Abstract
Signal transduction across biological membranes is central to life. This process generally happens through communication between different domains and hierarchical coupling of information. Here, we review structural and thermodynamic principles behind transmembrane (TM) signal transduction and discuss common themes. Communication between signaling domains can be understood in terms of thermodynamic and kinetic principles, and complex signaling patterns can arise from simple wiring of thermodynamically coupled domains. We relate this to functions of several signal transduction systems: the M2 proton channel from influenza A virus, potassium channels, integrin receptors, and bacterial kinases. We also discuss key features in the structural rearrangements responsible for signal transduction in these systems.
Keywords: signal transduction, allostery, transmembrane signaling, receptor, ion channel
INTRODUCTION
In this review, we explore how the binding of ligands to proteins induces functional conformational changes. Ligand-induced conformational changes are fundamental to many aspects of protein function; for example, when an enzyme binds its substrate, the act of binding induces changes in the protein's structure and dynamics to enable catalysis (1–3). Thus, it is often difficult to separate binding from catalysis. Similarly, binding of an allosteric ligand can lead to the propagation of conformational changes over long distances. We focus primarily on the generation and transmission of conformational changes in membrane proteins; we first introduce the types and limits of coupled, ligand-induced conformational changes. Next, we examine in quantitative terms the effects of different coupling mechanisms.
We then explore how ligand binding affects the structures and functions of various membrane proteins. We compare how the binding of multiple protons and potassium ions to the M2 proton channel of influenza A and the potassium channel, respectively, is used to generate high specificity, to organize the conduction pathway, and to orchestrate functionally essential large-scale conformational changes. We then explore the mutual influence and signaling between the transmembrane (TM) domains and other membrane-associated or water-soluble domains or proteins in the context of these channels, bacterial histidine kinases, and integrins, exploring the mechanisms by which multiple proteins, small molecules, metal ions, phospholipids, and posttranslational modifications influence their activation and signaling.
COUPLING OF LIGAND BINDING AND ENVIRONMENT TO CONFORMATION
Ligand binding generally leads to changes in both structure and dynamics, with increases and decreases in dynamics often occurring at different sites of the same protein (4). The effect of ligand binding on protein activity can be viewed in light of classical thermodynamic concepts of allostery, induced fit or conformational selection, and cooperativity (1–6). For simplicity, we consider a two-state transition between an active and a resting state. The experimentally observed change in activity depends on several factors including: (a) the free-energy difference or “energy gap” between the active and resting states, which determines the fraction of the protein in the activated state in the absence of the ligand; (b) the affinity of the protein for the ligand and their concentrations; and (c) the ability of the ligand to induce a transition when fully bound. Less than complete activation occurs, even when the ligand is fully bound if it binds to both the activated and the resting conformations. In this case, the extent of activation relates to the relative affinity of the ligand for the activated versus resting state. These concepts of coupled equilibria are well studied and understood. Relating these to such phenomena as partial activation, potentiation, and synergistic ligands is a simple matter of algebraic book keeping, as shown below.
In the next section, we explore in more quantitative terms how these simple binding phenomena lead to a variety of activation curves when there are multiple ligands capable of activating the protein of interest. In such cases, distinct ligand-binding sites at different locations can “couple” to give a variety of outcomes; the binding of one can alter the affinity of the other for the protein, and they can have additive or nonadditive effects on the degree of maximal activation upon ligand saturation.
The coupling of binding and functional transitions refers to the phenomenon by which the binding of a ligand to one site influences the structure, dynamics, and binding affinity at a second site, which relates to the protein's energy gap and the ratio of affinities of the regulatory ligand for the activated versus the resting states. Given these simple requirements, it is not surprising that coupling can be achieved in different ways. One special case is when the binding of a ligand induces a change in association state. When no change in association is observed, it is often implicitly assumed that signaling occurs via “hard connections” in which helices shift, tilt, or change register, mechanically driving a conformational change that propagates across a bilayer. A second model involves communication between subunits, which can entail large-scale or subtle changes in interactions between abutting domains or ones connected by flexible linkers. These different modes of communication can lead to different outcomes when one measures the effects of mutations at positions between the binding sites. For example, if a domain shifts as a rigid body, the search for a “pathway” from one location to the other might give rise to different results than if the transition of structural and dynamic information occurs by correlated motions of side chains that propagate in a two-state manner between the two sites.
Finally, both the magnitude as well as the sign of the energy gap of a TM protein depend on the membrane environment. Any shift in structure that changes the exposure of specific residues or the width (cross-sectional surface area) of a protein at a given position in the membrane will make the energy gap sensitive to the physical properties of the bilayer. Hence, mechanosensitive channels switch on in response to changes in bilayer tension (7). Potassium channels respond to the TM electrical potential, and the location and coupling of cytoplasmic loops and helices change in response to the fraction of phosphoinositides, sphingomyelin, and cholesterol in the bilayer (8–11). Indeed, the hydrophobic length of TM helices is reflected in the width of the bilayers in the organelles to which they localize. Additionally, once located in a given membrane, there is tight coupling between the lipid environment and the conformation of the protein as it, for example, partitions into cholesterol/sphingomyelin-rich phases (12, 13).
SIMPLE MODELS FOR ENERGETIC COUPLING
Energy Gap
It is convenient to think of functional proteins as having at least two thermodynamic states—a resting state and an active state. The free-energy gap between these, ΔGgap = Gactive – Ginactive (where Gactive and Ginactive are the free energies of the active and inactive states, respectively), determines the equilibrium fraction of active protein as . R is the gas constant, and T is the absolute temperature. Because the largest possible fraction of the protein in the active state is 1, this also determines the maximal attainable degree of activation owing to binding of any ligand or perturbation, as A = 1 + eΔGgap/RT. For example, if ΔGgap is 0.41 kcal/mol, then at room temperature, the background fraction of the active state is 1/3. Therefore, even the best activating agent can only result in a threefold increase in activity. However, if ΔGgap is 6.82 kcal/mol, the maximal degree of activation is 105.
A ligand causes activation when it binds with higher affinity to the active relative to the inactive state (see Figure 1a,b). If KA and KI are the dissociation constants for the ligand binding to the active and inactive states, respectively, then the ligand is activating if KA < KI. Moreover, the fraction KI/KA determines the activating potential of the ligand. When the system is saturated with ligand, the free-energy difference between active and inactive states becomes: . For an ideal activating ligand, KI approaches infinity, such that ligand saturation leads to complete activation, and the degree of maximal activation (relative to background) is only limited by the intrinsic ΔGgap. If a ligand binds to both states, the maximum degree of activation is , and is limited by both the intrinsic ΔGgap and the ratio of equilibrium binding constants KI/KA (see Figure 1b). For a given protein (and a fixed ΔGgap), this ratio of equilibrium binding constants could differ for different ligands, leading to varying degrees of activation upon ligand saturation. The specifics of a biological process determine how large ΔGgap needs to be and how it is modulated by binding. Large ΔGgap and KI/KA lead to a high degree of maximal activation (Figure 1b). A large ΔGgap is also useful in situations with multiple effectors that act in combination.
Figure 1.
Simple linked-equilibrium models. (a) A thermodynamic cycle illustrating the coupling between activation of a two-state protein and ligand binding. (b) Activation profiles of a two-state protein as a function of an agonist ligand concentration. Ratio of dissociation constants for the inactive and active states, KI/KA, and the intrinsic energy gap, ΔGgap, determine maximal attainable activation. (c) A diagram of a simple signal-transduction molecule. Two domains, a receptor domain and a cytoplasmic functional domain, each with two states, are thermodynamically coupled, with the receptor modulated by an activating ligand. All eight states of the system are explicitly shown. Free-energy differences between ligand-bound states (back side of the cube) are omitted for simplicity, but follow simply from thermodynamic cycles. L indicates the concentration of the cytoplasmic ligand. In a and c, equilibrium free-energy differences correspond to the direction of the arrow with which they are shown. (d ) Activation profiles of the system in panel c as a function of the energy gap of the cytoplasmic domain, , and coupling free energy of the receptor to the cytoplasmic domain, . KA and KI are set to 10 nM and 20 μM, respectively, and the energy gap of the receptor domain, , is set to 3.0 kcal/mol. The effective energy gap for this system can be shown to be . Thus, if is zero (i.e., there is no coupling between the receptor and cytoplasmic domains), the overall gap is just , but as long as is negative (i.e., activation of the receptor promotes the active state of the functional domain), higher values of contribute to a higher overall energy gap. Similarly, tighter coupling (lower ) leads to a higher overall gap and larger degree of maximal activation.
Environment
ΔGgap can be modulated by environmental variables, such as the membrane composition. Although it may be possible to treat membrane effects as ensembles of binding sites, we treat them as environmental factors modulating ΔGgap in the simplest case. For example, voltage-dependent potassium channels exist in equilibrium between “on” and “off” conformations that differ not only in their conductance characteristics, but also in the number of charged groups on the two sides of the electrically impermeable membrane. The on-to-off conformational change involves the movement of 12 charged groups across the membrane, corresponding to ~0.28 kcal/mol/mV. Thus, abrupt switching occurs over a 10 mV increment in the membrane potential (14), such that if at resting state the population of active channel is 1%, a 10-mV change in potential leads to a 50-fold activation.
Coupling
The activation states of adjoining protein domains can be coupled. For example, once the receptor domain of a TM signaling protein shifts into its active state, this information is transmitted onto the functional cytoplasmic end of the molecule. This is sometimes referred to as allostery and is equivalent to stating that one end of the molecule is thermodynamically coupled to the other. This coupling depends on the specifics of the molecule's structure and dynamics but can be thought of as arising from differences in interdomain interaction free energies when in different states. This interdomain coupling or allostery can therefore be captured with a thermodynamic coupling free energy parameter, ΔΔGc, which determines the extent to which the different domains experience each other's states.1
Simple Model 1
Consider the simple model of a TM signaling molecule in Figure 1c. A two-state extracellular receptor domain, with an energy gap , receives input from a signaling ligand, which binds preferentially to the active state of the receptor. The receptor domain is thermodynamically coupled, via the coupling free energy parameter , to the cytoplasmic domain, which carries out the function of the system in its active state. The intrinsic activity of the cytoplasmic domain is controlled by its own energy gap, . Figure 1d shows how the activity profile of this system (e.g., level of activation as a function of ligand concentration) depends on and . Because the function of the overall system is based on the activity of the cytoplasmic domain, high values of its gap lead to low levels of background activity, thus more switch-like behavior (compare profiles at low and high in Figure 1c). However, because the two domains are coupled, the energy gap of the receptor domain also influences the level of activation. In fact, the energy gaps of the two domains can be thought of as jointly modulating the overall effective energy gap of the system, as shown in Figure 1c.
