Abstract
Objective
To assess carotid artery intima media thickness (CIMT) change over two years in overweight Latino adolescents and examine its relationship to cardiometabolic risk.
Study design
72 healthy overweight male and female Latino adolescents (mean age: 14.5±1.7 yrs; mean BMI: 31.5±6.9 kg/m2) were evaluated at baseline and 2 years later for: CIMT by high resolution B-mode ultrasound, the metabolic syndrome and its features, body composition by DEXA and MRI, and glucose/insulin measures by fasting blood, and oral and intravenous glucose tolerance tests.
Results
Baseline CIMT did not differ from 2-year follow-up; however 38 participants increased CIMT (0.017±0.003mm; +2.8%) and 34 decreased (-0.019±0.002mm; −3.1%). ANCOVA analyses showed that participants with CIMT progression had higher baseline LDL-cholesterol and total cholesterol (91.3±3.4 and 150.3±3.9mg/dL) compared with those with CIMT regression (78.1±3.6 and 135.6±4.2mg/dL, p<0.05), independent of sex, baseline CIMT, age, and height. In multivariate regression, LDL-cholesterol was the sole predictor of CIMT progression, but the effect was small (odds of CIMT progression increased by 3% for each 1 mg/dL higher baseline LDL-cholesterol [95% CI: 1.004-1.006, p=0.03].
Conclusions
These results indicate a high variability in the magnitude of CIMT change in growing overweight Latino youth and support the use of LDL-cholesterol to assess sub-clinical atherosclerosis risk in this population.
Keywords: Obesity, Cardiovascular disease risk, Ultrasound imaging
The increasing prevalence of pediatric childhood obesity (1) warrants investigation into the link between obesity and atherosclerosis risk in youth. The Latino population in the US is rapidly growing and has shown a variety of obesity related co-morbidities, such as hypertension and diabetes, placing them at high-risk for cardiovascular disorders. Latino children have a high prevalence of obesity (1), insulin resistance (2), metabolic syndrome (3) and impaired fasting glucose/pre-diabetes (4), all of which may contribute to the early development of atherosclerosis.
Carotid artery intima media thickness (CIMT) is a noninvasive measure of subclinical atherosclerosis. Increased CIMT has been shown to start in youth and its correlates are similar to those in adulthood. Yet, the progression of CIMT in youth has not been widely examined. Increased thickness of the carotid artery, and its associated cardiovascular disease events in adulthood, has various inter-related predictors such as obesity, male sex, metabolic dysfunction, dyslipidemias and hypertension. Studies over the past decade have shown similar relationships of elevated CIMT and traditional cardiometabolic risk factors in children and young adults (5-14). We have previously shown that children with persistent metabolic syndrome over a 3-year period exhibited a higher CIMT than those who never had the metabolic syndrome (15). Of the metabolic syndrome components, high blood pressure and high waist circumference were the features most highly associated with higher CIMT (15).
The first objective of this study was to assess the change of CIMT over a 2-year period. Next, we assessed the relationship of CIMT progression to potential CIMT predictors, including adiposity, the metabolic syndrome and its individual features, lipids and insulin resistance. Finally, we examined any potential sex differences in these associations. We hypothesized that baseline systolic blood pressure, abdominal adiposity and insulin resistance would predict CIMT progression.
METHODS
Participants were enrolled in the Study of Latino Adolescents at Risk for Diabetes (SOLAR), a longitudinal study exploring metabolic risk factors for type 2 diabetes. Study participants satisfied the following criteria for inclusion at the initial baseline visit: 8-13 years of age, Latino ethnicity (i.e., parents and grandparents of Latino descent), age- and sex-specific BMI ≥ 85th percentile, positive family history for type 2 diabetes, and absence of diabetes as assessed by an oral glucose tolerance test (OGTT). Participants were excluded if they were using a medication or diagnosed with a condition known to influence body composition or insulin / glucose metabolism. Prior to testing procedures, written informed consent from parents and assent from the children were obtained. This investigation was approved by the Institutional Review Board of the University of Southern California. To be included in this analysis, SOLAR participants must have had a baseline CIMT in 2006 (n=123) and a repeat CIMT measure in 2008 (n=73) and all measures of cardiometabolic risk. One participant was diagnosed with diabetes and was excluded from this analysis (n=72). The drop-out rate we evidenced was due to: 1) loosing contact with the participant and their families, 2) participants moving or leaving to attend college, and 3) disinterest in study after 6-8 years of annual participation. We conducted an analysis on this sub-group of excluded participants and observed no significant physical or metabolic differences between the excluded (n=51) and included (n=72) participants.
