Abstract
Context
Antipsychotic drugs have been linked to QT-interval prolongation, a presumed marker of cardiac risk, and torsade de pointes.
Objective
To examine the associations between antipsychotics and 1) outpatient-originated sudden cardiac death and ventricular arrhythmia (SD/VA) and 2) all-cause death.
Design
Two retrospective cohort studies
Setting
Medicaid programs of California, Florida, New York, Ohio and Pennsylvania.
Patients
Incident antipsychotic users aged 30–75 years.
Main Outcome Measures
1) Incident, first-listed emergency department or principal inpatient SD/VA diagnoses; and 2) death reported in the Social Security Administration Death Master File.
Results
Among 459,614 incident antipsychotic users, the incidences of SD/VA and death were 3.4 and 35.1 per 1,000 person-years, respectively. Compared to olanzapine as the referent, adjusted hazard ratios (HRs) for SD/VA were 2.06 (95% CI, 1.20–3.53) for chlorpromazine, 1.72 (1.28–2.31) for haloperidol, and 0.73 (0.57–0.93) for quetiapine. Adjusted HRs for perphenazine and risperidone were consistent with unity. In a subanalysis limited to first prescription exposures, HRs for chlorpromazine and haloperidol were further elevated (2.54 [1.07–5.99] and 2.68 [1.59–4.53], respectively), with the latter exhibiting a dose-response relationship. Results for death were similar.
Conclusions
Haloperidol and chlorpromazine had less favorable cardiac safety profiles than olanzapine. Among atypical agents, risperidone had a similar cardiac safety profile to olanzapine, whereas quetiapine was associated with 30% and 20% lower risks of SD/VA and death, respectively, compared to olanzapine. These measured risks do not correlate well with average QT prolongation, further supporting the notion that average QT prolongation may be a poor surrogate of antipsychotic arrhythmogenicity.
Keywords: Antipsychotic agents, Cardiac arrhythmias, Cohort studies, Death, International classification of diseases, Medicaid, Pharmacoepidemiology, Proportional hazards models, Sudden death, Torsades de pointes
Introduction
Drug-induced sudden cardiac death (SD) and ventricular arrhythmia (VA) are major public health concerns that continue to be of interest to patients, clinicians, and regulators. Potential mechanisms include electrophysiological disturbances in cardiac depolarization and/or repolarization, myocardial ischemia, and myocarditis [1]. Electrocardiographic QT-interval prolongation is currently the most frequent cause of withdrawal or relabeling of marketed drugs [2]. CredibleMeds, developed by the University of Arizona Center for Education and Research on Therapeutics (AZ CERT), names more than 125 potentially arrhythmogenic drugs based on evidence or suspicion that each drug prolongs the QT interval and/or causes torsade de pointes (TdP) [3]. However, putative markers of risk, such as a drug’s ability to prolong the QT interval or inhibit cardiac ion channel currents, may not be reliable predictors of arrhythmogenicity in clinical use [4]. Therefore, outcome studies in clinical populations are crucial to quantify risk and inform our understanding of the etiology of drug-induced arrhythmias.
Antipsychotics have long been linked to electrocardiographic abnormalities, beginning with case reports in the early 1960s [5–8]. Subsequently, numerous studies have explored their effects on the QT interval, their effects in inhibiting the delayed rectifier potassium channel (IKr) encoded by the human ether-a-go-go related gene (hERG), and differences in antipsychotic drug concentration distribution between the myocardium and plasma [9–16]. Given that these measures, among others, have been used as surrogates for arrhythmia risk, CredibleMeds has classified 14 antipsychotics as torsadogenic [3]. Further, the entire therapeutic class (including both conventional and atypical agents) carries a boxed warning referencing SD [17], thioridazine carries an additional boxed warning describing its proarrhythmic effects [18], and many other antipsychotics reference QT prolongation/TdP in their label [19].
A number of studies have examined risks of clinical cardiovascular outcomes and death associated with antipsychotics [20–46], although few of these studies have reported agent-specific risks. This is important given that antipsychotics’ effects on putative markers of arrhythmogenicity differ greatly, even within conventional vs. atypical designations and within pharmacologic subclass (e.g., phenothiazines) [47,48]. We therefore conducted this study to compare antipsychotics with regard to risk of SD/VA. We also wished to examine all-cause death, given that some cases of SD/VA may not be identified as such in health care data. In the process, we would be testing whether published rankings of the antipsychotics by degree of QT prolongation predicted their clinical effects.
Materials and Methods
Overview and study population
We performed two incident-user cohort studies of the association between antipsychotics and SD/VA (primary outcome) and all-cause death (secondary outcome) within a large population of Medicaid and Medicaid-Medicare enrollees aged 30–75. The study excluded persons under age 30 because SD/VA is extremely rare in such individuals and may have a different cause [49]. Persons greater than age 75 were excluded, as significant competing comorbidities may influence causes of death in this group. Our study cohorts consisted exclusively of person-time exposed to: chlorpromazine, fluphenazine, haloperidol, loxapine, mesoridazine, molindone, perphenazine, pimozide, thioridazine, thiothixene, or trifluoperazine (conventional agents); or aripiprazole, clozapine, olanzapine, quetiapine, risperidone, or ziprasidone (atypical agents). To limit the potential for confounding by indication, no antipsychotic-unexposed groups were included. Rather, olanzapine was chosen as the reference exposure because: 1) it is not linked to substantive QT prolongation in the literature [50], supported by that fact that it had the smallest increase in mean corrected QT from baseline (1.7 milliseconds) among six antipsychotics evaluated in a randomized crossover trial in humans [51]; 2) it had the lowest IKr blocking potency among seven antipsychotics evaluated in murine cells [11]; 3) it does not alter heart rate variability, a predictor of sudden death [52]; 4) it served as the reference exposure in the Ziprasidone Observational Study of Cardiac Outcomes (ZODIAC) large simple trial [50]; and 5) it is used extensively in Medicaid enrollees.
The data for this study included that of the Medicaid programs of California, Florida, New York, Ohio, and Pennsylvania from 1999–2003, obtained from the Centers for Medicare and Medicaid Services (CMS) [53]. These states comprise about 21 million Medicaid enrollees at any one time (~38% of the United States Medicaid population) [54], and 32 million enrollees cumulatively, who contributed nearly 72 million person-years (p-y) of observation. Because ~15–17% of Medicaid beneficiaries were co-enrolled in Medicare during this period [55], our data also included Medicare claims for all dually-eligible persons in these states to ensure a more complete picture of their medical care. We linked these datasets to the Social Security Administration Death Master File (SSA-DMF) [56] to identify deaths.
The University of Pennsylvania’s Committee on Studies Involving Human Beings approved this study.
