Abstract
The direct estimate of 46,000 snakebite deaths in India in 2005 (1 for every 2 HIV/AIDS deaths), based on verbal autopsies, renders unrealistic the total of only 47,000 snakebite deaths in the whole world in 2010, obtained indirectly as part of the “Global Burden of Disease 2010” study. Persistent underestimation of its true morbidity and mortality has made snakebite the most neglected of all the WHO's “neglected tropical diseases”, downgrading its public health importance. Strategies to address this neglect should include the improvement of antivenom, the only specific antidote to envenoming. To accommodate increased understanding of geographical intraspecific variation in venom composition and the range of snake species that are medically important in India, the design of antivenoms (choice of venom sources and species coverage) should be reconsidered. Methods of preclinical and clinical testing should be improved. The relatively new science of venomics involves techniques and strategies for assessing the toxin composition of snake venoms directly through proteomics-centred approaches or indirectly via high-throughput venom gland transcriptomics and bioinformatic analysis. Antivenomics is translational venomics: a proteomics-based protocol to quantify the extent of cross-reactivity of antivenoms against homologous and heterologous venoms. These approaches could revolutionize the preclinical assessment of antivenom efficacy, leading to a new generation of antivenoms that are clinically more effective.
Keywords: Antivenoms, cobra, envenoming, krait, preclinical efficacy, Russell's viper, snakebite, venom, venomics
Introduction
When most people involved in public health think of neglected tropical diseases (NTDs) that affect the lives of men, women and children throughout the developing economies of the world, their minds usually turn to a conflagration of tropical infectious diseases including guinea worm, leishmaniasis, dengue, onchocerciasis, Chagas disease, leprosy and lymphatic filariasis. The World Health Organization (WHO) agrees. Supported by drug makers and special interest groups, WHO initiated a coordinated effort to address a number of these diseases in 2005. That effort has steadily mushroomed, resulting in publication of a landmark report on these problems in 2010, that gave rise to a strategic plan for eradication and control efforts1. Today WHO lists 17 NTDs on its website as targets for eradication. At the WHO's World Health Assembly on 27th May 2013, resolution EB132.R7 was adopted, urging member States to expand their programmes to prevent, control, eliminate and eradicate all 17 of these NTDs2. But what few people will know is that in March 2009, WHO added snakebite to its official list of NTDs, and then quietly demoted it, along with podoconiosis and strongyloidiasis to a separate listing of what it calls “other neglected conditions”; a list that is included in none of WHO's plans to eradicate NTDs, or even mentioned in either its 2010 report, or the recently available 2013 report1,3. Such a move seems to be the way in which the public health community considers snakebite, begrudgingly admitting to it, but then quietly relegating it to the category of being too difficult to achieve, despite the reality that this forgotten tropical disease continues to impose a tremendous cost of suffering and chronic disability on many of the world's poorest and most disenfranchised communities4.
Persuading the global community to take serious notice of the extent to which families and even whole communities are fractured by snakebites is a challenge that is being taken up by scientists, doctors and others who have been confronted by the tragedy of lives lost or bodies crippled by what is essentially a preventable and treatable illness. In 2008, members of the international toxinology community established the Global Snakebite Initiative (GSI), since when its members have worked towards developing an integrated new approach to improving therapeutic management of snakebite, and addressing issues ranging from prevention, to health-worker training and victim rehabilitation5,6,7. At the core of the GSI strategy is the desire to couple modern proteomic, immunological, pharmacological and molecular biological techniques to the quest for improved therapeutics and the understanding of the underlying pathophysiology and biomechanics of snakebite injuries7,8,9,10. Among the links being forged by the GSI, are partnerships with Indian researchers and institutions interested in the challenge of improving both the treatment of snakebite in India, and the outcomes for the million or more people bitten each year.
India has had a long and tumultuous relationship with serpents, which are both revered and feared. Some of the earliest reports of snakebite from India during British colonial rule spoke of fatalities in the tens of thousands. Sir Joseph Fayrer who championed the destruction of India's snakes as the best means to curb human deaths, wrote in a report to Nature published on December 28, 1882, that snakebite had caused the deaths of 11,416 people across seven administrative regions representing about half of what was then British India (including modern Burma and Pakistan) in 1869, but he believed this to be an under-estimate of the true burden11. Fayrer believed between 150,000 and 200,000 deaths due to snakebite had occurred in India from 1870-1882, citing 19,060 human deaths in 1880 and 18,610 in 1881. During this two year period the government paid out 23,623 rupees in bounties for 467,744 snakes killed under eradication programmes that Fayrer had fought to have instituted. Numerically, the highest numbers of fatalities in 1880/81 were recorded in the heavily populated Bengal (19,272), and the North-West Provinces/Aoudh (9,733), which also had the highest mortality rates relative to population size (15.93/100,000/yr and 11.04/100,000/yr, respectively) along with the Central Provinces (11.50/100,000/yr) and the small population of Ajmere-Marwara (10.30/100,000/yr)12. Using population data from the time provided in a letter to the British Medical Journal by Dr Edward Nicholson in 188312, the mortality rate for snakebite right across British India for 1880/81 averaged 10 deaths per 100,000/yr, a figure which if true today would equate to more than 163,504 deaths/yr across the former British India (excluding Sri Lanka) of which more than 122,000 would occur in present day India. Fayrer's claim of up to 200,000 deaths from 1870-1882 is supported by what were said to be incomplete annual figures from the British Administration in India13. It reported that 21,391 deaths occurred in 1875, 19,279 in 1876 and 22,125 in 1882. The 1876 figure is cited as the lowest number between 1875-1882. Thirty years of reports published in 1913 continued to put the annual death toll from snakebite at more than 20,000 per year14. In 1909, there were 21,634 deaths across the British colonies, and 22,478 were recording in 1910, with 7,767 of these occurring in Bengal14. Bombay (now Mumbai) saw far fewer snakebites than Bengal. In 1902 and 1903 there were 1,288 and 1,074 deaths from snakebites, respectively representing 16.7 per cent of all the deaths from injury in that 2 year period15. In 1910, snakebites caused 1,247 deaths in Bombay11. In 1924, there were 19,867 deaths from snakebites reported across British India, accounting for 2.35 per cent of all deaths recorded in the Public Health Commissioners annual report to government16.
This review presents a modern summary of snakebite in India, and discusses how real improvements in the quality of snake antivenoms as front-line therapeutics can be achieved by embracing the opportunities presented by modern scientific technologies when applied to this age-old problem.
The global burden of snakebites
The recently published Global Burden of Disease 2010 estimated, from a large number of different sources, that the global total of deaths from animal contact (venomous) decreased from 54,900 (95% uncertainty intervals 30,100-89,300) in 1990 to 47,000 (25,600-84,700) in 2010 and that deaths from “other neglected tropical diseases”, which include snakebites http://www.who.int/neglected_diseases/diseases/en/)17, increased from 22,900 (14,300-29,500) in 1990 to 23,700 (16,600 - 30,900) in 201018. The absurd inadequacy of these estimates is immediately apparent if one considers the direct estimate of 46,000 (99% confidence interval 41,000 - 51,000) snakebite deaths in India in 200519. Over the last half century, there have been a few attempts to discover the incidence of snakebite and its associated morbidity and mortality worldwide. In 1954, Swaroop and Grabb, using national hospital returns, suggested half a million bites with 30,000-40,000 deaths each year but they had no data from China, USSR, or Central Europe20. In 1998, Chippaux used results of household surveys, together with hospital and health authority data to increase these figures almost five-fold to 5 million bites, 125,000 deaths and 100,000 severe sequelae each year21. Evaluation of overall disease burden using the concept of disability adjusted life years (DALYs) produced the high estimate of 2 million DALYs per year for sub-Saharan Africa17. In the most recent WHO-sponsored effort, Kasturiratne et al22 dredged the so-called “grey literature”, combining these suspect sources with hospital returns and by extrapolation between adjacent (and some not-so-adjacent) countries produced a figure of 420,549–1,841,158 envenomings (bites in which venom was injected) and 19,886 - 93,945 deaths each year. Assuming that envenoming occurred in about one in every four snakebites, they estimated that between 1.2 and 5.5 million snakebites might occur annually. In all likelihood the only way in which the true burden will ever be known is if snakebites are made a reportable event by governments willing to invest in properly defining the impact of snakebite on public health and community well-being.
Snakebite incidence and mortality in India
With the growth of human populations in South Asia since Sir Joseph Fayrer and others produced their first calculations of snakebite impact more than 130 years ago, one might expect that snakebite figures in India would have climbed with the burgeoning of humanity. Community-based surveys in Burdwan district, West Bengal23, and a neighbouring area just across the border in Nepal's eastern Terai24, found surprisingly high annual incidences of snakebite deaths, 16.4 and 162 per 100,000 population per annum, respectively. However, between 2004 and 2009, hospitals in 31 of the 35 States and Union Territories in India reported an average of only 1,350 snakebite deaths each year to the Government of India http://cbhidghs.nic.in/index2.asp?slid=985&sublinkid=694). These national totals were lower than older figures from single States. For example, in 1971, 1,476 snakebite deaths were recorded in Bombay Province, Maharashtra State and Ratnagiri district25. Controversy about the true burden of snakebite mortality in India should at last have been resolved by results of the Registrar General of India's “Million Death Study” (MDS)19. All causes of all deaths were ascertained in 6,671 randomly chosen sample areas, each including about 1000 people. Verbal autopsy was used to identify snakebite deaths. This involves a structured interview of the relatives or close associates of the deceased conducted by non-medical staff with central medical coding by at least two doctors. Verbal autopsy is reliable for snakebite because the fatal event is distinctive, dramatic and therefore memorable. However, some deaths may have been missed since some victims of nocturnal krait bites do not realize that they have been bitten and present with mysterious ‘early morning paralysis’ or seizures that would not be associated with snakebite26. In 2005, an estimated 46,000 people (99% CI 41,000 - 51,000) died of snakebite in India, approximately one for every two HIV/AIDS deaths19. Assuming that there are 100 non-fatal bites for each fatal bite (from results of a community-based study in Bangladesh)27 there could be as many as 4.6 million snakebites in India each year. The MDS results indicate which States of India are worst affected: the highest numbers of deaths were in Uttar Pradesh (8,700), Andhra Pradesh (5,200), and Bihar (4,500), while Andhra Pradesh had the highest incidence of mortality due to snakebite (6.2/100 000 population/year)19. Snakebite accounts for 3 per cent of all deaths in children of the age of 5-14 yr. Ninety seven per cent of the victims of snakebite die in rural areas, 77 per cent of them outside health facilities, because they preferred traditional therapy from tantriks, vaidyas and ojhas. These figures should encourage the Ministry of Health to reassess the public health priority of snakebite and deploy its resources where these are most needed.
Approaches to preventing snakebites
Snakebite in India is an occupational and environmental disease mainly affecting rural agricultural workers and their families. Prevention is all important. Like any form of health promotion, community education by all available means and media is crucial. The aim is to alert communities to the types of environments most frequented by dangerous species, and to advise them how to avoid being bitten. For example, cobras are most commonly found near water or in irrigated paddy fields, while Russell's vipers frequent these areas especially at times of harvest when rodents are most abundant. The most dangerous time of year is usually during the monsoon and hot weather. Many bites are inflicted as people walk home or collect firewood in the dark after dusk or before dawn. Safer walking and safer working can be achieved by wearing protective shoes, boots or gloves and by carrying a light after dark. The risk of nocturnal krait bites can be mitigated by sleeping in a hammock, on a raised bed or under a well-tucked-in mosquito net28. Communities should be persuaded to abandon their preference for traditional treatments and, instead, to transfer bite victims to dispensaries, clinics or hospitals as quickly as possible. Steps must be taken to improve the medical treatment of snakebite (including the effectiveness and safety of antivenoms) to generate confidence in conventional medicine. Where there are no roads and limited means of transport, village-based motorcycle volunteers have proved effective for transporting obstetrical emergencies in Sierre Leone and patients with neurotoxic snakebites in the eastern Terai of Nepal “SK Sharma, personal communication”29. Once the patient has arrived in hospital, prevention of morbidity and mortality will depend on the training of medical staff and provision of necessary equipment, antivenom and other drugs. Guidelines for the SEARO region, including India, have been published by WHO30.
Indian snakes of medical relevance and the design of Indian polyvalent antivenoms
India and its surrounding seas are inhabited by more than 60 species of venomous snakes some of which are sufficiently common to cause frequent bites and whose venoms are capable of severe envenoming31. Four species, common krait (Bungarus caeruleus) (Fig. 1a), spectacled cobra (Naja naja) (Fig. 2a), Russell's viper (Daboia russelii) (Fig. 3a), and saw-scaled viper (Echis carinatus) (Fig. 4a) are distributed throughout virtually the whole country and have long been recognized as the most important causes of bites, deaths and disability. Over the past century, venoms of only these four species have been used by Indian manufacturers to raise polyvalent antivenoms (also called anti-snake venoms, ASV, or anti-snakebite serums) for national use. However, there have been increasing concerns about the design of these antivenoms, due in part to recognition that many more species of venomous snakes are implicated in envenoming across India and also to ongoing concerns about the safety and effectiveness of products that have gained a reputation for being poorly manufactured in some cases, and poorly effective in published clinical studies of snakebites32.
