Abstract
Herpes simplex virus type-1 (HSV-1) causes significant health problems from periodical skin and corneal lesions to encephalitis. We report here that an aqueous extract preparation from the barks of neem plant Azardirachta indica acts as a potent entry inhibitor against HSV-1 infection into natural target cells. The extract from neem bark (NBE) significantly blocked HSV-1 entry into cells at concentrations ranging from 50 to 100 μg/ml. The blocking activity of NBE was observed when the extract was pre-incubated with the virus but not with the target cells suggesting a direct anti-HSV-1 property of the neem bark. Further, virions treated with NBE failed to bind the cells which implicate a role of NBE as an attachment step blocker. Cells treated with NBE also inhibited HSV-1 glycoprotein mediated cell to cell fusion and polykaryocytes formation suggesting an additional role of NBE at the viral fusion step. These finding open a potential new avenue for the development of NBE as a novel anti-herpetic microbicide.
Keywords: Azardirachta indica, neem, plant antiviral, herpes simplex type-1, virus-cell interaction
INTRODUCTION
Herpes simplex virus (HSV) infections are extremely widespread in the human population. The virus causes a broad range of diseases ranging from labial herpes, ocular keratitis, genital disease and encephalitis (Liesegang et al., 1989; Roizman and Sears, 1996; Whitley et al., 1998; Whitely and Kimberlin, 1997). The herpetic infection is a major cause of morbidity especially in immunocompromised patients. Following initial infection in epithelial cells, HSV establishes latency in the host sensory nerve ganglia (Knickelbein et al., 2008; Snyder et al., 2008; Spear, 1993). Over 90 % of the trigeminal ganglia examined in a post-mortem study of the American population contained HSV type-1 (HSV-1) (Hill et al., 2008). The virus emerges sporadically from latency and causes lesions on mucosal epithelium, skin, and the cornea, among other locations. Prolonged or multiple recurrent episodes of corneal infections can result in vision impairment or blindness, due to the development of herpetic stromal keratitis (HSK) (Kaye et al., 2000). HSK accounts for 20–48% of all recurrent ocular HSV infections leading to significant vision loss (Liesegang et al., 1989; Liesegang, 2001). HSV infection may also lead to other diseases including retinitis, meningitis, and encephalitis.
HSV entry into host cells initiates the primary infection. It is a multi-step process that is initiated by specific interaction of viral envelope glycoproteins and host cell surface receptors (Spear, 1993; Spear et al., 2000). Both HSV-1 and highly related HSV-2 use envelope glycoproteins B and C (gB and gC, respectively) to mediate their initial attachment to cell surface heparan sulfate proteoglycans (HSPG) (WuDunn and Spear, 1989; Shieh et al., 1992; Herold et al., 1991). The initial binding of HSV to HSPG likely precedes a conformational change that brings viral glycoprotein D (gD) to the binding domain of host cell surface gD receptors (Whitbeck et al., 1999; Krummenacher et al., 1998, 1999, 2000). Thereafter, a concerted action involving gD, its receptor, three additional HSV glycoproteins; gB, gH, and gL, and possibly an additional gH co-receptor trigger fusion of the viral envelope with the plasma membrane of host cells (Scanlan et al., 2003; Spear and Longnecker, 2003; Perez-Romero et al., 2005). Subsequently viral capsids and tegument proteins are released into the cytoplasm of the host cell.
HSV gD receptors are cell-surface molecules derived from three structurally unrelated families. These include a member of the tumor necrosis factor (TNF) receptor family, two members of the nectin family of receptors, and the product of certain 3-O sulfotransferases (3-OSTs) called 3-O-sulfated heparan sulfate (3-OS HS) (Shukla et al., 1999; Shukla and Spear, 2001; Spear and Longnecker, 2003). Herpesvirus entry mediator (HVEM or TNFRSF14) principally mediates the entry of HSV-1 and HSV-2 (Montgomery et al., 1996; Marsters et al., 1997; Kwon et al., 1997) into human T lymphocytes and is expressed in many fetal and adult human tissues including the lung, liver, kidney, and lymphoid tissues (Montgomery et al., 1996). We recently reported the requirement of HVEM receptor for HSV-2 entry in ocular cells of human origin (Tiwari et al., 2007). Nectin-1 and nectin-2, also known as herpesvirus entry proteins C and B (HveC and HveB) respectively are cell-adhesion molecules that belong to the immunoglobulin superfamily (Cocchi et al., 1998; Milne et al., 2001; Shukla et al., 2000). Both nectin-1 and nectin-2 mediate entry of HSV-1 and HSV-2, but only nectin-1 mediates bovineherpesvirus-1 (BHV-1) entry (Martinez and Spear, 2002; Warner et al., 1998). HSV-1 entry mediated activity of nectin-2 is limited to some mutant strains only (Warner et al., 1998; Lopez et al., 2000). Nectin-1 is extensively expressed in human cells of epithelial and neuronal origin (Richart et al., 2003; Tiwari et al., 2008), while nectin-2 is widely expressed in many human tissues, but only with limited expression in neuronal cells and keratinocytes. The non-protein receptor, 3-OS HS, is expressed in multiple human cell lines (e.g. neuronal, endothelial cells and human corneal fibroblasts) and mediates entry of HSV-1, but not HSV-2 (Shukla et al., 1999; Shukla and Spear, 2001; Tiwari et al., 2004; Tiwari et al., 2006; Tiwari et al., 2007).
The bark of neem plant [Azardirachata indica Linn (Meliaceae)] has been widely used as a traditional medicine for many centuries in tropical countries. Earlier studies have indicated that stem bark of neem contains some substance with strong anti-inflammatory activity (Murthy et al., 1978). Various preparations of neem obtained from its different parts have been found to exert antibacterial, antiviral, ant malarial, antioxidant, antifungal, anti mutagenic, anticarcinogenic, contraceptive and antiulcer activity (Subapryya and Nagini, 2005). The previous reports have documented that neem extracts significantly inhibited the polio virus, HIV, coxackie B group virus, and dengue virus at early step of viral genome replication (Badam et al., 1999; Parida et al., 2002; Rai and Sethi, 1972, Rao et al., 1969, Reddy and Sethi, 1974; Upadhyay et al., 1993; Sai Ram et al., 2000). Additionally, neem extract act as a vriucidal agent against coxsackiervirus virus B-4 as suggested by virus inactivation and yield reduction assay besides interfering at an early event of its replication cycle (Badam et al., 1999). Similarly, virus inhibition and RT-PCR assays confirmed inhibitory potential of neem extract at virus replication step for Dengue virus type-2 (Parida et al., 2002).
Inhibition of HSV-1 at the stage of viral entry provides a unique opportunity for therapeutic intervention. In this present study we report in vitro anti HSV-1 activity of neem bark extract (NBE) in the natural target cells. The pre-incubation of HSV-1 virus with NBE significantly blocked viral attachment and entry process. In addition, NBE further blocked the viral spread during cell-to-cell fusion assay and polykaryocytes formation. This finding opens a potential new avenue for the development of NBE as anti-herpetic microbicide.