Simple Model 2
The TM domain may have two or more states, especially because many TM domains are dimeric, naturally leading to the possibility of multiple low-energy conformations or changes of local association. In Figure 2, we consider the case of the TM domain having its own active and inactive states, with the state of the receptor coupled to that of the TM, which is in turn coupled to the state of the cytoplasmic domain. Figure 2b illustrates how the activation profile depends on the new parameters in this model—the energy gap of the TM domain and its coupling to the cytoplasmic domain. Whereas larger energy gaps of the cytoplasmic domain always lead to larger overall energy gaps of the system (i.e., a larger degree of maximal activation), this is not necessarily the case for the energy gap of the TM domain (see Figure 2b). The effect of increasing can go in either direction, depending on the other parameters. Intuitively, this is because unlike , does not directly modulate the activity of the functional cytoplasmic domain, but rather does so through thermodynamic coupling. Increasing leads to lowering of the absolute active fraction of the cytoplasmic domain by virtue of the two domains being coupled. This serves to silence background activity in the resting state, whereas in the presence of an activating ligand, it limits the maximal fraction of active protein. The overall consequence depends on which of these two effects dominates. Because the TM domain lowers activity of the resting state, it may provide higher levels of activation compared to a system without a two-state TM domain (Figure 2b). This illustrates the advantage of having multiple loosely coupled domains—it allows for strong switching behavior without resorting to large energy barriers between active and inactive states. Intermediate domains also provide additional opportunities for modulation of the signal. Additional environmental conditions, such as the state of the membrane or the presence of specific ligands, can be integrated into the final output of the signaling system.
Figure 2.
A more complex model of a signaling molecule. (a) In addition to the receptor and functional cytoplasmic domains as in Figure 1, here the transmembrane (TM) domain is explicitly modeled as having two states. The intrinsic fraction of TM domain in the active state is defined by , and it is coupled to the cytoplasmic domain via . The 16 states of the system along with the corresponding free energies are shown in the table on the right. (b) The dependency of activation profiles on and . KA and KI are set to 10 nM and 20 μM, respectively, and values of other parameters are shown. The line with circles demonstrates the case where no two-state TM domain is present, and the receptor domain couples directly to the cytoplasmic domain with the same as in other models.
Simple Model 3
Extracellular and intracellular signals can be integrated by the same receptor. The VirA/VirG two-component system from Agrobacterium tumefaciens integrates multiple inputs with both its extracellular and cytoplasmic domains, including plant phenolic signaling molecules, monosaccharides, and pH (15). This can be modeled with the addition of a two-state cytoplasmic linker domain (Figure 3a). An intracellular ligand, such as a phenolic molecule, binds stronger to the active state than the inactive one (ligand C in Figure 3a), whereas a different ligand, such as a sugar, modulates the states of the receptor domain (ligand L in Figure 3a). Both the maximal activation as well as the concentration of half-maximal activity (IC50) change for one ligand in response to different concentrations of the other (Figure 3b). Such cumulative modulation of activity by both ligands effectively amounts to signal integration. It can be used by the cell to perform certain actions only in cases, for example, where both ligands are present at certain concentrations. In this case, both ligands were chosen to bind more tightly to the active states of their respective domains, but repressing signals can also modulate receptor function.
Figure 3.
A realistic model of signal transduction, inspired by the VirA protein of the VirA/VirG two-component system. (a) In addition to the domains and states in the model presented in Figure 2, there is a cytoplasmic linker domain, with its own active and inactive states, which is modulated by the signaling ligand C. A different ligand, L, modulates the receptor. The total number of states in this system is 64, and for clarity only the fully inactive and the fully active states are diagrammed. (b) Activation, relative to the resting state, is plotted against an increasing concentration of ligand L in the presence of different concentrations of C. This illustrates the ability to integrate signals from multiple ligands. Though this model does not explicitly treat the receptor as being dimeric (as is the case for the VirA protein), it can be easily extended to do so. In this case, the binding of a ligand may or may not be coupled with receptor dimerization, and there may exist cooperativity (negative or positive) between the two ligand-binding sites. Potential scenarios that can be explored include the following: Binding of a single ligand may push the system toward the active state, whereas binding two ligands symmetrically may favor the inactive state; alternatively, the receptor may signal only when two ligands are bound; or binding of the second ligand may proceed independently of the first one.
THERMODYNAMIC AND STRUCTURAL COUPLING UNDERLYING ION BINDING SPECIFICITY, CONDUCTION, AND GATING OF THE M2 PROTON CHANNEL AND K+ CHANNELS
In this section, we explore the mechanism by which environmental variables and permeant ion binding couple to the structural, dynamic, and functional properties of two protein families: (a) the K+ channels typified by KcsA and the voltage-gated K+ channels (16–18) and (b) viroporins typified by the M2 proton channel of the influenza A virus (19, 20). Despite large differences in overall structure, they share many similarities.
Both have dual gates to regulate ion diffusion into versus through the channel.
They achieve high selectivity by binding multiple copies of the permeant ions.
Motions of the gates are thermodynamically coupled.
Both M2 and K+ channels are blocked by positively charged hydrophobic alkylamines.
The structure and conduction properties of K+ channels have been extensively reviewed (16–18). However, the structural basis for the energetic coupling associated with the opening and closing of its two gates is only now becoming clear (21–34). Similarly, the structural mechanism of the activity of the M2 proton channel has progressed dramatically in recent years (35–40).
Biological Functions and Structural Biology of Influenza A Virus M2
M2 is a small multifunctional membrane protein, which is essential to the life cycle of the influenza A virus (41–44). This proton channel was originally discovered as the target of the antiviral channel-blocking drugs amantadine and rimantadine. The channel conducts protons into the interior of the virus entrapped within acidifying endosomes and is essential for the uncoating of the viral RNA from the matrix protein. M2 delays acidification of the late Golgi in some strains of the virus. Its channel activity and other independent functions are compartmentalized into remarkably compact sequences consisting of a short N-terminal region that is important for incorporation into the virion (45); a TM helix that is necessary and sufficient for tetramerization, drug binding, and proton channel formation (46–49); a C-terminal amphiphilic helix that is not required for channel formation or drug-binding (46), but is involved in membrane localization, budding, and scission (43); and a C-terminal tail that interacts with the matrix protein, M1 (50). Mutagenesis and electrophysiological measurements of full-length M2 in oocytes showed that drug-resistant mutations occur at pore-lining residues of the N-terminal portion of the TM helix (51–53). A recent crystal structure of the amantadine-bound M2 TM domain (37) showed electron densities in the N-terminal pore region. A secondary site on the outside of the protein can be observed when the drug is present at very high concentrations in micelles (38) or bilayers (19), but electrophysiological studies and the drug sensitivities of reverse-engineered viruses showed that this site does not contribute to the pharmacological inhibition of the channel (51–53).
The membrane-interactive domain of M2 is a 40-residue region (47) consisting of the TM pore-forming domain, followed by the basic amphiphilic helix (43, 54). The structure of the pore domain has been studied by X-ray crystallography (37, 40) and solid-state NMR (SSNMR) (19), resulting in structures of varying resolution. The highest-resolution structure is a 1.65-Å crystal structure (40) of the TM domain of M2 from micelles, which complements earlier structures obtained under different conditions at intermediate resolutions (2.0 to 3.5 Å) (37). This peptide has also been studied by aligned and magic-angle-spinning NMR (49, 55), culminating in a structure of the amantadine-peptide complex in phospholipid bilayers (19), with a resolution on par with that of solution NMR. Solution NMR (38, 56) and recent SSNMR studies (35) have focused on the structure of a fragment that includes the TM and the cytoplasmic helix. Although these studies are in reasonable agreement on the overall arrangement of the helices in the TM region, they place the cytoplasmic helices in different orientations; in the solution structure (56), the cytoplasmic helix forms a tetrameric bundle extending into the cytoplasm, whereas the SSNMR structure places the cytoplasmic helix against the C-terminal end of the bundle exposed to the head-group region of the bilayer (35). Extensive mutagenesis (43, 46, 54) has shown that the cytoplasmic helix contributes to the localization and budding of viruses; however, simultaneous mutation of five residues that mediate the interactions proposed to be formed by the cytoplasmic helix in the SSNMR or the solution structures have no discernable effect on the expression, insertion, conduction, selectivity, or drug binding of the full-length proteins (43, 46, 54). This is consistent with the fact that the cytoplasmic helical segment has a function distinct from channel formation and acts in a largely autonomous manner (43). A synthetic 16-residue peptide with this sequence alone is capable of inducing budding in giant unilamellar vesicles (43) and mutation of this sequence inhibited budding of transfected M2 protein in vivo.
Ion Binding and Flux through M2 and K+ Channels
The flux of ions through channels is very tightly regulated to prevent leakage, which would be disastrous for the life of an organism. For example, voltage-gated channels are tightly regulated over a very narrow range of TM electrical potential (16), and both the pH dependency of activation and the maximal conduction of the M2 channel are closely matched to not exceed the requirements for a given strain of virus to minimize toxicity to its host cell (51).
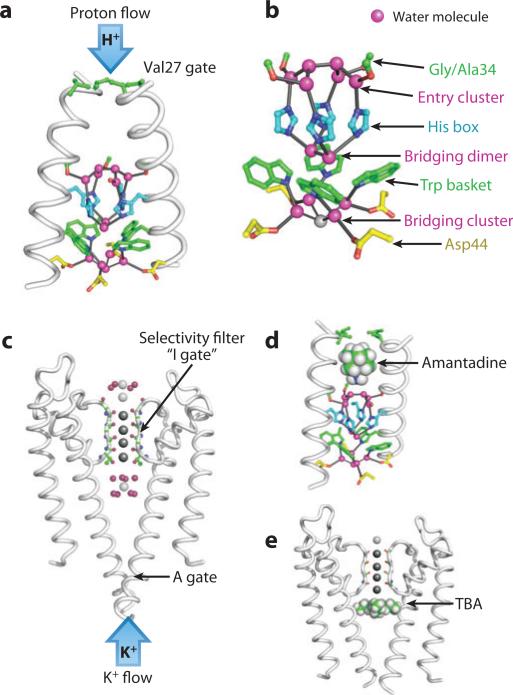
Figure 4 illustrates the ion-conducting pores of the K+ channel, KcsA (57, 58), in comparison with the M2 proton channel (40). In both, ions first encounter a hydrophobic gate formed by the coalescence of pore-lining helices; the activation or “A gate” of KcsA is in a closed conformation, while the Val27 gate of M2 is partially open. In KcsA, an ion next encounters a central aqueous cavity, followed by a selectivity filter with sites that can bind four ions, albeit not simultaneously. Finally, a water-lined cluster of water molecules marks the exit.
Figure 4.
The ion-conducting pores of M2 and KcsA. Both are drawn with their cytoplasmic domain oriented downward. The physiological flow of ions is inward (down) for M2 versus outward (up) for KcsA. (a) A high-resolution crystal structure of M2 [Protein Data Bank (PDB) code 3LBW] showing the overall structure, and (b) a blow-up of the ordered pore-lining side chains and water molecules. In all five panels, water molecules are shown as small magenta spheres, and the identity of the various side chains are annotated in panel b. (c) The structure of KcsA (PDB code 1K4C) with the potassium ions in the selectivity filter shown in dark gray. Additional potassium ions are shown in gray, and waters of hydration in magenta. (d and e) The structures of amantadine and tetrabutylammonium (TBA) bound to M2 and KcsA (PDB code 2HVK for the latter), respectively. The structure of M2 in panel d is from the solid-state NMR structure (2KQT) with the water from 3LBW docked in to show the approximate locations of water molecules.
