Abstract
Lifestyle modifications, including adoption of the Dietary Approaches to Stop Hypertension (DASH) dietary pattern, weight loss in individuals who are overweight or obese, and physical activity, are effective in the prevention and treatment of hypertension. A healthy lifestyle may also have beneficial effects on metabolic abnormalities, such as insulin resistance, that are associated with high blood pressure. This review examines the independent and combined effects of the DASH diet and weight loss plus exercise on blood pressure and insulin sensitivity, with a focus on recently published results from the ENCORE study. Our data suggest that the DASH eating plan alone lowers blood pressure in overweight individuals with higher than optimal blood pressure, but significant improvements in insulin sensitivity are observed only when the DASH diet is implemented as part of a more comprehensive lifestyle modification program that includes exercise and weight loss.
Keywords: Hypertension, Blood pressure, Diabetes, Insulin sensitivity, DASH diet, Exercise, Physical activity, Weight loss, Lifestyle modification
Introduction
High blood pressure is a major risk factor for cardiovascular disease and contributes to the risk of adverse events in a continuous, graded, and independent fashion [1–4]. Patients with hypertension are also predisposed to the development of insulin resistance and diabetes, in part through an association with excess weight [5–7]. The combination of high blood pressure and diabetes—each a powerful atherogenic risk factor—places patients in “double jeopardy” for coronary heart disease, stroke, heart failure, and chronic kidney disease, greatly increasing the risk of these adverse consequences of hypertension [5, 8–11].
Although elevated blood pressure can be lowered pharmacologically, antihypertensive medications may be costly, often must be used in combination to achieve adequate blood pressure control, and can be associated with adverse effects that impair quality of life and reduce adherence [12, 13]. Moreover, metabolic abnormalities associated with hypertension, including insulin resistance, may be exacerbated by some medications [14]. For these reasons, lifestyle interventions are preferred as the initial approach to treating most patients with uncomplicated high blood pressure.
The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-7) [15] recommends that lifestyle modifications, such as weight loss and regular aerobic exercise, be the initial strategy for lowering high blood pressure. It specifically recommends the Dietary Approaches to Stop Hypertension (DASH) diet, a diet rich in fiber, fruits, vegetables, and low-fat dairy products that is also low in fat. This review examines the independent and combined effects of the DASH eating plan and weight loss plus exercise on blood pressure and insulin sensitivity, with a focus on recently published data from the ENCORE study.
Effects of Weight-Loss Diets and Exercise on Insulin Sensitivity and the Development of Diabetes
Previous randomized trials of lifestyle interventions have demonstrated that increasing physical activity combined with a diet to encourage weight loss can decrease the incidence of type 2 diabetes in susceptible individuals. In the Diabetes Prevention Program, for example, nondiabetic persons with elevated fasting and post-load plasma glucose concentrations were randomized to a diet and exercise intervention (with a goal of at least 7% weight loss and a minimum of 150 minutes of physical activity weekly), metformin (850 mg twice daily), or placebo [16]. The lifestyle intervention was more effective than metformin in reducing the risk of diabetes over the 2.8 years of follow-up; the incidence rates of diabetes were 11.0, 7.8, and 4.8 cases per 100 person-years in the placebo, metformin, and lifestyle groups, respectively. The Finnish Diabetes Prevention Study compared an intensive lifestyle intervention (targeting a reduction in weight of at least 5% and moderate exercise for at least 30 minutes per day) to diet and exercise advice, and found that the intensive intervention reduced the incidence of diabetes by 58% over a mean of 3.2 years of follow-up [17]. A recent meta-analysis describes the results of these and other randomized trials that included exercise plus diet and standard recommendation arms [18••]. The exercise interventions varied from trial to trial, ranging from advice to promote physical activity to supervised exercise programs of differing intensities. Diet interventions generally focused on caloric restriction, reduced fat intake, and increased fiber consumption. Overall, the exercise plus diet interventions resulted in significant weight loss and reduced the risk of diabetes by 37%.
Three large trials of lifestyle interventions for the prevention of diabetes randomized patients to weight-loss diet only, exercise only, diet plus exercise, or control, thus allowing a comparison of the independent effects of diet and exercise on glucose metabolism. In the Da Qing Impaired Glucose Tolerance and Diabetes Study [19], subjects with impaired glucose tolerance randomized to the diet-only group were counseled to consume more vegetables, limit consumption of alcohol, and reduce intake of simple sugars; those with a body mass index (BMI) greater than 25 kg/m2 also reduced their calorie consumption. Participants in the exercise intervention group were taught and encouraged to increase their leisure-time physical activity. The dietary intervention resulted in a 31% reduction in the incidence of diabetes over 6 years. Fasting and 2-hour postprandial plasma glucose levels both increased less in the diet arm than in the control group. Of note, the incidence of diabetes was not changed by dietary modification in lean participants (who did not reduce caloric intake). The exercise intervention also lowered the cumulative incidence of diabetes (by 46%) and ameliorated the increase in glucose levels.
