Abstract
Background
Families of children with attention deficit hyperactivity disorder (ADHD) report more negative family relationships than families of children without ADHD. Questions remain as to the role of genetic factors underlying associations between family relationships and children’s ADHD symptoms, and the role of children’s ADHD symptoms as an evocative influence on the quality of relationships experienced within such families. Utilizing the attributes of two genetically sensitive research designs, the present study examined associations between biologically related and non-biologically related maternal ADHD symptoms, parenting practices, child impulsivity/activation, and child ADHD symptoms. The combined attributes of the study designs permit assessment of associations while controlling for passive genotype-environment correlation and directly examining evocative genotype-environment correlation (rGE); two relatively under examined confounds of past research in this area.
Methods
A cross-sectional adoption-at-conception design (Cardiff IVF Study; C-IVF) and a longitudinal adoption-at-birth design (Early Growth and Development Study; EGDS) were used. The C-IVF sample included 160 mothers and children (age 5–8 years). The EGDS sample included 320 linked sets of adopted children (age 6 years), adoptive-, and biologically-related mothers. Questionnaires were used to assess maternal ADHD symptoms, parenting practices, child impulsivity/activation, and child ADHD symptoms. A cross-rater approach was used across measures of maternal behavior (mother reports) and child ADHD symptoms (father reports).
Results
Significant associations were revealed between rearing mother ADHD symptoms, hostile parenting behavior, and child ADHD symptoms in both samples. Because both samples consisted of genetically-unrelated mothers and children, passive rGE was removed as a possible explanatory factor underlying these associations. Further, path analysis revealed evidence for evocative rGE processes in the longitudinal adoption-at-birth study (EGDS) from biologically-related maternal ADHD symptoms to biologically-unrelated maternal hostile parenting through early disrupted child behavior (impulsivity/activation), with maternal hostile parenting and disrupted child behavior associated with later child ADHD symptoms, controlling for concurrent adoptive mother ADHD symptoms.
Conclusions
Results highlight the importance of genetically-influenced child ADHD-related temperamental attributes on genetically-unrelated maternal hostility that in turn links to later child ADHD symptoms. Implications for intervention programs focusing on early family processes and the precursors of child ADHD symptoms are discussed.
Keywords: ADHD, parenting, gene-environment correlation, adoption
Biological and Rearing Mother Influences on Child ADHD Symptoms: Revisiting the Developmental Interface between Nature and Nurture
Attention Deficit Hyperactivity Disorder (ADHD), whether defined as a diagnostic category or dimensionally, is known to be highly heritable (Thapar, Cooper, Eyre, & Langley, 2012). However, like all psychopathology, non-inherited factors also contribute (Lifford, Harold, & Thapar, 2009). Although many pre-, peri-, and postnatal environmental factors have been associated with ADHD symptoms, it has been challenging to demonstrate which environmental factors have causal risk effects (Thapar et al., 2012). Family relationships, particularly negative parenting practices, are known to contribute to different forms of psychopathology (Harold, Elam, Lewis, Rice, & Thapar, 2012), with families of children with ADHD reporting higher rates of conflict within the family and more negative parent-child relationships (Barkley, 1998). However, it remains unclear whether specific family conditions (e.g., hostile parenting) have risk or worsening effects on ADHD as two primary alternative explanations may account for this association. First, the association between family relationship factors and child ADHD symptoms may be explained by genes shared between parents and their biologically related children. Second, this relationship may be explained by child ADHD symptoms affecting family relationship interaction patterns, rather than the other way around. Very few studies have utilized research designs that allow genetic and environmental factors to be disentangled effectively and yield the possibility of examining child-on-parent effects (reverse causation) in exploring the etiological synergies between childhood ADHD symptoms and negative family relationships. The present study uses data from two novel genetically sensitive research designs to advance understanding of the interplay between mother-child family interaction patterns and child ADHD symptoms, while addressing each of these caveats of past research.
Family Influences on Children’s ADHD Symptoms
The role of genetic and family relationship influences on the course of child ADHD symptoms is plausible given evidence from multiple research designs, including classic twin designs (Burt, 2009; 2010; Greven, Rijsdijk & Plomin, 2011), genomic studies (Williams et al., 2011), community longitudinal studies (Lifford et al., 2009), and experimental treatment studies (Wells et al., 2000). Clinical and population studies, including longitudinal and treatment studies, have shown especially robust associations between negative parent-child relationships and child ADHD symptoms (see Deault, 2010). However, treatment and longitudinal studies have also provided evidence that negative parent-child relationships may arise as a result of child ADHD symptoms (Lifford et al., 2009; Schachar et al., 1987). Very few studies in this area have used designs that control for the effects of inherited confounders and consider child-on-parent effects. One notable exception to this general dearth of knowledge derives from a series of studies utilizing a quasi-experimental design with children from a Romanian orphanage in which extreme, albeit rare, forms of adversity increased the risks of ADHD symptoms and quasi-autistic symptoms (Stevens, Sonuga-Barke, Asherson, Kreppner, & Rutter, 2006).
The vast majority of research on family relationship influences and childhood outcomes has been conducted with biologically related parents (mothers) and children. However, such studies make it difficult to ascertain whether associations between family-level variables (e.g., parenting) and child outcomes (e.g., ADHD) represent environmental effects or shared genetic influences (Plomin, DeFries, & Loehlin, 1977). In biological families, associations between characteristics of the parent and characteristics of the child may result from shared genetic characteristics that simultaneously influence both the trait in the parent and child. That is, genes may not only affect the specific index of psychopathology considered (e.g., ADHD), but may also affect the rearing environment that children experience (e.g., parenting practices). This overlap of influence has been defined as genotype-environment correlation (rGE).
