Abstract
Loss of heterozygosity (LOH) is a common type of genomic alterations in ovarian cancer. Analyzing 74,415 copy neutral LOH events in 513 serous ovarian adenocarcinomas samples from the Cancer Genome Atlas, we report that the frequency of LOH events increases with age. Similar trend is observed for chromosome 17, which is frequently implicated in ovarian cancer. The results are consistent when we analyze data from the Boston High-grade serous cancer (HGSC) cohort. We further show that germ line and somatic mutations in BRCA1 (in chromosome 17) and BRCA2 (in chromosome 13) loci are not necessary to establish the pattern. We also report significant age-related changes in expression patterns for several genes in the homologous recombination (HR) pathway such as BRCA1, RAD50, RAD52, XRCC2, XRCC3, and MRE11A in these patient samples. Furthermore, we develop a metric for pathway-level imbalance, and show that increased imbalance in the HR pathway i.e. increase in expression of some HR genes and decrease in expression of others - is common, and correlates significantly with the frequency of LOH events in the patient samples. Taken together, it is highly likely that aging and deregulation of HR pathway contribute to the increased incidence of copy-neutral loss of heterozygosity in ovarian cancer patients.
INTRODUCTION
Ovarian cancer is one of the most deadly cancers that affects women (Ries, et al. 2007), and the 5 year survival rate for all stages of ovarian cancer is typically poor (~47%). It has caused ~14,000 deaths in the United States in 2010 alone (Jemal, et al. 2010). Ovarian cancer is typically more common in older patients – age adjusted rate increases with age, and the older patients are more likely to have advanced disease at the time of initial diagnosis (Yancik 1993). Systematic investigation by the Cancer Genome Atlas and other studies reported extensive genomic alterations in ovarian cancer patients (Kan, et al. 2010; Ruark, et al. 2013; 2011). Loss of heterozygosity (LOH) is a common class of genomic alterations observed in ovarian cancer genomes, which occurs due to heterozygous deletion of one allele, or duplication of a maternal or paternal chromosome or chromosomal region and concurrent loss of the other allele; the latter is known as copy neutral LOH. Several historic studies have suggested that not only the age-adjusted incidence rate (Yancik 1993), but also molecular profiles including the pattern of LOH might differ between younger and older patients. For instance, Garcia et al. reported that ovarian cancer patients with chr17q-LOH-positive tumors were older than those with chr17q-LOH-negative tumors (mean ages 67 and 49, respectively) (Garcia, et al. 2000). But a genome-wide systematic assessment of copy neutral LOH patterns in a large cohort of patients was not possible until recently.
Meanwhile, in model organisms, it has been demonstrated that the incidence of LOH events and the efficiency of DNA double strand repair mechanisms change with aging. Increasing evidence suggest that the efficiency of these repair pathways, and more interestingly, the preference for a given repair pathway over another can change with age (Chen, et al. 2007). For instance, using Rr3, a repair reporter system in Drosophila, Preston et al. showed that the incidence of homologous repair increased gradually from 14% in young individuals to more than 60% in old ones, whereas two other repair pathways showed a age-related decrease, and that the proportion of long conversion tracts also increased in older flies (Preston, et al. 2006). In budding yeast, there is a significant increase in the frequency of loss of heterozygosity (LOH) events in older diploid yeast mother cells (McMurray and Gottschling 2003), suggesting age-dependent abnormalities in the HR pathway. Consistent with this observation, it was reported that NHEJ, which is an alternative to the HR pathway for DSB repair, is more efficient and accurate in young human fibroblast cells than in older cells (Seluanov, et al. 2004). These findings have led to a speculation that age-dependent changes in DNA repair contribute to increased LOH events in aging organisms, and that the findings from these model organisms could be extended to humans as well (Carr and Gottschling 2008).
Availability of systematic genomic, transcriptomic and clinical data for 529 well-characterized high grade serous ovarian cancer samples from the Cancer Genome Atlas (TCGA 2011) has allowed us to investigate age-related changes in the incidence of copy-neutral loss of heterozygosity comprehensively. Here we investigate whether the frequency of LOH events increases with age, and whether germ line and somatic mutations in BRCA1 and BRCA2 loci are necessary to establish the pattern. We validate the findings in another cohort. Furthermore, since imperfect repair of DNA double strand break by HR pathway leads to LOH, we also examine whether age related changes in the expression of key HR pathway genes could explain the pattern.