Eligible person-time
We included all person-time beginning with a prescription for a drug under study and ending with the earliest of the prescription claim’s days supply field, 30 days, or filling a subsequent prescription for a study drug (in which a new observation would begin), provided that no outcome of interest (described below) had yet been observed. We assumed that each antipsychotic prescription lasted for a maximum of 30 days because Medicaid prescriptions for such drugs in these states tend to be dispensed in 30-day increments [24], as we confirmed by examining frequency distributions of: 1) the pharmacist-entered days supply field; and 2) the number of days between subsequent antipsychotic prescriptions for the same enrollee. An exception to our 30-day assumption was implemented for clozapine claims, as this agent was dispensed in 7-day increments, consistent with dispensing procedures outlined in its label [57]. We excluded person-time defined by prescriptions for multiple different antipsychotics filled on the same date; this served to focus the study population to monotherapy-treated persons via efforts to exclude the non-recommended practice of purposeful antipsychotic polypharmacy [58].
Beneficiaries were eligible to contribute person-time to one or more of the 17 antipsychotic-specific exposure groups named above, if the beneficiary was an incident user of the respective antipsychotic. An incident user was defined as a beneficiary without a prescription claim for the given antipsychotic in the six months prior to the prescription claim of interest.
We performed additional, secondary analyses excluding person-time contributed by 1) second and later prescriptions for a given agent, to elucidate the effect in the initial prescription period and 2) enrollees with cancer, as such persons may disenroll from their Medicaid benefit soon after their cancer diagnosis, leading to incomplete follow-up [59].
Ascertainment of exposure, dose, and covariates
The antipsychotic agent of the prescription that contributed the relevant person-time determined exposure. The examination of daily antipsychotic dose, an analysis limited to the primary outcome, was facilitated by converting agent-specific dosages into chlorpromazine dose equivalents [60–62], then assuming that the prescription was consumed over the days supply. To ensure comparability among drugs when examining dose-response relationships, results were reported using low (< 100 mg chlorpromazine equivalents), medium (100–299 mg chlorpromazine equivalents), and high (≥ 300 mg chlorpromazine equivalents) dose categories [33].
As the concomitant administration of some antipsychotics and metabolic clearance inhibitors may lead to functional high-dose exposures of antipsychotics, and QT prolongation/TdP may be more likely in this setting [63], we examined (for the primary outcome) the association between each antipsychotic and SD/VA in persons receiving clinically-relevant cytochrome P-450 (CYP) 2D6 and 3A4 inhibitors. Each of these isozymes affects the metabolism of at least one of the antipsychotics under study [64] (see appendix Table A1). To isolate an effect of CYP inhibition on the antipsychotic’s pharmacokinetics rather than a direct arrhythmogenic effect the CYP inhibitor itself, we considered CYP inhibitors with known or suspected arrhythmogenic effects separately from those having no such effects.
We defined three types of potential confounding variables: 1) chronic diseases, defined as a diagnosis ever before the current study prescription; 2) drug markers of chronic disease, defined as a prescription ever before the current study prescription; and 3) current drugs, defined as a prescription in the 28 days prior through 10 days after the current study prescription. International Classification of Diseases (ICD-9) codes and National Drug Codes utilized are available from the authors.
Ascertainment of primary and secondary outcomes
As our goal was to study the effects of antipsychotics in an ambulatory population, our primary outcome of interest was an outpatient-originating SD or VA event precipitating hospital presentation. While VA cases are documented arrhythmic events, SD cases are generally considered undocumented arrhythmias (i.e., sudden and presumed arrhythmic) [65]. Therefore, our use of a composite SD/VA outcome is consistent with the high correlation between arrhythmic death and sudden death in the general community [66]. Incident SD/VA outcomes were identified in emergency department (ED) and inpatient Medicaid and Medicare claims having at least one of the following discharge ICD-9 diagnoses in a first-listed or principal position: paroxysmal ventricular tachycardia (427.1), ventricular fibrillation and flutter (427.4), ventricular fibrillation (427.41), ventricular flutter (427.42), cardiac arrest (427.5), sudden death (798), instantaneous death (798.1), or death occurring in less than 24 hours from onset of symptoms, not otherwise explained (798.2). We previously validated this algorithm, finding a positive predictive value (PPV) of 85% (95% confidence interval [CI] 78–91%) for identifying outpatient-originating SD/VA not due to extrinsic (e.g., traumatic) causes [67,68]. Although literal terminology would suggest otherwise, not all SD events result in the death of an individual, as some persons with SD are revived [69]. Therefore, we conducted a subanalysis limited to persons dying the day of or the day following their event date (i.e., fatal SD/VA, a subset of SD/VA).
Our secondary outcome of interest was all-cause death. In an effort to elucidate potential cardiac effects of antipsychotics on death, we conducted a subanalysis limited to persons dying as a result of a cardiac cause (i.e., cardiovascular death, a sub-outcome of death), as determined by cardiac diagnoses in a principal position on an inpatient claim or first-listed position on an ED claim occurring on the day of or day before the death date. This definition was adapted from that used by Ray et al. [33].
Statistical analysis
We first calculated unadjusted incidence rates (with 95% CIs) of each outcome for each antipsychotic. For drugs with at least 10 outcomes, we then used proportional hazards regression models to calculate adjusted hazard ratios (HRs) for the associations between each antipsychotic of interest and each outcome, using olanzapine as the referent. Potential confounding factors were evaluated using a change-in-estimate approach and were included in the adjusted model if a given covariate’s introduction changed the HR of interest by ≥ 10%. Analyses were conducted using SAS v9.1 (SAS Institute Inc.: Cary, North Carolina) and Stata v9.2 (StataCorp: College Station, Texas).
Role of the funding source
The National Institutes of Health and the Agency for Healthcare Research and Quality supported this study. Other than suggestions made by peer reviewers during the grant review process, the funding sources had no role in the study’s design, conduct, or interpretation.
Results
Incidence
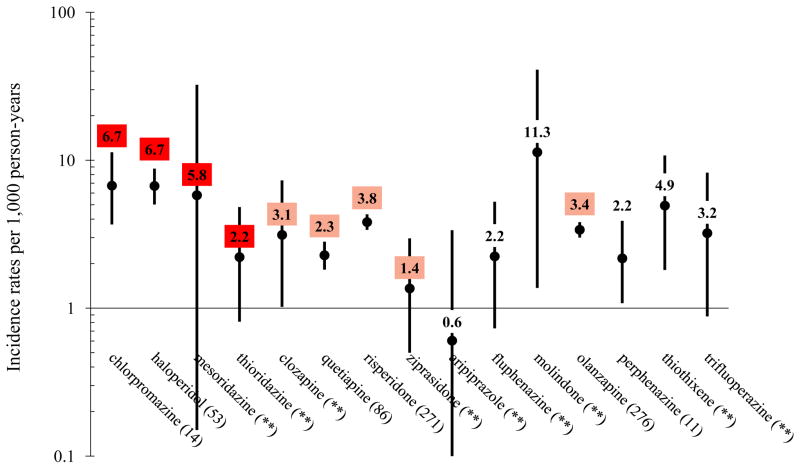
In the primary outcome cohort, we identified 459,614 incident antipsychotic users contributing 221,164 p-y of observation during which 747 incident SD/VAs occurred, for a crude incidence of 3.4 per 1,000 p-y. Agent-specific rates of SD/VA are presented in Figure 1. Of these 747 SD/VA events, 483 (65%) were fatal, for a crude incidence of 2.2 per 1,000 p-y. In the all-cause death (secondary outcome) cohort, we identified 462,163 incident antipsychotic users contributing 233,993 p-y of observation during which 8,207 all-cause deaths occurred, for a crude incidence rate of 35.1 per 1,000 p-y. Agent specific rates of death are presented in appendix Table A2. Of these 8,207 deaths, 742 (9%) occurred the same day or the day after an ED visit or hospitalization for a cardiac diagnosis, for a crude incidence of 3.2 per 1,000 p-y.