Details of the longitudinal study protocol and design have been previously described (3, 15). In brief, participants attended two annual visits to at the USC General Clinical Research Center. On the first visit, participants received a comprehensive medical history and physical examination by a licensed health care provider. Clinical staff then collected vital signs, blood pressure in triplicate and performed a 2-hour oral glucose tolerance test (OGTT). Approximately 7-14 days following the outpatient visit, participants were admitted for an inpatient visit at the USC GCRC for their second visit. They were examined once more by a licensed health care provider and were given dinner. Following a supervised, overnight fast, a 3-hour modified frequently-sampled intravenous glucose tolerance test (FSIVGTT, with 13 time points) was performed by certified nursing and phlebotomy staff. Detailed information on glucose, insulin measures, and lipid assays (15). We defined the metabolic syndrome with criteria similar to the Adult Treatment Panel (21) that we adapted for pediatric populations (3).
Body composition measures and CIMT measure were performed at either visit based on availability of the participant and staff. Total body composition was completed by dual-energy x-ray absorptiometry and magnetic resonance imaging was used to assess abdominal adiposity. Using a GE 1.5 Signa LX-Ecospeed with 1.5 Tesla magnet (GE Healthcare, Piscataway, New Jersery), a single-slice axial TR 400/16 view of the abdomen at the level of the umbilicus was analyzed for cross-sectional area of visceral and subcutaneous abdominal adipose tissue.
CIMT was determined at the USC Atherosclerosis Research Unit Core Imaging and Reading Center as previously described (16-20). High resolution B-mode ultrasound images were obtained using a Seimens Acuson CV70 (13 MHz linear array) imager. C-IMT was measured from computer processed images of the right distal common carotid artery approximately 1-2 cm from the bifurcation into external and internal carotids. The same sonographer and reader performed all baseline and follow-up CIMT measures and she was blinded to all participant information. The intra-class correlation coefficient for CIMT measures was 0.96 (95% CI: 0.93-0.97).
Statistical Analysis
In order to assess the change of the dependent variable (CIMT), each participant’s CIMT change was calculated by taking the difference of the 2-year follow-up CIMT from the baseline CIMT. In preliminary analyses, CIMT change was used as a continuous variable while in ANCOVA analyses, participants were placed in one of 3 groups: Non-progression (CIMT change ≤ 0.000mm), Normal Progression (CIMT change > 0.000mm and <0.01mm) or Advanced Progression (CIMT ≥0.01mm). Advanced CIMT Progression was based on a conservative cut-off using normal rates of CIMT progression in healthy adults (approximately 0.005mm/year hence, ≥0.01mm over 2 years was deemed advanced progression). For logistic regression, CIMT progression was defined to include any CIMT progression (CIMT ≥0.01mm) or Advanced CIMT progression only (CIMT ≥0.01mm). The independent variables (adiposity measures, metabolic syndrome, blood pressure, lipids and measures of glucose/insulin metabolism) were measured at baseline and follow-up CIMT assessments. The Shapiro-Wilkes W test was used to test the Gaussian distribution of residual values of all continuous variables, none of which deviated from normality (p>0.05).
For descriptive analysis, independent t-test tests were performed to assess mean physical and metabolic characteristics by sex and then at baseline and 2-year follow-up. This followed by a preliminary analysis of simple and partial correlations of CIMT change with each independent variable at baseline. This analysis was repeated to examine the relationship between CIMT change and changes in adiposity (BMI, BMI z-score, total fat mass, visceral and subcutaneous adiposity), blood pressure, lipids (triglycerides, LDL-, HDL- and total cholesterol), and glucose and insulin indices (fasting and 2-hour glucose and insulin, insulin sensitivity, acute insulin response, disposition index, glucose and insulin AUC). Analysis of covariance (ANCOVA) was used to compare differences of each cardiometabolic risk factor at baseline (as well as 2-year change) by CIMT progression groups. A priori covariates included sex, baseline age and height due to their documented effects on CIMT progression. Baseline measures were used to adjust for any analysis of 2-year change in the variables of interest.
Significant associations were further examined by multivariate linear regression and by 2-way ANCOVA for the metabolic syndrome. Interaction terms were used to test whether sex significantly modified the relationship between each of the cardiovascular risk factors and change in CIMT, while adjusting for baseline CIMT, age and height.
