Abstract
The concept of autonomic balance views autonomic states along a bipolar continuum from sympathetic (S) to parasympathetic (P) dominance, whereas regulatory capacity models emphasize overall autonomic flexibility as a marker of the capacity for regulation. These two concepts were evaluated for their utility in characterizing patterns of autonomic control. Measures of P (high frequency heart rate variability, HF) and S (pre-ejection period, PEP) cardiac control were obtained. A measure of cardiac autonomic balance (CAB) was derived as the difference in the normalized P index minus the S index, and a measure of cardiac autonomic regulation (CAR) was derived as the normalized P index plus the S index. Results reveal that CAR, but not CAB, was a significant predictor of the prior occurrence of a myocardial infarction, net of demographic and other variables, whereas CAB, but not CAR, was a significant predictor of concurrent diabetes.
Descriptors: sympathetic, parasympathetic, pre-ejection period, heart rate variability, respiratory sinus arrhythmia, cardiac control, autonomic balance, myocardial infarction, diabetes
The concept of autonomic balance has an extensive history. Eppinger and Hess (1915) proposed that individuals are constitutionally disposed toward either sympathetic (sympathicotonia) or parasympathetic (vagotonia) dominance and that these dispositions may bias toward distinct psychosomatic disorders (e.g., hypertension and asthma, respectively). Wenger (1966) confirmed individual differences in a measure of autonomic balance (Ā), but reported this metric shows a normal, rather than a dichotomous, distribution across individuals.
Autonomic balance models continue to be espoused in the contemporary literature, both as individual difference characteristics, and as predictors of health outcomes. One example is the sympathovagal balance metric derived from measures of heart rate variability (Malliani, 2005; Malliani & Montano, 2002). Specifically, high frequency (HF) heart rate variability, in the respiratory frequency band, provides a relatively pure index of parasympathetic cardiac control, whereas low frequency (LF) variability reflects a combination of sympathetic and parasympathetic influences (see Berntson et al., 1993a, 1997; Malik, 1996). Based on this and other empirical findings, Malliani and colleagues suggest that the ratio of LF to HF variability may index the relative autonomic balance, extending from sympathetic to parasympathetic dominance (see Malliani, 2005; Malliani & Montano, 2002). Although this metric has been challenged on both conceptual and empirical grounds (Eckberg, 1997), it continues to be widely employed as a metric of sympathovagal balance. Among the more salient issues with the concept of sympathovagal balance is the finding that the autonomic branches are not invariably reciprocally controlled. Although a reciprocal relationship may be seen with lower-level orthostatic and other reflexes, psychological processes and higher neurobehavioral substrates are more flexible and can yield independent activation or even coactivation of the two autonomic divisions (Berntson, Cacioppo & Quigley, 1991, 1993b, 1994; Berntson & Cacioppo, 2007).
Although there may be individual differences in the relative contributions of sympathetic and parasympathetic branches, it is not at all certain that sympathovagal balance is even a regulated dimension. Moreover, despite powerful homeostatic controls over cardiovascular parameters such as blood pressure, it is clear that even regulated dimensions are not characterized by fixed, invariant levels. Rather, it is alterations in these dimensions (e.g., blood pressure, heart rate, myocardial contractility, etc) that permit an adaptive cardiovascular response to perturbations such as orthostatic stress, exercise, or fight/flight responses. This pattern of regulatory flexibility has been termed allostatic (McEwen & Wingfield, 2003; Sterling & Eyer, 1981) or allodynamic (Berntson & Cacioppo, 2007) regulation, and is conceptualized as a means of achieving “stability through change” (Sterling & Eyer, 1988, p 631). According to this view, the critical dimension of autonomic regulation may not be autonomic balance, per se, but autonomic flexibility or regulatory capacity that permits an organism to adaptively deal with changing demands.
Diminished HF heart rate variability reflective of lower parasympathetic cardiac control, for example, has been reported to be a significant risk factor for recovery following myocardial infarctions (Balanescu et al., 2004; Kleiger et al., 2005; Malik 1996; Tsuji et al., 1996; Liao, 1997) and is also a predictor of hypertension after controlling for age and other risk factors (Masi et al., 2007). This might be consistent with a shift toward sympathetic dominance along a bipolar axis. However, it is also the case that diminished low frequency variability (which includes sympathetic contributions) may be an equivalent or even superior risk stratifier (Kleiger et al., 2005; Malik 1996; Tsuji et al., 1996; Liao, 1997) and is also predictive of the development of hypertension (Singh et al., 1998). Hemingway et al. (2005) report that low heart rate variability, especially LF power, may be a critical mediator of the relations between low social status, metabolic syndrome, and increased cardiac risk. These findings are more consistent with the view that an important dimension of autonomic control may be the overall level of autonomic regulation and that autonomic variability, rather than or in addition to sympathovagal balance, may be a relevant parameter in cardiovascular health (Aubert & Ramaekers, 1999).
Low overall heart rate variability is linked not only with cardiovascular disorders, but has been reported to be a risk factor for all-cause mortality and morbidity (Dekker et al., 1997; Gang & Malik, 2003). Reduced “autonomic flexibility”, reflected by low heart rate variability, has been reported in conditions as disparate as anxiety and dyspepsia (Friedman, 2007; Friedman & Thayer, 1998; Hoehn-Saric & McLeod, 2000; Thayer & Sternberg, 2006). These findings suggest that a relevant health determinant may be the overall autonomic regulatory capacity, which supports flexible adjustments in the face of adaptive challenges.
Cardiovascular disease is the leading cause of mortality and morbidity in the United States, and more than 1 million Americans suffer a myocardial infarction (MI) each year (National Center for Health Statistics, see Thom, 2004). High sympathetic cardiac control is a known risk factor for myocardial ischemia (MI) and for survival following MI (Airaksinen, 1999; Billman, 2006a, b; Schwartz et al., 1992; Hohnloser, 2005) and drugs that block sympathetic actions (primarily beta adrenergic blockers) are a common treatment strategy after MI (Hohnloser, 2005). In contrast, the parasympathetic system exerts antifibrillary actions (Airaksinen, 1999), and parasympathetic activity may be a positive indicator for recovery after MI (Billman, 2006a; Manfrini et al., 2003). It remains uncertain, however, whether the health correlates of autonomic regulation are more in line with an autonomic balance model or the regulatory capacity model.
Diabetes mellitus is a prevalent metabolic disorder with an increasing incidence, currently afflicting about 10% of the population-- a percentage that approximately doubles with advancing age (National Diabetes Information Clearinghouse; The National Institute of Diabetes and Digestive and Kidney Diseases). Type 1 diabetes is generally attributable to an autoimmune assault on insulin-producing pancreatic Beta cells, whereas type 2 diabetes is associated with the development of insulin resistance. Even in Type 2 diabetes, however, there is a progressive loss of Beta cells and growing evidence suggests immune and inflammatory reactions contribute to both types of diabetes (Pearl-Yafe et al., 2007; Rosenbloom, 2003).
In parallel to its influence on cardiovascular function, the autonomic nervous system plays an important role in insulin regulation and may impact diabetes. In addition to the direct innervation of adipose tissue (see Romijn & Fliers, 2005), the parasympathetic and sympathetic innervations of the pancreas stimulate and inhibit insulin secretion, respectively (Ahren, 2000; Campfield & Smith, 1983). The autonomic nervous system may also impact insulin regulation indirectly, by modulation of immune and inflammatory reactions directed toward Beta cells. Sympathetic adrenergic modulation has complex effects on the immune system and can either enhance or suppress the release of pro-inflammatory cytokines such as tumor necrosis factor (TNF) and interleukin 1 (IL1), whereas the parasympathetic division represents an important component of what has been termed the cholinergic anti-inflammatory system (Elenkov et al., 2000; Tracey, 2007). These immuno-modulatory actions of the autonomic nervous system may play an important role in the development of diabetes.