MATERIALS AND METHOS
Plant extract
The stock (1 mg/ml) of neem bark powder (Thera Neem; Organi X South Inc. FL) was dissolved in 1× dilution of phosphate buffer saline (PBS) in a 50 ml tube and kept on a rotator shaker at 4°C for overnight. Red brown supernatant obtained after centrifugation at 3000 rpm (10 min) was filtered through 0.22 μm membrane filter (Millipore, US) and stored at −20°C until used.
Cells, plasmids and viruses
Wild-type Chinese hamster ovary (CHO-K1) cells provided by P. G. Spear (Northwestern University, Chicago) were grown in Ham’s F12 (Invitrogen Corp., Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS), while African green monkey kidney (Vero) cells were grown in Dulbecco’s Modified Eagles Medium (DMEM) (Invitrogen) supplemented with 5% FBS. CHO-IEβ-8 cells, obtained by stable transfection of CHO-K1 cells with pMLP01, express the Escherichia coli lacZ gene under control of the HSV-1 ICP4 promoter (Montgomery et al., 1996). Cultures of HeLa and retinal pigment epithelial (RPE) cells were grown in L-glutamine containing DMEM (Invitrogen Corp.) supplemented with 10% FBS. As previously described, cultures of human corneal fibroblasts (CF; provided by Dr. Beatrice Yue, University of Illinois at Chicago) were grown in DMEM media supplemented with 10% FBS and 5% calf serum (CS) (Tiwari et al., 2006). Recombinant β-galactosidase-expressing HSV-1(KOS) gL86 were used. Dr. P.G. Spear also provided additional strains of HSV-1 viruses (F, MP, 17; Dean et al., 1994) as well as the plasmids expressing HVEM (pBec10), nectin-1 (pBG38) and 3-OST-3 (pDS43) used throughout this study. GFP expressing HSV-1 (K26GFP) was provided by P. Desai (Johns Hopkins University, Baltimore). The viral stocks were propagated at low multiplicity of infection (MOI) in complementing cell lines, tittered on Vero cells and stored at −80°C.
Cytotoxic assay
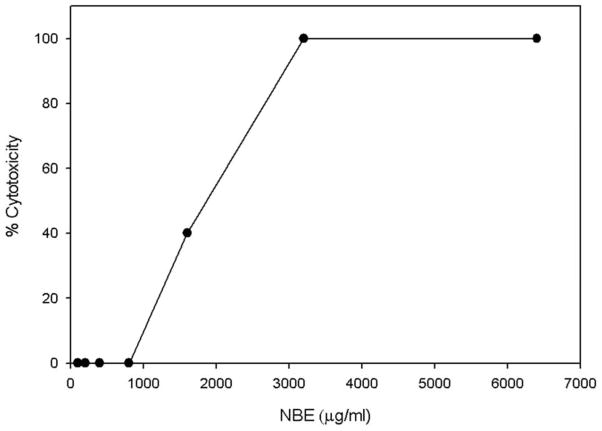
The cytotoxicity level of NBE to Vero cells was evaluated to ensure that it showed no cytotoix effect on cell viability at concentration that blocked HSV-1 infection. The assay was performed using 96-well microtiter plates. Increasing concentration of (10–6400 μg/ml) of the NBE were added to monolayers of Vero cells in triplicate and the plates were incubated at 37°C in a 5% CO2. After 6 hrs, MTT (3-[4,-5-dimethylthiazol-2-yl]-2-diphenyltetrazolium bromide) was added as previously described. The absorbance at 570 nm was measured using a 96-well plate ELISA reader. The 50% cytotoxic concentration (CC50) of NBE was calculated as previously described (Weislow et al., 1989).
Viral entry assay
Viral entry assays were based on quantitation of β-galactosidase expressed from the viral genome in which β-galactosidase expression is inducible by HSV infection. Cells were transiently transfected in 6-well tissue culture dishes, using Lipofectamine 2000 (Invitrogen) with plasmids expressing HSV-1 entry receptors (necitn-1, HVEM and 3-OST-3 expression plasmids) at 1.5 μg per well in 1 ml. At 24 hr post-transfection, cells were re-plated in 96-well tissue culture dishes (2 × 104 cells per well) at least 16 hr prior to infection. Cells were washed and exposed to serially diluted pre-incubated virus with NBE or with 1 × PBS at two fold dilutions for 2 hr at room temperature. In parallel experiments the cells were also pre-incubated with NBE for 2 hr at room temperature before infected with virus. Later the cells were washed with 1 × PBS and allowed 6 hr at 37 °C before solubilization in 100 μl of PBS containing 0.5% NP-40 and the β-galactosidase substrate, o-nitro-phenyl-β-D-galactopyranoside (ONPG; ImmunoPure, PIERCE, Rockford, IL, 3 mg/ml). The enzymatic activity was monitored at 410 nm by spectrophotometry (Molecular Devices spectra MAX 190, Sunnyvale, CA) at several time points after the addition of ONPG in order to define the interval over which the generation of the product was linear with time. Inhibitory effect of NBE on HSV-1 entry in cells were also confirmed by 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal) staining. The cells were grown in Lab-Tek chamber slides. After 6 hr of infection with reporter virus treated with NBE or left untreated with1 × PBS, cells were washed with PBS and fixed with 2% formaldehyde and 0.2% glutaradehyde at room temperature for 15 min. The cells were then washed with PBS and permeabilized with 2 mM MgCl2, 0.01% deoxycholate and 0.02% Nonidet NP-40 for 15 min. After rinsing with PBS, 1.5 ml of 1.0 mg/ml X-gal in ferricyanide buffer was added to each well and the blue color developed in the cells was examined. Microscopy was performed using 20 × objective on an inverted microscope (Zeiss, Axiovert 100M). The slide book version 3.0 was used for images. All experiments were repeated a minimum of three times unless otherwise noted.
Viral binding assay
Purified green fluorescent protein (GFP)-expressing HSV-1 (K26-GFP)pre-incubated with NBE (50–100 μg/ml) or with 1 × PBS for 90 min at room temperature were challenged to the gD receptor expressing CHO-K1 cells or naturally susceptible cells (HeLa, Vero and human CF) grown in 96-well assay plates (BD Falcon). All cells were incubated at 4 °C for 1 hr, washed five times to remove unbound virus, and finally replaced with warm medium for further incubation. Viral binding measured as relative fluorescence units (RFU) per treatment was determined using GENios Pro plate reader (TECAN) at 480-nm excitation and 520-nm emission spectrum. Measurements of three replicates of NBE treated and untreated samples were performed. Data were expressed as mean ± SD.
