Abstract
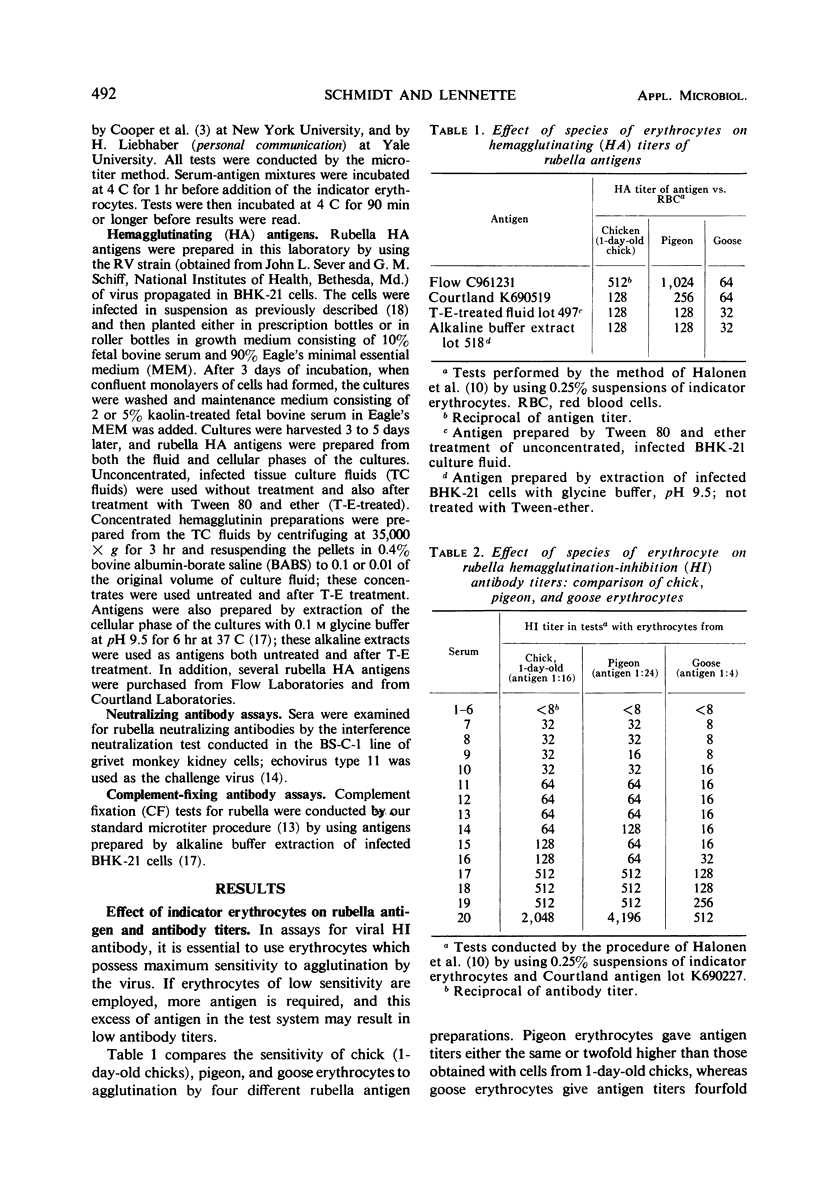
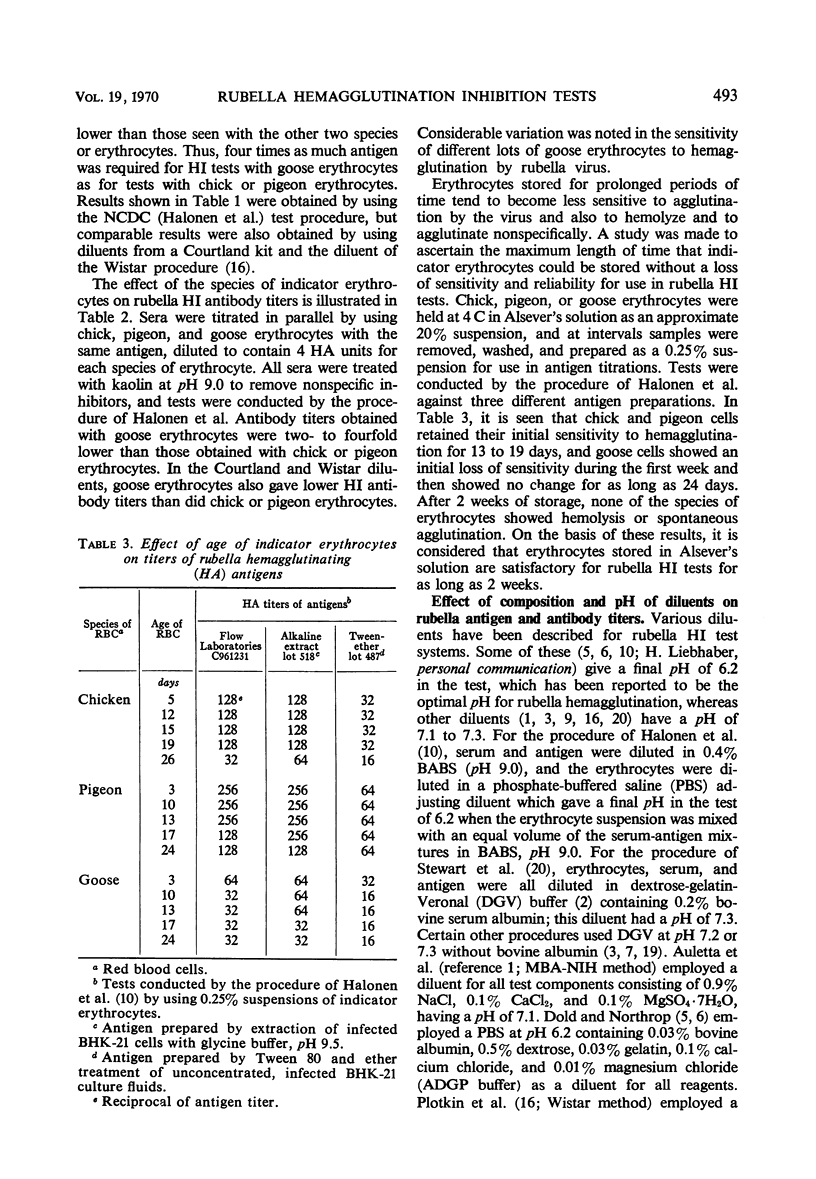
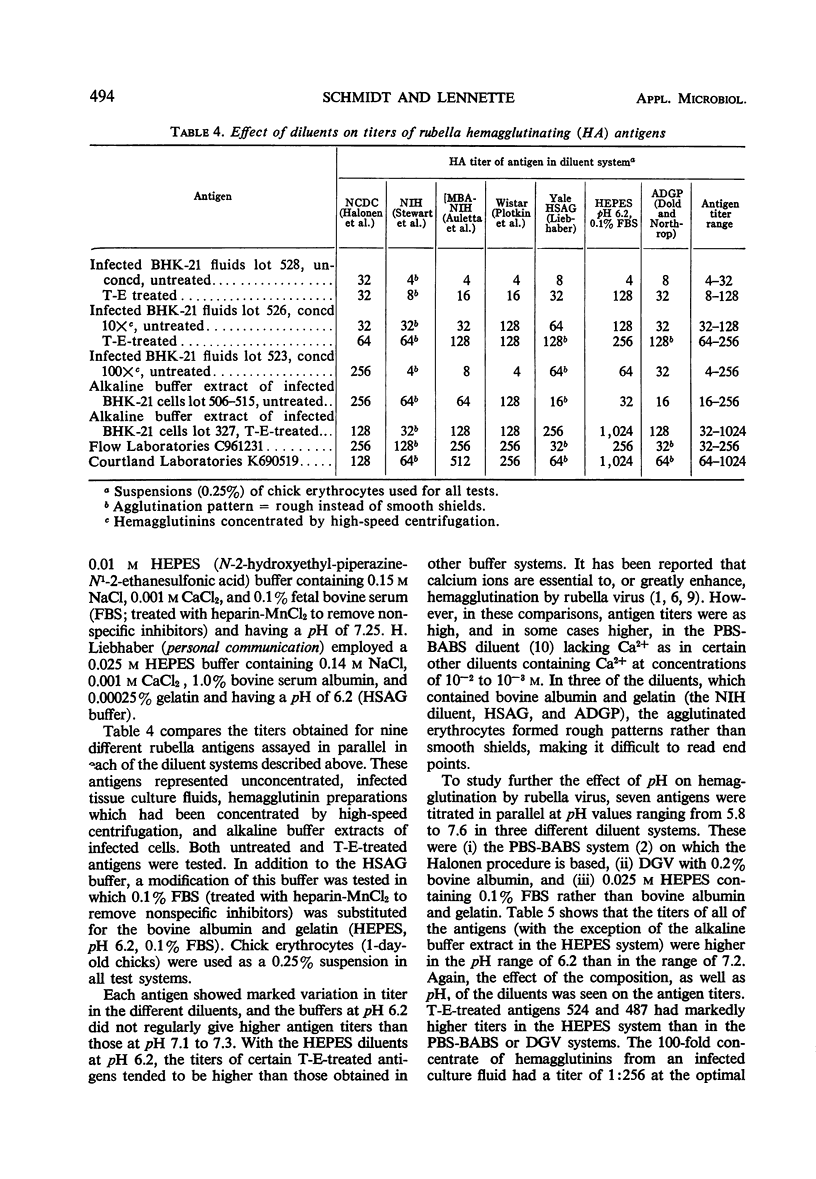
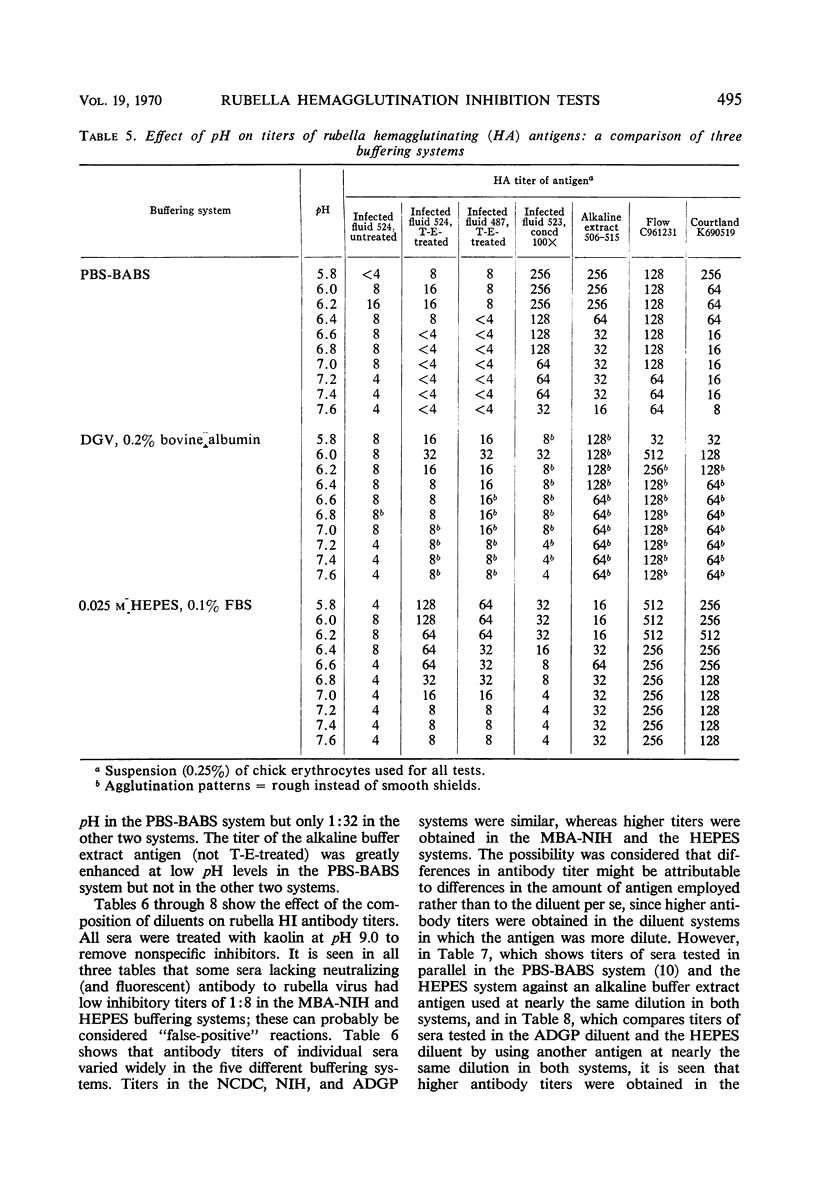
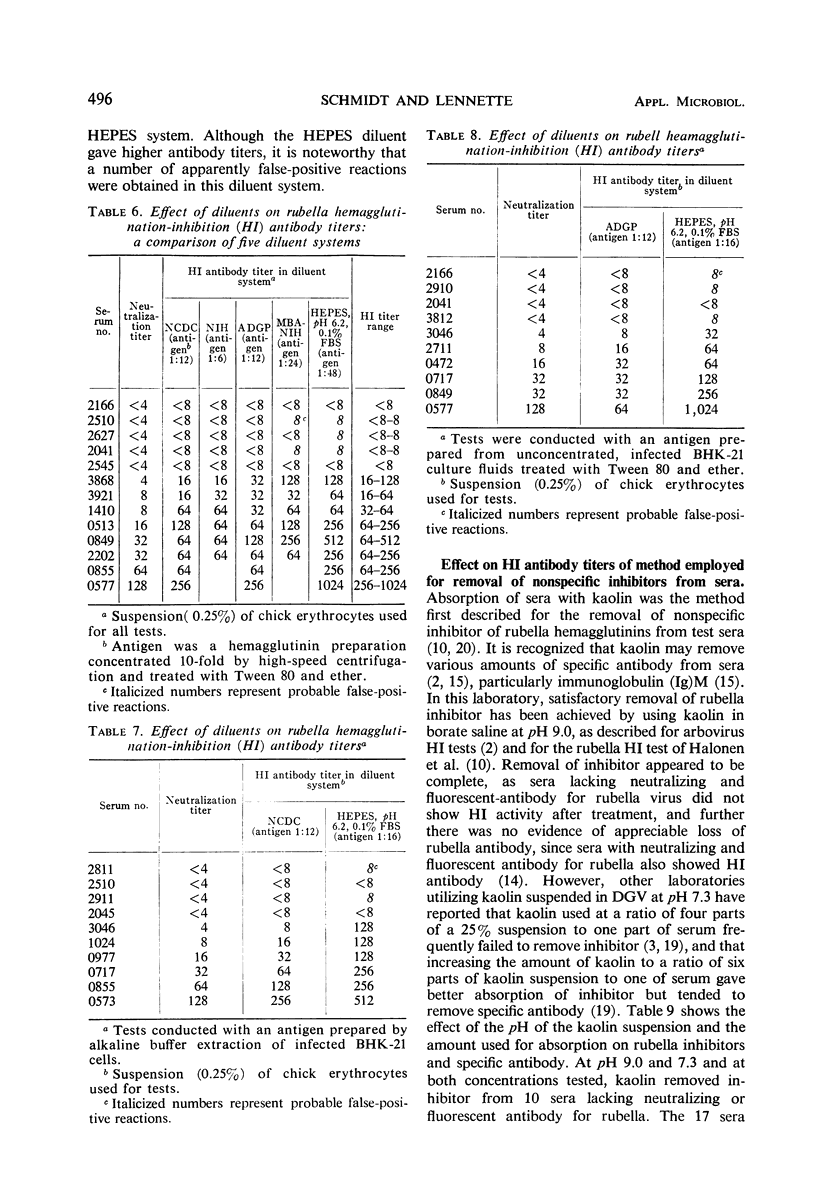
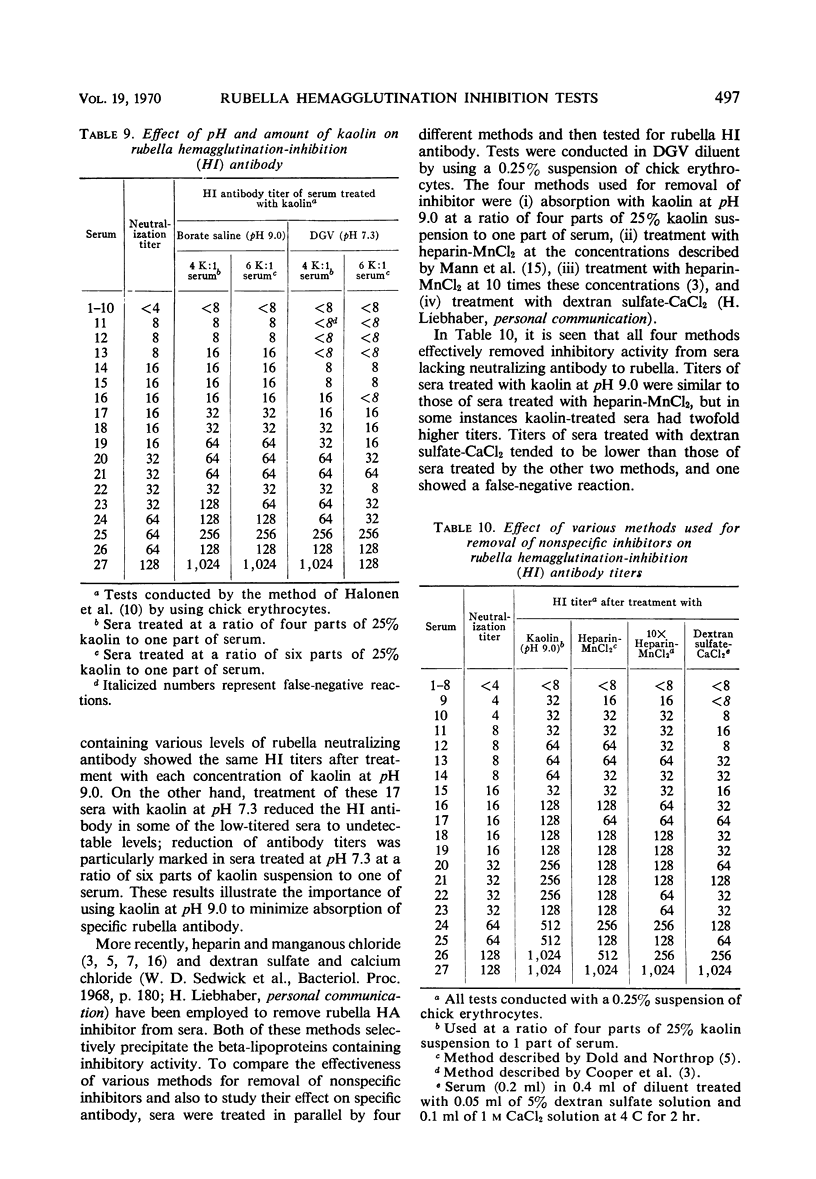
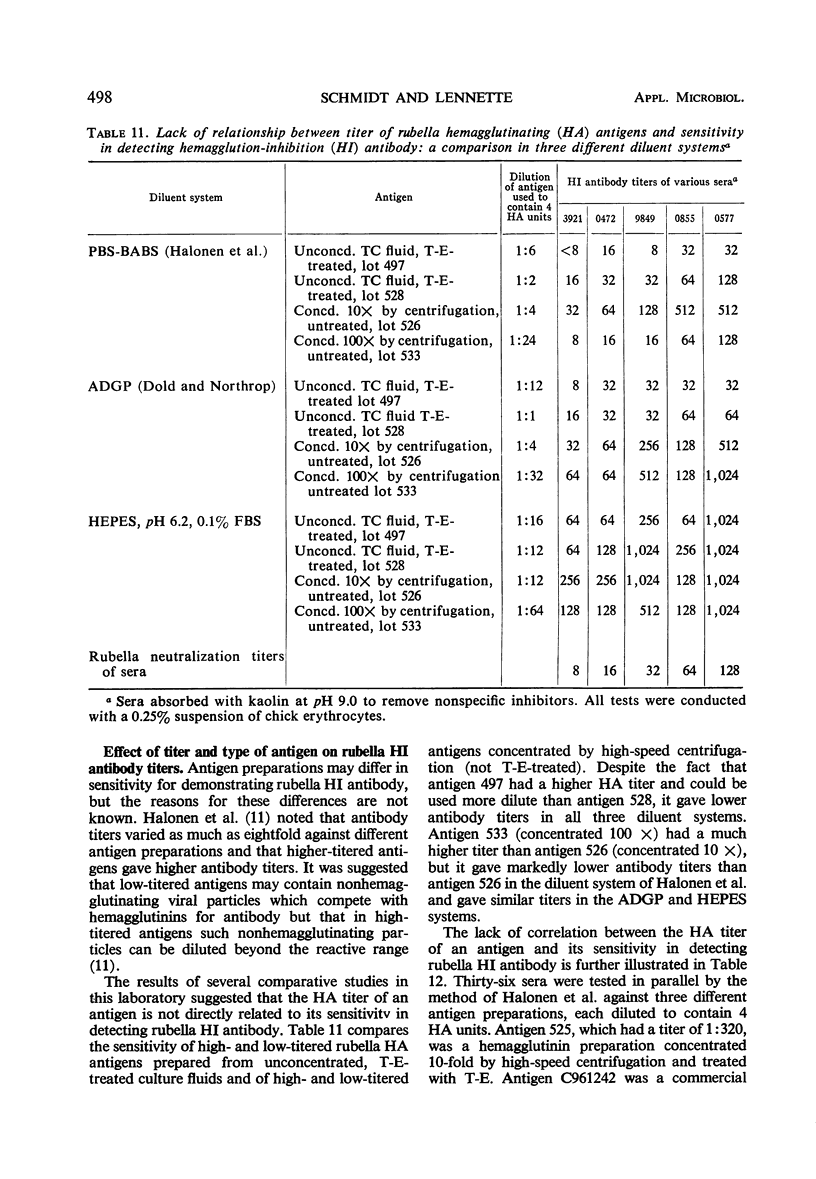
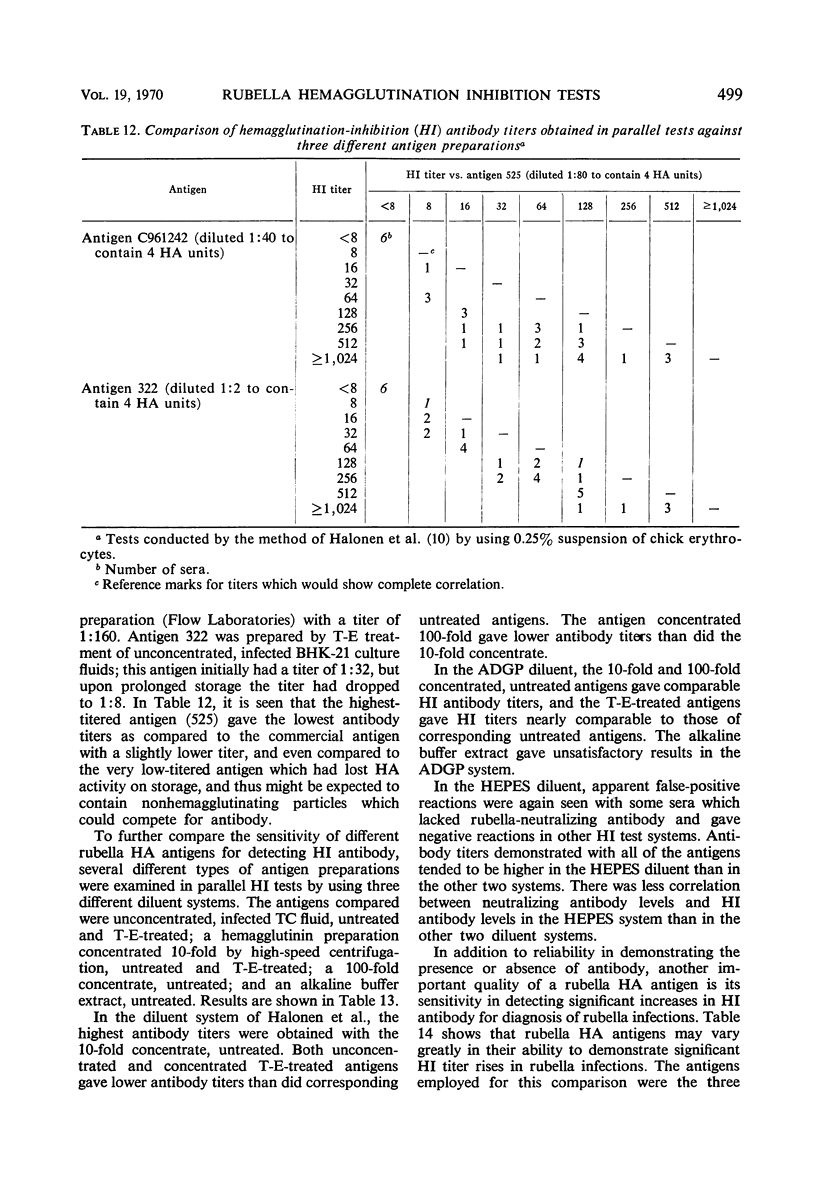
A systematic study was made of certain variables of the rubella hemagglutination-inhibition (HI) test system and their effect on antigen and antibody titers. Erythrocytes from pigeons and 1-day-old chicks gave similar antigen and antibody titers, but goose erythrocytes gave lower titers. Indicator erythrocytes could be stored in Alsever's solution at 4 C for as long as 2 weeks without losing sensitivity