Diabetes has generally been considered to be associated with a hyper-sympathetic state, at least prior to the development of diabetic neuropathies (Perin, Maule & Quadri, 2001; Tentolouris, Liatis & Katsilambros, 2006). There may also be a concurrent hypoactive parasympathetic system. Insulin resistance has been hypothesized to be attributable, at least in part, to diminished parasympathetic control (Lautt, 1999, 2004). Diabetics or first degree relatives of diabetics often show low parasympathetic control, as indexed by respiratory sinus arrhythmia, and the degree of insulin resistance correlates negatively with parasympathetic control in non-diabetics (see Masi et al., 2007 for review and data). Insulin resistance can be created experimentally by parasympathectomy (see Fliers et al., 2003), and has been shown to be reduced by cholinergic agonists in both animal and human studies (Ahren, Sauerberg & Thomsen, 1999; Lautt et al, 2004). This pattern of hyper-sympathetic and hypo-parasympathetic regulation might be best represented as a shift in autonomic balance.
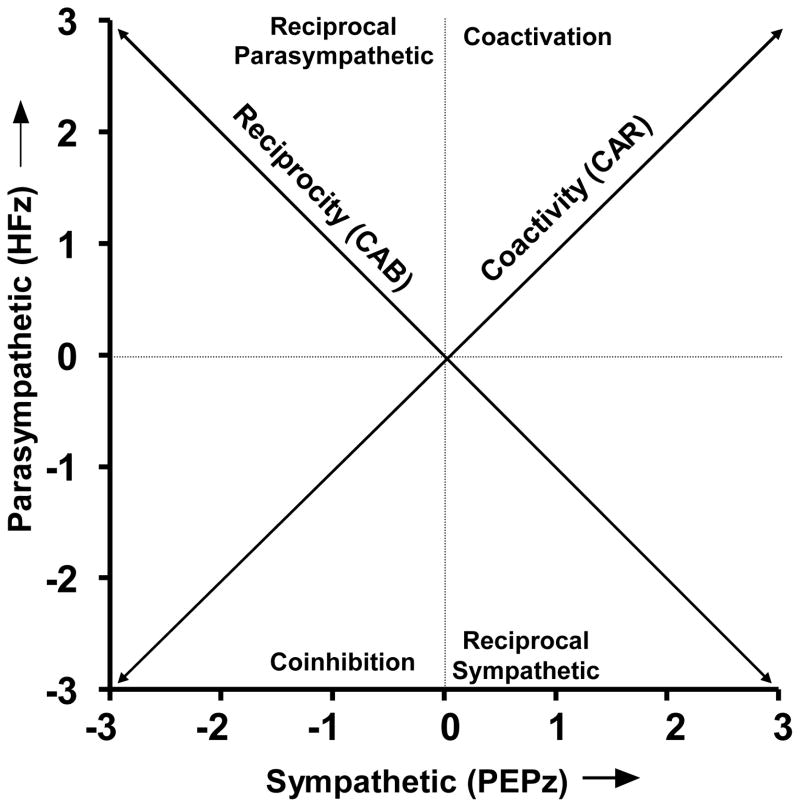
The present study evaluated the potential utility of the autonomic balance and the regulatory capacity models in studies of health and disease in a population based sample (CHASRS; Cacioppo et al., 2005). Based on a bivariate model of autonomic space (see Figure 1, Berntson, Cacioppo & Quigley, 1990, 1993b, 1994), two metrics were developed to represent the balance and regulatory capacity models. These metrics were derived from measures of relative sympathetic and parasympathetic activity. Pre-ejection period (PEP) was employed as a measure of sympathetic cardiac control (Berntson et al., 1994; Cacioppo et al., 1994; Sherwood et al., 1990), and respiratory sinus arrhythmia or high frequency heart rate variability (HF) was employed as a metric of parasympathetic control (Berntson et al., 1993a; 1997; Malik, 1996). Cardiac Autonomic Balance (CAB) was operationalized as the difference between the normalized sympathetic and parasympathetic estimates. CAB was designed to provide a measure of autonomic balance along a parasympathetic-sympathetic dimension (the reciprocal diagonal of Figure 1). In contrast, overall Cardiac Autonomic Regulation (CAR) was taken as the sum of the normalized sympathetic and parasympathetic cardiac controls. This was intended to capture the extent of coactivation of the parasympathetic and sympathetic systems (along the coactivity diagonal in Figure 1). Results revealed that CAR, but not CAB, was a significant predictor of the prior occurrence of a MI, where as CAB, but not CAR, was a predictor of diabetes.
Figure 1.
Bivariate representation of autonomic space, with a parasympathetic ordinate (estimated by normalized HF) and a sympathetic abscissa (estimated by normalized PEP). The 0,0 intersection illustrates the mean position along both dimensions, the diagonal of reciprocity represents a bipolar, model of reciprocally controlled autonomic divisions. The diagonal of coactivity illustrates another dimension of regulation, which more effectively indexes the aggregate or total autonomic activity. The axes and quadrants of this graphic represent the major modes of autonomic control as defined by Berntson et al., 1991. The locations along the parasympathetic and sympathetic axes represent patterns of independent parasympathetic and sympathetic control, respectively. The quadrants represent the remaining four modes of control (reciprocal parasympathetic, reciprocal sympathetic, coactivation and coinhibition.
Methods
Study Population
Data for this study were collected in years 1–3 of the Chicago Health, Aging and Social Relations Study (CHASRS), a longitudinal, population based study of persons born between 1935 and 1952. The target population was non-Hispanic Caucasian, African American, and non-Black Latino American persons between the ages of 50 an 68 living in Cook County, IL, who were English-speaking and sufficiently ambulatory to come to the University of Chicago for a daylong visit to the laboratory. The sample was selected using a multistage probability design in which African Americans and Latino Americans were over sampled and gender equality maintained. Data for individual participants were averaged over the three year period, to increase reliability. In cases where data points were missing from one or two years, the participant’s score was based on the available data. Across variables, 60–83% of the participant’s had scores for all three years, 88–89% had scores for at least two years. The final sample size was 229.
Procedure
Participants arrived at the laboratory between 8 a.m. and 9 a.m. They provided informed consent and then began a day of assessments that included standard psychological surveys, interviews, lunch, and a cardiovascular protocol.
Cardiovascular activity was measured prior to lunch for all participants. Experimenters attached sensors for electrocardiograph, impedance cardiograph, and blood pressure recording. Participants were then seated in a comfortable padded chair. During a 15-min adaptation period, participants completed questionnaires while experimenters established good signal quality. Participants then sat quietly for an additional five min prior to a four-min recording of baseline cardiovascular activity. Although this was nominally a baseline measure, subjects were likely moderately stressed by the overall procedure which included blood draws.
Cardiovascular Measures
Primary cardiovascular measures of sympathetic and parasympathetic cardiac control, respectively, were pre-ejection period (PEP) and high frequency (0.15– 0.4 Hz) heart rate variability (HF). PEP, derived from impedance cardiography, is commonly used as a measure of sympathetic cardiac control (Berntson et al., 1994; Cacioppo et al., 1994; Sherwood et al., 1990). It has previously been shown to have good long-term (over 1 year) temporal consistency (Burleson et al., 2003), and was found to be highly reliable over the three years of the present study (Cronbach alpha = .79). Although within-subjects changes in psychophysiological variables (such as PEP) often have fewer sources of extraneous variance than between-subjects differences, individual differences in PEP have been shown to be highly predictive of cardiac sympathetic control as indexed by the “gold standard” of pharmacological blockades (r = .82; Cacioppo et al., 1994).
