Abstract
Animal models to study the causes and consequences of obesity during infancy in humans would be valuable. In this study we examine the patterns of fat mass gain from birth to 12 months in common marmosets (Callithrix jacchus). Lean and fat mass was measured by quantitative magnetic resonance at 1, 2, 6, and 12 months for 31 marmosets, 15 considered Normal and 16 considered Fat (>14% body fat) at 12 months. Animals were fed either the regular colony diet mix or a high-fat variation. Subjects classified as Fat at 12 months already had greater lean mass (198.4±6.2g vs 174.0±6.8g, p=.013) and fat mass (45.5±5.0g vs 24.9±3.4g, p=.002) by 6 months. Body mass did not differ between groups prior to 6 months, however, by 1 month Fat infants had greater percent body fat. Percent body fat decreased between 1 and 12 months in Normal subjects; in Fat subjects it increased. The high-fat diet was associated with body fat >14% at 6 months (p=.049), but not at 12 months. This shift was due to 3 subjects on the normal diet changing from Normal to Fat between 6 and 12 months. Although maternal prepregnancy adiposity did not differ, overall, between Normal and Fat subjects, the subjects Normal at 6 and Fat at 12 months all had Fat mothers. Therefore, diet and maternal obesity appear to have potentially independent effects that may also vary with developmental age. Although birth weight did not differ between groups, it was associated with fat mass gain from 1 to 6 months in animals with >14% body fat at 6 months of age (r=.612, p=.026); but not in 6 month old animals with < 14% body fat (r=-.012, p=.964). Excess adiposity in captive marmosets develops by 1 month. Birth weight is associated with adiposity in animals vulnerable to obesity.
Keywords: Primates, body composition, adiposity, childhood obesity
Introduction
Childhood overweight and obesity is a growing concern in the world with an average rate of 11.7% (95% CI: 8.9 – 15.3%) in developed countries and 6.1% (95% CI: 5.0 – 7.2%) in developing nations [de Onis et al., 2010]. There are an estimated 43 million overweight or obese children under the age of 5 years in the world [de Onis et al., 2010]. The proportion of preschool-aged (2-4 years old) children in the U.S. classified as obese is approximately 15% [CDC, 2009]. Among 4 year olds the estimated prevalence of obesity in the U.S. is over 18%, ranging from 12.8% in Asian Americans to 31.2% in American Indians [Anderson & Whitaker, 2009]. In Leeds, United Kingdom 27.1% of children aged 3 – 14 years old were considered overweight with 12.6% classified as obese [Fraser & Edwards, 2010]. Childhood obesity is associated with an increased risk of adult obesity and of early life occurrence of diseases that have been associated historically with middle- and late-age in humans, such as Type II diabetes [Gardner et al., 2009].
Many common animal models may provide a relatively poor comparison to humans in terms of early life body fat acquisition [Kuzawa, 1998]. Humans are proposed to have substantially higher body fat in infancy and early childhood than found in other primates or in many commonly used mammalian models, such as mice and rats. Human infants average 15% fat, relative to body weight at birth, and percentage body fat increases to a peak of around 25% at 6-8 months – the point at which weaning generally begins. Domestic and research mammals for which data are available generally have only 1-5% body fat at birth. Kuzawa [1998] proposes that the likely evolutionary basis of this difference lies in greater adiposity associated with the energy reserves required to meet the demands of the large human brain paired with the likelihood of nutritional disruption during the weaning period. However, data on nonhuman primate body composition in early life is relatively limited, with published data cited in Kuzawa's comparative analysis limited only to the baboon [Lewis et al., 1983].
Many, if not most, nonhuman primate species display overweight and obese phenotypes when kept in a captive environment, with ad lib access to food and relatively low energy requirements [Kemnitz, 1984; Bodkin et al., 1993; Hansen & Bodkin, 1998; West & York, 1998; Comuzzie et al., 2003; Wagner et al., 2006; Kavanagh et al., 2007; Tardif et al., 2009]. However, the relation of these adult phenotypes to infant and juvenile growth patterns has not been examined for most species.
The common marmoset, a small New World monkey, offers opportunities in this area of research. Associated with its small body size is a relatively fast life history. Marmoset infants begin weaning at around 30 days of age and are completely weaned by around 70-80 days of age. They begin puberty between 11 and 14 months of age and are fully reproductively competent by around 18 months of age. Stable adult weights are generally attained by two years of age [Tardif et al., 2003]. In the wild, adult marmosets average 320 to 336 grams [Arujo et al., 2000]. The average weight of adult captive animals ranges from 283 to 530 grams, with most animals historically being in the range of 350-400 grams [Tardif et al., 2009]. However, we have observed a consistent increase in the number of high-weight, high-fat animals in our colony over a 14 year period. Mean early adult weight in our colony is now close to 400g, and the proportion of adult animals with body weights above 450g has greatly increased.
Recent publications, by our group and others, have described phenotypes associated with obesity in the marmoset, including metabolic dysfunction and dyslipidemia [Tardif et al., 2009; Wachtman et al., 2010]. For example, adult marmosets with body fat percentages above the 80th percentile (14% body fat for males, 17% for females) had significantly elevated glycated hemoglobin (HbA1c) and fasting glucose, triglycerides and very low-density lipoproteins (VLDL). We reported on growth in body weight in marmosets destined to be obese by early adulthood. Marmosets that would eventually reach the 90th percentile of early adult weight were distinguished by higher weights at birth as well as during the pre-weaning and post-weaning periods of infancy. The birth weight differences, while statistically significant, were minor (3%) while the differences in the peri-weaning period had increased to 15.5%. Thus, approximately half of the eventual relative weight difference between “obese” and “normal” adult marmosets had been attained by 6 months of age [Tardif et al., 2009]. However, we had no information on body composition within this population.