METHODS
We obtained data on loss of heterozygosity (LOH), gene expression, and clinical information for high-grade serous ovarian adenocarcinomas (serous cystadenocarcinomas) from the Cancer Genome Atlas (TCGA 2011). All data were mapped to the human genome version hg18/NCBI36. All statistical analyses were performed in R.
We performed extensive quality control steps to process the LOH data (Supplementary Figure SF1). LOH status was determined by Hudson Alpha Institute for Biotechnology using Illumina Human1MDuo SNP chip as a part of the TCGA initiative. There were 529 samples with LOH data in the initial dataset. We checked for systematic errors and batch effects. The samples with plate-ID 0535 had significantly more LOH events and log2 signal intensity compared to all other samples in the dataset, which could be due to biological reasons or systematic errors, and were excluded from further analysis. In addition, several samples had extremely large number of potential LOH events compared to all other samples; they was excluded from further analyses as well. The final filtered dataset had 513 ovarian adenocarcinomas samples.
We then applied two filters to identify the most likely copy-neutral LOH events. First, we excluded the heterozygous deletion-mediated LOH events. Copy number alteration status for the ovarian cancer samples were analyzed the Agilent Human Genome CGH244A microarray at Memorial Sloan Kettering Cancer Center, and processed using published protocols (TCGA 2011). We flagged those LOH events as heterozygous deletion-mediated, which overlapped with deletion events (aCGH log2 signal intensity ratio < −0.20) for at least 80% of its length, and excluded from the analysis. The final list of the LOH events was mostly insensitive to the choice of threshold for copy number detection. Second, the LOH log2 signal intensity distribution in the original dataset had a range from 0.00 to 1.00, with a prominent dip near 0.075. The LOH events with log2 value <0.075 could be potentially spurious cases, although we can't rule out effects of genetic heterogeneity. In any case, we considered only the LOH events with log2 values >0.075 for final analysis. We also repeated the analyses with LOH events having log2 value >0.1 and >0.2, and got similar results. After using these two filters, the final dataset had 74,415 copy neutral LOH events in 513 samples.
We obtained gene expression data for these samples from the Cancer Genome Atlas (TCGA 2011). Gene expression was analyzed using Affymetrix U133A microarray at the Broad Institute, and processed using published protocols. Germ line and somatic mutation data for BRCA1 and BRCA2 were obtained from the Supplementary Table S8.1 and S8.2 of the TCGA ovarian adenocarcinoma paper (TCGA 2011).
The processed LOH calls and per SNP copy number calls for the Boston High-grade serous cancer (HGSC) cohort were previously described (Wang, et al. 2012). We focused only on the samples with stage ≥III and grade ≥3. For each LOH region, we counted the number of marker SNPs in those LOH region (N), and the number of such SNPs with allelic copy number <1.9 (n). If n/N was ≥0.8, we postulated that LOH to be deletion mediated; while the remaining ones (n/N <0.8) as copy neutral LOH. We only considered the likely copy neutral LOH events with size >10kb. There were 569 LOH events in 33 samples in the filtered dataset.
To estimate pathway-level imbalance, we first analyzed expression data of a set of genes (e.g. HR pathway genes) in the tumor samples alongside that in one (or average) normal sample. For each gene (i) in each sample (j), we calculated the normalized difference in rank between its expression level in the sample with that in the normal control (dij = rankitumor-j – rankinormal/number of samples). If a sample has relatively high expression (compared to control) for all the genes (i in 1:N) in the set, average diji=1:N will be high and variance (e.g. inter-quartile range, IQR(diji=1:N)) would be small. Similarly, for a sample with relatively low (or moderate) expression for all the genes in the set, will have low (or intermediate) average diji=1:N and small variance. In contrast, a sample with relatively high expression of some genes (high dij), and low expression in some other (low dij) will have a high IQR(diji=1:N). We have used IQR(diji=1:N) as a score for pathway-level imbalance in tumor samples. This method is non-parametric, and hence unaffected by outliers. It also does not depend on the actual expression level of the genes, or the number of genes (preferably 4 or more genes) in the dataset.