Two primary configurations of rGE—evocative rGE and passive rGE—have been highlighted in past research. Evocative rGE suggests that genetically influenced child characteristics evoke patterned responses such as hostility or negativity from a parent (see Ge et al., 1996). Passive rGE suggests that associations between parent and child characteristics (hostile parenting and child ADHD symptoms) result from common underlying genetic factors that simultaneously influence behavioral traits in both parent and child (Jaffe & Price, 2007).
Traditional Approaches to Examining Genotype-Environment Correlation
Passive and evocative rGE have been highlighted in past research using the twin design (Horwitz & Neiderhiser, 2011), and a variation thereof known as the children-of-twins (CoT) design (D’Onofrio, 2005). Some studies suggest that passive rGE is not an evident component underlying associations between features of parenting and adolescent antisocial behavior (Caspi et al., 2004). However, evidence of passive rGE cannot be ruled out in most genetically informed studies, specifically in relation to links between parenting behavior and child adjustment. Examination of evocative rGE has been facilitated by longitudinal designs where genetically-influenced twin behaviors are found to predict later parenting. Using this design, evocative effects have been found between toddlers’ difficult temperament and behavior on mothers’ hostile parenting (Forget-Dubois et al., 2007), with twin studies of older children and adolescents also finding evidence of passive and evocative rGE effects underlying associations between family relationship patterns and child outcomes (Neiderhiser et al., 2007; Neiderhiser et al., 2004).
The CoT and the extended CoT designs also provide evidence for passive and evocative rGE, with one study identifying an environmental component to harsh punishment as a causal link to child externalizing behavior (Lynch et al., 2006). Further, in adolescent samples, evocative rGE was present between adolescent internalizing problems and maternal emotional over-involvement (Narusyte et al., 2008), and between adolescent externalizing behavior and maternal criticism (Narusyte et al., 2011). Thus, twin studies and their derivatives have provided important insights into the relative role of passive and evocative rGE across a range of parenting and child behaviors. However, twin studies fundamentally rely on samples of genetically-related parents and children. This allows derivation of estimates of genetic and environmental contributions underlying this association, but not unequivocal separation of genetic and environmental factors underlying associations between family level variables and child outcomes (or vice versa).
Utilizing the Advantages of Natural Experimental Research Designs
Utilizing research designs that permit separation of passive rGE from family relationship and child outcome associations and that permit examination of evocative rGE has significant implications for understanding associations between patterns of family interaction and child development. We offer two complementary study designs that accommodate this unique opportunity. The first study is an adoption-at-birth design with a sample of families with domestic infant adoptions; the second study is an adoption-at-conception design using a sample of families with children conceived through in vitro fertilization (IVF). Both studies allow examination of the interplay between family relationship variables and child outcomes where the potential confounding influence of passive rGE is controlled, with the former study also permitting examination of evocative rGE processes.
Children conceived via assisted reproductive technologies may be genetically related to both parents (homologous IVF), the mother only (sperm donation), the father only (egg donation), or to neither parent (embryo donation). A further category exists where both parents are genetically related to the child but the intrauterine environment is provided by a genetically unrelated surrogate (gestational surrogacy). The current study focuses specifically on mothers who are genetically unrelated to their offspring (egg and embryo donation), and therefore constitutes an adoption-at-conception design because rearing mothers undergo gamete “adoption” prenatally.
By using a sample of children adopted-at-birth and children conceived through IVF and their genetically-unrelated rearing mothers, the influence of genetic confounds on family-level variables that arise postnatally (passive rGE) is eliminated in examining the pattern of association between mother-child interaction patterns and non-biological offspring behavioral outcomes. In addition, a noteworthy opportunity is provided through the adoption-at-birth design to also examine the role of evocative rGE processes that may underlie links between child behavior and the maternal provided rearing environment experienced by children (child-on-rearing mother effects).
The Present Study
The present study examined associations between maternal hostility and child ADHD symptoms using a cross-sectional, rearing mother-focused adoption-at-conception design (Cardiff IVF Study; C-IVF), and a longitudinal adoption-at-birth design (the Early Growth and Development Study; EGDS). Initial analyses examined associations between rearing mother ADHD symptoms, maternal hostility (hostile parenting), and child ADHD symptoms using both study designs, whereby passive rGE was controlled across all analyses. Additional analyses using the longitudinal EGDS adoption-at-birth design examined the role of biological mother ADHD symptoms on biologically-related child behavior problems (impulsivity/activation; consonant with early ADHD type behaviors) at age 4.5 years, and the role of child impulsivity/activation on child ADHD symptoms at age 6 years, controlling for concurrent rearing (adoptive) mother ADHD symptoms.
To our knowledge, this is the first study to jointly incorporate the unique attributes of these two study designs to examine the interplay between specific family interaction patterns and child psychopathology, allowing the confound of passive rGE to be controlled, while also permitting simultaneous examination of child-on-parent effects stemming from child genetically-influenced risk behaviors on maternal hostility toward the child (evocative rGE).
Method
Participants and Procedures
Sample 1: Early Growth and Development Study (EGDS)
Participants were a subsample (n = 320) of 361 linked sets of adopted children, adoptive mothers and fathers, and biological mothers from Cohort I of the EGDS. The current subsample included all mother-father families who had data on at least one of the measures used in the present analyses. Participants were recruited between 2003 and 2006 through 33 adoption agencies located in 10 states spanning the Northwest, Mid-Atlantic and Southwest regions of the United States. Participating agencies reflected the full range of adoption agencies in the United States: public, private, religious, secular, those favoring open adoptions and those favoring closed adoptions. Eligibility criteria included: (1) domestic adoption placement; (2) placement occurring within 3 months postpartum; (3) non-relative placement; (4) no known major medical conditions such as extreme prematurity or extensive medical surgeries; and (5) biological and adoptive parents able to understand English at the eighth-grade level. Data were collected by home visit assessments and online questionnaires.