Multiple logistic regression analysis was employed to determine the relative contribution of the baseline predictors (independent continuous variables in model 1: fasting insulin, glucose effectiveness and LDL-cholesterol) to the CIMT progression (CIMT was coded as a binary dependent variable, CIMT non-progression and CIMT progression), while considering the confounding effects of sex, baseline age and height. The second model included the same independent variables but used CIMT coded as a binary variable with CIMT non-progression and Advanced CIMT progression. Data were analyzed using SPSS for Mac version 16.0 (SPSS Inc., Chicago, IL), with an a priori significance level of p<0.05.
RESULTS
The distribution of each participant’s change in CIMT showed that of the 72 participants, 38 showed an increase in CIMT (mean increase: 0.017±0.003mm, +2.8%) and 34 participants showed a decrease in CIMT (mean decrease: −0.019±0.002mm, −3.1%).
Descriptive statistics of physical and metabolic characteristics of 72 Latino adolescents are shown in Table I. Body composition or cardiometabolic risk characteristics did not significantly differ between baseline and 2-year follow-up, with the exception of fasting glucose that was higher at the baseline measure (p<0.05). Males were significantly taller and had higher total lean tissue mass than females (p<0.001). Systolic and diastolic blood pressures were higher in males than females (p<0.05). HDL-cholesterol was significantly lower in males than in females (p<0.05). Females had a lower disposition index than males (p<0.05). CIMT did not differ by sex but females had a lower maximum carotid diameter than males (p<0.05).
Table 1.
Descriptive table of physical and metabolic characteristics at baseline and 2-year follow-up (n=72)
| Baseline | 2-year follow- up |
p-value | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age(years) | 14.6±1.7 | 16.6±1.7 | <0.001 |
| Gender (Male/Female) | 38/34 | 38/34 | NS |
| Body compostition measures | |||
| Height (cm) | 163.0±8.9 | 166.2±8.3 | NS |
| Weight (kg) | 84.8±22.5 | 89.8±23.1 | NS |
| BMI (kg/m2) | 31.7±6.9 | 32.3±6.9 | NS |
| Total Lean Tissue Mass (kg) | 50.3±10.8 | 53.9±11.0 | NS |
| Total Fat Mass (kg) | 29.1±1.2 | 29.7±12.1 | NS |
| Visceral Adipose Tissue (cm2) | 40.9±27.2 | 35.9±25.0 | NS |
| Subcutaneous Adipose Tissue (cm2) | 389.2±167.9 | 430.2±201.0 | NS |
| Cardiometabolic risk factors | |||
| Wasit Circumference (cm) | 93.0±14.0 | 95.3±15.1 | NS |
| Systolic blood pressure (mmHg) | 115.0±10.3 | 116.1±10.9 | NS |
| Diastolic blood pressure (mmHg) | 64.2±5.7 | 65.6±6.4 | NS |
| HDL Cholesterol (mg/dL) | 37.4±8.4 | 38.0±8.6 | NS |
| Triglycerides (mg.dL) | 104.5±49.6 | 93.5±52.3 | NS |
| Fasting Glucose (mg/dL) | 88.3±7.6 | 85.8±8.0 | 0.02 |
| 2-hr Glucose (mg/dL) | 116.8±21.2 | 113.9±7.6 | NS |
| LDL-cholesterol (mg/dL) | 85.4±21.6 | 84.9±25.1 | NS |
| Total cholesterol (mg/dL) | 143.7±24.5 | 141.5±28.0 | NS |
| Fasting Insulin | 14.6±9.8 | 16.5±17.1 | NS |
| Insulin IAUC (nmol/min/L) | 283.9±194.9 | 244.0±165.9 | NS |
| Insulin sensitivity ((×10−4/min−1/μU/mL | 1.64±1.01 | 1.77±0.87 | NS |
| Acute Insulin Response (μU/mL)−1 | 1553±1088 | 1323±700 | NS |
| Disposition index (×10−4/min−1 | 1972±967 | 2014±950 | NS |
| Glucose effectiveness (%per minute) | 0.016±0.007 | 0.015±0.006 | NS |
| Sub-clinical measures of atherosclerosis | |||
| CIMT (mm) | 0.605±0.059 | 0.605±0.055 | NS |
| Maximum diameter (mm) | 7.33±0.57 | 7.40±0.58 | NS |
| Minimum diameter (mm) | 6.38±0.52 | 6.44±0.54 | NS |
Simple and partial correlations between baseline cardiovascular risk factors and CIMT change revealed that LDL-cholesterol, fasting insulin and insulin AUC were positively correlated to change in CIMT (r=0.21 – 0.24, p<0.05) with glucose effectiveness the only factor negatively correlated with change in CIMT (r= −0.30, p=0.01). Only the inverse relationship between glucose effectiveness and CIMT change remained significant after adjustment for sex, age, height, and baseline CIMT (Figure, r= −0.30, p=0.01). Correlations between baseline body composition (including abdominal adiposity), insulin sensitivity and blood pressure with CIMT change were not significant (range: −0.03 to 0.13, p>0.05). All correlations between change in any given cardiometabolic risk factor and CIMT change were also not significant and there were no significant sex interactions independent of baseline CIMT (range: −0.12 to 0.09, p>0.05).