RESULTS
We first obtained the loss of heterozygosity (LOH) data for 529 high-grade serous ovarian adenocarcinomas (serous cystadenocarcinomas) from the Cancer Genome Atlas (TCGA) (TCGA 2011). We performed extensive quality control steps to identify most likely copy neutral LOH events (see Methods and Supplementary Figure SF1 for more details). In brief, we first checked for systematic errors arising from batch, plate, or center-specific effects in the microarray-based LOH calls, and removed the samples with systematic biases, or those with a considerable excess of LOH calls. We then overlaid the copy number alteration data for the same samples, and excluded those LOH events, which are likely to arise due to heterozygous deletion. Finally we applied a semi-supervised LOH log2 signal intensity cut-off (≤0.075) to exclude those with a low signal to noise ratio. The final dataset had 74,415 copy neutral LOH events in 513 samples. We also obtained the age of initial diagnosis data from the clinical data repository at the TCGA (TCGA 2011). The age of the patients in the filtered dataset ranged from 30 to 89, with a median of 59. In particular, 196 patients were of age ≤55 years (pre or peri-menopausal), and 317 were >55 years old (post-menopausal).
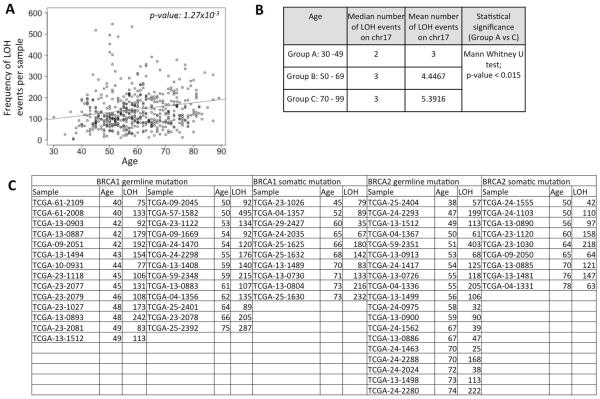
We plotted the number of LOH events per individual against the age those patients. We found that the number of LOH events was significantly higher in older patients (Figure 1A, p-value <0.005). The results were similar when we used alternative threshold for LOH log2 signal ratio of 0.1 and 0.2 (Supplementary Figure SF2), suggesting that our results were insensitive to the choice of threshold.
Figure 1.
A) Scatterplot showing the distribution of loss of heterozygosity (LOH) frequency for the patients in the TCGA cohort against their age. The trend line for regression is shown in dark grey. The p-value is shown at the top right corner. B) Frequency of LOH events affecting chr17 in patients of different age group. C) LOH frequency in patients with germ line or somatic mutations in BRCA1 or BRCA2. TCGA-13-1512 had germ line mutation in both BRCA1 and BRCA2.
Loss of heterozygosity on chr17 is frequent in epithelial ovarian tumors(Eccles, et al. 1992). We examined whether the frequency of LOH events affecting chr17 also change with age, and found that while the younger patients (age: 30–49) had fewer LOH events (mean: 3 events) on chr17, those between 50 and 69 (mean: 4.44 events), and older patients (≥70 years; mean: 5.39 events) had more LOH events on chr17 (Figure 1B), and this difference was significant (30–49 years vs ≥70 years; Mann Whitney U test; p-value: 0.015). Therefore, increased likelihood of chr17 LOH is also a trend associated with older patients. We did not find significant difference in survival between the patients who had LOH in chr17 and those who did not. The lack of statistical significance could also be confounded by the relatively small sample size.
We then repeated the analyses using data from the Boston High-grade serous cancer (HGSC) cohort (Wang, et al. 2012). We found that the frequency of copy neutral LOH events significantly increased in the older patients (Figure 2A; p-value: 0.028). Moreover, the patients with chr17 LOH were typically older compared to those who had no LOH (Figure 2B), but it was not statistically significant due to small size of the dataset.
Figure 2.
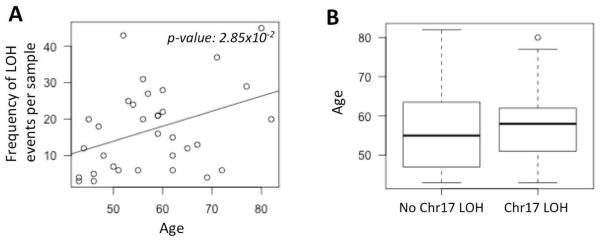
A) Scatterplot showing the distribution of loss of heterozygosity (LOH) frequency for the patients in the Boston HGSC cohort against their age. The trend line for regression is shown in dark grey. The p-value is shown at the top right corner. B) Boxplot showing the age of the patients who had chr17 LOH against those who did not.
We also analyzed the LOH events in the TCGA glioblastoma samples (TCGA 2008), and found that the age related patterns and also the genome-wide distribution of LOH events in glioblastoma were different from that reported for the ovarian cancer (Supplementary text Stx1 and Supplementary Figure SF3). It might be due to the distinct biology of glioblastoma, but we could not rule out biases due to technical issues.