For the present study, data from the second through fifth assessment were used: biological mother ADHD symptoms were measured at child age 18 months and 4.5 years; child impulsivity/activation and maternal hostility were measured at 4.5 years; and rearing mother and child ADHD symptoms were measured at 6 years. Child ADHD symptoms were assessed when children were 6 years old (M = 5.99, SD = 0.17) so as to be comparable in age and stage of educational experience to the C-IVF sample. Fifty-seven percent of the children were male. The median child age at adoption placement was 2 days. Adoptive parents were typically college educated, middle to upper class families (single-parents and same sex couples were excluded from the present study). Adoptive mother and adoptive father mean ages were 38 (SD = 5.5) and 38 (SD = 5.8), respectively, at the birth of the child. The ethnicity of adoptive mothers and fathers was: 91% and 90% Caucasian, 4% and 5% African American, 3% and 2% Hispanic or Latino, 1% and 1% multiracial, 1% and 1% Asian, <1% and 0% American Indian or Alaskan Native, and 1% and 1% unknown or unreported, respectively. For full demographic information refer to Leve et al. (2013).
Sample 2: Cardiff IVF Study
Participants were a subset of families who had conceived a child through one of the assisted reproductive methods described previously and were subsequently recruited through a number of different fertility clinics that agreed to participate (18 clinics in the United Kingdom and one in the United States). Families with children born between 1994 and 2002 (child aged 4 to 10 years) following successful artificial reproductive treatment were considered eligible for the study and were contacted via mail from the clinic on behalf of the research team. For the purposes of comparison between conception groups (not a focus of the present study), it was required that gamete donors and surrogates were unrelated to either rearing parent. All data were collected by mailed questionnaires sent to families by each participating clinic.
For the present study, the sample included genetically-unrelated mothers and their 5–8 year old children (n = 160). The sample included an approximately even proportion of boys (50.01%) and girls (49.99%) (M = 6.47 years, SD = .83), so as to be comparable in age and stage of educational experience to the EGDS sample. Mother and father mean ages were 35 (SD = 4.73) and 38 (SD = 6.58), respectively, at the birth of the child. The number of families in each conception group for genetically unrelated mothers was 135 IVF with egg donation and 25 IVF with embryo donation. The ethnicity of mothers and fathers was: 91% and 89% Welsh, English, Scottish, or Irish; 2% and 1% other European; <1% and <1% African or Afro-Caribbean; 1% and <1% Bangladeshi, Indian, or Pakistani; <1% and <1% South East Asian, other ethnicity; 1% and 1% unknown; and 5% and 7% unreported, respectively.
Measures
Where possible, comparable measures were utilized across the two research designs employed in the present study.
Biological and Rearing Mother ADHD Symptoms
Biologically related and unrelated (rearing/adoptive) mothers in both studies completed the Barkley’s Adult ADHD scale (Barkley & Murphy, 1998) regarding their impulsive, hyperactive, and inattentive behavior during the past 12 months. Mothers reported on a 4-point scale ranging from ‘never’ to ‘very often’, with higher scores indicating greater hyperactive or inattentive behavior. The 18-item scale included items such as “fidget with hands or feet or squirm in seat”. Internal consistency estimates were good in both studies (C-IVF α = .84; EGDS biological mother α = .90, EGDS adoptive mother α = .85).
Within the EGDS study, biological mothers also completed the Adult Temperament Questionnaire (Rothbart, Ahadi, & Evans, 2000) regarding their negative affect, extraversion, effortful control, and orienting sensitivity. Mothers reported on a 7-point scale ranging from ‘extremely untrue’ to ‘extremely true’, with higher scores indicating greater levels of trait behavior. The 5-item attention control subscale was used in the present study, and included items such as, “It’s often hard for me to alternate between two different tasks”. The internal consistency estimate was good (α = .73). The attention control subscale of the Adult Temperament Questionnaire (assessed at child age 18 months) and the Barkley’s Adult ADHD scale (assessed at child age 4.5 years) were found to be moderately correlated (r =.48). The scales were thus standardized and summed into a single measure of biological mother ADHD symptoms, thereby representing a measure of symptom continuity for biological mothers utilizing the very unique longitudinal attributes of this study design. Internal consistency for the combined measure was good (α = .88).
Maternal Hostility to Child (Rearing Mother)
Genetically-unrelated mothers in both studies completed the Iowa Family Interaction Rating Scales (Melby et al., 1993) regarding their parenting behaviors. Mothers reported on their own hostile behaviors toward their child on a 7-point scale ranging from ‘never’ to ‘always’ with high scores indicating greater hostility. In EGDS, a 5-item hostility subscale from this measure was administered; in the C-IVF study, a 4-item hostility subscale was administered. Items overlapped between samples and included items such as “how often did you get angry at him/her”, and “how often did you argue with him/her when you disagreed about something.” Internal consistency estimates were good for mothers and fathers in both studies (C-IVF α = .79; EGDS α = .91).
Child Impulsivity and Behavioral Activation
Rearing mothers in the EGDS study completed the Children’s Behavior Questionnaire (CBQ; Rothbart, Ahadi, Hershey, & Fisher, 2001). Mothers reported on a range of child reactions in various scenarios over the past six months on a 7-point scale ranging from ‘extremely untrue’ to ‘extremely true.’ The 13-item impulsivity scale was used in the present study and included items such as “usually rushes into an activity without thinking about it.” Internal consistency estimates were good (α =.78).