At subsaturating ion concentrations, the rate of flux through the open form of a pore generally scales with the ion concentration on the side from which it is diffusing, multiplied by a second-order rate constant (16). Because ions can flow in both directions, a net flux of a given species is observed only in the presence of a TM chemical or electrical gradient. The second-order rate constant for diffusion of ions through K+ channels is near the diffusion-controlled limit (~108 M–1 s–1), resulting in rates approaching 108 ions/s under physiological electrochemical gradients (16). The selectivity filter determines the ability of K+ channels to exhibit such high rates while maintaining exquisite selectivity for K+ ions over Na+. Two potassium ions (separated by one molecule of water) can stably occupy the four binding sites at a given time, and they oscillate between “1 and 3” and “2 and 4” configurations with nearly equal occupancy (57, 59–62). The conducting conformation of the selectivity filter binds these K+ relatively tightly, with a dissociation constant about two orders of magnitude lower than that of the physiological ion concentration (59), leading to specific stabilization as in Figure 1a,b. The binding interaction with K+ provides a sufficiently strong driving force to induce a conformation featuring a convergent and periodic array of close-packed carbonyl groups (and the side chain of Thr75), creating four sites with nearly perfectly matched energetics in the 1 and 3 versus 2 and 4 configurations. Movement of a third K+ from a site in the aqueous cavity just below the selectivity filter (lower gray ion in Figure 4c) into the selectivity filter leads to a repulsion and rapid expulsion of an ion to the cellular exterior. The entire process takes place in approximately 10 ns, indicating the absence of high-energy barriers along the path. Thus, the negative cooperativity between two tight-binding sites and a third weak site provides both high selectivity as well as very rapid K+ diffusion. The site is also highly selective; when there is only Na+ in solution, the channel defaults to a nonconductive conformation, which is collapsed at sites 2 and 3. The theoretical basis for this process in terms of the relative contributions of electrostatics, hydration, sterics, and entropy remains an area of active investigation (63–67).
The M2 proton channel conducts protons with an overall second-order rate constant of about 107 to 108 M–1 s–1 near pH 6 (68–71). Given the very low proton concentration at this pH, this rate constant translates to a turnover rate of about 100 ions/s. Although seemingly slow, this rate is approximately that expected for the diffusion-controlled flux of a proton through a pore with the dimensions of the M2 channel (20, 68). The precise rate of proton flux through M2 varies in a sequence-dependent manner within the viral genomic context (51, 72, 73). The rate of proton flux through M2 reaches an upper limit at low pH (74, 75), indicating that a process other than proton diffusion into the channel becomes rate limiting when the diffusion of protons into the channel is rapid. Equilibrium studies show that M2 binds two protons per tetrameric channel at His37 with high affinity (76). The pKa of these two histidines is around 8 or approximately two orders of magnitude higher than the physiological pH under which the channel functions. Thus, binding of the first two protons specifically drives the conformational ensemble toward the conducting state (Figure 1a,b). The third pKa of His37 is near 6, matching the midpoint of the pH-proton flux profile (74, 75). In the shuttle model of proton conduction (77), the protein alternates between the +2 and +3 states, and the saturation of proton flux at low pH has been associated with the rate-limiting deprotonation of His37 (and any accompanying conformational changes). An order of magnitude estimate of the rate of deprotonation can be obtained on the basis of the above-mentioned fact that protons arrive at a rate of approximately 108 M–1s–1, and the third pKa of His37, which predicts an off rate of approximately 100 s–1 in good agreement with experimental values (68–71). M2 promotes diffusion of protons from the acidic environment of an endosome (pHout) into the viral interior (pHin), and electrophysiological measurements show that proton flux is greater with low pHout and high pHin than in the reverse situation for M2 from most strains of influenza A virus (70, 74, 75, 78). Trp41, which interacts with His37, is required for this asymmetric conductance (78). At longer times when pHin and pHout are both low, a low level of cation flux is observed, preventing the buildup of the electrical gradient, which would otherwise result as protons accumulate in the interior of the virus (71).
Structural studies of M2 in the neutral to +2 state provide an explanation for M2's asymmetrical proton flux. The high-resolution structure (1.65 Å of the protein in what appears to be the +2 state) shows a pore filled with cascading layers of water molecules, interleaved with amino acid side chains, which provide a pathway for Grotthuss diffusion of protons through “wires” of hydrogen-bonded waters and side chains (40). Protons gain access to the channel through the Val27 gate. Passing through a region of disordered water, a proton arrives at an “entry cluster” of water molecules, consisting of a triangular prism of six waters whose square base is anchored through hydrogen bonds to the Nδ of His37 and the carbonyl oxygen of Gly34 (Ala34 in the crystallized mutant). The His residues engage in a “His-box” interaction similar to aromatic boxes, with adjacent rings interacting in an edge-face manner. There is no direct hydrogen bonding between the imidazoles, but instead, they are connected via a highly structured network of water molecules.
On the cytoplasmic side of the His box, its Nε nitrogens bind a tight “bridging” dimer of water molecules that are well situated to mediate a π-cation interaction with the electron-rich rings of Trp41 residues. The Trp41 rings are stabilized in a basket-like structure via a third cluster of water molecules, which bridges the indole NH of Trp41 with the carboxylate of Asp44 and completes the path to the viral interior. The basic arrangement of the His box was first predicted in a model of the protein based on mutagenesis of the pore-forming region (77), and it is also seen in the solution NMR structures (38) as well as in a high-resolution SSNMR structure of the amantadine complex (19). An alternate orientation of the His residues has been suggested, but this arrangement was based on an SSNMR model built exclusively from measurements of the orientations of backbone amides, which did not incorporate any experimental distance restraints or information concerning the geometric arrangement of side chains (35). In most structures, the Trp41 basket is well oriented to inhibit reverse flow of protons out of an acidified virus.
Based on their high pKas (8), the binding of the first two protons to His37 thermodynamically stabilizes the His37 box in much the same way that binding of the first two K+ ions stabilize the selectivity filter of KcsaA, whereas binding of a third permeant ion occurs with a lower affinity (pKa ≈ 6) and increases the lability of the protons attached to the His box. SSNMR studies show that this third protonation event increases the dynamics of His37 and the hydrogen-bonding of its Nε to water molecules on the cytoplasmic side of the His box (36). Solution NMR studies show increased mobility of the overall structure and more rapid dynamics of Trp41 (38). Finally, crystal structures that appear to be representative of the intermediate- and low-pH states show a dramatic increase in the aqueous accessibility of the site (37).
Although these features are now clear, important mechanistic questions remain. One is how protons diffuse to the His box, and another is how the protein stabilizes a high degree of charge in such a narrow enclosure. In the predominantly neutral state, the upper portion of the channel is lined by hydrogen-bond accepting carbonyl groups of Ala30, Gly34, and the deprotonated lone pairs of His37. Thus, water molecules in the channel experience a dearth of hydrogen bond donating groups and are highly polarized to bring protons into the channel, kinetically and thermodynamically favoring protonation of His37. As charge density accumulates, it is stabilized by the clusters of waters bound to hydrogen-bond accepting carbonyls of Gly34 and the electron-rich ring of Trp41. A second question is how protons exit from the His box through the Trp basket into the virus interior. The mechanism of transfer of the third proton from the water-His cluster depends on whether the third proton is fully transferred to His37 as in the shuttle model (35, 36, 79). In this model, the addition of a proton from the outside to the Nδ of His37 is followed by release of the proton to the interior of the virus. A ring flip or water-mediated tautomerization repositions a lone pore on His37 to accept another proton from outside the virus, beginning another step of a cyclic process. A gated channel model instead envisions opening of a pore lined by a chain of water molecules (80), and a hybrid model involves rapid delocalization of the proton throughout the water/His cluster (40). Experimental data favor formal transfer of the proton to and from the His box/water cluster during each turnover, and this remains a topic of current investigation.
Coupling between Drug and Permeant Ion Binding and Large-Scale Conformational Changes
In the classical model for nerve impluse transmission, now largely defined at atomic resolution (81), a perturbation of the TM electrical potential induces a change in the ΔGgap and a concomitant conformational change of a “voltage sensor,” which places a mechanical force on the K+ channel inner activation gate. This force leads to a shift in the A gate inner pore helix (strong coupling or large magnitude of ΔΔGc, Figure 2b), opening the lower section of the pore (Figure 4). Prior to this step, the selectivity filter is already in its conducting state, loaded with two K+ ions. Thus, opening of the A gate results in rapid conversion of the channel from the “C,O” to the “O,O” state (the letters respectively designate the state of the A gate and the selectivity filter) that enables ion transmission. The channel then spontaneously closes by proceeding through two other conformational states (8, 21–24, 26, 31, 82, 83); opening of the helices at the lower end of the bundle places a strain on the open conformation of the selectivity filter, promoting its deformation to a nonconducting open-inactivated (O,I) conformation in a process known as C-type inactivation (a second N-type inactivation process involves binding of an N-terminal peptide to the open pore, which blocks transmission by impeding proton transport and promoting C-type inactivation). As the voltage sensor to the hyperpolarized conformation, the A gate relaxes too, generating the C,I state, which now promotes reorganization of the selectivity filter, regenerating the resting C,O form of the channel. Because these transitions are kinetically and thermodynamically coupled, inactivation is highly modulated by permeant ions and pore blockers. Also, although motion through the cycle occurs in response to changes in membrane polarization for the voltage-gated channel, in the case of the KcsA channel, the transitions occur spontaneously at acidic pH, driven only by thermal energy (84, 85).
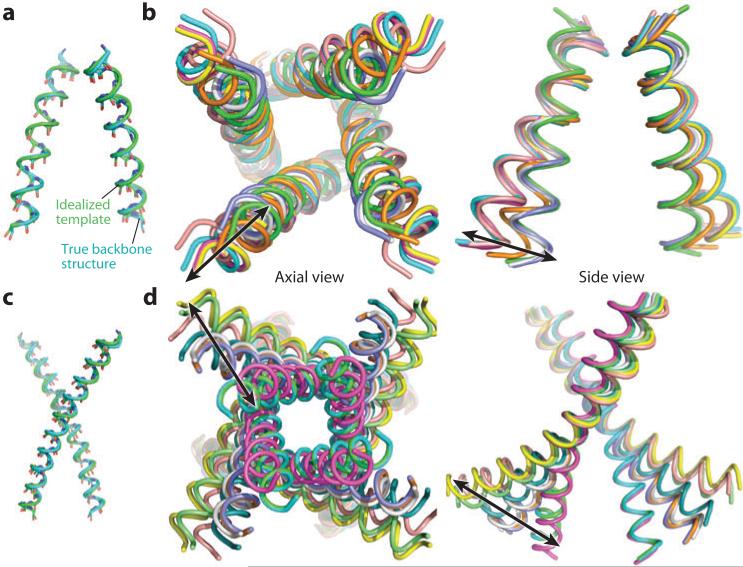
Recently, a variant of the KcsA channel was crystallized in a number of different conformations, providing a view of the same channel in various states of activation (23, 24) and extending earlier structural work on the Na+/K+ channel (32). As expected from the energetic coupling, opening of the A gate at the lower end of the channel induces a nonconducting (inactivated) conformation of the selectivity filter and vice versa (as expected from the model in Figure 1c). We have used a generalized geometric model (86) to examine the conformational transitions of the channel helical bundle over the entire family of 10 structures (see Figure 5). The pore-forming bundles can be described to less than 1-Å resolution by simple motions representing a helical bending motion and a change in the “pitch angle” representing the tilt of the helix relative to the bundle axis, as anticipated from previous studies (28, 32). Both a change in bending by up to 25° and rigid-body tilting of up to 26° occur. These motions occur within a plane parallel to the perimeter of the bundle. The pivot point occurs near the top of the selectivity filter, resulting in dramatic motions near the A gate. More subtle—but nevertheless significant—shifts near the pivot point destabilize the packing of the helices against the open selectivity filter, promoting a conformational change of the filter to its inactivated state. The change involves a network of side chains (29, 31), including Phe103, which undergoes a rotameric shift toward Thr75 of the selectivity filter and Ile100 in the neighboring subunit. All interactions appear to be necessary to maintain a large value of ΔGgap, but Phe103 appears to help translate changes in the structure of the inner helical bundle to changes in the kinetic and thermodynamic stability of the selectivity filter (and vice versa). Indeed, this coupling is lost in mutants in which Phe103 is replaced by a small side chain, and the mutations of residues at homologous positions in mammalian channels have similar effects (24). Other regions of key importance include a carboxyl-carboxylate pair, which directly modulates the selectivity filter, found between Glu71 and Asp80 (82, 87) and a patch of ionic residues, which modulates the stability of the inner pore helix in the open versus closed conformations, located near the cytoplasmic end of the bundle (88). Interestingly, the transition occurs in a nearly fully cooperative process involving a coordinated movement of the four subunits (27, 89).