Fig. 1.
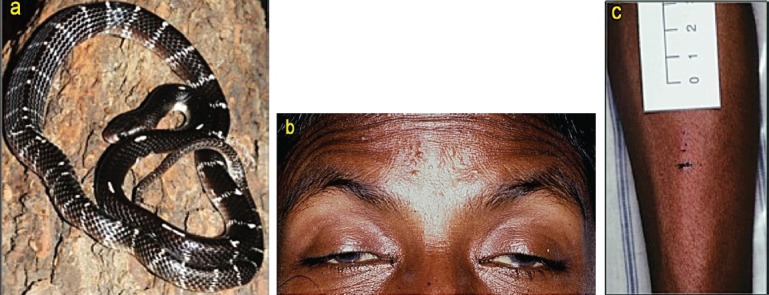
Envenoming by common krait (Bungarus caeruleus): (a) the snake (specimen from Pune, Maharashtra); (b) bilateral ptosis, external ophthalmoplegia and facial paralysis; (c) fang and tooth puncture marks without local swelling in the same patient as 1b.
Fig. 2.
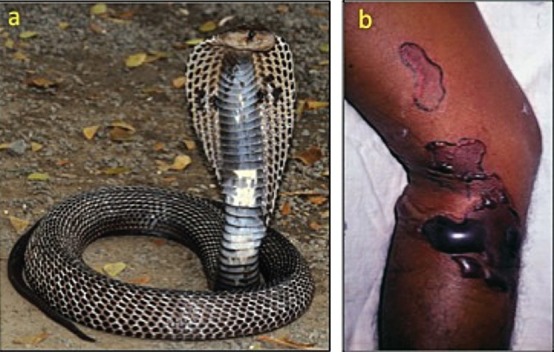
Envenoming by common cobra (Naja naja): (a) the snake (specimen from Pune, Maharashtra); (b) local swelling, blistering and demarcated areas of dermonecrosis in a patient.
Fig. 3.
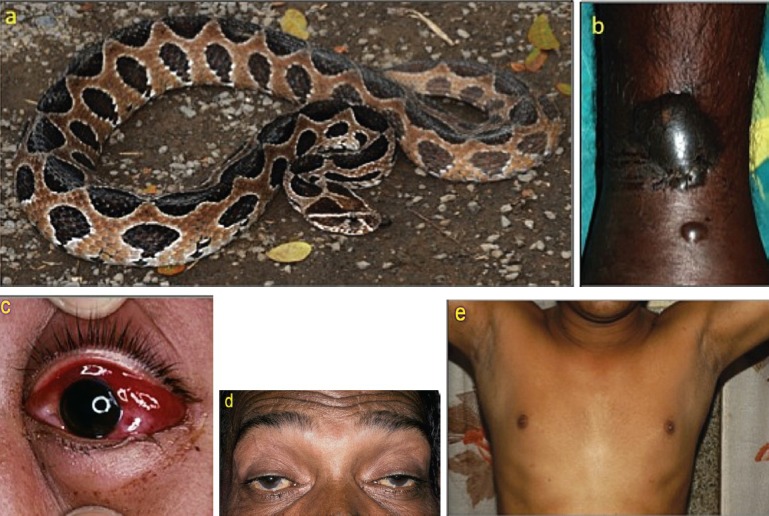
Envenoming by Russell's viper (Daboia russelii): (a) the snake (specimen from Pune, Maharashtra); (b) local swelling and blistering (Kerala); (c) chemosis, a sign of generalized increase in capillary permeability; (d) bilateral ptosis, external ophthalmoplegia and facial paralysis; (e) chronic pan-hypopituitarism (loss of secondary sexual hair and gynaecomastia) following a bite 3 years previously (Kerala).
Fig. 4.
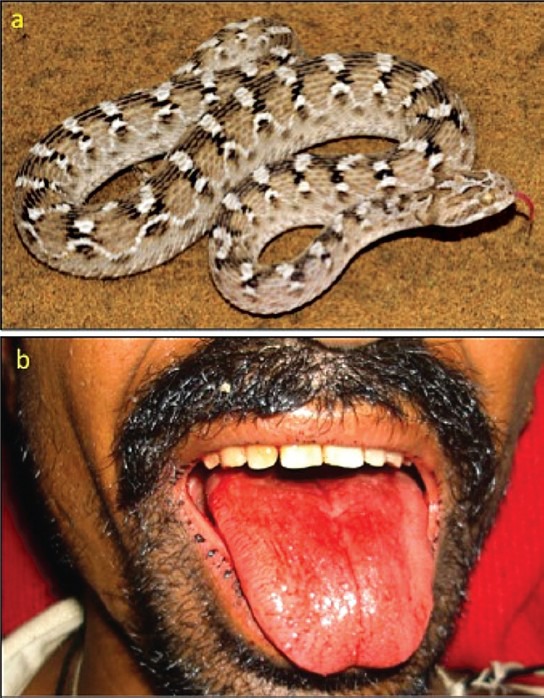
Envenoming by northern saw-scaled viper (Echis carinatus sochureki): (a) the snake (specimen from Bikaner, Rajasthan; (b) Bleeding from the gums (Bikaner) (courtesy of Dr PD Tanwar).
Which species are responsible for snakebite envenoming in India?
Apart from the widely distributed “big four”, there are a number of other species occurring in localized areas of the country that are now known to be capable of causing severe envenoming32 :
Monocellate cobra (N. kaouthia) - occurs in north-eastern India, in many parts of which it causes more bites than N. naja with equally severe consequences.
Central Asian or Oxus cobra (N. oxiana) - is confined to the far north (Kashmir and Himachal Pradesh) where it is rare. No bites have been reported in India but it has proved dangerous in other parts of its range.
Kraits (Bungarus) - taxonomic revisions have elevated two former sub-species to the status of full species. Wall's krait (Bungarus walli) occurs in the north-east and the Sind krait (B. sindanus) in the west. Bungarus sindanus has caused life-threatening envenoming in Maharashtra33 and Rajastan (Dr PD Tanwar, personal communication, February 2011). Bungarus walli has caused fatalities in Nepal and Bangladesh (unpublished data of Aniruddha Ghose, Md A. Faiz, Sharma SK, Harris JB, Kuch U, Warrell DA in preparation). The greater black krait (B. niger) occurs in the far north-east of India. In Bangladesh, it has caused fatal envenoming through generalized rhabdomyolysis and acute kidney injury (AKI), as well as by more familiar paralytic effects34.
King cobra (Ophiophagus hannah) - occurs in the Western Ghats and north-east India. It is the largest and potentially the most dangerous of all venomous snakes but is rare and elusive, causing very few bites and deaths35.
Levantine or snub-nosed viper (Macrovipera lebetina) - this large and dangerous species is confined to Jammu and Kashmir at altitudes above 900 m and has caused only one recorded bite in India35.
Pit-vipers (Crotalinae) - among the 15 Indian species, the hump-nosed pit-viper (Hypnale hypnale) (Fig. 5a) of the south-west coast and Western Ghats, where it has been misidentified as E. carinatus, has caused local necrosis, coagulopathy, bleeding and AKI37. The Himalayan pit-viper (Gloydius himalayanus) of the western Himalayas, bamboo pit-viper (Trimeresurus gramineus) of the Western and Eastern Ghats, large-scaled pit-viper (Peltopelor macrolepis) of the south-west37, mountain pit-viper (Ovophis monticola) of north and north-east and northern white-lipped green pit-viper (Cryptelytrops septentrionalis), red-tailed pit-viper (C. erythrurus) and some other arboreal green pit-vipers of the north-east, can cause local swelling, coagulopathy, bruising and bleeding. Envenoming by the Malabar pit-viper (Trimeresurus malabaricus) of the Western Ghats38 can cause local necrosis. The venoms of none of these species are covered by Indian polyvalent antivenoms.
Fig. 5.

Envenoming by hump-nosed viper (Hypnale hypnale): (a) the snake (specimen from Thattekkad, Kerala); (b) marked local swelling after a bite on the foot (Angamaly, Kerala).
Sea-snakes (Hydrophiinae, Laticaudinae) - there have been a few reported cases of sea snake bites around the coast of India39 (Fig. 6).
Fig. 6.

Beaked sea snake (Enhydrina schistosa): the snake (courtesy of Mark O’shea).
Clinical phenotypes of envenoming by Indian snakes
Elapid envenoming is characterized by neurotoxicity, first evident as ptosis and external ophthalmoplegia (Fig. 1b) then involving muscles innervated by the cranial nerves in a descending pattern followed by the axial muscles of the trunk, resulting in respiratory paralysis and finally, if respiration is supported, generalized flaccid paralysis, partially sparing only the extremities. Krait bites are almost always inflicted on people sleeping indoors on the ground. Local envenoming is absent or minimal (Fig. 1c) and there is usually no local pain. However, severe crescendo abdominal pain may be a presenting symptom. Destructive, irreversible neurotoxicity that does not respond to antivenom is a feature of krait envenoming. Cobra bite envenoming causes, in addition, local envenoming: pain, swelling, regional lymphadenopathy, blistering and necrosis (Fig. 2b). Russell's viper bite envenoming causes marked local envenoming (Fig. 3b) and a variety of systemic manifestations: shock, coagulopathy, bleeding and AKI. In some parts of the country such as Kerala, there is generally increased capillary permeability (Fig. 3c). In others, such as Tamil Nadu, there is neuro-myotoxicity (Fig. 3d). The risk of anterior pituitary infarction resulting in fatal acute pituitary adrenal failure or chronic pan-hypopituitarism occurs in southern India. Saw-scaled viper bites are usually less severe, lacking the risk of AKI, permeability and pituitary infarction but associated with local necrosis, coagulopathy and bleeding (Fig. 4b). Hump-nosed viper bites are associated with local envenoming (Fig. 5b), coagulopathy, bleeding, microangiopathic haemolysis and AKI. Bites by other Indian pit-vipers can cause local envenoming resulting in necrosis, coagulopathy, bleeding rarely if ever in AKI. Generalised rhabdomyolysis causing myalgia followed by myoglobinuria and AKI is a feature of sea snakebite envenoming.
Is Indian polyvalent antivenom equally effective in treating bites by the “big four” species throughout the whole of India?
The phenomenon of intra-species variation in venom composition has been well documented in the cases of many species of viperid and elapid snakes40,41. Geographical diversity in venom antigenicity is the most important consideration in antivenom design. In India, there is evidence of such variation in the venoms of N. naja and D. russelii42,43 and yet 80 per cent of the venom currently used to raise Indian polyvalent antivenoms is collected by the Irula cooperative from snakes inhabiting one small area around Mahabalipuram in Tamil Nadu (http://www.madrascrocodilebank.org/cms/conservation-and-research-2/the-irulas/). There is growing clinical suspicion that Indian antivenom may be less effective in some areas distant from the source of immunizing venoms. In Rajasthan, envenoming by the larger northern sub-species of the saw-scaled viper (Echis carinatus sochureki) requires larger doses of antivenom than for the smaller southern sub-species (E. c. carinatus)44. In Maharashtra and northern Kerala, antivenom appears to be less effective in the treatment of D. russelii envenoming. For example, in Kozhikode, treatment with generally accepted initial doses of 10-20 vials of polyvalent antivenom within a few hours of the bite failed to prevent development of the syndrome of generalized increase in capillary permeability (capillary leak syndrome) (Dr Remi Thomas - personal communication, February 2012).
Should the classic Indian polyvalent antivenoms be redesigned to provide broader cover?
Because of geographical intra-species variation in venom composition and hence immunogenicity, the current practice of using venom from restricted geographical source(s) may have the result that envenoming even by the traditional ‘big four’ species may not be adequately treated by Indian polyvalent antivenoms. Ideally, venom pools including samples from a wide geographical range should be used for immunization. The range of species against which polyvalent antivenom is effective should also be expanded to cover some of the additional species. In a recent study, two Indian polyvalent antivenoms manufactured by Vins Bioproducts and Bharat Serums and Vaccines showed different levels of neutralization for both Indian and south-east Asian Naja and Bungarus venoms, indicating that polyvalent antivenoms produced in India, using the same source of venom, should not be assumed to be identical in their therapeutic potential45. In reconsidering their future strategy for antivenom design, the Indian authorities might decide to continue with one polyvalent antivenom with expanded range to cover the whole country or perhaps three new regional antivenoms. For example, NE (North-East) antivenom might include, in addition to the big four, N. kaouthia, B. walli, B. niger and one of the pit-vipers among its immunizing venoms; NW (North-West) - N. oxiana, B. sindanus, E. c. sochureki and M. lebetina; SW (South-West)- B. sindanus, H. hypnale, T. malabaricus and T. gramineus.
Toolbox for the preclinical assessment of antivenom efficacy
An ideal therapeutic antivenom should contain antibodies that can neutralize the widest possible range of medically important toxins to give the widest polyspecific coverage, using as few specific venoms as possible in its production. The large variation in the composition of snake venoms, both at the inter- and intra- species levels, has implications for the design of venom mixtures for immunization of animals for antivenom production and in the analysis of antivenom neutralizing efficacy at the preclinical level. The introduction of a new antivenom in a particular geographical setting should be preceded by a rigorous analysis of its preclinical efficacy against the venoms of the most relevant snakes in the region. The single most important test to assess neutralization of venoms by antivenoms is the mouse lethality test but, depending on the toxicological profile of the venom being tested, additional assays should also be included.