In this longitudinal analysis of age-specific body composition and relative gains in fat versus lean mass, we investigated the development of adiposity in captive common marmosets from birth through 12 months of age. This study was undertaken to determine: 1) the age at which patterns of growth begin to diverge between marmosets destined to be fat versus lean, 2) if animals that were considered fat at 12 months of age could be distinguished from their leaner counterparts at earlier ages, in terms of body mass or relative and absolute fat acquisition during infancy – i.e. how predictive is fatness or leanness at earlier ages to body composition at 12 months, and 3) how fatness at each age was related to maternal adiposity or exposure to higher-fat diets. The study also afforded the opportunity to determine if the body composition of this species reflects the low relative body fat in infancy that is suggested to be true for nonhuman primates [Kuzawa, 1998].
Methods
Study Population
The study population of 33 marmoset infants was comprised of 20 litters from 13 dams housed at the Southwest National Primate Research Center (SNPRC), San Antonio TX (see Table 1). The study was conducted between January 2008 and July 2011. The research was approved by the Animal Care and Use Committee of SNPRC, and adhered to the American Society of Primatologists principles for the ethical treatment of primates. Basic details on housing and husbandry have been previously described [Tardif & Bales, 2004]. All infants were housed with their parents plus older offspring. Animals were fed either the standard diet mix fed to the entire colony (Normal mix) or a modification of that mix that included Normal mix plus the standard diets formulated with increased fat content (High-fat mix). Marmosets begin to consume solid food at around 30 days of age; are completely weaned at somewhere between 70 and 80 days of age; and begin puberty at 11-14 months of age. Based upon a comparison of this weaning and maturation pattern to humans, a 30 day old marmoset may be considered equivalent to a 5-8 month old human infant, a 180 day (6 month) old marmoset may be considered equivalent to a prepubertal child – i.e. a juvenile, and a 12 month old marmoset similar to a human adolescent.
Table 1.
Dams in the study. Dams were roughly evenly divided among fat/normal and on a high fat diet or normal diet mix.
| Dam ID | Number of litters | Number of infants | Diet | Dam Fat or Normal |
|---|---|---|---|---|
| 616 | 2 | 3 | Normal mix | Normal |
| 629 | 1 | 1 | Normal mix | Normal |
| 920 | 2 | 4 | Normal mix | Normal |
| 1014 | 1 | 2 | Normal mix | Normal |
| 494 | 2 | 4 | High fat mix | Normal |
| 738 | 2 | 4 | High fat mix | Normal |
| 765 | 2 | 3 | High fat mix | Normal |
| 536 | 2 | 4 | Normal mix | Fat |
| 688 | 2 | 2 | Normal mix | Fat |
| 438 | 1 | 1 | High fat mix | Fat |
| 586 | 1 | 2 | High fat mix | Fat |
| 778 | 1 | 1 | High fat mix | Fat |
| 900 | 1 | 2 | High fat mix | Fat |
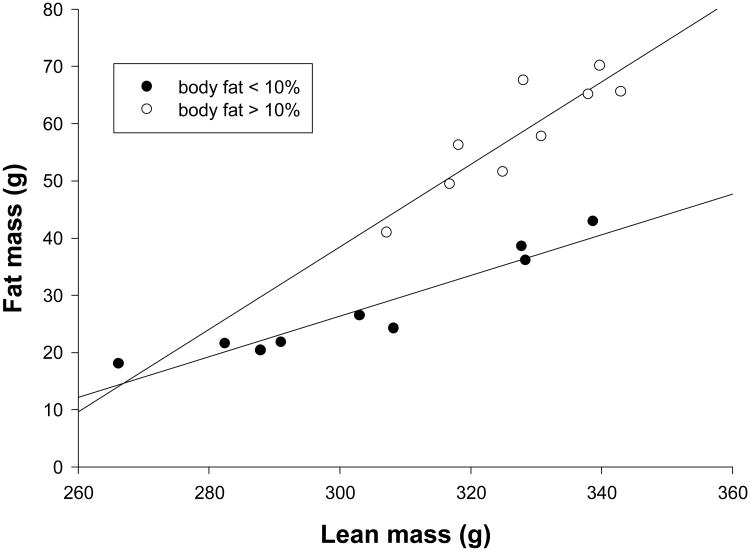
All dams in this study were between 3 and 6 years of age and had produced at least one successful litter prior to the birth of the infants in this study. Prepregnancy maternal body mass was recorded and lean and fat mass measured by quantitative magnetic resonance imaging (QMR) – see description, below. The mothers were considered Normal if their percent body fat was at or below 10%, and Fat if their percent body fat was greater than 10%. This percent fat was chosen because dams above 10% body fat had a significantly different relationship between fat and lean mass than did dams with less than 10% body fat (Figure 1).
Figure 1.
Maternal fat mass versus maternal lean mass. Dams with > 10% body fat had a significantly steeper slope for the relationship between fat and lean mass (0.721±0.145 versus 0.338±0.054, t=2.748, df=19, p=0.013) than did dams with < 10% body fat.
Data Collection
We collected weight and body composition data from birth through 12 months on total body mass and lean and fat mass for 31 common marmosets and additional data through 6 months of age for two animals that died from acute causes before reaching one year. Infants were weighed to the nearest gram within 36 hours of birth (Day 1 wt), and then at approximately 1, 2, 3, 6 and 12 months of age. In addition, opportunistic body weights were recorded approximately every week. Body composition (lean and fat mass) was assessed at approximately 1, 2, 6 and 12 months of age via quantitative magnetic resonance imaging (QMR) using two Echo Medical Systems (Houston, TX) MRI units – one accommodating animals up to approximately 80 grams and the other accommodating animals from approximately 100 to 800 grams. This system has been extensively validated for mice [Tinsley et al., 2004] and the detection methodology used in the marmoset system is identical to that used in the mouse system, with only the volume of the homogenous magnetic region differing. Unsedated animals were placed in a plastic tube, which was then inserted into the magnetic chamber. Scans took < 2 min, on average, for each animal.