Figure 5.
(a,b) Parametric views of structural changes in the pore-forming helices of the M2 protein channel and (c,d ) the KcsA channel. Pore-forming helix bundles (residues 25–46 for M2 and 86–122 in KcsA; for the latter, some structures lacked the N-terminal section of this region) were described via the arched bundle parameterization (outlined in Reference 86). In total, 8 structures were analyzed for M2 [Protein Data Bank (PDB) codes 2RLF, 3C9J, 2KQT, 2L0J, 3LBW, and 3 symmetric structures derived from 3 different helices of the asymmetric structure 3BKD], and 10 structures were analyzed for KcsA (PDB codes 1R3J, 2HVK, 3EFF, 3F5W, 3F7V, 3F7Y, 3FB5, 3FB6, 3FB7, and 3FB8). Essential features were identified by forcing progressively more of the parameters to have the same values for all structures. Panels a and c illustrate the accuracy of the parametric representation by comparing true backbone structures (cyan) with idealized templates (green) for representative bundles from M2 and KcsA, respectively. An advantage of the mathematically modeled structures is the unambiguous alignment frame they provide. (b) Axial and side views (for the latter, only two opposing helices shown) of superposed parameterized models of all eight M2 structures clearly illustrate the dominant modes of motion (illustrated with arrows). Essential parameters of variation for these structures are the radius of curvature of helical arches, helix tilt angle, and bundle radius (86), with the possible exception of the solution NMR structure 2RLF [whereas all structures were fit within 1.0-Å root mean square deviation (RMSD) with just these three parameters, the first model in 2RLF produced an RMSD of 1.66 Å]. (d ) Axial and side views (for the latter, only two opposing helices are shown) of superposed parameterized models of all 10 KcsA structures. The essential parameters of variation for these structures are radius and direction of curvature of helical arches, and helix pitch angle (86).
The recent structure of KcsA with its N-terminal cytoplasmic helix intact (33) revealed a well-defined four-helix bundle that projects approximately 70 Å toward the cytoplasm, confirming an earlier site-directed electron para-magnetic resonance study (90, 91). This helical bundle stabilizes the protein and modulates gating by stabilizing the closed form of the channel. Of particular interest is the relationship between the stability of the bundle and the gating of the channel (92, 93).
A similar structural analysis of the entire set of crystallographic, solution, and SSNMR structures of the pore-forming segment of the M2 proton channel showed similar motions, although the shifts in the helices were less extreme than in KcsA. The pivot point occurs near the top of the bundle, such that tilting primarily affects the C-terminal end. A slight (up to 12°) bending of the helices tends to cause variable left-handed supercoiling, minimizing the divergence of the helices in the most bent structures. Although the structures were solved in a variety of environments, at varying pH and with different length constructs, they vary in a continuous manner along a single trajectory, suggesting movement along a smooth functionally relevant energy landscape.
There is a general trend for the structures to diverge near the C terminus with decreasing pH, and hence protonation of His37. A small dilation of the N terminus accompanies the C-terminal dilation. A potential caveat is that the protonation states are not always unambiguously defined by the pH and the pKas of His37. Thus, it is reassuring that molecular dynamics simulations from the groups of Klein, Cross, and Zhou showed the same trend, as the charge state of the four His residues was varied (94–96). Furthermore, solution and SSNMR studies show a greater degree of conformational mobility and hydration of the bundle as the degree of protonation increases (36, 97, 98). A second potential caveat is that the micelle environment used for the crystal structures might allow larger-scale fluctuations than observed in bilayers (99). However, the high-resolution crystal structure is the most tightly packed of the various structures, and the motions between the structures occur along a common trajectory, suggesting that protonation is strongly coupled to motions of the TM helices.
Finally, it is interesting to examine the amplitude and timescale of the motions of M2 in comparison to the gating motions of KcsA. Overall, the range of conformational changes seen for M2 are smaller than those of the K+ channel. For M2, the diameter of the channel measured at Trp41 increases by 7 Å from the most compact to the widest structure, whereas the corresponding change is in excess of 15 Å for the K+ channel. Moreover, the timescale of conformational gating in the K+ channel depends on the membrane environment, pH, and voltage (26, 31, 100), but at low pH, the gating transitions occur on the millisecond time frame (84, 85). Finally, the KcsA is structurally more complex than M2, having not only a membrane-binding helix at one terminus but also an extended helical bundle at the other terminus (33). By comparison, M2 has a short unstructured N-terminal segment (which is entirely deleted in the corresponding M2 protein from the influenza B virus) and a C-terminal surface helix, which has no discernable effect on channel activity (43).
NMR studies of M2 show that in bilayers and micelles the amides’ resonances are broadened near physiological pH (38, 101, 102), indicative of helical motions on the same microsecond to millisecond time regime as the gating seen in K+ channels. Side chain dynamics also show motions in the microsecond regime (36, 38, 103). However, an important distinction between the two proteins is the faster flux of ions through KcsA. During a 10-ms opening, approximately 105 ions are conducted through KcsA, whereas M2 would pass approximately a single proton during this same time period (69, 71). Thus, proton conduction through M2 appears to occur amid conformational fluctuations, suggesting a transporter-like mechanism in which N-terminal opening in the underprotonated state encourages the entry of exterior water, while conformational changes following protonation promote release of the proton into the virus interior (37, 94, 95). Thus, it is important to devise experiments that test the dynamical changes associated with protonation/deprotonation under conditions in which the channel can be shown to be conducting protons.
Finally, alkylammonium drugs are known to inhibit both the K+ channels as well as M2. Because channel-blockers bind preferentially to specific states of the channel, they have significant effects on the extent and kinetics of various inactivation processes, as well as directly blocking the pathway of ion conduction (104–106). Thus, they have at times been considered to act via conformational selection, allosteric binding, or physical occlusion, but the latter is a dramatic and immediate mechanism by which they can achieve complete inhibition. Tetrabutylammonium (TBA) binds at the entry to KcsA's selectivity filter (Figure 4e) in a manner quite similar to the binding of amantadine (adamantylamine) to M2, which lies proximal to the entry water cluster/His box (19). In both cases, there is little voltage dependence to the inhibition, which is consistent with the location of the drugs just prior to their respective selectivity filters over which the electrical potential is known to decay quite steeply (46, 104, 107). In KcsA, TBA displaces the K+ hydrate near the entry of the selectivity filter (Figure 4e), and drug binding displaces protons from His37 in M2 (46, 76). Figure 4d illustrates the SSNMR structure of the M2 channel with the water molecules seen in the high-resolution structure superimposed to show their approximate positions. The ammonium group lies within hydrogen-bonding distance of the entry cluster, such that binding not only blocks access of protons to the center, but also lowers the pKa of His37 (46, 76, 101). Interestingly, it has been possible to design higher-affinity drugs on the basis of this analysis (108, 109).
INSIDE-TO-OUTSIDE COUPLING IN INTEGRIN RECEPTORS
Integrins are α-β heterodimeric adhesion receptors that mediate interactions between the extracellular environment and the intracellular cytoskeleton (110–114), demonstrating how bidirectional TM signal transduction is fine-tuned by multiple coupled equilibria in the extracellular, TM, and cytosolic domains (Figure 6a). Integrin ectodomains contain a large globular N-terminal ligand binding “head” and distinct C-terminal α- and β-“legs” that cross the plasma membrane as single TM domains and terminate in short cytoplasmic domains that bind intracellular molecules. The inactive integrin adopts a bent conformation, placing the head in proximity to the membrane and the distal legs. Upon activation, an extended conformation is favored and the α- and β-legs, TM, and cytoplasmic domains separate.
Figure 6.
Thermodynamic model of integrin signaling. (a) At the coarsest level, extracellular, transmembrane (TM), and intracellular domains of integrins can be thought of as being either active or inactive, giving rise to eight coupled states as illustrated. The various effector molecules modulate integrin state by preferentially binding to select domain conformations. “Inside-out” signaling refers to modulation of the extracellular domain conformation via interactions between an integrin's cytosolic tails and intracellular signaling molecules. “Outside-in” events sometimes refer to the activation of oligomerization-dependent signaling pathways subsequent to binding polyvalent ligands. However, keeping with our model, here it refers to the ability of extracellular ligands to affect the conformation and function of intracellular domains. (b) Using reasonable values for the energy gaps and coupling constants associated with these states as well as equilibrium binding constants for the active and inactive states of different effector molecules, one can interrogate the thermodynamics of integrin signaling in a coarse, semiapproximate manner. Shown here is the level of integrin activation (separation of TM/tail regions) in response to increasing amounts of an activating antibody as a function of Mn2+ concentration. This illustrates outside-in signaling (the level of activation is shown on the z-axis and also indicated by the pseudocolor). (c,d ) Inside-out signaling as illustrated by the fraction of extracellular domain bound to native ligand as a function of different amounts of active intracellular talin and kindlin. The binding affinity of the ligand as well as the amounts of talin/kindlin all couple to determine the activation state of the integrin.
Interactions between the α- and β-chain legs, TM, and membrane-proximal cytosolic domains stabilize the inactive integrin. Single point mutations can result in receptor activation (115–118), and membrane-proximal αβ disulfide cross-linking completely abrogates TM signal transduction in either direction (119, 120). Although structural studies suggest that there is a distinct TM interface, the membrane-proximal regions are dynamic (118, 120–123), befitting the need for rapid signal transduction.