The Oslo Diet and Exercise Study enrolled sedentary, nondiabetic individuals with BMI greater than 24 kg/m2 [20]. Dietary counseling was individualized for each person and encouraged reduction in caloric intake; increased consumption of fish, vegetables, and fiber-rich complex carbohydrates; and decreased intake of total and saturated fat and sugar. The exercise intervention included supervised workouts three times weekly at 60% to 80% of maximum heart rate. Weight at 12 months decreased by 6.8 kg in the diet group and increased by 1.1 kg in the control group. The weight loss in the diet intervention was associated with a decrease in fasting glucose and insulin levels and an improvement in insulin sensitivity as measured by the homeostasis model. Participants in the exercise-alone intervention had no significant weight loss and did not exhibit significant decreases in fasting glucose or insulin levels or improvement in insulin sensitivity.
In a third study conducted by Wing et al. [21], the effects of lifestyle interventions were examined in a US cohort of overweight individuals with a family history of diabetes. Subjects in the diet group received instruction in a low-fat, low-calorie diet from a multidisciplinary team of counsellors. Participants in the exercise intervention were encouraged to gradually increase their levels of physical activity to 1500 calories per week. In both treatment arms, subjects attended weekly group sessions for the first 6 months, then biweekly meetings for 6 months, and then two 6-week refresher courses in the second year of the trial. Participants in the diet condition lost 9.1 kg in the first 6 months of the program and exhibited significant decreases in fasting glucose and insulin levels. At the end of 24 months, however, these individuals were only 2.1 kg lighter than at baseline, and the improvements in glucose and insulin levels were not maintained. Those in the exercise group had a more modest weight loss (2.1 kg) at 6 months and no significant change in biochemical measures of glucose tolerance and insulin sensitivity. Weight loss from baseline to 2 years was a strong independent predictor of absence of diabetes.
These and other studies demonstrate that intervention strategies that emphasize caloric restriction and achieve weight loss lead to improved insulin sensitivity and decrease the risk of diabetes in vulnerable populations [22–24]. It is less clear whether limiting specific macronutrients such as fat and refined carbohydrates retards the development of diabetes independent of a reduction in calories [25–27]. Similarly, studies examining the effects of exercise on glucose metabolism have produced mixed results [28–34]. Physical activity may have a beneficial acute effect: studies of both healthy adults and patients with type 2 diabetes have shown that improved insulin sensitivity is maintained for at least 6 hours after a single bout of exercise, but it may be diminished within 60 hours after the final exercise training session [35–38]. In general, studies demonstrating improvements in glucose metabolism after longer-term exercise training have not established that these effects are attributable to exercise independent of weight loss.
Effects of the DASH Diet on Blood Pressure
Recent research examining dietary strategies to lower blood pressure has focused on the effects of healthful dietary patterns, rather than on the benefits of specific nutrients or single foods. The DASH diet and similar dietary patterns emphasize fruits, vegetables, and low-fat dairy products; include whole grains, poultry, fish, and nuts; and minimize red meat, sweets, and beverages containing sugar. Although no specific nutrient is identified as the key element in blood pressure reduction, these diets are rich in potassium, magnesium, calcium, and fiber, and have a low content of saturated fat.
The DASH eating plan has been shown to be effective in lowering blood pressure in a series of well-designed clinical trials. In a landmark study, the DASH Collaborative Group established the efficacy of the DASH diet in 459 adults with systolic blood pressure less than 160 mm Hg and diastolic blood pressure 80 to 95 mm Hg [39]. Importantly, weight was kept constant by manipulating calorie consumption in this controlled feeding study. The DASH diet reduced systolic blood pressure by 5.5 mm Hg and diastolic blood pressure by 3.0 mm Hg more than a control diet; the reductions were even greater (11.4 mm Hg/5.5 mm Hg) in those with hypertension. The dietary intervention lowered blood pressure regardless of the participants’ age, gender, BMI, or physical activity levels, and was particularly effective in African Americans [40]. A subsequent trial demonstrated that the DASH diet lowered blood pressure at each of three levels of sodium intake and that reduction of dietary sodium and the DASH diet were more effective in combination than separately [41].