HF heart rate variability is a rhythmical fluctuation of heart rate in the respiratory frequency band (respiratory sinus arrhythmia), and has been shown to be a relatively pure index of parasympathetic control (see Berntson et al., 1993a, 1997; Malik, 1996). AS for PEP, HF has previously been shown to have good long-term temporal consistency (Burleson et al., 2003), and was found to be highly reliable over the three years of the present study (Cronbach alpha = .85). Moreover, individual differences in HF have been shown to be predictive of differences in cardiac vagal control as indexed by pharmacological blockade studies (r = .90, Fouad et al., 1984; r = .90, Hayano et al., 1991; r = .50, Grossman & Kollai, 1993).
The electrocardiogram (ECG) was obtained using the standard lead II configuration. The impedance cardiogram was obtained using the standard tetrapolar electrode system and procedures described elsewhere (Sherwood et al., 1990). The ECG and basal thoracic impedance (Z0) were measured using a Biopac MP100 system (ECG100 and EB1100 modules, respectively; Biopac Systems, Inc., Santa Barbara, CA). ECG and Z0 were digitized at 1000 Hz.
Custom software (Mindware, Gahanna, OH) was used to analyze the dZ/dt waveforms to obtain impedance-derived measures (i.e., PEP). The same software was used to verify, edit, and summarize cardiovascular data. For each subject, ECG and impedance data were ensemble averaged for each minute to produce estimates of the PEP. PEP was quantified as the time interval in milliseconds from the onset of the ECG Q wave to the B point of the dZ/dt wave (Sherwood et al., 1990). The B point was localized by an improved method, based on large and salient features of the waveform, which has high within and between scorer reliabilities (Lozano et al., 2007). Minute by minute means were then averaged over the 4 min baseline period.
HF heart rate variability was derived by spectral analysis (Mindware Impedance Cardiography system, Gahanna, OH) of the interbeat interval (RR) series derived from the ECG, following procedures specified by Berntson et al. (1997). Briefly, the RR interval series was time sampled at 4 Hz (with interpolation) to yield an equal interval time series. This time series was detrended (2nd order polynomial), end-tapered, and submitted to an FFT. HF spectral power was then integrated over the respiratory frequency band (0.15–0.4 Hz). Respiratory measures were also obtained to ensure that the respiratory rates were within the analytical band. If respiratory rates fell below the HF cutoff, data from that minute were excluded from analysis. This was an issue in only two cases and, for each, resulted in a single minute (of 4) being excluded.
Because respiration amplitude and depth can influence HF measures, we ran preliminary analyses to insure that these respiratory parameters did not correlate with the derived metrics (CAR and CAB). No significant correlations were observed between respiratory parameters and the experimental measures. In addition, there were no significant differences in respiratory parameters between subjects in the MI or diabetes groups and remaining participants.
Based on a bivariate model of autonomic space (see Figure 1), two measures of autonomic control were derived from HF and PEP. An index of autonomic balance, Cardiac Autonomic Balance (CAB), was derived as the difference between normalized values of parasympathetic control (HF) and sympathetic control (PEP). CAR reflects the location along a bipolar model of autonomic balance (the reciprocal diagonal of Figure 1). A metric of overall Autonomic Cardiac Regulation (CAR) was derived as the sum of the normalized values of HF and PEP. CAR represents a metric of overall autonomic activity or coactivation (along the coactivity diagonal in Figure 1). Normalization of HP and PEP was necessary because of the wide differences in means and scaling among these measures. Normalization was accomplished by transforming individual HP and PEP values to z-scores, so all normalized values are expressed in standard deviations relative to the population means. In addition, because increased sympathetic control is associated with shortened PEP values, PEP was multiplied by −1 (-PEP), in order to invert the relationship to a positive association. Consequently, Cardiac Autonomic Balance (CAB) = HFz – (-PEPz), and Cardiac Autonomic Regulation (CAR) = HFz + (-PEPz).
Although HF and PEP each provide measures of autonomic cardiac control (parasympathetic and sympathetic, respectively), they are based on distinct (chronotropic vs. ionotropic) functional dimensions. This appears reasonable as physiological studies reveal a functional coupling of the sympathetic control of cardiac inotropy and chronotropy, as evidenced by the existence of common brainstem regulatory systems and by covariation of these dimensions with both central (brain) or peripheral (sympathetic nerve) stimulation (Campos & McAllen, 1999). In accord with these considerations, Cacioppo et al. (1994) found that individual differences in the inotropic state of the heart, as indexed by PEP, were highly correlated (r = .82) with sympathetic effects on the chronotropic state as measured by autonomic blockades.
As an ancillary analysis, we also derived low frequency (LF) heart rate variability (0.05–0.15 Hz). Both sympathetic and parasympathetic branches contribute to variability at this frequency, but the LF/HF ratio has been proposed as a measure of sympathovagal balance (Malliani 2005; Malliani & Montano, 2002). Although this metric has been challenged on both conceptual and empirical grounds (Berntson et al., 1997; Eckberg, 1997), it continues to be employed as a metric of sympathovagal balance. For completeness, we also include it here.
Health Measures
As an overall measure of health, participants completed the RAND 36-item Health Survey short form (SF-36; see Ware and Sherbourne, 1992). This survey taps eight health concepts: physical functioning, bodily pain, role limitations due to physical health problems, role limitations due to personal or emotional problems, emotional well-being, social functioning, energy/fatigue, and general health perceptions. The survey yields two major summary scales, physical health (PH) and mental health (MH), and a pain (BP) subscale of the PH.
In addition to the SF-36, we also gathered more specific health information by a questionnaire/interview adaptation of the Charlson index (Katz et al., 1996) that asks about presence/absence of a wide range of medical conditions, including myocardial infarction and diabetes. Although these were self-report data, some corroboration is available from the fact that blood glucose was significantly correlated with the report of diabetes (r = .63, p < .001).
Data Analysis
Linear regression analyses were used to test the magnitude of the effects of predictor variables on health status. Point-biserial correlations were used with dichotomous variables. All predictor variables were standardized to a mean of 0 and a standard deviation of 1 in order to represent potentially substantive individual differences in these characteristics. The exception was for variables such as gender and marital status, which were coded as categorical variables. We report unstandardized coefficients throughout, which are therefore interpretable as the magnitude of change in the score of the criterion variable associated with a 1 standard deviation increase in each psychosocial predictor, and with a 1 unit increase for each demographic and control variable. A set of linear regression models were used in which the control variables were added to the regression equation to test the individual associations between each of the predictor variables independent of demographic characteristics (i.e. age, gender, education, income and ethnicity) known or likely to influence the outcome measure. Logit regression was employed for the analysis of myocardial infarction and diabetes, which were coded either as a yes or no.
Covariates
Demographic covariates were gender, ethnicity, age, education (years of schooling), and household income. Household income was reported in 12 categories ranging from less than 5,000 to more than 200,000; to achieve a more continuous distribution, we used the log-transformed median of each category in analyses. Missing values for education (6 subjects) and household income (13 subjects) were replaced with means from the corresponding gender by ethnic group combination.
Body mass index (BMI), calculated as weight in kg/(height in m) 2, served as a covariate in analyses of cardiovascular and health status variables. Forty percent of participants were on vasoactive medications, 5% were on volume active medications, and an additional 11% were on both types of medication. A point-biserial correlation showed that the likelihood of being on cardiovascular medications did not differ as a function of CAR or CAB. Nevertheless, cardiovascular medications were held constant in critical regression models to permit an assessment of the independent effects on outcomes. No reported effects were changed by the addition of any covariates.