We then examined whether germ line and somatic mutation status of BRCA1 and BRCA2 contributed to the observed trend in ovarian cancer. 27 and 10 TCGA ovarian cancer samples had BRCA1 germ line and somatic mutations, respectively; 20 and 9 had BRC2 germ line and somatic mutations, respectively; 2 samples had both BRCA1 and BRCA2 mutations (TCGA 2011). We first repeated the analysis for the samples wild type for BRCA1 and BRCA2, and found consistent results, i.e. the number of LOH events increased in older patients. Next, focusing on the samples with BRCA1 or BRCA2 germ line and somatic mutations we observed similar age associated increase (Figure 1C); even though individual datasets were too small to test for statistical significance meaningfully. For instance, among the patients with BRCA1 germ line mutations, those with age ≤49 years (group A according to the convention used in Figure 1B) had a mean LOH frequency of 132.7 while those with age ≥50 years (group B and C combined) had a mean LOH frequency of 175.9. Similarly, among the patients with BRCA2 germ line mutations, those with age ≤49 and ≥50 years had mean LOH frequencies of 44.6 and 61.5, respectively. The Boston HGSC cohort was too small for a comparable analysis. Taken together, our findings suggest that in ovarian cancer the number of LOH events increases with age, and that BRCA1 and BRCA2 mutation are not necessary to establish the pattern.
LOH events arise due to incorrect repair of DNA double strand breaks by homologous recombination (HR) pathway (Chapman, et al. 2012; TCGA 2011). So, we then investigated whether expression pattern of the genes in the HR pathway (BRCA1, BRCA2, RAD50, RAD51, RAD52, RAD54B, RAD54L, XRCC2, XRCC3, and MRE11A; Figure 3) also change systematically with age. We grouped the samples by age: 30–49 years, 50–70 years, and 70–89 years, and plotted the distribution of expression-level of these genes. We found that expression level of BRCA1, RAD52, and XRCC2 significantly increased with age (Mann Whitney U test; adjusted p-value <0.05), while it significantly decreased with age for RAD50, XRCC3, and MRE11A (Mann Whitney U test; adjusted p-value <0.05). We found similar trends when using linear models (expression ~ age), suggesting that our findings are not sensitive to the choice of age thresholds. Collectively, these findings suggest that, several genes in the HR pathway show systematic change in expression with age in ovarian cancer patients.
Figure 3.
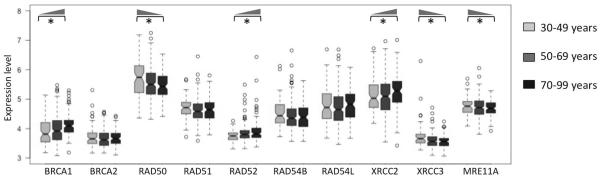
Change in expression level of the key homologous recombination pathway genes in young (30–49 years, light grey), intermediate (50–69 years, dark grey) and older (70–99 years, black) patients. Those with adjusted p-value <0.05 are shown with asterisk, and the wedges show the direction of increase in expression.
We then asked whether the increase in frequency of LOH events in older ovarian cancer patients could be due to age-related change in expression of any of the HR pathway genes. At this end, we developed a multivariate model testing for association between LOH and age of the patients and also expression of these 11 genes simultaneously. We found that the frequency of LOH events was significantly correlated with age (p-value: 0.00789); but expression of none of the HR pathway genes (except RAD54L, p-value: 0.01) was significantly related to the frequency of LOH events (p-value >0.05; Supplementary Figure SF4). This suggests that, even though the expression of some of the HR genes changes with age, increase in the frequency of LOH events in older patients cannot directly be inferred from altered expression of the HR pathway genes.