The DASH diet is also effective in lowering blood pressure when implemented as part of a more comprehensive lifestyle modification program in an outpatient setting. In the PREMIER trial, 810 adults with higher-than-optimal blood pressure (systolic blood pressure 120–159 mm Hg, diastolic blood pressure 85–90 mm Hg) were randomized to one of three 6-month interventions: 1) advice only; 2) an established intervention, which included a behavioral intervention to achieve weight loss in those who were overweight, reduced sodium intake, increased physical activity, and limited alcohol intake in those who drank alcohol; and 3) an intervention that implemented the established lifestyle modifications plus the DASH diet [42]. Both active interventions resulted in significant weight loss (4.9 kg in the established-intervention group and 5.8 kg in the established-plus-DASH group, compared with a loss of 1.1 kg in the advice-only group), as well as improved fitness and lower dietary sodium intake; the established-plus-DASH intervention also increased fruit, vegetable, and dairy consumption. The net reduction in systolic blood pressure (relative to advice only) was 3.7 mm Hg in the established group and 4.3 mm Hg in the established-plus-DASH group (P<0.001 for reductions with each intervention vs advice only; P=0.43 for established vs established-plus-DASH).
More recently, our research group published results of the ENCORE study (Exercise and Nutritional Interventions for Cardiovascular Health), a trial that examined the independent and combined effects on blood pressure of the DASH diet and weight loss plus exercise [43•]. In ENCORE, 144 overweight or obese patients with higher than optimal blood pressure (systolic blood pressure 130–159 mm Hg or diastolic blood pressure 85–99 mm Hg) were randomized for 4 months to a usual-diet control group, the DASH diet alone, or the DASH diet in combination with a weight management intervention (consisting of a cognitive behavioral weight loss program and supervised aerobic exercise). Participants in the DASH diet plus weight management group lost weight (8.7 kg) and exhibited a significant increase in aerobic capacity, whereas participants assigned to the DASH diet alone or usual care maintained their weight and had no improvement in fitness levels. Relative to the control group, the DASH diet resulted in a decrease in systolic blood pressure of 7.8 mm Hg, and an even greater decrease (12.7 mm Hg) when combined with exercise and weight loss.
Effects of the DASH Diet on Insulin Sensitivity
Although the efficacy of the DASH diet in reducing blood pressure is well established, less is known about the metabolic effects of this dietary pattern, including its influence on insulin sensitivity. Observational data from the Insulin Resistance Atherosclerosis Study suggest that the DASH diet may prevent the development of diabetes is some individuals [44]. Habitual dietary intake at baseline was assessed using a semiquantitative, 114-item food frequency interview, from which a DASH adherence score was derived. A significant inverse association of the DASH score with incident diabetes over 5 years of follow-up was observed in whites, but not in blacks or Hispanics and not in the study cohort as a whole.
In an ancillary study of PREMIER, insulin sensitivity was measured in a subset of 52 participants using the frequently sampled IV glucose tolerance test with minimal model analysis [45]. Over the course of the 6-month intervention, the insulin sensitivity index was unchanged in the advice-only group, improved from 2.32 to 2.97 with the established intervention (P=0.146 in comparison with advice only), and improved from 1.96 to 2.95 in the patients randomized to the established intervention plus DASH diet (P=0.047 for established plus DASH vs advice only; P=0.616 for established plus DASH vs established). The results of this small study suggest that the DASH dietary pattern in combination with a comprehensive lifestyle modification program for hypertension may improve insulin sensitivity. Whether the DASH diet provides incremental benefit over exercise and weight loss is uncertain, however, as participants in the established-plus-DASH group tended to lose more weight than those receiving the established intervention, and the changes in insulin sensitivity index were not significantly different between these two groups. In another secondary analysis of PREMIER, the established and established-plus-DASH interventions both led to significant decreases in fasting insulin levels and in the homeostasis model index of insulin resistance [46]. However, there were no differences in the insulin resistance index between the active intervention arms.