The “loose” dynamic interactions between the α- and β-chains near the membrane give way to an extensive interface between globular portions of the extracellular domain, which can be perturbed via mutagenesis (124). Thermodynamic coupling occurs throughout the globular ectodomain. Crystallization with and without ligand resulted in active-extended (125) and inactive-bent (126, 127) conformations, respectively. Soaking the inactive crystals with a ligand resulted in only small-scale changes in the binding site without leg extension owing to crystal contact constraints (126). Without such constraints, anti-integrin drugs can generate adverse side effects by activating the receptor and generating immune reactive epitopes or ligand-induced binding sites (LIBSs) in the integrin legs (114). Small-molecule drugs can uncouple subdomains as well. Allosteric inhibitors that compete for interdomain contacts generate functionally split integrins in which the head is locked into an inactive conformation, but distal regions generate active LIBSs (127). LIBS antibodies also shift the integrin equilibrium to an active conformation. Constraining the integrin C termini stabilizes the bent conformation and the inactive ligand binding site, but the addition of LIBS antibodies and Mn2+ (a second allosteric activator) shifts the head domain equilibrium to an active state despite the distal constraints (128). Demonstrating bidirectionallity, LIBS antibodies induce TM separation when not constrained (116). The combination of a LIBS antibody and a second activator, Mn2+, further increased activation, but Mn2+ alone did not significantly affect TM separation. Therefore, as in the case of VirA/VirG (Figure 3), multiple stimuli are integrated to produce different intracellular responses (modeled in Figure 6b).
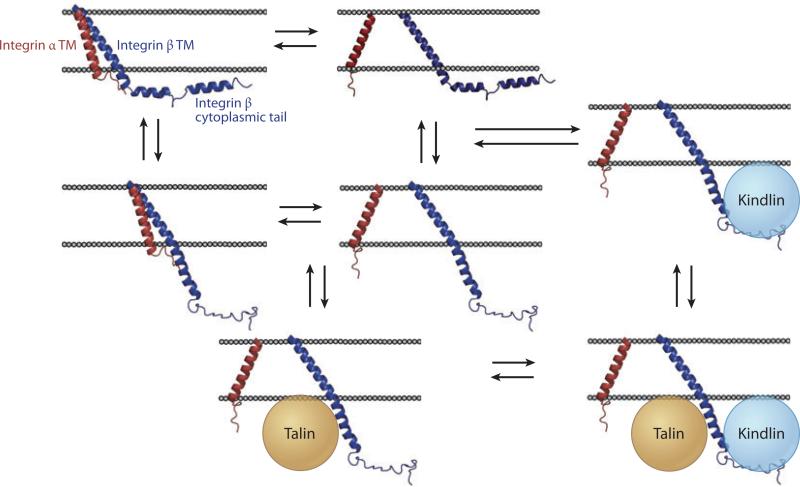
Although the integrin cytoplasmic domain is much shorter (~5–6 kDa for β-integrins) than the ectodomain, it integrates multiple signals to extracellular function. In spite of the fact that there is convincing evidence of a defined αIIbβ3 TM interface, the membrane-proximal and cytoplasmic domains have not provided consistent structures (118, 122, 129–132). Recently, to capture the αIIbβ3 inactive conformation, a disulfide cross-link was introduced between the αIIb and β3 membrane-proximal domains (133). The αIIb cytoplasmic domain was disordered, but caused a kink in β3 that was predicted to stabilize the interaction of two β3 cytoplasmic helices with the bilayer. Significantly, these dynamic membrane interacting helices contain the primary motifs that bind the evolutionarily related talin and kindlin families of proteins (reviewed in References 113 and 134). Figure 7 outlines a simple linked equilibrium model, which is quantitatively described in Figure 6c,d, illustrating how coupling between talin- and kindlin-binding motifs allows differentiation between ligands with varying affinities for the receptor, effectively recapitulating an intermediate affinity state without the need for distinct extracellular conformations (128, 135).
Figure 7.
Coupled equilibria linking integrin transmembrane (TM) interactions to cytosolic events. Integrin αβ TM heterodimers (left) stabilize inactive ectodomain conformations, and TM separation (right) stabilizes active ectodomain conformations. The αβ heterodimer stabilizes interactions between the β-cytoplasmic domain and the inner leaflet of the bilayers (top). Cytosolic proteins talin and kindlin shift the equilibrium toward an active conformation by stabilizing the extended cytoplasmic domain and separated TM domains (bottom right). Loss of either talin-1 or kindlin-3 in mouse platelets results in bleeding and nearly complete loss of inside-out integrin activation (138, 139). Similarly, patients who lack kindlin-3 suffer from bleeding and immune impairments due to loss of αIIbβ3 and β2 integrin functions, respectively (140–144). Although platelets that lack talin and kindlin-3 are physiologically impaired, they are capable of partial integrin activation in the presence of very strong agonists. Also, large excesses of talin can activate integrins in the absence of kindlins (145), coexpression of kindlins and talin result in significantly increased activation (146, 147), and overexpression of kindlins alone does not significantly activate integrins. This equilibrium can further be expanded via phosphorylation of integrin interaction motifs (148, 149) or competition with other signaling molecules, such as filamins (150, 151).
KINETIC CONSIDERATIONS
The extensive conformational changes involved in integrin activation illustrate how coupled equilibria can affect the kinetics of receptor-ligand interactions. Platelets are surrounded by a large excess of soluble fibrinogen, yet they do not spontaneously activate αIIbβ3, whereas a small-molecule Arg-Gly-Asp (RGD) ligand can shift the integrin equilibrium toward activation. This active state is long-lived, on the order of hours, in purified integrin (136), suggesting that, although the small-molecule binding site is kinetically accessible, the bent conformation is not kinetically accessible to a large fibrinogen molecule. Recent surface plasmon resonance analyses demonstrate that inactive αIIbβ3 does not bind soluble fibrinogen without kinetic priming by a small-molecule ligand (137). Even in its primed state, the on rate is relatively slow and entropically disfavored, which the authors attribute to the fact that αIIbβ3 binds to disordered regions in addition to the central RGD sequence. The local equilibria that regulate activation of an individual integrin participate in larger monomermultimer equilibria driven by polymeric ligands, the cytoskeleton, and mechanical force (138, 139), potentially giving integrin clusters unique kinetic properties under tension as well.
The control of the oligomeric state and conformation is crucial to a range of signaling receptors, including cytokine and growth factor receptors (140, 141), and has been proposed to play a role in fine-tuning G protein–coupled receptor signal transduction (142). Receptor oligomerization can be simply accounted for as a thermodynamic state in a larger equilibrium. However, it may also produce kinetic effects by potentially kinetically uncoupling ligand-induced conformational states from signal transduction until oligomerization has occurred. Different signaling events can be encoded in such equilibria as in the case of members of the epidermal growth factor receptor family, which equilibriate to populations of monomers and preformed dimers (143) and demonstrate allosteric regulation that may allow discrimination of various ligands to produce different signals (144).
CONCLUSIONS
With the expanding number of high-resolution membrane-protein structures, especially in different functional states, structural decomposition of the events involved in signal transduction is increasingly possible. Common structural mechanisms have emerged. For example, despite large structural and functional differences, the M2 proton channel and the KcsA K+ channel share striking similarities in their mechanisms of ion selectivity and rearrangements responsible for gating and switching between different states of activation. Similarly, although the details of signaling through various two-component bacterial kinases and adhesion protein receptors differ, common principles underlie recurring themes. These systems can be modulated with effector molecules, both natural and synthetic, that perturb the native equilibrium and elicit manifold responses. Chemical probes, in combination with biophysical, structural, cellular, and organismic approaches, are being increasingly used to deconstruct these complex equilibria ultimately toward the goals of engineering new therapeutics or biotechnological processes.
M2: the M2 proton channel from influenza A virus
Energy gap: the difference in free energy between active and inactive states
A gate: activation gate of the potassium channel, formed by the C-terminal helix that lines the pore
SUMMARY POINTS.
Binding of ligands to membrane proteins induces functional conformational changes that are thermodynamically and kinetically communicated between different protein modules.
There are significant commonalities in the structural features, often involving perturbations in helix-packing geometries, that carry the signal across the membrane.
The coupling between extracellular, TM, and intracellular domains can be understood with thermodynamic models, using basic concepts, such as the energy gap between active and inactive states and interdomain coupling energies.
Communication can be mediated with subtle structural rearrangement between states that are in equilibrium with one another and does not require hard connections with protein domains mechanically driving a conformational change that propagates across a bilayer.
Thermodynamic coupling of different domains allows for the sensing and integration of multiple inputs and generation of distinct signals. This can produce either switch-like behavior or result in more graded responses, as appropriate for the biological system.
FUTURE ISSUES.
Emerging experimental findings concerning the structural basis of gating and selectivity in K+ channels are now laying a firm foundation for in-depth theoretical and computational investigations of these processes.
Systems and single-molecule level studies can be integrated to provide quantitative descriptions of signal transduction.
What are the amplitudes of side chain and backbone fluctuations in M2 and how do they couple to its pH activation and proton transport?
Design of new M2 inhibitors that address the problem of drug resistance is of critical importance, as this will be aided by the availability of amantadine-M2 structures.
The C-terminal cytoplasmic helix of M2 is emerging as a functionally rich element, with the recent discovery that it acts to aid in localization and viral filament formation as well as in promoting budding and scission (43, 54). The structural and biophysical bases for these activities are interesting topics for further investigation.
ACKNOWLEDGMENTS
We acknowledge funding from National Institutes of Health grant numbers 5F32GM084631, GM54616, GM56432, and AI74571.
Footnotes
Coupling free energy is the effective free energy of interaction between two domains of a protein when both are in the same state of activation (both active or both inactive) relative to their interaction free energy when they are in different activation states (one active and the other inactive).
