstrations of social entrainment came from studies on beavers who over-winter as family groups in burrows under the snow and are only infrequently exposed to light (Bovet and Oertli, 1974). Similarly, there is good evidence for social entrainment among cave-dwelling bats that are largely isolated from environmental cycles in light and temperature (Marimuthu et al., 1981). It may be then that the search for concrete examples of social entrainment is best focused on species that experience isolation from the geophysical cues that typically provide a consistent and reliable temporal cue for the synchronization of circadian clocks. It would be of interest to examine the question of social entrainment, for example, in the numerous species of cave dwelling insects.
It is worth emphasizing that social interactions may have important consequences for temporal organization of behavior that do not involve direct entrainment of the circadian system. Social contact can clearly have important masking effects that can re-shape temporal patterns of behavior and may also modulate the entrainment response to alternative zeitgebers in the environment. The focus of past behavioral studies of circadian organization has been almost exclusively on the study of individuals in isolation, but recent efforts to examine behavior in a social context have uncovered effects that are both interesting and profound (e.g., Fujii et al., 2007; Lin and Lee, 1996). These data suggest that much is yet to be discovered about the relationship between social interaction and circadian control in the regulation of behavior.
Acknowledgements
We thank Mr. Scott Brown for technical assistance. This research was supported by NIH Grant No. MH069836. The project described was supported by Award Number MH069836 from the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
References
- Barrett RK, Page TL. Effects of light on circadian pacemaker development 1. The freerunning period. J. Comp. Physiol. A. 1989;165:41–49. doi: 10.1007/BF00613798. [DOI] [PubMed] [Google Scholar]
- Batschelet E. Circular Statistics in Biology. Academic Press; New York: 1981. p. 371. [Google Scholar]
- Bovet J, Oertli EF. Free-running circadian activity rhythms in free-living beaver (Castor canadensis) J. Comp. Physiol. A. 1974;92:1–10. [Google Scholar]
- Cornwell PB. The Cockroach (Volume 1): A Laboratory Insect and Industrial Pest. Hutchinson & Company; London: 1968. p. 391. [Google Scholar]
- Crowley M, Bovet J. Social synchronization of circadian-rhythms in deer mice (Peromyscus maniculatus) Behav. Ecol. and Sociobiol. 1980;7:99–105. [Google Scholar]
- Davidson AJ, Menaker M. Birds of a feather flock together-sometimes: social synchronization of circadian rhythms. Curr. Opinion in Neurobiol. 2003;13:765–769. doi: 10.1016/j.conb.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Davidson AJ, Tataroglu O, Menaker M. Circadian Rhythms. Elsevier Academic Press Inc; San Diego: 2005. Circadian effects of timed meals (and other rewards) pp. 509–523. [DOI] [PubMed] [Google Scholar]
- Davis FC, Stice S, Menaker M. Activity and reproductive state in the hamster: independent control by social stimuli and a circadian pacemaker. Physiol. Behav. 1987;40:583–590. doi: 10.1016/0031-9384(87)90101-6. [DOI] [PubMed] [Google Scholar]
- Feillet CA, Albrecht U, Challet E. ”Feeding time” for the brain: A matter of clocks. J. Physiol. Paris. 2006;100:252–260. doi: 10.1016/j.jphysparis.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Fujii S, Krishnan P, Hardin P, Amrein H. Nocturnal male sex drive in Drosophila. Curr. Biol. 2007;17:244–251. doi: 10.1016/j.cub.2006.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harano K, Sasaki M, Sasaki K. Effects of reproductive state on rhythmicity, locomotor activity and body weight in the European honeybee, Apis mellifera queens (Hymenoptera, Apini) Sociobiol. 2007;50:189–200. [Google Scholar]
- Johnson CH. Testing the adaptive value of circadian systems. Methods in Enzymol. 2005;393:818–837. doi: 10.1016/S0076-6879(05)93043-7. [DOI] [PubMed] [Google Scholar]