Viral glycoprotein induced cell-to-cell fusion assay
In this experiment, the CHO-K1 cells (grown in F-12 Ham, Invitrogen) designated “effector” cells were co-transfected with plasmids expressing four HSV-1(KOS) glycoproteins, pPEP98 (gB), pPEP99 (gD), pPEP100 (gH) and pPEP101 (gL), along with the plasmid pT7EMCLuc that expresses firefly luciferase gene under the T7 promoter (Pertel et al., 2001). Wild-type CHO-K1 cells express cell surface HS but lack functional gD receptors, therefore transiently transfected with HSV-1 entry receptors. Wild type CHO-K1 cultured cells expressing HSV-1 entry receptors (nectin-1, HVEM and 3-OST-3) considered as “target cells” were co-transfected with pCAGT7 that expresses T7 RNA polymerase using chicken actin promoter and CMV enhancer. The untreated effector cells expressing pT7EMCLuc and HSV-1 essential glycoproteins and the target cells expressing gD receptors transfected with T7 RNA polymerase were used as the positive control. Effector cells pre-incubated with NBE (50–100 μg/ml) were used for the test. For fusion, at 18 hr post transfection, the target and the effector cells were mixed together (1:1 ratio) and co-cultivated in 24- well dishes. The activation of the reporter luciferase gene as a measure of cell fusion was examined using reporter lysis assay (Promega) at 24 hr post mixing.
Quantification of polykaryocytes
In this experiment CHO-K1 effector cells expressing four HSV-1(KOS) glycoproteins (gB, gD, gH-gL) along with the plasmid pT7EMCLuc that expresses firefly luciferase gene under the T7 promoter were either pre-incubated with NBE (50 μg/ml) or with 1 × PBS for 90 min. This incubation was followed by co-culture of effector cells with target cells expressing gD receptor (nectin-1, HVEM and 3-OST-3) with pCAGT7 that expresses T7 RNA polymerase using chicken actin promoter and CMV enhancer. Both populations of effector and target cells were cultured in 1:1 ratio for 24 hrs. The cells were then fixed and Gimesa stained for microscopic visualization at 40 × magnification. A group of multinucleate cells (10–15 joint cells) were scored positive for polykaryocytes formation as previously described (Tiwari et al., 2007).
RESULTS
Neem bark extracts (NBE) significantly blocks HSV-1 entry into glycoprotein D (gD) receptor expressing CHO-K1 cells
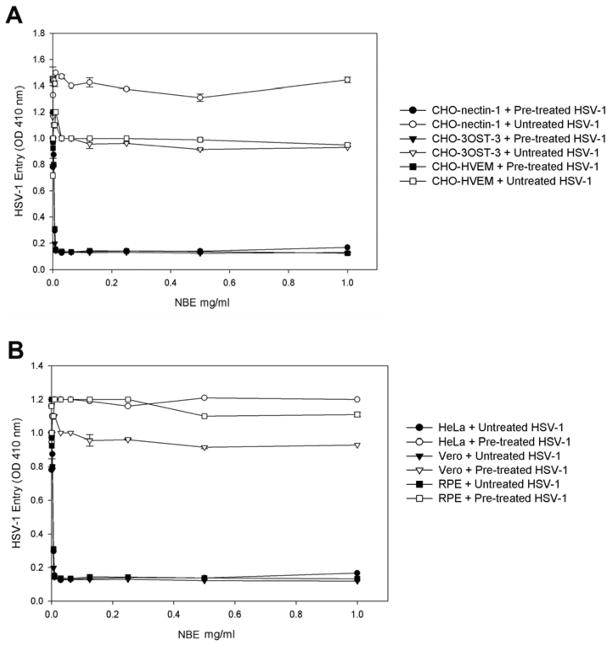
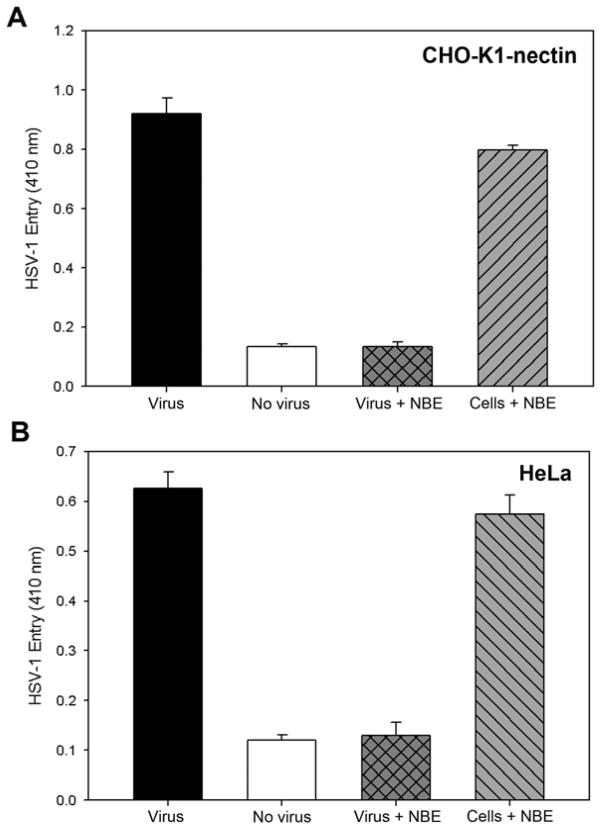
First, to assay for cytotoxic effects of NBE, Vero cells were incubated with increasing amounts (10–6400 μg/ml) of the plant extract. The viability of the treated cultures was investigated using the MTT method. The result indicated that extract concentrations up to 1000 μg/ml did not impair at all the cell viability (Fig. 1). Next, we determined the effect of NBE on HSV-1 entry into target cells. Chinese hamster ovary (CHO-K1) cells expressing gD receptors (nectin-1, HVEM or 3-OST-3) were infected with β-galactosidase expressing HSV-1 reporter virus (gL86) pretreated with NBE or mock treated. As shown in Fig. 2, pre-treatment with NBE significantly blocked HSV-1 entry in a dose dependent manner in gD receptor expressing CHO-K1 cells. In contrast, the control cells treated with 1× PBS (untreated) showed HSV-1 entry. The blocking activity of NBE was pronounced even at lower concentrations (0.1 mg/ml or 100 μg/ml) and as our results indicate, effective with all gD receptors used.
Figure 1. Cytotoxicity of neem bark extract (NBE) from A. indica on Vero cells monlayers.
Data are expressed as percent cytotoxicity (OD570 test/control) ×100. The NBE concentration at or below 1000 μg/ml was not significantly toxic to cells.
Figure 2. Neem bark extract (NBE) significantly blocks herpes simplex virus type-1 (HSV-1) entry into Chinese hamster ovary (CHO-K1) cells expressing glycoprotein D (gD) receptors.
(A). In this experiment, β-galactosidase-expressing recombinant virus HSV-1 (KOS) gL86 (25 pfu/cell) was pre-incubated for 90 min at room temperature with NBE at indicated concentrations or mock-incubated with 1 × phosphate buffer saline (PBS). Subsequently the virus was incubated with CHO-K1 cells expressing gD receptors: nectin-1, HVEM and 3-OST-3 expressing cells. After 6 hr, ONPG assay was performed. In this and other figures each value shown is the mean of three or more determinations (± SD). HSV-1 treated with 1 ×PBS was used as a control.
(B). Evaluation of HSV-1 entry blocking activity of NBE into natural target cells. Naturally susceptible cells (HeLa, Vero and retinal pigment epithelial; RPE) were used in this experiment. The β-galactosidase-expressing recombinant virus HSV-1 (KOS) gL86 (25 pfu/cell) was pre-incubated for 90 min at room temperature with NBE at indicated concentrations or mock treated with 1 × phosphate buffer saline (PBS). The virus was then incubated with HeLa, Vero and RPE cells. After 6 hr, ONPG assay was performed.