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Csermely P, Palotai R, Nussinov R. Induced fit, conformational selection and independent dynamic segments: an extended view of binding events. Trends Biochem. Sci. 2010;35:539–46. doi: 10.1016/j.tibs.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monod J. From enzymatic adaptation to allosteric transitions. Science. 1966;154:475–83. doi: 10.1126/science.154.3748.475. [DOI] [PubMed] [Google Scholar]
- 3.Monod J, Wyman J, Changeux JP. On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 4.Boehr DD, Nussinov R, Wright PE. The role of dynamic conformational ensembles in biomolecular recognition. Nat. Chem. Biol. 2009;5:789–96. doi: 10.1038/nchembio.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koshland DE, Jr, Nemethy G, Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966;5:365–85. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- 6.Hill AV. The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J. Physiol. 1922;40:iv–vii. [Google Scholar]
- 7.Liu Z, Gandhi CS, Rees DC. Structure of a tetrameric MscL in an expanded intermediate state. Nature. 2009;461:120–24. doi: 10.1038/nature08277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt D, Cross SR, MacKinnon R. A gating model for the archeal voltage-dependent K+ channel KvAP in DPhPC and POPE:POPG decane lipid bilayers. J. Mol. Biol. 2009;390:902–12. doi: 10.1016/j.jmb.2009.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt D, Jiang QX, MacKinnon R. Phospholipids and the origin of cationic gating charges in voltage sensors. Nature. 2006;444:775–79. doi: 10.1038/nature05416. [DOI] [PubMed] [Google Scholar]
- 10.Nishida M, Cadene M, Chait BT, MacKinnon R. Crystal structure of a Kir3.1-prokaryotic Kir channel chimera. EMBO J. 2007;26:4005–15. doi: 10.1038/sj.emboj.7601828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo W, Cady SD, Hong M. Immobilization of the influenza A M2 transmembrane peptide in virus envelope-mimetic lipid membranes: a solid-state NMR investigation. Biochemistry. 2009;48:6361–68. doi: 10.1021/bi900716s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schroeder C, Heider H, Moncke-Buchner E, Lin TI. The influenza virus ion channel and maturation cofactor M2 is a cholesterol-binding protein. Eur. Biophys. J. 2005;34:52–66. doi: 10.1007/s00249-004-0424-1. [DOI] [PubMed] [Google Scholar]
- 13.Schroeder C. Cholesterol-binding viral proteins in virus entry and morphogenesis. Subcell. Biochem. 2010;51:77–108. doi: 10.1007/978-90-481-8622-8_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zagotta WN, Hoshi T, Aldrich RW. Shaker potassium channel gating. III: Evaluation of kinetic models for activation. J. Gen. Physiol. 1994;103:321–62. doi: 10.1085/jgp.103.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin YH, Gao R, Binns AN, Lynn DG. Capturing the VirA/VirG TCS of Agrobacterium tumefaciens. Adv. Exp. Med. Biol. 2008;631:161–77. doi: 10.1007/978-0-387-78885-2_11. [DOI] [PubMed] [Google Scholar]
- 16.Hille B. Ionic Channels of Excitable Membranes. Sinauer; Sunderland, MA: 1992. [Google Scholar]
- 17.Gouaux E, Mackinnon R. Principles of selective ion transport in channels and pumps. Science. 2005;310:1461–65. doi: 10.1126/science.1113666. [DOI] [PubMed] [Google Scholar]
- 18.MacKinnon R. Potassium channels. FEBS Lett. 2003;555:62–65. doi: 10.1016/s0014-5793(03)01104-9. [DOI] [PubMed] [Google Scholar]
- 19.Cady SD, Schmidt-Rohr K, Wang J, Soto CS, DeGrado WF, Hong M. Structure of the amantadine binding site of influenza M2 proton channels in lipid bilayers. Nature. 2010;463:689–92. doi: 10.1038/nature08722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou HX. Diffusion-influenced transport of ions across a transmembrane channel with an internal binding site. J. Phys. Chem. Lett. 2010;1:1973–76. doi: 10.1021/jz100683t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panyi G, Deutsch C. Probing the cavity of the slow inactivated conformation of shaker potassium channels. J. Gen. Physiol. 2007;129:403–18. doi: 10.1085/jgp.200709758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ray EC, Deutsch C. A trapped intracellular cation modulates K+ channel recovery from slow inactivation. J. Gen. Physiol. 2006;128:203–17. doi: 10.1085/jgp.200609561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuello LG, Jogini V, Cortes DM, Perozo E. Structural mechanism of C-type inactivation in K+ channels. Nature. 2010;466:203–8. doi: 10.1038/nature09153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuello LG, Jogini V, Cortes DM, Pan AC, Gagnon DG, et al. Structural basis for the coupling between activation and inactivation gates in K+ channels. Nature. 2010;466:272–75. doi: 10.1038/nature09136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuello LG, Cortes DM, Jogini V, Sompornpisut A, Perozo E. A molecular mechanism for proton-dependent gating in KcsA. FEBS Lett. 2010;584:1126–32. doi: 10.1016/j.febslet.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imai S, Osawa M, Takeuchi K, Shimada I. Structural basis underlying the dual gate properties of KcsA. Proc. Natl. Acad. Sci. USA. 2010;107:6216–21. doi: 10.1073/pnas.0911270107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rotem D, Mason A, Bayley H. Inactivation of the KcsA potassium channel explored with heterotetramers. J. Gen. Physiol. 2010;135:29–42. doi: 10.1085/jgp.200910305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miloshevsky GV, Jordan PC. Open-state conformation of the KcsA K+ channel: Monte Carlo normal mode following simulations. Structure. 2007;15:1654–62. doi: 10.1016/j.str.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 29.Haliloglu T, Ben-Tal N. Cooperative transition between open and closed conformations in potassium channels. PLoS Comput. Biol. 2008;4:e1000164. doi: 10.1371/journal.pcbi.1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimizu H, Iwamoto M, Konno T, Nihei A, Sasaki YC, Oiki S. Global twisting motion of single molecular KcsA potassium channel upon gating. Cell. 2008;132:67–78. doi: 10.1016/j.cell.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 31.Ader C, Schneider R, Hornig S, Velisetty P, Vardanyan V, et al. Coupling of activation and inactivation gate in a K+-channel: potassium and ligand sensitivity. EMBO J. 2009;28:2825–34. doi: 10.1038/emboj.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alam A, Jiang Y. High-resolution structure of the open NaK channel. Nat. Struct. Mol. Biol. 2009;16:30–34. doi: 10.1038/nsmb.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uysal S, Vasquez V, Tereshko V, Esaki K, Fellouse FA, et al. Crystal structure of full-length KcsA in its closed conformation. Proc. Natl. Acad. Sci. USA. 2009;106:6644–49. doi: 10.1073/pnas.0810663106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao LL, Wu A, Bi LJ, Liu P, Zhang XE, et al. Length-dependent regulation of the Kv1.2 channel activation by its C-terminus. Mol. Membr. Biol. 2009;26:186–93. doi: 10.1080/09687680802714741. [DOI] [PubMed] [Google Scholar]
- 35.Sharma M, Yi M, Dong H, Qin H, Peterson E, et al. Insight into the mechanism of the influenza A proton channel from a structure in a lipid bilayer. Science. 2010;330:509–12. doi: 10.1126/science.1191750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu F, Luo W, Hong M. Mechanisms of proton conduction and gating in influenza M2 proton channels from solid-state NMR. Science. 2010;330:505–8. doi: 10.1126/science.1191714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stouffer AL, Acharya R, Salom D, Levine AS, Di Costanzo L, et al. Structural basis for the function and inhibition of an influenza virus proton channel. Nature. 2008;451:596–99. doi: 10.1038/nature06528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schnell JR, Chou JJ. Structure and mechanism of the M2 proton channel of influenza A virus. Nature. 2008;451:591–95. doi: 10.1038/nature06531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Acharya A, Carnevale V, Fiorin G, Levine BG, Polishchuk A, et al. Structure and mechanism of proton transport through the transmembrane tetrameric M2 protein bundle of the influenza A virus. Proc. Natl. Acad. Sci. USA. 2010;107:15075–80. doi: 10.1073/pnas.1007071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Acharya R, Carnevale V, Fiorin G, Levine BG, Polishchuk AL, et al. Structure and mechanism of proton transport through the transmembrane tetrameric M2 protein bundle of the influenza A virus. Proc. Natl. Acad. Sci. USA. 2010;107:15075–80. doi: 10.1073/pnas.1007071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinto LH, Lamb RA. Influenza virus proton channels. Photochem. Photobiol. Sci. 2006;5:629–32. doi: 10.1039/b517734k. [DOI] [PubMed] [Google Scholar]
- 42.Rossman JS, Lamb RA. Autophagy, apoptosis, and the influenza virus M2 protein. Cell Host Microbe. 2009;6:299–300. doi: 10.1016/j.chom.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 43.Rossman JS, Jing X, Leser GP, Lamb RA. Influenza virus M2 protein mediates ESCRT-independent membrane scission. Cell. 2010;142:902–13. doi: 10.1016/j.cell.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gannage M, Dormann D, Albrecht R, Dengjel J, Torossi T, et al. Matrix protein 2 of influenza A virus blocks autophagosome fusion with lysosomes. Cell Host Microbe. 2009;6:367–80. doi: 10.1016/j.chom.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park EK, Castrucci MR, Portner A, Kawaoka Y. The M2 ectodomain is important for its incorporation into influenza A virions. J. Virol. 1998;72:2449–55. doi: 10.1128/jvi.72.3.2449-2455.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma C, Polishchuk AL, Ohigashi Y, Stouffer AL, Schon A, et al. Identification of the functional core of the influenza A virus A/M2 proton-selective ion channel. Proc. Natl. Acad. Sci. USA. 2009;106:12283–88. doi: 10.1073/pnas.0905726106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kochendoerfer GG, Salom D, Lear JD, Wilk-Orescan R, Kent SB, DeGrado WF. Total chemical synthesis of the integral membrane protein influenza A virus M2: role of its C-terminal domain in tetramer assembly. Biochemistry. 1999;38:11905–13. doi: 10.1021/bi990720m. [DOI] [PubMed] [Google Scholar]
- 48.Salom D, Hill BR, Lear JD, DeGrado WF. pH-dependent tetramerization and amantadine binding of the transmembrane helix of M2 from the influenza A virus. Biochemistry. 2000;39:14160–70. doi: 10.1021/bi001799u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cady SD, Luo W, Hu F, Hong M. Structure and function of the influenza A M2 proton channel. Biochemistry. 2009;48:7356–64. doi: 10.1021/bi9008837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCown MF, Pekosz A. Distinct domains of the influenza A virus M2 protein cytoplasmic tail mediate binding to the M1 protein and facilitate infectious virus production. J. Virol. 2006;80:8178–89. doi: 10.1128/JVI.00627-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balannik V, Carnevale V, Fiorin G, Levine BG, Lamb RA, et al. Functional studies and modeling of pore-lining residue mutants of the influenza A virus M2 ion channel. Biochemistry. 2010;49:696–708. doi: 10.1021/bi901799k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jing X, Ma C, Ohigashi Y, Oliveira FA, Jardetzky TS, et al. Functional studies indicate amantadine binds to the pore of the influenza A virus M2 proton-selective ion channel. Proc. Natl. Acad. Sci. USA. 2008;105:10967–72. doi: 10.1073/pnas.0804958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohigashi Y, Ma C, Jing X, Balannick V, Pinto LH, Lamb RA. An amantadine-sensitive chimeric BM2 ion channel of influenza B virus has implications for the mechanism of drug inhibition. Proc. Natl. Acad. Sci. USA. 2009;106:18775–79. doi: 10.1073/pnas.0910584106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rossman JS, Jing X, Leser GP, Balannik V, Pinto LH, Lamb RA. Influenza virus M2 ion channel protein is necessary for filamentous virion formation. J. Virol. 2010;84:5078–88. doi: 10.1128/JVI.00119-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu J, Asbury T, Achuthan S, Li C, Bertram R, et al. Backbone structure of the amantadine-blocked trans-membrane domain M2 proton channel from influenza A virus. Biophys. J. 2007;92:4335–43. doi: 10.1529/biophysj.106.090183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pielak RM, Chou JJ. Solution NMR structure of the V27A drug resistant mutant of influenza A M2 channel. Biochem. Biophys. Res. Commun. 2010;401:58–63. doi: 10.1016/j.bbrc.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou Y, MacKinnon R. The occupancy of ions in the K+ selectivity filter: charge balance and coupling of ion binding to a protein conformational change underlie high conduction rates. J. Mol. Biol. 2003;333:965–75. doi: 10.1016/j.jmb.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 58.Zhou Y, Morais-Cabral JH, Kaufman A, MacKinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature. 2001;414:43–48. doi: 10.1038/35102009. [DOI] [PubMed] [Google Scholar]