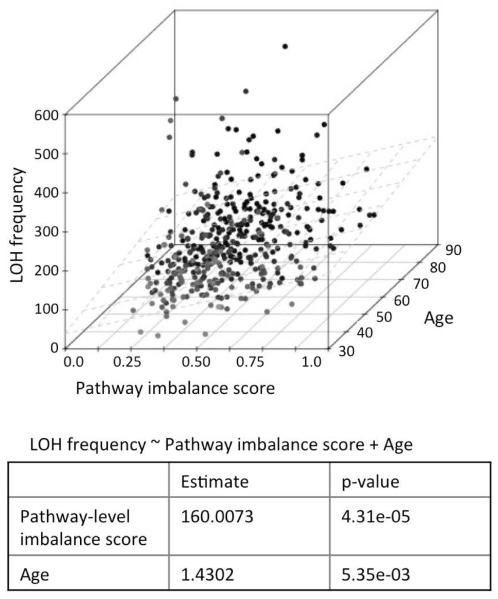
Our findings guided us to revisit the age-related changes in expression of the HR pathway genes from another angle. When some HR genes increased in expression with age, others decreased – led us to investigate whether the balance within the HR pathway might be affected. We developed a non-parametric model for estimating pathway-level imbalance, and assigned a score to each sample (see Methods for details), such that (i) a sample with concerted increase (or decrease or no change) in expression of most of the genes in a pathway relative to normal samples would have a low score, while (ii) a sample with considerable increase in expression of some genes and decrease in expression of some other genes in the pathway will have a high score. The non-parametric pathway imbalance score is conceptually similar to pathway-level variance estimators(Glaab and Schneider 2012), but it is robust against outliers and actual expression level of the genes. Using a multivariate model (LOH ~ age + pathway imbalance score), we found that both age and pathway imbalance score were significantly associated with the frequency of LOH events (Figure 4; p-value <0.006 in both cases). Furthermore, using partial correlation analysis, we found that the effect of age on LOH frequency is independent of the pathway imbalance score (Spearman partial correlation; p-value: 1.14e–4), and on the other hand, the effect of pathway imbalance score on LOH is also independent of age (Spearman partial correlation; p-value: 1.9e–06). We also obtained similar results using an alternative, parametric approach for estimating pathway-level imbalance (Supplementary text STx2). Taken together, even though one needs to be careful while inferring causality from correlation, in the light of published reports (Chen, et al. 2007; Kirkwood 2005; Preston, et al. 2006), it is tempting to suggest that both aging and deregulation of the HR pathway are likely to contribute to an increase in LOH frequency in ovarian cancer patients.
Figure 4.
3D scatter plot showing joint distribution of the frequency of loss of heterozygosity (LOH) events per sample with the pathway imbalance score and age at diagnosis of the patients. The regression plane (LOH ~ pathway imbalance score + Age) is shown in light grey. In the 3D plot, darker points are further away compared to those with a lighter shade.
DISCUSSION
Here, we performed the first systematic survey of the frequency of LOH events in a large cohort of more than 500 ovarian cancer patients, and reported that the incidence of loss of heterozygosity is higher in older ovarian cancer patients. We also found that chr17, which is commonly implicated in ovarian cancer also shows similar trend, and that BRCA1 and BRCA2 mutation are not necessary to establish the pattern. We also found age-related changes in expression in several key HR pathway genes (increase: BRCA1, RAD52, and XRCC2; decrease: RAD50, XRCC3, and MRE11A), and reported that in addition to age, deregulation of the HR pathway contributes to an increase in LOH events. Our results are robust towards statistical approaches and choice of datasets.
Our findings are concurrent with the report that ovarian cancer patients with chr17q-LOH-positive tumors are older than those with chr17q-LOH-negative tumors(Garcia, et al. 2000), and also suggest that the increased LOH frequency not only affects chr17, but is a genome-wide phenomenon. Furthermore, age-related changes in expression of the HR-pathway genes are consistent with the fact that increased deregulation of DNA repair pathways is common in older individuals - as reported in model systems(Carr and Gottschling 2008; Chen, et al. 2007; McMurray and Gottschling 2003; Preston, et al. 2006; Seluanov, et al. 2004; 2011). Previously, Preston et al. reported that the age-related changes in DNA repair in fruit fly did not imply an overall increase or decrease in repair capacity, but instead followed a change in preference for non-homologous end-joining and single-strand annealing in younger individuals to predominantly homologous recombination-driven repair in older ones(Preston, et al. 2006). It is suspected that similar mechanisms might be operating in human tissues as well(Carr and Gottschling 2008), contributing to age-related increase in LOH frequency in different types of cancer. But, as reported in the gastric mucosa (Moragoda, et al. 2002), it may not be a universal rule and one can expect to find tissue-specific patterns. Why is HR-mediated repair relatively more frequent in older individuals? According to the `antagonistic pleiotropy' hypothesis(Kirkwood 2005), during early development simpler and quicker end-joining processes allow rapid repair of DNA breaks and faster growth, which in turn might offer a significant competitive advantage for most species, even if that comes at the cost of increased mutation burden with deleterious consequences later in life(Engels, et al. 2007).