Mothers also completed the Behavioral Inhibition Scale/Behavioral Activation Scale about the child (BIS/BAS; Blair, Peters, & Granger, 2004). Mothers reported on the three BAS subscales of child drive, reward responsiveness, and fun seeking on a 7-point scale ranging from ‘extremely untrue’ to ‘extremely true.’ The drive subscale included the item “when my child wants something, he/she goes all out to get it”, the reward responsiveness scale included “when good things happen to my child, it affects him/her strongly”, and the fun seeking scale included “my child craves excitement and new sensations.” Internal consistency estimates for the three scales were good (drive α = .81, reward responsiveness α = .71, fun seeking α = .70). The CBQ impulsivity subscale was found to significantly correlate with the three BAS subscales (r = .29 to .66). The scales were standardized and summed into a single measure representing child impulsivity and activation which had good internal consistency (α = .87).
Child ADHD symptoms
Fathers in both studies completed the Conner’s Abbreviated Parent Questionnaire (Conners, 1997) regarding the child’s hyperactive and inattentive behavior. Father’s reported on a 4-point scale ranging from ‘not at all’ to ‘very much’ with higher scores indicating greater hyperactivity and inattentiveness. The 10-item scale included items such as “often fidgets or squirms in seat.” Internal consistency estimates were good in both studies (C-IVF α = .86; EGDS α = .89). Father reports were employed to overcome reliance on maternal reports across all other primary theoretical measures.
Statistical Analyses
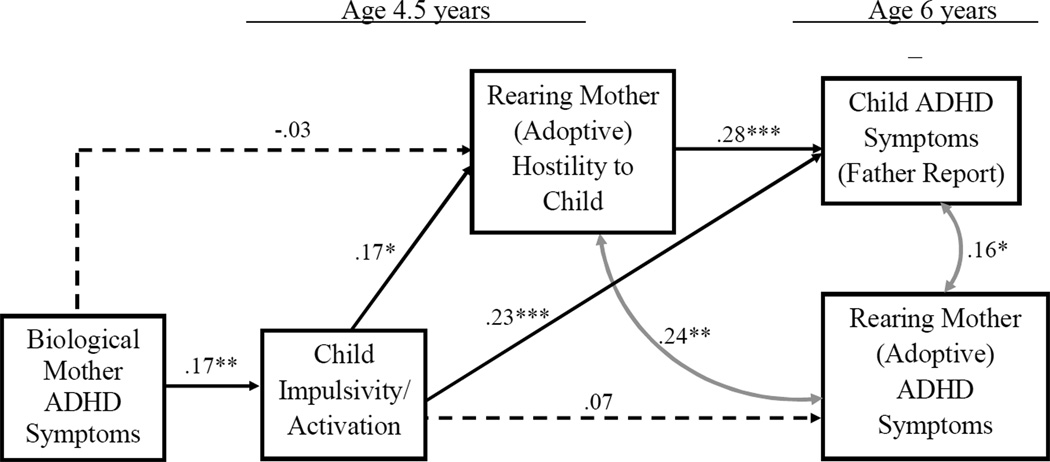
Analyses were conducted in two primary steps. First, correlations between primary theoretical variables were initially examined across the EGDS and C-IVF samples, whereby passive rGE was controlled. Second, path analysis using structural equation modeling (SEM; Muthén, & Muthén, 2007) was used to extend these analyses whereby evocative rGE processes were additionally examined using the EGDS longitudinal adoption-at-birth design. All relevant statistical assumptions inherent to the application of SEM (e.g., multivariate normalcy) were initially examined and affirmed. A number of variables (i.e., child ADHD symptoms in C-IVF; biological mother ADHD symptoms and adoptive mother hostility in EGDS) were transformed to correct for moderate but significant skew. Specific to the theoretical model examined for the EGDS sample (see Figure 1), relevant adoption covariates were included to control for their possible effect on associations across child and parent measures (e.g., adoption openness (contact with biological parents) and pregnancy complications).
Figure 1.
Model results for the EGDS sample examining the relation between biological mother ADHD, child impulsivity/activation, maternal hostility from the rearing (adoptive), child ADHD symptoms, and rearing (adoptive) mother ADHD symptoms. χ2 = 9.08, df = 10, AGFI=0.99, RMSEA = 0.00. Significant indirect pathways: biological mother ADHD symptoms via child impulsivity/activation to maternal hostility (β = .03, p < .05), and child ADHD symptoms (β = .04, p < .05); child impulsivity/activation via maternal hostility to child ADHD symptoms (β = .05, p < .05). *p < .05; **p < .01, ***p < 001.
Results
Rearing Mother ADHD Symptoms, Maternal Hostility, and Child ADHD Symptoms: Controlling for Passive rGE
Intercorrelations, means, and standard deviations are presented in Table 1 for both samples. Significant associations were apparent between rearing mothers’ reports of their own ADHD symptoms and fathers’ report of child ADHD symptoms (C-IVF, r = .37, p < .001; EGDS, r = .24, p < .001), as well as between rearing mothers’ report of maternal hostility and fathers’ report of child ADHD symptoms (C-IVF, r = .32, p < .001; EGDS, r = .33, p < .001). Rearing mothers’ report of ADHD symptoms was also correlated with maternal hostility (C-IVF, r = .29, p < .001; EGDS, r = .27, p < .01). Further associations were present within the EGDS sample; biological mother ADHD symptoms were significantly related to child impulsivity/activation (r = .17, p < .01), which in turn was significantly related to both maternal hostility in adoptive mothers (r = .17, p < .05), and the child’s own ADHD symptoms (r = .28, p < .001). Substantively, these results highlight the pattern of association between maternal hostility and child ADHD symptoms across both samples when passive rGE is controlled as a factor underlying observed associations.
Table 1.