in hemagglutination (HA) and HI tests. Antigen titers varied by eightfold or more in different diluent systems; titers were generally higher at pH 6.2 than at pH 7.2. A diluent without Ca2+ gave antigen titers as high as those obtained in diluents with added Ca2+ ions. Antibody titers also varied in different diluent systems. HEPES diluents at pH 6.2 gave higher antibody titers than those obtained in other diluents, but occasional “false-positive” inhibition reactions were seen. Kaolin suspended in borate saline at pH 9.0 effectively removed inhibitor from sera without absorbing specific antibody, but at pH 7.3 it removed various amounts of specific antibody. Antibody titers of sera treated with kaolin at pH 9.0 were similar to those of sera treated with heparin-MnCl2; treatment with dextran sulfate-CaCl2 gave lower antibody titers. Antigens varied widely in sensitivity for detecting HI antibody and in the ability to detect diagnostically significant increases in antibody. Sensitivity in detecting antibody was not related to the HA titer of the antigens. Tween-ether-treated antigens gave lower antibody titers but were more reliable than corresponding untreated antigens for serological diagnosis of infection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auletta A. E., Gitnick G. L., Whitmire C. E., Sever J. L. An improved diluent for rubella hemagglutination and hemagglutination-inhibition tests. Appl Microbiol. 1968 May;16(5):691–694. doi: 10.1128/am.16.5.691-694.