It was recently reported that expression of key HR pathway genes such as BRCA1, MRE11, RAD51, but not BRCA2, declines in single mouse and human oocytes, and that specific knockdown of BRCA1, MRE11, RAD51, and ATM expression increased DNA double strand breaks and reduced survival(Titus, et al. 2013). We found that the expression of these genes changed in age-specific manner in ovarian cancer patients. While deregulated HR pathway is causally implicated in increased incidence of loss of heterzygosity and incorrect repair of DNA double strand break (Chapman, et al. 2012), it is however difficult to pinpoint whether deregulated DNA repair pathways are causes or consequences of tumorigenesis and aging. Increased cell to cell stochastic variation in gene expression is a hallmark of aging (Bahar, et al. 2006), which could be due to the increasing burden of somatic mutations in apparently healthy individuals (De 2011), or it might arise as a result of specific deregulation of certain metabolic pathways (Cluett and Melzer 2009). Age-associated telomere shortening could be another confounding factor (Eissenberg 2013). Therefore, further work is warranted. In addition, the prevalence of ovarian cancer, and also the frequencies of BRCA mutations differ between patients from different ethnic background. In the future, it will be informative to take into account the ethnic background of the patients, and test whether the age-related genome-wide LOH patterns differ between different ethnicities.
Nevertheless, our findings have important implications for ovarian cancer biology and treatment of the patients. In general, ovarian cancer is more common in older patients- more than 48% of all ovarian cancers occur in women in the age group 65 years and older; the age-adjusted incidence rates also increases with age and older patients are more likely to have advanced disease at the time of initial diagnosis (Yancik 1993). While the patients of age less than 45 years have 5 year survival rate of 82%, only 33% of those of age 65 years or more survive more than 5 years (Pignata and Vermorken 2004; Ries 1993; Riggs 1995). Furthermore, the older persons are at a higher risk for therapeutic complications than the younger patients (Balducci and Corcoran 2000; Pignata and Vermorken 2004). The molecular bases of these differences between older and younger patients are poorly understood. Some of the complications might arise due to the increase in LOH events we observe. It was recently reported that the LOH of BRCA1 locus might be involved in brain metastasis from ovarian cancer (Sekine, et al. 2013), and that the loss of heterozygosity at multiple markers in cell free DNA of the patients could be indicative of lymph node metastasis (Schwarzenbach, et al. 2012). However, further work is necessary to establish the relationship between LOH and metastasis firmly. Copy neutral LOH mediated gene conversion can potentially replace wild-type alleles with recessive deleterious alleles, leading to increased risk of manifestation of recessive deleterious traits, complicating the resulting phenotype in the affected individuals. This can be a particularly complicated issue for ovarian cancer genomes that have high frequency of LOH events. Given that many LOH events are megabases in size, such changes have the potential to affect many genomic loci. Given the complex relationship of ovarian cancer with several other diseases (e.g. obesity) (Olsen, et al. 2007), loss of heterozygosity at loci harboring the disease causing variants have the potential to compound the phenotypic consequences (e.g. increasing BMI or cross-talk between diseases) during the course of tumorigenesis, which in turn can affect treatment strategies. Another possibility is that, error-prone repair of these sites of genomic alterations contributes to increased incidence of somatic point mutations (De and Babu 2010); a subset of those in protein coding genes or conserved functional elements (De, et al. 2008) could affect important cellular functions leading to additional complications in these patients. Thus, increased incidence of LOH in older ovarian cancer patients may have important implications for disease progression and treatment.
Supplementary Material
Acknowledgements
The authors thank David Schwartz, Mark Geraci, James DeGregori, Ivana Yang, and the anonymous reviewers for insightful discussions and critical comments.