It was not possible to obtain QMR assessments on live neonates because of concerns regarding disrupting parental care to the point that infant survival might be compromised. However, QMR assessments were made of fat and lean mass of two infants who died at birth to obtain an indication of % fat at birth. One infant was stillborn and the second died from acute effects of trauma. Both infants were full term and were within the normal birth weight range.
Based on our previously published results for fat mass in marmosets [Tardif et al., 2009] the subjects in this study were divided into Normal (less than 14% body fat) and Fat (more than 14% body fat) at both 6 and 12 months of age. Young marmosets generally have higher percent body fat than do fully adult animals [Tardif & Power, unpublished data].
Analysis
The following variables were compared in the 12 month old Normal versus Fat subjects: weight at days 1, 30, 60, 90, and 180; body fat (absolute and relative to lean mass) at days 30, 60 and 180; and fat and lean mass gain from approximately day 30 to 180. Values are given as mean ± SEM. Differences between the two groups in body mass, lean and fat mass, percent body fat, and the ratio of fat-to-lean were tested by ANOVA. The relationship between Day 1 weight and fat gain was examined by correlation. The effects of maternal adiposity, diet, and infant sex on body mass and lean and fat mass were examined by ANCOVA with age as the covariate.
To further explore differences in growth patterns between Normal and Fat infant marmosets we examined the full set of weight data points (planned and opportunistic) using ANCOVA and linear regression. Body mass and age were transformed by the natural logarithm function, and the difference in ln(body mass) between the two groups was tested using ANCOVA, with ln(age) as the covariate. Linear regression of the transformed parameters was used to estimate the exponent of growth and 95% confidence interval for each group.
Correlation and chi-square were used to examine the association between adiposity at 6 months and 12 months of age. Chi-square, ANOVA and ANCOVA (with birth weight as the covariate) were used to examine the effects of diet and maternal adiposity on offspring adiposity at 1, 2, 6 and 12 months of age.
Results
All 33 infants were mother-reared; 13 litters were reared as twins and 7 as singletons. Based on our criteria of 14% body fat, at 12 months of age 15 infants were considered to be Normal and 16 considered to be Fat. There was no effect of sex, with 8 males and 8 females in the Fat group and 5 males and 10 females in the Normal group, or litter size (p=.930). In only two of the 12 twin litters (both from the same dam) was there discordance between the twins; otherwise both twins were either Normal or Fat at 12 months. Six dams produced a single litter, producing 4 Fat and 4 Normal subjects. Seven dams produced two litters in this study (Table 1), producing 12 Fat and 11 Normal subjects at 12 months of age. Four of those dams produced either all Fat or all Normal infants in both litters; two dams produced a litter of Fat infants and a litter of Normal infants, and a single female contributed both of the litters with Normal and Fat infants.
Relation of Diet and Maternal Adiposity to Birth Weight
Dams on the High-fat mix produced larger infants at birth (infant day 1 body mass of 30.5±0.5 g versus 28.6±0.6 g, F=6.179, df=1,31, p=.019). Infant day 1 body mass was positively associated with maternal adiposity as measured by either the ratio of maternal fat-to-lean mass (r=.453, p=.008) or by maternal percent body fat (r=.426, p=.013), and Fat dams produced larger infants (30.9±0.7 g versus 28.9±0.5 g, F=6.808, df=1,31, p=.014). Dam adiposity did not differ between diets (based on 20 litters, Normal mix mean Dam body fat = 9.1±1.1%, High-fat mix mean Dam body fat = 10.5±1.1%, F=0.857, df=1,18, p=.367). A two-way ANOVA determined that both dam adiposity (F=8.466, df=1,29, p=.007) and diet (F=7.070, df=1,29, p=.013) independently (dam X diet, p=.899) affected infant day 1 body mass, with Fat dams and High-fat diet mix both associated with larger infants at birth.
Relation of Diet to Offspring Adiposity
Diet had a significant effect on juvenile adiposity at 6 months of age. Ten of 17 juveniles offered the High-fat mix had greater than 14% body fat at 6 months as opposed to four of 16 animals offered the Normal mix (Chi-square = 3.860, df=1, p=.049). Juveniles offered the High-fat mix had greater gains in lean (0.99±0.05 g/day vs 0.86±0.03 g/day, F(1,27)=5.685, p=.024) and fat mass (0.25±0.04 g/day vs 0.12±0.02 g/day, F(1,27)=9.067, p=.006) from 1 to 6 months, and a greater ratio of fat-to-lean gain (0.236±.035 vs .139±.014, F(1,27)=7.695, p=.010). At 12 months of age, however, there was no longer an effect of diet.
Relation of Maternal Adiposity to Offspring Adiposity
Maternal adiposity was not associated with infant body mass or adiposity at 1, 2, 6 or 12 months of age. Maternal prepregnancy weight did not differ between marmosets categorized as Normal and Fat at either 6 months (400.7±10.3g versus 407.2±10.8g, p = .671) or at 12 months (396.3±12.1g versus 406.7±10.0g, p=.508) and neither did any measure of maternal adiposity. Mothers with prepregnancy body fat above 10% produced 7 Fat and 3 Normal juveniles at 12 months of age; for mothers with body fat below 10% the numbers were 9 Fat and 12 Normal (Chi-square=1.998, df=1, p=.157).