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- Cooper L. Z., Matters B., Rosenblum J. K., Krugman S. Experience with a modified rubella hemagglutination inhibition antibody test. JAMA. 1969 Jan 6;207(1):89–93. [PubMed] [Google Scholar]
- Deibel R., Cohen S. M., Ducharme C. P. Serology of rubella. Virus neutralization, immunofluorescence in BHK21 cells, and hemagglutination inhibition. N Y State J Med. 1968 Jun 1;68(11):1355–1362. [PubMed] [Google Scholar]
- Dold H. J., Northrop R. L. Analysis of the diluents used for rubella virus hemagglutination and hemagglutination-inhibition tests. Appl Microbiol. 1969 Aug;18(2):221–227. doi: 10.1128/am.18.2.221-227.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dold H. J., Northrop R. L. The nonspecific inhibitors of rubella-virus hemagglutination. Proc Soc Exp Biol Med. 1968 Jun;128(2):577–581. doi: 10.3181/00379727-128-33071. [DOI] [PubMed] [Google Scholar]
- Feldman H. A. Removal by heparin-MnCl2 of nonspecific rubella hemagglutinin serum inhibitor. Proc Soc Exp Biol Med. 1968 Feb;127(2):570–573. doi: 10.3181/00379727-127-32743. [DOI] [PubMed] [Google Scholar]
- Field A. M., Vandervelde E. M., Thompson K. M., Hutchinson D. N. A comparison of the haemagglutination-inhibition test and the neutralisation test for the detection of rubella antibody. Lancet. 1967 Jul 22;2(7508):182–184. doi: 10.1016/s0140-6736(67)90006-2. [DOI] [PubMed] [Google Scholar]