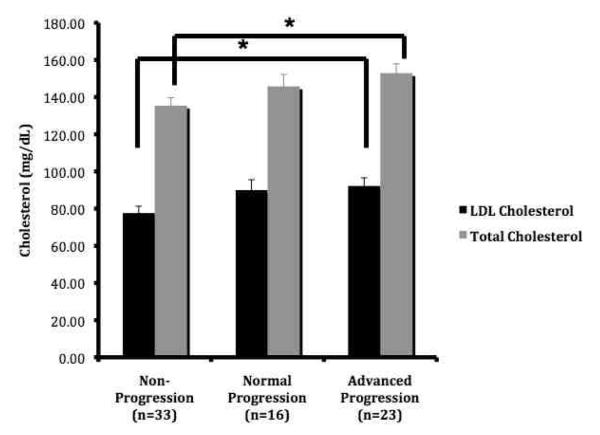
Figure 1.
Participants with advanced CIMT progression had significantly higher baseline LDL- and total cholesterol
ANCOVA is adjusted for sex, baseline age, Tanner stage, CIMT, HDL-cholesterol and height. *p<0.05
Participants with the metabolic syndrome at baseline showed an increase in CIMT change compared with those who did not have the metabolic syndrome, but this difference did not reach significance (ANOVA, 0.007±0.005 vs. −0.003±0.003mm, p=0.16). Sex did not modify the effect of CIMT change between participants with or without the metabolic syndrome (2-way ANOVA interaction, p>0.05). Descriptive statistics of physical and metabolic characteristics of by CIMT progression group are shown in Table II. The baseline LDL- and total cholesterol were significantly higher in the Advanced CIMT Progression Group (92.6 ±4.4 and 152.9±5.1 mg/dL) versus those in the CIMT Non-progression group (77.7±3.7 and 135.5±4.2mg/dL, p<0.05), independent of sex, baseline CIMT, age, height and HDL-cholesterol. All other cardiometabolic risk factors had no association with the 3 CIMT progression groups (p>0.05, data not shown).
Table 2.
Descriptive table of physical and metabolic characteristics at baseline by CIMT progression group
| Baseline Characteristics | Non- Progression (n–33) |
Normal Progression (n–16) |
Advanced Progression (n–23) |
|---|---|---|---|
| Age(years) | 14.8±1.7 | 13.6±1.8 | 14.8±14* |
| Maturation stage (by Tanner) | |||
| 1/2 | 3 | 4 | 1 |
| 3 | 7 | 3 | 4 |
| 4/5 | 23 | 9 | 18 |
| Body composition | |||
| Height (cm) | 165.2±8.0 | 157.5±103 | 163.5±7.8* |
| Weight (kg) | 86.4±21.4 | 80.6±25.9 | 84.2±21.8 |
| BMI (kg/m2) | 31.7±7.6 | 31.8±6.8 | 31.2±6.1 |
| BMI z-score | 1.86±0.73 | 2.09±0.51 | 1.87±0.55 |
| Total Lean Tissue Mass (kg) | 52.3±9.4 | 45.2±11.6 | 50.5±11.6 |
| Total Fat Mass (kg) | 28.2±12.8 | 27.9±8.8 | 30.4±11.7 |
| Cardiometabolic risk factors | |||
| Waist Circumference (cm) | 93.5±15.2 | 93.5±14.4 | 91.3±13.8 |
| Systolic blood pressure (mmHg) | 117.4±10.0 | 113.1±9.6 | 113.3±11.1 |
| Diastolic blood pressure (mmHg) | 64.0±5.7 | 63.8±5.3 | 64.8±6.1 |
| HDL Cholesterol (mg/dL) | 37.6±9.7 | 35.1±5.2 | 38.9±8.3 |
| Triglycerides (mg/dL) | 96.2±48.9 | 113.0±55.9 | 109.5±47.2 |
| LDL-cholesterol (mg/dL) | 78.6±23.0 | 87.2±19.1 | 92.7±18.7* |
| Total cholesterol (mg/dL) | 146.0±24.5 | 140.4±24.4 | 140.4±24.4 |
| Fasting Glucose (mg/dL) | 87.6±8.2 | 89.2±7.3 | 88.6±7.1 |
| 2-hr Glucose (mg/dl) | 112.2±18.8 | 120.1±24.3 | 120.9±21.6 |
| HbA1c (%) | 5.3±0.3 | 5.3±0.4 | 5.3±0.3 |
| Fasting Insulin (μU/mL) | 12.8±6.4 | 15.4±13.5 | 16.0±10.6 |
| Insulin AUC (nmol/min/L) | 261±150 | 350±241 | 351±251 |
| Insulin sensitivity ((×10−4/min−1/μU/mL)) | 1.76±1.05 | 1.39±0.61 | 1.69±1.16 |
| Acute Insulin Response (μU/mL)−1 | 1543±1187 | 1756±960 | 1454±1063 |
| Dispostition index (×10−4/min−1) | 1996±1043 | 2214±918 | 1831±852 |
| Glucose effectiveness (% per min) | 0.016±0.008 | 0.017±0.008 | 0.017±0.008 |