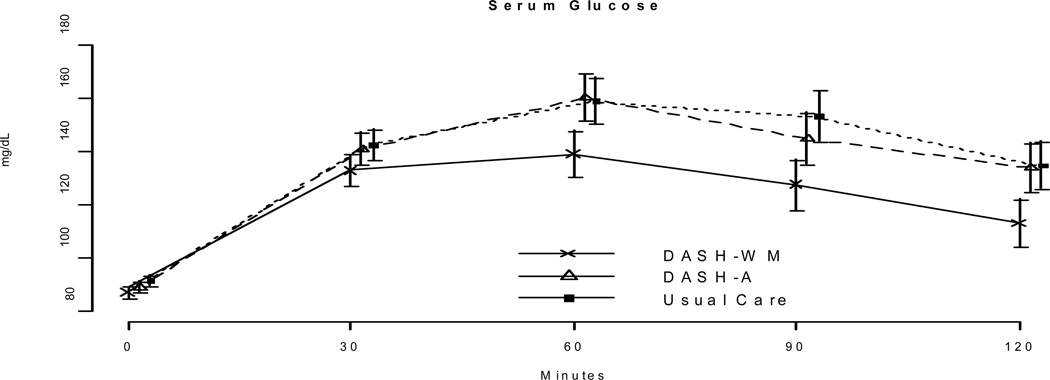
The design of the ENCORE study permitted an assessment of the effects on insulin sensitivity of the DASH diet alone and in combination with weight loss [47•]. Glucose tolerance tests using an oral glucose load of 75 g were performed at baseline and at the conclusion of the 4-month interventions. Glucose tolerance was assessed by calculating the area under the glucose concentration curve. Insulin sensitivity was estimated using the quantitative insulin sensitivity check index (QUICKI), as described by Katz et al. [48]; and also by using the Insulin Sensitivity Index (ISI0,120), a method based on dynamic glucose and insulin levels [49]. Both of these surrogate measures provide estimates of insulin sensitivity that correlate closely with glucose clamp measurements and are predictive of the onset of type 2 diabetes [50].
Compared with usual-care participants, those who completed the DASH plus weight management intervention showed lower fasting glucose and insulin levels and lower values for area under the glucose concentration curve (Table 1). The DASH plus weight management participants also exhibited greater insulin sensitivity, as measured by both QUICKI and ISI0,120, compared with either DASH-alone or usual-care participants (Fig. 1). The DASH-alone group did not differ from the usual-care group on any measure of glucose metabolism. Thus, although participants in the DASH plus weight management condition achieved significant improvements in glucose tolerance and insulin sensitivity, no change in these metabolic parameters was noted in response to the DASH diet alone.
Table 1.
Background characteristics of the sample
| DASH-WM N=49 |
DASH-A N = 46 |
UC N = 49 |
All N = 144 |
|
|---|---|---|---|---|
| Age (years) | 52.3 (10) | 51.8 (10) | 51.8 (9) | 52.0 (10) |
| Gender: Female | 69% (34) | 63% (29) | 69% (34) | 67% (97) |
| Ethnicity | ||||
| Caucasian | 69% (34) | 50% (23) | 59% (29) | 60% (86) |
| African American | 31% (15) | 48% (22) | 39% (19) | 39% (56) |
| Asian | 0% (0) | 2% (1) | 2% (1) | 1% (2) |
| Level of Education | ||||
| High School | 31% (15) | 30% (14) | 42% (20) | 34% (49) |
| Some College | 8% (4) | 9% (4) | 14% (7) | 11% (15) |
| Completed College | 29% (14) | 30% (14) | 18% (9) | 22% (32) |
| Post-Graduate School | 20% (10) | 28% (13) | 20% (10) | 24% (34) |
| Other | 12% (6) | 13% (6) | 2% (1) | 9% (13) |
| Weight (kg) | 93.9 (14) | 93.0 (14) | 92.6 (15) | 93.1 (14.1) |
| BMI (kg/m2) | 33.5 (4.4) | 32.8 (3.4) | 33.0 (3.9) | 33.1 (3.9) |
Values are mean (standard deviation) for continuous variables and group percent (n) for categories.
Figure 1.
Indices of insulin sensitivity, adjusted for age, sex, ethnicity, and pretreatment insulin sensitivity values: comparison of posttreatment means and 95% confidence intervals in the three arms of the ENCORE study. For the Insulin Sensitivity Index (ISI0,120), contrasts were significant for DASH plus weight management (DASH+WM) versus usual care (P 0.026) and for DASH+WM versus DASH alone (P=0.031). For the quantitative insulin sensitivity check index (QUICKI), contrasts were also significant for DASH+WM versus usual care (P<0.001) and for DASH+WM versus DASH alone (P<0.001).
Based upon their glucose levels 2 hours following the oral glucose load, ENCORE participants were classified as diabetic (>199 mg/dL), prediabetic (141–199 mg/dl), or normal (<140 mg/dL). Overall, 72% (n=13) of the 18 participants in the DASH plus weight management group who were either prediabetic or diabetic at study entry improved by at least one category over the course of the trial, compared with 54% (7/13) randomized to DASH alone and 44% (8/18) in usual care. Among participants who were not diabetic or were prediabetic upon study entry, diabetic risk status worsened in only 2% (1/44) of participants in the DASH plus weight management intervention, versus 16% (7/43) in the DASH-alone group and 11% (5/46) in usual care.