Intercorrelations, Means, and Standard Deviations (SD) Among Constructs for EGDS Sample (Lower Diagonal, n = 320) and the C-IVF Sample (Upper Diagonal, n = 160)
| Variable | (1) | (2) | (3) | (4) | (5) | Mean | SD |
|---|---|---|---|---|---|---|---|
| (1) Biological Mother ADHD Symptoms | ---- | 7.29 | 2.18 | ||||
| (2) Child Impulsivity/Activation | .17** | ---- | 73.32 | 8.92 | |||
| (3) Maternal Hostility | −.02 | .17* | ---- | .29*** | .32*** | 11.81 | 4.96 |
| (4) Rearing Mother ADHD Symptoms | −.05 | .07 | .27** | ---- | .37*** | 8.16 | 5.53 |
| (5) Child ADHD Symptoms (father-rated) | .01 | .28*** | .33*** | .24*** | ---- | 8.01 | 5.91 |
| Mean | 11.95 | 8.94 | 8.09 | ||||
| SD | 3.54 | 6.14 | 5.89 | ||||
Note. EGDS sample, rearing/adoptive mother; IVF sample, genetically unrelated rearing mother.
p <.05,
p < .01,
p < .001.
Biological Mother ADHD Symptoms, Child Impulsivity/Activation, and Rearing (Adoptive) Mother ADHD Symptoms: Examining Evocative rGE
In order to illuminate the pattern of association linking maternal hostility and child ADHD symptoms noted above, analyses were taken one step further using the EGDS longitudinal adoption-at-birth design. Path analysis was used to examine the role of biological mother ADHD as an influence on adopted children’s early behavior problems (impulsivity/activation) and associations with maternal hostility in adoptive mothers, thereby allowing examination of evocative rGE processes (see Figure 1). Maternal hostility was in turn hypothesized to predict children’s later ADHD symptoms, controlling for adoptive mothers’ own ADHD symptoms. Within the present study sample, 320 cases were available for analysis, of which 212 had complete data. The Little’s test of missing data indicated that data were missing completely at random (MCAR) , X2 (239) = 253.64, p = .25. Multiple imputation with data augmentation was used to generate values for missing data across individual variables using NORM 2.03 (Schafer, 1997). Subsequently generated datasets were then tested using Mplus version 6 (see Muthen & Muthen, 2007).
As presented in Figure 1, biological mother ADHD symptoms significantly predicted child impulsivity/activation at age 4.5 years (β=.17, p<.01), which in turn predicted maternal hostility from adoptive mother (β=.17, p<.05), and child ADHD symptoms (β=.23, p<.001; age 6 years). The pattern of association reported in Table 1 was also replicated when specific covariates were controlled across the EGDS analyses such that maternal hostility from adoptive mother at child age 4.5 years was associated with child ADHD symptoms at age 6 years (β=.28, p<.01), controlling for concurrent adoptive mother ADHD symptoms (r=.16, p<.05, with relevant variable covariates also controlled across these associations). Importantly, in relation to tests of hypothesized evocative rGE processes, significant indirect pathways were observed from biological mother ADHD symptoms via child impulsivity/activation to maternal hostility from adoptive mothers (β=.03, p<.05), and child ADHD symptoms (β=.04, p<.05). A significant indirect pathway was also observed from child impulsivity/activation via maternal hostility to child ADHD symptoms (β=.05, p<.05). Fit indices indicated an excellent fit for the specified model (χ2 =9.08, df = 10, GFI=0.99, RMSEA = 0.00).
Discussion
The overall aim of the present study was to examine the association between a specific feature of children’s rearing environment (maternal hostility) and child ADHD symptoms. The use of two adoption-based study designs – adoption-at-conception and adoption-at-birth – in which the rearing mother was genetically unrelated to the child allowed examination of this association, controlling for the possible confounding presence of passive rGE across all analyses. Further, the longitudinal adoption-at-birth design allowed examination of evocative rGE processes underlying associations between maternal hostility in rearing (adoptive) mothers and child ADHD symptoms. The results uniquely highlight two findings of clinical and developmental significance: (1) the salience of hostile maternal parenting behavior on the course of children’s ADHD symptoms; and (2) the role of early disrupted child behavior (impulsivity/activation) as an influence through which genetically influenced child attributes influence biologically-unrelated mothers’ hostility, which in turn is predictive of children’s later ADHD symptoms.
In the present study, significant associations between maternal hostility in rearing mothers and child ADHD, and between rearing mothers’ ADHD symptoms and child ADHD symptoms were identified in both samples. Because all of the children in this study were genetically unrelated to their rearing mother, these associations cannot be attributed to shared genetic influences (i.e., cannot result from passive rGE). This pattern of results was replicated across both studies despite the different nature of the samples and the different countries that the participants resided in, suggesting that the pattern of association linking mother-child interaction and child ADHD symptoms is quite robust.
A second key finding of the present study is the presence of evocative rGE effects. Specifically, biological mother ADHD symptoms that index genetic risk had an indirect effect on maternal hostility in adoptive mothers via child impulsivity/activation. In other words, genetic influences on early disrupted child behavior marked by child impulsivity/activation evoked hostility from the rearing mother toward her child. Child impulsivity/activation also had a direct effect on later ADHD symptoms, with rearing mother hostility predicting higher child ADHD symptoms. Substantively, the path from biological mother ADHD symptoms to child impulsivity/activation suggests that such child behaviors might be among the earliest manifestations of genetic influences on child ADHD, and provides a clue to the mechanisms underlying biological-based intergenerational transmission of ADHD within families. To our knowledge, this is the first study to identify specific evocative effects on child ADHD symptoms, and specific child behaviors whereby such effects are transmitted.