Two logistic regression models (Table III) were used to determine the independent predictors of CIMT progression, which was evident in 53% of the sample. In model 1, LDL-cholesterol was the sole predictor of any type of CIMT progression (CIMT>0.000mm), where the odds for CIMT progression over a 2-year period was 1.03 for each 1 mg/dL higher baseline LDL-cholesterol [95% CI 1.004-1.006, p=0.03]. Model 2 shows that LDL-cholesterol was a marginally significant predictor of advanced CIMT progression.
Table 3.
Determinants of CIMT progression and advanced progression using mulitvariate logistic regression
| OR | 95%CI | P-value | |
|---|---|---|---|
|
Model 1: Predictors of CIMT
Progression |
|||
| Male | 0.83 | 0.23-3.09 | 0.79 |
| Age | 0.98 | 0.66-1.44 | 0.91 |
| Height | 0.92 | 0.84-1.01 | 0.07 |
| Baseline CIMT (per 0.1mm) | 0.50 | 0.00-1.5 | 0.14 |
| Fastin Insulin | 1.02 | 0.96-1.08 | 0.63 |
| Glucose Effectiveness (per 0.001%/min) | 0.50 | 0.00-2.5 | 0.57 |
| LDL-cholesterol | 1.03 | 1.003-1.006 | 0.03 |
|
Model 2: Predictors of Advance CIMT
Progression |
|||
| Gender | 2.90 | 0.72-11.74 | 0.14 |
| Age | 1.11 | 0.75-1.65 | 0.59 |
| Height | 1.01 | 0.93-1.17 | 0.69 |
| Baseline CIMT (per 0.1) | 0.02 | 0.00-5.8 | 0.24 |
| Fasting Insulin | 1.00 | 0.94-1.07 | 0.99 |
| Glucose Effectiveness (per 0.001%/min) | 2.86 | 0.00-2.0 | 0.79 |
| LDL-cholesterol | 1.03 | 0.99-1.06 | 0.06 |
DISCUSSION
This study examined CIMT progression during childhood growth and development and its cardiometabolic predictors in healthy, overweight Latino youth. Our results indicate that change in CIMT was highly variable. Moreover, 36% of participants showed progression beyond that of the physiological norm (Advanced CIMT Progression Group with CIMT ≥0.01mm over 2 years). Participants in the advanced CIMT progression group had significantly higher baseline LDL and total cholesterol than those in the CIMT non-progression group. Another predictor of CIMT change was baseline glucose effectiveness, which had a negative relationship with CIMT change independent of sex and baseline CIMT, age and height. Finally, the odds of CIMT progression increased 1.03 times (or 3%) for each 1mg/dL increase of baseline LDL-cholesterol, independent of glucose effectiveness and other covariates. These results highlight the baseline effects of LDL-cholesterol in youth that results in advanced CIMT progression.
Contrary to our hypothesis, baseline systolic blood pressure, abdominal adiposity or insulin sensitivity were not associated with change in CIMT over a 2-year period. We have previously shown that Latino children with persistent high blood pressure and high waist circumference, along with children with persistent metabolic syndrome, had a higher CIMT than those children without these risk factors. The cumulative effects of the metabolic syndrome, high blood pressure and waist circumference resulted in elevated CIMT. Our present report shows that these same risk factors were not associated with the rate of CIMT progression. Instead, LDL-cholesterol was the defining cardiometabolic risk factor translated into more CIMT progression. Together, these two studies suggest that multiple risk factors are involved in both elevated CIMT and CIMT progression and that all of these risk factors are important in determining atherosclerosis risk in overweight Latino youth.