Mass and Body Composition from Birth to 6 Months
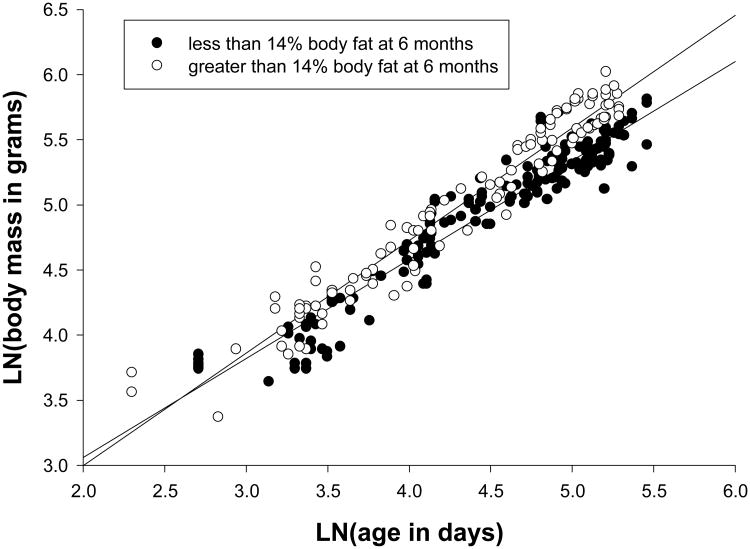
Mean Day 1 wt for all infants was 29.6g and did not differ between the Normal and Fat 6 month old groups. Similarly, the two groups were not significantly different in body mass at either 1, 2 or 3 months of age (Table 2). However, the relationship between age and body mass through 6 months of age was significantly different between marmosets destined to be Fat (>14% body fat) versus Normal at 12 months of age (F(1,253)=4.473, p=.035). The exponent of growth of total body mass was 0.810 (.769 - .850; R2 = 0.922) in Fat subjects and 0.755 (.712 - .798; R2 = 0.910) in Normal subjects. Juveniles later classified as Fat at 12 months weighed more by 6 months of age (mean body mass 274g, range = 162 - 348g vs 227g, range = 176 - 343g, F(1,29)=9.080, p=.005). This difference in body mass did not appear until after the animals were 3 months of age (Figure 2). Table 2 provides comparative data on weights from 1 month to 12 months of age in marmosets representing the feral condition in terms of maximum weight attainment [de Castro Leão et al., 2009] versus the Normal and Fat subjects in the present study. The Normal animals were within 20% of the predictions of de Castro Leao and colleagues [2009] at each time point. Although the Fat animals were within 13% of the prediction through 3 months of age, they began to deviate from the prediction after 3 months of age and were 37% and 47% greater in mass than predicted at 6 and 12 months, respectively.
Table 2.
Comparison of infant weigh ts in present study to predictions from a situation more closely mirroring feral conditions.
| Age | Present study – infants < 14% fat at 12 months N = 15 | Present study – infants > 14% fat at 12 months N = 16 | Weight prediction from ref 17 |
|---|---|---|---|
| Birth | 29.7 ± 0.8g | 29.5 ± 0.4g | |
| 1 month | 62.5 ± 2.6g | 61.8 ± 2.4g | 70 |
| 2 months | 113.6 ± 6.0g | 111.4 ± 5.9g | 100 |
| 3 months | 177.1 ± 8.6g | 167.7 ± 7.4g | 140 |
| 6 months | 226.6 ± 10.3g* | 273.6 ± 11.6g* | 200 |
| 12 months | 257.5 ± 9.8* | 402.9 ± 12.0* | 275 |
indicates values were statistically different; P<.01)
Figure 2.
Growth in common marmosets from birth to 6 months of age in animals that had body fat greater than 14% at 6 months of age (open circles) and body fat less than 14% at 6 months (filled circles).
Fat subjects had greater percent body fat and ratio of fat-to-lean mass at 1 month of age, despite no significant difference in body mass (Table 3). At 2 months of age these differences were no longer significant. Note that the ratio of fat-to-lean mass and percent fat mass declined for both groups between 1 and 2 months (Table 3). Marmosets categorized as Fat at 12 months had greater lean mass by 6 months of age (198g vs 174g, F(1,29)=7.063, P=.013) in addition to greater fat mass (46g vs 25g, F(1,29)=11.345, p=.002). The mean daily gains in lean (1.06±0.03 g/day vs 0.81±0.02 g/day, F(1,27)=41.863, p<.001) and fat mass (0.30±0.03 g/day vs 0.09±0.01 g/day, F(1,27)=60.675, p<.001) over the first 6 months of life were greater in Fat subjects. The relationship between fat and lean mass changed between 2 and 6 months; in Normal subjects it stayed roughly constant while in Fat subjects it increased (Table 3).
Table 3.
Mean lean and fat mass, and the ratio of fat-to-lean and percent body fat at 1, 2 and 6 months of age in common marmoset infants classified as either Normal (<14% body fat) or Fat (>14% body fat) at 12 months of age. P values for the comparisons are given under the results for the Fat subjects. Four infants were not measured at 1 month and one at 2 months due to technical problems with the equipment.