NBE significantly blocks HSV-1 entry into naturally susceptible cells
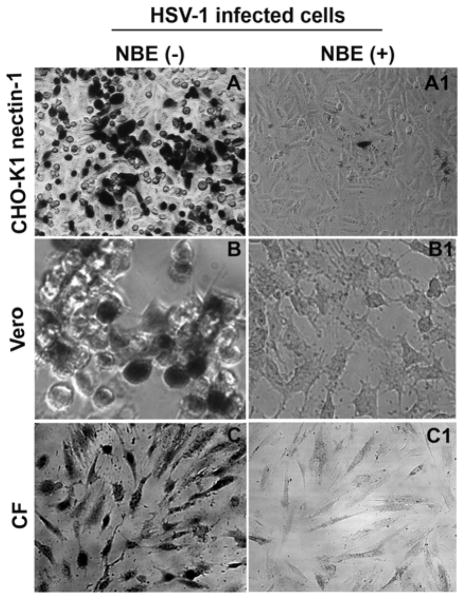
To confirm blocking activity of NBE on HSV-1 entry, we used natural susceptible cells such as HeLa, Vero and retinal pigment epithelial (RPE) cells. Recently we showed that RPE cells express gD receptor nectin-1 to allow HSV-1 entry (Tiwari et al., 2008). As shown in Fig. 2 (panel B) compared to the mock treated cells, the HSV-1 treated with NBE (0.1 mg/ml or 100 μg/ml) or even lower dilutions showed significant blocking of HSV-1 entry in all cell types tested. These results were consistent with the previous observations made with CHO-K1 cells expressing gD receptors. We further confirmed the above results by X-gal assay. In this experiment we used both CHO-K1 cells expressing nectin-1, HeLa cells and primary cultures of human corneal fiborbalsts (CF). HeLa cells express all known gD receptors while CF exclusively express HVEM and 3-OST-3 (Tiwari et al., 2005; Tiwari et al., 2006; Tiwari et al., 2008). As demonstrated in Figure 3, the HSV-1 treatment with NBE significantly reduced the number of blue cells in CHO-K1 cells expressing nectin-1, HeLa and CF cells (panels A1–C1). In contrast, mock-treated virions were able to infect fine as 100% cells turned blue (panels A–C). Taken together, the results highlight the HSV-1 entry blocking ability of NBE, which appears to be receptor independent.
Figure 3. Confirmation of entry inhibition by X-gal (1.0 mg/ml) staining.
Infection of nectin-1-expressing CHO-K1, Vero and primary corneal fibroblasts (CF) with HSV-1 (KOS gL86 at 25 pfu/cell) virus pre-incubated (50 μg/ml) for 90 min with NBE (panels A1, B1, and C1) is shown (panels A1, B1and C1). Cells infected with identical dosage of virus incubated with 1 × PBS was used as control (panels A, B and C). Dark (blue) cells represent viral entry. Microscopy was performed using a 20 × objective on Zeiss Axiovert 100. The slide book version 3.0 was used for image processing.
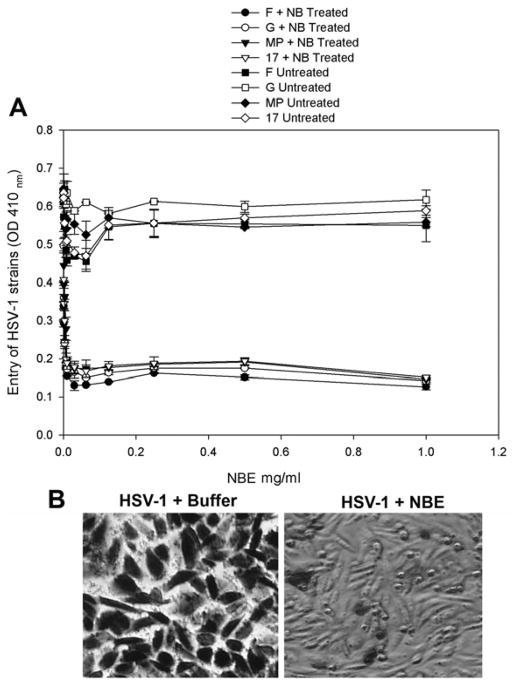
Anti-HSV-1 entry activity of NBE is not viral strain specific
The next question was to evaluate the broader significance of NBE as an anti-HSV agent. We therefore, tested the ability of NBE to block viral entry in different but more virulent strains of HSV-1 (F, G, MP and 17 strains) (Dean et al., 1994). Here we used nectin-1 expressing CHO Ig8 cells that express β-galactosidase upon viral entry (Montgomery et al., 1996). The high virulence HSV-1 strains were pre-incubated with NBE and were subsequently used to infect the cells. The results again showed that NBE blocked entry of different HSV-1 strains in a dosage dependent manner as evident by the ONPG-based entry assay (Fig. 4; panel A). Similarly, X-gal assay supported the above results as the untreated virions were able to infect virtually all the cells while the NBE treated F-strain HSV-1 virions were unable to infect cells (Fig. 4; panel B).
Figure 4. HSV-1 entry blocking activity of NBE is not viral-strain specific.
Different strains of HSV-1 (F, G, MP and 17 strains of HSV-1 at 25 pfu/cell) were either pre-incubated with 1 × PBS (control) or with NBE at indicated concentrations for 90 min at room temperature. The two pools of viruses were incubated on CHO Ig8 cells that express β-galactosidase upon viral entry. The viral entry blocking was measured by ONPG assay (panel A) or by X-gal assay (panel B) as previously described. In X-gal assay (panel B), F-strain of HSV-1 is shown.
NBE interacts with HSV-1 envelope glycoproteins
We next determined whether the inhibitory activity of NBE on HSV-1 entry was attributed to target cells or viral particles. CHO-K1 cells expressing nectin-1 receptor and the natural target HeLa cells were pre-incubated with NBE or mock treated with 1 × PBS for 90 min followed by HSV-1 infection at 25 pfu/cell. In parallel we used NBE treated (100 μg/ml) HSV-1 (KOS) gL86 to infect CHO-nectin-1 and HeLa cells. Interestingly, as shown in Figure 5, pre-incubation of CHO-K1 expressing nectin-1 (panel A) and HeLa cells (panel B) with NBE had a minor or no effect on HSV-1 entry, while virus incubation with NBE significantly blocked HSV-1 entry into both CHO-K1 cells expressing nectin-1 (panel A) and HeLa cells (panel B). This data suggesting that anti-HSV-1 activity of NBE is due to the effect on viral particles.
Figure 5. Pre-incubation of NBE with HSV-1 virions but not with the target cells significantly blocks HSV-1 entry.