Neutralization of lethality assay
Lethality is the single most important effect to be tested when analysing venom toxicity and its neutralization by antivenoms. Thus, the gold standard for the assessment of preclinical antivenom efficacy is the neutralization of lethal activity of venoms46. First, the overall toxicity of venom, the median lethal dose (LD50), is determined, usually using the mouse model. To this end, serial doses of venom, diluted in saline solution, are injected in mice of a determined weight, either by the intravenous (i.v.) or the intraperitoneal (i.p.) routes. Deaths occurring during a predefined time span (24 or 48 h) are recorded and the LD50 is estimated by statistical methods such as Spearman-Karber47, probits48 or non-parametric procedures. LD50 corresponds to the venom dose which induces death in 50 per cent of the injected mice; it can be expressed as μg venom per mouse or as μg venom per gram of animal weight. For the neutralization assays, a fixed dose of venom, known as the ‘challenge dose’, which corresponds to a defined number of LD50s (usually from 3 to 6 depending on the laboratory), is selected. This fixed venom solution is mixed with various dilutions of the antivenom, in order to achieve several antivenom/venom ratios, and the mixtures are incubated (usually for 30 min at 37°C). Control samples include venom incubated with saline solution instead of antivenom. Aliquots of the mixtures are then injected into mice and deaths are recorded. Neutralization is expressed as the median effective dose (ED50), i.e. the antivenom/venom ratio in which 50 per cent of the injected mice survive. ED50 can be expressed in various ways, which varies depending on the laboratory, i.e. milligram of venom neutralized per millilitre antivenom, millilitre antivenom required to neutralize one milligram venom, or number of LD50 s of venom neutralized per millilitre antivenom. The assessment of the ability of antivenoms to neutralize lethality is routinely performed in the manufacturers’ quality control laboratories and by national regulatory agencies, as part of regular analyses of the antivenoms being manufactured, purchased and distributed. Unfortunately, some countries rely mostly on data reported by the manufacturers, rather than on control exerted by regulatory agencies.
Towards a more complete assessment of the preclinical efficacy of antivenoms
The neutralization of venom lethality is, and will remain, the gold standard in the preclinical testing of antivenom efficacy46. However, the study of the biochemical and toxicological complexity of snake venoms has shown clearly that, besides lethality, the venoms of many species induce additional toxic activities that play a key role in the pathophysiology of human envenoming. For example, envenomings by viperid snakes in many regions around the world are characterized by complex local tissue damage (myonecrosis, dermo-necrosis, haemorrhage, blistering) and by systemic disturbances (haemorrhage, coagulopathy, cardiovascular shock, acute kidney injury). Therefore, it has been argued that a more detailed analysis of the preclinical efficacy of antivenoms should encompass, in addition to lethality, the neutralization of these other clinically relevant effects46,49,50. Several simple in vivo and in vitro laboratory assays have been developed for the quantitative assessment of haemorrhagic, myotoxic, dermo-necrotic, coagulant, and defibrinogenating activities, among others51,52,53. Thus, although the routine quality control of antivenoms involves the neutralization of lethal activity, when a new antivenom is being developed, or when an existing antivenom is introduced to a new geographical setting, a comprehensive preclinical analysis of neutralizing efficacy should be performed against the most relevant toxic effects of the most important snake venoms in that particular region. This allows a rigorous testing of whether the antivenom is effective, not only against venom-induced lethality, but also against other clinically-relevant effects. A brief description of the fundamentals of these additional tests follows:
Haemorrhagic activity: The most widely used method is based on the intradermal injection of venom solutions followed, several hours later, by the measurement of the area of the haemorrhagic spot in the inner side of the skin. The original method was described in rabbits54, and has been adapted for use in rats51 and mice55. Venom activity is expressed as the minimum haemorrhagic dose (MHD), which corresponds to the dose of venom that induces a haemorrhagic halo of 10 mm diameter55. More recently, the analysis of systemic haemorrhage has been performed by the study of pulmonary haemorrhage. Mice were injected i.v. with venom and, one hour later, the animals were sacrificed and the thoracic cavity exposed for observation of haemorrhagic spots on the surface of the lungs. The minimum pulmonary haemorrhagic dose (MPHD) corresponds to the lowest amount of venom that induces haemorrhagic spots in the lungs of all mice injected56.
Myotoxic activity: Venom-induced skeletal muscle necrosis can be assessed by histological examination of muscle tissue injected with venom. Mice receive an intramuscular injection of venom solution, for example, in the gastrocnemius muscle, are sacrificed after 24 h, and the injected muscle dissected out, placed in fixative solution (e.g. formalin), and processed for histological analysis. The number of necrotic cells and the total number of muscle cells are quantified by microscopic assessment, and the myotoxic effect is expressed as the necrotic index, i.e. the ratio of necrotic muscle fibres to total muscle fibres57. Venom activity can be expressed as the dose inducing a necrotic index of 0.5. Since histological analysis is time consuming and not available in many laboratories, an alternative and highly convenient test is based on the quantification of the plasma activity of the enzyme creatine kinase (CK), which is present in large amounts in muscle tissue and is released when the plasma membrane of muscle cells is disrupted by the action of venom myotoxins52. The minimum myotoxic dose (MMD) is defined as the dose of venom that increases the plasma CK activity four times as compared to mice injected with saline solution58.
Dermo-necrotic activity: This effect is assessed in either rats or mice by carrying out intradermal injections of venom solutions, followed by the measurement of the necrotic area in the inner side of the skin 72 hour after venom injection51.
Coagulant activity: In vitro coagulant activity of venoms is assessed by the addition of various doses of venom to samples of citrated human plasma, followed by the determination of clotting time. Activity is expressed as the minimum coagulant dose (MCD), defined as the dose of venom that induces clotting in 60 sec51,53. For assessing thrombin-like activity of venoms, a similar test is performed on fibrinogen solutions instead of plasma51.
Defibrinogenating activity: It is assessed in rats or mice by intravenous injection of venom solutions. After a defined period of time, a blood sample is collected, placed in a glass tube, and incubated at room temperature for observation of clot formation. Activity is expressed as the minimum defibrinogenating dose (MDD), defined as the dose of venom that induces incoagulability in all animals injected51,53.
Other tests: In addition to the most frequently performed tests described above, other toxic activities can be also assessed, such as oedema-forming activity59, neurotoxic activity using nerve-muscle preparations ex vivo60, and thrombocytopenic effect61. Likewise, the analysis of neutralization of venom enzyme activities has been also investigated, such as neutralization of proteinase, phospholipase A2 and hyaluronidase activities55,62,63. As in the case of neutralization of lethality, a ‘challenge dose’ of venom is selected and a solution of a fixed dose of venom is mixed with various dilutions of antivenom, followed by incubation (30 min at 37°C). Aliquots of the mixtures are then tested in the corresponding assay systems described; controls of venom solutions incubated with saline solution instead of antivenom are included. Neutralization is expressed as ED50, i.e. the antivenom/venom ratio at which the effect of venom is neutralized to a 50 per cent64. In the case of coagulant and defibrinogenating activities, neutralization is expressed as effective dose (ED), defined as the antivenom/venom ratio at which the clotting time of plasma is prolonged three times when compared to plasma incubated with venom alone (for coagulant activity) or as the antivenom/venom ratio at which blood clots in all animals injected (for defibrinogenating effect)53.
Selection of tests for preclinical evaluation of antivenoms should be based on the toxicological profile of venoms
Since snake venoms are highly variable in their toxicological profiles, the selection of laboratory tests to be implemented for the preclinical evaluation of antivenom efficacy should be based on the toxicological profile of venoms and on the predominant clinical manifestations of envenomings. The following are some examples:
(i) Venoms of elapid snakes exerting predominantly neurotoxic effect: The venoms of a large variety of elapid snakes exert, both at the clinical and the experimental levels, a predominant neurotoxic effect, based on the action of either presynaptically-acting phospholipase A2 (PLA2s), such as β-bungarotoxin from species of Bungarus, or post-synaptically-acting neurotoxins of the ‘three finger family’, known as α-neurotoxins, which bind with strong affinity to the nicotinic cholinergic receptor at the motor end-plate. These neurotoxins act at the peripheral nervous system and generate flaccid paralysis which, when affects respiratory muscles, might result in respiratory paralysis and death65. The preclinical efficacy of antivenoms against these venoms can be assessed by the neutralization of lethal effect (the ED50 test), since the end result of neurotoxicity is death. Thus, in the cases of venoms of many species of cobras (Naja sp.) and kraits (Bungarus sp.), neutralization of lethality suffices to test the preclinical efficacy of antivenoms45.
(ii) Venoms of viperid snakes: Despite presenting significant variations in venom composition and activities between and within species, viperid venoms are generally characterized for inducing local tissue damage (i.e. haemorrhage, myonecrosis and oedema) and systemic alterations associated with haemorrhage, coagulopathy, cardiovascular disturbances and renal damage65. Therefore, the preclinical assessment of antivenoms against viperid venoms should include the analysis of lethal, haemorrhagic, myotoxic, coagulant, and defibrinogenating activities of venoms66. This would be the case of the venoms of Echis sp., Daboia sp. and Hypnale sp. in the Indian subcontinent.
(iii) Other venoms: Venoms of a number of spitting cobras in Africa and Asia induce predominantly a local necrotising effect in humans65,67. In these cases, antivenoms should be assessed for their capacity to neutralize lethality and dermo-necrosis68. Sea snake venoms induce, in addition to neurotoxic effect, a systemic myotoxic action which might result in rhabdomyolysis. Hence, antivenoms should be tested for the neutralization of lethal and myotoxic activities66. On the other hand, the venoms of some Australian land elapids induce neurotoxicity, myotoxicity and coagulopathy; consequently, preclinical evaluation of antivenoms should include the neutralization of these effects69.
When an antivenom, which is being developed or introduced to a new geographical setting for the first time, is shown to be effective at the preclinical level, subsequent routine quality control of its efficacy can be based on the neutralization of lethality only.
The issue of venom variability and the preparation of venom pools
A key aspect in the preclinical assessment of antivenom efficacy is the quality and representativeness of the venoms used to test neutralization. There may be a large intraspecific variability in venom composition, especially in species of wide geographical distribution, such as Daboia russelii in Asia43, Bitis arietans in sub-Saharan Africa70 and Bothrops atrox in South America71. In these and in similar cases, it is necessary to ensure that the venoms utilized correspond to representative pools prepared from specimens distributed in different geographical locations40,46. These venom pools should be prepared from specimens acquired from right across the range of the species, that have been correctly identified, since the traceability of the venoms for preclinical testing is of paramount relevance. Ideally, each country should have snake collections and laboratories for maintenance of snakes and for venom collection and storage, following appropriate standardised protocols46 and, the preclinical assessment of antivenom efficacy should be carried out with venoms from specimens collected in the region where this antivenom is intended to be used. This task demands national and international cooperation from health authorities, National Regulatory Agencies, independent scientists and manufacturers to ensure the qualification of snake collections and venom sources and the adequacy of selected preclinical tests for the species venoms concerned. Furthermore, protocols for quality control of venoms used in antivenom production and evaluation should be also implemented according to the national and international standards46.
The need for the development of in vitro tests for assessing antivenom preclinical efficacy
Many of the tests used for the preclinical evaluation of antivenoms involve the use of experimental animals, mostly mice. Since venoms induce pain and other effects in these animals, there is a growing concern about this issue and interest in finding suitable surrogate in vitro tests that could be used in the evaluation of antivenoms. One of the main drawbacks for achieving this goal has to do with the great biochemical and toxicological complexity of venoms, in which several relevant effects are induced by different venom components66. Despite this limitation, some advances have been achieved, such as determining procoagulant activity in vitro40, and using enzyme immunoassays whose results correlate with the neutralization of lethality72,73,74. However, compared to the murine ED50 assay, the neuromuscular preparation assay requires a high degree of skill and experience to perform it well, and the technique is not easily reproducible from one laboratory to another. In addition, the use of chicken embryos at a stage prior to the development of pain sensitivity has been proposed as an alternative to rodent tests for assessing lethal and haemorrhagic activities of viperid venoms75. The search for alternative in vitro tests for assessing the neutralizing ability of antivenoms is an area of research and development that deserves urgent attention. In particular, at the start of the 21st century, developments in proteomics-centred approaches for assessing the detailed composition of venoms (venomics) and the immunochemical profile of antivenoms (antivenomics) are likely to revolutionise the design and pre-clinical assessment of antivenoms, especially in predicting paraspecific neutralization. The operational principles of these approaches are discussed below.