The relationship between LDL-cholesterol and CIMT has been well-documented in adults but is still ambiguous in studies of healthy children; 2 studies have observed this relationship (22, 23) whereas 2 others have not (24, 25). We have shown a statistically significant relationship with LDL-cholesterol and CIMT progression after only 2 years in overweight Latino youth with a family history of type 2 diabetes. Our data showed that small changes in LDL-cholesterol at baseline were still sufficient to observe differences between CIMT progressors and non-progressors. It is important to note that only 5 participants (all male) had a clinically abnormal level of LDL-cholesterol level (above the 90th percentile for sex and age) (26) and 3 of these 5 participants had family histories of hypercholesterolemia. Studies have shown that children with a familial hypercholesterolemia consistently had increased CIMT compared with healthy controls (22-24). Based on these studies, we repeated our analyses excluding the 5 participants with abnormal high LDL-cholesterol. We found that baseline LDL- and total cholesterol was still higher in the Advanced CIMT Progression Group (89.3±4.1 mg/dL) versus those in the CIMT Non-progression group (76.5±3.3 mg/dL), after controlling for covariates but the difference was marginally significant (p=0.055), likely caused by a decrease in power.
The findings of our study demonstrate the clinical importance of pediatric risk assessment for sub-clinical atherosclerosis. Currently, there is no screening of subclinical atherosclerosis in high-risk youth, yet there have been recommendations made for asymptomatic adults above the age of 45, in other words, individuals who would theoretically have normative CIMT measures similar to the overweight youth we study. The Primary Prevention Writing Group III for the AHA Prevention Conference V stated the potential value of risk assessment when CIMT measures were used in conjunction with traditional risk factor assessments (27). Given the growing literature on the early development of atherosclerosis in children, along with added predisposition to metabolic disorders, there may be reasons not to extend this recommendation to children. With respect to treatment, studies have reported improved vascular function and thickness in children that participated in interventions targeted for obesity reduction (28-30). These results support the notion that all overweight children should have the opportunity to engage in physical activity and diet interventions in order to gain health benefits.
The strengths of this study include its longitudinal measures of subclinical atherosclerosis using the same sonographer and reader of the ultrasound images. Assessment of cardiovascular risk was done with clinical measures of total and regional body composition (DEXA and MRI scans) and direct measures of insulin sensitivity (FSIVGTT with minimal modeling). In addition, simple and clinically applicable measures using fasting blood were also employed. The use of a homogeneous sample of understudied minority youth contributes to the strengths of this report, but it also limits the generalizability of the results to overweight Latino adolescents with a family history of type 2 diabetes.
Several limitations must also be discussed. First, the moderate sample size and a short time frame of this longitudinal study may have impeded any other predictors of CIMT progression, particularly when one considers the large number of predictors analyzed. This limitation was addressed to some extent by building the multivariate statistical models with only a chosen number of predictors. Another limitation related to this small and highly specific group of overweight Latino youth was the narrow range of BMI change that this children incurred during the 2-year time frame. Most studies of subclinical atherosclerosis in overweight children have shown a relationship between adiposity and CIMT. In contrast, our study has been limited to a small BMI change and we were unable to show this relationship. Broadening the BMI range by adding lean participants would be beneficial to further examine the effects of BMI and adiposity. Lastly, we were unable to statistically control for other important covariates such as physical activity data or for C-reactive protein and other cardiovascular biomarkers, as we did not collect these data or assay blood for these proteins.
These findings extend our previous findings in which persistent metabolic syndrome, high blood pressure and high waist circumference were related to CIMT and suggest that LDL-cholesterol contributes to CIMT and its progression in overweight Latino youth.
Acknowledgments
We would like to thank the staff of the University of Southern California/Los Angeles County GCRC and the SOLAR staff over the past 6 years. Our gratitude is especially extended to the loyal participants and their families for their continued participation.