In this experiment, target CHO-K1 cell expressing HSV-1 gD receptor nectin-1 (panel A) or a natural target HeLa cells (panel B) were either pre-incubated for 90 min with 50 μg/ml NBE or treated with 1 × phosphate buffer saline (PBS as a control) before viral infection. In parallel experiment β-galactosidase-expressing recombinant virus HSV-1 (KOS) gL86 (25 pfu/cell) was similarly pre-incubated with NBE. The four sets of combinations (virus alone, no virus, pre-incubated virus with NBE and pre-incubated target cells with NBE) were used to infect CHO-K1 cells expressing nectin-1 (A) or naturally target HeLa cells (B) for 2 hrs at room temperature followed by washing of cells with PBS. After 4 hr, ONPG assay was performed. Each bar represents the value of three independent experiments.
NBE significantly affects HSV-1 binding to the cells
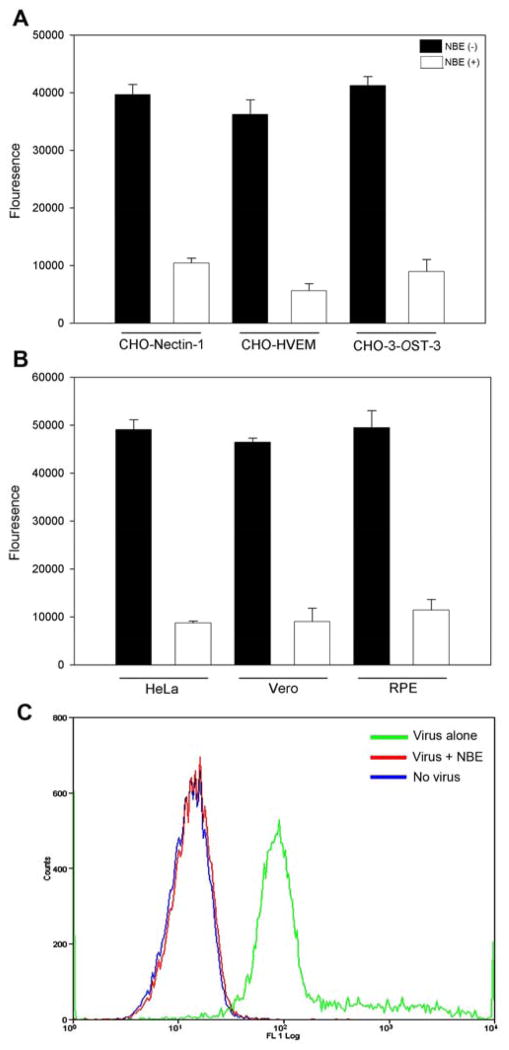
Because NBE blocked HSV-1 entry, we next tested its ability to affect viral binding to the cells. To determine the difference between NBE (100 μg/ml) treated viruses versus untreated ones on viral attachment or binding we used GFP-expressing HSV-1 (K26GFP). As shown in Fig. 6 the GFP signal on cell surface was significantly weaker when virus was treated with NBE in both gD expressing CHO-K1 cells (panel A) and natural target cells (panel B). The untreated virus had very high viral binding read out in the cell types. This data indicated that NBE significantly affected viral entry at the attachment step. In a parallel experiment similar findings were obtained using flow cytometry (Fig. 6; panel C).
Figure 6. NBE significantly inhibits HSV-1 binding to the target cells (A–C).
Green fluorescent protein (GFP) expressing HSV-1 (K26 GFP at 25 pfu/cell) was pre-incubated with NBE (50 μg/ml; represent as white bar) or with 1 × PBS (represent as black bar) for 90 min at room temperature. The mixture was allowed to incubate with CHO-K1 cells expressing gD receptors; nectin-1, HVEM and 3-OST-3 (panel A) or natural target cells; HeLa, Vero and RPE cells (panel B) for 1 hr at room temperature followed by a quick citrate buffer treatment to remove unbound viruses. The fluorescent output as a result of viral binding to the cells was recorded using Tecan spectrophotometer is presented. Each value shown is the mean of three or more determinations (± SD). GFP-expressing HSV-1 pre-incubated with 1 × PBS was used as a control. (C). HSV-1 binding to cells in presence of NBE was quantified using flow cytometry analysis. GFP expressing HSV-1 virions (25 pfu/cell) pre-incubated with NBE (50 μg/ml) or treated with 1 ×PBS were cold bound to HeLa cells at 4°C. Uninfected RPE cells were used as a negative control. Cells were examined by fluorescence-activated cell sorter (FACS) analysis after 35 min of incubation with GFP-expressing HSV-1. Note that the NBE-pretreatment to GFP virions significantly impaired the ability to bind the cells (Red). Mock treated GFP-HSV-1 virions were able to bind cells (Green). Uninfected HeLa cells were used as background control (blue).
NBE treatment inhibits HSV-1 glycoprotein mediated cell to cell fusion and polykaryocytyes formation
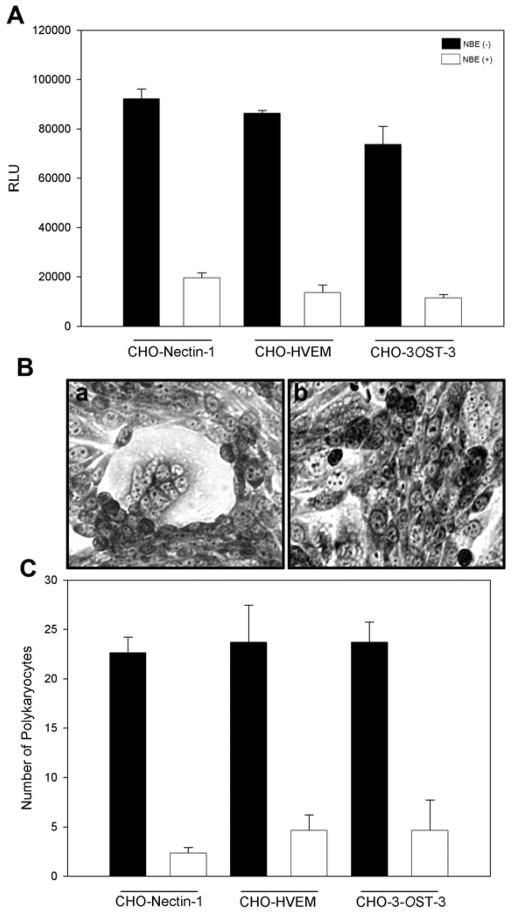
Finally, we tested the effect of NBE during HSV-1 glycoproteins mediated cell to cell fusion. Cell to cell fusion has been studied to demonstrate the viral and cellular requirements during virus-cell interactions and also as means of viral spread. We sought to determine whether interaction between NBE and HSV-1 envelope glycoproteins that are essential for viral entry affects cell to cell fusion. Surprisingly, effector cells expressing HSV-1 glycoproteins treated with NBE (100 μg/ml) impaired the cell to cell fusion in both CHO-K1 cells expressing gD specific receptors (Fig. 7; white bars in panel A). In parallel the control untreated effector cells co-cultured with target cells had high fusion (Fig. 7; black bars in panel A). This response was further observed when polykaryocytes formation was estimated. Again NBE treated-effector cells failed to form polykaryons when co- cultured with target cells (Fig. 7; panel B; right panel), while in control untreated effector cells efficiently showed larger polykaryons (Fig. 7; panel B; left panel). Similar counts in polykaryons were noticed in NBE-treated cells (Fig. 7; panel C; white bars) versus mock treated cells (Fig. 7; panel C; black bars). Fusion of the membrane between virion envelop and plasma membrane of the target cell requires glycoproteins (gB, gD, gH-gL) or in combination of all of them (Roizman and Sears, 1996; Spear, 1993). Our results indicate that the presence of NBE significantly reduced the viral attachment and penetration. We therefore propose that NBE possibly interferes with viral envelope glycoproteins thereby preventing the binding and fusion process.