Figure 1.
Crude incidence rates of sudden cardiac death/ventricular arrhythmia (N = 747 events) among antipsychotics users, by drug*,†
*Values in parentheses following each antipsychotic drug name are numbers of incident sudden cardiac death/ventricular arrhythmia events occurring during person-time of exposure to the given drug (i.e., N events); a double asterisk (**) has replaced each N-value ≤ 10, in accordance with CMS privacy policies. Pimozide and loxapine were excluded from the figure, as neither group had any events in very limited person-time (137 and 377 person-years, respectively).
†The color-shading is reflective of CredibleMeds ratings of drugs’ QT-prolonging/TdP-inducing potentials, and is consistent with their coloring scheme: a) red = drugs with a risk of TdP; b) pink = drugs with a possible risk of TdP; and c) not shaded = drugs not rated. Of note, olanzapine was transitioned from a non-rated drug to a drug with possible risk (i.e., pink) on 01/04/2013.
As 11 and four of the 17 study antipsychotics had < 10 primary and secondary events respectively, they were subsequently excluded from the multivariable analyses of their respective outcomes. Appendix Table A3 shows characteristics of users of the remaining six antipsychotics in the primary outcome cohort. Users were predominantly female and non-elders, and included considerable numbers of minorities, as is representative of the base Medicaid population under study. Users of antipsychotics were rather similar, other than in nursing home residence and certain indication-specific (e.g., chlorpromazine’s use in hiccough) and formulation-specific (e.g., perphenazine’s availability with an antidepressant in a combination pill) instances.
Modeled results
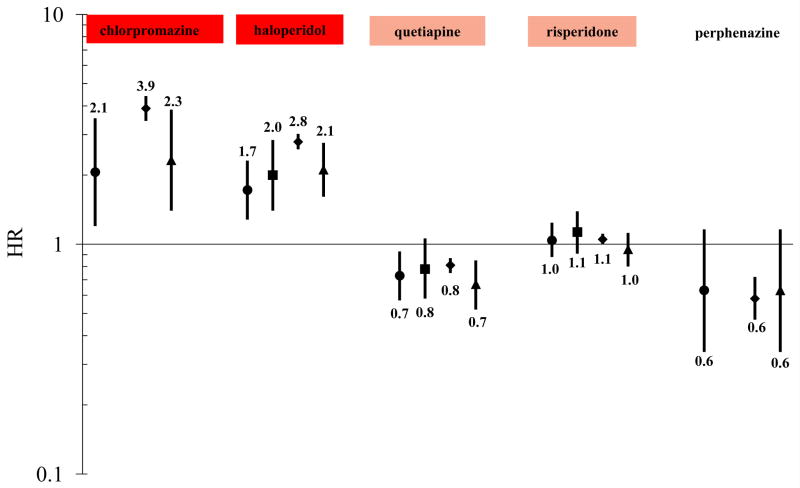
Unadjusted HRs for individual antipsychotics and SD/VA (compared to an olanzapine referent) were minimally different from adjusted estimates and therefore are not presented for sake of brevity (data available from authors). Adjusted HRs for SD/VA (compared to an olanzapine referent) were: 2.06 (1.20–3.53) for chlorpromazine; 1.72 (1.28–2.31) for haloperidol; 0.63 (0.34–1.16) for perphenazine; 0.73 (0.57–0.93) for quetiapine; and 1.04 (0.88–1.24) for risperidone (Figure 2, ●). Adjusted HRs for individual antipsychotics and all-cause death (compared to an olanzapine referent) were: 0.42 (0.27- 0.65) for aripiprazole; 3.90 (3.44–4.41) for chlorpromazine; 0.58 (0.39- 0.89) for clozapine; 1.13 (0.85–1.50) for fluphenazine; 2.79 (2.59–3.02) for haloperidol; 0.58 (0.47–0.72) for perphenazine; 0.81 (0.75–0.87) for quetiapine; 1.05 (1.00–1.11) for risperidone; 0.73 (0.56–0.96) for thioridazine; 1.12 (0.80–1.57) for thiothixene; 0.46 (0.27–0.78) for trifluoperazine; and 0.60 (0.46–0.78) for ziprasidone. See Figure 2 for death effect estimates (◆), limited to antipsychotics also evaluated for the primary outcome. Figure 2 further depicts adjusted HRs for aforementioned sub-outcomes, fatal SD/VA (■) and cardiovascular death (▲), limited to antipsychotics also evaluated for the primary outcome. Subanalyses excluding beneficiaries with cancer yielded similar results for drugs examined in each outcome (data available from authors). A presentation of adjusted HRs for antipsychotics examined for at least one of the study outcomes, without regard to their ability to be evaluated for the primary outcome, can be found in appendix Figure A1.
Figure 2.
Change-in-estimate adjusted* HRs for sudden cardiac death/ventricular arrhythmia, fatal sudden cardiac death/ventricular arrhythmia, death, and cardiovascular death—each versus olanzapine as the reference exposure†
Outcome key: ● SD/VA ■ Fatal SD/VA ◆ Death ▲ Cardiovascular death
*SD/VA model adjusted for age, sex, race, state of residence, nursing home residence, prior diagnosis of organic psychosis, and current exposure to cyclic and related antidepressant; Fatal SD/VA model adjusted for age, sex, race, state of residence, and nursing home residence; Death model adjusted for age, sex, race, state of residence, nursing home residence, prior diagnosis of heart failure/cardiomyopathy, prior diagnosis of kidney disease, prior diagnosis of organic psychosis, prior diagnosis of other psychoses, loop diuretic drug marker of disease, and oral corticosteroid drug marker of disease; Cardiovascular death model adjusted for age, sex, race, state of residence, nursing home residence, prior diagnosis of organic psychosis, and current exposure to cyclic and related antidepressant.
†The color-shading is reflective of CredibleMeds ratings of drugs’ QT-prolonging/TdP-inducing potentials, and is consistent with their coloring scheme: a) red = drugs with a risk of TdP; b) pink = drugs with a possible risk of TdP; and c) not shaded = drugs not rated. Of note, olanzapine was transitioned from a non-rated drug to a drug with possible risk (i.e., pink) on 01/04/2013.