Results
Participant Characteristics
Demographic and other characteristics of the participants are illustrated in Table 1. Table 2 shows a breakdown of autonomic parameters by age and gender.
Table 1.
Participant Characteristics (Means ± SEM)
| Overall (n =229) | Males (n = 109) | Female (n=120) | |
|---|---|---|---|
| Age | 57.43 ± (.29) | 57.53 ± (.46) | 57.34 ± (.39) |
| Income | 67,122 ± (3829) | 77,875 ± (6025) | 57,501 ± ($4700) |
| Education | 13.27 ± (.21) | 13.16 ± (.31) | 13.37 ± (.28) |
| Married/Cohabitating | 140 (61%) | 82 (75%) | 58 (48%) |
| Ethnicity | |||
| Caucasians | 82 (36%) | 39 (36%) | 43 (36%) |
| African Americans | 81 (36%) | 37 (34%) | 44 (37%) |
| Hispanics | 66 (28%) | 33 (30%) | 33 (28%) |
|
| |||
| SF-36 Health | 63.98 (.98) | 64.67 (1.4) | 63.35 (1.4) |
| BMI | 31.50 ± (.46) | 31.17± (.61) | 31.81 ± (.69) |
| Myocardial Ischemia | 11 (5%) | 6 (6%) | 5 (4%) |
| Diabetes | 40 (17%) | 17 (16%) | 23 (19%) |
Table 2.
Autonomic measures and derived indices as a function of age and gender
| CAR | CAB | HR | MAP | PEP HF | LF | LF+HF | LF/HF | |
|---|---|---|---|---|---|---|---|---|
| Overall | 0 (.03) | 0 (.1) | 65.3 (.7) | 90.7 (.7) | 103.0 (1) 5.0 (.1) | 5.7 (.1) | 10.68(.1) | 1.2 (.02) |
| Male | −.22 (.10) | −.02 (.1) | 65.4 (1.0) | 92.8 (1.0) | 105.0 (1.6) 4.8 (.1) | 5.9 (.1) | 10.67(.1) | 1.2 (.03) |
| Female | .20 (.08) | .02 (.1) | 65.3 (.9) | 88.8 (1.0) | 101.5 (1.2) 5.2 (.1) | 5.2 (.1) | 10.67(.1) | 1.1 (.02) |
| Age (med. split) | ||||||||
| 50 – 57 | .12 (.10) | .03 (.1) | 64.6 (.9) | 90.1 (1.0) | 102.3 (1.4) 5.1 (.1) | 5.8 (.1) | 10.82(.2) | 1.2 (.03) |
| 58 – 68 | −.12 (.08) | −.03 (.1) | 66.1 (1.0) | 91.3 (1.2) | 103.7 (1.3) 4.9 (.1) | 5.6 (.1) | 10.53(.2) | 1.2 (.02) |
Parasympathetic and Sympathetic Measures in Bivariate Autonomic Space
The distribution of HF scores, indexing parasympathetic control, and PEP scores, indexing sympathetic control, are illustrated in Figure 2. As is apparent, the bivariate distribution does not cluster along the reciprocal diagonal as would be expected of reciprocally controlled autonomic branches in accord with a bipolar model of autonomic control. Although error variance and non-autonomic determinants would add variance and scatter, in fact there was no significant correlation between the HFz and PEPz measures. A metric along the reciprocal diagonal (CAB) could capture a component of the variance in this distribution, but an additional component could be captured by a metric tapping the other (coactivity) diagonal (CAR).
Figure 2.
Distribution of HFz and PEPz scores across the CHASRS population, and relation to the derived CAR and CAB metrics. The overall distribution deviates considerably from the reciprocal diagonal representing a bipolar model. Individuals in the Reciprocal Parasympathetic quadrant would have relatively high CAB scores, whereas those in the Reciprocal Sympathetic quadrant would have relatively low CAB scores. An additional dimension is reflected along the coactivity diagonal. Individuals in the Coactivation quadrant would have relatively high CAR scores, whereas those in the Coinhibition quadrant would have relatively low CAR scores.
Derived Regulatory Models: CAB and CAR
Table 3 illustrates the intercorrelations among the autonomic measures and the derived metrics of CAB and CAR. Consistent with its derivation as a point along a parasympathetic-sympathetic continuum, CAB is positively correlated with parasympathetic control (as indexed by HF) and inversely correlated with sympathetic control (as indexed by –PEP). As expected it is also negatively correlated with heart rate, as high CAB values mark higher parasympathetic and lower sympathetic cardiac control. CAB is also correlated with LF/HF, a putative marker of sympathovagal balance, although this correlation accounts for only 25% of the variance in these measures. This correlation is negative, as high CAB values denote high parasympathetic control whereas high LF/HF values putatively indicate high sympathetic control.
Table 3.
Relations of CAR and CAB to autonomic measures
| CAR | CAB | HR | MAP | PEP1 | HF | LF | LF+HF | LF/HF | |
|---|---|---|---|---|---|---|---|---|---|
| CAR (r) | - | .02 | −.36** | −.08 | −.67** | .68** | .34** | .54** | −.42** |
| CAB (r) | .02 | - | −.50** | −.06 | .73** | .73** | .47** | .63** | −.47** |
Higher sympathetic control would be reflected in lower PEP values
p < .01
p < .001
Normalized values of the parasympathetic measure (HF) and the sympathetic index (-PEP), were not significantly correlated across individuals (r(223) = −.08, p = .23). This does not in accord with a bipolar model of individual differences in autonomic regulation, where activities in the two autonomic branches would be expected to be negatively correlated. Although CAB may characterize individual differences in patterns of autonomic control in some contexts and conditions, autonomic control may deviate from a bipolar model. Consequently, the alternative metric CAR may reflect distinct aspects of autonomic regulation.
In contrast to CAB, but consistent with its derivation, CAR is positively correlated with both parasympathetic and sympathetic control. CAR is negatively correlated with heart rate, in keeping with the relative predominance of parasympathetic control of the heart under basal (sitting) conditions (e.g., Cacioppo et al., 1994). This is also the probable basis for the observed negative correlation between CAR and LF/HF ratio. As expected, there was no significant correlation between CAB and CAR (see Table 3).
Table 4 illustrates the values of autonomic parameters for high and low levels of CAB and CAR. As expected from its derivation, individuals with high CAB showed higher parasympathetic cardiac control (as indexed by higher HF values), lower levels of sympathetic control (as indexed by longer PEP values), lower heart rate and lower LF/HF ratio. Based on the derivation of CAR, individuals with high CAR displayed a distinctly different pattern. High CAR subjects showed higher parasympathetic control (as indexed by higher HF values) and higher sympathetic control (as evidenced by lower PEP values) as well as lower LF/HF ratios. They also showed lower heart rate, consistent with the finding that parasympathetic control is typically greater than sympathetic control under resting conditions.
Table 4.