- 59.Lockless SW, Zhou M, MacKinnon R. Structural and thermodynamic properties of selective ion binding in a K+ channel. PLoS Biol. 2007;5:e121. doi: 10.1371/journal.pbio.0050121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou M, MacKinnon R. A mutant KcsA K+ channel with altered conduction properties and selectivity filter ion distribution. J. Mol. Biol. 2004;338:839–46. doi: 10.1016/j.jmb.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 61.Morais-Cabral JH, Zhou Y, MacKinnon R. Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature. 2001;414:37–42. doi: 10.1038/35102000. [DOI] [PubMed] [Google Scholar]
- 62.Berneche S, Roux B. Energetics of ion conduction through the K+ channel. Nature. 2001;414:73–77. doi: 10.1038/35102067. [DOI] [PubMed] [Google Scholar]
- 63.Egwolf B, Roux B. Ion selectivity of the KcsA channel: a perspective from multi-ion free energy landscapes. J. Mol. Biol. 2010;401:831–42. doi: 10.1016/j.jmb.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dixit PD, Merchant S, Asthagiri D. Ion selectivity in the KcsA potassium channel from the perspective of the ion binding site. Biophys. J. 2009;96:2138–45. doi: 10.1016/j.bpj.2008.12.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Domene C, Furini S. Dynamics, energetics, and selectivity of the low-K+ KcsA channel structure. J. Mol. Biol. 2009;389:637–45. doi: 10.1016/j.jmb.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 66.Kraszewski S, Boiteux C, Ramseyer C, Girardet C. Determination of the charge profile in the KcsA selectivity filter using ab initio calculations and molecular dynamics simulations. Phys. Chem. Chem. Phys. 2009;11:8606–13. doi: 10.1039/b905991a. [DOI] [PubMed] [Google Scholar]
- 67.Thompson AN, Kim I, Panosian TD, Iverson TM, Allen TW, Nimigean CM. Mechanism of potassium-channel selectivity revealed by Na+ and Li+ binding sites within the KcsA pore. Nat. Struct. Mol. Biol. 2009;16:1317–24. doi: 10.1038/nsmb.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Decoursey TE. Voltage-gated proton channels and other proton transfer pathways. Physiol. Rev. 2003;83:475–579. doi: 10.1152/physrev.00028.2002. [DOI] [PubMed] [Google Scholar]
- 69.Mould JA, Li H- C, Dudlak CS, Lear JD, Pekosz A, et al. Mechanism for proton conduction of the M2 ion channel of influenza A virus. J. Biol. Chem. 2000;275:8592–99. doi: 10.1074/jbc.275.12.8592. [DOI] [PubMed] [Google Scholar]
- 70.Mould JA, Drury JE, Frings SM, Kaupp UB, Pekosz A, et al. Permeation and activation of the M2 ion channel of influenza A virus. J. Biol. Chem. 2000;275:31038–50. doi: 10.1074/jbc.M003663200. [DOI] [PubMed] [Google Scholar]
- 71.Leiding T, Wang J, Martinsson J, DeGrado WF, Arskold SP. Proton and cation transport activity of the M2 proton channel from influenza A virus. Proc. Natl. Acad. Sci. USA. 2010;107:15409–14. doi: 10.1073/pnas.1009997107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chizhmakov IV, Ogden DC, Geraghty FM, Hayhurst A, Skinner A, et al. Differences in conductance of M2 proton channels of two influenza viruses at low and high pH. J. Physiol. 2003;546:427–38. doi: 10.1113/jphysiol.2002.028910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Czabotar PE, Martin SR, Hay AJ. Studies of structural changes in the M2 proton channel of influenza A virus by tryptophan fluorescence. Virus Res. 2004;99:57–61. doi: 10.1016/j.virusres.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 74.Chizhmakov IV, Geraghty FM, Ogden DC, Hayhurst A, Antoniou M, Hay AJ. Selective proton permeability and pH regulation of the influenza virus M2 channel expressed in mouse erythroleukaemia cells. J. Physiol. 1996;494:329–36. doi: 10.1113/jphysiol.1996.sp021495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang C, Lamb RA, Pinto LH. Activation of the M(2) ion channel of influenza virus: a role for the transmembrane domain histidine residue. Biophys. J. 1995;69:1363–71. doi: 10.1016/S0006-3495(95)80003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu J, Fu R, Nishimura K, Zhang L, Zhou HX, et al. Histidines, heart of the hydrogen ion channel from influenza A virus: toward an understanding of conductance and proton selectivity. Proc. Natl. Acad. Sci. USA. 2006;103:6865–70. doi: 10.1073/pnas.0601944103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pinto LH, Dieckmann GR, Gandhi CS, Papworth CG, Braman J, et al. A functionally defined model for the M2 proton channel of influenza A virus suggests a mechanism for its ion selectivity. Proc. Natl. Acad. Sci. USA. 1997;94:11301–6. doi: 10.1073/pnas.94.21.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang Y, Zaitseva F, Lamb RA, Pinto LH. The gate of the influenza virus M2 proton channel is formed by a single tryptophan residue. J. Biol. Chem. 2002;277:39880–86. doi: 10.1074/jbc.M206582200. [DOI] [PubMed] [Google Scholar]
- 79.Dieckmann GR, McRorie DK, Lear JD, Sharp KA, DeGrado WF, Pecoraro VL. The role of protonation and metal chelation preferences in defining the properties of mercury-binding coiled coils. J. Mol. Biol. 1998;280:897–912. doi: 10.1006/jmbi.1998.1891. [DOI] [PubMed] [Google Scholar]
- 80.Chen H, Wu Y, Voth GA. Proton transport behavior through the influenza A M2 channel: insights from molecular simulation. Biophys. J. 2007;93:3470–79. doi: 10.1529/biophysj.107.105742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Long SB, Tao X, Campbell EB, MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450:376–82. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- 82.Cordero-Morales JF, Jogini V, Lewis A, Vasquez V, Cortes DM, et al. Molecular driving forces determining potassium channel slow inactivation. Nat. Struct. Mol. Biol. 2007;14:1062–69. doi: 10.1038/nsmb1309. [DOI] [PubMed] [Google Scholar]
- 83.Ader C, Schneider R, Hornig S, Velisetty P, Wilson EM, et al. A structural link between inactivation and block of a K+ channel. Nat. Struct. Mol. Biol. 2008;15:605–12. doi: 10.1038/nsmb.1430. [DOI] [PubMed] [Google Scholar]
- 84.Chakrapani S, Cordero-Morales JF, Perozo E. A quantitative description of KcsA gating II: single-channel currents. J. Gen. Physiol. 2007;130:479–96. doi: 10.1085/jgp.200709844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chakrapani S, Cordero-Morales JF, Perozo E. A quantitative description of KcsA gating I: macroscopic currents. J. Gen. Physiol. 2007;130:465–78. doi: 10.1085/jgp.200709843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grigoryan G, DeGrado WF. Probing designability via a generalized model of helical bundle geometry. J. Mol. Biol. 2011;405(4):1079–100. doi: 10.1016/j.jmb.2010.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bucher D, Guidoni L, Rothlisberger U. The protonation state of the Glu-71/Asp-80 residues in the KcsA potassium channel: a first-principles QM/MM molecular dynamics study. Biophys. J. 2007;93:2315–24. doi: 10.1529/biophysj.106.102509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thompson AN, Posson DJ, Parsa PV, Nimigean CM. Molecular mechanism of pH sensing in KcsA potassium channels. Proc. Natl. Acad. Sci. USA. 2008;105:6900–5. doi: 10.1073/pnas.0800873105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blunck R, McGuire H, Hyde HC, Bezanilla F. Fluorescence detection of the movement of single KcsA subunits reveals cooperativity. Proc. Natl. Acad. Sci. USA. 2008;105:20263–68. doi: 10.1073/pnas.0807056106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Perozo E, Kloda A, Cortes DM, Martinac B. Site-directed spin-labeling analysis of reconstituted Mscl in the closed state. J. Gen. Physiol. 2001;118:193–206. doi: 10.1085/jgp.118.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cortes DM, Cuello LG, Perozo E. Molecular architecture of full-length KcsA: role of cytoplasmic domains in ion permeation and activation gating. J. Gen. Physiol. 2001;117:165–80. doi: 10.1085/jgp.117.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yuchi Z, Pau VP, Lu BX, Junop M, Yang DS. An engineered right-handed coiled coil domain imparts extreme thermostability to the KcsA channel. FEBS J. 2009;276:6236–46. doi: 10.1111/j.1742-4658.2009.07327.x. [DOI] [PubMed] [Google Scholar]
- 93.Hirano M, Takeuchi Y, Aoki T, Yanagida T, Ide T. Rearrangements in the KcsA cytoplasmic domain underlie its gating. J. Biol. Chem. 2010;285:3777–83. doi: 10.1074/jbc.M109.084368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yi M, Cross TA, Zhou HX. Conformational heterogeneity of the M2 proton channel and a structural model for channel activation. Proc. Natl. Acad. Sci. USA. 2009;106:13311–16. doi: 10.1073/pnas.0906553106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Khurana E, Dal Peraro M, DeVane R, Vemparala S, DeGrado WF, Klein ML. Molecular dynamics calculations suggest a conduction mechanism for the M2 proton channel from influenza A virus. Proc. Natl. Acad. Sci. USA. 2009;106:1069–74. doi: 10.1073/pnas.0811720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Khurana E, Devane RH, Dal Peraro M, Klein ML. Computational study of drug binding to the membrane-bound tetrameric M2 peptide bundle from influenza A virus. Biochim. Biophys. Acta. 2011;1808(2):530–37. doi: 10.1016/j.bbamem.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Luo W, Hong M. Conformational changes of an ion channel detected through water-protein interactions using solid-state NMR spectroscopy. J. Am. Chem. Soc. 2010;132:2378–84. doi: 10.1021/ja9096219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hu F, Luo W, Cady SD, Hong M. Conformational plasticity of the influenza A M2 transmembrane helix in lipid bilayers under varying pH, drug binding, and membrane thickness Biochim. Biophys. Acta. 2010;1808:415–23. doi: 10.1016/j.bbamem.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cross TA, Sharma M, Yi M, Zhou HX. Influence of solubilizing environments on membrane protein structures. Trends Biochem. Sci. 2011;36(2):117–25. doi: 10.1016/j.tibs.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bhate MP, Wylie BJ, Tian L, McDermott AE. Conformational dynamics in the selectivity filter of KcsA in response to potassium ion concentration. J. Mol. Biol. 2010;401:155–66. doi: 10.1016/j.jmb.2010.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hu J, Fu R, Cross TA. The chemical and dynamical influence of the anti-viral drug amantadine on the M2 proton channel transmembrane domain. Biophys. J. 2007;93:276–83. doi: 10.1529/biophysj.106.102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li C, Qin H, Gao FP, Cross TA. Solid-state NMR characterization of conformational plasticity within the transmembrane domain of the influenza A M2 proton channel. Biochim. Biophys. Acta. 2007;1768:3162–70. doi: 10.1016/j.bbamem.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Boehr DD, McElheny D, Dyson HJ, Wright PE. Millisecond timescale fluctuations in dihydrofolate reductase are exquisitely sensitive to the bound ligands. Proc. Natl. Acad. Sci. USA. 2010;107:1373–78. doi: 10.1073/pnas.0914163107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yohannan S, Hu Y, Zhou Y. Crystallographic study of the tetrabutylammonium block to the KcsA K+ channel. J. Mol. Biol. 2007;366:806–14. doi: 10.1016/j.jmb.2006.11.081. [DOI] [PubMed] [Google Scholar]