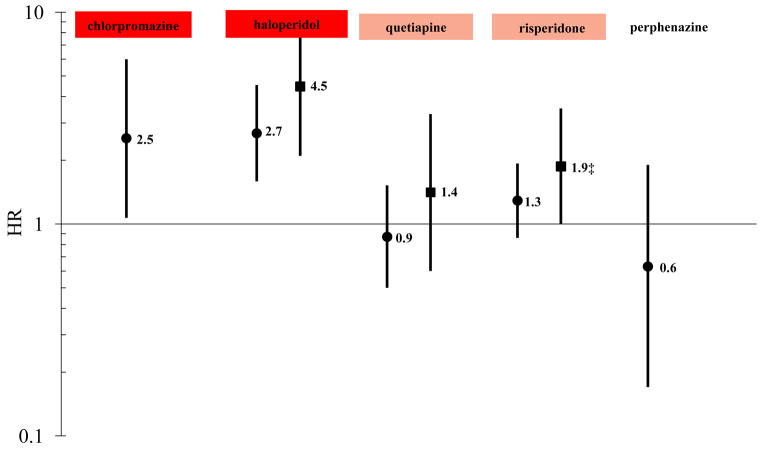
Results of the subanalysis limited to first antipsychotic prescription exposures are presented in Figure 3. Of note, compared to the analyses including both first and later antipsychotic prescriptions, HRs of SD/VA for first prescription exposures to chlorpromazine, haloperidol, quetiapine, and risperidone were greater by 19%, 59%, 29%, and 30%, respectively. HRs of fatal SD/VA for first prescription exposures to haloperidol, quetiapine, and risperidone were greater by 225%, 75%, and 73%, respectively. Chlorpromazine and perphenazine had too few fatal SD/VA events during first prescription exposures to be examined.
Figure 3.
Change-in-estimate adjusted* HRs for sudden cardiac death/ventricular arrhythmia and fatal sudden cardiac death/ventricular arrhythmia, limited to first antipsychotic prescription exposures—each versus olanzapine as the reference exposure†
Outcome key: ● SD/VA ■ Fatal SD/VA
* SD/VA first antipsychotic prescription model adjusted for age, sex, race, state of residence, nursing home residence, prior diagnosis of organic psychosis, and current exposure to cyclic and related antidepressant; Fatal SD/VA first antipsychotic prescription model adjusted for age, sex, race, state of residence, and nursing home residence.
† The color-shading is reflective of CredibleMeds ratings of drugs’ QT-prolonging/TdP-inducing potentials, and is consistent with their coloring scheme: a) red = drugs with a risk of TdP; b) pink = drugs with a possible risk of TdP; and c) not shaded = drugs not rated. Of note, olanzapine was transitioned from a non-rated drug to a drug with possible risk (i.e., pink) on 01/04/2013.
‡ Lower bound of 95% CI = 1.00 when rounded to two decimal places, but p < 0.05
Analyses examining antipsychotic dose and concomitantly-prescribed CYP inhibitors
For each of the six antipsychotics examined for the primary outcome, high-dose exposure was not significantly associated with an increased risk of SD/VA compared to a low-dose referent (data available from authors). For four of these six antipsychotics, we were able to examine the impact of first prescription exposures by dose. While HRs for medium- and high-dose olanzapine, quetiapine, and risperidone first prescription exposures were consistent with unity (compared to a low-dose referent), first prescription haloperidol exposure demonstrated a significant dose-response relationship (HR medium-dose=1.37, 0.36–5.23; HR high-dose=4.55, 1.22–16.94; p-value for linear trend=0.03). These models were adjusted for 37 covariates listed in appendix Table A4.
For each of the six antipsychotics examined for the primary outcome, concomitant exposures to clinically relevant CYP450 inhibitors did not increase the risk of SD/VA (data available from authors), even for antipsychotics such as haloperidol and risperidone that are major substrates of such pathways.
Discussion
Antipsychotics may have widely variable effects on intermediaries along the hypothesized hERG affinity-to-SD pathway [13], including differences in ion channel inhibition, receptor blockade and selectivity, action potential duration, early after depolarization, and QT prolongation. Average QT prolongation has been of particular interest to regulators and drug developers as evidenced by guidance documents on the conduct of QT studies [70,71] and prescribing recommendations based on QT prolongation [72]. See appendix Table A5 for average effects on the QT interval for antipsychotics under study. As average QT prolongation and other intermediaries may or may not truly correlate with a given antipsychotic’s propensity for causing clinically important arrhythmias, the conduct of epidemiologic studies of clinical outcomes of these agents, rather than surrogates, is crucial to understanding their safety in real-life use. Our study found that haloperidol and chlorpromazine were associated with ~2-fold greater risks of SD/VA and 3- and 4-fold greater risks of death (respectively), compared to olanzapine. Further, the risks of SD/VA were even more prominent upon first prescription exposures. Quetiapine was associated with 30% and 20% lower risks of SD/VA and death, respectively, compared to olanzapine. Perphenazine also appeared to be associated with lower risks of these outcomes, but this may have been driven by its comparatively low utilization by users residing in nursing homes and/or with diagnosed mental health disorders. Of note, numerous other conventional and atypical antipsychotics slated for study, but excluded because of low numbers of primary outcome events, were sparsely utilized despite this being the largest known epidemiologic study of antipsychotic-induced arrhythmia to date.
Some aspects of these results agree and others conflict with prior research conducted in the Medicaid population. Our prior study [25], using 1993–1996 data from three Medicaid states (a timeframe that does not overlap with the current study), found an elevated risk of SD/VA for haloperidol (rate ratio [RR]=2.2, 1.7–3.0) vs. an antipsychotic-unexposed glaucoma patient referent—a result consistent with our current findings. In contrast to the current study, our prior study identified an elevated risk of SD/VA with risperidone (RR=3.1, 2.2–4.5). However, our subsequent validation studies [67,68] have shown us that the outcome definition used in our prior study likely had a suboptimal PPV, as it was not limited to principal diagnoses.
A prior study by Ray et al. [34], using 1988–1993 Medicaid data from Tennessee, utilized a different method for ascertaining SD and found elevated risks with chlorpromazine and haloperidol (RR=3.64, 1.36–9.74 and RR=1.90, 1.10–3.30, respectively), compared to a nonuser referent. Our results are generally consistent with these findings. A more recent study by Ray et al. [33], using 1999–2005 Tennessee Medicaid data, found increased risks of SD for haloperidol, olanzapine, quetiapine, and risperidone compared to a non-user referent. Notably, olanzapine——the drug used as the referent in our study——was found to have an incidence rate ratio (IRR) =1.99 (1.41–2.79) vs. non-users. To facilitate a more direct comparison between our study and theirs, we reset Ray et al’s reference group from non-users to olanzapine, using his published results to generate the following IRRs. After doing so, our results were consistent for quetiapine (IRR~0.75); equivocal for risperidone (IRR~1.25); and contradictory for haloperidol (IRR~0.70).
Our study has several strengths. First, it is the largest study to date of this question. Second, it uses an outcome previously well validated in the population in which it is being used. Third, the results have face validity, given the agreement between our effect estimates and ratings of drugs with a TdP risk by CredibleMeds experts. Further, although we were unable to examine ziprasidone’s SD/VA risk in adjusted models because of limited events during its person-time, its IRR (vs. olanzapine) was 0.41. This result is consistent with the findings of ZODIAC (RR=0.67 for ziprasidone vs. olanzapine for SD) [50], thereby further corroborating our results.