International cooperative projects should be implemented to assess the preclinical efficacy of antivenoms on a worldwide basis
The analysis of the neutralizing ability of antivenoms at the preclinical level has been the focus of a number of collaborative research projects in Latin America. As a consequence, a large body of evidence has accumulated and, on this basis, the public health authorities in the region are able to make informed decisions on antivenom acquisition and deployment in several countries. Examples are studies performed in Argentina76, Brasil77,78, Perú58, Colombia79, Ecuador80, Costa Rica55,77 and Guatemala81. Recently, a large collaborative regional project evaluated several antivenoms against the venoms of the medically most important Bothrops species in the region82. In contrast, studies on the preclinical efficacy of antivenoms in Africa and Asia have been scarce and there is a large gap in our knowledge of the cross-neutralization of viperid and elapid snake venoms by antivenoms used in these continents. For instance, several antivenoms are distributed in sub-Saharan Africa, but only a few studies have reported data on their preclinical neutralizing profile83,84,85,86 ; moreover, venoms from snakes of many African countries have not been tested for their neutralization by antivenoms. This situation jeopardises efforts to bring antivenoms to large areas of Africa and Asia since the introduction of antivenoms for clinical use without preclinical information on their efficacy, is not recommended.
The solution to this problem demands concerted international efforts involving diverse partners, including herpetologists, toxinologists, antivenom manufacturers, epidemiologists and public health authorities, among others. The development and strengthening of local groups, in universities and ministries of health in developing countries, in the preclinical testing of antivenoms are of paramount importance and should be promoted through national and international workshops and training programmes. Likewise, the establishment of snake collection facilities and laboratories responsible for obtaining venoms are required to ensure the proper identification and quality assurance of venoms to be used in preclinical testing. Research laboratories working on venom proteomics and other classical and “omics” aspects of toxinology are essential to generate detailed knowledge of venom composition and variability.
The Global Snakebite Initiative (GSI, http://www.snakebiteinitiative.org), with the support of the International Society on Toxinology (IST, http://www.toxinology.org), has proposed an international strategy to improve our knowledge of the composition of the medically most important venoms, as well as of the ability of currently available antivenoms to neutralize these venoms at the preclinical level7. This knowledge may pave the way for the design of novel, knowledge-based immunizing mixtures with the aim of generating polyspecific antivenoms of broad efficacy, particularly in sub-Saharan Africa and Asia. Already, GSI has developed protocols for Indian researchers to enable them to collect very high quality venom samples in the field, and return these to the laboratory for analysis without degradation. Efforts are now focusing on ensuring that these venoms are used in well-designed, relevant assays to measure preclinical effectiveness of current Indian antivenoms. A pilot study, examining venom variation in Russell's vipers from different locations in India, and the ability of commercial antivenoms to neutralize all of the venoms has been designed and local researchers have begun collecting the venom samples (personal communication of Chellam R, Martin G, Whitaker R, et al, Madras Crocodile Bank Trust).
Proteomic tools for exploring the venom proteome
Snake venomics: Research on venoms has been continuously enhanced by advances in technology. Advances in instrumentation and high-throughput “omics” methodologies have fuelled an expansion of the scope of biological studies from simple biochemical analyses involving a few molecules at a time to the systematic study of whole genomes, transcriptomes, and proteomes. Specifically, the last decade has witnessed the development of techniques and strategies for assessing the toxin composition of snake venoms (“venomics”), directly (through proteomics-centred approaches)8,87,88 or indirectly (via high-throughput venom gland transcriptomics and bioinformatic analysis)89,90,91 in a relatively rapid and cost-effective manner. Bottom-up proteomics remains the workhorse for venomic analysis. Standard approaches include the tryptic digestion of the proteins into peptides, previously separated through single- or multi-dimensional protocols combining electrophoretic and chromatographic techniques87,88. These are then sequentially isolated in the mass spectrometer and sequenced by analysis of their fragmentation pattern. Parent proteins can then be identified by database searching strategies92. Proteomics-centred venomics requires homologous searchable databases for exploiting fully its analytical capabilities93,94,95,96. However, the slow progress in the generation of genomic and transcriptomic databases precludes the application of automated high-throughput proteomic approaches to study venoms. In addition, in the absence of reliable automatic de novo sequencing mass spectrometric algorithms, snake venom proteome investigation remains a laborious task. Recent contributions to the literature of venom proteomics emphasised that sample de-complexation before mass spectrometry represented the best approach for maximizing proteome coverage97. Fractionation via multi-dimensional techniques and strategies for the depletion of high abundance proteins, as well as the enrichment of extremely low abundance proteins to mine the venom proteome more effectively, have been investigated98,99. Clearly, if sufficient pre-mass spectrometric and mass spectrometric efforts are applied, thorough proteomic coverage can be achieved by an experienced researcher.
Our snake venomics approach7,8,9,100 (Fig. 7) includes an initial step of fractionation of the crude venom by reverse-phase (RP) HPLC followed by the initial characterization of each protein fraction by a combination of N-terminal sequencing, SDS-PAGE, and mass spectrometric determination of the molecular masses, and eventually the cysteine (-SH and S-S) content, of the isolated components. RP-HPLC allows the quantitative recovery of all venom components present in the molecular mass range of 7–150 kDa that can be separated by conventional 2DE. Further, the initial part of the acetonitrile gradient of the reverse-phase chromatography resolves peptides and small proteins (0.4–7 kDa), which would not be recovered from a 2DE separation. Monitoring the eluate at the absorption wavelength of the peptide bond (190-230 nm) represents a reliable method for quantifying the relative abundance of the different venom components in the reverse-phase chromatogram8,9. In-gel digestion, peptide mass fingerprinting, and de novo peptide sequencing by collision-induced dissociation (CID)-tandem mass spectrometry (MS/MS) coupled to database search by BLAST analysis allow the unambiguous assignment to known or unknown protein families of all venom toxin bands visualized in a Coomassie blue-stained SDS-polyacrylamide gel. Sequence coverage becomes an important issue to differentiate between the different classes of multi-domain proteins, such as the haemorrhagic Zn2+-dependent metalloproteinases (SVMP), whose pharmacological activities are modulated by their distinct domain structures101.
Fig. 7.
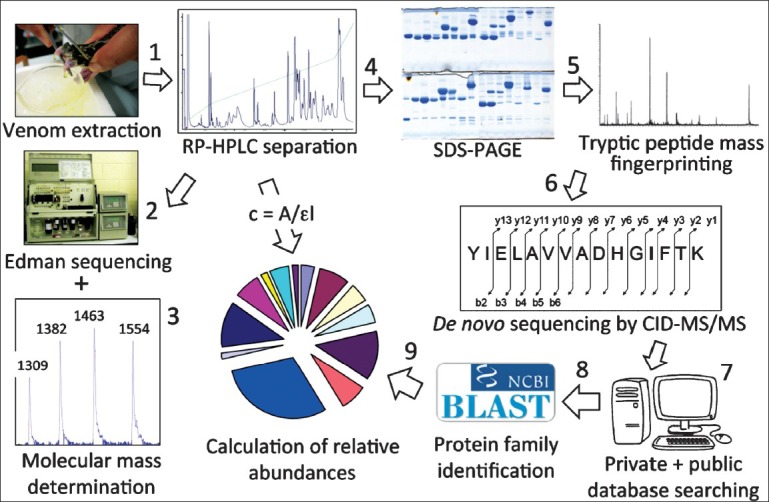
Venomics workflow. The bottom-up venomics approach includes an initial step of fractionation of the crude venom by reverse-phase (RP) HPLC (1) followed by the initial characterisation of each protein fraction by means of N-terminal sequencing (2), mass determination by MALDI- or ESI-MS (3), and SDS-PAGE (4). Tryptic peptides generated by in-gel digestion of electrophoretic bands are subjected to peptide mass fingerprinting (5), and de novo peptide sequencing by collision-induced dissociation (CID)-tandem mass spectrometry (MS/MS) (6). MS/MS-derived amino acid sequences are assigned to toxins/toxin families by database searching (7) and BLAST analysis (8). Structural assignments and the relative abundance of the venom's toxin families are integrated into a pie chart (9). Pie charts offer a simple way of displaying the toxin profile of a venom and represent an intuitive method for comparing quantitative data across different taxa.
Venomics analyses involving nearly 100 taxa have revealed that snake venoms are mixtures of pharmacologically active proteins and peptides synthesized from several tens to a few hundred of unique gene products89,90,91, which, however, belong to only a handful (<13) of toxin families8,9,10,87,88. A major goal of bottom-up venomics is to measure the full chemical space within which the pathological effects of snakebite envenoming are manifested. Another prime challenge of venomics is the formulation of rigorous biological hypotheses based on the information gathered by the discovery-based proteomics platform. In this respect, it is worth recalling the words of the prominent geneticist and evolutionary biologist, Theodosius G. Dobzhansky: “Nothing in biology makes sense except in the light of evolution”102. Understanding the molecular mechanisms and evolutionary trends that underlie inter- and intra-specific venom variation provides insights into snakebite pathology103,104,105,106,107,108,109,110. The occurrence of variability in the biochemical composition of venoms and in the symptomatology after envenomation by snakes from different geographical locations and age has long been appreciated by herpetologists and toxinologists38,111, although detailed comparative analyses were scarce in the literature before 2008. A specific question that we have assessed in recent years by venomics includes the occurrence and onset of geographical and ontogenetic variations in Bothrops asper107, B. atrox71, the Crotalus durissus complex105,112, C. tigris113, C. scutulatus106, Sistrurus miliarius barbouri114, and across genus Lachesis115. Emerging views of these, and other continuing studies indicate that (i) geographical variability is a general phenomenon and phenotypic variation across the population encompasses two distinct but overlapping causal factors: individual and ontogenetic variations. The occurrence of intraspecific individual allopatric variability highlights the concept that a species should be considered as a group of meta-populations; (ii) ontogenetic changes follow genus-specific trends71,107,112,113,114,115; and (iii) within a species range, paedomorphic and ontogenetic venom phenotypes often occur in geographically differentiated areas71,107,116,117.
Translational venomics: antivenomics
A robust knowledge of the toxin composition of venoms and the recognition of convergent and divergent trends along venom evolution is of fundamental importance in the selection of species and specimens for the manufacture of improved therapeutic antivenoms. This is particularly relevant for highly adaptable and widely distributed species, in which allopatric venom variability represents a source of diversity of the pathological effects of envenoming. To help antivenom design and to assess the range of possible clinical application of currently commercial or experimental mono- and polyspecific antivenoms, we have developed, since 2008, “antivenomics”118. Antivenomics is a proteomics-based protocol to quantify the extent of cross-reactivity of antivenoms against homologous and heterologous venoms110,119,120. First generation antivenomics consisted of the immuno-depletion of toxins upon incubation of whole venom with antivenom followed by the addition of a secondary antibody. Antigen-antibody complexes immuno-depleted from the reaction mixture contain the toxins against which antibodies in the antivenom are directed. By contrast, venom components that remain in the supernatant are those which failed to raise antibodies in the antivenom, or which triggered the production of low-affinity antibodies. These components can be easily identified by comparison of reverse-phase HPLC separation of the non-precipitated fraction with the HPLC pattern of the whole venom previously characterized by venomics. Second generation antivenomics119 is an affinity chromatography protocol to investigate the immuno-capturing ability of immobilized F(ab′)2120,121 or IgG123 antibody molecules followed by the proteomic characterization of the bound and the non-bound venom components (Fig. 8). Antivenomics provides qualitative and quantitative information on both the set of toxins bearing antivenom-recognized epitopes and those toxins exhibiting poor immunoreactivity. Its ease of use, reproducibility, sensitivity, and low cost suggest the possibility that antivenomics might supplant the use of immunoassays and Western blots, the most popular techniques for assessing the immunoreactivity of antibodies. However, the immuno-chemical detection of blotted proteins provides a Yes/No response: a given protein is either recognized or not by the antivenom, and it is essentially a non-quantitative technique.
Fig. 8.
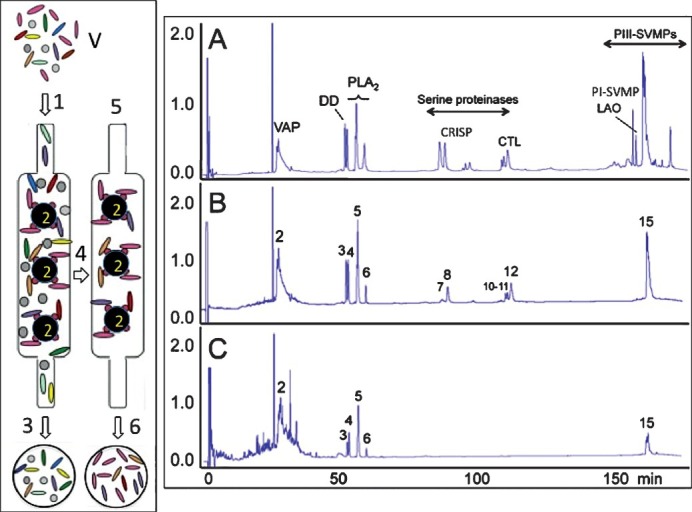
Antivenomics workflow. Left panel, whole venome (V) is applied to an immunoaffinity column (1) packed with antivenom antibodies immobilized onto Sepharose beads (2). After eluting the non-binding fraction (3), the column is thoroughly washed with equilibration buffer (4) and the immunocaptured proteins eluted (6) with elution buffer (5). Right display, panels A-C show, respectively, reverse-phase chromatographic separations of the components of whole venom, the fraction retained and subsequently recovered from the antivenom affinity matrix, and the non-immunocaptured venom fraction. Proteins within the immunocaptured and the flow-through fractions are identified by the venomics approach schematically displayed in Fig. 7.