Supported by NIDDK (grant R01-DK59211 to M.G.), General Clinical Research Center for Health Resources (grant M01 RR 00043), and NIDDK fellowship (F31-DK081276 to C.T.-C. under the sponsorship of M.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Flegal KM, Ogden CL, Carroll MD. Prevalence and trends in overweight in Mexican-american adults and children. Nutr Rev. 2004;62:S144–8. doi: 10.1111/j.1753-4887.2004.tb00085.x. [DOI] [PubMed] [Google Scholar]
- 2.Goran MI, Bergman RN, Cruz ML, Watanabe R. Insulin resistance and associated compensatory responses in african-american and Hispanic children. Diabetes Care. 2002;25:2184–90. doi: 10.2337/diacare.25.12.2184. [DOI] [PubMed] [Google Scholar]
- 3.Cruz ML, Weigensberg MJ, Huang TT, Ball G, Shaibi GQ, Goran MI. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrinol Metab. 2004;89:108–13. doi: 10.1210/jc.2003-031188. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez BL, Fujimoto WY, Mayer-Davis EJ, Imperatore G, Williams DE, Bell RA, et al. Prevalence of cardiovascular disease risk factors in U.S. children and adolescents with diabetes: the SEARCH for diabetes in youth study. Diabetes Care. 2006;29:1891–6. doi: 10.2337/dc06-0310. [DOI] [PubMed] [Google Scholar]
- 5.Reinehr T, Kiess W, de Sousa G, Stoffel-Wagner B, Wunsch R. Intima media thickness in childhood obesity: relations to inflammatory marker, glucose metabolism, and blood pressure. Metabolism. 2006;55:113–8. doi: 10.1016/j.metabol.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 6.Zhu W, Huang X, He J, Li M, Neubauer H. Arterial intima-media thickening and endothelial dysfunction in obese Chinese children. Eur J Pediatr. 2005;164:337–44. doi: 10.1007/s00431-005-1642-y. [DOI] [PubMed] [Google Scholar]
- 7.Osika W, Dangardt F, Montgomery SM, Volkmann R, Gan LM, Friberg P. Sex differences in peripheral artery intima, media and intima media thickness in children and adolescents. Atherosclerosis. 2009;203:172–7. doi: 10.1016/j.atherosclerosis.2008.05.054. [DOI] [PubMed] [Google Scholar]
- 8.Bohm B, Hartmann K, Buck M, Oberhoffer R. Sex differences of carotid intima-media thickness in healthy children and adolescents. Atherosclerosis. 2009;206:458–63. doi: 10.1016/j.atherosclerosis.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Atabek ME, Pirgon O, Kivrak AS. Evidence for association between insulin resistance and premature carotid atherosclerosis in childhood obesity. Pediatr Res. 2007;61:345–9. doi: 10.1203/pdr.0b013e318030d206. [DOI] [PubMed] [Google Scholar]
- 10.Reinehr T, Wunsch R, de Sousa G, Toschke AM. Relationship between metabolic syndrome definitions for children and adolescents and intima-media thickness. Atherosclerosis. 2008;199:193–200. doi: 10.1016/j.atherosclerosis.2007.09.041. [DOI] [PubMed] [Google Scholar]
- 11.Wiegman A, de Groot E, Hutten BA, Rodenburg J, Gort J, Bakker HD, et al. Arterial intima-media thickness in children heterozygous for familial hypercholesterolaemia. Lancet. 2004;363:369–70. doi: 10.1016/S0140-6736(04)15467-6. [DOI] [PubMed] [Google Scholar]
- 12.Morrison KM, Dyal L, Conner W, Helden E, Newkirk L, Yusuf S, et al. Cardiovascular risk factors and non-invasive assessment of subclinical atherosclerosis in youth. Atherosclerosis. 2010;208:501–5. doi: 10.1016/j.atherosclerosis.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 13.Stabouli S, Kotsis V, Papamichael C, Constantopoulos A, Zakopoulos N. Adolescent obesity is associated with high ambulatory blood pressure and increased carotid intimal-medial thickness. J Pediatr. 2005;147:651–6. doi: 10.1016/j.jpeds.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Lim SM, Kim HC, Lee HS, Lee JY, Suh M, Ahn SV. Association between blood pressure and carotid intima-media thickness. J Pediatr. 2009;154:667–71. doi: 10.1016/j.jpeds.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 15.Toledo-Corral CM, Ventura EE, Hodis HN, Weigensberg MJ, Lane CJ, Li Y, et al. Persistence of the metabolic syndrome and its influence on carotid artery intima media thickness in overweight Latino children. Atherosclerosis. 2009;206:594–8. doi: 10.1016/j.atherosclerosis.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selzer RH, Hodis HN, Kwong-Fu H, Mack WJ, Lee PL, Liu CR, et al. Evaluation of computerized edge tracking for quantifying intima-media thickness of the common carotid artery from B-mode ultrasound images. Atherosclerosis. 1994;111:1–11. doi: 10.1016/0021-9150(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 17.Selzer RH, Mack WJ, Lee PL, Kwong-Fu H, Hodis HN. Improved common carotid elasticity and intima-media thickness measurements from computer analysis of sequential ultrasound frames. Atherosclerosis. 2001;154:185–93. doi: 10.1016/s0021-9150(00)00461-5. [DOI] [PubMed] [Google Scholar]