These results should be interpreted with the following limitations in mind. First, an important limitation of any non-randomized pharmacoepidemiologic study is the potential for confounding by indication. We attempted to limit this potential by studying only person-time with exposure to an antipsychotic and by measuring and adjusting for a number of potential confounding factors. Nevertheless, we cannot rule out the possibility that unmeasured factors may have contributed to the observed associations.
Second is the potential for incomplete ascertainment of outcomes. For example, because this study relied upon ED and inpatient diagnoses, it would have missed fatal events that did not result in presentation to a hospital. However, studies suggest that 69–80% of persons experiencing an out-of-hospital cardiac arrest [73,74] and up to 88% of persons experiencing a witnessed ventricular tachycardia survive to hospital admission [75], although literature estimates do vary by year and subject age [76]. Furthermore, trends suggest that survival until hospital admission among such persons is increasing over time [77]. An approach by which out-of-hospital events could be captured, via use of death certificate diagnoses alone, has been shown to have a poor PPV for identifying SD/VA [78–80].
Third, because of both low utilization and a low number of SD/VA events, we were unable to estimate measures of association for a number of drugs of interest.
Finally, the ascertainment of certain covariates within administrative billing claims (such as Medicaid and Medicare) may be incomplete. In particular, ascertaining a beneficiary’s smoking status and alcohol/substance use or abuse is notoriously difficult, and was principally limited to related or surrogate diagnoses appearing on encounter claims (e.g., alcoholic cirrhosis as a surrogate for an alcohol user). Given our reliance on proxies for these difficult-to-measure covariates, occurrences of such conditions were likely under-ascertained [81].
Our study found that haloperidol and chlorpromazine were associated with an approximate doubling of the risk of SD/VA and tripling to quadrupling of the risk of death vs. olanzapine——suggesting that these conventional antipsychotics have a less favorable cardiac safety profile than olanzapine. Among atypical agents, risperidone showed a similar cardiac safety profile to olanzapine, whereas quetiapine was associated with 30% and 20% lower risks of SD/VA and death, respectively, vs. olanzapine. As expected, these risks do not necessarily correlate with published estimates of the duration of average QT prolongation in the presence of individual antipsychotics [15,51,82], further supporting the notion that average prolongation of the QT interval is a poor surrogate of a drug’s arrhythmogenic potential [83]. Given this, it is prudent to remain cognizant of other mechanisms by which antipsychotics may induce arrhythmia [84], including: autonomic dysregulation, such as sympathetic hyperactivity [85] (e.g., elevated plasma norepinephrine levels with chlorpromazine); vascular effects, such as changes in blood pressure (e.g., hypertension with clozapine) [86]; other cardiac effects, such as a reduction in heart rate variability (e.g., thioridazine) [87]; inhibitory potential of IKr that is modifiable by interactions with cardiac adrenergic receptors [84]; direct cardiotoxic effects (e.g., myocarditis with haloperidol) [88]; and indirect effects on metabolism, such as weight gain. Although further research is needed to elucidate mechanism, our results provide clinicians with agent-specific risks of serious arrhythmogenic outcomes among antipsychotics users, a population already at higher baseline risk of premature death due to cardiovascular disease by nature of their illness [89].
Acknowledgments
The authors wish to thank Geralyn Barosso, MPH, MS from the Research Data Assistance Center at the University of Minnesota’s School of Public Health for her expertise in the use of Centers for Medicare and Medicaid Services claims. We thank Rajat Deo, MD of the University of Pennsylvania’s Division of Cardiovascular Medicine for his expertise in cardiac electrophysiology. We thank Qing Liu, BS, Hopiy Kim, BS and Fei Wan, MS of the University of Pennsylvania’s Biostatistics Analysis Center for their programmatic work. Finally, we thank Hanieh Razzaghi, MPH of the University of Pennsylvania’s Center for Clinical Epidemiology and Biostatistics and Center for Pharmacoepidemiology Research and Training for her assistance in literature reviews and interpreting results.
Funding/support: This study was supported by the following grants from the U.S. Department of Health & Human Services: National Institutes of Health (5R01HL076697, 2R01AG025152, 8UL1TR000003, 3UL1RR024134) and Agency for Healthcare Research and Quality (5U18HS010389, 5U18HS016946). Other than suggestions made during the grant’s peer-review process, none of the funders had a role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Previous presentation: A portion of the results described herein were presented, in abstract form, at the 26th International Conference on Pharmacoepidemiology & Therapeutic Risk Management (Brighton, England, United Kingdom) in August 2010.
Potential conflicts: Dr. Leonard has no conflicts of interest to report. Ms. Freeman is partially supported by research funds from AstraZeneca and Bristol-Myers Squibb, each unrelated to this topic. Mr. Newcomb has no conflicts of interest to report. Dr. Bilker has no conflicts of interest to report. Dr. Kimmel has received funding and done consulting for several pharmaceutical companies including Novartis, GlaxoSmithKline, Pfizer and Merck, and received an honorarium from Ortho-McNeil, all unrelated to this topic. Dr. Strom has received research funding from AstraZeneca and Bristol-Myers Squibb, and consulted for Bristol-Myers Squibb, GlaxoSmithKline, Pfizer, and Teva, all unrelated to this topic. Dr. Hennessy has received funding from AstraZeneca and Bristol-Myers Squibb, and consulted for AstraZeneca, Endo, Novartis, and Wyeth, all unrelated to this topic.