- Klowden MJ. The check is in the male: Male mosquitoes affect female physiology and behavior. J. Am. Mosquito Cont. Assoc. 1999;15:213–220. [PubMed] [Google Scholar]
- Levine JD, Funes P, Dowse HB, Hall JC. Resetting the circadian clock by social experience in Drosophila melanogaster. Science. 2002;298:2010–2012. doi: 10.1126/science.1076008. [DOI] [PubMed] [Google Scholar]
- Lin TM, Lee HJ. The expression of locomotor circadian rhythm in female German cockroach, Blattella germanica (L) Chronobiol. Int. 1996;13:81–91. doi: 10.3109/07420529609037072. [DOI] [PubMed] [Google Scholar]
- Marimuthu G, Rajan S, Chandrashekaran MK. Social entrainment of the circadian-rhythm in the flight activity of the microchiropteran bat Hipposideros speoris. Behav. Ecol. and Sociobiol. 1981;8:147–150. [Google Scholar]
- McCluskey E, Carter CE. Loss of rhythmic activity in female ants caused by mating. Comp Biochem. Physiol. 1969;31:217–8. doi: 10.1016/0010-406x(69)91650-8. [DOI] [PubMed] [Google Scholar]
- Menaker M, Eskin A. Entrainment of circadian rhythms by sound in Passer domesticus. Science. 1966;154:1579–80. doi: 10.1126/science.154.3756.1579. [DOI] [PubMed] [Google Scholar]
- Moore AJ, Reagan NL, Haynes KF. Conditional signaling strategies: effects of ontogeny, social experience and social status on the pheromonal signal of male cockroaches. An. Behav. 1995;50:191–202. [Google Scholar]
- Moore D. Honey bee circadian clocks: behavioral control from individual workers to whole-colony rhythms. J. Insect Physiol. 2001;47:843–857. [Google Scholar]
- Mote MI, Black KR. Action spectrum and threshold sensitivity of entrainment of circadian running activity in the cockroach. Photochem. Photobiol. 1981;34:257–265. [Google Scholar]
- Page TL, Block GD. Circadian rhythmicity in cockroaches - effects of early post-embryonic development and aging. Physiol. Entomol. 1980;5:271–281. [Google Scholar]
- Page TL, Koelling E. Circadian rhythm in the olfactory response in the antennae controlled by the optic lobe in the cockroach. J. Insect Physiol. 2003;49:697–707. doi: 10.1016/s0022-1910(03)00071-4. [DOI] [PubMed] [Google Scholar]
- Page TL. Circadian organization in the cockroach. In: Huber I, et al., editors. Cockroaches as Models for Neurobiology: Applications in Biomedical Research. Vol. II. CRC Press; Boca Raton: 1990. pp. 224–245. [Google Scholar]
- Portaluppi F, Touitou Y, Smolensky MH. Ethical and methodological standards for laboratory and medical biological rhythm research. Chronobiol. Int. 2008;25:999–1016. doi: 10.1080/07420520802544530. [DOI] [PubMed] [Google Scholar]
- Rajaratnam SM, Redman JR. Entrainment of activity rhythms to temperature cycles in diurnal palm squirrels. Physiol. Behav. 1996;63:271–277. doi: 10.1016/s0031-9384(97)00440-x. [DOI] [PubMed] [Google Scholar]
- Roth LM, Barth RH. Sense organs employed by cockroaches in mating behavior. Behav. 1967;28:58–94. [Google Scholar]
- Rymer J, Bauernfeind AL, Brown S, Page TL. Circadian rhythms in the mating behavior of the cockroach, Leucophaea maderae. J Biol. Rhythms. 2007;22:43–57. doi: 10.1177/0748730406295462. [DOI] [PubMed] [Google Scholar]
- Silvegren G, Lofstedt C, Rosen WQ. Circadian mating activity and effect of pheromone pre-exposure on pheromone response rhythms in the moth Spodoptera littoralis. J. Insect Physiol. 2005;51:277–286. doi: 10.1016/j.jinsphys.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Smith AF, Schal C. Circadian calling behavior of the adult female brown-banded cockroach, Supella longipalpa (f) (Dictyoptera, Blattellidae) J Insect Behav. 1991;4:1–14. [Google Scholar]
- Sreng L. Cockroach mating behaviors, sex-pheromones, and abdominal glands (Dictyoptera, Blaberidae) J. Insect Behav. 1993;6:715–735. [Google Scholar]
- Viswanathan N, Chandrashekaran MK. Cycles of presence and absence of mother mouse entrain the circadian clock of pups. Nature. 1985;317:530–531. doi: 10.1038/317530a0. [DOI] [PubMed] [Google Scholar]
- Zhukovskaya MI. Circadian-rhythm of sex-pheromone perception in the male American cockroach, Periplaneta americana L. J. Insect Physiol. 1995;41:941–946. [Google Scholar]