| 1 month | 2 months | 6 months | ||||
|---|---|---|---|---|---|---|
| Normal N = 16 | Fat N = 13 | Normal N = 19 | FatN = 13 | Normal N = 19 | Fat N = 14 | |
| Lean mass | 49.6±2.3g | 48.8±1.7g ns | 91.4±3.9g | 87.7±3.8g ns | 174.0±6.8g | 198.4±6.2g p = .013 |
| Fat mass | 8.6±0.5g | 9.7±0.6g ns | 12.8±1.3g | 13.9±1.4g ns | 24.9±3.4g | 45.5±5.0g p = .002 |
| Fat:lean mass | .173±005 | .197±008 p = .023 | .138±.010 | .156±012 ns | .139±013 | .222±.020 p = .002 |
| Percent fat | 14.3±0.3% | 15.9±0.6% p = .033 | 11.0±0.7% | 12.4±0.9% ns | 10.6±0.9% | 15.9±1.2% p = .002 |
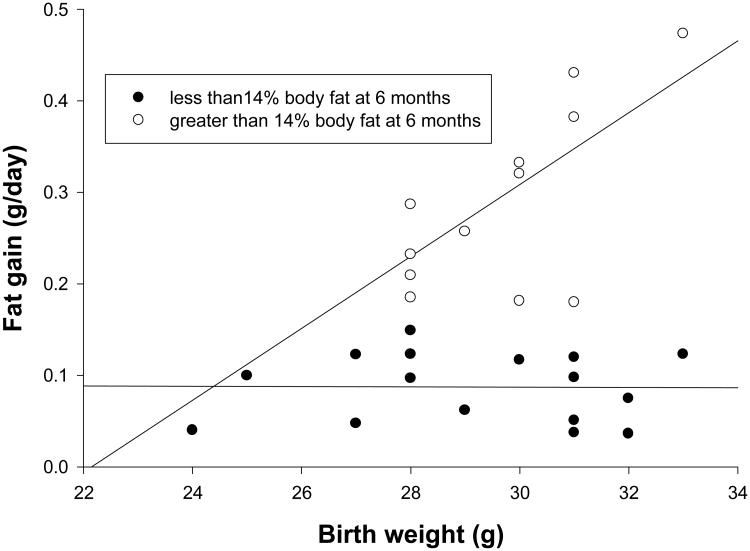
Neither Day 1 weight, diet, nor maternal adiposity was significantly associated with infant adiposity at 1 or 2 months of age (ANCOVA, p>.1 for all three). At 6 months diet was the sole significant factor (F=4.195, df=1,28, p=.05). However, interestingly, Day 1 weight was associated with the gain in fat mass from 1 to 6 months in subjects classified as Fat at 6 months (r=.612, p=.026); but not in Normal subjects (r = -.012, p=.964; Figure 3).
Figure 3.
Gain in fat mass (g/day) between 1 and 6 months by birth weight (Day 1 weight) in grams for juvenile marmosets with greater than 14% body fat at 6 months (open circles) and less than 14% body fat at 6 months (filled circles).
Mass and Body Composition from 6 to 12 months
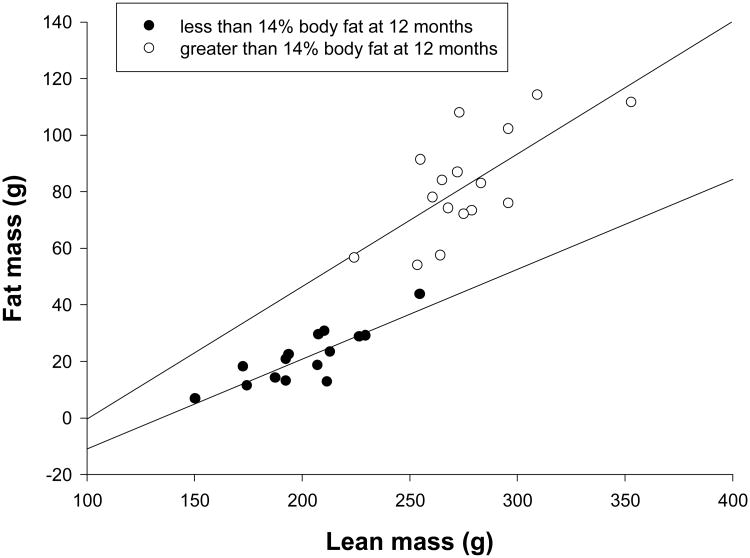
At 12 months of age 16 of the 31 animals had body fat greater than 14%. These Fat animals had significantly greater body mass (Table 2), lean mass (276.9±7.1g versus 201.8±6.6g, F=59.435, df=1,29, p<.001) and fat mass (82.6±4.8g versus 21.4±2.5g, F=124.863, df=1,29, p<.001) than Normal subjects. The regressions between fat and lean body mass were significant for both Fat (R2=.489, p=.003, β=.468±.128) and Normal (R2=.717, p<.001, β=.318±.055) animals (Figure 4). The coefficient relating lean to fat mass in the Normal group was virtually identical to the coefficient for Normal dams (β=.338±.054, Figure 2), suggesting that these animals had a normal adult pattern to lean and fat mass. Although the coefficients were not significantly different between Fat and Normal subjects (p=.302), ANCOVA results found that lean mass was a significant covariate (F=29.755, df=1,28, p<.001) and that the two groups differed in fat mass (F=20.779, df=1,28, p<.001). Neither Day 1 weight, diet, nor maternal adiposity was significantly associated with adiposity at 12 months of age (ANCOVA, p>.1 for all three).
Figure 4.
The relationship between lean and fat mass for Fat and Normal subjects at 12 months of age.
Percent body fat at 6 months was associated with percent body fat at 12 months (r=.587, p=.001). Of the 13 animals with greater than 14% body fat at 6 months of age 12 had greater than 14% body fat at 12 months with the sole individual declining to under 14% body fat suffering a bout of diarrhea at 9-10 months of age that resulted in lost weight; 14 of 18 animals with less than 14% body fat at 6 months remained under 14% body fat at 12 months (Chi-square = 11.342, df=1, p=.001). All four of the animals Normal at 6 months and Fta at 12 months had Fat mothers (mean maternal body fat = 14.3±1.0%, range: 12.9 – 17.0%). This association of maternal adiposity with a risk of increased body fat between 6 and 12 months of age was significant (p=.049). Three of these four animals were fed the Normal mix.