Autonomic Measures in Individuals with high and low (median split) CAR and CAB
| CAR | CAB | HR | MAP | PEP | HF | LF | LF+HF | LF/HF | |
|---|---|---|---|---|---|---|---|---|---|
| CAR | |||||||||
| Low | −.78(.04) | 07(.15) | 67.47(1) | 91.9(1) | 111.4(1.2) | 4.42(.1) | 5.41(.1) | 9.83(.2) | 1.26(.03) |
| High | .78(.06) | −.08(.13) | 62.74 (.8) | 89.8(1) | 94.58(1.1) | 5.53(.1) | 5.93(.1) | 11.5(.2) | 1.09(.02) |
| CAB | |||||||||
| Low | .02(.10) | −1.2(.09) | 68.96(.9) | 91.06(1.1) | 94.78(1.2) | 4.26(.1) | 5.23(.1) | 9.53(.2) | 1.27(.03) |
| High | −.02(.08) | 1.1 (.08) | 61.26(.9) | 90.62(1.1) | 111.0(1.1) | 5.68(.1) | 6.07(.1) | 11.8(.1) | 1.08(.01) |
Relation to Health Status
CAR, but not CAB, was significantly correlated with the overall SF-36 index of General Health (r(220) = .15, p = 0.02). This finding was followed up in regression analyses, which revealed that CAR (but not CAB) predicts SF-36 General Health after controlling for demographics and BMI (R2 change = .04, Beta = .20, t (206) = 2.76, p = .006). In analyses of SF-36 subscales, net of demographics and BMI, CAR was a significant predictor of Physical Health (R2 change = .02, Beta = .15, t (193) = 2.12, p = .03) and Pain (R2 change = .02, Beta = .16, t (206) = 2.25, p = .03), but not Mental Health. CAR, but not CAB, is also an inverse predictor of the total number of adverse health items endorsed in the health questionnaire, controlling for demographics and BMI (R2 change = .04, p = .006. Beta = −.20, t (194) = 2.80, p = .006).
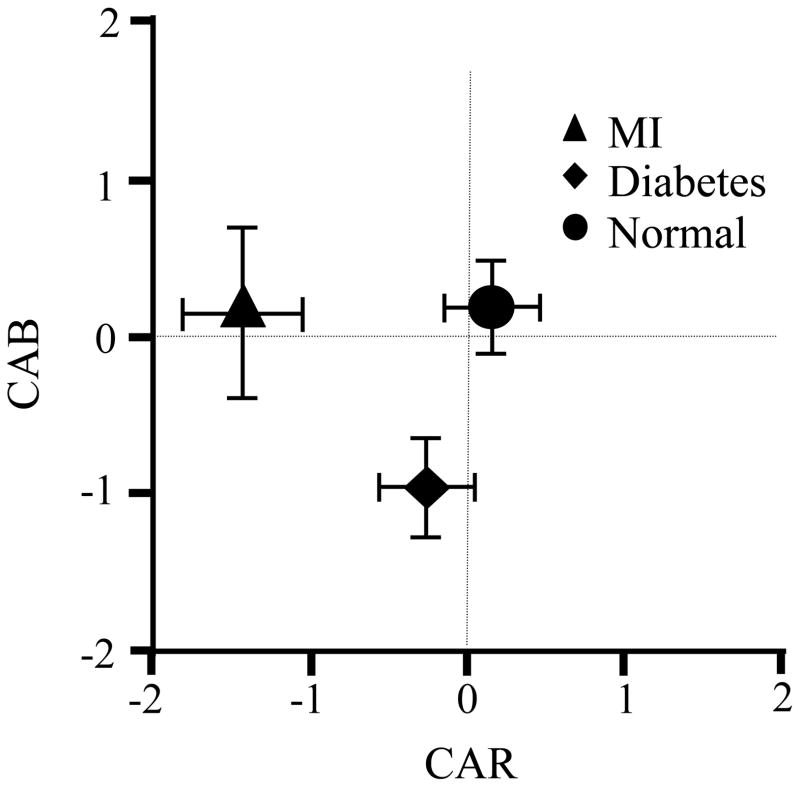
The relations between CAB, CAR and specific health conditions were further evaluated in correlational (point biserial) analyses, which revealed that the presence of diabetes and the prior occurrence of a MI were associated with distinct patterns of autonomic control. As illustrated in Figure 1 and Table 5, diabetes was negatively correlated with CAB, consistent with the literature which suggests a hyper-sympathetic state and diminished parasympathetic control in this condition. This is also consistent with the individual measures of parasympathetic (HF) and sympathetic (PEP) cardiac control, and with the significant correlation with higher heart rate. The LF/HF ratio has been previously proposed as a measure of autonomic balance, although it has been challenged on both empirical and conceptual grounds (Berntson & Cacioppo, 1999; Eckberg, 1997). The LF/HF ratio was correlated with CAB, as well as with CAR, but it was not significantly associated with the occurrence of diabetes.
Table 5.
Autonomic Variables and Health Status
| CAR | CAB | HR | MAP | PEP1 | HF | LF | LF+HF | LF/HF | SF-36 Health | |
|---|---|---|---|---|---|---|---|---|---|---|
| Means (SE) | ||||||||||
| MI (n=11) | −1.5 (.3) | .14 (.6) | 67.8 (3.3) | 91.9 (3.2) | 114.8 (4.4) | 4.1 (.4) | 4.9 (.3) | 9.04(.6) | 1.3 (.10) | |
| Diabetes (n=40) | −.25(.3) | −.92(.2) | 71.3 (1.9) | 93.8 (1.8) | 98.7 (2.2) | 4.4 (.2) | 5.1 (.2) | 9.48(.4) | 1.2 (.05) | |
| Neither (n=178) | .06 (.1) | .12(.1) | 64.2 (.70) | 90.0 (.8) | 103.2 (1.1) | 5.1 (.1) | 5.8 (.1) | 10.97(.1) | 1.1 (.02) | |
| Correlations | ||||||||||
| MI (r) (n=11) | −.26** | .06 | .02 | .01 | −.19* | −.14* | .06 | −.15*** | .06 | .18** |
| Diabetes (r) (n=40) | −.06 | −.29** | .27** | .12 | .15* | −.25** | −.25** | −.28** | .09 | −.21** |
Higher sympathetic control would be reflected in lower PEP values
p < .01
p < .01
p < .01
As is apparent in Table 5, a different pattern of autonomic control was seen in individuals who had a prior MI. MI was negatively correlated with CAR, but was not significantly related to CAB. This is consistent with the suggestion that overall autonomic control of the heart may be a positive stratifier for cardiac health (Tsuji et al., 1996; Liao et al., 1997; Hemingway et al., 2005). Indeed, there was a negative association between MI and parasympathetic as well as sympathetic cardiac control (see Table 5). Although high sympathetic control in isolation may be a risk factor, concurrent parasympathetic control may serve as a synergistic positive health buffer, and may be more advantageous than minimal autonomic control from either branch.
ANCOVA and Regression Analyses
Additional analyses were pursued to further examine the relationship between CAB and CAR and health status, as well as potential mediators of these relations.
MI
Analysis of Co-Variance (ANCOVA), with age and gender held constant, revealed significant group differences in CAR for MI vs. non-MI participants, with MI being associated with lower CAR (MI = −.51 +/− .08 vs. non-MI = .02 +/− .03; F(1,223) = 7.41, p = .007).
A logit regression revealed that CAR was a significant negative predictor of MI, with demographics (age, gender, marital status, education and income) held constant. For every one unit increase in CAR, the odds of MI (vs. non-MI) decreased by a factor of .11 (Beta = −.2.2, Wald = 5.66, p = .02). Moreover, CAR continued as a significant predictor even after the prior entry of HF and PEP, the metrics from which it was derived. In contrast, CAB, LF/HF and HR were not significant predictors.
Diabetes
ANCOVA, with age and gender held constant, revealed significant group differences in CAB for diabetes vs. non-diabetes comparisons (diabetes = −.97 +/− .22 vs. nondiabetics = .17 +/− .10; F(1,223) = 19.06, p = <.001).
A logit regression revealed that CAB was a significant negative predictor of diabetes, with demographics (age, gender, marital status, education and income) held constant. Low CAB (high sympathetic and low parasympathetic cardiac control) is more likely to characterize individuals with diabetes. For every one unit increase in CAB, the odds of diabetes decreased by a factor of .61 (Beta = −.50, Wald = 9.06, p = .003). CAB continued as a significant predictor even after the prior entry of HF and PEP, the metrics from which it was derived. In contrast, neither LF/HF nor CAR was a significant predictor of diabetes.