Implications for Clinical Practice
At least two primary avenues for management of child ADHD symptoms are suggested by the present findings. First, the association between rearing mothers’ parenting hostility and child ADHD symptoms suggests that early interventions targeting mother directed hostility toward children, especially those with temperamental attributes of impulsivity/activation that appear to evoke such maternal responses, may strengthen the efficacy of intervention programs aimed at reducing child ADHD symptom levels in the general population. Numerous existing evidence-based programs targeting child conduct problems include such a focus (e.g., Dishion et al., 2008; Webster-Stratton, Reid, & Hammond, 2001), and National Institutes for Health and Care Excellence (NICE, UK) and European guidance recommends such programs for the initial treatment of ADHD. These findings suggest that interventions could start much earlier in life. However, the evocative pathway identified from child impulsivity/behavioral activation to adoptive mother hostility suggests that parents may also be trained to better identify specific behavioral attributes in their child early that evoke hostile maternal responses that, in turn, promote continuity of ADHD problems later. These approaches might ultimately help reduce the expression of ADHD symptoms in children in the general population at a period in their development (school entry) where such symptoms can have long-term effects on multiple developmental domains, including family and peer relationships, academic attainment, as well as future emotional and behavioral development (Barkley, 2002; Hoza, 2007; Thapar et al., 2007).
Limitations and Future Directions
Several limitations of the present study should be noted. First, the C-IVF study was cross-sectional in nature, and therefore the directionality of association between mother and child variables cannot be fully disentangled. However, developmental theory along with the longitudinal nature of the EGDS study provides support for the direction of effects as hypothesized. Nonetheless, neither study measured maternal hostility at a developmental period after the child ADHD assessment; additional research is needed to examine potential bi-directional associations later in development. Second, results need to be replicated with more diverse and high-risk families to test the generalizability of the present findings to other populations. Third, all measures were derived from parent-report. However, we included mother report of her ADHD symptoms and her parenting hostility, and father report of child ADHD, thus reducing the likelihood that associations were solely the result of rater bias. In addition, in the EGDS, biological mothers provided ratings of their own ADHD symptoms. Fourth, mothers’ ADHD symptoms were measured (rather than fathers’ symptoms), even though ADHD diagnoses are more prevalent in males as compared to females. This may have reduced (or increased) the magnitude of associations between mother and child ADHD symptoms. Additionally, we did not examine the role of father-child relations in these analyses. Recent research suggests that patterns of interaction and child outcomes may vary across mother-child and father-child relationships (Lamb, 2004; Harold et al., 2013).Finally, this study measured ADHD symptoms and for parents focused on current symptoms, rather than diagnoses. It is unknown whether the familial associations found in the present study will generalize to clinical populations.
These limitations notwithstanding, the present study suggests a two-way process through which family relationship patterns affect child ADHD symptoms. First, hostility directed by mothers to children may promote a transition from early impulsivity related temperamental attributes in children to an increase in child ADHD symptoms. Second, as uniquely evidenced by the present study, genetically informed early behavioral attributes in children may evoke more negative parenting from mothers, which in turn affects children’s ADHD symptoms. Interventions aimed at ameliorating the developmental precursors to ADHD in the general population may therefore target not only hostile parenting behaviors consonant with elevated ADHD symptoms in children, but specific parenting responses more likely to be evoked by early behavioral attributes in children at genetic risk for ADHD.
Key Points.
Maternal parenting practices influence child ADHD symptoms in the general population, and we know from the present study that these associations cannot be solely explained by common genetic factors.
Genetically influenced ADHD attributes in children evoke more hostile parenting practices from mothers.
Interventions that reduce the activation of more negative parenting practices evoked by children at genetic risk for ADHD may result in a reduction of children’s ADHD symptom expression/development over time
Acknowledgements
Early Growth and Development Study: This project was supported by R01 HD042608, National Institute of Child Health & Human Development (NICHD), NIDA, and OBSSR, National Institutes of Health (NIH); U.S. PHS (Principal Investigators: David Reiss and Leslie D. Leve). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver NICHD or the NIH. Additional support was provided by P30 DA023920, R01 DA020585, and R01 MH092118. We thank the birth and adoptive parents who participated in this study and the adoption agencies who helped with the recruitment of study participants. Special gratitude is given to Rand Conger, John Reid, Xiaojia Ge, Jody Ganiban, and Laura Scaramella, who contributed to the larger study aims. Cardiff IVF study: We thank the families who participated in this study and our fertility center and clinic collaborators. We thank Dale Hay, Jacky Boivin, Marianne van den Bree of Cardiff University, Frances Rice of University College London, the late Xiaojia Ge of the University of Minnesota for assistance in the study design, and Allyson Lewis and Val Russell, also of Cardiff University, for antenatal data collection and administrative support. This study was supported by a Wellcome Trust Showcase Award and a Wellcome Trust Project grant along with a Project Grant award from the Nuffield Foundation.The authors have declared that they have no competing or potential conflicts of interest.
This article was invited by the journal, for which the principal author has been offered a small honorarium payment towards personal expenses.