Neonatal adiposity
The post mortem percent body fat at day 1 was 12.04% for an infant weighing 25 g and 18.36% for an infant weighing 28 g. Percent body fat at weaning initiation (day 30) was about 15% in the infants in this study, similar to the post mortem neonatal data. At two months of age percent body fat had declined to about 12%. By 6 months of age the mean value had further decreased in Normal infants to less than 11% but had increased in Fat infants to about 16% (Table 3). In comparison, human neonates are reported to have approximately 15% body fat, increasing to 25% at weaning initiation and then declining post weaning to about 16% prepuberty [Kuzawa, 1998].
Discussion
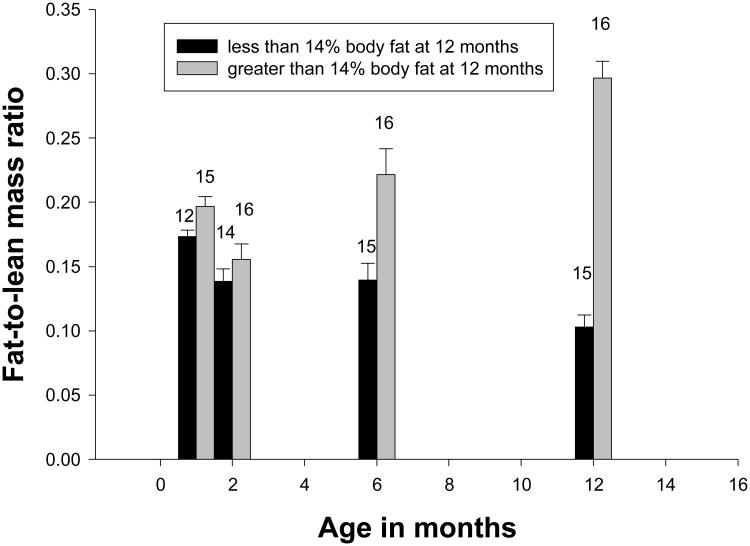
While not distinguishable by birth weight, by 12 months of age the subjects in our study had separated into two distinguishable groups, with marmosets over 14% body fat having greater lean as well as fat mass. Normal and Fat marmosets at 12 months of age could not be distinguished by body mass until after 3 months of age, a point at which the infants are weaned from milk and are subsisting exclusively on solid food. However, excess adiposity in captive marmosets appears to develop by 1 month of age, as evidenced by the greater fat-to-lean mass ratio at that age in subjects Fat at 12 months (Table 3; Figure 5). This suggests that, despite similar total body mass for most of early life, the two groups differed in growth patterns from an early age, with the infants destined to be designated Fat at 12 months gaining more fat-per-lean tissue. Subsequently, these animals appeared to have greater lean mass growth as well as greater fat deposition, such that they rapidly diverged in both lean and fat mass from the Normal juveniles. Despite the greater lean body mass growth, the ratio of fat-to-lean mass gain remained elevated in Fat subjects. Indeed, while the ratio of fat-to-lean mass declined with age in the Normal animals, in the Fat animals the ratio declined slightly between 1 and 2 months of age, and then increased at 6 and 12 months of age (Figure 5). Thus, it would appear that the infants in this study who became Fat at 12 months differed from their Normal conspecifics in two patterns of early growth. In the first month of life more fat was deposited for every gram of lean body mass; then, at some age between two and six months, growth of both lean and fat mass became higher than for the Normal animals. When compared to our previously characterized population of adult marmosets [Tardif et al., 2009] the Normal subjects by 6 months of age had a fat-to-lean ratio similar to non-obese adults (.126 versus .133) while the Fat juveniles had a fat-to-lean ratio similar to that of obese adults (.261 versus .267). By 12 months of age mean fat-to-lean mass ratio in the Fat marmosets from this study had increased to 0.297.
Figure 5.
The fat-lean-mass ratio with SEM for Normal and Fat animals at 1, 2, 6 and 12 months. Animals defined to be Normal had body fat < 14% and animals defined to be Fat had body fat > 14% at 12 months of age. Numbers above each bar are the number of subjects measured. Groups differed at p<.05 for ages 1, 6 and 12 months, but not at 2 months of age.
Adiposity at 6 months of age is predictive of adiposity at 12 months. Most (26 of 31) subjects were categorized identically between 6 and 12 months. One female subject changed from Fat to Normal. This individual lost weight at about 9-10 months of age due to a bout of diarrhea, and did not regain the weight she lost. Her twin sister remained Fat. All four subjects that went from Normal to Fat were offspring of mothers with greater than 10% body fat, ranging from 12.9% to 17.0%. Three of these infants were on the Normal diet mix. Maternal adiposity appeared to affect birth weight but had no direct association with adiposity later in life. Although diet had a significant effect on adiposity at 6 months, it was no longer significant at 12 months. About half of the subjects on either diet were above 14% body fat at 12 months. It would appear that access to a high-fat diet increases the incidence of early obesity; but that infants of fat mothers without access to a high-fat diet still have a higher likelihood of becoming obese at 12 months. Diet and maternal obesity appear to have potentially independent effects that may also vary with developmental age.
Birth weight was not associated with fat mass gain in Normal subjects; however, it was significantly associated with fat gain in the Fat subjects at 6 months (Figure 3). Both the High-fat mix diet and maternal body fat above 10% independently were associated with higher birth weight. These results suggest that some aspect of birth condition may play a role in determining patterns of infant growth, implying that in utero effects, through both maternal diet and adiposity, influence post natal growth. With these data we cannot distinguish between in utero conditions having different consequences for Normal versus Fat juveniles, or if some other early life experience during lactation changes the relationships between maternal adiposity, birth condition and growth between 1 and 12 months in either Normal or Fat juveniles. All we can say is that the initial divergence in growth between these two groups occurs during lactation; but that the eventual growth pattern in Fat juveniles may be affected by gestational conditions, as evidenced by the effect of birth weight on adiposity at 6 months in this group.