Despite its recent introduction, the usefulness and validity of antivenomics to complement the in vivo standard preclinical assays of neutralization of lethality and toxic activities by antivenoms has been extensively demonstrated68,105,120,121,122,123. Antivenomics thus provides a ground for rationalizing the paraspecificity of antivenoms thereby expanding their potential clinical range.
Current venomics analyses of Indian snakes of medical relevance
The common krait (Bungarus caeruleus) (Fig. 1a), the Indian cobra (Naja naja) (Fig. 2a), Russell's viper (Daboia russelii) (Fig. 3a), and the saw-scaled viper (Echis carinatus) (Fig. 4a), collectively called the “Big 4”, are considered to be the major endemic venomous snakes responsible for fatality in the Indian subcontinent124. The efficient clinical management of snakebites requires timely administration of appropriate antivenoms that neutralize the numerous toxins contained in the snake venom. Antivenoms against the “big four”, produced by several Indian firms http://apps.who.int/bloodproducts/snakeantivenoms/database/default.htm) are available. However, detailed studies on the immunoreactive properties of these polyvalent antivenoms have not been performed. To complicate matters, the emergence of the hump-nosed pit viper (Hypnale hypnale) (Fig. 5a) and other species as snakes of medical significance in south-western India36,124, has rendered the “Big 4” obsolete and incomplete.
Snake venomics of the eastern Russell's viper (Daboia siamensis) and its relation to pharmacological activities have been addressed by Risch and coworkers125. D. siamensis is found in Burma, Thailand, Cambodia, parts of China, Taiwan and Indonesia126,127. The clinical manifestations of Russell's viper bite vary from one region to another within Southeast Asia, including India43,128,129,130. Some studies on the biochemical properties of Russell's viper venom from eastern India that correlate with the distinct clinico-pathological manifestation in Russell's viper bites have been reported130. In particular, non-enzymatic peptides present in Daboia russelii (Eastern region) venom, and which render commercial polyvalent antivenom ineffective, have been detected41. The identity of these venom components has not been determined. In addition, there is a dearth of knowledge about the qualitative and quantitative protein composition of D. russelii venom across the Indian peninsula.
The Indian cobra, Naja naja, and the common krait, Bungarus caeruleus (Elapidae) cause severe neuromuscular paralysis as a result of the blockade of neuromuscular transmission. Toxins from cobra venom predominantly act postsynaptically, whereas those from krait venom mainly act presynaptically131. The common krait is regarded as the most dangerous species of venomous snake in the Indian subcontinent. Chemical and pharmacological characterization of toxic polypeptides from the venom of B. caeruleus have been long reported131,132. The major neurotoxic component of krait venom is β-bungarotoxin, a heterodimeric basic PLA2 which has high affinity for presynaptic neuromuscular receptors133. However, a global picture of the toxin composition and geographical variability of B. caeruleus venom is lacking. Similarly, although there are 32 UniProtKB/TrEMBL entries of Indian cobra venom toxins, the relative contribution of these proteins to the whole venom remains undisclosed. Furthermore, regional variation in venom composition and toxicity has also been described for Indian N. naja134,135: eastern N. naja venom is neurotoxic (being PLA2 molecules largely responsible for the toxicity) and procoagulant (in vitro, not clinically), whereas western region venom is myotoxic and anticoagulant (in vitro, not clinically)132. Region-specific neutralization of Indian cobra (Naja naja) venom by polyclonal antibody raised against the eastern venom has been published42. However, the identity of the geographically variable molecules and the immunoreactivity pattern of available antivenoms towards regional venoms deserve future studies.
A large proportion of the 71 E. carinatus venom protein sequences available in the UniProtKB/TrEMBL database http://www.expasy.org) has been derived by venom gland transcriptome surveys using cDNA libraries constructed from ten wild-caught specimens of E. carinatus sochureki from Sharjah, UAE136,137. Their expression and relative abundance in the venom have not been addressed. The phylogeny of the medically important, and taxonomically unresolved, viper genus Echis has been investigated by Pook and colleagues138. The results showed that the populations of the genus fall into four main clades: the Echis carinatus, E. coloratus, E. ocellatus and E. pyramidum groups. The E. carinatus group includes haplotypes from India (E. c. carinatus), Pakistan and the northeastern Arabian Peninsula (E. c. sochureki) that form a cluster without clear phylogeographical structure. More basal sister haplotypes originate from western and southern India. Whether E. c. sochureki should be recognised as distinct from southern Indian E. c. carinatus at species or subspecies level remains an open question. Although the low divergence between northwestern Indian, Pakistani, northeastern Arabian and Central Asian E. carinatus populations indicated that these haplotypes first diverged approximately 0.9 Mya138, the link between the transcriptomic data gathered from the E. c. sochureki specimens from Sharjah, UAE and the venom proteome composition of the Indian populations of E. c. carinatus (South Indian saw-scaled viper; peninsular India) remains obscure.
Snakes of the genus Hypnale are of local medical importance in Sri Lanka and southwestern India and pathological effects of the venom of Sri Lankan Hypnale in animals was the subject of some early studies139. Recently, Maduwage and colleagues140 have investigated the biochemical and pharmacological properties of the venoms from the three Hypnale species in Sri Lanka. Cytotoxicity appeared to be the most potent effect of these venoms, which had similar reverse-phase chromatographic toxin profiles. Indian polyvalent antivenom raised against Naja naja, Daboia russellii, Bungarus caeruleus and Echis carinatus did not neutralize the venom effects140. Kumar and Sabitha141 have also reported that available Indian anti-snake venom in central Kerala does not cover envenomation by H. hypnale. Cross-neutralization in vitro and in a rodent model of H. hypnale venom by polyvalent and monovalent antivenoms against Malayan pit viper (Calloselasma rhodostoma) venom, a sister taxon of H. hypnale142, has been documented143. Establishing the molecular basis for this finding requires detailed venomics and antivenomics investigation.
Remarks and perspectives
An essential defining characteristic for a biological hypothesis is its ability to generate testable predictions. Developments in venomics and antivenomics are likely to revolutionize the design and preclinical assessment of antivenoms by bringing to bear technologies that enable us to peel back the layers of imprecision that exist in current methodologies, and get to heart of the problem by being able to design and test new antivenom preparations developed using these dynamic platforms. Antivenomics represents a knowledge-based approach to help to design improved venom-based immunogen mixtures, and to predict paraspecific neutralization by antivenom preparations to the level of species-specific toxins. In India, antivenom production began at the Central Research Institute, Kasauli during the 1920s. There are now several producers manufacturing Indian polyvalent antivenoms, all of which follow a standard set out in the Indian Pharmacopoeia requiring products to neutralize 0.6 mg/ml Naja naja venom, 0.6 mg/ml Daboia russelii venom, 0.45 mg/ml Bungarus caeruleus venom and 0.45 mg/ml Echis carinatus venom. This potency is much lower than the standards that applied during the early 1950s when Indian antivenoms were required to neutralize 4.0 mg/ml Daboia russelii venom and 2.0 mg/ml Naja naja venom144. This low potency demands that patients be given initial doses of at least 10 vials. In some cases, considerably larger doses are administered. A study in Chandigarh found that patients received an average of 51.2 viahls (512 ml) of Indian polyvalent antivenom for bites by Naja sp., and Bungarus sp., and an average of 32 vials (320 ml) to treat Daboia russelii or Echis carinatus envenoming. At the extreme, some patients received as many as 130 vials (1.3 litres) for bites by Russell's vipers; while up to 190 vials (1.9 litres) were administered after a neurotoxic snakebite145. Such high doses of immunoglobulin carry the risk of inducing severe dose-related adverse reactions, including anaphylaxis146,147,148. Clearly, new strategies are needed to meet the challenge of neutralizing large injected doses of venom without compromising patient safety. Production of higher potency antivenoms will be required. Some of the snake species that occur in India produce large amounts of venom; Asian cobras (Naja sp.) have yields ranging from 58-742 mg149, kraits can produce 8-60 mg150 and Russell's vipers venom yields a range from 21-268 mg151. A new antivenom, recently produced in Costa Rica for the treatment of Papuan taipan (Oxyuranus scutellatus) envenoming in Papua New Guinea, has addressed the problem of balancing antivenom potency and dose against the average venom yield (120 mg) of the species involved. A single 40 ml vial of antivenom, with a potency standard of at least 3 mg/ml and total protein of just 4.59±0.9 g/dl, should allow an initial treatment dose of 1 vial to neutralize at least 120 mg O. scutellatus venom69. Modern proteomic and antivenomic methods have been employed in the characterization of this venom and in the preclinical evaluation of this new antivenom. Such an approach could easily be used to design and produce a high potency polyvalent antivenom for use in India. The new antivenom could be evaluated preclinically and then subjected to clinical trial. This evidence might persuade Indian regulators to adopt new specifications for antivenom in the Indian Pharmacopoeia.
Acknowledgment
Funding for the projects (to JJC) was provided by grant BFU2007-61563 and BFU2010-17373 from the Ministerios de Educación y Ciencia and Ciencia é Innovación, Madrid; CRUSA-CSIC (2007CR0004 and 2009CR0021); and CYTED (206AC0281). Travelling between Spain and Costa Rica was financed by Acciones Integradas 2006CR0010 between CSIC and the University of Costa Rica (UCR).
References
- 1.World Health Organisation. Working to overcome the global impact of neglected tropical diseases: First WHO report on neglected tropical diseases. In: Crompton DWT, Peters P, editors. Geneva: Department for the Control of Neglected Tropical Diseases, WHO; 2010. p. 172. [Google Scholar]
- 2. http://www.who.int/neglected_diseases/EB132_R7_en.pdf .
- 3.World Health Organisation. Sustaining the drive to overcome the global impact of neglected tropical diseases: Second WHO report on neglected tropical diseases. In: Crompton DWT, editor. WHO, Geneva: Department for the Control of Neglected Tropical Diseases; 2013. p. 172. [Google Scholar]
- 4.Harrison RA, Hargreaves A, Wagstaff SC, Faragher B, Lalloo DG. Snake envenoming: a disease of poverty. PLoS Negl Trop Dis. 2009;3:e569. doi: 10.1371/journal.pntd.0000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams DJ, Gutiérrez JM, Harrison R, Warrell DA, White J, Winkel KD, et al. The Global Snake Bite Initiative: an antidote for snake bite. Lancet. 2010;375:89–91. doi: 10.1016/S0140-6736(09)61159-4. [DOI] [PubMed] [Google Scholar]
- 6.Gutierrez JM, Williams DJ, Fan HW, Warrell DA. Snakebite envenoming from a global perspective: Towards an integrated approach. Toxicon. 2010;56:1223–35. doi: 10.1016/j.toxicon.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 7.Williams DJ, Gutiérrez JM, Calvete JJ, Wüster W, Ratanabanangkoon K, Paiva O, et al. Ending the drought: new strategies for improving the flow of affordable, effective antivenoms in Asia and Africa. J Proteomics. 2011;74:1735–67. doi: 10.1016/j.jprot.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 8.Calvete JJ. Proteomic tools against the neglected pathology of snake bite envenoming. Expert Rev Proteomics. 2011;8:739–58. doi: 10.1586/epr.11.61. [DOI] [PubMed] [Google Scholar]
- 9.Calvete JJ, Juárez P, Sanz L. Snake venomics. Strategy and applications. J Mass Spectrom. 2007;42:1405–14. doi: 10.1002/jms.1242. [DOI] [PubMed] [Google Scholar]
- 10.Calvete JJ. Snake venomics, antivenomics, and venom phenotyping: ménage à trois of proteomic tools aimed at understanding the biodiversity of venoms. In: Kini RM, Markland FS, McLane MA, Morita T, editors. Toxins and hemostasis: from bench to bedside. Dordrecht (The Netherlands): Springer; 2010. pp. 45–72. [Google Scholar]
- 11.Fayrer J. Destruction of life in India by poisonous snakes. Nature. 1882;27:205–8. [Google Scholar]
- 12.Nicholson E. Statistics of deaths from snake-bite. BMJ. 1883;2:448–9. [Google Scholar]
- 13.The mortality from snakes and wild animals in India. BMJ. 1884;2:1088. [Google Scholar]
- 14.Snakes and wild animals in India. BMJ. 1913;1:964. [Google Scholar]
- 15.India: Reports from Bombay. Deaths from Injuries in Bombay Presidency, 1892-1903.Also from Dysentery and Diarrhea during the Same Period. Public Health Rep. 1904;19:2529–30. [Google Scholar]
- 16.India: Indian Sanitary Report. BMJ. 1927;1:538–9. [Google Scholar]
- 17.Geneva: WHO; 2007. World Health Organization. Rabies and envenoming a neglected public health issue. Report of a consultative meeting. Available from: http://www.who.int/bloodproducts/animal_sera/Rabies.pdf . [Google Scholar]
- 18.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohapatra B, Warrell DA, Suraweera W, Bhatia P, Dhingra N, Jotkar RM, et al. Million Death Study Collaborators. Snakebite mortality in India: A nationally representative mortality survey. PLoS Negl Trop Dis. 2011;5:e1018. doi: 10.1371/journal.pntd.0001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swaroop S, Grab B. Snakebite mortality in the world. Bull World Health Organ. 1954;10:35–76. [PMC free article] [PubMed] [Google Scholar]
- 21.Chippaux JP. Snake-bites: appraisal of the global situation. Bull World Health Organ. 1998;76:515–24. [PMC free article] [PubMed] [Google Scholar]
- 22.Kasturiratne A, Wickremasinghe AR, de Silva N, Gunawardena NK, Pathmeswaran A, Premaratna R, et al. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5:e218. doi: 10.1371/journal.pmed.0050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hati AK, Mandal M, De MK, Mukherjee H, Hati RN. Epidemiology of snake bite in the district of Burdwan, West Bengal. J Indian Med Assoc. 1992;90:145–7. [PubMed] [Google Scholar]
- 24.Sharma SK, Chappuis F, Jha N, Bovier PA, Loutan L, Koirala S, et al. Impact of snake bites and determinants of fatal outcomes in southeastern Nepal. Am J Trop Med Hyg. 2004;71:234–8. [PubMed] [Google Scholar]
- 25.Sawai Y. Snakebites in the past and present in India. The Snake. 1975;7:17–22. [Google Scholar]
- 26.Saini RK, Singh S, Sharma S, Rampal V, Manhas AS, Gupta VK, et al. Snake bite poisoning presenting as early morning neuroparalytic syndrome in jhuggi dwellers. J Assoc Physicians India. 1986;34:415–7. [PubMed] [Google Scholar]
- 27.Rahman R, Faiz MA, Selim S, Rahman B, Basher A, Jones A, et al. Annual incidence of snake bite in rural Bangladesh. PLoS Negl Trop Dis. 2010;4:e860. doi: 10.1371/journal.pntd.0000860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chappuis F, Sharma SK, Jha N, Loutan L, Bovier PA. Protection against snake bites by sleeping under a bed net in southeastern Nepal. Am J Trop Med Hyg. 2007;77:197–9. [PubMed] [Google Scholar]
- 29.Sharma SK, Bovier P, Jha N, Alirol E, Loutan L, Chappuis F. Effectiveness of Rapid Transport of Victims and Community Health Education on Snake Bite Fatalities in Rural Nepal. Am J Trop Med Hyg. 2013 doi: 10.4269/ajtmh.12-0750. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warrell DA. Guidelines for the management of snakebites. WHO Regional Office for South-East Asia. 2010. Available from: http://www.searo.who.int/entity/emergencies/documents/9789290223774/en/index.html .