- 18.Hodis HN, Mack WJ, LaBree L, Selzer RH, Liu CR, Liu CH, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128:262–9. doi: 10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 19.Xiang AH, Azen SP, Buchanan TA, Raffel LJ, Tan S, Cheng LS, et al. Heritability of subclinical atherosclerosis in Latino families ascertained through a hypertensive parent. Arterioscler Thromb Vasc Biol. 2002;22:843–8. doi: 10.1161/01.atv.0000015329.15481.e8. [DOI] [PubMed] [Google Scholar]
- 20.Hodis HN, Mack WJ, LaBree L, Mahrer PR, Sevanian A, Liu CR, et al. Alpha-tocopherol supplementation in healthy individuals reduces low-density lipoprotein oxidation but not atherosclerosis: the Vitamin E Atherosclerosis Prevention Study (VEAPS) Circulation. 2002;106:1453–9. doi: 10.1161/01.cir.0000029092.99946.08. [DOI] [PubMed] [Google Scholar]
- 21.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Jama. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 22.Tonstad S, Joakimsen O, Stensland-Bugge E, Leren TP, Ose L, Russell D, et al. Risk factors related to carotid intima-media thickness and plaque in children with familial hypercholesterolemia and control subjects. Arterioscler Thromb Vasc Biol. 1996;16:984–91. doi: 10.1161/01.atv.16.8.984. [DOI] [PubMed] [Google Scholar]
- 23.Virkola K, Pesonen E, Akerblom HK, Siimes MA. Cholesterol and carotid artery wall in children and adolescents with familial hypercholesterolaemia: a controlled study by ultrasound. Acta Paediatr. 1997;86:1203–7. doi: 10.1111/j.1651-2227.1997.tb14847.x. [DOI] [PubMed] [Google Scholar]
- 24.Lavrencic A, Kosmina B, Keber I, Videcnik V, Keber D. Carotid intima-media thickness in young patients with familial hypercholesterolaemia. Heart. 1996;76:321–5. doi: 10.1136/hrt.76.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pauciullo P, Iannuzzi A, Sartorio R, Irace C, Covetti G, Di Costanzo A, et al. Increased intima-media thickness of the common carotid artery in hypercholesterolemic children. Arterioscler Thromb. 1994;14:1075–9. doi: 10.1161/01.atv.14.7.1075. [DOI] [PubMed] [Google Scholar]
- 26.Hickman TB, Briefel RR, Carroll MD, Rifkind BM, Cleeman JI, Maurer KR, et al. Distributions and trends of serum lipid levels among United States children and adolescents ages 4-19 years: data from the Third National Health and Nutrition Examination Survey. Prev Med. 1998;27:879–90. doi: 10.1006/pmed.1998.0376. [DOI] [PubMed] [Google Scholar]
- 27.Greenland P, Abrams J, Aurigemma GP, Bond MG, Clark LT, Criqui MH, et al. Prevention Conference V: Beyond secondary prevention: identifying the high-risk patient for primary prevention: noninvasive tests of atherosclerotic burden: Writing Group III. Circulation. 2000;101:E16–22. doi: 10.1161/01.cir.101.1.e16. [DOI] [PubMed] [Google Scholar]
- 28.Farpour-Lambert NJ, Aggoun Y, Marchand LM, Martin XE, Herrmann FR, Beghetti M. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol. 2009;54:2396–406. doi: 10.1016/j.jacc.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 29.Iannuzzi A, Licenziati MR, Vacca M, De Marco D, Cinquegrana G, Laccetti M, et al. Comparison of two diets of varying glycemic index on carotid subclinical atherosclerosis in obese children. Heart Vessels. 2009;24:419–24. doi: 10.1007/s00380-008-1138-6. [DOI] [PubMed] [Google Scholar]
- 30.Kleber M, Schaefer A, Winkel K, Hoffmann D, Wunsch R, Kersting M, et al. Lifestyle intervention “Obeldicks Mini” for obese children aged 4 to 7 years. Klin Padiatr. 2009;221:290–4. doi: 10.1055/s-0029-1234129. [DOI] [PubMed] [Google Scholar]