Conclusions
Adoption of the DASH dietary pattern is effective in lowering blood pressure in individuals with higher than optimal blood pressures. Whether the DASH diet also improves metabolic abnormalities associated with hypertension, such as insulin resistance, is less clear. A number of trials have demonstrated that increasing exercise combined with a diet to encourage weight loss can reduce the incidence of diabetes in susceptible individuals. The key element of these interventions is probably caloric restriction: though studies examining the influence of dietary composition or physical activity have yielded mixed results, weight loss consistently results in improved glucose metabolism. Data from the ENCORE study suggest that the DASH eating plan alone lowers blood pressure in overweight individuals with high blood pressure, but significant improvements in insulin sensitivity are observed only when the DASH diet is implemented as part of a more comprehensive lifestyle modification program that includes exercise and weight loss.
Table 2.
Body Composition (DEXA) measures before and after treatment
| Treatment Group | P-value from pairwise comparison after treatment | ||||||
|---|---|---|---|---|---|---|---|
| Variable | DASH-WM | DASH-A | UC | DASH-WM vs. DASH-A |
DASH-WM vs. UC |
DASH-A vs UC |
|
| Total % Body Fat | Before | 37.6 (35.5, 39.7) |
35.4 (33.2, 37.5) |
36.4 (34.3, 38.5) |
|||
| After | 33.1 (32.4, 33.8) |
36.2 (35.5, 36.8) |
36.9 (36.2, 37.6) |
< .001 | < .001 | .293 | |
| Total Lean Body Mass (Kg) |
Before | 56.0 (53.0, 59.1) |
57.5 (54.2, 60.7) |
56.7 (53.7, 59.8) |
|||
| After | 54.3 (53.8, 54.9) |
56.8 (56.2, 57.4) |
56.5 (55.9, 57.0) |
< .001 | < .001 | .694 | |
| Total Trunk Fat (Kg) |
Before | 17.7 (16.4, 19.0) |
16.2 (14.9, 17.5) |
16.9 (15.6, 18.2) |
|||
| After | 13.6 (13.1, 14.1) |
16.6 (16.1, 17.1) |
17.1 (16.7, 17.6) |
< .001 | < .001 | .217 | |
Values are mean and 95% confidence interval. Values after treatment are adjusted for pretreatment levels of outcome variable, age, gender, and ethnicity. P-values are adjusted using Tukey’s Honestly Significant Difference procedure.
Table 3.
Glucose and insulin values before and after treatment
| Treatment Group | P-value from pairwise comparison after treatment | ||||||
|---|---|---|---|---|---|---|---|
| Variable | DASH-WM | DASH-A | UC | DASH-WM vs. DASH-A |
DASH-WM vs. UC |
DASH-A vs UC |
|
| Fasting glucose (mg/dl) | Before | 89.4 (86.4, 92.3) |
90.4 (87.3, 93.5) |
91.3 (88.4, 94.3) |
|||
| After | 87.2 (85.1, 89.3) |
89.4 (87.3, 91.5) |
91.9 (89.9, 93.9) |
.324 | .006 | .214 | |
| Fasting insulin (µU/ml) | Before | 18.1 (15.7, 20.4) |
16.6 (14.2, 19.0) |
18.0 (15.7, 20.3) |
|||
| After | 12.5 (10.8, 14.3) |
17.6 (15.9, 19.4) |
18.6 (16.9, 20.2) |
< .001 | < .001 | .711 | |
| Glucose AUC (mg/dl·minutes) | Before | 6057 (5221, 6893) |
6087 (5224, 6951) |
6345 (5508, 7181) |
|||
| After | 4947 (4340, 5554) |
6238 (5637, 6838) |
6334 (5756, 6912) |
.011 | .005 | .958 | |
| ISI0,120 (mg·L2/mmol·mU·min) | Before | 74.4 (67.1, 81.8) |
70.9 (63.5, 78.3) |
66.0 (58.8, 73.2) |
|||
| After | 75.3 (71.8, 78.8) |
68.7 (65.1, 72.3) |
68.8 (65.4, 72.2) |
.031 | .026 | .981 | |
| QUICKI | Before | 0.319 (0.313, 0.325) |
0.319 (0.313, 0.325) |
0.315 (0.309, 0.321) |
|||
| After | 0.334 (0.329, 0.339) |
0.318 (0.313, 0.323) |
0.316 (0.312, 0.321) |
< .001 | < .001 | .850 | |
Values are mean and 95% confidence interval. Values after treatment are adjusted for pretreatment levels of outcome variable, age, gender, and ethnicity. P-values are adjusted using Tukey’s Honestly Significant Difference procedure.
Table 4.