References
- 1.Timour Q, Frassati D, Descotes J, Chevalier P, Christé G, et al. Sudden death of cardiac origin and psychotropic drugs. Front Pharmacol. 2012;3:76. doi: 10.3389/fphar.2012.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Letsas KP, Tsikrikas S, Letsas GP, Sideris A. Drug-induced proarrhythmia: QT interval prolongation and torsades de pointes. Hospital Chronicles. 2011;6:118–122. [Google Scholar]
- 3.Arizona Center for Education and Research on Therapeutics: QT drug lists by risk groups. 2013. [Google Scholar]
- 4.Sala M, Lazzaretti M, De Vidovich G, Caverzasi E, Barale F, et al. Electrophysiological changes of cardiac function during antidepressant treatment. Ther Adv Cardiovasc Dis. 2009;3:29–43. doi: 10.1177/1753944708096282. [DOI] [PubMed] [Google Scholar]
- 5.Kelly HG, Fay JE, Laverty SG. Thioridazine Hydrochloride (Mellaril): Its Effect On The Electrocardiogram and a Report of Two Fatalities with Electrocardiographic Abnormalities. Can Med Assoc J. 1963;89:546–554. [PMC free article] [PubMed] [Google Scholar]
- 6.Baeckman H, Elosuo R. Electrocardiographic Findings in Connection with a Clinical Trial of Chlorpromazine. With Particular Reference to T Wave Changes and The Duration of Ventricular Activity. Ann Med Intern Fenn. 1964;53:1–8. [PubMed] [Google Scholar]
- 7.Ban Ta, Stjean A. The Effect of Phenothiazines on the Electrocardiogram. Can Med Assoc J. 1964;91:537–540. [PMC free article] [PubMed] [Google Scholar]
- 8.Desautels S, Filteau C, St-Jean A. Ventricular Tachycardia Associated With Administration Of Thioridazine Hydrochloride (Mellaril): Report of a Case with a Favourable Outcome. Can Med Assoc J. 1964;90:1030–1031. [PMC free article] [PubMed] [Google Scholar]
- 9.Nielsen J, Graff C, Kanters JK, Toft E, Taylor D, et al. Assessing QT interval prolongation and its associated risks with antipsychotics. CNS Drugs. 2011;25:473–490. doi: 10.2165/11587800-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Stöllberger C, Huber JO, Finsterer J. Antipsychotic drugs and QT prolongation. Int Clin Psychopharmacol. 2005;20:243–251. doi: 10.1097/01.yic.0000166405.49473.70. [DOI] [PubMed] [Google Scholar]
- 11.Pfizer: FDA psychopharmacological drugs advisory committee, briefing document for Zeldox® capsules (ziprasidone HCl) 2000. [Google Scholar]
- 12.Calderone V, Testai L, Martinotti E, Del Tacca M, Breschi MC. Drug-induced block of cardiac HERG potassium channels and development of torsade de pointes arrhythmias: the case of antipsychotics. J Pharm Pharmacol. 2005;57:151–161. doi: 10.1211/0022357055272. [DOI] [PubMed] [Google Scholar]
- 13.Titier K, Girodet PO, Verdoux H, Molimard M, Bégaud B, et al. Atypical antipsychotics: from potassium channels to torsade de pointes and sudden death. Drug Saf. 2005;28:35–51. doi: 10.2165/00002018-200528010-00003. [DOI] [PubMed] [Google Scholar]
- 14.Ekins S, Crumb WJ, Sarazan RD, Wikel JH, Wrighton SA. Three-dimensional quantitative structure-activity relationship for inhibition of human ether-a-go-go-related gene potassium channel. J Pharmacol Exp Ther. 2002;301:427–434. doi: 10.1124/jpet.301.2.427. [DOI] [PubMed] [Google Scholar]
- 15.Kongsamut S, Kang J, Chen XL, Roehr J, Rampe D. A comparison of the receptor binding and HERG channel affinities for a series of antipsychotic drugs. Eur J Pharmacol. 2002;450:37–41. doi: 10.1016/s0014-2999(02)02074-5. [DOI] [PubMed] [Google Scholar]
- 16.Titier K, Canal M, Déridet E, Abouelfath A, Gromb S, et al. Determination of myocardium to plasma concentration ratios of five antipsychotic drugs: comparison with their ability to induce arrhythmia and sudden death in clinical practice. Toxicol Appl Pharmacol. 2004;199:52–60. doi: 10.1016/j.taap.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Food and Drug Administration. Information for healthcare professionals: Conventional antipsychotics. 2008. [Google Scholar]
- 18.Food and Drug Administration. Mellaril (thioridazine HCl) dear healthcare professional letter, Jul 2000. 2000. [Google Scholar]
- 19.Drug Facts & Comparisons: Antipsychotic agents. 2013. [Google Scholar]
- 20.Curkendall SM, Mo J, Glasser DB, Rose Stang M, Jones JK. Cardiovascular disease in patients with schizophrenia in Saskatchewan, Canada. J Clin Psychiatry. 2004;65:715–720. doi: 10.4088/jcp.v65n0519. [DOI] [PubMed] [Google Scholar]
- 21.De Bruin ML, Hoes AW, Leufkens HG. QTc-prolonging drugs and hospitalizations for cardiac arrhythmias. Am J Cardiol. 2003;91:59–62. doi: 10.1016/s0002-9149(02)02998-3. [DOI] [PubMed] [Google Scholar]
- 22.Enger C, Weatherby L, Reynolds RF, Glasser DB, Walker AM. Serious cardiovascular events and mortality among patients with schizophrenia. J Nerv Ment Dis. 2004;192:19–27. doi: 10.1097/01.nmd.0000105996.62105.07. [DOI] [PubMed] [Google Scholar]
- 23.Gill SS, Bronskill SE, Normand SL, Anderson GM, Sykora K, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146:775–786. doi: 10.7326/0003-4819-146-11-200706050-00006. [DOI] [PubMed] [Google Scholar]
- 24.Hennessy S, Bilker WB, Knauss JS, Kimmel SE, Margolis DJ, et al. Comparative cardiac safety of low-dose thioridazine and low-dose haloperidol. Br J Clin Pharmacol. 2004;58:81–87. doi: 10.1111/j.1365-2125.2004.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hennessy S, Bilker WB, Knauss JS, Margolis DJ, Kimmel SE, et al. Cardiac arrest and ventricular arrhythmia in patients taking antipsychotic drugs: cohort study using administrative data. BMJ. 2002;325:1070. doi: 10.1136/bmj.325.7372.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hollis J, Forrester L, Brodaty H, Touyz S, Cumming R, et al. Risk of death associated with antipsychotic drug dispensing in residential aged care facilities. Aust N Z J Psychiatry. 2007;41:751–758. doi: 10.1080/00048670701519864. [DOI] [PubMed] [Google Scholar]
- 27.Honkola J, Hookana E, Malinen S, Kaikkonen KS, Junttila MJ, et al. Psychotropic medications and the risk of sudden cardiac death during an acute coronary event. Eur Heart J. 2012;33:745–751. doi: 10.1093/eurheartj/ehr368. [DOI] [PubMed] [Google Scholar]
- 28.Huybrechts KF, Rothman KJ, Silliman RA, Brookhart MA, Schneeweiss S. Risk of death and hospital admission for major medical events after initiation of psychotropic medications in older adults admitted to nursing homes. CMAJ. 2011;183:E411–419. doi: 10.1503/cmaj.101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jolly K, Gammage MD, Cheng KK, Bradburn P, Banting MV, et al. Sudden death in patients receiving drugs tending to prolong the QT interval. Br J Clin Pharmacol. 2009;68:743–751. doi: 10.1111/j.1365-2125.2009.03496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kales HC, Valenstein M, Kim HM, McCarthy JF, Ganoczy D, et al. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry. 2007;164:1568–1576. doi: 10.1176/appi.ajp.2007.06101710. [DOI] [PubMed] [Google Scholar]