- Halonen P. E., Ryan J. M., Stewart J. A. Rubella hemagglutinin prepared with alkaline extraction of virus grown in suspension culture of BHK-21 cells. Proc Soc Exp Biol Med. 1967 May;125(1):162–167. doi: 10.3181/00379727-125-32038. [DOI] [PubMed] [Google Scholar]
- Halonen P. E., Stewart J. A., Hall A. D. Rubella hemagglutinin prepared in serum free suspension culture of BHK-21 cells. Ann Med Exp Biol Fenn. 1967;45(2):182–185. [PubMed] [Google Scholar]
- Herrmann K. L., Halonen P. E., Stewart J. A., Casey H. L., Ryan J. M., Hall A. D., Caswell K. E. Evaluation of serological techniques for titration of rubella antibody. Am J Public Health Nations Health. 1969 Feb;59(2):297–304. doi: 10.2105/ajph.59.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennette E. H., Schmidt N. J., Magoffin R. L. The hemagglutination inhibition test for rubella: a comparison of its sensitivity to that of neutralization, complement fixation and fluorescent antibody tests for diagnosis of infection and determination of immunity status. J Immunol. 1967 Oct;99(4):785–793. [PubMed] [Google Scholar]
- Mann J. J., Rossen R. D., Lehrich J. R., Kasel J. A. The effect of kaolin on immunoglobulins: an improved technique to remove the nonspecific serum inhibitor of reovirus hemagglutination. J Immunol. 1967 Jun;98(6):1136–1142. [PubMed] [Google Scholar]
- Plotkin S. A., Bechtel D. J., Sedwick W. D. A simple method for removal of rubella hemagglutination inhibitors from serum adaptable to finger-tip blood. Am J Epidemiol. 1968 Nov;88(3):301–304. doi: 10.1093/oxfordjournals.aje.a120888. [DOI] [PubMed] [Google Scholar]
- Schmidt N. J., Lennette E. H., Gee P. S., Dennis J. Physical and immunologic properties of rubella antigens. J Immunol. 1968 Apr;100(4):851–857. [PubMed] [Google Scholar]
- Schmidt N. J., Lennette E. H. Rubella complement-fixing antigens derived from the fluid and cellular phases of infected BHK-21 cells: extraction of cell-associated antigen with alkaline buffers. J Immunol. 1966 Dec;97(6):815–821. [PubMed] [Google Scholar]
- Stewart G. L., Parkman P. D., Hopps H. E., Douglas R. D., Hamilton J. P., Meyer H. M., Jr Rubella-virus hemagglutination-inhibition test. N Engl J Med. 1967 Mar 9;276(10):554–557. doi: 10.1056/NEJM196703092761006. [DOI] [PubMed] [Google Scholar]