Figure 7. NBE significantly impairs HSV-1 glycoproteins induced cell to cell fusion and polykaryocytes formation.
(A). The “effector CHO-K1 cells” expressing HSV-1 glycoproteins (gB, gD, gH-gL) along with T7 plasmid were pre-incubated with 100 μg/ml NBE or 1 × PBS for 90 min. The two pools of effector cells (NBE treated and PBS treated) were mixed with CHO-K1 cells expressing luciferase gene along with specific gD receptors; nectin-1, HVEM and 3-OST-3. Membrane fusion as a means of viral spread was detected by monitoring the luciferase activity. Black bars and white bars represent 1× PBS treated and NBE treated cells respectively. Error bars represent standard deviations. * P< 0.05, one way ANOVA. (B). Microscopic visualization of polykaryocyte impairments by NBE. The effector CHO-K1 cells expressing four essential HSV-1 glycoproteins (gB, gD, gH-gL) were pre-incubated with either NBE or 1 × PBS for 90 min before they were co-cultured in 1:1 ratio for 24 hrs. The cells were fixed for 20 min. and then stained with Gimesa stain (Fluka) for 20 min. Shown are photographs of representative cells (Zeiss Axiovert 200) pictured under microscope at 40 × objective. The left panel shows cell fusion and polykaryocytes formation in the absence of NBE, the right panel shows significant inhibition of polykaryocytes formation in the presence of NBE.
(C). In parallel polykaryocytes formation was also quantified by counting polykaryons (group of 15 cells). The panel shows number of polykaryons in mock-treated cells (black bars) versus NBE treated cells (white bars). The values shown were from one representative experiment performed in triplicate.
DISCUSSION
Our understanding on the molecular mechanisms of viral entry into cell provides a unique opportunity to generate novel ways of preventing viral cell interactions for novel therapeutic interventions (Dimitrov, 2004; Sieczkarski and Whittaker, 2005). We used an aqueous extract from neem bark (NBE) from A. indica to test its ability to block HSV-1 entry. Plants have been used as folk remedies, and many are now being collected and examined in an attempt to identify possible source of antiviral agents. It has been suggested that natural products are preferable to synthetic compounds (Vanden Berghe et al., 1986; Vlietinck and Vanden Berghe, 1991). A large number of small molecules, like phenolics, polyphenols, terpenes, flavinoids, and sugar containing compounds from plants have been reported against herpes simplex viruses (Khan et al., 2005). NBE is widely used to prepare a number of skin and personal hygiene products. It is a multi functional as well as multi utility natural product and without any side effects. The bark contains 3.43% protein, 0.68% alkaloids and 4.16% minerals. Polysaccharides in NBE possess anti-tumor as well as anti-inflammatory properties.
This study identifies a potent anti-HSV activity in aqueous extracts (NBE) from dried bark of neem plant. NBE significantly inhibited HSV-1 entry and viral glycoprotein mediated cell-to-cell fusion in a cell culture model. Further, the anti-HSV-1 activity of NBE was not limited to particular receptor or the viral strain tested. Our results showed that HSV-1 entry was significantly blocked in CHO-K1 cells expressing either protein receptors (nectin-1 and or HVEM) or a sugar receptor (3-OST-3 modified 3OS HS). Similar blocking was also observed in a natural target CF cells isolated from human cornea which expresses 3OS HS (Tiwari et al., 2006). HSV-1 is involved in several ocular diseases (Liesegang, 2001) including herpes stromal kertatitis which is leading cause of corneal blindness. Therefore, anti-HSV plant extract have significant relevance in infectious epithelial keratitis, stromal keratitis and endothelitis.
Interestingly, the blocking activity of NBE was only seen when HSV-1 virions were pre- incubated with the extract as compared to pre-incubation with target cells. This result suggested two important points; 1, the tested concentrations of NBE was not cytotoxic to the cells as the viral entry was quantified when NBE was pre-incubated with the target cells, and 2, the inhibitory effect of NBE targets the virions as virucidal effect was seen when the extract was incubated with the virus (Fig. 5). Future studies will be needed to isolate, detect and characterize the active molecule present in NBE which is responsible for anti-herpes activity. Whether the activity of A. indica is against all herpesvirus infections or is specific to HSV-1 needs to be tested. In addition, NBE-anti herpes activity may be evaluated against individual HSV-1 glycoprotein. Further characterization of the molecule and its toxicity will aid in the development of the therapeutic agents against herpes infection.
Acknowledgments
We thank Dr. P.G. Spear (Northwestern University, Chicago) for providing reagents. An institutional grant support from Western University of Health Sciences (WUHS) to V.T. is acknowledged. D.S. is a recipient of Lew Wasserman Merit award from Research to Prevent Blindness, USA.