Serum lipids before and after treatment
| Treatment Group | P-value from pairwise comparison after treatment | ||||||
|---|---|---|---|---|---|---|---|
| Variable | DASH-WM | DASH-A | UC | DASH-WM vs. DASH-A |
DASH-WM vs. UC |
DASH-A vs UC |
|
| Total Cholesterol (mg/dl) | Before | 209 (198, 220) |
199 (188, 211) |
206 (195, 217) |
|||
| After | 184 (177, 199) |
199 (192, 205) |
205 (199, 211) |
.008 | < .001 | .364 | |
| Low Density Lipoprotein-Cholesterol (mg/dl) | Before | 128 (118, 138) |
122 (112, 132) |
126 (116, 136) |
|||
| After | 112 (106, 117) |
122 (116, 127) |
125 (119, 130) |
.054 | .005 | .715 | |
| High Density Lipoprotein-Cholesterol (mg/dl) | Before | 55 (50, 59) |
53 (49, 57) |
55 (51, 59) |
|||
| After | 54 (52, 55) |
51 (50, 53) |
54 (53, 56) |
.115 | .911 | .047 | |
| Triglycerides (mg/dl) | Before | 133 (116, 149) |
122 (106, 139) |
122 (106, 139) |
|||
| After | 93 (81, 106) |
129 (117, 142) |
130 (118, 142) |
< .001 | < .001 | .900 | |
Values are mean and 95% confidence interval. Values after treatment are adjusted for pretreatment levels of outcome variable, age, gender, and ethnicity. P-values are adjusted using Tukey’s Honestly Significant Difference procedure.
Acknowledgments
Supported by grants from the National Heart, Lung, and Blood Institute (HL074103) and the General Clinical Research Center, National Institutes of Health (M01-RR-30).
Footnotes
Disclosure
No potential conflicts of interest relevant to this article were reported.
Contributor Information
Alan L. Hinderliter, Department of Medicine, Division of Cardiology, CB #7075, Burnett Womack Building, University of North Carolina, Chapel Hill, NC 27599-7075, USA. hinderli@med.unc.edu
Michael A. Babyak, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Campus Box 3119, Durham, NC 27710, USA. babya001@mc.duke.edu
Andrew Sherwood, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Campus Box 3119, Durham, NC 27710, USA. sher002@mc.duke.edu.
James A. Blumenthal, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Campus Box 3119, Durham, NC 27710, USA. blume003@mc.duke.edu
References and Recommended Reading
Recently published papers of interest have been highlighted as
• Of importance
•• Of major importance
- 1.Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 2.Vokonas PS, Kannel WB. Epidemiology of coronary heart disease in the elderly. In: Tresh DD, Aronow WS, editors. Cardiovascular Disease in the Elderly. edn 2. New York: Marcel Dekker; 1999. pp. 139–164. [Google Scholar]
- 3.MacMahon S, Peto R, Cutler J, et al. Blood pressure, stroke and coronary heart disease. Part 1: prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335:765–774. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB, Neaton JD, Wentworth, et al. Overall and coronary heart disease mortality rates in relation to major risk factors in 325,348 men screened for the MRFIT. Am Heart J. 1986;112:825–836. doi: 10.1016/0002-8703(86)90481-3. [DOI] [PubMed] [Google Scholar]
- 5.Kannel WB, Wilson PWF, Zhang TJ. The epidemiology of impaired glucose tolerance and hypertension. Am Heart J. 1991;121:1268–1273. doi: 10.1016/0002-8703(91)90432-h. [DOI] [PubMed] [Google Scholar]
- 6.Julius S, Jamerson K, Mejia A, et al. The association of borderline hypertension with target organ changes and higher coronary risk: Tecumseh Blood Pressure Study. JAMA. 1990;264:354–358. [PubMed] [Google Scholar]
- 7.Nguyen NT, Magno CP, Lane KT, et al. Association of hypertension, diabetes, dyslipidemia, and metabolic syndrome with obesity: findings from the National Health And Nutrition Examination Survey, 1999 to 2004. J Am Coll Surg. 2008;207:928–934. doi: 10.1016/j.jamcollsurg.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 8.Almgren T, Wilhelmsen L, Samuelsson O, et al. Diabetes in treated hypertension is common and carries a high cardiovascular risk: results from a 28-year follow-up. J Hypertens. 2007;25:1311–1317. doi: 10.1097/HJH.0b013e328122dd58. [DOI] [PubMed] [Google Scholar]
- 9.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 10.Hypertension in Diabetes Study Group. HDS 2: Increased risk of cardiovascular complications in hypertensive type 2 diabetic patients. J Hypertens. 1993;11:319–325. doi: 10.1097/00004872-199303000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Dunder K, Lind L, Zethelius B, et al. Increase in blood glucose concentration during antihypertensive treatment as a predictor of myocardial infarction: population based cohort study. BMJ. 2003;326:681–684. doi: 10.1136/bmj.326.7391.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neal B, MacMahon S, Chapman N. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Blood Pressure Lowering Treatment Trialists' Collaboration. Lancet. 2000;356:1955–1964. doi: 10.1016/s0140-6736(00)03307-9. [DOI] [PubMed] [Google Scholar]
- 13.Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326:1427–1431. doi: 10.1136/bmj.326.7404.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet. 2007;369:201–207. doi: 10.1016/S0140-6736(07)60108-1. [DOI] [PubMed] [Google Scholar]
- 15.Chobanian AV, Bakris GL, Black HR, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 16.Knowler WC, Barrett-Connor E, Fowler SE. Diabetes Prevention Program Research Group: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- Orozco LJ, Buchleitner AM, Gimenez-Perez G, et al. Exercise or exercise and diet for preventing type 2 diabetes mellitus [review] Cochrane Database Syst Rev. 2008;(3):CD003054. doi: 10.1002/14651858.CD003054.pub3. This review summarizes the published literature describing the effects of exercise and weight loss on insulin sensitivity and the development of diabetes.