- 31.Whitaker R, Captain A. Chennai: Draco Books; 2004. Snakes of India: The Field Guide; p. 495. [Google Scholar]
- 32.Warrell DA. Snake bite: A neglected problem in twenty-first century India. Natl Med J India. 2011;24:321–4. [PubMed] [Google Scholar]
- 33.Pillai LV, Ambike D, Husainy S, Khaire A, Captain A, Kuch U, et al. Severe Neurotoxic Envenoming and Cardiac Complications after the Bite of a ‘Sind Krait’ (Bungarus cf. sindanus) in Maharashtra, India. Trop Med Health. 2012;40:103–8. doi: 10.2149/tmh.2012-08c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faiz A, Ghose A, Ahsan F, Rahman R, Amin R, Hassan MU, et al. The greater black krait (Bungarus niger), a newly recognized cause of neuro-myotoxic snake bite envenoming in Bangladesh. Brain. 2010;133:3181–93. doi: 10.1093/brain/awq265. [DOI] [PubMed] [Google Scholar]
- 35.Sharma LR, Lal V, Simpson ID. Snakes of medical significance in India: the first reported case of envenoming by the Levantine viper (Macrovipera lebetina) Wilderness Environ Med. 2008;19:195–8. doi: 10.1580/07-WEME-CR-175.1. [DOI] [PubMed] [Google Scholar]
- 36.Joseph JK, Simpson ID, Menon NC, Jose MP, Kulkarni KJ, Raghavendra GB, et al. First authenticated cases of life threatening envenoming by the hump-nosed pit viper (Hypnale hypnale) in India. Trans R Soc Trop Med Hyg. 2007;101:85–90. doi: 10.1016/j.trstmh.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Whitaker R. Pit viper [Trimeresurus macrolepis (Beddome)] bites at a South Indian tea estate. J Bombay Nat Hist Soc. 1973;70:207–8. [Google Scholar]
- 38.Warrell DA. Geographical and intraspecies variation in the clinical manifestations of envenoming by snakes. In: Thorpe RS, Wüster W, Malhotra A, editors. Venomous snakes. Ecology, evolution and snakebite. Oxford: Clarendon Press; 1987. pp. 189–203. [Google Scholar]
- 39.Warrell DA. Sea snake bites in the Asia-Pacific region. In: Gopalakrishnakone P, editor. Sea Snake Toxinology. Singapore: Singapore University Press; 1994. pp. 1–36. [Google Scholar]
- 40.Gutiérrez JM, Lomonte B, León G, Alape-Girón A, Flores-Diaz M, Sanz L, et al. Snake venomics and antivenomics: Proteomic tools in the design and control of antivenoms for the treatment of snakebite envenoming. J Proteomics. 2009;72:165–82. doi: 10.1016/j.jprot.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 41.Kumar AV, Gowda TV. Novel non-enzymatic toxic peptide of Daboia russelii (Eastern region) venom render commercial polyvalent antivenom ineffective. Toxicon. 2006;47:398–408. doi: 10.1016/j.toxicon.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Shashidharamurthy R, Kemparaju K. Region-specific neutralization of Indian cobra (Naja naja) venom by polyclonal antibody raised against the eastern regional venom: A comparative study of the venoms from three different geographical distributions. Int Immunopharmacol. 2007;7:61–9. doi: 10.1016/j.intimp.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 43.Prasad NB, Uma B, Bhatt SK, Gowda VT. Comparative characterisation of Russell's viper (Daboia/Vipera russelli) venoms from different regions of the Indian peninsula. Biochim Biophys Acta. 1999;1428:121–36. doi: 10.1016/s0304-4165(99)00053-7. [DOI] [PubMed] [Google Scholar]
- 44.Kochar DK, Tanwar PD, Norris RL, Sabir M, Nayak KC, Agrawal TD, et al. Rediscovery of severe saw-scaled viper (Echis sochureki) envenoming in the Thar desert region of Rajasthan, India. Wilderness Environ Med. 2007;18:75–85. doi: 10.1580/06-WEME-OR-078R.1. [DOI] [PubMed] [Google Scholar]
- 45.Leong PK, Tan NH, Fung SY, Sim SM. Cross neutralisation of Southeast Asian cobra and krait venoms by Indian polyvalent antivenoms. Trans R Soc Trop Med Hyg. 2012;106:731–7. doi: 10.1016/j.trstmh.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 46.World Health Organization. Geneva: World Health Organization; 2010. WHO Guidelines for the production, control and regulation of snake antivenom immunoglobulins. Available from: www.who.int/bloodproducts/snake_antivenoms/snakeantivenomguide/en . [Google Scholar]
- 47.World Health Organization. Geneva: WHO Offset Publication; 1981. Progress in the characterization of venoms and standardization of antivenoms. No. 58. [PubMed] [Google Scholar]
- 48.Finney DJ. Cambridge: Cambridge University Press; 1971. Probit Analysis. [Google Scholar]
- 49.Theakston RDG. Characterization of venoms and standardization of antivenoms. In: Harris JB, editor. Natural Toxins. Animal, Plant and Microbial. Oxford: Clarendon Press; 1986. pp. 287–303. [Google Scholar]
- 50.Gutiérrez JM, Rojas G, Bogarín G, Lomonte B. Evaluation of the neutralizing ability of antivenoms for the treatment of snake bite envenoming in Central America. In: Bon C, Goyffon M, editors. Envenomings and their treatments. Lyon: Fondation Marcel Mérieux; 1996. pp. 223–31. [Google Scholar]
- 51.Theakston RDG, Reid HA. Development of simple standard assay procedures for the characterization of snake venom. Bull World Health Organ. 1983;61:949–56. [PMC free article] [PubMed] [Google Scholar]
- 52.Gutiérrez JM, Arroyo O, Bolaños R. Myonecrosis, hemorrhage and edema induced by the venom of Bothrops asper in white mice. Toxicon. 1980;18:603–10. doi: 10.1016/0041-0101(80)90087-2. [DOI] [PubMed] [Google Scholar]
- 53.Gené JA, Roy A, Rojas G, Gutiérrez JM, Cerdas L. Comparative study on coagulant, defibrinating, fibrinolytic and fibrinogenolytic activities of Costa Rican crotaline snake venoms and their neutralization by a polyvalent antivenom. Toxicon. 1989;27:841–8. doi: 10.1016/0041-0101(89)90096-2. [DOI] [PubMed] [Google Scholar]
- 54.Kondo H, Kondo S, Ikezawa I, Murata R, Ohsaka A. Studies of the quantitative method for the determination of hemorrhagic activity of Habu snake venom. Japan J Med Sci Biol. 1960;13:43–51. doi: 10.7883/yoken1952.13.43. [DOI] [PubMed] [Google Scholar]
- 55.Gutiérrez JM, Gené JA, Rojas G, Cerdas L. Neutralization of proteolytic and hemorrhagic activities of Costa Rican snake venoms by a polyvalent antivenom. Toxicon. 1985;23:887–93. doi: 10.1016/0041-0101(85)90380-0. [DOI] [PubMed] [Google Scholar]
- 56.Escalante T, Núñez J, Moura da Silva AM, Rucavado A, Theakston RDG, Gutiérrez JM, et al. Pulmonary hemorrhage induced by jararhagin, a metalloproteinase from Bothropsjararaca venom. Toxicol Appl Pharmacol. 2003;193:17–28. doi: 10.1016/s0041-008x(03)00337-5. [DOI] [PubMed] [Google Scholar]
- 57.Teixeira CFP, Zamunér SR, Zuliani JP, Fernandes CM, Cruz-Hofling MA, Fenandes I, et al. Neutrophils do not contribute to local tissue damage, but play a key role in skeletal muscle regeneration, in mice injected with Bothrops asper snake venom. Muscle Nerve. 2003;28:449–59. doi: 10.1002/mus.10453. [DOI] [PubMed] [Google Scholar]
- 58.Rojas E, Quesada L, Arce V, Lomonte B, Rojas G, Gutiérrez JM, et al. Neutralization of four Peruvian Bothrops sp snake venoms by polyvalent antivenoms produced in Perú and Costa Rica: preclinical assessment. Acta Trop. 2005;93:85–95. doi: 10.1016/j.actatropica.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 59.Gutiérrez JM, Rojas G, Lomonte B, Gené JA, Cerdas L. Comparative study of the edema-forming activity of Costa Rican snake venoms and its neutralization by a polyvalent antivenom. Comp Biochem Physiol. 1986;85C:171–5. doi: 10.1016/0742-8413(86)90069-1. [DOI] [PubMed] [Google Scholar]
- 60.Barfaraz A, Harvey AL. The use of the chick biventer cervicis preparation to assess the protective activity of six international reference antivenoms on the neuromuscular effects of snake venoms in vitro. Toxicon. 1994;32:267–72. doi: 10.1016/0041-0101(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 61.Rucavado A, Soto M, Escalante T, Loría GD, Arni RD, Gutiérrez JM, et al. Thrombocytopenia and platelet hypoaggregation induced by Bothrops asper snake venom. Toxins involved and their contribution to metalloproteinase-induced pulmonary hemorrhage. ThrombHaemost. 2005;94:123–31. doi: 10.1160/TH05-02-0112. [DOI] [PubMed] [Google Scholar]
- 62.Gutiérrez J, Sanz L, Escolano J, Fernandez J, Lomonte B, Angulo Y, et al. Snake venomics of the Lesser Antillean pit vipers Bothrops caribbaeus and Bothrops lanceolatus. Correlation with toxicological activities and immunoreactivity of a heterologous antivenom. J Proteome Res. 2008;7:4396–408. doi: 10.1021/pr8003826. [DOI] [PubMed] [Google Scholar]
- 63.Gené JA, Gómez M, Gutiérrez JM, Cerdas L. Neutralization of hyaluronidase and indirect hemolytic activities of Costa Rican snake venoms by a polyvalent antivenom. Toxicon. 1985;23:1015–8. doi: 10.1016/0041-0101(85)90397-6. [DOI] [PubMed] [Google Scholar]
- 64.Gutiérrez JM, Rojas G, Lomonte B, Gené JA, Chaves F, Alvarado J, et al. Standardization of assays for testing the neutralizing ability of antivenoms. Toxicon. 1990;28:1127–9. doi: 10.1016/0041-0101(90)90110-s. [DOI] [PubMed] [Google Scholar]
- 65.Warrell DA. Snake bite. Lancet. 2010;375:77–88. doi: 10.1016/S0140-6736(09)61754-2. [DOI] [PubMed] [Google Scholar]
- 66.Gutiérrez JM, Solano G, Pla D, Herrera M, Segura A, Villalta M, et al. Assessing the preclinical efficacy of antivenoms: From the lethality neutralization assay to antivenomics. Toxicon. 2013;64:60–9. doi: 10.1016/j.toxicon.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 67.Warrell DA. Clinical toxicology of snakebite in Africa and the Middle East/Arabian Peninsula. In: Meier J, White J, editors. Handbook of Clinical Toxicology of Animal Venoms and Poisons. Boca Raton (Florida): CRC Press; 1995. pp. 433–92. [Google Scholar]
- 68.Petras D, Sanz L, Segura A, Herrera M, Villalta M, Solano D, et al. Snake venomics of African spitting cobras: toxin composition and assessment of congeneric cross-reactivity of the pan-African EchiTAb-Plus-ICP® antivenom by antivenomics and neutralization approaches. J Proteome Res. 2011;10:1266–80. doi: 10.1021/pr101040f. [DOI] [PubMed] [Google Scholar]
- 69.Vargas M, Segura A, Herrera M, Villalta M, Estrada R, Cerdas M, et al. Preclinical evaluation of caprylic acid-fractionated IgG antivenom for the treatment of Taipan (Oxyuranus scutellatus) envenoming in Papua New Guinea. PLoS Negl Trop Dis. 2011;5:e1144. doi: 10.1371/journal.pntd.0001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Currier RB, Harrison RA, Rowley PD, Laing GD, Wagstaff SC. Intra-specific variation in venom of the African puff adder (Bitis arietans): Differential expression and activity of snake venom metalloproteinases (SVMPs) Toxicon. 2010;55:864–73. doi: 10.1016/j.toxicon.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 71.Calvete JJ, Sanz L, Pérez A, Borges A, Vargas AM, Lomonte B, et al. Snake population venomics and antivenomics of Bothrops atrox: paedomorphism along its transamazonian dispersal and implications of geographic venom variability on snakebite management. J Proteomics. 2011;74:510–27. doi: 10.1016/j.jprot.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 72.Rial A, Morais V, Rossi S, Massaldi H. A new ELISA or determination of potency in snake antivenoms. Toxicon. 2006;4:462–6. doi: 10.1016/j.toxicon.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 73.Maria WS, Pacheco BG, Barbosa CF, Velarde DT, Chavez-Olórtegui C. Determination of the neutralizing potency of horse antibothropic and anticrotalic antivenoms in blood samples collected on filter paper. Toxicon. 2001;39:1607–9. doi: 10.1016/s0041-0101(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 74.Theakston RDG, Reid HA. Enzyme-linked immunosorbent assay (ELISA) in assessing antivenom potency. Toxicon. 1979;17:511–5. doi: 10.1016/0041-0101(79)90284-8. [DOI] [PubMed] [Google Scholar]