- 31.Liperoti R, Gambassi G, Lapane KL, Chiang C, Pedone C, et al. Conventional and atypical antipsychotics and the risk of hospitalization for ventricular arrhythmias or cardiac arrest. Arch Intern Med. 2005;165:696–701. doi: 10.1001/archinte.165.6.696. [DOI] [PubMed] [Google Scholar]
- 32.Mehta S, Chen H, Johnson M, Aparasu RR. Risk of serious cardiac events in older adults using antipsychotic agents. Am J Geriatr Pharmacother. 2011;9:120–132. doi: 10.1016/j.amjopharm.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360:225–235. doi: 10.1056/NEJMoa0806994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ray WA, Meredith S, Thapa PB, Meador KG, Hall K, et al. Antipsychotics and the risk of sudden cardiac death. Arch Gen Psychiatry. 2001;58:1161–1167. doi: 10.1001/archpsyc.58.12.1161. [DOI] [PubMed] [Google Scholar]
- 35.Reilly JG, Ayis SA, Ferrier IN, Jones SJ, Thomas SH. Thioridazine and sudden unexplained death in psychiatric in-patients. Br J Psychiatry. 2002;180:515–522. doi: 10.1192/bjp.180.6.515. [DOI] [PubMed] [Google Scholar]
- 36.Schneeweiss S, Setoguchi S, Brookhart A, Dormuth C, Wang PS. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ. 2007;176:627–632. doi: 10.1503/cmaj.061250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294:1934–1943. doi: 10.1001/jama.294.15.1934. [DOI] [PubMed] [Google Scholar]
- 38.Setoguchi S, Wang PS, Brookhart MA, Canning CF, Kaci L, et al. Potential causes of higher mortality in elderly users of conventional and atypical antipsychotic medications. J Am Geriatr Soc. 2008;56:1644–1650. doi: 10.1111/j.1532-5415.2008.01839.x. [DOI] [PubMed] [Google Scholar]
- 39.Straus SM, Bleumink GS, Dieleman JP, van der Lei J, ‘t Jong GW, et al. Antipsychotics and the risk of sudden cardiac death. Arch Intern Med. 2004;164:1293–1297. doi: 10.1001/archinte.164.12.1293. [DOI] [PubMed] [Google Scholar]
- 40.Thomas SH, Drici MD, Hall GC, Crocq MA, Everitt B, et al. Safety of sertindole versus risperidone in schizophrenia: principal results of the sertindole cohort prospective study (SCoP) Acta Psychiatr Scand. 2010;122:345–355. doi: 10.1111/j.1600-0447.2010.01563.x. [DOI] [PubMed] [Google Scholar]
- 41.Tiihonen J, Lönnqvist J, Wahlbeck K, Klaukka T, Niskanen L, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study) Lancet. 2009;374:620–627. doi: 10.1016/S0140-6736(09)60742-X. [DOI] [PubMed] [Google Scholar]
- 42.van Noord C, Sturkenboom MC, Straus SM, Witteman JC, Stricker BH. Non-cardiovascular drugs that inhibit hERG-encoded potassium channels and risk of sudden cardiac death. Heart. 2011;97:215–220. doi: 10.1136/hrt.2009.188367. [DOI] [PubMed] [Google Scholar]
- 43.Walker AM, Lanza LL, Arellano F, Rothman KJ. Mortality in current and former users of clozapine. Epidemiology. 1997;8:671–677. doi: 10.1097/00001648-199710000-00010. [DOI] [PubMed] [Google Scholar]
- 44.Wang PS, Schneeweiss S, Setoguchi S, Patrick A, Avorn J, et al. Ventricular arrhythmias and cerebrovascular events in the elderly using conventional and atypical antipsychotic medications. J Clin Psychopharmacol. 2007;27:707–710. doi: 10.1097/JCP.0b013e31815a882b. [DOI] [PubMed] [Google Scholar]
- 45.Wang PS, Schneeweiss S, Avorn J, Fischer MA, Mogun H, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353:2335–2341. doi: 10.1056/NEJMoa052827. [DOI] [PubMed] [Google Scholar]
- 46.Wilton LV, Heeley EL, Pickering RM, Shakir SA. Comparative study of mortality rates and cardiac dysrhythmias in post-marketing surveillance studies of sertindole and two other atypical antipsychotic drugs, risperidone and olanzapine. J Psychopharmacol. 2001;15:120–126. doi: 10.1177/026988110101500212. [DOI] [PubMed] [Google Scholar]
- 47.Crumb WJ, Jr, Ekins S, Sarazan RD, Wikel JH, Wrighton SA, et al. Effects of antipsychotic drugs on I(to), I (Na), I (sus), I (K1), and hERG: QT prolongation, structure activity relationship, and network analysis. Pharm Res. 2006;23:1133–1143. doi: 10.1007/s11095-006-0070-7. [DOI] [PubMed] [Google Scholar]
- 48.Lindström E, Farde L, Eberhard J, Haverkamp W. QTc interval prolongation and antipsychotic drug treatments: focus on sertindole. Int J Neuropsychopharmacol. 2005;8:615–629. doi: 10.1017/S1461145705005250. [DOI] [PubMed] [Google Scholar]
- 49.Liberthson RR. Sudden death from cardiac causes in children and young adults. N Engl J Med. 1996;334:1039–1044. doi: 10.1056/NEJM199604183341607. [DOI] [PubMed] [Google Scholar]
- 50.Strom BL, Eng SM, Faich G, Reynolds RF, D’Agostino RB, et al. Comparative mortality associated with ziprasidone and olanzapine in real-world use among 18,154 patients with schizophrenia: The Ziprasidone Observational Study of Cardiac Outcomes (ZODIAC) Am J Psychiatry. 2011;168:193–201. doi: 10.1176/appi.ajp.2010.08040484. [DOI] [PubMed] [Google Scholar]
- 51.Harrigan EP, Miceli JJ, Anziano R, Watsky E, Reeves KR, et al. A randomized evaluation of the effects of six antipsychotic agents on QTc, in the absence and presence of metabolic inhibition. J Clin Psychopharmacol. 2004;24:62–69. doi: 10.1097/01.jcp.0000104913.75206.62. [DOI] [PubMed] [Google Scholar]
- 52.Hempel RJ, Tulen JH, van Beveren NJ, Röder CH, Hengeveld MW. Cardiovascular variability during treatment with haloperidol, olanzapine or risperidone in recent-onset schizophrenia. J Psychopharmacol. 2009;23:697–707. doi: 10.1177/0269881108091254. [DOI] [PubMed] [Google Scholar]
- 53.Hennessy S, Carson JL, Ray WA, Strom BL. Medicaid databases. In: Strom BL, editor. Pharmacoepidemiology. Sussex: John Wiley; 2005. [Google Scholar]
- 54.Centers for Medicare & Medicaid Services. MSIS tables. [Google Scholar]
- 55.Medicare Payment Advisory Commission. Report to the congress: New approaches in Medicare. 2004. [Google Scholar]
- 56.National Technical Information Service. Social security administration’s death master file. 2011. [Google Scholar]
- 57.Drug Facts & Comparisons: Clozapine. 2013. [Google Scholar]
- 58.Buchanan RW, Kreyenbuhl J, Kelly DL, Noel JM, Boggs DL, et al. The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36:71–93. doi: 10.1093/schbul/sbp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramsey SD, Zeliadt SB, Richardson LC, Pollack L, Linden H, et al. Disenrollment from Medicaid after recent cancer diagnosis. Med Care. 2008;46:49–57. doi: 10.1097/MLR.0b013e318158ec7f. [DOI] [PubMed] [Google Scholar]