This result is in keeping with the literature suggesting exaggerated sympathetic and diminished parasympathetic control in diabetes. This was further confirmed by ANCOVA analyses (with age and gender held constant), which revealed significantly higher sympathetic control (as indexed by lower PEP) in diabetes (see Table 5; F(1,226) = 5.68, p = .02). In addition, there was significantly lower parasympathetic control (as indexed by lower HF) in diabetics (see Table 5; F(1,226) = 12.91, p < .001). Both of these findings are also consistent with the higher heart rates in diabetics (see Table 5; F(1,229) = 15.94, p = <.001).
Two participants had both diabetes and a prior MI. Although this was not a sufficient sample for statistical analysis, consistent with the findings above, these participants had both low overall cardiac regulatory capacity (CAR = −0.62 +/− 0.28 SE, relative to the overall mean of zero), as well as a low (sympathetic) autonomic balance (CAB = −2.12 +/− 0.29 SE, relative to the overall mean of zero). Thus they fit both predictive autonomic patterns.
Discussion
The present study found that two distinct models of autonomic control may tap into patterns of regulation that relate differentially to health and disease. The classic bipolar concept of autonomic balance (Cardiac Autonomic Balance or CAB) was instantiated as the normalized value of parasympathetic cardiac control (HF) minus the normalized value of sympathetic cardiac control (-PEP). In contrast, a model of total autonomic regulation (Cardiac Autonomic Regulation or CAR) was quantified as the total normalized parasympathetic value plus the normalized sympathetic value.
CAR, but not CAB, was a significant predictor of overall health status as measured by the SF-36 General Health, net of demographics and BMI. High parasympathetic activity is generally considered to reflect a positive health state or reduced health risk (Masi et al., 2007; Thayer & Sternberg, 2006; Kleiger et al., 2005; Malik 1996; Porges, 2006), whereas high sympathetic activity can be a clear risk factor (Airaksinen, 1999; Billman, 2006a, b; Schwartz et al., 1992; Hohnloser, 2005). High parasympathetic control, however, may serve to buffer the health consequences of sympathetic activity. This is in keeping with the observations that high overall heart rate variability including high LF heart rate variability (which includes both sympathetic and parasympathetic contributions) is a predictor of positive outcome after MI (Kleiger et al., 2005; Malik 1996; Tsuji et al., 1996; Liao et al., 1997). According to this view, high overall heart rate variability may index a high cardiac regulatory capacity (in both sympathetic and parasympathetic branches), which may have positive health benefits. In contrast, low overall heart rate variability has been reported to be a risk factor for all cause mortality and morbidity (Dekker et al., 1997; Gang & Malik, 2003).
Consistent with this view, the measure of total cardiac regulatory capacity (CAR) was negatively associated with the prior incidence of MI, and that association continued after holding constant demographics, BMI, and cardiovascular medications. As expected, PEP (reflecting high sympathetic cardiac control) was a positive predictor of the prior occurrence of an MI, but accounted for less than half of the variance accounted for by CAR. The negative relationship between HF and MI also likely contributed to the predictive power of CAR. However, CAR continued as a significant predictor of MI even after HF and PEP were held constant in the regression analysis. This indicates that the CAR metric may reflect a physiological state that is more relevant to health than the independent sympathetic or parasympathetic controls, or the autonomic balance between these controls as indexed by CAB (or LF/HF ratio). Because the highest values of CAR were associated with high sympathetic control, which is itself a risk factor for MI, the associated high parasympathetic control may serve to buffer the sympathetic risk.
The question arises as to whether a prior MI may result in a chronic decrease contractility and thereby prolong PEP and lower the estimate of sympathetic cardiac control, independent of sympathetic autonomic regulation. Participants with prior MIs, however, also showed lower LF heart rate variability which includes both sympathetic and parasympathetic control, as well as lower HF and total heart rate variability (with no change in the LF/HF ratio). In addition, the heart rates of MI participants did not differ from normal subjects (see Table 5). Together, these findings suggest a more generalized reduction in cardiac sympathetic as well as parasympathetic control, based on measures of sinoatrial function that are not sensitive to variations in contractility. Moreover, the finding that CAR predicts overall health status (SF-16), and continues to do so even after the MI participants are excluded, suggests that the health relevance of CAR is not a simple artifact of myocardial disease. Nevertheless, a possible contribution of the disease state to the obtained metrics remains a possibility and that is a limitation of cross-sectional studies. One approach to this issue is through prospective studies, in which the autonomic patterns of regulation can be identified before the development of clinical pathology. This issue will be further addressed in future years, where longitudinal, prospective analyses can be pursued with the CHASRS population.
An additional limitation of the present study is the relatively small number of subjects in the MI group. The fact that significant predictions could be obtained, despite this small sample size, suggests that the CAR metric may offer an important experimental tool. Additional MIs are likely to be seen as this population is followed over subsequent years, and the ability of CAR to predict the occurrence of MI prospectively will be important to demonstrate.
In contrast to MI, diabetes was more closely related to a pattern of high sympathetic and low parasympathetic activity that was statistically captured by the autonomic balance metric, CAB. This is in keeping with the existing literature on autonomic regulation and diabetes (Perin et al., 2001; Tentolouris et al., 2006; Lautt et al., 2001; Masi et al., 2007; Fliers et al., 2003; Ahren et al., 1999; Lautt et al, 2004). Together, these findings support the suggestion that different patterns or dimensions of autonomic regulation may differentially impact, or be impacted by, health status. As discussed in the introduction, the literature does offer a rationale for how these autonomic patterns may dispose toward specific aspects of health or disease. It is not possible, however, to infer cause and effect in a cross sectional study. It may very well be the case that the disease states contribute to the autonomic patterns, or that there is a reciprocal interplay between diseases and the pattern of autonomic control. An example of the latter comes from the literature on diabetes, wherein reduced parasympathetic control can promote insulin resistance and hyperglycemia and hyperglycemia can contribute to autonomic neuropathies that may further compromise parasympathetic control (see Lautt, 1999, 2004).
Again, one approach to the cause and effect issue would be through longitudinal studies to determine whether specific autonomic patterns antedate the onset of particular disease states and whether they can predict the occurrence of those states. These studies will be pursued as the CHASRS study is ongoing, and longitudinal data will ultimately become available.
In summary, autonomic measurement models impose constraints on the kind of data representations we analyze, and an inappropriate model may not organize empirical findings to optimally reveal lawful relations. The bipolar autonomic balance model, for example, may be useful in some contexts (e.g. with orthostatic reflexes) and relevant to some health conditions (e.g. diabetes). It is not a comprehensive model, however, and may not afford capture autonomic patterns, such as coactivation and autonomic regulatory capacity, which may appear in other contexts (e.g. psychological stressors) and other disease states (such as MI). The availability of quantitative metrics of both CAB and CAR may provide important tools in the study of psychosomatic relations.
Figure 3.
CAR and CAB in disease states. Data points illustrate means and standard errors of CAR and CAB as a function of participant group. Compared to other participants, subjects with a prior myocardial infarction (MI) had lower CAR scores, indicating lower overall cardiac regulatory capacity, but were not highly deviate on CAB. In contrast, those with diabetes showed a lower CAB score, reflective of a predominant sympathetic balance, but were not highly deviant on CAB.