Supported by: NCI Physical Sciences Oncology Center pilot grant, American Cancer Society grant, and University of Colorado School of Medicine startup funds (to SD); Ovarian Cancer Research Fund Liz Tilberis grant (MAS)
References
- Bahar R, Hartmann CH, Rodriguez KA, Denny AD, Busuttil RA, Dolle ME, Calder RB, Chisholm GB, Pollock BH, Klein CA, Vijg J. Increased cell-to-cell variation in gene expression in ageing mouse heart. Nature. 2006;441(7096):1011–1014. doi: 10.1038/nature04844. [DOI] [PubMed] [Google Scholar]
- Balducci L, Corcoran MB. Antineoplastic chemotherapy of the older cancer patient. Hematol Oncol Clin North Am. 2000;14(1):193–212. x–xi. doi: 10.1016/s0889-8588(05)70284-7. [DOI] [PubMed] [Google Scholar]
- Carr LL, Gottschling DE. Does age influence loss of heterozygosity? Exp Gerontol. 2008;43(3):123–129. doi: 10.1016/j.exger.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Chapman JR, Taylor MR, Boulton SJ. Playing the end game: DNA double-strand break repair pathway choice. Mol Cell. 2012;47(4):497–510. doi: 10.1016/j.molcel.2012.07.029. [DOI] [PubMed] [Google Scholar]
- Chen JH, Hales CN, Ozanne SE. DNA damage, cellular senescence and organismal ageing: causal or correlative? Nucleic Acids Res. 2007;35(22):7417–7428. doi: 10.1093/nar/gkm681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluett C, Melzer D. Human genetic variations: Beacons on the pathways to successful ageing. Mech Ageing Dev. 2009;130(9):553–563. doi: 10.1016/j.mad.2009.06.009. [DOI] [PubMed] [Google Scholar]
- De S. Somatic mosaicism in healthy human tissues. Trends Genet. 2011;27(6):217–223. doi: 10.1016/j.tig.2011.03.002. [DOI] [PubMed] [Google Scholar]
- De S, Babu MM. A time-invariant principle of genome evolution. Proc Natl Acad Sci U S A. 2010;107(29):13004–13009. doi: 10.1073/pnas.0914454107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De S, Lopez-Bigas N, Teichmann SA. Patterns of evolutionary constraints on genes in humans. BMC Evol Biol. 2008;8:275. doi: 10.1186/1471-2148-8-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles DM, Russell SE, Haites NE, Atkinson R, Bell DW, Gruber L, Hickey I, Kelly K, Kitchener H, Leonard R, et al. Early loss of heterozygosity on 17q in ovarian cancer. The Abe Ovarian Cancer Genetics Group. Oncogene. 1992;7(10):2069–2072. [PubMed] [Google Scholar]
- Eissenberg JC. Telomeres, cancer & aging: live long & prosper? Mo Med. 2013;110(1):11–16. [PMC free article] [PubMed] [Google Scholar]
- Engels WR, Johnson-Schlitz D, Flores C, White L, Preston CR. A third link connecting aging with double strand break repair. Cell Cycle. 2007;6(2):131–135. doi: 10.4161/cc.6.2.3758. [DOI] [PubMed] [Google Scholar]
- Garcia A, Bussaglia E, Machin P, Matias-Guiu X, Prat J. Loss of heterozygosity on chromosome 17q in epithelial ovarian tumors: association with carcinomas with serous differentiation. Int J Gynecol Pathol. 2000;19(2):152–157. doi: 10.1097/00004347-200004000-00009. [DOI] [PubMed] [Google Scholar]
- Glaab E, Schneider R. PathVar: analysis of gene and protein expression variance in cellular pathways using microarray data. Bioinformatics. 2012;28(3):446–447. doi: 10.1093/bioinformatics/btr656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Kan Z, Jaiswal BS, Stinson J, Janakiraman V, Bhatt D, Stern HM, Yue P, Haverty PM, Bourgon R, Zheng J, Moorhead M, Chaudhuri S, Tomsho LP, Peters BA, Pujara K, Cordes S, Davis DP, Carlton VE, Yuan W, Li L, Wang W, Eigenbrot C, Kaminker JS, Eberhard DA, Waring P, Schuster SC, Modrusan Z, Zhang Z, Stokoe D, de Sauvage FJ, Faham M, Seshagiri S. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature. 2010;466(7308):869–873. doi: 10.1038/nature09208. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120(4):437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- McMurray MA, Gottschling DE. An age-induced switch to a hyper-recombinational state. Science. 2003;301(5641):1908–1911. doi: 10.1126/science.1087706. [DOI] [PubMed] [Google Scholar]
- Moragoda L, Jaszewski R, Kulkarni P, Majumdar AP. Age-associated loss of heterozygosity of tumor suppressor genes in the gastric mucosa of humans. Am J Physiol Gastrointest Liver Physiol. 2002;282(6):G932–936. doi: 10.1152/ajpgi.00312.2001. [DOI] [PubMed] [Google Scholar]