One of the surprising findings of this study was the relatively high percent body fat measured in two neonates measured post mortem (12% and 18% fat) and throughout infancy even among the Normal study infants (Table 3). There is a paucity of published information on body fat at birth or throughout infancy in nonhuman primates. A finding of 3% body fat at birth in baboons [Lewis et al., 1983] led Kuzawa [1989] to propose that nonhuman primates likely mirrored other nonhuman mammals in having quite low fat mass at birth. However, the findings of this study raise questions as to whether all nonhuman primates display low fat masses at birth when compared to humans and points out the value of comparative data from additional nonhuman primate species. Marmosets, at least, appear to have significant amounts of body fat as infants.
One caution regarding the results of this study is that the QMR methodology has not been validated against chemical extraction for marmosets. Marmosets are valuable animals, and in this longitudinal study we had insufficient subjects to sacrifice animals for chemical analysis. The pattern of estimated fat mass by QMR with total body mass in adult marmosets is consistent with results we have previously obtained using estimation of body water by stable isotope dilution [Power et al., 2001], however, the adult animals for the earlier study were lower in body weight, on average, than the adult animals we measured by QMR and reported previously [Tardif et al., 2009]. We are not aware of any other data on lean and fat mass in infant and juvenile marmosets that could be used for comparison. However, there are a number of studies that compare QMR findings on both live and post-mortem rodent adult samples, with chemical analysis. Nixon and colleagues [2010] reported that QMR performed postmortem (as was done on our newborn marmosets) slightly overestimated both fat and lean values relative to live QMR. Tinsley and colleagues [2004] reported that QMR and DEXA estimated body fat values in rodents that exceeded those of chemical analysis by around 20%. Therefore, differences in methods of assessing body fat mass may account for some of the differences between studies. However, even with a potential overestimate of 20% by QMR compared to chemical analysis, the percent body fat of the neonatal marmosets would far exceed the value reported in the literature for baboons [Lewis et al., 1983].
Although our results suggest that marmoset infants may resemble human infants in adiposity, humans are reported to differ from other mammals in the type of adipose tissue as well as the amount. Brown adipose tissue accounts for a relatively small amount of adipose tissue in human neonates, but comprises a substantial majority of neonatal fat in nonhuman mammals [Hahn & Novak, 1975]. Juvenile and adult marmosets have significant depots of brown fat [Rothwell & Stock, 1985]. In addition to verifying the suggested high adiposity of marmoset infants, an important area for future research will be the assessment of the relative amounts of brown versus white adipose tissue in marmosets, as well as in other nonhuman primate infants.
Previously, we found that female marmosets were more likely to become obese as adults than were males [Tardif et al., 2009]. This was not true of our subjects in this study; there were roughly equal numbers of fat males and females at 6 and 12 months of age. However, puberty is not attained until after 12 months in the marmoset. Continued surveillance of these subjects may yet show a sex difference post puberty. Among adult females in our previous study [Tardif et al., 2009] birth weight was associated with weight at a later age. This was not true of adult male marmosets; however, there were relatively few obese adult males in our sample. In this study, rather than a sex difference in the effect of birth weight on later fat gain, we found that a relationship existed among infants that put on excessive fat by 6 month, regardless of sex. It is possible that our previous result did not represent a true sex difference in the association between birth condition and later adiposity; but rather there is such an association among individuals that become obese, male or female, that was not evident in males due to the few obese males in our previous sample.
Conclusions
In this study we have identified key time periods (0 - 1 month and 2 – 5 months) during which growth patterns diverge between normal weight infant marmosets and their fatter conspecifics. Excess adiposity in marmosets can be detected as early as 1 month of age. Between 2 and 6 months of age marmosets that become obese grow faster (higher lean mass) and begin to increase in percent body fat, the opposite pattern from normal growth. We also found an effect of access to a high-fat diet on the likelihood of becoming obese at an early age. The association between birth weight, maternal diet and adiposity, and the association between birth weight and adiposity in the subset of infants that do become fat at 6 months indicate that studies examining in utero conditions would be of value in understanding why some marmoset infants grow so dramatically faster and fatter from an early age.
Marmoset infants may provide a useful animal model for the development of early-life obesity in humans. Their level of adiposity as infants is closer to human than other anthropoid primate models appear to be. Certainly a significant proportion of captive marmoset infants become essentially obese by 6 months of age. Our previous studies on adult marmosets demonstrated that adult obesity is associated with lipid and glucose metabolism characteristics resembling metabolic syndrome in humans [Tardif et al., 2009] implying that early obesity may have similar metabolic consequences to those found in humans.
Acknowledgments
We thank Donna Layne-Colon and Joselyn Artavia for their assistance in this research. This research was funded by PHS grant R01 DK077639. This research was conducted in full accordance with the laws of the United States and under protocols approved by the Animal Care and Use Committee of the Southwest National Primate Research Center.