References
- Ahren B. Autonomic regulation of islet hormone secretion--implications for health and disease. Diabetologia. 2000;43:393–410. doi: 10.1007/s001250051322. [DOI] [PubMed] [Google Scholar]
- Ahren B, Sauerberg P, Thomsen C. Increased insulin secretion and normalisation of glucose tolerance by cholinergic agonism in high-fat fed C57BL/6 J mice. American Journal of Physiology. 1999;277:E93–E102. doi: 10.1152/ajpendo.1999.277.1.E93. [DOI] [PubMed] [Google Scholar]
- Airaksinen KE. Autonomic mechanisms and sudden death after abrupt coronary occlusion. Annals of Medicine. 1999;31:240–5. doi: 10.3109/07853899908995886. [DOI] [PubMed] [Google Scholar]
- Aubert AE, Ramaekers D. Neurocardiology: the benefits of irregularity. The basics of methodology, physiology and current clinical applications. Acta Cardiologica. 1999;54:107–20. [PubMed] [Google Scholar]
- Balanescu S, Corlan AD, Dorobantu M, Gherasim L. Prognostic value of heart rate variability after acute myocardial infarction. Medical Science Monitor. 2004;10:CR307–15. [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, Van der Molen MW. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT. Heart rate variability: A neuroscientific perspective for further studies. Cardiac Electrophysiology Review. 1999;3:279–282. [Google Scholar]
- Berntson GG, Cacioppo JT. Integrative physiology: Homeostasis, allostasis and the orchestration of systemic physiology. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. 3. Cambridge, UK: Cambridge University Press; 2007. pp. 433–452. [Google Scholar]
- Berntson GG, Cacioppo JT, Binkley PF, Uchino BN, Quigley KS, Fieldstone A. Autonomic cardiac control: III. Psychological stress and cardiac response in autonomic space as revealed by pharmacological blockades. Psychophysiology. 1994;31:599–608. doi: 10.1111/j.1469-8986.1994.tb02352.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Autonomic Determinism: The modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychological Review. 1991;98:459–487. doi: 10.1037/0033-295x.98.4.459. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Respiratory sinus arrhythmia: Autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology. 1993a;30:183–196. doi: 10.1111/j.1469-8986.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Cardiac psychophysiology and autonomic space in humans: Empirical perspectives and conceptual implications. Psychological Bulletin. 1993b;114:296–322. doi: 10.1037/0033-2909.114.2.296. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS, Fabro VJ. Autonomic space and psychophysiological response. Psychophysiology. 1994;31:44–61. doi: 10.1111/j.1469-8986.1994.tb01024.x. [DOI] [PubMed] [Google Scholar]
- Billman GE. A comprehensive review and analysis of 25 years of data from an in vivo canine model of sudden cardiac death: implications for future anti-arrhythmic drug development. Pharmacology & Therapeutics. 2006a;111:808–35. doi: 10.1016/j.pharmthera.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Billman GE. Heart rate response to onset of exercise: evidence for enhanced cardiac sympathetic activity in animals susceptible to ventricular fibrillation. American Journal of Physiology: Heart & Circulatory Physiology. 2006b;291:H429–35. doi: 10.1152/ajpheart.00020.2006. [DOI] [PubMed] [Google Scholar]
- Burleson MH, Poehlmann KM, Hawkley LC, Ernst JM, Berntson GG, Malarkey WB, Kiecolt-Glaser JK, Glaser R, Cacioppo JT. Neuroendocrine and cardiovascular reactivity to stress in mid-aged and older women: Long term temporal consistency of individual differences. Psychophysiology. 2003;40:358–369. doi: 10.1111/1469-8986.00039. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Binkley PF, Quigley KS, Uchino BN, Fieldstone A. Autonomic cardiac control. II. Basal response, noninvasive indices, and autonomic space as revealed by autonomic blockades. Psychophysiology. 1994;31:586–598. doi: 10.1111/j.1469-8986.1994.tb02351.x. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Rickett EM, Masi CM. Sociality, spirituality, and meaning-making: Chicago health, aging, and social relations study (CHASRS) Review of General Psychology. 2005;9:143–155. [Google Scholar]
- Cacioppo JT, Hughes ME, Waite LJ, Hawkley LC, Thisted RA. Loneliness as a specific risk factor for depressive symptoms: Cross sectional and longitudinal analyses. Psychology and Aging. 2006;21:140–151. doi: 10.1037/0882-7974.21.1.140. [DOI] [PubMed] [Google Scholar]
- Campfield LA, Smith FJ. Neural control of insulin secretion: interaction of norepinephrine and acetylcholine. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 1983;244:R629–R634. doi: 10.1152/ajpregu.1983.244.5.R629. [DOI] [PubMed] [Google Scholar]
- Campos RR, McAllen RM. Cardiac inotropic, chronotropic, and dromotropic actions of subretrofacial neurons of cat RVLM. American Journal of Physiology. 1999;276:R1102–1111. doi: 10.1152/ajpregu.1999.276.4.R1102. [DOI] [PubMed] [Google Scholar]
- Dekker JM, Schouten EG, Klootwijk P, Pool J, Swenne CA, Kromhout D. Heart rate variability from short electrocardiographic recordings predicts mortality from all causes in middle-aged and elderly men: the Zutphen Study. American Journal of Epidemiology. 1997;145:899–908. doi: 10.1093/oxfordjournals.aje.a009049. [DOI] [PubMed] [Google Scholar]
- Eckberg DL. Sympathovagal balance: a critical appraisal. Circulation. 1997;96:3224–32. doi: 10.1161/01.cir.96.9.3224. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacological Reviews. 2000;52:595–638. [PubMed] [Google Scholar]
- Eppinger H, Hess L. Vagotonia Ment Nerv Dis Monogr No 20. New York: Nervous & Mental Disease Publishing Company; 1915. [Google Scholar]
- Fliers E, Kreier F, Voshol PJ, Havekes LM, Sauerwein HP, Kalsbeek A, Buijs RM, Romijn JA. White adipose tissue: getting nervous. Journal of Neuroendocrinology. 2003;15:1005–1010. doi: 10.1046/j.1365-2826.2003.01096.x. [DOI] [PubMed] [Google Scholar]
- Fouad FM, Tarazi RC, Ferrario CM, Fighaly S, Alicandri C. Assessment of parasympathetic control of heart rate by a noninvasive method. American Journal of Physiology. 1984;246:H838–42. doi: 10.1152/ajpheart.1984.246.6.H838. [DOI] [PubMed] [Google Scholar]
- Fraser GE. A comparison of first event coronary heart disease rates in two contrasting California populations. Journal of Nutrition, Health and Aging. 2005;9:53–8. [PubMed] [Google Scholar]
- Friedman BH. An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biological Psychology. 2007;74:185–99. doi: 10.1016/j.biopsycho.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Friedman BH, Thayer JF. Anxiety and autonomic flexibility: a cardiovascular approach. Biological Psychology. 1998;49:303–23. doi: 10.1016/s0301-0511(98)00051-9. [DOI] [PubMed] [Google Scholar]
- Gang Y, Malik M. Heart rate variability analysis in general medicine. Indian Pacing and Electrophysiology Jorunal. 2003;3:34–40. [PMC free article] [PubMed] [Google Scholar]
- Goldberg LR. The development of markers for the BigFive factor structure. Psychological Assessment. 1992;4:26–42. [Google Scholar]
- Grossman P, Kollai M. Respiratory sinus arrhythmia, cardiac vagal tone, and respiration: within- and between-individual relations. Psychophysiology. 1993;30:486–495. doi: 10.1111/j.1469-8986.1993.tb02072.x. [DOI] [PubMed] [Google Scholar]