- 75.Sells P. Animal experimentation in snake venom research and in vitro alternatives. Toxicon. 2003;42:115–33. doi: 10.1016/s0041-0101(03)00125-9. [DOI] [PubMed] [Google Scholar]
- 76.de Roodt A, Dolab JA, Fernández T, Segre L, Hajos EE. Cross-reactivity and heterologous neutralisation of crotaline antivenoms used in Argentina. Toxicon. 1998;36:1025–38. doi: 10.1016/s0041-0101(97)00111-6. [DOI] [PubMed] [Google Scholar]
- 77.Bogarín G, Morais JF, Yamaguchi IK, Stephano MA, Marcelino JR, Nishikawa AK, et al. Neutralization of crotaline snake venoms from Central and South America by antivenoms produced in Brazil and Costa Rica. Toxicon. 2000;38:1429–41. doi: 10.1016/s0041-0101(99)00236-6. [DOI] [PubMed] [Google Scholar]
- 78.Camey KU, Velarde DT, Sánchez EF. Pharmacological characterization and neutralization of the venoms used in the production of Bothropic antivenom in Brazil. Toxicon. 2002;40:501–9. doi: 10.1016/s0041-0101(01)00245-8. [DOI] [PubMed] [Google Scholar]
- 79.Otero R, Núñez V, Osorio RG, Gutiérrez JM, Giraldo CA, Posada LE, et al. Ability of six Latin American antivenoms to neutralize the venom of mapaná equis (Bothrops atrox) from Antioquia and Chocó (Colombia) Toxicon. 1995;33:809–15. doi: 10.1016/0041-0101(95)00009-b. [DOI] [PubMed] [Google Scholar]
- 80.Theakston RDG, Laing GD, Fielding CM, Lascano AF, Touzet JM, Vallejo F, et al. Treatment of snake bites by Bothrops species and Lachesis muta in Ecuador: laboratory screening of candidate antivenoms. Trans R Soc Trop Med Hyg. 1995;89:550–4. doi: 10.1016/0035-9203(95)90105-1. [DOI] [PubMed] [Google Scholar]
- 81.Saravia P, Rojas E, Escalante T, Arce V, Chaves E, Velásquez R, et al. The venom of Bothropsasper from Guatemala: toxic activities and neutralization by antivenoms. Toxicon. 2001;329:401–5. doi: 10.1016/s0041-0101(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 82.Segura A, Castillo MC, Núñez V, Yarlequé A, Goncalves LRC, Villalta M, et al. Preclinical assessment of the neutralizing capacity of antivenoms produced in six Latin American countries against medically-relevant Bothrops snake venoms. Toxicon. 2010;56:980–9. doi: 10.1016/j.toxicon.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 83.Laing GD, Renjifo JM, Ruiz F, Harrison RA, Nasidi A, Gutiérrez JM, et al. A new Pan African polyspecific antivenom developed in response to the antivenom crisis in Africa. Toxicon. 2003;42:35–41. doi: 10.1016/s0041-0101(03)00098-9. [DOI] [PubMed] [Google Scholar]
- 84.Segura A, Villalta M, Herrera M, León G, Harrison R, Durfa N, et al. Preclinical assessment of the efficacy of a new antivenom (EchiTAb-Plus-ICP®) for the treatment of viper envenoming in sub-Saharan Africa. Toxicon. 2010;55:369–74. doi: 10.1016/j.toxicon.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 85.Ramos-Cerrillo B, de Roodt AR, Chippaux J-P, Olguín L, Casasola A, Guzmán G, et al. Characterization of a new polyvalent antivenom (Antivipmyn Africa) against African vipers and elapids. Toxicon. 2008;52:881–8. doi: 10.1016/j.toxicon.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 86.Casewell NR, Cook DA, Wagstaff SC, Nasidi A, Durfa N, Wüster W, et al. Pre-clinical assays predict pan-African Echis viper efficacy for a species-specific antivenom. PLoS Negl Trop Dis. 2010;4:e851. doi: 10.1371/journal.pntd.0000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Georgieva D, Arni RK, Betzel C. Proteome analysis of snake venom toxins: pharmacological insights. Expert Rev Proteomics. 2008;5:787–97. doi: 10.1586/14789450.5.6.787. [DOI] [PubMed] [Google Scholar]
- 88.Fox JW, Serrano SMT. Exploring snake venom proteomes: multifaceted analyses for complex toxin mixtures. Proteomics. 2008;8:909–20. doi: 10.1002/pmic.200700777. [DOI] [PubMed] [Google Scholar]
- 89.Durban J, Juárez P, Angulo Y, Lomonte B, Flores-Díaz M, Alape-Girón A, et al. Profiling the venom gland transcriptomes of Costa Rican snakes by 454 pyrosequencing. BMC Genomics. 2011;12:259. doi: 10.1186/1471-2164-12-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rokyta DR, Wray KP, Lemmon AR, Lemmon EM, Caudle SB. A high-throughput venom-gland transcriptome for the Eastern Diamondback Rattlesnake (Crotalusadamanteus) and evidence for pervasive positive selection across toxin classes. Toxicon. 2011;57:657–71. doi: 10.1016/j.toxicon.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 91.Rokyta DR, Lemmon AR, Margres MJ, Aronow K. The venom-gland transcriptome of the eastern diamondback rattlesnake (Crotalus adamanteus) BMC Genomics. 2012;13:312. doi: 10.1186/1471-2164-13-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cottrell JS. Protein identification using MS/MS data. J Proteomics. 2011;74:1842–51. doi: 10.1016/j.jprot.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 93.Wagstaff SC, Sanz L, Juárez P, Harrison RA, Calvete JJ. Combined snake venomics and venom gland transcriptomic analysis of the ocellated carpet viper, Echis ocellatus. J Proteomics. 2009;71:609–23. doi: 10.1016/j.jprot.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 94.OmPraba G, Chapeaurouge A, Doley R, Devi KR, Padmanaban P, Venkatraman C, et al. Identification of a novel family of snake venom proteins veficolins from Cerberus rynchops using a venom gland transcriptomics and proteomics approach. J Proteome Res. 2010;9:1882–93. doi: 10.1021/pr901044x. [DOI] [PubMed] [Google Scholar]
- 95.Corrêa-Netto C, Junqueira-de-Azevedo IL, Silva DA, Ho PL, Leitão-de-Araújo M, Alver ML, et al. Snake venomics and venom gland transcriptomic analysis of Brazilian coral snakes, Micrurus altirostris and M. corallinus. J Proteomics. 2011;74:1795–809. doi: 10.1016/j.jprot.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 96.Rodrigues RS, Boldrini-França J, Fonseca FP, de la Torre P, Henrique-Silva F, Sanz L, et al. Combined snake venomics and venom gland transcriptomic analysis of Bothropoidespauloensis. J Proteomics. 2012;75:2707–20. doi: 10.1016/j.jprot.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 97.Fox JW, Ma L, Nelson K, Sherman NE, Serrano SMT. Comparison of indirect and direct approaches using ion-trap and Fourier transform ion cyclotron resonance mass spectrometry for exploring viperid venom proteomes. Toxicon. 2006;47:700–14. doi: 10.1016/j.toxicon.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 98.Calvete JJ, Fasoli E, Sanz L, Boschetti E, Righetti PG. Exploring the venom proteome of the western diamondback rattlesnake, Crotalus atrox, via snake venomics and combinatorial peptide ligand library approaches. J Proteome Res. 2009;8:3055–67. doi: 10.1021/pr900249q. [DOI] [PubMed] [Google Scholar]
- 99.Fasoli E, Sanz L, Wagstaff S, Harrison RA, Righetti PG, Calvete JJ, et al. Exploring the venom proteome of the African puff adder, Bitis arietans, using a combinatorial peptide ligand library approach at different pHs. J Proteomics. 2010;73:932–42. doi: 10.1016/j.jprot.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 100.Calvete JJ, Sanz L, Angulo Y, Lomonte B, Gutiérrez JM. Venoms, venomics, antivenomics. FEBS Lett. 2009;583:1736–43. doi: 10.1016/j.febslet.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 101.Fox JW, Serrano SMT. Structural considerations of the snake venom metalloproteinases, key members of the M12 reprolysin family of metalloproteinases. Toxicon. 2005;45:969–85. doi: 10.1016/j.toxicon.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 102.Dobzhansky T. Nothing in biology makes sense except in the light of evolution. Am Biol Teach. 1973;35:125–9. [Google Scholar]
- 103.Angulo Y, Escolano J, Lomonte B, Gutiérrez JM, Sanz L, Calvete JJ. Snake venomics of Central American pitvipers. Clues for rationalizing the distinct envenomation profiles of Atropoides nummifer and Atropoides picadoi. J Proteome Res. 2008;7:708–19. doi: 10.1021/pr700610z. [DOI] [PubMed] [Google Scholar]
- 104.Gutiérrez JM, Sanz L, Flores-Díaz M, Figueroa L, Madrigal M, Herrera M, et al. Impact of regional variation in Bothrops asper snake venom on the design of antivenoms: integrating antivenomics and neutralization approaches. J Proteome Res. 2010;9:564–77. doi: 10.1021/pr9009518. [DOI] [PubMed] [Google Scholar]
- 105.Boldrini-França J, Corrêa-Netto C, Silva MMS, Rodrigues RS, De La Torre P, Pérez A, et al. Snake venomics and antivenomics of Crotalus durissus subspecies from Brazil: assessment of geographic variation and its implication on snakebite management. J Proteomics. 2010;73:1758–76. doi: 10.1016/j.jprot.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 106.Massey DJ, Calvete JJ, Sánchez EE, Sanz L, Richards K, Curtis R, et al. Venom variability and envenoming severity outcomes of the Crotalus scutulatus scutulatus (Mojave rattlesnake) from Southern Arizona. J Proteomics. 2012;75:2576–87. doi: 10.1016/j.jprot.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 107.Alape-Girón A, Sanz L, Escolano J, Flores-Díaz M, Madrigal M, Sasa M, et al. Snake venomics of the lancehead pitviper Bothrops asper. Geographic, individual and ontogenetic variations. J Proteome Res. 2008;7:3556–71. doi: 10.1021/pr800332p. [DOI] [PubMed] [Google Scholar]
- 108.Lomonte B, Rey-Suárez P, Tsai W-C, Angulo Y, Sasa M, Gutiérrez JM, et al. Snake venomics of the pitvipers Porthidium nasutum, Porthidium ophryomegas, and Cerrophidion godmani from Costa Rica: toxicological and taxonomical insights. J Proteomics. 2012;75:1675–89. doi: 10.1016/j.jprot.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 109.Herrera M, Fernández J, Vargas M, Villalta M, Segura A, León G, et al. Comparative proteomic analysis of the venom of the snake taipan, Oxyuranus scutellatus, from Papua New Guinea and Australia: Role of neurotoxic and procoagulant effects in venom toxicity. J Proteomics. 2012;75:2128–40. doi: 10.1016/j.jprot.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 110.Lomonte B, Tsai W-C, Bonilla F, Solórzano A, Solano G, Angulo Y, et al. Snake venomics and toxicological profiling of the arboreal pitviper Bothriechis supraciliaris from Costa Rica. Toxicon. 2012;59:592–9. doi: 10.1016/j.toxicon.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 111.Chippaux J-P, Williams V, White J. Snake venom variavility: methods of study, results and interpretation. Toxicon. 1991;29:1279–303. doi: 10.1016/0041-0101(91)90116-9. [DOI] [PubMed] [Google Scholar]