- 105.Yellen G. Permeation in potassium channels: implications for channel structure. Annu. Rev. Biophys. Biophys. Chem. 1987;16:227–46. doi: 10.1146/annurev.bb.16.060187.001303. [DOI] [PubMed] [Google Scholar]
- 106.Baukrowitz T, Yellen G. Two functionally distinct subsites for the binding of internal blockers to the pore of voltage-activated K+ channels. Proc. Natl. Acad. Sci. USA. 1996;93:13357–61. doi: 10.1073/pnas.93.23.13357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gandhi CS, Shuck K, Lear JD, Dieckmann GR, DeGrado WF, et al. Cu(II) inhibition of the proton translocation machinery of the influenza A virus M2 protein. J. Biol. Chem. 1999;274:5474–82. doi: 10.1074/jbc.274.9.5474. [DOI] [PubMed] [Google Scholar]
- 108.Balannik V, Wang J, Ohigashi Y, Jing X, Magavern E, et al. Design and pharmacological characterization of inhibitors of amantadine-resistant mutants of the M2 ion channel of influenza A virus. Biochemistry. 2009;48:11872–82. doi: 10.1021/bi9014488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang J, Cady SD, Balannik V, Pinto LH, DeGrado WF, Hong M. Discovery of spiro-piperidine inhibitors and their modulation of the dynamics of the M2 proton channel from influenza A virus. J. Am. Chem. Soc. 2009;131:8066–76. doi: 10.1021/ja900063s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 2007;25:619–47. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bennett JS, Moore DT. Regulation of platelet beta 3 integrins. Haematologica. 2010;95:1049–51. doi: 10.3324/haematol.2010.024893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bennett JS, Berger BW, Billings PC. The structure and function of platelet integrins. J. Thromb. Haemost. 2009;7(Suppl. 1):200–5. doi: 10.1111/j.1538-7836.2009.03378.x. [DOI] [PubMed] [Google Scholar]
- 113.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat. Rev. Mol. Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cox D, Brennan M, Moran N. Integrins as therapeutic targets: lessons and opportunities. Nat. Rev. Drug Discov. 2010;9:804–20. doi: 10.1038/nrd3266. [DOI] [PubMed] [Google Scholar]
- 115.Luo BH, Carman CV, Takagi J, Springer TA. Disrupting integrin transmembrane domain heterodimerization increases ligand binding affinity, not valency or clustering. Proc. Natl. Acad. Sci. USA. 2005;102:3679–84. doi: 10.1073/pnas.0409440102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim M, Carman CV, Springer TA. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science. 2003;301:1720–25. doi: 10.1126/science.1084174. [DOI] [PubMed] [Google Scholar]
- 117.Berger BW, Kulp DW, Span LM, DeGrado JL, Billings PC, et al. Consensus motif for integrin transmembrane helix association. Proc. Natl. Acad. Sci. USA. 2010;107:703–8. doi: 10.1073/pnas.0910873107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhu J, Luo BH, Barth P, Schonbrun J, Baker D, Springer TA. The structure of a receptor with two associating transmembrane domains on the cell surface: integrin alphaIIbbeta3. Mol. Cell. 2009;34:234–49. doi: 10.1016/j.molcel.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Luo BH, Springer TA, Takagi J. A specific interface between integrin transmembrane helices and affinity for ligand. PLoS Biol. 2004;2:e153. doi: 10.1371/journal.pbio.0020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhu J, Carman CV, Kim M, Shimaoka M, Springer TA, Luo BH. Requirement of alpha and beta subunit transmembrane helix separation for integrin outside-in signaling. Blood. 2007;110:2475–83. doi: 10.1182/blood-2007-03-080077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Metcalf DG, Kulp DW, Bennett JS, DeGrado WF. Multiple approaches converge on the structure of the integrin alphaIIb/beta3 transmembrane heterodimer. J. Mol. Biol. 2009;392:1087–101. doi: 10.1016/j.jmb.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yang J, Ma YQ, Page RC, Misra S, Plow EF, Qin J. Structure of an integrin alphaIIb beta3 transmembrane-cytoplasmic heterocomplex provides insight into integrin activation. Proc. Natl. Acad. Sci. USA. 2009;106:17729–34. doi: 10.1073/pnas.0909589106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xie C, Zhu J, Chen X, Mi L, Nishida N, Springer TA. Structure of an integrin with an alphaI domain, complement receptor type 4. EMBO J. 2010;29:666–79. doi: 10.1038/emboj.2009.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Donald JE, Zhu H, Litvinov RI, DeGrado WF, Bennett JS. Identification of interacting hotspots in the beta3 integrin stalk using comprehensive interface design. J. Biol. Chem. 2010;285:38658–65. doi: 10.1074/jbc.M110.170670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xiao T, Takagi J, Coller BS, Wang JH, Springer TA. Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature. 2004;432:59–67. doi: 10.1038/nature02976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xiong JP, Stehle T, Zhang R, Joachimiak A, Frech M, et al. Crystal structure of the extracellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science. 2002;296:151–55. doi: 10.1126/science.1069040. [DOI] [PubMed] [Google Scholar]
- 127.Shimaoka M, Salas A, Yang W, Weitz-Schmidt G, Springer TA. Small molecule integrin antagonists that bind to the beta2 subunit I-like domain and activate signals in one direction and block them in the other. Immunity. 2003;19:391–402. doi: 10.1016/s1074-7613(03)00238-3. [DOI] [PubMed] [Google Scholar]
- 128.Chen X, Xie C, Nishida N, Li Z, Walz T, Springer TA. Requirement of open headpiece conformation for activation of leukocyte integrin alphaXbeta2. Proc. Natl. Acad. Sci. USA. 2010;107:14727–32. doi: 10.1073/pnas.1008663107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vinogradova O, Velyvis A, Velyviene A, Hu B, Haas T, et al. A structural mechanism of integrin alpha(IIb)beta(3) “inside-out” activation as regulated by its cytoplasmic face. Cell. 2002;110:587–97. doi: 10.1016/s0092-8674(02)00906-6. [DOI] [PubMed] [Google Scholar]
- 130.Weljie AM, Hwang PM, Vogel HJ. Solution structures of the cytoplasmic tail complex from platelet integrin alpha IIb- and beta 3-subunits. Proc. Natl. Acad. Sci. USA. 2002;99:5878–83. doi: 10.1073/pnas.092515799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ulmer TS, Yaspan B, Ginsberg MH, Campbell ID. NMR analysis of structure and dynamics of the cytosolic tails of integrin alpha IIb beta 3 in aqueous solution. Biochemistry. 2001;40:7498–508. doi: 10.1021/bi010338l. [DOI] [PubMed] [Google Scholar]
- 132.Vinogradova O, Vaynberg J, Kong X, Haas TA, Plow EF, Qin J. Membrane-mediated structural transitions at the cytoplasmic face during integrin activation. Proc. Natl. Acad. Sci. USA. 2004;101:4094–99. doi: 10.1073/pnas.0400742101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Metcalf DG, Moore DT, Wu Y, Kielec JM, Molnar K, et al. NMR analysis of the αIIbβ3 cytoplasmic interaction suggests a mechanism for integrin regulation. Proc. Natl. Acad. Sci. USA. 2010;107:22481–86. doi: 10.1073/pnas.1015545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Moser M, Legate KR, Zent R, Fassler R. The tail of integrins, talin, and kindlins. Science. 2009;324:895–99. doi: 10.1126/science.1163865. [DOI] [PubMed] [Google Scholar]
- 135.Shimaoka M, Xiao T, Liu JH, Yang Y, Dong Y, et al. Structures of the alpha L I domain and its complex with ICAM-1 reveal a shape-shifting pathway for integrin regulation. Cell. 2003;112:99–111. doi: 10.1016/s0092-8674(02)01257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hantgan RR, Stahle MC. Integrin priming dynamics: mechanisms of integrin antagonist-promoted alphaIIbbeta3:PAC-1 molecular recognition. Biochemistry. 2009;48:8355–65. doi: 10.1021/bi900475k. [DOI] [PubMed] [Google Scholar]
- 137.Hantgan RR, Stahle MC, Lord ST. Dynamic regulation of fibrinogen: integrin alphaIIbbeta3 binding. Biochemistry. 2010;49:9217–25. doi: 10.1021/bi1009858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Puklin-Faucher E, Sheetz MP. The mechanical integrin cycle. J. Cell Sci. 2009;122:179–86. doi: 10.1242/jcs.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Friedland JC, Lee MH, Boettiger D. Mechanically activated integrin switch controls alpha5beta1 function. Science. 2009;323:642–44. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]
- 140.Wang X, Lupardus P, Laporte SL, Garcia KC. Structural biology of shared cytokine receptors. Annu. Rev. Immunol. 2009;27:29–60. doi: 10.1146/annurev.immunol.24.021605.090616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–34. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lohse MJ. Dimerization in GPCR mobility and signaling. Curr. Opin. Pharmacol. 2010;10:53–58. doi: 10.1016/j.coph.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 143.Chung I, Akita R, Vandlen R, Toomre D, Schlessinger J, Mellman I. Spatial control of EGF receptor activation by reversible dimerization on living cells. Nature. 2010;464:783–87. doi: 10.1038/nature08827. [DOI] [PubMed] [Google Scholar]
- 144.Alvarado D, Klein DE, Lemmon MA. Structural basis for negative cooperativity in growth factor binding to an EGF receptor. Cell. 2010;142:568–79. doi: 10.1016/j.cell.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ye F, Hu G, Taylor D, Ratnikov B, Bobkov AA, et al. Recreation of the terminal events in physiological integrin activation. J. Cell. Biol. 2010;188:157–73. doi: 10.1083/jcb.200908045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ma YQ, Qin J, Wu C, Plow EF. Kindlin-2 (Mig-2): a co-activator of beta3 integrins. J. Cell. Biol. 2008;181:439–46. doi: 10.1083/jcb.200710196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Harburger DS, Bouaouina M, Calderwood DA. Kindlin-1 and -2 directly bind the C-terminal region of beta integrin cytoplasmic tails and exert integrin-specific activation effects. J. Biol. Chem. 2009;284:11485–97. doi: 10.1074/jbc.M809233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bledzka K, Bialkowska K, Nie H, Qin J, Byzova T, et al. Tyrosine phosphorylation of integrin beta3 regulates kindlin-2 binding and integrin activation. J. Biol. Chem. 2010;285:30370–74. doi: 10.1074/jbc.C110.134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Anthis NJ, Haling JR, Oxzley CL, Memo M, Wegener KL, et al. Beta integrin tyrosine phosphorylation is a conserved mechanism for regulating talin-induced integrin activation. J. Biol. Chem. 2009;284:36700–10. doi: 10.1074/jbc.M109.061275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kiema T, Lad Y, Jiang P, Oxley CL, Baldassarre M, et al. The molecular basis of filamin binding to integrins and competition with talin. Mol. Cell. 2006;21:337–47. doi: 10.1016/j.molcel.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 151.Takala H, Nurminen E, Nurmi SM, Aatonen M, Strandin T, et al. Beta2 integrin phosphorylation on Thr758 acts as a molecular switch to regulate 14-3-3 and filamin binding. Blood. 2008;112:1853–62. doi: 10.1182/blood-2007-12-127795. [DOI] [PubMed] [Google Scholar]