References
- Badam L, Joshi SP, Bedekar SS. ‘In vitro’ antiviral activity of neem (Azadirachta indica. A. Juss) leaf extract against group B coxackieviruses. J Cimmun Dis. 1999;31:79–90. [PubMed] [Google Scholar]
- Cocchi F, Menotti L, Mirandola P, Lopez M, Campadelli-Fiume G. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J Virol. 1998;72:9992–10002. doi: 10.1128/jvi.72.12.9992-10002.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campadelli-Fiume G, Cocchi F, Menotti L, Lopez M. The novel receptors that mediate the entry of herpes simplex viruses and animal alphaherpesviruses into cells. Rev Med Virol. 2000;10:305–319. doi: 10.1002/1099-1654(200009/10)10:5<305::aid-rmv286>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Clement C, Tiwari V, Scanlan PM, Valyi-Nagy T, Yue BYJT, Shukla D. A novel role for phagocytosis-like uptake in herpes simplex virus entry. J Cell Biol. 2006;174:1009–1021. doi: 10.1083/jcb.200509155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly SA, Whitbeck JC, Rux AH, Krummenacher C, van Drunen Little-van den Hurk S, Cohen GH, Eisenberg RJ. Glycoprotein D homologs in herpes simplex virus type 1, pseudorabies virus, and bovine herpes virus type 1 bind directly to human HveC (Nectin-1) with different affinities. Virology. 2001;280:7–18. doi: 10.1006/viro.2000.0747. [DOI] [PubMed] [Google Scholar]
- Dean HJ, Terhune S, Shieh MT, Susmarski N, Spear PG. Single amino acid substitutions in gD of herpes simplex virus 1 confer resistant to gD-mediated interference and cause cell type-dependent alterations in infectivity. Virology. 1994;199:67–80. doi: 10.1006/viro.1994.1098. [DOI] [PubMed] [Google Scholar]
- Dimitrov DS. Virus entry: Molecular mechanism and biomedical applications. Nature Rev. 2004;2:109–122. doi: 10.1038/nrmicro817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold BC, WuDunn D, Soltys N, Spear PG. Glycoprotein C of herpes simplex virus type 1 plays a principle role in the adsorption of virus to cells and in infectivity. J Virol. 1991;65:1090–1098. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JM, Ball MJ, Neumann DM, Azcuv AM, Bhattacharjee PS, Bouhanik S, Clement C, Lukiw WJ, Foster TP, Kumar M, Kafman HE, Thompson HW. The high prevalence of herpes simplex virus type 1 DNA in human trigeminal ganglia is not a function of age or gender. J Virol. 2008;85:8230–8234. doi: 10.1128/JVI.00686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye SB, Baker K, Bonshek R, Maseruka H, Grinfeld E, Tullo A, Easty DL, Hart CA. Human herpesviruses in the cornea. Br J Opthalmol. 2000;84:563–571. doi: 10.1136/bjo.84.6.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MTH, Ather A, Thompson KD, Gambari R. Extracts and molecules from medicinal plants against herpes simplex viruses. Antiviral Res. 2005;67:107–119. doi: 10.1016/j.antiviral.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science. 2008;322:268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummenacher C, Nicola AV, Whitbeck JC, Lou H, Hou W, Lambris JD, Geraghty RJ, Spear PG, Cohen GH, Eisenberg RJ. Herpes simplex virus glycoprotein D can bind to poliovirus receptor related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J Virol. 1998;72:7064–7074. doi: 10.1128/jvi.72.9.7064-7074.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummenacher C, Rux AH, Whitbeck JC, Ponce-de-Leon M, Lou H, Baribaud I, Hou W, Zou C, Geraghty RJ, Spear PG, Eisenberg RJ, Cohen GH. The first immunoglobulin-like domain of HveC is sufficient to bind herpes simplex virus gD with full affinity while the third domain is involved in oligomerization of HveC. J Virol. 1999;73:8127–8137. doi: 10.1128/jvi.73.10.8127-8137.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummenacher C, Baribaud I, Ponce de Leon M, Whitbeck JC, Lou H, Cohen GH, Eisenberg RJ. Localization of a binding site for herpes simplex virus glycoprotein D on herpesvirus entry mediator C by using antireceptor monoclonal antibodies. J Virol. 2000;74:10863–10872. doi: 10.1128/jvi.74.23.10863-10872.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon BS, Tan KB, Ni J, Oh KO, Lee ZH, Kim YJ, Wang S, Gentz R, Yu GL, Harrop J, Lyn SD, Silverman C, Porter TG, Truneh A, Young PR. A newly identified member of the tumor necrosis factor receptor superfamily with a wide tissue distribution and involvement in lymphocyte activation. J Biol Chem. 1997;272:14272–14276. doi: 10.1074/jbc.272.22.14272. [DOI] [PubMed] [Google Scholar]
- Liesegang TJ, Melton LJ, Daly PJ, Ilstrup DM. Epidemiology of ocular herpes simplex. Incidence in Rochester, Minn, 1950 through 1982. Arch Ophthalmol. 1989;107:1155–1159. doi: 10.1001/archopht.1989.01070020221029. [DOI] [PubMed] [Google Scholar]
- Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20:1–13. doi: 10.1097/00003226-200101000-00001. [DOI] [PubMed] [Google Scholar]
- Lopez M, Cocchi F, Menotti L, Avitabile E, Dubreuil P, Campadelli-Fiume G. Nectin2α (PRR2α or HveB) and nectin2δ are low-efficiency mediators for entry of herpes simplex virus mutants carrying the Leu25Pro substitution in glycoprotein D. J Virol. 2000;74:1267–1274. doi: 10.1128/jvi.74.3.1267-1274.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsters SA, Ayres TM, Skubatch M, Gary CL, Rothe M, Ashkenazi A. Herpesvirus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-kB and AP-1. J Biol Chem. 1997;272:14029–14032. doi: 10.1074/jbc.272.22.14029. [DOI] [PubMed] [Google Scholar]
- Martinez WM, Spear PG. Amino acid substitution in the V domain of nectin-1 (HveC) that impair entry activity for herpes simplex virus type 1 and 2 but not for pseudorabies virus or bovine herpesvirus 1. J Virol. 2002;76:7255–7262. doi: 10.1128/JVI.76.14.7255-7262.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne RS, Connolly SA, Krummenacher C, Eisenberg RJ, Cohen GH. Porcine, HveC, a member of the highly conserved HveC/nectin 1 family, is a functional alphaherpesvirus receptor. Virology. 2001;281:315–328. doi: 10.1006/viro.2000.0798. [DOI] [PubMed] [Google Scholar]
- Montgomery RI, Warner MS, Lum BJ, Spear PG. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- Murthy KS, Rao DN, Rao DK, Murthy LBS. A preliminary study on hypoglycemic and anti-hyperglycemic effects of Azadirachta indica. Ind J Pharmacology. 1978;10:247–250. [Google Scholar]
- Parida MM, Upadhyay C, Pandya G, Jana AM. Inhbitrory potential of neem (Azadirachta indica Juss) leaves on dengue virus type-2 replciation. J Ethnopharmacol. 2002;79 (2):273–278. doi: 10.1016/s0378-8741(01)00395-6. [DOI] [PubMed] [Google Scholar]
- Perez-Romero P, Perez A, Capul A, Montgomery R, Fuller AO. Herpes simplex virus entry mediator associates in infected cells in a complex with viral proteins gD and at least gH. J Virol. 2005;79:4540–4544. doi: 10.1128/JVI.79.7.4540-4544.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertel P, Fridberg A, Parish M, Spear PG. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH–gL requires a gD receptor but not necessarily heparan sulfate. Virology. 2001;279:313–324. doi: 10.1006/viro.2000.0713. [DOI] [PubMed] [Google Scholar]