- 112.Calvete JJ, Sanz L, Cid P, De La Torre P, Flores-Díaz M, dos Santos MC, et al. Snake venomics of the Central American rattlesnake Crotalus simus and the South American Crotalus durissus complex points to neurotoxicity as an adaptive paedomorphic trend along Crotalus dispersal in South America. J Proteome Res. 2010;9:528–44. doi: 10.1021/pr9008749. [DOI] [PubMed] [Google Scholar]
- 113.Calvete JJ, Pérez A, Lomonte B, Sánchez EE, Sanz L. Snake venomics of Crotalustigris: the minimalist toxin arsenal of the deadliest neartic rattlesnake venom. Evolutionary clues for generating a pan-specific antivenom against crotalid type II venoms. J Proteome Res. 2012;11:1382–90. doi: 10.1021/pr201021d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gibbs HL, Sanz L, Chiucchi JE, Farrell TM, Calvete JJ. Proteomic analysis of ontogenetic and diet-related changes in venom composition of juvenile and adult Dusky Pigmy rattlesnakes (Sistrurus miliarius barbouri) J Proteomics. 2011;74:2169–79. doi: 10.1016/j.jprot.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 115.Madrigal M, Sanz L, Flores-Diaz M, Sasa M, Núñez V, Alape-Girón A, et al. Snake venomics across genus Lachesis. Ontogenetic changes in the venom composition of L. stenophrys and comparative proteomics of the venoms of adult L. melanocephala and L. acrochorda. J Proteomics. 2012;77:280–97. doi: 10.1016/j.jprot.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 116.Wooten JA, Gibbs HL. Niche divergence and lineage diversification among closely related Sistrurus rattlesnakes. J Evol Biol. 2012;25:317–28. doi: 10.1111/j.1420-9101.2011.02426.x. [DOI] [PubMed] [Google Scholar]
- 117.Núñez V, Cid P, Sanz L, De La Torre P, Angulo Y, Lomonte B, et al. Snake venomics and antivenomics of Bothrops atrox venoms from Colombia and the Amazon regions of Brazil, Perú and Ecuador suggest the occurrence of geographic variation of venom phenotype by a trend towards paedomorphism. J Proteomics. 2009;73:57–78. doi: 10.1016/j.jprot.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 118.Lomonte B, Escolano J, Fernández J, Sanz L, Angulo Y, Gutiérrez JM, et al. Snake venomics and antivenomics of the arboreal neotropical pitvipers Bothriechis lateralis and Bothriechis schlegelii. J Proteome Res. 2008;7:2445–57. doi: 10.1021/pr8000139. [DOI] [PubMed] [Google Scholar]
- 119.Pla D, Gutiérrez JM, Calvete JJ. Second generation snake antivenomics: comparing immunoaffinity and immunodepletion protocols. Toxicon. 2012;60:688–99. doi: 10.1016/j.toxicon.2012.04.342. [DOI] [PubMed] [Google Scholar]
- 120.Calvete JJ. Antivenomics and venom phenotyping: A marriage of convenience to address the performance and range of clinical use of antivenoms. Toxicon. 2012;56:1284–91. doi: 10.1016/j.toxicon.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 121.Fahmi L, Makran B, Pla D, Sanz L, Oukkache N, Lkhider M, et al. Venomics and antivenomics profiles of North African Cerastes cerastes and C. vipera populations reveals a potentially important therapeutic weakness. J Proteomics. 2012;75:2442–53. doi: 10.1016/j.jprot.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 122.Makran B, Fahmi L, Pla D, Sanz L, Oukkache N, Lkhider M, et al. Snake venomics of Macrovipera mauritanica from Morocco, and assessment of the para-specific immunoreactivity of an experimental monospecific and a commercial antivenoms. J Proteomics. 2012;75:2431–41. doi: 10.1016/j.jprot.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 123.Calvete JJ, Cid P, Sanz L, Segura A, Villalta M, Herrera M, et al. Antivenomic assessment of the immunological reactivity of EchiTAb-Plus-ICP, an antivenom for the treatment of snakebite envenoming in sub-Saharan Africa. Am J Trop Med Hyg. 2010;82:1194–201. doi: 10.4269/ajtmh.2010.09-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Simpson ID, Norris NL. Snakes of medical importance in India: is the concept of the “Big 4” still relevant and useful? Wilderness Environ Med. 2007;18:2–9. doi: 10.1580/06-weme-co-023r1.1. [DOI] [PubMed] [Google Scholar]
- 125.Risch M, Georgieva D, von Bergen M, Jehmlich N, Genov N, Arni RK, et al. Snake venomics of the Siamese Russell's viper (Daboia russelii siamensis)- relation to pharmacological activities. J Proteomics. 2009;72:256–69. doi: 10.1016/j.jprot.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 126.Warrell DA. Russell's viper: biology, venom and treatment of bites. Trans R Soc Trop Med Hyg. 1989;83:732–40. doi: 10.1016/0035-9203(89)90311-8. [DOI] [PubMed] [Google Scholar]
- 127.Campbell JA, Lamar WW. Ithaca (NY) and London: Comstock Publishing Associates; 2004. The Venomous Reptiles of the Western Hemisphere. [Google Scholar]
- 128.Jayanthi JP, Gowda TV. Geographical variation in India in the composition and lethal potency of Russell's viper (Vipera russelii) venom. Toxicon. 1988;26:257–64. doi: 10.1016/0041-0101(88)90216-4. [DOI] [PubMed] [Google Scholar]
- 129.Mukherje AK, Ghosal SK, Maity CR. Some biochemical properties of Russell's viper (Daboia russelii) venom from Eastern India: correlation with clinico-pathological manifestation in Russell's viper bite. Toxicon. 2000;38:163–75. doi: 10.1016/s0041-0101(99)00125-7. [DOI] [PubMed] [Google Scholar]
- 130.Harris JB. Snake venoms in science and clinical medicine. Neuropharmacological aspects of the activity of snake venoms. Trans R Soc Trop Med Hyg. 1989;83:745–7. doi: 10.1016/0035-9203(89)90313-1. [DOI] [PubMed] [Google Scholar]
- 131.Bon C, Changeux J-P. Chemical and pharmacological characterization of toxic polypeptides from the venom of Bungarus caeruleus. Eur J Biochem. 1977;74:31–42. doi: 10.1111/j.1432-1033.1977.tb11363.x. [DOI] [PubMed] [Google Scholar]
- 132.Abe T, Alemá S, Miledi R. Isolation and characterization of presynaptically acting neurotoxins from the venom of Bungarus snakes. Eur J Biochem. 1977;80:1–12. doi: 10.1111/j.1432-1033.1977.tb11849.x. [DOI] [PubMed] [Google Scholar]
- 133.Rowan EG. What does β-bungarotoxin do at the neuromuscular junction? Toxicon. 2001;39:107–18. doi: 10.1016/s0041-0101(00)00159-8. [DOI] [PubMed] [Google Scholar]
- 134.Shashidharamurthy R, Jagadeesha DK, Girish KS, Kemparaju K. Variation in biochemical and pharmacological properties of Indian cobra (Naja naja) venom due to geographical distribution. Mol Cell Biochem. 2002;229:93–101. doi: 10.1023/a:1017972511272. [DOI] [PubMed] [Google Scholar]
- 135.Mukherjee AK, Maity CR. Biochemical composition, lethality and pathophysiology of venom from two cobras, N. naja and N. kaouthia. Comp Biochem Physiol B. 2002;131:125–32. doi: 10.1016/s1096-4959(01)00473-0. [DOI] [PubMed] [Google Scholar]
- 136.Casewell NR, Harrison RA, Wüster W, Wagstaff SC. Comparative venom gland transcriptome surveys of the saw-scaled vipers (Viperidae: Echis) reveal substantial intra-family gene diversity and novel venom transcripts. BMC Genomics. 2009;10:564. doi: 10.1186/1471-2164-10-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Casewell NR, Wagstaff SC, Harrison RA, Wüster W. Gene Tree parsimony of multilocus snake venom protein families reveals species tree conflict as a result of multiple parallel gene loss. Mol Biol Evol. 2011;28:1157–72. doi: 10.1093/molbev/msq302. [DOI] [PubMed] [Google Scholar]
- 138.Pook CE, Joger U, Stümpel N, Wüster W. When continents collide: Phylogeny, historical biogeography and systematics of the medically important viper genus Echis (Squamata: Serpentes: Viperidae) Mol Phylogenet Evol. 2009;53:792–807. doi: 10.1016/j.ympev.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 139.Davy J. London: Longman, Hurst, Rees and Brown; 1821. An account of the interior of Ceylon. [Google Scholar]
- 140.Maduwage K, Hodgson WC, Konstantakopoulos C, O’Leary MA, Gawarammana I, Isbister GK. The in vitro toxicity of venoms from South Asian Hump-nosed pit vipers (Viperidae: Hypnale) J Venom Res. 2011;2:17–23. [PMC free article] [PubMed] [Google Scholar]
- 141.Kumar V, Sabitha P. Inadequacy of present polyspecific anti snake venom - a study from central Kerala. Indian J Pediatr. 2011;78:1125–8. doi: 10.1007/s12098-011-0396-y. [DOI] [PubMed] [Google Scholar]
- 142.Vidal N, Lecointre G. Weighting and congruence: a case study based on three mitochondrial genes in pit vipers. Mol Phylogenet Evol. 1998;9:366–74. doi: 10.1006/mpev.1998.0509. [DOI] [PubMed] [Google Scholar]
- 143.Tan CH, Leong PK, Fung SY, Sim SM, Ponnudurai G, Ariaratnam C, et al. Cross neutralization of Hypnale hypnale (hump-nosed pit viper) venom by polyvalent and monovalent Malayan pit viper antivenoms in vitro and in a rodent model. Acta Trop. 2011;117:119–24. doi: 10.1016/j.actatropica.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 144.Grasset E. Survey of assay methods of antivenins; immunological factors influencing antivenin standardization. Bull World Health Organ. 1957;16:79–122. [PMC free article] [PubMed] [Google Scholar]
- 145.Sharma N, Chauhan S, Faruqui S, Bhat P, Varma S. Snake envenomation in a north Indian hospital. Emerg Med J. 2005;22:118–20. doi: 10.1136/emj.2003.008458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ariaratnam CA, Sjostrom L, Raziek Z, Kularatne SA, Arachchi RW, Sheriff MH, et al. An open, randomized comparative trial of two antivenoms for the treatment of envenoming by SriLankan Russell's viper (Daboia russelii russelii) Trans R SocTrop Med Hyg. 2001;95:74–80. doi: 10.1016/s0035-9203(01)90339-6. [DOI] [PubMed] [Google Scholar]
- 147.Phillips RE, Theakston RD, Warrell DA, Galigedara Y, Abeysekera DT, Dissanayaka P, et al. Paralysis, rhabdomyolysis and haemolysis caused by bites of Russell's viper (Vipera russelli pulchella) in Sri Lanka: failure of Indian (Haffkine) antivenom. Q J Med. 1988;68:691–715. [PubMed] [Google Scholar]
- 148.Theakston RD, Phillips RE, Warrell DA, Galagedera Y, Abeysekera DT, Dissanayaka P, et al. Envenoming by the common krait (Bungarus caeruleus) and Sri Lankan cobra (Naja naja naja): efficacy and complications of therapy with Haffkineantivenom. Trans R Soc Trop Med Hyg. 1990;84:301–8. doi: 10.1016/0035-9203(90)90297-r. [DOI] [PubMed] [Google Scholar]
- 149.Mirtschin PJ, Dunstan N, Hough B, Hamilton E, Klein S, Lucas J. Venom yields from Australian and some other species of snakes. Ecotoxicology. 2006;15:531–8. doi: 10.1007/s10646-006-0089-x. [DOI] [PubMed] [Google Scholar]
- 150.Prasarnpun S, Walsh J, Awad SS, Harris JB. Envenoming bites by kraits: the biological basis of treatment resistant neuromuscular paralysis. Brain. 2005;128:2987–96. doi: 10.1093/brain/awh642. [DOI] [PubMed] [Google Scholar]
- 151.Pe T, Cho KA. Amount of venom injected by Russell's viper (Vipera russelli) Toxicon. 1986;24:730–3. doi: 10.1016/0041-0101(86)90037-1. [DOI] [PubMed] [Google Scholar]