- Hayano J, Sakakibara Y, Yamada A, Yamada M, Mukai S, Fujinami T, Yokoyama K, Watanabe Y, Takata K. Accuracy of assessment of cardiac vagal tone by heart rate variability in normal subjects. American Journal of Cardiology. 1991;67:199–204. doi: 10.1016/0002-9149(91)90445-q. [DOI] [PubMed] [Google Scholar]
- Hemingway H, Shipley M, Brunner E, Britton A, Malik M, Marmot M. Does autonomic function link social position to coronary risk? The Whitehall II study. Circulation. 2005;111:3071–3077. doi: 10.1161/CIRCULATIONAHA.104.497347. [DOI] [PubMed] [Google Scholar]
- Hoehn-Saric R, McLeod DR. Anxiety and arousal: physiological changes and their perception. Journal of Affective Disorders. 2000;61:217–224. doi: 10.1016/s0165-0327(00)00339-6. [DOI] [PubMed] [Google Scholar]
- Hohnloser SH. Ventricular arrhythmias: antiadrenergic therapy for the patient with coronary artery disease. Journal of Cardiovascular Pharmacology and Therapeutics. 2005;10(Suppl 1):S23–31. doi: 10.1177/10742484050100i404. [DOI] [PubMed] [Google Scholar]
- Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical review? Medical Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- Kleiger RE, Stein PK, Bigger JT., Jr Heart rate variability: measurement and clinical utility. Annals of Noninvasive Electrocardiology. 2005;10:88–101. doi: 10.1111/j.1542-474X.2005.10101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautt WW. The HISS story overview: a novel hepatic neurohumoral regulation of peripheral insulin sensitivity in health and diabetes. Canadian Journal of Physiology and Pharmacology. 1999;77:553–562. [PubMed] [Google Scholar]
- Lautt WW. A new paradigm for diabetes and obesity: the hepatic insulin sensitizing substance (HISS) hypothesis. Journal of Pharmacological Science. 2004;95:9–17. doi: 10.1254/jphs.95.9. [DOI] [PubMed] [Google Scholar]
- Lautt WW, Macedo MP, Sadri P, Legare DJ, Reid MA, Guarino MP. Pharmaceutical reversal of insulin resistance. Proceedings of the Western Pharmacological Society. 2004;47:30–32. [PubMed] [Google Scholar]
- Lautt WW, Macedo MP, Sadri P, Takayama S, Duarte Ramos F, Legare DJ. Hepatic parasympathetic (HISS) control of insulin sensitivity determined by feeding and fasting. American Journal of Physiology: Gastrointestinal and Liver Physiology. 2001;281:G29–36. doi: 10.1152/ajpgi.2001.281.1.G29. [DOI] [PubMed] [Google Scholar]
- Liao D, Cai J, Rosamond WD, Barnes RW, Hutchinson RG, Whitsel EA, Rautaharju P, Heiss G. Cardiac autonomic function and incident coronary heart disease: a population-based case-cohort study: the ARIC Study: Atherosclerosis Risk in Communities Study. American Journal of Epidemiology. 1997;145:696–706. doi: 10.1093/aje/145.8.696. [DOI] [PubMed] [Google Scholar]
- Lozano D, Norman G, Knox D, Wood BL, Miller BD, Emery CL, Berntson GG. Where to B in dZ/dt. Psychophysiology. 2007;44:113–119. doi: 10.1111/j.1469-8986.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- Malik M. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Malliani A. Heart rate variability: from bench to bedside. European Journal of Internal Medicine. 2005;16:12–20. doi: 10.1016/j.ejim.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Malliani A, Montano N. Heart rate variability as a clinical tool. Ital Heart J. 2002;3:439–45. [PubMed] [Google Scholar]
- Manfrini O, Pizzi C, Trere D, Fontana F, Bugiardini R. Parasympathetic failure and risk of subsequent coronary events in unstable angina and non-ST-segment elevation myocardial infarction. European Heart Journal. 2003;24:1560–6. doi: 10.1016/s0195-668x(03)00345-2. [DOI] [PubMed] [Google Scholar]
- Masi CM, Hawkley LC, Rickett EM, Cacioppo JT. Respiratory sinus arrhythmia and diseases of aging: Obesity, diabetes mellitus, and hypertension. Biological Psychology. 2007;74:212–223. doi: 10.1016/j.biopsycho.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Hormones & Behavior. 1998;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- National Diabetes Information Clearinghouse. NIH publication 99–3926. Vol. 19. Bethesda, Md: National Institute of Diabetes and Digestive and Kidney Diseases; 1999. Diabetes Statistics. [Google Scholar]
- Pearl-Yafe M, Kaminitz A, Yolcu ES, Yaniv I, Stein J, Askenasy N. Pancreatic islets under attack: cellular and molecular effectors. Current Pharmaceutical Design. 2007;13:749–60. doi: 10.2174/138161207780249155. [DOI] [PubMed] [Google Scholar]
- Perin PC, Maule S, Quadri R. Sympathetic nervous system, diabetes, and hypertension. Clinical and Experimental Hypertension. 2001;23:45–55. doi: 10.1081/ceh-100001196. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romijn JA, Fliers E. Sympathetic and parasympathetic innervation of adipose tissue: metabolic implications. Current Opinion in Clinical Nutrition and Metabolic Care. 2005;8:440–444. doi: 10.1097/01.mco.0000172586.09762.55. [DOI] [PubMed] [Google Scholar]
- Rosenbloom AL. Obesity, Insulin Resistance, beta-Cell Autoimmunity, and the Changing Clinical Epidemiology of Childhood Diabetes. Diabetes Care. 2003;26:2954–2956. doi: 10.2337/diacare.26.10.2954. [DOI] [PubMed] [Google Scholar]
- Schwartz P, La Rovere M, Vanoli E. Autonomic nervous system and sudden cardiac death: experimental basis and clinical observations for post-myocardial infarction risk stratification. Circulation. 1992;85 (Suppl 1):177–191. [PubMed] [Google Scholar]
- Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, Van Doornen LJ. Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27:1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- Singh JP, Larson MG, Tsuji H, Evans JC, O’Donnell CJ, Levy D. Reduced heart rate variability and new-onset hypertension: insights into pathogenesis of hypertension: the Framingham Heart Study. Hypertension. 1998;32:293–7. doi: 10.1161/01.hyp.32.2.293. [DOI] [PubMed] [Google Scholar]
- Sterling P, Eyer J. In: Handbook of Life Stress, Cognition and Health. Fisher S, Reason J, editors. New York: John Wiley Ltd; 1988. p. 631. [Google Scholar]
- Sterling P, Eyer J. Biological basis of stress-related mortality. Social Science & Medicine. 1981;15:3–42. doi: 10.1016/0271-5384(81)90061-2. [DOI] [PubMed] [Google Scholar]
- Tentolouris N, Liatis S, Katsilambros N. Sympathetic system activity in obesity and metabolic syndrome. Annals of the New York Academy of Sciences. 2006;1083:129–52. doi: 10.1196/annals.1367.010. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Sternberg E. Beyond heart rate variability: vagal regulation of allostatic systems. Annals of the New York Academy of Sciences. 2006;1088:361–72. doi: 10.1196/annals.1366.014. [DOI] [PubMed] [Google Scholar]
- Thom T, editor. Morbidity and Mortality: 2004 Chart Book on Cardiovascular, Lung and Blood Diseases. Bethesda: National Heart, Lung, and Blood Institute; 2004. [Google Scholar]
- Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. Journal of Clinical Investigation. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji H, Larson MG, Venditti FJ, Jr, Manders ES, Evans JC, Feldman CL, Levy D. Impact of reduced heart rate variability on risk for cardiac events: the Framingham Heart Study. Circulation. 1996;94:2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36): I. Conceptual Framework and Item Selection. Medical Care. 1992;30:473–483. [PubMed] [Google Scholar]
- Wenger MA. Studies of autonomic balance: a summary. Psychophysiology. 1966;2:173–86. doi: 10.1111/j.1469-8986.1966.tb02641.x. [DOI] [PubMed] [Google Scholar]