Footnotes
Conflict of interest: No conflict of interest. Abstract
References
- Allison PD. Missing Data. Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- Barkley RA. Major life activity and health outcomes associated with Attention-Deficit/Hyperactivity Disorder. Journal of Clinical Psychiatry. 2002;63:10–15. [PubMed] [Google Scholar]
- Barkley RA, Murphy KR. Attention-Deficit Hyperactivity Disorder: A handbook for diagnosis and treatment. 3rd ed. New York, NY: Guilford Press; 2006. [Google Scholar]
- Blair C, Peters R, Granger D. Physiological and neuropsychological correlates of approach/withdrawal tendencies in preschool: Further examination of the Behavioral Inhibition System/Behavioral Activation System Scales for young children. Developmental Psychobiology. 2004;45:113–124. doi: 10.1002/dev.20022. [DOI] [PubMed] [Google Scholar]
- Burt SA. Are there shared environmental influences on attention-deficit/hyperactivity disorder? Reply to Wood, Buitelaar, Rijsdijk, Asherson, & Kuntsi (2010) Psychological Bulletin. 2010;136:341–343. doi: 10.1037/a0019116. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Morgan J, Rutter M, Taylor A, Arseneault L, et al. Maternal expressed emotion predicts children's antisocial behavior problems: using monozygotic-twin differences to identify environmental effects on behavioral development. Developmental Psychology. 2004;40:149–161. doi: 10.1037/0012-1649.40.2.149. [DOI] [PubMed] [Google Scholar]
- Conners CK. Conners' Rating Scales-Revised. North Tonawanda, NY: Multi-Health Systems, Inc; 1997. [Google Scholar]
- Deault LC. A systematic review of parenting in relation to the development of comorbidities and functional impairments in children with Attention-Deficit/Hyperactivity Disorder (ADHD) Child Psychiatry and Human Development. 2010;41:168–192. doi: 10.1007/s10578-009-0159-4. [DOI] [PubMed] [Google Scholar]
- Dishion TJ, Connell A, Weaver C, Shaw D, Gardner F, Melvin W. The Family Check-Up with high-risk indigent families: Preventing problem behavior by increasing parents’ positive behavior support in early childhood. Child Development. 2008;79:1395–1414. doi: 10.1111/j.1467-8624.2008.01195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio BM. The children of twins design. In: Everitt B, Howell D, editors. Encyclopedia of Behavior Statistics. New York: Wile; 2005. pp. 256–258. [Google Scholar]
- Fabiano GA, Pelham WE, Jr, Coles EK, Gnagy EM, Chronis-Tuscano A, et al. A meta-analysis of behavioral treatments for attention-deficit/hyperactivity disorder. Clinical Psychology Review. 2009;29:129–140. doi: 10.1016/j.cpr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Forget-Dubois N, Boivin M, Dionne G, Pierce T, Tremblay RE, Pérusse D. A longitudinal twin study of the genetic and environmental etiology of maternal hostile-reactive behavior during infancy and toddlerhood. Infant Behavior and Development. 2007;30:453–465. doi: 10.1016/j.infbeh.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Ge X, Conger RD, Cadoret RJ, Neiderhiser JM, Yates W, Troughton E, et al. The developmental interface between nature and nurture: A mutual influence model of child antisocial behavior and parent behaviors. Developmental Psychology. 1996;32:574–589. [Google Scholar]
- Greven C, Rijsdijk F, Plomin R. A twin study of ADHD symptoms in early adolescence: Hyperactivity-impulsivity and inattentiveness show substantial genetic overlap but also genetic susceptibility. Journal of Abnormal Child Psychology. 2011;39:265–275. doi: 10.1007/s10802-010-9451-9. [DOI] [PubMed] [Google Scholar]
- Harold GT, Elam KK, Lewis G, Rice F, Thapar A. Interparental conflict, parent psychopathology, hostile parenting and child antisocial behavior: Examining the role of maternal versus paternal influences using a novel genetically sensitive research design. Development and Psychopathology. 2012;24:1283–1295. doi: 10.1017/S0954579412000703. [DOI] [PubMed] [Google Scholar]
- Harold GT, Leve LD, Elam KE, Thapar A, Neiderhiser JM, Natsuaki MN, Shaw DS, Reiss D. The nature of nurture: Disentangling passive genotype-environment correlation from family relationship influences on children’s externalizing problems. Journal of Family Psychology. 2013;27:12–21. doi: 10.1037/a0031190. [PMC: 3576129] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold GT, Rice F, Hay DF, Boivin J, Van DBM, Thapar A. Familial transmission of depression and antisocial behavior symptoms: disentangling the contribution of inherited and environmental factors and testing the mediating role of parenting. Psychological Medicine. 2010;41:1–11. doi: 10.1017/S0033291710001753. [DOI] [PubMed] [Google Scholar]
- Hinshaw SP, Owens EB, Wells KC, Kraemer HC, Abikoff HB, Arnold E, et al. Family processes and treatment outcome in the MTA: Negative/Ineffective parenting practices in relation to multimodal treatment. Journal of Abnormal Child Psychology. 2000;28:555–568. doi: 10.1023/a:1005183115230. [DOI] [PubMed] [Google Scholar]
- Horwitz BN, Neiderhiser JM. Gene-environment interplay, family relationships, and child adjustment. Journal of Marriage and Family. 2011;73:804–816. doi: 10.1111/j.1741-3737.2011.00846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoza B. Peer functioning in children with ADHD. Journal of Pediatric Psychology. 2007;32:655–663. doi: 10.1093/jpepsy/jsm024. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Caspi A, Moffitt TE, Taylor A. Physical maltreatment victim to antisocial child: Evidence of an environmentally mediated process. Journal of Abnormal Psychology. 2004;113:44–55. doi: 10.1037/0021-843X.113.1.44. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Price TS. Gene-environment correlations: A review of the evidence and implications for prevention of mental illness. Molecular Psychiatry. 2007;12:432–442. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb ME. The role of the father in child development. 4th Edn. Hoboken, NJ: John Wiley & Sons; 2004. [Google Scholar]