- 60.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 61.Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Supplement to: Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360:225–235. doi: 10.1056/NEJMoa0806994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nosè M, Tansella M, Thornicroft G, Schene A, Becker T, et al. Is the Defined Daily Dose system a reliable tool for standardizing antipsychotic dosages? Int Clin Psychopharmacol. 2008;23:287–290. doi: 10.1097/YIC.0b013e328303ac75. [DOI] [PubMed] [Google Scholar]
- 63.Zemrak WR, Kenna GA. Association of antipsychotic and antidepressant drugs with Q-T interval prolongation. Am J Health Syst Pharm. 2008;65:1029–1038. doi: 10.2146/ajhp070279. [DOI] [PubMed] [Google Scholar]
- 64.Flockhart DA. Drug interactions: Cytochrome P450 drug interaction table. [Google Scholar]
- 65.McMurray J. Cardiology Scientific Update. 2002. Prophylactic class III antiarrhythmic drug therapy after myocardial infarction: ALIVE or dead? [Google Scholar]
- 66.Abildstrom S, Køber L, Torp-Pedersen C. Epidemiology of arrhythmic and sudden death in the chronic phase of ischemic heart disease. CEPR. 1999;3:177–179. [Google Scholar]
- 67.Hennessy S, Leonard CE, Freeman CP, Deo R, Newcomb C, et al. Validation of diagnostic codes for outpatient-originating sudden cardiac death and ventricular arrhythmia in Medicaid and Medicare claims data. Pharmacoepidemiol Drug Saf. 2010;19:555–562. doi: 10.1002/pds.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mines D. Commentary on the validation studies of sudden cardiac death and ventricular arrhythmia by Hennessy et al. and Chung et al. Pharmacoepidemiol Drug Saf. 2010;19:573–575. doi: 10.1002/pds.1913. [DOI] [PubMed] [Google Scholar]
- 69.Cummins RO, Ornato JP, Thies WH, Pepe PE. Improving survival from sudden cardiac arrest: the “chain of survival” concept. A statement for health professionals from the Advanced Cardiac Life Support Subcommittee and the Emergency Cardiac Care Committee, American Heart Association. Circulation. 1991;83:1832–1847. doi: 10.1161/01.cir.83.5.1832. [DOI] [PubMed] [Google Scholar]
- 70.Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. Nonclinical evaluation of the potential for delayed ventricular repolarization (QT interval prolongation) by human pharmaceuticals. 2005;S7B:1–10. [PubMed] [Google Scholar]
- 71.Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. Clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. 2005;E14:1–15. [Google Scholar]
- 72.Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry. 2013;14:2–44. doi: 10.3109/15622975.2012.739708. [DOI] [PubMed] [Google Scholar]
- 73.Bunch TJ, White RD, Bruce GK, Hammill SC, Gersh BJ, et al. Prediction of short- and long-term outcomes by electrocardiography in survivors of out-of-hospital cardiac arrest. Resuscitation. 2004;63:137–143. doi: 10.1016/j.resuscitation.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 74.Manfredini R, Portaluppi F, Grandi E, Fersini C, Gallerani M. Out-of-hospital sudden death referring to an emergency department. J Clin Epidemiol. 1996;49:865–868. doi: 10.1016/0895-4356(96)00114-x. [DOI] [PubMed] [Google Scholar]
- 75.Myerburg RJ, Kessler KM, Zaman L, Conde CA, Castellanos A. Survivors of prehospital cardiac arrest. JAMA. 1982;247:1485–1490. [PubMed] [Google Scholar]
- 76.Kim C, Fahrenbruch CE, Cobb LA, Eisenberg MS. Out-of-hospital cardiac arrest in men and women. Circulation. 2001;104:2699–2703. doi: 10.1161/hc4701.099784. [DOI] [PubMed] [Google Scholar]
- 77.Bunch TJ, Hammill SC, White RD. Outcomes after ventricular fibrillation out-of-hospital cardiac arrest: expanding the chain of survival. Mayo Clin Proc. 2005;80:774–782. doi: 10.1016/S0025-6196(11)61532-2. [DOI] [PubMed] [Google Scholar]
- 78.Chugh SS, Jui J, Gunson K, Stecker EC, John BT, et al. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Coll Cardiol. 2004;44:1268–1275. doi: 10.1016/j.jacc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 79.Fox CS, Evans JC, Larson MG, Lloyd-Jones DM, O’Donnell CJ, et al. A comparison of death certificate out-of-hospital coronary heart disease death with physician-adjudicated sudden cardiac death. Am J Cardiol. 2005;95:856–859. doi: 10.1016/j.amjcard.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 80.Iribarren C, Crow RS, Hannan PJ, Jacobs DR, Jr, Luepker RV. Validation of death certificate diagnosis of out-of-hospital sudden cardiac death. Am J Cardiol. 1998;82:50–53. doi: 10.1016/s0002-9149(98)00240-9. [DOI] [PubMed] [Google Scholar]
- 81.Hennessy S, Freeman CP, Cunningham F. In: US government claims databases, in Pharmacoepidemiology. Strom BL, Kimmel SE, Hennessy S, editors. Wiley-Blackwell; 2012. p. 209. [Google Scholar]
- 82.Glassman AH, Bigger JT., Jr Antipsychotic drugs: prolonged QTc interval, torsade de pointes, and sudden death. Am J Psychiatry. 2001;158:1774–1782. doi: 10.1176/appi.ajp.158.11.1774. [DOI] [PubMed] [Google Scholar]
- 83.Kowey PR, Malik M. The QT interval as it relates to the safety of non-cardiac drugs. Eur Heart J Suppl. 2007;9:G3–G8. [Google Scholar]
- 84.Leung JY, Barr AM, Procyshyn RM, Honer WG, Pang CC. Cardiovascular side-effects of antipsychotic drugs: the role of the autonomic nervous system. Pharmacol Ther. 2012;135:113–122. doi: 10.1016/j.pharmthera.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 85.Agelink MW, Majewski T, Wurthmann C, Lukas K, Ullrich H, et al. Effects of newer atypical antipsychotics on autonomic neurocardiac function: a comparison between amisulpride, olanzapine, sertindole, and clozapine. J Clin Psychopharmacol. 2001;21:8–13. doi: 10.1097/00004714-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 86.Woo YS, Kim W, Chae JH, Yoon BH, Bahk WM. Blood pressure changes during clozapine or olanzapine treatment in Korean schizophrenic patients. World J Biol Psychiatry. 2009;10:420–425. doi: 10.1080/15622970801910399. [DOI] [PubMed] [Google Scholar]
- 87.Silke B, Campbell C, King DJ. The potential cardiotoxicity of antipsychotic drugs as assessed by heart rate variability. J Psychopharmacol. 2002;16:355–360. doi: 10.1177/026988110201600410. [DOI] [PubMed] [Google Scholar]
- 88.Bhatia MS, Gupta R, Dhawan J. Myocarditis after overdose of conventional antipsychotics. World J Biol Psychiatry. 2009;10:606–608. doi: 10.1080/15622970701678815. [DOI] [PubMed] [Google Scholar]
- 89.Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298:1794–1796. doi: 10.1001/jama.298.15.1794. [DOI] [PubMed] [Google Scholar]