- 19.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 20.Torjesen PA, Birkeland KI, Anderssen SA, et al. Lifestyle changes may reverse development of the insulin resistance syndrome. The Oslo Diet and Exercise Study: a randomized trial. Diabetes Care. 1997;20:26–31. doi: 10.2337/diacare.20.1.26. [DOI] [PubMed] [Google Scholar]
- 21.Wing RR, Venditti E, Jakicic JM, et al. Lifestyle intervention in overweight individuals with a family history of diabetes. Diabetes Care. 1998;21:350–359. doi: 10.2337/diacare.21.3.350. [DOI] [PubMed] [Google Scholar]
- 22.Goodpaster BH, Kelley DE, Wing RR, et al. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes. 1999;48:839–847. doi: 10.2337/diabetes.48.4.839. [DOI] [PubMed] [Google Scholar]
- 23.Rice B, Janssen I, Hudson R, Ross R. Effects of aerobic or resistance exercise and/or diet on glucose tolerance and plasma insulin levels in obese men. Diabetes Care. 1999;22:684–691. doi: 10.2337/diacare.22.5.684. [DOI] [PubMed] [Google Scholar]
- 24.Dengel DR, Pratley RE, Hagberg JM, et al. Distinct effects of aerobic exercise training and weight loss on glucose homeostasis in obese sedentary men. J Appl Physiol. 1996;81:318–325. doi: 10.1152/jappl.1996.81.1.318. [DOI] [PubMed] [Google Scholar]
- 25.Tinker LF, Bonds DE, Margolis KL, et al. Low-fat dietary pattern and risk of treated diabetes mellitus on postmenopausal women: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. Arch Intern Med. 2008;168:1500–1511. doi: 10.1001/archinte.168.14.1500. [DOI] [PubMed] [Google Scholar]
- 26.Palmer JR, Boggs DA, Krishnan S, et al. Sugar-sweetened beverages and incidence of type 2 diabetes mellitus in African American women. Arch Intern Med. 2008;168:1487–1492. doi: 10.1001/archinte.168.14.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feinglos MN, Totten SE. Are you what you eat, or how much you eat? The case of type 2 diabetes mellitus. Arch Intern Med. 2008;168:1485–1486. doi: 10.1001/archinte.168.14.1485. [DOI] [PubMed] [Google Scholar]
- 28.Yamanouchi K, Shinozaki T, Chikada K, et al. Daily walking combined with diet therapy is a useful means for obese NIDDM patients not only to reduce body weight but also to improve insulin sensitivity. Diabetes Care. 1995;18:775–778. doi: 10.2337/diacare.18.6.775. [DOI] [PubMed] [Google Scholar]
- 29.Schneider SH, Khachadurian AK, Amorosa LF, et al. Ten-year experience with an exercise-based outpatient life-style modification program in the treatment of diabetes mellitus. Diabetes Care. 1992;15:1800–1810. doi: 10.2337/diacare.15.11.1800. [DOI] [PubMed] [Google Scholar]
- 30.Vanninen E, Uusitupa M, Siitonen O, et al. Habitual physical activity, aerobic capacity and metabolic control in patients with newly-diagnosed type 2 (non-insulin-dependent) diabetes mellitus: effect of 1-year diet and exercise intervention. Diabetologia. 1992;35:340–346. doi: 10.1007/BF00401201. [DOI] [PubMed] [Google Scholar]
- 31.Trovati M, Carta Q, Cavalot F, et al. Influence of physical training on blood glucose control, glucose tolerance, insulin secretion, and insulin action in non-insulin-dependent diabetic patients. Diabetes Care. 1984;7:416–420. doi: 10.2337/diacare.7.5.416. [DOI] [PubMed] [Google Scholar]
- 32.Poirier P, Tremblay A, Broderick T, et al. Impact of moderate aerobic exercise training on insulin sensitivity in type 2 diabetic men treated with oral hypoglycemic agents: Is insulin sensitivity enhanced only in nonobese subjects? Med Sci Monit. 2002;8:CR59–CR65. [PubMed] [Google Scholar]
- 33.Cuff DJ, Meneilly GS, Martin A, et al. Effective exercise modality to reduce insulin resistance in women with type 2 diabetes. Diabetes Care. 2003;26:2977–2982. doi: 10.2337/diacare.26.11.2977. [DOI] [PubMed] [Google Scholar]
- 34.Blumenthal JA, Sherwood A, Gullette ECD, et al. Exercise and weight loss reduce blood pressure in men and women with mild hypertension. Arch Intern Med. 2000;160:1942–1958. doi: 10.1001/archinte.160.13.1947. [DOI] [PubMed] [Google Scholar]