- Olsen CM, Green AC, Whiteman DC, Sadeghi S, Kolahdooz F, Webb PM. Obesity and the risk of epithelial ovarian cancer: a systematic review and meta-analysis. Eur J Cancer. 2007;43(4):690–709. doi: 10.1016/j.ejca.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Pignata S, Vermorken JB. Ovarian cancer in the elderly. Crit Rev Oncol Hematol. 2004;49(1):77–86. doi: 10.1016/s1040-8428(03)00100-8. [DOI] [PubMed] [Google Scholar]
- Preston CR, Flores C, Engels WR. Age-dependent usage of double-strand-break repair pathways. Curr Biol. 2006;16(20):2009–2015. doi: 10.1016/j.cub.2006.08.058. [DOI] [PubMed] [Google Scholar]
- Ries LA. Ovarian cancer. Survival and treatment differences by age. Cancer. 1993;71(2 Suppl):524–529. doi: 10.1002/cncr.2820710206. [DOI] [PubMed] [Google Scholar]
- Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J. Patient and Tumor Characteristics. National Cancer Institute, SEER Program; 2007. SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988–2001. NIH Pub. No. 07-6215. [Google Scholar]
- Riggs JE. Rising ovarian cancer mortality in the elderly: a manifestation of differential survival. Gynecol Oncol. 1995;58(1):64–67. doi: 10.1006/gyno.1995.1184. [DOI] [PubMed] [Google Scholar]
- Ruark E, Snape K, Humburg P, Loveday C, Bajrami I, Brough R, Rodrigues DN, Renwick A, Seal S, Ramsay E, Duarte Sdel V, Rivas MA, Warren-Perry M, Zachariou A, Campion-Flora A, Hanks S, Murray A, Pour NA, Douglas J, Gregory L, Rimmer A, Walker NM, Yang TP, Adlard JW, Barwell J, Berg J, Brady AF, Brewer C, Brice G, Chapman C, Cook J, Davidson R, Donaldson A, Douglas F, Eccles D, Evans DG, Greenhalgh L, Henderson A, Izatt L, Kumar A, Lalloo F, Miedzybrodzka Z, Morrison PJ, Paterson J, Porteous M, Rogers MT, Shanley S, Walker L, Gore M, Houlston R, Brown MA, Caufield MJ, Deloukas P, McCarthy MI, Todd JA, Turnbull C, Reis-Filho JS, Ashworth A, Antoniou AC, Lord CJ, Donnelly P, Rahman N. Mosaic PPM1D mutations are associated with predisposition to breast and ovarian cancer. Nature. 2013;493(7432):406–410. doi: 10.1038/nature11725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzenbach H, Eichelser C, Kropidlowski J, Janni W, Rack B, Pantel K. Loss of heterozygosity at tumor suppressor genes detectable on fractionated circulating cell-free tumor DNA as indicator of breast cancer progression. Clin Cancer Res. 2012;18(20):5719–5730. doi: 10.1158/1078-0432.CCR-12-0142. [DOI] [PubMed] [Google Scholar]
- Sekine M, Yoshihara K, Komata D, Haino K, Nishino K, Tanaka K. Increased incidence of brain metastases in BRCA1-related ovarian cancers. J Obstet Gynaecol Res. 2013;39(1):292–296. doi: 10.1111/j.1447-0756.2012.01961.x. [DOI] [PubMed] [Google Scholar]
- Seluanov A, Mittelman D, Pereira-Smith OM, Wilson JH, Gorbunova V. DNA end joining becomes less efficient and more error-prone during cellular senescence. Proc Natl Acad Sci USA. 2004;101(20):7624–7629. doi: 10.1073/pnas.0400726101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TCGA Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TCGA Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, Dickler M, Robson M, Moy F, Goswami S, Oktay K. Impairment of BRCA1-Related DNA Double-Strand Break Repair Leads to Ovarian Aging in Mice and Humans. Sci Transl Med. 2013;5(172):172ra121. doi: 10.1126/scitranslmed.3004925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZC, Birkbak NJ, Culhane AC, Drapkin R, Fatima A, Tian R, Schwede M, Alsop K, Daniels KE, Piao H, Liu J, Etemadmoghadam D, Miron A, Salvesen HB, Mitchell G, DeFazio A, Quackenbush J, Berkowitz RS, Iglehart JD, Bowtell DD, Matulonis UA. Profiles of genomic instability in high-grade serous ovarian cancer predict treatment outcome. Clin Cancer Res. 2012;18(20):5806–5815. doi: 10.1158/1078-0432.CCR-12-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancik R. Ovarian cancer. Age contrasts in incidence, histology, disease stage at diagnosis, and mortality. Cancer. 1993;71(2 Suppl):517–523. doi: 10.1002/cncr.2820710205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.