Literature cited
- Anderson SE, Whitaker RC. Prevalence of obesity among US preschool children in different racial and ethnic groups. Archives of Pediatric and Adolescent Medicine. 2009;163:344–348. doi: 10.1001/archpediatrics.2009.18. [DOI] [PubMed] [Google Scholar]
- Araujo A, Arruda M, Alencar A, Albuquerque F, Nascimento MC, Yamamoto ME. Body weight of wild and captive common marmosets. International Journal of Primatology. 2000;21:317–324. [Google Scholar]
- Bodkin N, Hannah J, Ortmeyer H, Hansen BC. Central obesity in rhesus monkeys: association with hyperinsulinemia, insulin resistance and hypertriglyceridemia? International Journal of Obesity and Related Metabolic Disorders. 1993;17:53–61. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Obesity prevalence among low-income, preschool-aged children – United States, 1998-2008. MMWR. 2009;58:769–773. [PubMed] [Google Scholar]
- Comuzzie AG, Cole SA, Martin L, Dee Carey K, Mahaney MC, Blangero J, VandeBerg JL. The baboon as a nonhuman primate model for the study of the genetics of obesity. Obesity. 2003;11:75–80. doi: 10.1038/oby.2003.12. [DOI] [PubMed] [Google Scholar]
- de Castro Leão A, Duarte Dória Neto A, de Sousa MB. New developmental stages for common marmosets (Callithrix jacchus) using mass and age variables obtained by K-means algorithm and self-organizing maps (SOM) Comput Biol Med. 2009;39:853–859. doi: 10.1016/j.compbiomed.2009.05.009. [DOI] [PubMed] [Google Scholar]
- de Onis M, Blössner, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. American Journal of Clinical Nutrition. 2010;92:1257–1264. doi: 10.3945/ajcn.2010.29786. [DOI] [PubMed] [Google Scholar]
- Fraser LK, Edwards KL. The association between the geography of fast food outlets and childhood obesity rates in Leeds, UK. Health Place. 2010;16:1124–1128. doi: 10.1016/j.healthplace.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Gardner DSL, Hosking J, Metcalf BS, Jefrey AN, Voss LD, Wilkins TJ. Contribution of early weight gain to childhood overweight and metabolic health: A longitudinal study (Early Bird 36) Pediatrics. 2009;123:e67–e73. doi: 10.1542/peds.2008-1292. [DOI] [PubMed] [Google Scholar]
- Hahn P, Novak M. Development of brown and white adipose tissue. Journal of Lipid Research. 1975;16:79–91. [PubMed] [Google Scholar]
- Hansen B, Bodkin N. Primary prevention of diabetes mellitus by prevention of obesity in monkeys. Diabetes. 1993;42:1809–1814. doi: 10.2337/diab.42.12.1809. [DOI] [PubMed] [Google Scholar]
- Kavanagh K, Fairbanks LA, Bailey JN, Jorgensen MJ, Wilson M, Zhang L, Rudel LL, Wagner JD. Characterization and heritability of obesity and associated risk factors in vervet monkeys. Obesity. 2007;15:1666–1674. doi: 10.1038/oby.2007.199. [DOI] [PubMed] [Google Scholar]
- Kemnitz J. Obesity in macaques: spontaneous and induced. Advances in Veterinary Science and Comparative Medicine. 1984;28:81–114. doi: 10.1016/b978-0-12-039228-5.50009-7. [DOI] [PubMed] [Google Scholar]
- Kuzawa CW. Adipose tissue in human infancy and childhood: an evolutionary perspective. Yearbook of Physical Anthropology. 1998;27:177–209. doi: 10.1002/(sici)1096-8644(1998)107:27+<177::aid-ajpa7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Lewis DS, Bertrand HA, Masoro EJ, McGill HC, Jr, Carey KD, McMahan CA. Preweaning nutrition and fat development in baboons. Journal of Nutrition. 1983;113:2253–2259. doi: 10.1093/jn/113.11.2253. [DOI] [PubMed] [Google Scholar]
- Nixon JP, Zhang M, Wang C, et al. Evaluation of a quantitative magnetic resonance imaging system for whole body composition analysis in rodents. Obesity. 2010;18:1652–1659. doi: 10.1038/oby.2009.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power RA, Power ML, Layne DG, Jaquish CE, Oftedal OT, Tardif SD. Relations among measures of body composition, age, and sex in the common marmoset monkey (Callithrix jacchus) Comparative Medicine Med. 2001;51:218–223. [PubMed] [Google Scholar]
- Rothwell NJ, Stock MJ. Thermogenic capacity and brown adipose tissue activity in the common marmoset. Comparative Biochemistry and Physiology. 1985;85:683–686. doi: 10.1016/0300-9629(85)91047-3. [DOI] [PubMed] [Google Scholar]
- Tardif S, Bales K. Relations among birth condition, maternal condition and postnatal growth in captive common marmosets (Callithrix jacchus) American Journal of Primatology. 2004;62:83–94. doi: 10.1002/ajp.20009. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Power ML, Ross CN, Rutherford JN, Layne-Colon DG, Paulik MA. Characterization of obese phenotypes in a small nonhuman primate, the common marmoset (Callithrix jacchus) Obesity. 2009;17:1499–1505. doi: 10.1038/oby.2009.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif SD, Smucny DA, Abbott DH, Mansfield K, Schults-Darken N, Yamamoto ME. Reproduction in captive common marmosets (Callithrix jacchus) Comparative Medicine. 2003;53:365–368. [PubMed] [Google Scholar]
- Tinsley FC, Taicher GZ, Heiman ML. Evaluation of a quantitative magnetic resonance method for mouse whole body composition analysis. Obesity Research. 2004;12:150–160. doi: 10.1038/oby.2004.20. [DOI] [PubMed] [Google Scholar]
- Wachtman LM, Kramer JA, Miller AD, Hachey AM, Curran EH, Mansfield KG. Differential contribution of dietary fat and monosaccharide to metabolic syndrome in the common marmoset (Callithrix jacchus) Obesity. 2011;19:1145–1156. doi: 10.1038/oby.2010.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JE, Kavanagh K, Ward GM, Auerbach BJ, Harwood HJ, Jr, Kaplan JR. Old world primate models of type 2 diabetes mellitus. Institute of Laboratory Animal Resources Journal. 2006;47:259–271. doi: 10.1093/ilar.47.3.259. [DOI] [PubMed] [Google Scholar]
- West D, York B. Dietary fat, genetic predisposition and obesity: lessons from animal models. American Journal of Clinical Nutrition. 1998;67:505S–512S. doi: 10.1093/ajcn/67.3.505S. [DOI] [PubMed] [Google Scholar]