- 35.Mikines KJ, Sonne B, Tronier B, Galbo H. Effects of training and detraining on dose-response relationship between glucose and insulin secretion. Am J Physiol. 1989;256(5 Pt 1):E588–E596. doi: 10.1152/ajpendo.1989.256.5.E588. [DOI] [PubMed] [Google Scholar]
- 36.Devlin JT, Hirshman M, Horton ED, Horton ES. Enhanced peripheral and splanchnic insulin sensitivity in NIDDM men after single bout of exercise. Diabetes. 1987;36:434–439. doi: 10.2337/diab.36.4.434. [DOI] [PubMed] [Google Scholar]
- 37.Burstein R, Polychronakos C, Toews CJ, et al. Acute reversal of the enhanced insulin action in trained athletes. Association with insulin receptor changes. Diabetes. 1985;34:756–760. doi: 10.2337/diab.34.8.756. [DOI] [PubMed] [Google Scholar]
- 38.Giacca A, Groenewoud Y, Tsui E, et al. Glucose production, utilization, and cycling in response to moderate exercise in obese subjects with type 2 diabetes and mild hyperglycemia. Diabetes. 1998;47:1763–1770. doi: 10.2337/diabetes.47.11.1763. [DOI] [PubMed] [Google Scholar]
- 39.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 40.Svetkey LP, Simons-Morton D, Vollmer WM, et al. Effects of dietary patterns on blood pressure: subgroup analysis of the dietary approaches to stop hypertension (DASH) randomized clinical trial. Arch Intern Med. 1999;159:285–293. doi: 10.1001/archinte.159.3.285. [DOI] [PubMed] [Google Scholar]
- 41.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 42.Appel LJ, Champagne CM, Harsha DW, et al. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. 2003;289:2083–2093. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak MA, Hinderliter A, et al. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Arch Intern Med. 2010;170:126–135. doi: 10.1001/archinternmed.2009.470. This paper describes the main results of the ENCORE study. In overweight and obese persons with above-normal blood pressure, the DASH diet lowered blood pressure, but the addition of exercise and weight loss resulted in even larger blood pressure reductions, greater improvements in vascular and autonomic function, and reduced left ventricular mass.
- 44.Liese AD, Nichols M, Sun X, et al. Adherence to the DASH diet is inversely associated with incidence of type 2 diabetes: the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2009;32:1434–1436. doi: 10.2337/dc09-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ard JD, Grambow SC, Liu D, et al. The effect of the PREMIER interventions on insulin sensitivity. Diabetes Care. 2004;27:340–347. doi: 10.2337/diacare.27.2.340. [DOI] [PubMed] [Google Scholar]
- 46.Lien LF, Brown AJ, Ard JD, et al. Effects of PREMIER lifestyle modifications on participants with and without the metabolic syndrome. Hypertension. 2007;50:609–616. doi: 10.1161/HYPERTENSIONAHA.107.089458. [DOI] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak MA, Sherwood A, et al. Effects of the Dietary Approaches to Stop Hypertension diet alone and in combination with exercise and caloric restriction on insulin sensitivity and lipids. Hypertension. 2010;55:1199–1205. doi: 10.1161/HYPERTENSIONAHA.109.149153. In this secondary analysis of the ENCORE study, the DASH diet alone reduced blood pressure, but improvements in insulin sensitivity and lipid levels were noted only when the DASH diet was combined with caloric restriction and exercise.
- 48.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 49.Gutt M, Davis CL, Spitzer SB, et al. Validation of the insulin sensitivity index (ISI(0,120)): comparison with other measures. Diabetes Res Clin Pract. 2000;47:177–184. doi: 10.1016/s0168-8227(99)00116-3. [DOI] [PubMed] [Google Scholar]
- 50.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–E26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]