- Rai AR, Sethi M. Screening of some plants for their activity against vaccinia and fowl pox viruses. Indian Journal of Animal Sciences. 1972;42:1066–1070. [Google Scholar]
- Rao AR, Kumar SSV, Paramsivam TR, Kamalakshi S, Parashuraman AR, Shanta B. Study of antiviral activity of leaves of margosa tree on vaccinia and variola viruses-a preliminary report. Indian Journal of Medicinal Research. 1969;57:495–502. [PubMed] [Google Scholar]
- Reddy AB, Sethi MS. Antiviral effects of some indigenious plant extracts on vaccinia and fowl pox viruses on chick embryo fibroblasts. Indian Journal of Experimental Biology. 1974;12:572–579. [PubMed] [Google Scholar]
- Richart SM, Simpson SA, Krummenacher C, Whitbeck JC, Pizer LI, Cohen GH, Eisenberg RJ, Wilcox CL. Entry of herpes simplex virus type 1 into primary sensory neurons in vitro is mediated by nectin-1/HveC. J Virol. 2003;77:3307–3311. doi: 10.1128/JVI.77.5.3307-3311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B, Sears AE. Herpes simplex viruses and their replication. In: Fields BN, Knipe DM, Chanock RM, Hirsch MS, Melnick JL, Monath TP, Roizman B, editors. Virology. 3. Vol. 2. Lippincott-Raven Publishers; Philadelphia: 1996. pp. 2231–2295. [Google Scholar]
- Sai Ram M, Ilavazhagan G, Sharma SK, Dhanraj SA, Saresh B, Parida MM, Jan AM, Davendra K, Selvanmurthy W. Antimicrobial activity of a new vaginal contraceptive NIM. 76 from neem oil (Azadirachta indica) J Ethno. 2000;1:377–382. doi: 10.1016/s0378-8741(99)00211-1. [DOI] [PubMed] [Google Scholar]
- Scanlan PM, Tiwari V, Bommireddy S, Shukla D. Cellular expression of gH confers resistance to herpes simplex virus type-1 entry. Virology. 2003;312:14–24. doi: 10.1016/s0042-6822(03)00176-4. [DOI] [PubMed] [Google Scholar]
- Shieh MT, WuDunn D, Montgomery RI, Esko JD, Spear PG. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J Cell Biol. 1992;116:1273–1281. doi: 10.1083/jcb.116.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieczkarski SB, Whittaker GR. Viral entry. Curr Top Microbiol Immunol. 2005;285:1–23. doi: 10.1007/3-540-26764-6_1. [DOI] [PubMed] [Google Scholar]
- Spear PG. Entry of alphaherpesviruses into cells. Semin Virol. 1993;4:167–180. [Google Scholar]
- Spear PG, Longnecker R. Herpesvirus entry: an update. J Virol. 2003;77:10179–10185. doi: 10.1128/JVI.77.19.10179-10185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear PG, Eisenberg RJ, Cohen GH. Three classes of cell surface receptors for alphaherpesvirus entry. Virology. 2000;275:1–8. doi: 10.1006/viro.2000.0529. [DOI] [PubMed] [Google Scholar]
- Shukla D, Dal Canto MC, Rowe CL, Spear PG. Striking similarity of murine nectin-1α to human nectin-1α (Hve C) in sequence and activity as a glycoprotein D receptor for alphaherpesvirus entry. J Virol. 2000;74:11773–11781. doi: 10.1128/jvi.74.24.11773-11781.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla D, Liu J, Blaiklock P, Shworak NW, Bai X, Esko JD, Cohen GH, Eisenberg RJ, Rosenberg RD, Spear PG. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell. 1999;99:13–22. doi: 10.1016/s0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]
- Shukla D, Spear PG. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J Clin Invest. 2001;108:503–510. doi: 10.1172/JCI13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synder A, Polcicova K, Johson DC. Herpes simplex virus gE/gI and US9 proteins promote transport of both capsid and virion glycoproteins in nuronal axons. J Virol. 2008;82:10613–10624. doi: 10.1128/JVI.01241-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari V, Clement C, Duncan MB, Chen J, Liu J, Shukla D. A role for 3-O-sulfated heparin sulfate in cell fusion induced by herpes simplex virus type 1. J Gen Virol. 2004;85:805–809. doi: 10.1099/vir.0.19641-0. [DOI] [PubMed] [Google Scholar]
- Tiwari V, Clement C, Xu D, Valyi-Nagy T, Yue BY, Liu J, Shukla D. Role for 3-O-sulfated heparan sulfate as the receptor for herpes simplex virus type 1 entry into primary human corneal fibroblasts. J Virol. 2006;80:8970–8980. doi: 10.1128/JVI.00296-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari V, Clement C, Scanlan PM, Kowlessur D, Yue BY, Shukla D. A role for herpesvirus entry mediator as the receptor for herpes simplex virus 1 entry into primary human trabecular meshwork cells. J Virol. 2005;79:13173–13179. doi: 10.1128/JVI.79.20.13173-13179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari V, Shukla SY, Yue BY, Shukla D. Herpes simplex virus type 2 entry into cultured human corneal fibroblasts is mediated by herpesvirus entry mediator. J Gen Virol. 2007;88:2106–2110. doi: 10.1099/vir.0.82830-0. [DOI] [PubMed] [Google Scholar]
- Tiwari V, ten Dam GB, Yue BY, van Kuppevelt TH, Shukla D. Role of 3-O-sulfated heparan sulfate in virus-induced polykaryocyte formation. FEBS Lett. 2007;18:4468–4472. doi: 10.1016/j.febslet.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari V, Oh MJ, Kovacs M, Shukla SY, Valyi-Nagy T, Shukla D. Role for nectin-1 in herpes simplex virus 1 entry and spread in human retinal pigment epithelial cells. FEBS J. 2008;275:5272–5285. doi: 10.1111/j.1742-4658.2008.06655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay SN, Dhawan S, Garg S, Wali N, Tusker L, Anderson DJ. Immunomodulatory properties of neem. World Neem Conference; Banglore, India. 1993. p. abstract no. 80. [Google Scholar]
- Vanden Berghe DA, Vlietinck AJ, Van Hoof L. Plant products as potential antiviral agents. Bulletin Institute Pasteur. 1986;84:101–147. [Google Scholar]
- Vlietinck AJ, Vanden Berghe DA. Can ethnopharmacology contribute to the development of antiviral drugs? J Ethnopharmacology. 1991;32:141–153. doi: 10.1016/0378-8741(91)90112-q. [DOI] [PubMed] [Google Scholar]
- Warner MS, Geraghty RJ, Martinez WM, Montgomery RI, Whitbeck JC, Xu R, Eisenberg RJ, Cohen GH, Spear PG. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. Virology. 1998;246:179–189. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- Weislow OS, Kiser R, Fine DL, Bader J, Shoemaker RH, Boyd MR. New soluble-formaazan assay for HIV-1 cytopathic effects; application to high-flux screening of synthetic and natural products for AIDS-antiviral activity. J Natl Cancer Inst. 1989;81:577–586. doi: 10.1093/jnci/81.8.577. [DOI] [PubMed] [Google Scholar]
- Whitbeck JC, Muggeridge MI, Rux AH, Hou W, Krummenacher C, Lou H, Geelen VAN, Eisenberg RJ, Cohen GH. The major neutralizing antigenic site on herpes simplex virus glycoprotein D overlaps a receptor-binding domain. J Virol. 1999;73:9879–9890. doi: 10.1128/jvi.73.12.9879-9890.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley RJ, Kimberlin DW. Herpes simplex viruses. Clin Infect Dis. 1997;26:97–109. doi: 10.1086/514600. [DOI] [PubMed] [Google Scholar]
- Whitley RJ, Roizman B. Herpes simplex viruses. In: Richman DD, Whitely RJ, Hayden FG, editors. Clinical Virology. Churchill Livingstone; New York: 1997. pp. 380–382. [Google Scholar]
- Whitley RJ, Kimberlin DW, Roizman B. Herpes simplex viruses. Clin Infect Dis. 1998;26:541–553. doi: 10.1086/514600. [DOI] [PubMed] [Google Scholar]
- WuDunn D, Spear PG. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989;63:52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]