- Leve LD, Neiderhiser JM, Shaw DS, Ganiban J, Natsuaki MN, Reiss D. The Early Growth and Development Study: A prospective adoption study of child behavior from birth through middle childhood. Twin Research and Human Genetics. doi: 10.1017/thg.2012.126. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifford K, Harold G, Thapar A. Parent-child hostility and child ADHD symptoms. Journal of Child Psychology and Psychiatry. 2009;50:1468–1476. doi: 10.1111/j.1469-7610.2009.02107.x. [DOI] [PubMed] [Google Scholar]
- Lynch SK, Turkheimer E, D'Onofrio BM, Mendle J, Emery RE, Slutske WS, et al. A genetically informed study of the association between harsh punishment and offspring behavioral problems. Journal of Family Psychology. 2006;20:190–198. doi: 10.1037/0893-3200.20.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melby JN, Conger RD, Book R, Rueter M, Lucy L, Repinski D, et al. The Iowa Family Interaction Rating Scales. 2nd Edn. Unpublished manuscript, Iowa State University Center for Family Research in Rural Mental Health; 1993. [Google Scholar]
- Muthén L, Muthén B. Mplus User’s Guide. 5th Edition. Los Angeles, CA: Muthén and Muthén; 2007. [Google Scholar]
- Narusyte J, Neiderhiser JM, D’Onofrio BM, Reiss D, Spotts EL, Ganiban J, et al. Testing different types of genotype-environment correlation: An extended children-of-twins model. Developmental Psychology. 2008;44:1591–1603. doi: 10.1037/a0013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narusyte J, Neiderhiser JM, D'Onofrio BM, Reiss D, Spotts EL, Ganiban J, et al. Parental criticism and externalizing behavior problems in adolescents-the role of environment and genotype-environment correlation. Journal of Abnormal Psychology. 2011;120:365–376. doi: 10.1037/a0021815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiderhiser JM, Reiss D, Lichtenstein P, Spotts EL, Ganiban J. Father-adolescent relationships and the role of genotype-environment correlation. Journal of Family Psychology. 2007;21:560–571. doi: 10.1037/0893-3200.21.4.560. [DOI] [PubMed] [Google Scholar]
- Neiderhiser JM, Reiss D, Pedersen NL, Lichtenstein P, Spotts EL, Hansson K, et al. Genetic and Environmental Influences on Mothering of Adolescents: A Comparison of Two Samples. Developmental Psychology. 2004;40:335–351. doi: 10.1037/0012-1649.40.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, Loehlin JC. Genotype-environment interaction and correlation in the analysis of human behavior. Psychological Bulletin. 1977;84:309–322. [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, Evans DE. Temperament and personality: Origins and outcomes. Journal of Personality and Social Psychology. 2000;78:122–135. doi: 10.1037//0022-3514.78.1.122. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey KL, Fisher P. Investigations of temperament at three to seven years: The Children’s Behavior Questionnaire. Child Development. 2001;72:1394–1408. doi: 10.1111/1467-8624.00355. [DOI] [PubMed] [Google Scholar]
- Rutter M, Pickles A, Murray R, Eaves L. Testing hypotheses on environmental risk mechanisms. Psychological Bulletin. 2001;127:291–324. doi: 10.1037/0033-2909.127.3.291. [DOI] [PubMed] [Google Scholar]
- Schachar R, Taylor E, Wieselberg M, Thorley G, Rutter M. Changes in family function and relationships in children who respond to methylphenidate. Journal of the American Academy of Child and Adolescent Psychiatry. 1987;26:728–732. doi: 10.1097/00004583-198709000-00019. [DOI] [PubMed] [Google Scholar]
- Schafer JL. Analysis of Incomplete Multivariate Data. London: Chapman and Hall; 1997. [Google Scholar]
- Stevens S, Sonuga-Barke EJS, Asherson P, Kreppner J, Rutter M. A consideration of the potential role of genetic factors in individual differences in response to early institutional deprivation: the case of inattention/overactivity in the English and Romanian adoptees study. The Association for Child and Adolescent Mental Health, Occasional Papers, Genetics: Impact on Current Child and Adolescent Mental Health. 2006;25:66–72. [Google Scholar]
- Thapar A, Harold GT, Rice FJ, Langley K, O’Donovan M. The contribution of gene-environment interaction to psychopathology. Development and Psychopathology. 2007;19(4):989–1004. doi: 10.1017/S0954579407000491. [DOI] [PubMed] [Google Scholar]
- Thapar A, Cooper M, Eyre O, Langley K. Practitioner review: What have we learnt about the causes of ADHD? Journal of Child Psychology and Psychiatry. 2012 doi: 10.1111/j.1469-7610.2012.02611.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson SMR, Richels C, Michalek AP, Raymer A. Psychosocial treatments for ADHD: A systematic appraisal of the evidence. Journal of Attention Disorders, Epub ahead of print. 2012 doi: 10.1177/1087054712447857. [DOI] [PubMed] [Google Scholar]
- Webster-Stratton C, Reid JM, Hammond M. Preventing conduct problems, promoting soc0069al competence: A parent and teacher training partnership in Head Start. Journal of Clinical Child Psychology. 2001;30:283–302. doi: 10.1207/S15374424JCCP3003_2. [DOI] [PubMed] [Google Scholar]
- Wells KC, Epstein JN, Hinshaw SP, Conners CK, Klaric J, Abikoff HB, et al. Parenting and family stress treatment outcomes in Attention Deficit Hyperactivity Disorder (ADHD): An empirical analysis in the MTA Study. Journal of Abnormal Child Psychology. 2000;28:543–553. doi: 10.1023/a:1005131131159. [DOI] [PubMed] [Google Scholar]
- Williams NM, Zaharieva I, Martin A, Langley K, Mantripragada K, Fossdal R, et al. Rare chromosomal deletions and duplications in attention-deficit hyperactivity disorder: a genome-wide analysis. The Lancet. 2012;376:1401–1408. doi: 10.1016/S0140-6736(10)61109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]